Abstract
Introduction:
The association between red blood cell (RBC) transfusions and necrotizing enterocolitis (NEC), so-called transfusion-associated NEC (ta-NEC), was first described in 1987. However, further work is needed to confirm a causal relationship, elucidate underlying mechanisms, and develop possible strategies for prevention. We performed an extensive literature search in the databases PubMed, EMBASE, and Scopus.
Areas covered:
Although multiple retrospective human studies have strongly suggested an association between blood transfusions and subsequent occurrence of NEC, meta-analyses of randomized controlled trials (RCTs) testing RBC transfusion thresholds or the use of recombinant erythropoiesis-stimulating growth factors did not confirm an association of anemia with ta-NEC. These conflicting data necessitated the development of an animal model to elucidate mechanisms and causal factors. Data from this recent mouse model of ta-NEC highlighted the importance of sequential exposure to severe anemia followed by transfusion for development of ta-NEC.
Expert opinion:
This review summarizes current human and experimental data, highlights open questions, and suggests avenues for further research aimed at preventing ta-NEC in preterm infants. Further studies are required to delineate whether there is a tipping point, in terms of the level and duration of anemia, and to develop an effective strategy for blood management and the quality of RBC transfusions.
Keywords: Anemia, Necrotizing enterocolitis, Preterm infants, ta-NEC, TANEC, TRAGI, Transfusion
Introduction
Premature infants are a heavily transfused population.1,2 Up to 90% of extremely low birth weight infants, and approximately 60% of preterm infants born at <32 weeks of gestational age receive red blood cell (RBC) transfusions during the neonatal period.3 In several reports, up to 25–40% of infants who developed necrotizing enterocolitis (NEC) may have received RBC transfusion(s) in the preceding 48 hours, and this connection has been referred to as transfusion-associated NEC (ta-NEC).4–26 However, these results have not been conclusive in systematic reviews of case-control or observational studies.6,27–29 In contrast, ta-NEC has not been associated to anemia in RCTs on RBC transfusion thresholds and in studies on the efficacy of recombinant erythropoiesis-stimulating factors.30,31 These problems indicated a need for focused preclinical and clinical studies. However, the interest in the association between anemia and ta-NEC has been rekindled following a recent comprehensive animal study.32
Herein, we review the current data on ta-NEC and its possible mechanisms and then recommend future direction of research and clinical practice. We performed an extensive literature search in the databases PubMed, EMBASE, and Scopus. To minimize bias, key words from the medical subject heading thesaurus on PubMed were shortlisted prior to the actual search and combined with text words used in titles and abstracts.
RBC Transfusions as a Potential Trigger of NEC in Very Preterm Infants
The association between transfusions and NEC was first observed by McGrady et al.33 in 1987, who investigated an outbreak of 33 cases of NEC in their neonatal intensive care unit (NICU) and reported that many cases followed RBC transfusions (odds ratio [OR] = 15.1, confidence interval [CI]: [2.59–92.51]).33 In 1998, Bednarek et al.34 noted that NICUs with fewer transfusions had less NEC (OR = 0.3, CI: [0.1–0.8]). Several subsequent studies confirmed that 25–40% of very preterm infants who developed NEC had received one or more RBC transfusions in the preceding 2–48 hours, generally about 12 hours, before the onset of NEC.5,11,13,21,23,25,26,33–41 These studies suggested that ta-NEC occurred (a) in infants born at earlier gestational ages than those who developed NEC unrelated to transfusion;5,11,13,21,23,25,26,33–39 (b) at 3–5 weeks after birth, later than NEC unrelated to transfusions seen at 1–3 weeks;5,13,21,23,25,26,33–39 (c) in neonates who had received 1–3 RBC transfusions;5,25 and (d) in infants who may have had a higher acuity of illness prior to developing NEC.6,11,13,23,26,39,40 The postnatal age of infants who developed ta-NEC was higher than those who developed NEC without a temporally proximate transfusion.5,13,25 However, the storage age of donor blood transfused into infants who developed ta-NEC was not different from that in matched controls who did not develop NEC.4
Most studies of ta-NEC were based on a small number of patients, and therefore, lack generalizability and the statistical power to adjust for confounders. Therefore, there have been several efforts focused on meta-analysis of pooled data. In one of these early reports, Mohamed and Shah6 systematically reviewed 11 observational studies of NEC and confirmed increased odds of NEC within a 48-hour period following an RBC transfusion. Another meta-analysis of five studies showed increased risk of NEC following transfusions in the previous 48 hours (pooled OR = 3.91, 95% CI: [2.97–5.14]; I2 = 58%).5,11,21,25,42 A meta-analysis of four studies also showed increased risk of NEC (pooled OR = 2.01, 95% CI: [1.61–2.50]; I2 = 91%).25,38,43 Another study that combined seven case-control studies (480 blood transfusion cases, 2,845 control cases) showed similar results in a random-effects model (OR = 3.35, 95% CI: [1.54–7.27]).27 Sensitivity analysis showed an increased OR for NEC within 48 hours after transfusion at 4.21 (95% CI: [2.17–8.16]). The OR was 4.29 (95% CI: [1.39–13.24]) after factors such as gestational age and birth weight were deconfounded.27
The evidence for an association between RBC transfusions and NEC became less convincing following a comprehensive meta-analysis of 17 observational studies by Garg et al.,44 who did not find supportive results (OR = 0.96, 95% CI [0.53–1.71], p = 0.88) (Table 1). Rai et al.45 performed a meta-analysis with 10 studies, and actually noted a 45% reduction in the unadjusted odds of NEC in infants exposed to a recent RBC transfusion (OR = 0.55, 95% CI: [0.31–0.98]).
