ABSTRACT
Hantavirus-induced diseases are emerging zoonoses with endemic appearances and frequent outbreaks in different parts of the world. In humans, hantaviral pathology is characterized by the disruption of the endothelial cell barrier followed by increased capillary permeability, thrombocytopenia due to platelet activation/depletion and an overactive immune response. Genetic vulnerability due to certain human leukocyte antigen haplotypes is associated with disease severity. Typically, two different hantavirus-caused clinical syndromes have been reported: hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS). The primarily affected vascular beds differ in these two entities: renal medullary capillaries in HFRS caused by Old World hantaviruses and pulmonary capillaries in HCPS caused by New World hantaviruses. Disease severity in HFRS ranges from mild, e.g. Puumala virus-associated nephropathia epidemica, to moderate, e.g. Hantaan or Dobrava virus infections. HCPS leads to a severe acute respiratory distress syndrome with high mortality rates. Due to novel insights into organ tropism, hantavirus-associated pathophysiology and overlapping clinical features, HFRS and HCPS are believed to be interconnected syndromes frequently involving the kidneys. As there are no specific antiviral treatments or vaccines approved in Europe or the USA, only preventive measures and public awareness may minimize the risk of hantavirus infection. Treatment remains primarily supportive and, depending on disease severity, more invasive measures (e.g., renal replacement therapy, mechanical ventilation and extracorporeal membrane oxygenation) are needed.
Keywords: hantavirus cardiopulmonary syndrome, hantavirus disease, hemorrhagic fever with renal syndrome, kidney, nephropathia epidemica
INTRODUCTION
Hantavirus-associated diseases are emerging zoonoses that remain a clinical challenge with increasing incidence and multiple serious outbreak situations (Figure 1) [1–4]. Hantavirus infections were first recognized during the Korean War, when more than 3000 United Nation’s troops developed a syndrome typically comprising hemorrhagic fever and kidney failure. This clinical picture was eventually referred to as hemorrhagic fever with renal syndrome (HFRS). The causative viral pathogen, Hantaan virus (HTNV), was first discovered in its natural reservoir, the striped field mouse (Apodemus agrarius), in the late 1970s (Figure 2) [5]. Puumala virus (PUUV), causing a milder course of HFRS also named nephropathia epidemica, was identified in a different mouse strain, bank voles (Myodes glareolus), in the 1980s, although the disease had been known in Scandinavia since the 1930s [6]. Dobrava–Belgrade virus (DOBV) was first isolated from a field mouse (Apodemus flavicollis) in Slovenia in the 1990s and was recognized as a frequent hazard for soldiers during the Yugoslav wars [7–11]. Mortality due to HFRS ranges from <1% in PUUV infections to 10–15% in HTNV or DOBV infections [12–14].
FIGURE 1:
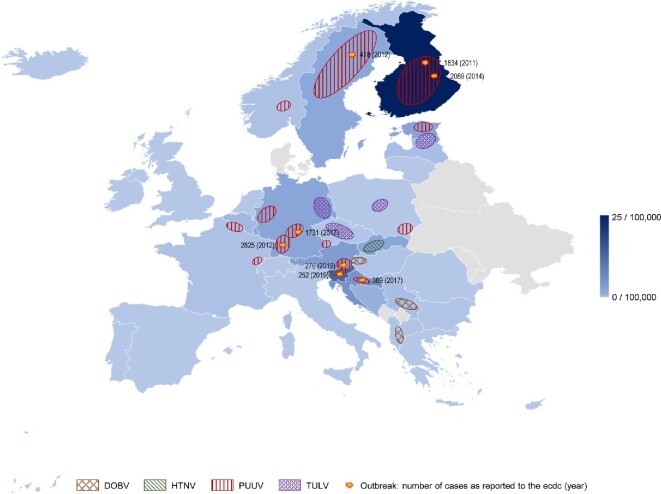
Epidemiology of hantavirus infections in Europe. Incidence for hantavirus infection in 2019 as recorded by the European Centre for Disease Prevention and Control (ECDC). More than 4000 cases of hantavirus disease were reported in Europe (0.8 cases per 100,000 population), with detection of PUUV as the causative pathogen in 98% of cases. Finland and Germany accounted for 69% of all reported cases. Distribution of PUUV, Dobrava virus (DOBV), HTNV and Tula virus (TULV) across Europe are depicted by colour. Recent outbreak situations as reported to the ECDC from 2011 to 2021 are indicated with approximately affected cases and year of the outbreak in parenthesis. European countries that do not report hantaviral infections to the ECDC are depicted in grey (Belarus, Denmark, Moldavia, Montenegro, Kosovo, Ukraine).
FIGURE 2:
European hosts for pathogenic hantaviruses. Source: Images were provided by Shutterstock.com: (A) Holger Kirk/Shutterstock.com, (B) Monika Surzin/Shutterstock.com, (C) Stephan Morris/Shutterstock.com, (D) corlaffra/Shutterstock.com and (E) Ryzhkov Sergey/Shutterstock.com.
