Abstract
Three strains of Sphingomonas were grown as biofilms and tested for binding of five fluorescently labeled lectins (Con A-type IV-TRITC or -Cy5, Pha-E-TRITC, PNA-TRITC, UEA 1-TRITC, and WGA-Texas red). Only ConA and WGA were significantly bound by the biofilms. Binding of the five lectins to artificial biofilms made of the commercially available Sphingomonas extracellular polysaccharides was similar to binding to living biofilms. Staining of the living and artificial biofilms by ConA might be explained as binding of the lectin to the terminal mannosyl and terminal glucosyl residues in the polysaccharides secreted by Sphingomonas as well as to the terminal mannosyl residue in glycosphingolipids. Staining of the biofilms by WGA could only be explained as binding to the Sphingomonas glycosphingolipid membrane, binding to the cell wall, or nonspecific binding. Glycoconjugation of ConA and WGA with the target sugars glucose and N-acetylglucosamine, respectively, was used as a method for evaluation of the specificity of the lectins towards Sphingomonas biofilms and Sphingomonas polysaccharides. Our results show that the binding of lectins to biofilms does not necessarily prove the presence of specific target sugars in the extracellular polymeric substances (EPS) in biofilms. The lectins may bind to non-EPS targets or adhere nonspecifically to components of the biofilm matrix.
Lectins are a group of diverse proteins which bind to specific configurations of sugar residues. The binding of lectins to sugar residues present in polysaccharides resembles the specific binding of antibodies to antigens (4). Lectins have been widely used to characterize surfaces of eucaryotic cells and polysaccharides. Recently, fluorescently labeled lectins have been applied in the study of biofilm formation and biofilm composition. Excretion of adhesive polymers during attachment of bacterial cells to surfaces has been described using a panel of fluorescent lectins (9, 14, 20). The formation of biofilms on living and nonliving surfaces has been investigated with lectins (17). Lectins in conjunction with confocal laser scanning microscopy (CLSM) have been valuable tools in the study of the three-dimensional structure of biofilms (12, 15) or of the composition of extracellular polymeric substances (EPS) involved in accumulation of chlorinated organic compounds (24). Strains of Sphingomonas spp. are known for their interesting catabolic capabilities to degrade a wide variety of environmentally hazardous compounds, including polycyclic aromatics (25), dioxine compounds (6), and chlorinated phenols (3). Theoretically, lectins may be used to study the interaction between Sphingomonas cells and environmental surfaces during biofilm formation or to investigate the interaction of EPS with organic compounds. The common approach has been to deduce the structure or composition of biofilm EPS on the basis of the specific binding of lectins to different sugar residues. In this study, we evaluate the use of lectins for the characterization of Sphingomonas biofilms by investigating the binding of five fluorescent lectins with known specificities to Sphingomonas biofilms and to industrially produced Sphingomonas exopolysaccharides (sphingans) with known molecular structures.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Sphingomonas paucimobilis EPA505 was obtained from J. Mueller (19), and Sphingomonas sp. strain LH128 and Sphingomonas sp. strain LB126 were received from L. Bastiaens (1). All strains were stored in 43% glycerol at −80°C. The bacteria were grown at room temperature in phosphate minimal medium supplemented with glucose as the sole carbon source (PMMG) containing (in grams/liter) the following: glucose, 2; Na2HPO4 · 2H2O, 0.875; KH2PO4, 0.1; (NH4)2SO4, 0.25; MgCl2 · 6H2O, 0.05; CaCl2 · 2H2O, 0.015; NaNO3, 0.018. The medium was amended with 5 ml of a trace element solution consisting of (in milligrams/liter) the following: Na-EDTA, 800; FeCl2, 300; MnCl2 · 4H2O, 10; CoCl2 · 6H2O, 4; CuSO4, 1; Na2MoO4 · 2H2O, 3; ZnCl2, 2; LiCl, 0.5; SnCl2 · 2H2O, 0.5; H3BO3, 1; KBr, 2; KI, 2; BaCl2, 0.5. Phosphate and glucose were autoclaved separately.