Table 1:
Summary of studies Included in the meta-analysis by Garg et al.
| Authors of study | Type of study | Gestational age | Birth weight | No. of infants case | No. of infants control | Hematocrit NEC | Odds ratio (OR, 95% confidence interval) RBC transfusion |
|---|---|---|---|---|---|---|---|
| Patel et al. | Case control | 27.9 ± 3.3 | 1015 ± 273 | 40 | 554 | For transfusion-0.4 (0.17–1.1), for anemia-5.9 (2–18; p = 0.001) | |
| AlFelah et al. | Retrospective case control | 28 | 1,042 | 40 | 112 | OR = 0.39, (0.18–0.84), p = 0.02 | |
| Sharma et al. | Case control | 27 ± 2 | 983 ± 333 | 42 | 42 | 1.4 (0.4–5.6) | |
| Wallenstein et al. | Retrospective cohort | 27 IQR 3 | 790 IQR 290 | 24 | 390 | 29 | 0.6 (0.2–1.7) |
| Bak et al. | Retrospective case control | 27.6 ± 2.2 | 1027 ± 343 | 18 | 162 | 46.9 ± 4.1 | 1.63 (1.14–2.3) |
| Gomez-Martin | Retrospective case-control | 30 | 30 | 1.5 (1.0–2.2) | |||
| Wan-Huen et al. | Case control | 26 ± 2.4 | 840 | 49 | 97 | 31.4 ± 3.7 | 3.0 (1.7–5.5) |
| Demirel et al. | Case control | 28.4 ± 1.4 | 1078 ± 236 | 96 | 551 | 30 ± 4.4 | OR not mentioned |
| Stritzke et al. | Case control | 25.8 ± 2.6 | 885 ± 446 | 927 | 2,781 | NA | 2.2 (1.8 0 2.8) |
| El-Dib et al. | Phase 1: Retrospective case-control; Phase 2: comparison study of incidence of NEC | 26.8 ± 2.5 | 935 ± 350 | 25 | 25 | NA | 5.1 (1.4–17.9) |
| Paul et al. | Case control | 26.8 ± 2.4 | 969 ± 239 | 33 | 30 | 28.6 ± 5.2 | 2.5 (0.8–8.3) |
| Singh et al. | Case control | 26.9 ± 2.5 | 969.7 ± 309.0 | 111 | 222 | 29.9 ± 5.6 | 5.6 (3.2–10.2) |
| Christensen et al. | Retrospective case control | 27 (26–28) | 981 (835–1,128) | 62 | 248 | Not mentioned | 11.8 (4.6–30.4) |
| Josephson et al. | Case control | 27.7 (25.7–30.7) | 1030 (740–1,410) | 93 | 91 | 31.8 ± 7.8 | 0.7 (0.4–1.3) |
| Binder et al. | <1,500 | 78 | 783 | 0.07 (0.03–0.14) | |||
| Harsono et al. | <1,500 | 43 | 2,080 | 0.3 (0.2–0.6) | |||
| Garg et al. | Retrospective cohort | 27.3 ± 2.5 | 992 ± 377 | 99 | 27.4 ± 4.5 | 2.83 (0.97–8.9) |
NEC, necrotizing enterocolitis; RBC, red blood cell; IQR, interquartile range; NA, not applicable
A prospective, matched-pair comparison of 42 NEC cases and their controls at three centers found no association between transfusions with NEC in the subsequent 48 h or 7 days.46 Elabiad et al.22 reviewed a large cohort of 3060 infants and identified 174 infants (5.7%) with NEC. They noted that 116 (67%) infants had been exposed to RBC transfusions; infants with BW ≤750; 751–1,000; 1,001–1,250; and 1,251–1,500 g had a relative risk of 0.14, 0.46, 1.83 and 1.78, respectively, to develop NEC following transfusions. They concluded that RBC transfusions significantly reduced the risk of NEC in ≤1,000 g infants, but noted a trend towards increased risk of NEC in infants with a birth weight of 1,001–1,500 g.
Anemia as Risk Factor for NEC in Very Preterm Infants
In a prospective, multicenter observational cohort study, Patel et al.12 evaluated 598 very low birthweight infants and noted that 44 (7.4%) developed NEC. In this cohort, however, 319 (53%) infants were exposed to RBC transfusions. The unadjusted cumulative incidence of NEC at 8 weeks after birth was 9.9% (95% CI [6.9–14.2%]), which was higher than the 4.6% (95% CI [2.6–8.0%], p = 0.02) incidence among those who were not transfused with RBCs (p = 0.02). In multivariable analysis, RBC transfusion in a given week was not significantly related to the rate of NEC (adjusted cause-specific hazard ratio 0.44 (95% CI [0.17–1.12]), p = 0.09). Based on the evaluation of 4,565 longitudinal measurements of hemoglobin concentrations (median = 7 g/dL per infant), they associated NEC with severe anemia (adjusted cause-specific hazard ratio 5.99 (95% CI [2.00–18.0]); p = 0.001), but not with RBC transfusions.12 A recent retrospective single-center cohort study in 207 extremely premature infants (23–27 wk gestation) identified a portion of 46% (13/28) of infants with ta-NEC and 54% (15/28) with non–ta-NEC. The incidence of ta-NEC, however, did not correlate with the number of antecedent pRBC transfusions or the pretransfusion median hemoglobin levels.40
These reports of the association of NEC with anemia were interesting. However, the impact of anemia on the risk of NEC remains uncertain. Two recent studies (ETTNO, TOP) compared liberal (higher) and restrictive (lower) RBC transfusion thresholds in extremely low birthweight infants, but did not find an association between NEC and low pretransfusion hematocrit/hemoglobin values.47 In the ETTNO trial, including 1013 infants, the absolute difference in the incidence of NEC (modified Bell stage ≥ IIa) in the liberal (hematocrit on day >21: <34% or <28% in critical or noncritical state) vs restrictive (hematocrit on day >21: <27% or <21% in critical or noncritical state) transfusion threshold was −0.9 (95% CI [−3.8–2.0]).48 The TOP trial included 1,824 infants; the adjusted relative risk for NEC (Bell’s stage ≥2) at high (weeks ≥3: 11.0 g/dl with or 10.0 g/dL without ventilator support) vs low (weeks ≥3: 8.5 g/dL with or 7.0 g/dL without ventilator support) hemoglobin thresholds was 0.95 (95% CI [0.73–1.25]).47
Some studies have evaluated the impact of recombinant human erythropoietin (rEpo), or its derivative darbepoietin, given to reduce anemia and the need for transfusions, on NEC. Ohlsson and Aher49 performed a meta-analysis to evaluate 3,643 preterm or low birth weight infants who had received early (at ≤8 days of age) received rEpo or darbepoetin vs others treated with placebo. As anticipated, early rEpo treatment reduced the numbers of RBC transfusions and donor exposures. A subanalysis of 15 studies reporting 2,639 infants showed that rEpo or darbepoetin administration reduced the rate of NEC (RR = 0.69, 95% CI [0.52–0.91]; p = 0.01; number needed to treat to benefit = 33). In another prospective (not double-blinded) randomized clinical trial of 1,285 infants, rEpo reduced the incidence of NEC (3% vs 5.4%, p = 0.027).50 The recent Preterm Erythropoietin Neuroprotection Trial (ett), a phase 3 RCT designed to assess the safety/efficacy of early high-dose rEpo for neuroprotection in 941 extremely preterm infants born at 24 weeks and 0 days to 27 weeks and 6 days of gestation, showed only a trend towards a decreased frequency of NEC in the rEpo group (RR = 0.87; 95% CI [0.60–1.27]; not significant).51