Apart from HFRS, there is the hantavirus cardiopulmonary syndrome (HCPS), with a high mortality rate of up to 40% in America [3, 15]. The causative hantavirus of HCPS, Sin Nombre (SNV), was identified within months after its first outbreak in the Four Corners Region in the USA [16, 17]. Other hantaviruses causing HCPS have been found in North and South America [18–21] over the years. Based on the geographical evolution, hantaviruses are separated into Old World viruses (e.g., HTNV, DOBV, PUUV) that commonly elicit HFRS and New World viruses [e.g., SNV, Andes virus (ANDV)] that commonly elicit HCPS (Table 1).
Table 1.
Human pathogenic and medically important hantaviruses according to the 2019 classification of hantavirids [264]
| Disease | ||||||
|---|---|---|---|---|---|---|
| HFRS | HCPS | Virus | Geographic distribution | Reservoir host | Commercial serological test | Comments |
| Old World hantaviruses | ||||||
| X | SEOV | Worldwide | Rat (Rattus) | X | Metropolitan distribution | |
| NEa | PUUV | Europe, Russia, Americas | Bank vole (Myodes glareolus) | X | Main European virus | |
| X | DOBV | Balkans | Yellow-necked mouse (Apodemus flavicollis) | X | ||
| X | TULV | Europe, Russia | Common vole (Microtus arvalis) | |||
| X | AMRV/SOOV | Far-East Russia | Korean field mouse (Apodemus peninsulae) | |||
| X | HTNV | China, Russia, Korea, Central Europe | Striped field mouse (Apodemus agrarius) | X | Main Asian virus | |
| X | Luxi virus (LUXV) | China | Yunnan red-backed vole (Eothenomys miletus) | |||
| X | THAIVb | Southeast Asia | Greater bandicoot rat (Bandicota indica) | |||
| X | SANGV | Africa | African Wood Mouse (Hylomyscus simus) | First African virus, spillover infections to bats | ||
| New World hantaviruses | ||||||
| X | Bayou virus (BAYV) | North America | Marsh rice rat (Oryzomys palustris) | |||
| X | Black Creek Canal virus (BCCV) | North America | Hispid cotton rat (Sigmodon hispidus) | |||
| X | New York virus (NYV) | North America | White-footed mouse (Peromyscus leucopus) | |||
| X | SNV | North America | Eastern deer mouse (Peromyscus maniculatus) | X | Main North American virus | |
| X | Choclo virus (CHOV) | Panama | Fulvous colilargo (Oligoryzomys fulvescens) | |||
| X | Araraquara virus (ARQV) | Brazil | Hairy-tailed bolo mouse (Bolomys lasiurus) | |||
| X | Anajatuba virus (ANJV) | South America | Fornes' colilargo (Oligoryzomys fornesi) | |||
| X | Castelo dos Sonhos virus (CASV) | South America | Brazilian colilargo (Oligoryzomys eliurus) | |||
| X | ANDV | Argentina, Chile | Long-tailed colilargo (Oligoryzomys longicaudatus) | X | Main South American virus, human-to-human transmission | |
| X | Bermejo virus (BMJV) | Argentina | Hairy-tailed bolo mouse (Bolomys Lasiurus) | |||
| X | Laguna Negra virus (LANV) | Argentina, Bolivia, Paraguay | Small vesper mouse (Calomys laucha) | |||
| X | Lechiguanas virus (LECV) | Argentina | Flavescent colilargo (Oligoryzomys flavescens) | |||
| X | Oran virus (ORNV) | Argentina | Long-tailed colilargo (Oligoryzomys longicaudatus) | |||
PUUV causes nephropathia epidemica, a mild form of HFRS. PUUV is the main European hantavirus, whereas HTNV is the main Asian hantavirus. ANDV and SNV are the major South and North American hantaviruses causing HCPS. Novel New World hantaviruses were detected recently with unknown pathogenicity.
So far only serological evidence reported.
NE: nephropathia epidemica.
Hantavirus infections are rare infectious diseases. However, hantavirus diseases emerge progressively, in particular in Europe, and new hantaviruses with yet unknown pathogenic impact are being discovered in different parts of the world, calling for joint efforts at the crossroads of nephrology, infectious diseases and public health [1, 2, 14, 22].