Cultivation of biofilms on microscope slides.
Single-species biofilms were grown on Cel-Line HTC printed microscope slides with six wells on each slide (Cel-Line Associates, Inc., Newfield, N.J.). The slides were sterilized overnight in an oven at 200°C and transferred to sterile petri dishes. One drop of a bacterial culture grown overnight in PMMG was added to each well, and the cells were allowed to attach to the surface for 1 h. PMMG was gently poured into the petri dishes to cover the slides with medium. The petri dishes with the slides were incubated on a slowly tilting table (Heidolph Duomax, Kelheim, Germany) for 3 days (LB126 and LH128) or 5 days (EPA505).
Binding of lectins to Sphingomonas biofilms.
Each strain was tested for binding of the five fluorescently labeled lectins: Con A-TRITC type IV (Canavalia ensiformis), Pha-E-TRITC (Phaseolus vulgaris), PNA-TRITC (Arachis hypogaea), UEA 1-TRITC (Ulex europaeus), all obtained from Sigma (Deisenhofen, Germany), and WGA-Texas red (Triticum vulgaris) obtained from Molecular Probes (Eugene, Oreg.). The affinity, the molecular weight, and the fluorophore content of the lectins are listed in Table 1. The biofilms were checked for contamination by streaking 10 μl of the overlying growth medium on Luria-Bertani plates. Slides with biofilms were washed in phosphate-buffered saline (PBS) (pH 6.8) consisting of (in grams/liter) the following: Na2HPO4, 0.2; NaH2PO4, 1.44; NaCl, 8.0; KCl, 0.2; CaCl2, 0.011; MnCl2, 0.013. The areas around the wells were blotted dry. Lectin stock solutions of 1.0 or 2.5 mg/ml were prepared by dissolving lectins in PBS containing 0.05% NaN3 to prevent microbial growth. Working solutions of 0.1 mg of lectin per ml were made by dilution of lectin stocks in PBS containing the nucleic acid stain PicoGreen (Molecular Probes) in a 1:500 dilution. Working solutions were centrifuged at 8,000 × g for 5 min before use. The supernatant (10 μl) was added to each well, and the slides were incubated for 15 min in the dark on a piece of wet paper in a petri dish. Biofilms incubated with PBS containing PicoGreen were used as controls. After incubation, each well was washed with 1 ml of PBS, the slides were immersed for 30 min in PBS in the dark, and slides were examined by epifluorescence microscopy.
TABLE 1.
Specificity of lectins
| Lectin | Fluorescent label | Specificity | Mol wt (103) | Cfluorophore/Clectin (mol/mol) |
|---|---|---|---|---|
| UEA1 | TRITC | α-l-Fucosea | 68 | 4.1 |
| Pha-E | TRITC | Galactoseb | 128 | 1.2 |
| ConA | TRITC | Terminal α-d-mannosec | 102 | 1.0 |
| Cy5 | Terminal α-d-glucosec | |||
| PNA | TRITC | β-Galactose(1→3)N-acetylgalactosaminea | 120 | 2.0 |
| WGA | Texas red | N-Acetyl-β-d-glucosamine,adN-acetylneuraminic acid,d and N-acetylmuraminic acide | 36 | 2.1 |
In order to check the washing efficiency and to eliminate the possibility of unspecific signals due to insufficient washing times, Sphingomonas sp. strain LH128 biofilms were grown on slides and stained with the lectins ConA and WGA. The control slides were transferred to fresh PBS every 30 min for a total of 30, 60, and 90 min in PBS in the dark and were prepared in such a way that all slides were ready for microscopy at the same time. Two wells were used for each treatment.
Lectins tagged with TRITC and Texas red were observed with Zeiss filter set No. 15 (excitation filter BP 546/12, beamsplitter FT 580, and emission filter LP 590). PicoGreen was observed with Zeiss filter set No. 09 (excitation filter BP 450-490, beamsplitter FT 510, and emission filter LP 520). The labeling procedure differs from other protocols (9, 12, 15, 16) by using 0.1 mg of lectin/ml rather than 1 to 5 mg/ml and also by using longer wash times.