We were not able to find consistent clinical evidence to determine whether anemia and/or RBC transfusions could be clearly associated with NEC. In search of insights, we therefore also revisited other analogous conditions marked by severe anemia with a need for transfusion(s), and the risk of intestinal injury. Twin-to-twin transfusion syndrome or hemolytic disease of the newborn are known to trigger intestinal injury, particularly after RBC transfusion.52–54
The importance of severe anemia in intestinal injury also finds credence in the typically delayed occurrence of ta-NEC beyond four weeks of postnatal age, when anemia of prematurity is often concordant with nutritional deficiencies and inflammatory conditions such as bronchopulmonary dysplasia.13 NEC-like bowel injury has also been seen in other populations of critically ill infants such as those receiving cardiopulmonary bypass or extracorporeal membrane oxygenation, particularly when they receive top-up transfusions to treat severe anemia.55 These findings are consistent with at least two clinical studies that have noted the importance of anemia in risk-stratification for ta-NEC.12,21 The association between packed RBC transfusion and splanchnic perfusion after feeds has been studied to understand ta-NEC better, but in multivariate analysis no overall association was found between splanchnic fractional tissue oxygen extraction (FTOE) and fasting perfusion in a multivariate repeated-measures model that accounted for transfusion epochs (primary analysis approach). However, exploratory analyses of postprandial changes in FTOEs undertaken for each transfusion epoch separately showed increasing postprandial FTOEs with repeated transfusions.56,57
More generally, close examination of the literature shows important differences when the observational studies were compared with the meta-analyses of RCTs. The interest in ta-NEC, as judged by the number of publications/year, began to wane, and this was possibly related to the lack of new insights from associative clinical studies. However, a recent preclinical study in newborn mice has shed some novel mechanistic insights and showed that severe anemia, followed by RBC transfusions may cause intestinal injury. This has rekindled the ta-NEC controversy. In the following sections, we recapitulate the experimental findings from this study.
A Murine Model of Transfusion-associated NEC
Maheshwari’s team developed a mouse model to evaluate the association between transfusions and NEC. They phlebotomized mouse pups on postnatal days 2, 4, 6, 8, and 10 to induce severe anemia (hematocrit 20–24%), and transfused these anemic pups and controls on postnatal day 11 with 20 mL/kg leukoreduced, packed (70%) RBCs that had been stored at 4°C for 7 days, recapitulating current transfusion practices in neonates.58 Control pups remained unharmed. However, the anemic-transfused pups developed intestinal injury in the ileocecal and mid-colonic segments between 18 and 28 h after transfusion. The histopathological features in the affected intestine were characteristic of NEC.59,60 Consistently, animals with intestinal injury showed increased plasma levels of the gut epithelial injury marker fatty acid-binding protein 2,61 loss of intestinal barrier function,62 and developed a severe systemic inflammatory response syndrome.
Both anemic and the anemia-transfused mice showed monocyte/macrophage infiltration in the affected intestine.63 RBC transfusions contained free hemoglobin and heme,64 which activated the newly recruited monocytes and macrophages in the intestine by activating Toll-like receptor 4-mediated signaling, redox cycling,65 and downstream NF-κB pathways.66 The role of Toll-like receptor 4 in NEC pathogenesis has been noted in other NEC models,67 and these seminal findings in ta-NEC highlighted the importance of these pathways as a unifying mechanism in NEC. The requirement of macrophages in ta-NEC was notable because the macrophage depletion prior to transfusions was protective. Blocking the NF-κB pathway in macrophages by administering specific inhibitory nanoparticles was also protective against ta-NEC.
The severity of anemia was important in murine ta-NEC. Mouse pups defined to have severe anemia (hematocrit 20–24%) developed more severe bowel injury as compared with those with moderate anemia (hematocrit 25–30%). The duration of anemia was also important; mice transfused on P10 (soon after the last phlebotomy) sustained less bowel damage than those transfused 24 hr later, on P11. If the transfused RBCs were leukoreduced, washed, and resuspended in saline prior to storage, the severity of ta-NEC was decreased. Pups that received multiple transfusions showed more severe injury. However, the duration of RBC storage (7 days vs 14 days) prior to transfusions did not change the severity of ta-NEC.
Discussion
A number of clinical studies suggests an association between RBC transfusions and NEC, although these findings have not been consistently proven in meta-analyses.4–26 In some premature infants, severe anemia may even be the predominant, possibly even sufficient, factor in the causation of NEC.12 However, the exact contribution of anemia and RBC transfusions to the development of bowel injury is not certain. In animal models, RBC transfusions can alter splanchnic autoregulation and cause at least transient intestinal ischemia.24,68 The murine model that we described above suggests that neither anemia nor RBC transfusions may be independently sufficient to cause NEC, but the risk may increase with a sequential exposure to these two factors. A dual-causation or possible multihit model may possibly explain the conflicting conclusions from many of the clinical studies and two of the meta-analyses.6,44 However, even though the experimental and laboratory findings in these animal studies are informative, there is a need for a cautious, refined approach to understand ta-NEC, thus using these findings only as a basis for designing confirmatory human studies.