Hantavirus ecology and epidemiology
Hantaviruses are carried and transmitted to humans by persistently infected rodents, insectivore hosts and bats. The geographic distribution and ecology of hantaviruses are therefore closely connected to their hosts. Myodes, Ratus and Apodemus are the primary rodent reservoirs of Old World hantaviruses, whereas New World hantaviruses are transmitted via Sigmodontines, a rodent subfamily of New World rats and mice [1]. Hantavirus species have been discovered in Africa, Asia and Europe, as well as North America and South America (Table 1) [1, 23, 24]. The evolution and distribution of hantavirus populations and the subsequent rate of infections are associated with climate change and disturbed rodent habitats caused by excessive agriculture and deforestation [25–27]. Increases in temperature, humidity and rainfall are directly associated with the incidence of hantaviral infections in different parts of the world [28–30]. For example, warm ambient temperature and abundant rainfall directly affect vegetation growth, boosting rodent populations [31, 32]. In Europe, rodent food availability and higher ambient temperatures in cold seasons have been reported to cause more frequent and severe outbreaks of hantavirus infections [30, 33]. Higher temperature is also correlated with increased rodent reproduction, sexual maturation and survival [34, 35]. The extent of rodent habitat growth and subsequent hantavirus distribution by long-term climate change is unclear (Table 2) [24]. Nevertheless, migration of humans due to climate change to areas less vulnerable to extreme weather events will increase rodent density and may even contribute to hantavirus disease in new regions [36]. Of note, the increase in atmospheric moisture as well as the increase in air temperature facilitates aerosolization and thus augments hantaviral infectivity [29, 37].
Table 2.
Research needs in hantavirus diseases
| Hantavirus ecology and epidemiology• Extension of host reservoir habitats due to climate change• Impact of human migration on hantaviral spread• Characterization of spillover infections, especially to bats, facilitating hantaviral reassortment and spread• Role of human-to-human transmission of ANDV and SNV in public health |
| Hantavirus structure and life cycle• Role of integrins for cell entry in pathogenic and apothegenic hantaviruses• Integrin gene polymorphisms in hantaviral attachment and the susceptibility for hantaviral infection |
| Hantavirus pathogenesis and immunopathology• Hantavirus cell tropism and organ-specific dysfunction in HRFS and HCPS• Generation of HFRS animal models reflecting human hantavirus disease• Local and systemic tissue damage caused by pathogenic mediators in hantavirus disease in in vivo models• Assessing causal attribution of involved pathogenic mediators using targeted approaches, i.e. antibodies• Pathomechanisms of AKI in hantavirus disease |
| Clinical presentation• Characterization of hantavirus disease beyond the dichotomous denominations of HFRS and HCPS• Revised taxonomy beyond HFRS and HCPS• Long-term kidney sequelae in DOBV and HTNV• Prognostic impact of pre-existing CKD, RRT, kidney transplantation and immune suppression in hantavirus disease• Phenotype, frequency and sequelae of kidney involvement in ANDV- and SNV-caused disease• Systematic analyses of overlapping features in pathogenesis, phenotype and treatment approaches between emerging viruses, i.e. SARS-CoV-2, Ebola virus and hantaviruses |
| Diagnosis• Definition of clinical and diagnostic criteria for hantavirus diseases• Prognosis-indicating scores facilitating risk stratification for the ER and ICU |
| Prevention• Development of EMA- and FDA-approved vaccines, i.e. on the basis of recent RNA vaccine technology• Development for preventive/precaution measures for public health (especially in endemic areas) |
| Therapy• Development of targeted antiviral pharmacological approaches examined in randomized controlled clinical trials |
AKI: acute kidney injury; CKD: chronic kidney disease; EMA: European Medicines Agency; ER: emergency room; FDA: US Food and Drug Administration; ICU: intensive care unit; RNA: ribonucleic acid.
Hantaviruses are circulating between chronically infected host reservoirs, whose infection is usually inapparent. Transmission to humans is caused by inhalation of contaminated aerosolized rodent excreta, leading to so-called spillover infections [3]. These spillover infections to humans or other animals, such as red fox, moose or domestic cat and dog, are a concern for public health, as they facilitate the evolution of new hantavirus species by natural reassortment (Table 2) [33, 38, 39]. The risk of hantavirus infections in humans mainly depends on the closeness to and frequency of contact with rodents. Therefore people with close exposure to rodent habitats, such as farmers, forestry workers, military personnel and zoologists are subject to a greater risk [40–42]. However, many cases are observed after sporadic cleansing of environments that are a habitat for rodents, like basements or attics, without a specific professional exposure.
The epidemiology of hantavirus-associated diseases depends on their host reservoirs’ distribution. In Europe, Tula virus (TULV), DOBV and PUUV are detected most frequently in their rodent reservoirs (Figure 2). PUUV is the most widely distributed hantavirus in Northern and Central Europe and usually causes mild disease (Figure 1, Table 1) [43]. However, PUUV still poses a threat due to its increasing incidence, with recurring outbreak situations and thousands of patients in epidemic years [2, 44, 45]. In contrast, Southeastern Europe is dominated by DOBV, resulting in a moderate–severe disease form of HFRS (Figure 2) [2]. Here, the Balkan region is primarily affected by DOBV, although its host distribution is greater [3, 46, 47]. Of note, DOBV has been emerging in Central and Eastern Europe in recent years [48–51]. TULV is dominantly circulating in the Tula region in Russia and leads to a mild disease course [52]. However, patient cases are found throughout Central Europe and the Baltic states (Figure 2) [52–55].