Preincubation of lectins with target sugars.
Binding sites of ConA were blocked with the target sugar d-glucose (4, 22), and the binding sites of WGA were blocked with the target sugar N-acetyl-d-glucosamine (23). Lectin stock solutions were diluted with sugar stock solutions to final concentrations of 1.0 or 0.1 mg/ml for lectins and 10 or 50 mg/ml for sugars (Table 2). The mixtures were incubated in the dark for 1 h, and the affinities of the lectins for EPA505 biofilms were assayed as described above, using the lectin-sugar solutions as working solutions. PicoGreen was included in the working solutions in a 1:500 dilution as a counterstain. Wells with no sugars, no lectins, or no sugars and no lectins were used as controls.
TABLE 2.
Effect of blocking ConA and WGA sugar binding sites with target sugars on binding of the lectins to EPA505 biofilmsa
| Lectin concn (mg/ml) | Target sugar concn (mg/ml) | Binding of ConA | Binding of WGA |
|---|---|---|---|
| 1.0 | 0 | +++ | +++ |
| 1.0 | 50 | +++ | +++ |
| 0.1 | 0 | ++ | ++ |
| 0.1 | 10 | + | + |
| 0.1 | 50 | + | + |
| 0 | 50 | − | − |
| 0 | 0 | − | − |
Ratings are as follows: +++, strong fluorescence; ++, medium fluorescence; +, low fluorescence; −, no fluorescence.
Binding of ConA and WGA to commercially available sphingans.
Three of the Sphingomonas exopolysaccharides (sphingans), gellan, welan, and rhamsan, were produced by Kelco, Inc. (San Diego, Calif.). Clarified gellan was purchased from Sigma under the trade name Phytagel. Industrial grade welan and rhamsan were a gift from Mikael Grathwohl (Ringsted og Semler A/S, Copenhagen, Denmark). The sphingans (250 μg per ml) were hydrated in Tris buffer (20 μM Tris-HCl, 10 μM CaCl2, pH 6.8) for 1 h and dissolved by autoclaving at 121°C for 5 min. The resulting solutions were centrifuged at 15,000 × g for 10 min. Printed microscope slides were coated with the sphingans by adding 50 μl of sphingan solution to each well and evaporating the water at 110°C for 2 h. The slides were washed in distilled water and stained with ConA and WGA as described above, omitting PicoGreen from the staining solutions.
Semiquantitative estimation of the binding of fluorescent lectins to biofilms or to commercially available polysaccharides.
The degree of binding of lectins to biofilms was estimated simply by looking at the biofilms with the microscope in the fluorescence mode. The fluorescence intensities were divided into groups showing strong fluorescence (+++), medium fluorescence (++), low fluorescence (+), and no fluorescence (−). In each experiment, a positive control slide (ConA, strong fluorescence) and a negative control slide (biofilm without lectin, no fluorescence) were compared to the biofilms under investigation. In the experiments with gellan, welan, and rhamsan, all three polysaccharides were compared to each other at the same time by switching back and forth between the slides. Biofilms were grown and screened at least twice. For replicates with divergent ratings, the experiments were done in triplicate.
Cultivation of biofilms in flow cells.
The bacteria were cultivated as continuous cultures in flow cells, which could be fitted directly on a microscope table for noninvasive analysis of the biofilms. Four parallel flow cells, each 4 by 7 by 40 mm, were drilled in stainless steel and fitted with short inlet and outlet tubes also made of stainless steel. The flow cells were assembled by applying silicone rubber around each channel and adding glass coverslips on top of the silicone. Silicone tubing was connected to the inlets and outlets, and the flow system was autoclaved. Medium was pumped through the sterile flow system with a multichannel peristaltic pump at a rate of 5 ml/h. The flow cells were inoculated by stopping the flow, clamping off the inlet tubes, and injecting 0.3 ml of overnight cultures into the flow cells through the inlet tubes by using 1-ml syringes with thin hypodermic needles. The flow of the medium was restarted after 1 h, and the flow cells were incubated for 3 days at room temperature.