There has been some difficulty in identifying the thresholds of low hemoglobin/hematocrit, at which the risk of NEC related to severe anemia may be higher than that related to corrective RBC transfusions.69–71 Four peer-reviewed national transfusion guidelines have been developed in Australia, Canada, and Europe (Fig. 1). These recommendations have found support from the broader scientific communities in neonatology, hematology, and transfusion medicine in their respective countries. In the United States, several single-center guidelines are in use that show some heterogeneity;13,48,72,73 this is highlighted in a post hoc analysis of data from the 19 participating sites in the preterm erythropoietin neuroprotection trial (PENUT).74 As discussed above, the adoption of clinical practices to minimize iatrogenic blood loss and possibly, (early) administration of rEpo/darbepoietin to prevent anemia, or other strategies to reduce the need for transfusions, is logical. However, further evidence is needed before firm recommendations can be made.75,76
Fig. 1:
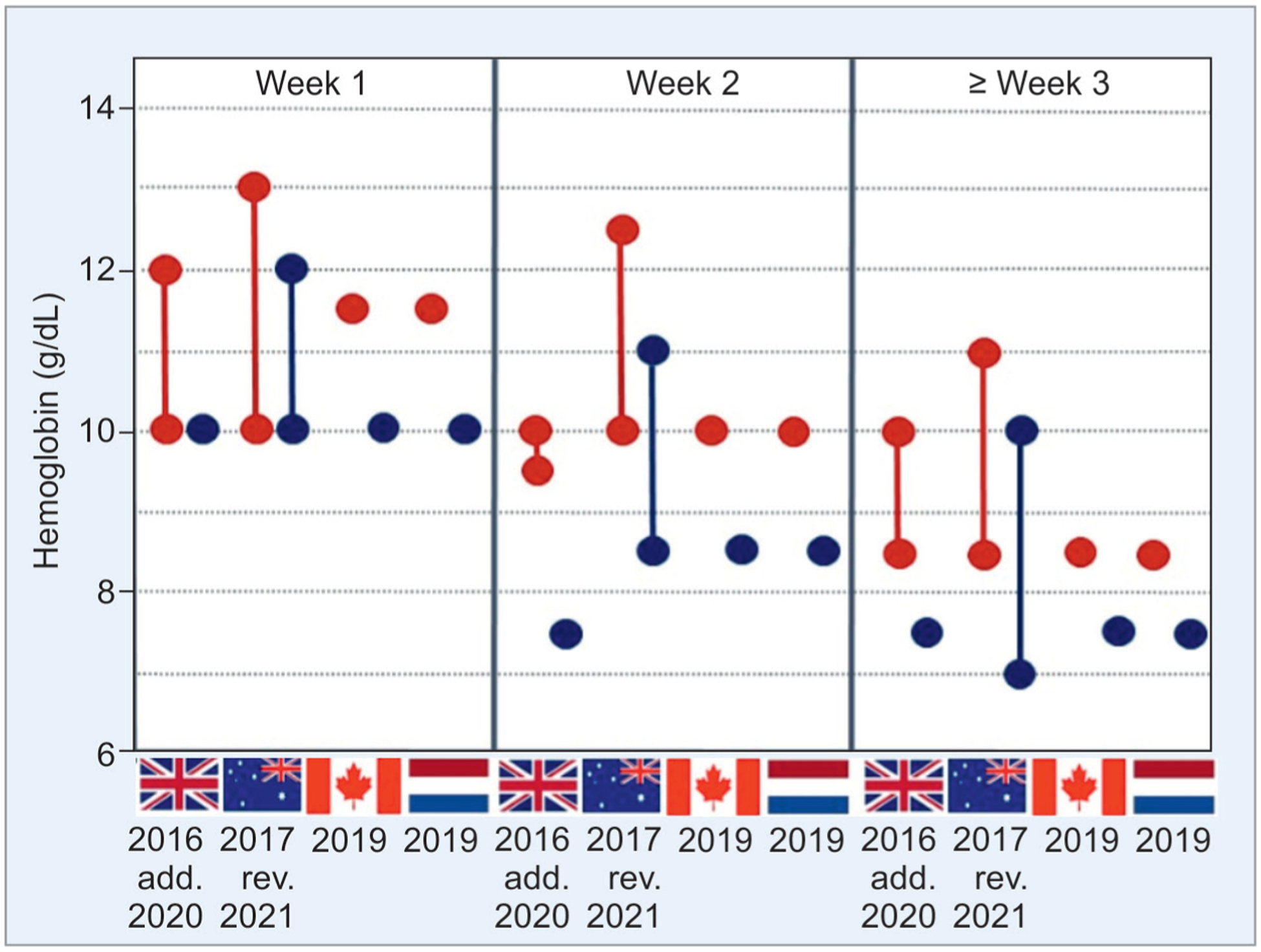
This graph summarizes current national guidelines (the United Kingdom, Australia, Canada, and the Netherlands as indicated by their flags) for RBC transfusions in VLBW infants with respect to age and the need of respiratory support (red dots). The wide variation in these recommendations highlights the need for further research to identify more definitive thresholds for transfusion. Blue dots indicate thresholds for infants without respiratory support. Recommended ranges or point thresholds of hemoglobin at which transfusions may be considered are shown. The year of publication is provided to visualize the trend towards more restrictive transfusion thresholds. Please note that we have not yet considered the impact of specific conditions such the type of blood sampling for measurement of hemoglobin values (vascular or capillary blood draws), precision of laboratory measurement, and the implications of physiological changes such as with altitude or the intravascular volume status
Eventually, a range, not clear tipping points, of hematocrit/hemoglobin levels may have to be considered based on individual physiological and environmental factors. The impact of the duration of anemia on subsequent risk of NEC also needs investigation. A combination of a transfusion threshold plus another marker of end organ oxygen delivery or perfusion may help to define better when to or when not to transfuse. Given the multifaceted nature of the problem, we see a need to combine data from multiple centers across the world to inform well-designed clinical trials.
While we ponder on these questions, we need to ask ourselves whether we can do anything immediately to minimize the serious morbidities and mortality associated with transfusion and possibly with ta-NEC. The answer to this, from our perspective, is in the affirmative. Even though we acknowledge the disparity between the observational evidence and the randomized trials and would consider the latter to provide more reliable evidence, data from the rodent model of ta-NEC and the available observational studies have led us to come to the following considerations for the “best clinical practice” at the present time.