Asia is the continent with the longest history of hantavirus infections. HTNV and the related Amur/Soochong virus (AMRV/SOOV) are found in China, Far East Russia and Korea, causing moderate forms of HFRS [1, 5, 56, 57]. China reports >10 000 cases and southern Korea ∼300–500 cases/year [58]. Further species with unknown pathogenicity, such as Thailand virus (THAIV), Imjin virus (MJNV) and Jeju virus (JJUV) have been recognized in different parts of Asia (Table 1) [1, 2, 59, 60].
Due to the cosmopolitan distribution of rats as its natural host, Seoul virus (SEOV) is considered to circulate worldwide [1]. SEOV is primarily reported from urban China; however, patient cases have also been observed occasionally in the USA and Europe [61–63]. Surveillance studies indicate that HFRS caused by rat-borne SEOV usually occurs in metropolitan areas and current trends in urbanization may further influence SEOV epidemiology [64].
New World hantaviruses were first discovered after an outbreak of an acute respiratory disease named HCPS in the USA in 1993 and SNV was found as a first causative pathogen [15, 17]. SNV is the main pathogen circulating in North America [2]. In contrast, ANDV is the dominant hantavirus in Latin America [65]. Of note, ANDV is the only known hantavirus that can be transmitted from human to human by superspreading events, further threatening public health and making pandemics a potential scenario [18–21, 66]. Recently, novel hantaviruses with unclear taxonomic status were discovered in their natural reservoirs in America, but little is known about their pathogenicity (Table 1) [1, 22].
Africa is the continent with the most recent scientific progress in hantavirus ecology and epidemiology. Fifteen years ago the first pathogenic hantavirus in Africa, Sangassou virus (SANGV), phylogenetically related to Old World hantaviruses, was isolated in Guinea [67, 68]. Novel hantavirus species have been discovered not only in rodents, but also in shrews and bats in Africa [2, 69, 70]. In particular, the role of bats as novel hosts facilitating easier spillover infections to humans reflects an additional concern for public health (Table 2) [3].
Taken together, the ecology and epidemiology of hantaviruses and their associated diseases are mainly reflected by their host reservoirs’ distribution. Hence HFRS caused by DOBV, HTNV and PUUV dominates Eurasia, whereas SNV- and ANDV-associated HCPS is primarily found in America (Table 1).
Hantavirus structure and life cycle
Hantaviruses, members of the Bunyaviridae family, are enveloped, single-stranded ribonucleic acid (ssRNA) viruses [71]. The total size of the viral RNA (vRNA) ranges from 11 845 nucleotides for HTNV to 12 317 nucleotides for SNV [1]. The viral genome is separated into three segments referred to as S (small), M (medium) and L (large), which share a 3′ terminal sequence and encode the nucleocapsid (N) protein, the glycoprotein precursors (GPCs) and the RNA-dependent RNA polymerase (RdRp) [23, 72]. The vRNA itself is encapsulated by the N protein, forming circular ribonucleoproteins (RNPs) [73]. The virion's surface consists of the two glycoproteins Gn and Gc (formerly G1 and G2, respectively) that mature from GPCs. The RdRp amplifies and transcribes vRNA and is essential for hantavirus replication (Figure 3). Hantaviruses can survive for >10 days at room temperature and for >18 days at +4°C outside cells, facilitating transmission and spillover infection [74].
FIGURE 3:
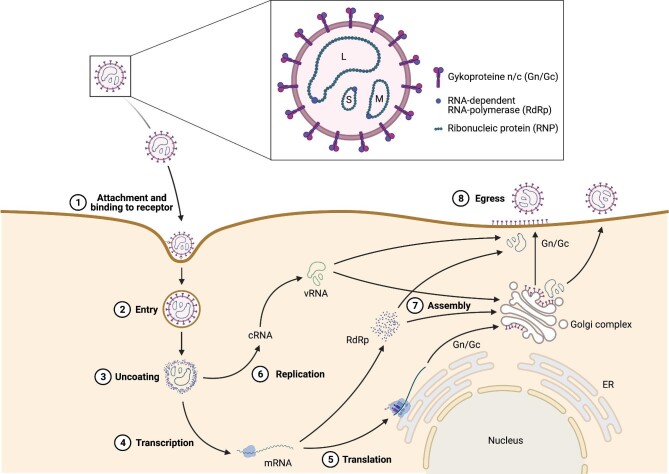
Hantavirus structure and life cycle. Hantavirus virions have a trisegmented single-stranded ribonucleid acid (ssRNA) genome, referred to as L (large), M (medium) and S (small) segments. The particle's surface consists of the glycoproteins Gn and Gc and viral RNA-dependent RNA polymerase (RdRp) is essential for hantavirus replication and transcription. The hantavirus life cycle consists of eight essential steps. (1) Hantaviruses attach to the host cell's surface by binding to surface receptors with their glycoproteins. (2) The virion particle enters the host cell either by Clathrin-dependent (Old World hantavirus) or -independent endocytosis (New World hantavirus). (3) Hantaviruses are uncoated in the host cell's endosomes and lysosomes, facilitating the release of the viral genome and proteins. (4) Viral RNA is transcribed by the RdRp and (5) viral mRNA is translated into viral proteins, hijacking the host cell's machinery. (6) vRNA is replicated by RdRp and (7) all viral components are put together at the Golgi apparatus (Old World hantavirus) or directly at the cell membrane (New World hantavirus). (8) Mature virion particles egress the host cell by fusion of the Golgi apparatus (Old World hantavirus) or the viral vesicle (New World hantavirus) with the cell membrane. ER: endoplasmic reticulum; Gc: C-glycoprotein; Gn: N-glycoprotein; L: large segment; M: medium segment; S: small segment; vRNA: viral RNA. Source: Figure created with Biorender.com.