Lectin staining of biofilms in flow cells.
ConA (Sigma) was tagged with the fluorophore Cy5 by using an Amersham Life Science FluoroLink Cy5 AB-Labelling Kit (Amersham Pharmacia Biotech UK, Buckinghamshire, England) as described by the manufacturer. The flow cells with the biofilms were washed with PBS for 30 min at a flow rate of 15 ml per h, and the liquid content of each flow cell was displaced by injection of 1.3 ml of lectin working solution through the inlet tubes. After 30 min of incubation, the flow cells were washed for 30 min at a flow rate of 15 ml per h. Stacks of digital images based on lectin and PicoGreen fluorescence were collected from selected areas of the biofilms with a CLSM 410 confocal laser scanning microscope coupled to an Axiovert 135M inverted microscope (both instruments from C. Zeiss, Jena, Germany). Images were generated using a 40×, 1.3 numerical aperture oil immersion lens, a pinhole value of 20 or 22, and a digital zoom factor of 2.5 or 5.0. WGA-Texas red images were collected using the 543-nm laser line and an LP590 emission filter. ConA-Cy5 was excited with the 633-nm laser line and collected using a LP 665 emission filter. PicoGreen images were obtained using the 488-nm laser line and a BP515-540 emission filter. The image dynamics were improved digitally with the computer program Adobe Photoshop 3.0.
RESULTS AND DISCUSSION
Binding of lectins to Sphingomonas biofilms and to commercially available sphingans.
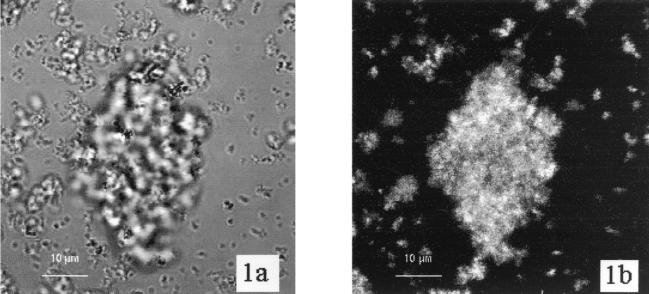
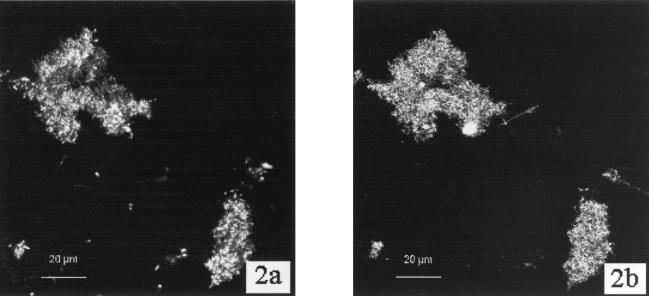
Biofilms of the three Sphingomonas strains were analyzed for binding of the five lectins shown in Table 1. The reproducibility of results was generally high. The binding of WGA to strain LH128 (Fig. 1) and of ConA to strain EPA505 (Fig. 2) demonstrate that fluorescence from WGA and ConA is closely associated with the cells and that the strongest fluorescence emanated from groups of cells and microcolonies. Control experiments with PicoGreen alone gave no signals with the CLSM settings used for WGA-Texas red and ConA-Cy5.
FIG. 1.
(A) CLSM transmission image of a microcolony of Sphingomonas sp. strain LH128 grown as a biofilm in a flow cell. (B) CLSM-extended depth of focus image of the same microscopic field showing fluorescence from the Texas red-labeled WGA lectin. The image was constructed from 15 xy sections with a vertical distance of 2 μm between successive images. Scale bar, 10 μm.
FIG. 2.
(A) CLSM-extended depth of focus image of two microcolonies of S. paucimobilis strain EPA505 stained with the nucleic acid stain PicoGreen. (B) CLSM-extended depth of focus image of the same microscopic field showing ConA-TRITC fluorescence. Both images are projections constructed from 20 xy sections with a vertical distance of 2 μm between successive images. Scale bar, 20 μm.