Expert Opinion
Current evidence favors minimizing the exposure of premature infants who may be at high risk of NEC, to prolonged periods of severe anemia. Four sets of national guidelines for RBC transfusions are currently available (Fig. 1),77–80 which show considerable differences. To understand the urgency of correcting anemia, we need further research to identify safe thresholds of hematocrit/hemoglobin.48 We should also continue to evaluate the potential benefits of rEpo and darbepoietin, at least in specific subsets (such as extremely low birth weight infants), if not in all at-risk infants. The exact thresholds of hematocrit/hemoglobin levels when RBC transfusions become necessary are still unclear. The animal models suggest that increased risk of NEC at hematocrit levels ≤24%,4 but some human studies suggest that organs such as the brain may be even less tolerant to anemia, with functional changes becoming evident at hematocrits ≤28%.81 RBC transfusion thresholds may have to be viewed as a range, not exact levels (Fig. 1), of hematocrit/hemoglobin based on the corrected gestational age, altitudes of residence, comorbidities, and the functional status of the microcirculation in various organ systems. Several interventions in a patient’s blood management are now possible to avoid severe anemia and to reduce the need for transfusions.
Thus, we conclude that only a minority of severely anemic premature infants develop ta-NEC.4 There is a need to identify the predisposing (genetic) and clinical factors that may increase the risk of ta-NEC. Considering the typically delayed onset of anemia of prematurity after birth, studies may need to focus on the identification of periods of increased vulnerability. Splanchnic vascular autoregulation deserves further evaluation. The availability of portable ultrasound machines may enhance the feasibility of such studies within the neonatal intensive care units.82 The association of ta-NEC with stimuli that increase intestinal oxygen consumption, such as feeding, need evaluation in high-risk infants.83 Murine and human studies have shown no harm in transfusions with RBCs stored for short periods,84 which is reassuring.85,86 However, further preclinical studies and models could be used to test potential interventions.84 We also do not believe irradiation to have a possible protective effect; current evidence suggests a pathophysiological role of macrophage-mediated innate, not lymphocyte-mediated adaptive immune response. The pathophysiological concept of anemia needs additional evaluation in premature infants.4 Fetal and adult hemoglobin have different oxygen carrying capacities. Therefore, low hematocrit levels in an infant who has previously received transfusions of RBC from adult donors may have different physiological implications than in a nontransfused infant.87 Thus, a detailed analysis infants recruited in the aforementioned clinical trials, who developed NEC/ta-NEC, would be of particular interest. The advantages and disadvantages of washing RBCs prior to storage or before transfusion needs study. Many studies suggest that washing may reduce the half-life of transfused RBCs.88 The findings in the murine model suggest that subtle hemolysis and extravasation of RBC contents may increase the risk of ta-NEC. The relationship between enteral feedings and ta-NEC needs more evaluation. Although the feeding during red cell transfusion (FEEDUR) trial, an open, multi-arm, parallel-group, single-center RCT did not show any difference in splanchnic oxygenation,89 the WHEAT (withholding enteral feeds around packed red cell transfusion) study90 may still yield important insights. Nutritional interventions, such as the use of pasteurized human donor milk as opposed to formula, enteral substitution of lactoferrin and/or L-arginine, and preventive application of multiple-strain probiotics deserve further investigation as strategies to reduce the rates of ta-NEC.30,91,92 Further study is needed to identify infants who might have ongoing subtle hemolysis either due to low-grade immune responses related to genetic defects or due to blood group incompatibility, as suggested by elevated reticulocyte counts and/or the presence of spherocytosis on peripheral blood smear.93 These infants may have anemia, activation of monocytes and macrophages, factors that have been found to be associated with ta-NEC. Effects of simultaneous transfusions with other blood products such as platelets, needs further evaluation as additional or synergistic risk factors for ta-NEC.94 In addition to larger, more definitive trials, some of the relevant questions in this domain may be amenable to scrutiny through use of big data and machine learning/AI.95–98
Article Highlight Box.
Several retrospective studies have associated RBC transfusions with necrotizing enterocolitis (ta-NEC) in very preterm infants.
Randomized controlled trials on transfusion thresholds and treatment with recombinant human erythropoietin or darbepoetin have not provided significant evidence for this association.
A murine model of RBC transfusion-associated NEC-like pathology showed that sequential exposure to anemia followed by RBC transfusion is a risk factor for ta-NEC.
Experimental and clinical data suggest that strategies of personalized blood management (including late cord clamping/milking and reduction of iatrogenic blood loss) in very preterm infants may help prevent ta-NEC.
Source of support:
This article was supported in parts by an Investigator Grant from the British Columbia Children’s Foundation (PML); and NIH awards DK116568 (IGDP); GM115428 (PMG); DK117296 (VS); HL124078 and HL133022 (AM).