Hantaviruses not only infect primarily endothelial cells, but also replicate in the epithelium—including podocytes and tubular cells in the kidney as well as alveolar cells in the lungs—macrophages, dendritic cells and lymphocytes [21, 75–79]. Figure 3 highlights the hantavirus life cycle. In vitro studies suggest that hantaviruses attach to integrins (αVβ3, α5β1, αMβ2 and αXβ2) on host cells through binding with their larger glycoprotein Gn [76, 80, 81]. β3- and β1-containing integrins are proposed as the major entry receptors for both virulent and avirulent Old and New World hantaviruses [80–82]. There is evidence that binding to α5β1 integrin instead of αVβ3 is associated with reduced virulence—as can be seen with the apathogenic Prospect Hill virus (PHV). On the other side, the African SANGV is the only pathogenic hantavirus known to bind to α5β1 and to cause hantavirus disease [83, 84]. Different nucleotide polymorphisms have been implicated in infection susceptibility in humans, calling for further research in hantaviral attachment (Table 2) [85, 86]. Of note, protocadherin-1 (PCDH1) was reported recently to be another critical determinant of attachment, entry and infection of New World but not Old World hantaviruses [87].
After cellular attachment, Old World hantaviruses enter host cells by clathrin-dependent endocytosis, whereas New World viruses employ different mechanisms of cell invasion simultaneously, including micropinocytosis, clathrin-independent receptor-mediated endocytosis and cholesterol- or caveolae-dependent endocytosis (Figure 3) [23, 88, 89]. After cell entry, viral particles are processed in endosomes or lysosomes, where they detach from their cellular receptors and uncoat as a consequence of decreasing pH. Afterwards, hantavirus RNPs are liberated into the cytoplasm by fusion of viral and endolysosomal membranes [88]. In the cytoplasm, vRNA is then transcribed to L, M and S messenger RNA (mRNA), enabling their translation into viral proteins that hijack the host machinery [1]. Additionally, vRNA is transcribed to complementary RNA (cRNA) using host-derived primers facilitating amplification and replication of vRNA [1]. Both transcription and replication processes of hantaviruses are performed by viral RdRP [23]. After replication of the hantavirus genome, the vRNA is encapsulated by the N protein. Then, further N proteins bind, resulting in larger RNPs [90, 91]. The different hantavirus components are finally assembled at the Golgi apparatus, or alternatively, as currently suggested for New World hantavirus, at the plasma membrane itself [92]. By fusion with the host's cell membrane, hantaviruses finally egress [93].
Hantavirus pathogenesis and immunopathology
Despite different causative pathogens and disease patterns, HFRS and HCPS have an overlapping pathophysiology consisting of increased vascular permeability, platelet activation and an overreacting host immune response (Figure 4, Table 3) [23, 46]. Differences in disease are reflected by the different vascular beds that are primarily infected—renal medulla or pulmonary capillaries. However, the mechanisms mediating cell tropism and organ-specific dysfunction during HFRS and HCPS remain unknown (Table 2) [94].
FIGURE 4:
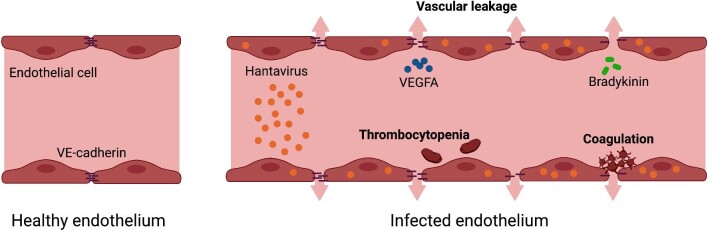
Hantavirus-caused pathogenesis is characterized by vascular leakage and platelet activation. Hantaviruses primarily infect endothelial cells, reducing their barrier function while increasing vascular permeability. Endothelial cell–cell contacts are disturbed by the downregulation of VE-cadherin in adherens junctions caused by vascular endothelial growth factor A (VEGFA) or bradykinin. Platelets are activated after hantavirus infection by either the direct interaction of viral glycoproteins and platelet integrin αIIβ3 or by endothelial cell damage releasing adhesive factors such as fibrinogen, fibronectin and von Willebrand factor. Hantavirus can additionally cause intravascular coagulation. Both activated platelets and coagulation contribute to thrombocytopenia. Source: Figure created with Biorender.com and figure concept adapted from Vaheri et al. [23].