The results of the lectin binding were similar among the strains (Table 3), showing strong fluorescence from ConA and medium fluorescence from WGA. In addition, Pha-E gave a weak but reproducible signal from biofilms of strain LB126 and strain EPA505, and PNA gave a weak signal from strain LB126. Controls without lectins were negative. Washing controls using strain LH128 showed that strong lectin-conferred fluorescence persisted even after repeated washing steps in PBS (Table 4). The relatively low fluorescence (++) seen for ConA after 30 min (Table 4) is difficult to explain but is probably due to random variation.
TABLE 3.
Binding of lectins to living Sphingomonas biofilmsa
| Lectin | Sphingomonas sp. strain LB126 | S. paucimobilis EPA505 | Sphingomona sp. strain LH128 |
|---|---|---|---|
| ConA | +++ | +++ | +++ |
| PNA | + | − | − |
| Uea1 | <+ | <+ | <+ |
| Pha-E | + | + | <+ |
| WGA | ++ | ++ | ++ |
| Control (without lectin) | − | − | − |
Ratings are as follows: +++, strong fluorescence; ++, medium fluorescence; +, low fluorescence; −, no fluorescence.
TABLE 4.
Binding of lectins to living Sphingomonas sp. strain LH128 biofilms using extended washing periodsa
| Washing time (min)b | ConA | WGA | Control (without lectin) |
|---|---|---|---|
| 30 | ++ | ++ | |
| 60 | +++ | ++ | − |
| 90 | +++ | ++ | − |
Ratings are as follows: +++, strong fluorescence; ++, medium fluorescence; +, low fluorescence; −, no fluorescence.
The slides were transferred to fresh PBS every 30 min.
We have estimated the binding of fluorescent lectins to biofilms by microscopically observing the biofilms in the fluorescence mode and dividing them into groups showing strong fluorescence, medium fluorescence, low fluorescence, and no fluorescence. The fluorescence conferred by bound ConA was considered to be a positive reference since it showed strong fluorescence in all experiments. In addition, a negative reference (biofilm without lectin) was also set up. All observed biofilms were always compared to the positive and negative references, and the evaluation of the fluorescence was based on parallel repeats of the experiments. Thus, even though this is a very subjective assay and should only be regarded as semiquantitative, it is a very quick and easy assay resulting in generally similar estimates among replicates.
The fluorophore-to-lectin ratio varied from 1.2 for PhaE to 4.1 for UEA-1 (Table 1), so one should be cautious when comparing the binding efficiencies of different lectins to biofilms of the same species by estimating fluorescence intensity (Table 3). This is especially true for WGA-Texas red, which has excitation and emission wavelengths which are different from those of TRITC and are suboptimal for the filter combinations used.
The binding of ConA and WGA to artificial biofilms of the sphingans gellan, welan, and rhamsan is shown in Table 5. Incubation of ConA with welan films resulted in high fluorescence, whereas incubation with gellan and rhamsan gave medium fluorescence. Incubation of WGA with welan, gelan, and rhamsan films showed medium fluorescence.
TABLE 5.
Binding of ConA and WGA to industrially produced sphingansa
| Sphingan | ConA | WGA | Control (without lectin) |
|---|---|---|---|
| Gellan | ++ | ++ | − |
| Welan | +++ | ++ | − |
| Rhamsan | ++ | ++ | − |
Ratings are as follows: +++, strong fluorescence; ++, medium fluorescence; −, no fluorescence.
The low fluorescence from PhaE-TRITC in the living and in the artificial biofilms could be a result of the low fluorophore/lectin ratio (Cfluorophore/Clectin = 1.2), but it also agrees well with low binding to sphingans since PhaE binds preferentially to galactose residues within polysaccharides (13) and consequently would not be expected to bind to Sphingomonas exopolysaccharides. The very low fluorescence from UEA-1 (Cfluorophore/Clectin = 4.1) and PNA (Cfluorophore/Clectin = 2.0), despite the high fluorophore-to-lectin ratios, clearly demonstrates that α-l-fucose and β-galactose(1→3)N-acetyl-galactosamine are not present in the biofilms of the three Sphingomonas species tested or in the artificial biofilms.