Footnotes
Conflict of interest: None
References
- 1.Valieva OA, Strandjord TP, Mayock DE, et al. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr 2009;155(3):331–337. e1. DOI: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.dos Santos AM, Guinsburg R, de Almeida MF, et al. Factors associated with red blood cell transfusions in very-low-birth-weight preterm infants in Brazilian neonatal units. BMC Pediatr 2015;15:113. DOI: 10.1186/s12887-015-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Lindern JS, Lopriore E. Management and prevention of neonatal anemia: current evidence and guidelines. Expert Rev Hematol 2014;7(2):195–202. DOI: 10.1586/17474086.2014.878225. [DOI] [PubMed] [Google Scholar]
- 4.Amin SC, Remon JI, Subbarao GC, et al. Association between red cell transfusions and necrotizing enterocolitis. J Matern Fetal Neonatal Med 2012;25(Suppl 5):85–89. DOI: 10.3109/14767058.2012.715465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion 2009;50(5):1106–1112. DOI: 10.1111/j.1537-2995.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics 2012;129(3):529–540. DOI: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 7.Hyung N, Campwala I, Boskovic DS, et al. The relationship of red blood cell transfusion to intestinal mucosal injury in premature infants. J Pediatr Surg 2017;52(7):1152–1155. DOI: 10.1016/j.jpedsurg.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Demirel G, Celik IH, Aksoy HT, et al. Transfusion-associated necrotising enterocolitis in very low birth weight premature infants. Transfus Med 2012;22(5):332–337. DOI: 10.1111/j.1365-3148.2012.01170.x. [DOI] [PubMed] [Google Scholar]
- 9.Stritzke AI, Smyth J, Synnes A, et al. Transfusion-associated necrotising enterocolitis in neonates. Arch Dis Child Fetal Neonatal Ed 2013;98(1):F10–14. DOI: 10.1136/fetalneonatal-2011-301282. [DOI] [PubMed] [Google Scholar]
- 10.Christensen RD, Lambert DK, Gordon PV, et al. Neonates presenting with bloody stools and eosinophilia can progress to two different types of necrotizing enterocolitis. J Perinatol 2012; 32(11):874–879. DOI: 10.1038/jp.2011.163. [DOI] [PubMed] [Google Scholar]
- 11.El-Dib M, Narang S, Lee E, et al. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol 2011;31(3): 183–187. DOI: 10.1038/jp.2010.157. [DOI] [PubMed] [Google Scholar]
- 12.Patel RM, Knezevic A, Shenvi N, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016;315(9):889–897. DOI: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josephson CD, Wesolowski A, Bao G, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J Pediatr 2010;157(6):972–978. DOI: 10.1016/j.jpeds.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg PM, Ravisankar S, Bian H, et al. Relationship between packed red blood cell transfusion and severe form of necrotizing enterocolitis: a case control study. Indian Pediatr 2015;52(12):1041–1045. DOI: 10.1007/s13312-015-0770-3. [DOI] [PubMed] [Google Scholar]
- 15.AlFaleh K, Al-Jebreen A, Baqays A, et al. Association of packed red blood cell transfusion and necrotizing enterocolitis in very low birth weight infants. J Neonatal Perinatal Med 2014;7(3):193–198. DOI: 10.3233/NPM-14814048. [DOI] [PubMed] [Google Scholar]
- 16.Baxi AC, Josephson CD, Iannucci GJ, et al. Necrotizing enterocolitis in infants with congenital heart disease: the role of red blood cell transfusions. Pediatr Cardiol 2014;35(6):1024–1029. DOI: 10.1007/s00246-014-0891-9. [DOI] [PubMed] [Google Scholar]
- 17.Derienzo C, Smith PB, Tanaka D, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev 2014;90(5):237–240. DOI: 10.1016/j.earlhumdev.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen RD, Wiedmeier SE, Baer VL, et al. Antecedents of Bell stage III necrotizing enterocolitis. J Perinatol 2010;30(1):54–57. DOI: 10.1038/jp.2009.93. [DOI] [PubMed] [Google Scholar]
- 19.Christensen RD, Baer VL, Del Vecchio A, et al. Unique risks of red blood cell transfusions in very-low-birth-weight neonates: associations between early transfusion and intraventricular hemorrhage and between late transfusion and necrotizing enterocolitis. J Matern Fetal Neonatal Med 2013;26(Suppl 2):60–63. DOI: 10.3109/14767058.2013.830495. [DOI] [PubMed] [Google Scholar]
- 20.Bak SY, Lee S, Park JH, et al. Analysis of the association between necrotizing enterocolitis and transfusion of red blood cell in very low birth weight preterm infants. Korean J Pediatr 2013;56(3):112–115. DOI: 10.3345/kjp.2013.56.3.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R, Visintainer PF, Frantz ID 3rd, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol 2011;31(3):176–182. DOI: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elabiad MT, Harsono M, Talati AJ, et al. Effect of birth weight on the association between necrotising enterocolitis and red blood cell transfusions in <=1500 g infants. BMJ Open 2013;3(11):e003823. DOI: 10.1136/bmjopen-2013-003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol 2006;23(8): 451–458. DOI: 10.1055/s-2006-951300. [DOI] [PubMed] [Google Scholar]
- 24.Marin T, Moore J, Kosmetatos N, et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion 2013;53(11):2650–2680. DOI: 10.1111/trf.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul DA, Mackley A, Novitsky A, et al. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics 2011;127(4):635–641. DOI: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 26.Blau J, Calo JM, Dozor D, et al. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr 2011;158(3):403–409. DOI: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Tao HK, Tang Q, Hei MY, et al. Meta-analysis of post-transfusion necrotizing enterocolitis in neonates [Chinese]. Zhonghua Er Ke Za Zhi 2013;51(5):336–339. [PubMed] [Google Scholar]
- 28.Hay S, Zupancic JA, Flannery DD, et al. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin Perinatol 2017;41(1):80–91. DOI: 10.1053/j.semperi.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Nickel RS, Josephson CD. Neonatal transfusion medicine: five major unanswered research questions for the twenty-first century. Clin Perinatol 2015;42(3):499–513. DOI: 10.1016/j.clp.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Bührer C, Fischer HS, Wellmann, S. Nutritional interventions to reduce rates of infection, necrotizing enterocolitis and mortality in very preterm infants. Pediatr Res 2020;87(2):371–377. DOI: 10.1038/s41390-019-0630-2. [DOI] [PubMed] [Google Scholar]
- 31.Guthmann F, Arlettaz Mieth RP, Bucher HU, et al. Short courses of dual-strain probiotics appear to be effective in reducing necrotising enterocolitis. Acta Paediatr 2016;105(3):255–259. DOI: 10.1111/apa.13280. [DOI] [PubMed] [Google Scholar]