Table 3.
Pathophysiology in response to hantavirus infection as discovered in cell culture, in vivo models and human biosamples
| Evidence reported in | |||||
|---|---|---|---|---|---|
| Pathogenic mechanisms in hantavirus disease | Cell culture | In vivo models | Humans | Comments | References |
| Increased vascular permeability | |||||
| VEGF-induced endothelial hyperpermeability | X | X | X | Orchestrated by a decrease in VE-cadherin and inactivation of the αVβ3-integrin–VEGFR2 complex | [98, 99, 105–107] |
| Bradykinin-induced capillary leakage | X | X | [109–111] | ||
| Cytokine-mediated hyperpermeability | X | X | [113–119] | ||
| Platelet activation | |||||
| Direct viral-caused platelet consumption | X | Interaction of viral glycoproteins and integrin αIIβ3 on platelets | [124, 125] | ||
| Endothelial cell injury causing platelet activation | X | X | Release of adhesive agents, such as fibrinogen, fibronectin, extracellular vesicle tissue factor and von Willebrand factor after endothelial infection | [23, 126, 127, 131, 132] | |
| Overreacting host immune response | |||||
| Reverse CD4+:CD8+ T-cell ratio | X | X | Causes further activation of pro-inflammatory cytokines | [1, 3, 151, 152, 156] | |
| Triggered T-cell immune response by HLA haplotypes | X | May explain interpersonal and regional differences in susceptibility and vulnerability | [153, 156–163] | ||
| Cytokine-mediated activation of innate and adaptive immune responses causing tissue damage | X | Distinct cytokine profiles in HFRS and HCPS; cytokine storm is a common central component in response in hemorrhagic fevers | [116, 118, 119, 138, 142–145] | ||
Increased vascular permeability, platelet activation and an overreacting host immune response are the central pathomechanisms in human disease caused by pathogenic Old World and New World hantaviruses.
The pathophysiology of capillary leakage is characterized by the disruption of endothelial cell–cell contacts. Hantaviruses primarily infect and replicate in endothelial cells of capillaries; however, there are few to no direct cytopathic effects to the endothelium as detected in histological samples [23, 95]. In contrast, it is hypothesized that the breakdown of endothelial cell-to-cell contact is mainly caused by the release of vasoactive factors, including vascular endothelial growth factors (VEGF), bradykinin and cytokines, rather than endothelial cell death [96, 97]. Mechanistically, released VEGF results in a decrease of VE-cadherin, a major component of adherens junctions in endothelial cells, in vitro [98, 99]. VEGF-induced capillary hyperpermeability may additionally be a direct consequence of hantavirus-caused inactivation of αVβ3 integrins followed by a decrease of VEGF receptor 2 (VEGFR2) on the cell surface [75, 100]. VEGFR2 and αVβ3 integrins normally form a complex affecting VEGFR2-directed permeability in response to VEGF [101]. Hantaviruses functionally block the VEGFR2/αVβ3 integrins complex that causes VEGF-orchestrated cellular permeability in vitro and in vivo [102–105]. In line with these findings, levels of VEGF and soluble VEGFR2 in serum and urine correlate with disease severity, indicated by low urine output and haemorrhages in PUUV- and DOBV-infected patients [106]. Moreover, as hypoxia additionally challenges VEGF production, elevated levels of VEGF are detected in pulmonary edema fluids of HCPS patients and correlate with HCPS disease severity [99]. Of note, vandetanib, a small-molecule antagonist of VEGFR2, leads to modest survival benefits in vivo as examined in the Syrian hamster model of ANDV-caused HCPS [107]. However, transfer of this treatment approach to the patient setting has not been successful due to the reported frequent severe adverse events of vandetanib in large-scale cancer trials on the one hand and the fragile, critically ill HCPS patients on the other [107, 108].
Furthermore, in vitro studies revealed activation of the plasma kallikrein–kinin system leading to an increase in bradykinin as another potential mechanism causing vascular permeability in hantavirus diseases [109, 110]. Successful treatment with the bradykinin receptor antagonist icatibant in case reports of severe PUUV infections underline the potential involvement of bradykinin in human disease [110–112]. Breakdown of cell-to-cell contacts is additionally caused by cytokines in vitro and in vivo [113–116]. For example, tumour necrosis factor (TNF)-α contributes to vascular permeability through the production of nitric oxide [117]. In particular, capillary leakage and extracellular matrix degradation are linked to certain cytokine profiles in serum samples in both HFRS and HCPS patients and may even predict disease severity [118, 119].