Bacteria belonging to the genus Sphingomonas are known to secrete acidic exopolysaccharides (sphingans) that contain a common repeating backbone (21). The repeating tetrasaccharide is [→3)-β-d-Glc-(1→4)-β-d-GlcA-(1→4)-β-d-Glc-(1→4)-α-l-Rha or l-Man(1→], where Glc is glucose, GlcA is glucuronic acid, Rha is rhamnose, and Man is mannose. In addition, some of the polysaccharides contain mono- or disaccharide side chains consisting of α-l-Man, α-l-Rha, α-d-Glc, or β-d-Glc (2, 18).
In our experiments, ConA did bind to biofilms of the three Sphingomonas strains as well as the three artificial sphingan films. ConA-conferred fluorescence was not affected by increased washing times (Table 4). Goldstein et al. (4) reported that ConA reacts only with terminal units of dextrans, and that there is a linear relationship between the extent of branching of dextrans, i.e., the number of terminal residues, and the degree of binding of ConA to the dextran (4). ConA did not react with linear polysaccharides, even though they possessed the requisite α-d-glucosyl units (4). Binding of ConA to artificial welan and rhamsan films is expected since these sphingans contains side chains with terminal mannosyl and glucosyl residues (2, 18). Gellan, on the other hand, is a linear polysaccharide, and binding of ConA to artificial gellan films must be either nonspecific or specific binding to nonsphingan material.
The genus Sphingomonas is characterized by the lack of a lipopolysaccharide membrane and the presence of a glycosphingolipid membrane consisting of dihydrosphingosine coupled to glucuronic acid or the tetrasaccharide [α-d-Man- (1→3)-α-d-Gal-(1→6)-α-d-GlcN-(1→4)-α-d-GlcA-(1→], where Gal is galactose (10, 11). Binding of ConA to living Sphingomonas biofilms and to artificial sphingan biofilms might be explained as binding to the terminal mannosyl and terminal glycosyl residues in the exopolysaccharides as well as binding to the terminal mannosyl residue in the sphingolipids.
Strong and reproducible binding of WGA to the tested Sphingomonas strains (Table 3), even after extended washing periods (Table 4), and to the tested sphingans (Table 5) is somewhat intriguing since WGA binds to N-acetylglucosamine and substituted N-acetylglucosamines (Table 1), but these sugars are not present in the repeating units of any of the published sphingans (2, 21). In fact, the WGA-conferred fluorescent signal intensities did not decrease with increasing washing times (Table 4). WGA either binds to non-EPS N-acetylglucosamine residues in the biofilms or binding of WGA is nonspecific. Alternative binding sites in the biofilms could include the glucosaminyl residues in the tetrasaccharide part of the Sphingomonas glycosphingolipid membrane or N-acetylglucosamine and N-acetylmuramic acid from the peptidoglycan cell wall. This raises the general question of whether binding of lectins to complex biofilms does not involve sugars present in the biofilm EPS, but rather binding to sugars present in the lipopolysaccharide membranes of the gram-negative organisms of Bacteria or binding to other non-EPS sites.
The glycosphingolipid tetrasaccharide also contains galactose, and it could be argued that if WGA binds to the glycosphingolipids then PhaE should also bind. However, the binding efficiency of PhaE to galactosyl residues is greatly influenced by neighboring sugar residues in the polysaccharide (13), which may inhibit the binding of PhaE to sites in the glycosphingolipid.
Preincubation of lectins with target sugars.
The lectins ConA and WGA were preincubated with target sugars to block the binding sites as a confirmation of the specificity of the lectin binding to the EPA505 biofilms (Table 2). There was no reduction in the binding of ConA and WGA when using 1 mg of lectin/ml and 50 mg of target sugars/ml compared to that of the controls without target sugars. This indicates that the target sugar-to-lectin ratio was not sufficiently high to block the binding sites of the lectins. Reducing the lectin concentration to 0.1 mg/ml yielded a reduction in the lectin signal intensity, compared to that of bound lectin at 1.0 mg/ml. Adding target sugars at concentrations of 10 and 50 mg/ml to the working solutions with 0.1 mg/ml lectin decreased the binding of the lectin, but surprisingly there was still some binding of the lectins even at a target sugar concentration of 50 mg/ml. This suggests that some lectin was bound nonspecifically to the biofilms.