- 32.MohanKumar K, Namachivayam K, Song T, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun 2019;10(1):3494. DOI: 10.1038/s41467-019-11199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrady GA, Rettig PJ, Istre GR, et al. An outbreak of necrotizing enterocolitis: association with transfusions of packed red blood cells. Am J Epidemiol 1987;126(6):1165–1172. DOI: 10.1093/oxfordjournals.aje.a114754. [DOI] [PubMed] [Google Scholar]
- 34.Bednarek FJ, Weisberger S, Richardson DK, et al. Variations in blood transfusions among newborn intensive care units. SNAP II Study Group. J Pediatr 1998;133(5):601–607. DOI: 10.1016/S0022-3476(98)70097-6. [DOI] [PubMed] [Google Scholar]
- 35.Carter BM, Holditch-Davis D, Tanaka D, et al. Relationship of neonatal treatments with the development of necrotizing enterocolitis in preterm infants. Nurs Res 2012;61(2):96–102. DOI: 10.1097/NNR.0b013e3182410d33. [DOI] [PubMed] [Google Scholar]
- 36.Couselo M, Aguar M, Ibáñez V, et al. Relation between packed red blood cell transfusion and severity of necrotizing enterocolitis in premature infants [Chinese]. Cir Pediatr 2011;24(3):137–141. [PubMed] [Google Scholar]
- 37.Ghirardello S, Lonati CA, Dusi E, et al. Necrotizing enterocolitis and red blood cell transfusion. J Pediatr 2011;159(2):354–355; author reply 355–356. DOI: 10.1016/j.jpeds.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Wan-Huen P, Bateman D, Shapiro DM, et al. Packed red blood cell transfusion is an independent risk factor for necrotizing enterocolitis in premature infants. J Perinatol 2013;33(10):786–790. DOI: 10.1038/jp.2013.60. [DOI] [PubMed] [Google Scholar]
- 39.Holder GL, Dohert DA, Patole SK. Elective red cell transfusion for anemia of prematurity and development of necrotizing enterocolitis in previously well preterm neonates: incidence and difficulties in proving a cause-effect association. J Neonat Perinat Med 2009;2(3):181–186. DOI: 10.3233/NPM-2009-0067. [DOI] [Google Scholar]
- 40.Stokes V, Rajai A, Mukherjee D, et al. Transfusion-associated necrotizing enterocolitis (NEC) in extremely preterm infants: experience of a tertiary neonatal center in UK. J Matern Fetal Neonatal Med 2021;20:1–6. DOI: 10.1080/14767058.2021.1874910. [DOI] [PubMed] [Google Scholar]
- 41.Lee EY, Kim SS, Park GY, et al. Effect of red blood cell transfusion on short-term outcomes in very low birth weight infants. Clin Exp Pediatr 2020;63(2):56–62. DOI: 10.3345/kjp.2019.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perciaccante JV, Young TE. Necrotizing enterocolitis associated with packed red blood cell transfusions in premature neonates. 2008. 5839.8. [Google Scholar]
- 43.Harsono M, et al. Are packed red blood cell transfusions protective against late onset necrotizing enterocolitis in very low birth weight infants? in E-PAS. 2011. p. 509. [Google Scholar]
- 44.Garg P, Pinotti R, Lal CV, et al. Transfusion-associated necrotizing enterocolitis in preterm infants: an updated meta-analysis of observational data. J Perinat Med 2018;46(6):677–685. DOI: 10.1515/jpm-2017-0162. [DOI] [PubMed] [Google Scholar]
- 45.Rai SE, Sidhu AK, Krishnan RJ. Transfusion-associated necrotizing enterocolitis re-evaluated: a systematic review and meta-analysis. J Perinat Med 2018;46(6):665–676. DOI: 10.1515/jpm-2017-0048. [DOI] [PubMed] [Google Scholar]
- 46.Sharma R, Kraemer DF, Torrazza RM, et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J Perinatol 2014;34(11):858–862. DOI: 10.1038/jp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006;149(3):301–307. DOI: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Franz AR, Engel C, Bassler D, et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA 2020;324(6):560–570. DOI: 10.1001/jama.2020.10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev 2020;2:CD004863. DOI: 10.1002/14651858.CD004863.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Song J, Sun H, et al. Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial. J Transl Med 2020;18(1):308. DOI: 10.1186/s12967-020-02459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juul SE, Comstock BA, Wadhawan R, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med 2020;382(3):233–243. DOI: 10.1056/NEJMoa1907423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schutzman DL, Porat R. Glucose-6-phosphate dehydrogenase deficiency: another risk factor for necrotizing enterocolitis? J Pediatr 2007;151(4):435–437. DOI: 10.1016/j.jpeds.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 53.Detlefsen B, Boemers TM, Schimke C. Necrotizing enterocolitis in premature twins with twin-to-twin transfusion syndrome. Eur J Pediatr Surg 2008;18(1):50–52. DOI: 10.1055/s-2007-965788. [DOI] [PubMed] [Google Scholar]
- 54.Ree IMC, de Grauw AM, Bekker V, et al. Necrotizing enterocolitis in haemolytic disease of the newborn: a retrospective cohort study. Vox Sang 2020;115(2):196–201. DOI: 10.1111/vox.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huybregts RA, de Vroege R, Jansen EK, et al. The association of hemodilution and transfusion of red blood cells with biochemical markers of splanchnic and renal injury during cardiopulmonary bypass. AnesthAnalg 2009;109(2):331–339. DOI: 10.1213/ane.0b013e3181ac52b2. [DOI] [PubMed] [Google Scholar]
- 56.Balegar VK, Jayawardhana M, Martin AJ, et al. Association of bolus feeding with splanchnic and cerebral oxygen utilization efficiency among premature infants with anemia and after blood transfusion. JAMA Netw Open 2020;3(2):e200149. DOI: 10.1001/jamanetworkopen.2020.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dave V, Brion LP, Campbell DE, et al. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol 2009;29(3):213–218. DOI: 10.1038/jp.2008.189. [DOI] [PubMed] [Google Scholar]
- 58.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115(21):4284–4292. DOI: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MohanKumar K, Namachivayam K, Cheng F, et al. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr Res 2016;81(1):99–112. DOI: 10.1038/pr.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng PC, Ang IL, Chiu RW, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest 2010;120(8):2989–3000. DOI: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MohanKumar K, Killingsworth CR, McIlwain RB, et al. Intestinal epithelial apoptosis initiates gut mucosal injury during extracorporeal membrane oxygenation in the newborn piglet. Lab Invest 2014;94(2):150–160. DOI: 10.1038/labinvest.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiou SR, Yu Y, Chen S, et al. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 2011;286(14): 12123–12132. DOI: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaynagetdinov R, Sherrill TP, Kendall PL, et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 2013;49(2):180–189. DOI: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol 2015;4: 193–199. DOI: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest 2012;122(4):1205–1208. DOI: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014;123(3):377–390. DOI: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for tlr4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007; 179(7):4808–4820. DOI: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 68.Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol 2009;26(2):99–105. DOI: 10.1055/s-0028-1090595. [DOI] [PubMed] [Google Scholar]