Interestingly, the ability of hantaviruses to infect endothelial cells is shared with many (emerging) viruses, i.e. severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), flaviruses (including dengue virus) and filoviruses (i.e., Ebola and Marburg viruses), although endothelial receptor, entry mechanism, mediators of disease and the extent of endothelial damage differ widely [100, 120–123]. Hence dysregulation in vascular function contributes significantly to pathophysiology in many life-threatening viral infections and our growing knowledge due to the current coronavirus disease 2019 (COVID-19) pandemic may promote research advances for hantavirus disease (Table 2) [100].
A common feature of hantavirus infection is thrombocytopenia; however, the exact pathomechanisms remain unknown [3, 23, 78]. Currently it is hypothesized that the adhesion of hantaviruses mediated by viral glycoproteins and integrin αIIβ3 on the surface of platelets contributes to platelet depletion [124, 125]. Also, hantavirus infection–derived endothelial cell injury may directly cause platelet activation through the release of adhesive agents, such as fibrinogen, fibronectin and von Willebrand factor [23, 126]. Moreover, hantaviruses promote intravascular coagulation, leading to an increase in thrombin and fibrinolysis that additionally may cause thrombocytopenia due to increased platelet consumption [127]. Of note, clinical criteria of disseminated intravascular coagulation (DIC) are fulfilled frequently in hantavirus patients, posing risks for bleeding and thromboembolic complications [128–130]. Platelet activation and elevated levels of tissue factor and extravesicular tissue factor expressed by both endothelial and immune cells are reported to promote this systemic procoagulant state [131–133]. Although the clinical picture consisting of renal injury and thrombocytopenia might suggest thrombotic microangiopathy, no histological or laboratory evidence for this entity has been reported. Hemolysis as well as schistocytes are usually not present.
In human kidneys, hantaviruses primarily replicate in the endothelial cells of the renal medulla [134]. However, hantaviruses may additionally infect other cell types in the kidney, i.e. tubular cells, podocytes and the glomerular endothelium as detected in kidney biopsy specimens of infected patients [135]. Of note, the barrier function in all these cell types can be altered due to the breakdown of cell–cell contacts, which may be an explanation for the observed proteinuria during hantavirus infection [135, 136]. The detection of viral antigen along with inflammatory cell infiltrations in the peritubular area indicates that viral replication and the host's immune response together cause tissue damage in human disease [137, 138]. The renal immunopathology is primarily characterized by an upregulation of pro-inflammatory cytokines, such as type I interferons (IFNs) and interleukin-8 (IL-8) in the distal nephron activating both innate and adaptive immune cells [139–141]. The further systemic immune response is driven by macrophages and antigen-specific cells, including CD4+ and CD8+ T cells, as well as antibody-producing B cells [1, 3, 23]. Inflammatory cytokines and chemokines, i.e. transforming growth factor (TGF)-β1, tumour necrosis factor (TNF)-α, IL-6 and IL-10, play double-sided roles in human hantavirus infection. On the one hand, immunosuppressive TGF-β1 acts protectively in the late phase of PUUV infection by limiting tissue damage caused by the immune system [142]. On the other hand, TNF-α, IL-1 and IL-6 are associated with fever and septic shock as well as the further induction of acute-phase proteins and increased levels of those cytokines can be detected in urine, plasma and tissue samples of severely infected patients [115, 116, 142, 143].
In line with this notion, disease severity in PUUV and DOBV infections is associated with elevated serum levels of TNF-α, IL-6 and IL-10 [115, 116, 143, 144]. Elevated urinary IL-6 levels in PUUV-induced HFRS indicate local renal production of IL-6 in addition to the systemic immune response [115]. With regard to HCPS, elevated serum IL-6 levels are directly linked to fatal outcomes in humans [145]. Interestingly, IL-6 production is an overlapping characteristic of viral hemorrhagic fevers, as high levels of IL-6 have been linked to disease severity in Ebola virus infection, dengue virus disease and Crimean–Congo hemorrhagic fever [146–148].
Another remarkable parallel can be drawn to the cytokine release syndrome (CRS), a toxic immune reaction upon cancer treatment with chimeric antigen receptor–modified T cells that leads to a systemic increase in IL-6 and IL-10 [149]. Symptoms of CRS and hantavirus disease highly overlap—in mild cases, flu-like symptoms; in severe cases, vascular leakage, coagulopathy, hypotension, acute kidney injury (AKI) and pulmonary edema—and blockage of the IL-6 receptor with tocilizumab reverses CRS [150]. This raises the question whether hantavirus-infected patients could benefit from similar treatment. However in vivo animal and human data in hantavirus disease are lacking and the full causal roles of the highlighted mediators on local tissue damage remain largely unaddressed [145].