Inactivation of antibodies by “blinding” the binding site with a specific hapten is often used in antibody assays as a control experiment or as a means to determine the activity of an antibody solution (e.g., see reference 5). Likewise, glycoconjugate-blinded lectins, in which the binding sites of the lectins are blocked with target sugars, are recommended by Hartmann et al. (7) as control experiments for nonspecific binding of lectins to biofilms. Michael and Smith (17) found that emission from ConA-fluorescein isothiocyanate (ConA-FITC) was undetectable following coincubation of biofilms with ConA-FITC and the sugar competitor α-methyl mannoside.
Glycoconjugation is indeed used as a standard method for determining the specificity of lectins towards various sugars and polysaccharides (4). On the product information sheet, Sigma informs that agglutination by ConA of a 1.3% suspension of red blood cells could be inhibited by N-acetyl-d-glucosamine, sucrose, fructose, mannose, glucose, or methyl α-d-mannopyranoside at concentrations of 78 to 625 μg/ml. We observed no reduction in binding of 1.0 mg of ConA or WGA per ml to biofilms in the presence of 10 mg of glucose or N-acetyl glucosamine per ml (Table 2). Reducing the lectin concentration to 0.1 mg/ml, which is 10 to 50 times less than the recommended concentration (16), and increasing the target sugar concentration to 50 mg/ml resulted only in partial inhibition of the lectin binding. Our interpretation of these findings is that part of the bound lectin, namely, the part which does not bind in the presence of target sugars, is bound to specific sugar residues in the biofilm. Another part, the part which binds in the presence of target sugars, is bound nonspecifically to the biofilm. Our results show that binding of lectins to Sphingomonas biofilms does not necessarily prove the presence of specific target sugars in the biofilm EPS. The lectins might be bound to non-EPS targets or bound nonspecifically to the biofilm matrix.
ACKNOWLEDGMENTS
We thank Dirk Springael for critically reading the manuscript and for suggestions.
This work was supported in part by the Research Center for Fundamental Studies of Aerobic Biological Wastewater Treatment (SFB 411, Munich, Germany) and the EU Biotech Program (Contract BIO4-CT97-2015).
REFERENCES
- 1.Bastiaens, L., D. Springael, P. Wattiau, H. Harms, R. deWachter, H. Verachtert, and L. Diels. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 66:1834–1843. [DOI] [PMC free article] [PubMed]
- 2.Chandrasekaran R, Radha A. Molecular architectures and functional properties of gellan gum and related polysaccharides. Trends Food Sci Technol. 1995;6:143–148. [Google Scholar]
- 3.Edere M, Crawford R, Herwig R, Orser C. PCP degradation is mediated by closely related strains of the genus Sphingomonas. Mol Ecol. 1997;6:39–49. doi: 10.1046/j.1365-294x.1997.00151.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein I J, Hollerman C E, Merrick J M. Protein-carbohydrate interaction. I. The interaction of polysaccharides with concanavalin A. Biochim Biophys Acta. 1965;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- 5.Hahn A, Frimmel F, Haisch A, Henkelmann G, Hock B. Immunolabelling of atrazine residues in soil. Z Pflanzenernaehr Bodenkd. 1992;155:203–208. [Google Scholar]
- 6.Halden R U, Halden B G, Dwyer D F. Removal of dibenzofuran, dibenzo-p-dioxin, and 2-chlorodibenzo-p-dioxin from soils inoculated with Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1999;65:2245–2249. doi: 10.1128/aem.65.5.2246-2249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann A, Lawrence J R, Assmus B, Schloter M. Detection of microbes by laser confocal microscopy. Mol Microb Ecol Manual. 1998;4.1.10:1–34. [Google Scholar]