- 69.Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev 2011(11):CD000512. DOI: 10.1002/14651858.CD000512.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Kirpalani H, Bell EF, Hintz SR, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med 2020;383(27):2639–2651. DOI: 10.1056/NEJMoa2020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol 2009;33(1):29–34. DOI: 10.1053/j.semperi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Christensen RD. Identifying neonates likely to benefit from a red blood cell transfusion. Transfusion 2012;52(2):217–218. DOI: 10.1111/j.1537-2995.2011.03408.x. [DOI] [PubMed] [Google Scholar]
- 73.Henry E, Christensen RD, Sheffield MJ, et al. Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates? J Perinatol 2015;35(2):132–136. DOI: 10.1038/jp.2014.171. [DOI] [PubMed] [Google Scholar]
- 74.Juul SE, Vu PT, Comstock BA, et al. Effect of high-dose erythropoietin on blood transfusions in extremely low gestational age neonates: post hoc analysis of a randomized clinical trial. JAMA Pediatr 2020;174(10):933–943. DOI: 10.1001/jamapediatrics.2020.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants. Pediatrics 2013;132(1):e119–e127. DOI: 10.1542/peds.2013-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel S, Ohls RK. Darbepoetin administration in term and preterm neonates. Clin Perinatol 2015;42(3):557–566. DOI: 10.1016/j.clp.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.New HV, Berryman J, Bolton-Maggs PH, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016;175(5):784–828. DOI: 10.1111/bjh.14233. [DOI] [PubMed] [Google Scholar]
- 78.Patient Blood Management Guidelines: Module 6, in Neonatal and Paediatrics. 2016, National Blood Authority, Canberra, Australia. [Google Scholar]
- 79.Services CB. Clinical guide to transfusion, in Chapter 13. Neonatal and Pediatric transfusion (W. Lau, ed.) 2019: Ottawa, Canada. [Google Scholar]
- 80.Heeger LE, Counsilman CE, Bekker V, et al. Restrictive guideline for red blood cell transfusions in preterm neonates: effect of a protocol change. Vox Sang 2019;114(1):57–62. DOI: 10.1111/vox.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitehead HV, Vesoulis ZA, Maheshwari A, et al. Anemia and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J Perinatol 2018;38(8):1022–1029. DOI: 10.1038/s41372-018-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashem RH, Mansi YA, Almasah NS, et al. Doppler ultrasound assessment of the splanchnic circulation in preterms with neonatal sepsis at risk for necrotizing enterocolitis. J Ultrasound 2017;20(1): 59–67. DOI: 10.1007/s40477-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szabo JS, Mayfield SR, Oh W, et al. Postprandial gastrointestinal blood flow and oxygen consumption: effects of hypoxemia in neonatal piglets. Pediatr Res 1987;21(1):93–98. DOI: 10.1203/00006450-198701000-00020. [DOI] [PubMed] [Google Scholar]
- 84.Fergusson D, Hutton B, Hogan DL, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev 2009;23(1):55–61. DOI: 10.1016/j.tmrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion 2009;49(8):1546–1553. DOI: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 2012;308(14): 1443–1451. DOI: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 87.Brown MS, Phipps RH, Dallman RH. Postnatal changes in fetal hemoglobin, oxygen affinity and 2,3-diphosphoglycerate in previously transfused preterm infants. Biol Neonate 1985;48(2):70–76. DOI: 10.1159/000242156. [DOI] [PubMed] [Google Scholar]
- 88.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med 2012;13(3):290–299. DOI: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schindler T, Yeo KT, Bolisetty S, et al. FEEding DURing red cell transfusion (FEEDUR RCT): a multi-arm randomised controlled trial. BMC Pediatr 2020;20(1):346. DOI: 10.1186/s12887-020-02233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gale C, Modi N, Jawad S, et al. The WHEAT pilot trial-WithHolding Enteral feeds Around packed red cell Transfusion to prevent necrotising enterocolitis in preterm neonates: a multicentre, electronic patient record (EPR), randomised controlled point-of-care pilot trial. BMJ Open 2019;9(9):e033543. DOI: 10.1136/bmjopen-2019-033543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014(4):CD005496. DOI: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 92.Chang HY, Chen JH, Chang JH, et al. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: an updated meta-analysis. PLOS ONE 2017;12(2):e0171579. DOI: 10.1371/journal.pone.0171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warren S, Schreiber JR, Epstein MF. Necrotizing enterocolitis and hemolysis associated with Clostridium perfringens. Am J Dis Child 1984;138(7):686–688. DOI: 10.1001/archpedi.1984.02140450068021. [DOI] [PubMed] [Google Scholar]
- 94.Fustolo-Gunnink SF, Roehr CC, Lieberman L, et al. Platelet and red cell transfusions for neonates: lifesavers or Trojan horses? Expert Rev Hematol 2019;12(10):797–800. DOI: 10.1080/17474086.2019.1657824. [DOI] [PubMed] [Google Scholar]
- 95.Bayne LE. Big data in neonatal health care: big reach, big reward? Crit Care Nurs Clin North Am 2018;30(4):481–497. DOI: 10.1016/j.cnc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Luo G, Stone BL, Johnson MD, et al. Automating construction of machine learning models with clinical big data: proposal rationale and methods. JMIR Res Protoc 2017;6(8):e175. DOI: 10.2196/resprot.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seghatchian J An introductory commentary on the use of artificial intelligence, machine learning and TQM, as novel computational tools in big data patterns or procedural analysis, in transfusion medicine. Transfus Apher Sci 2020;59(6):102985. DOI: 10.1016/j.transci.2020.102985. [DOI] [PubMed] [Google Scholar]
- 98.Pendry K The use of big data in transfusion medicine. Transfus Med 2015;25(3):129–137. DOI: 10.1111/tme.12223. [DOI] [PubMed] [Google Scholar]