- 8.Haugland R P. Lectin conjugates. In: Spence M T Z, editor. Handbook of fluorescent probes and research chemicals. Eugene, Oreg: Molecular Probes, Inc.; 1999. pp. 141–142. [Google Scholar]
- 9.Hood M, Smidt J. The examination of Seliberia stellata exopolymers using lectin assays. Microb Ecol. 1996;31:281–290. doi: 10.1007/BF00171572. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara K, Seydel U, Matsuura M, Danbara H, Rietschel E T, Zaehringer U. Chemical structure of glycosphingolipids isolated from Sphingomonas paucimobilis. FEBS Lett. 1991;292:107–110. doi: 10.1016/0014-5793(91)80845-t. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki S, Moriguchi R, Sekiya K, Nakai T, Ono E, Kume K, Kawahara K. The cell-envelope structure of the lipopolysaccharide-lacking gram-negative bacterium Sphingomonas paucimobilis. J Bacteriol. 1994;176:284–290. doi: 10.1128/jb.176.2.284-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolari M, Matilla K, Mikkola R, Salkinoja-Salonen M S. Community structure of biofilms on ennobled stainless steel in Baltic sea water. J Ind Microbiol Biotechnol. 1998;21:261–274. [Google Scholar]
- 13.Kornfeld R, Kornfeld S. The structure of a phytohemeagglutinin receptor site from human erythrocytes. J Biol Chem. 1970;245:2536–2545. [PubMed] [Google Scholar]
- 14.Langille S E, Weiner R M. Spatial and temporal deposition of Hyphomonas strain VP-6 capsules involved in biofilm formation. Appl Environ Microbiol. 1998;64:2906–2913. doi: 10.1128/aem.64.8.2906-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence J R, Neu T R, Swerhone G D W. Application of multiple parameter imaging for the quantification of algal, bacterial and exopolymer components of microbial biofilms. J Microbiol Methods. 1998;32:253–261. [Google Scholar]
- 16.Lawrence J R, Wolfaardt G M, Neu T R. The study of biofilms using confocal laser scanning microscopy. In: Wilkinson M H F, Schut F, editors. Digital image analysis of microbes: morphometry, fluorometry and motility techniques and applications. New York, N.Y: John Wiley and Sons Ltd.; 1998. pp. 431–465. [Google Scholar]
- 17.Michael T, Smith C M. Lectins probe molecular films in biofouling: characterization of early films on non-living and living surfaces. Mar Ecol Prog Ser. 1995;119:229–236. [Google Scholar]
- 18.Moorhouse R. Structure/property relationships of a family of microbial polysaccharides. In: Yalpani M, editor. Industrial polysaccharides. Genetic engineering, structure/property relations and applications. Amsterdam, The Netherlands: Elsevier; 1987. pp. 187–206. [Google Scholar]
- 19.Mueller J G, Chapman P J, Blattmann B O, Pritchard P H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu T R, Marshall K C. Microbial “footprints”—a new approach to adhesive polymers. Biofouling. 1991;3:101–112. [Google Scholar]
- 21.Pollock T J. Gellan-related polysaccharides and the genus Sphingomonas. J Gen Microbiol. 1993;139:1939–1955. [Google Scholar]
- 22.Reeke G, Becker J, Cunningham B, Gunther G, Wang J, Edelman G. Relationships between the structure and activities of concanavalin A. Ann N Y Acad Sci. 1974;234:369–382. doi: 10.1111/j.1749-6632.1974.tb53049.x. [DOI] [PubMed] [Google Scholar]
- 23.Voet D, Voet J G. Biochemie. Weinheim, Germany: Wiley-VCH; 1994. p. 247. [Google Scholar]
- 24.Wolfaardt G, Lawrence J. In situ characterization of biofilm exopolymers involved in the accumulation of chlorinated organics. Microb Ecol. 1998;35:213–223. doi: 10.1007/s002489900077. [DOI] [PubMed] [Google Scholar]
- 25.Ye D, Siddiqi M A, Maccubbin A E, Kumar S, Sikka H C. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ Sci Technol. 1996;30:136–142. [Google Scholar]