With regard to the adaptive immune system, hantaviruses increase the number of CD8+ T cells and reverse the CD4+:CD8+ T-cell ratio [3]. As a consequence, further pro-inflammatory cytokines are produced while regulatory cytokines are downregulated, enhancing the harmful effect of the immune system [151, 152]. Of interest, certain human leukocyte antigen (HLA) haplotypes are associated with severe disease courses in both HFRS and HCPS by triggering T-cell-mediated immune responses, especially of CD8+ T-cells [153–156]. This genetic vulnerability may explain interpersonal and regional differences in disease severity of both HFRS and HCPS in endemic areas [136]. For example, HLA alleles HLA-B*08, HLA-DRB1*0301 and HLA-DRB1*15 are associated with a severe form of PUUV infection, whereas HLA-B*27 has a benign prognosis [153, 157, 158]. In HTNV-caused HFRS, individuals with HLA-B*46 and HLA-B*46-DRB1*09 or HLA-B*51-DRB1*09 haplotypes are at a greater risk, whereas HLA-DRB1*12 is protective [159, 160]. With regard to HCPS, HLA-B*3501, HLA-DRB1*1402 and HLA-B*08 are negative prognostic factors in SNV- and ANDV-infected patients, respectively [156, 161]. In contrast, HLA-DRB1*15 and HLA-B*35-restricted memory T-cell responses are reported to be protective in ANDV-caused HCPS [161, 162]. Of note, different hantaviruses may be processed differently through the same HLA molecules, leading to either a mild or severe disease course as shown for HLA-DRB1*13 and HLA-B*35 in PUUV- and DOBV-caused HFRS [160, 163].
Small animal models recapitulating human hantavirus disease are scarce, as human pathogenic hantaviruses are maintained in nature by persisting rodent infection. For example, mice, rats and hamsters infected with HTNV, DOBV and PUUV fail to develop a human HFRS phenotype, whereas new born rodents that are infected within 3 days after birth die due to neurologic complications not recapitulating any disease characteristics resembling human disease [164]. The generation of HFRS animal models reflecting human hantavirus disease remains an urgent scientific need to fully understand hantaviral pathogenesis (Table 2). In contrast, Syrian hamsters infected with ANDV and SNV mimic human disease with regard to incubation time, rapid-progressing respiratory failure and pathological lung findings, but biosafety measurements make experimental design complex [165–167].
Clinical presentation
Common early symptoms in the prodromal phase of both HFRS and HCPS include myalgia, fatigue, abdominal pain, headache, high fever and other flu-like symptoms [168]. Although similar in pathogenesis, further observed clinical differences between HFRS and HCPS are the consequence of the primarily affected organs [3].
HFRS
HFRS is a generalized infection and its clinical course and outcome are dependent on the causative (Old World) hantavirus, although disease courses vary individually from subclinical to lethal.
The incubation phase of hantavirus ranges from 2 to 6 weeks [1]. Afterwards, the disease can be divided in five distinct phases: fever, hypotension, oliguria, polyuria and convalescence (Figure 5) [1, 3]. HFRS is characterized by the sudden onset of prodromal symptoms, such as myalgia, fatigue, abdominal pain, headache, high fever, blurred vision and other flu-like symptoms lasting 3–6 days. Somnolence is reported less frequently. The beginning of the hypotensive phase is characterized by hemorrhage (i.e. conjunctival suffusion, skin and mucosal petechiae, hematemesis, epistaxis, melaena, hematuria or even intracranial bleeding) due to thrombocytopenia, as well as hypotension or shock caused by vascular leakage. Of note, approximately one-third of case fatalities are related to an irreversible and fulminant shock [3]. Hemorrhage is also facilitated by thrombocytopenia, which is usually present in this stage and is the direct consequence of hantavirus-caused activation and depletion of platelets. The nadir in platelet count ranges from ∼70 × 109/L in PUUV to <30 × 109/L in DOBV- and HTNV-caused HFRS [46, 169, 170]. Bleeding events, typically reported as gastrointestinal bleeding, hematuria or hemoptysis are reported in ∼10% of HFRS cases. However, the incidence of bleeding events ranges from <5% in PUUV to 20% in DOBV and 35% in HTNV infections [171–173]. Microscopic hematuria is present in the vast majority of HFRS patients, whereas macroscopic hematuria is more frequently reported in DOBV and HTNV infections [174]. Hematuria usually derives from medullary or interstitial hemorrhages, i.e. is of intrarenal origin [175, 176]. DIC occurs in ∼30% of DOBV- or HTNV-infected patients as a consequence of an activated coagulation system and is a negative prognostic factor [127, 128, 177]. DIC is reported in ∼20% of PUUV patients according to the modified scoring system of the International Society of Thrombosis and Haemostasis; however, DIC is less severe in PUUV-caused disease [128, 177]. The hypotensive stage typically lasts 1–2 days and is followed by the often rapidly progressing oliguric AKI. With hemodynamic stabilization, renal function is eventually restored over a period of 3–7 days (Figure 5). The decrease in systemic blood pressure does not fully explain AKI, as a decline in kidney function may even occur without hypotension and measured blood pressures do not correlate with disease severity [96]. Intrarenal events—especially hantaviral-caused acute interstitial nephritis with cortical and peritubular capillaritis—are currently believed to contribute to the development of AKI; however, the exact mechanisms remain elusive and call for further research (Table 2) [96, 97, 176].
FIGURE 5:
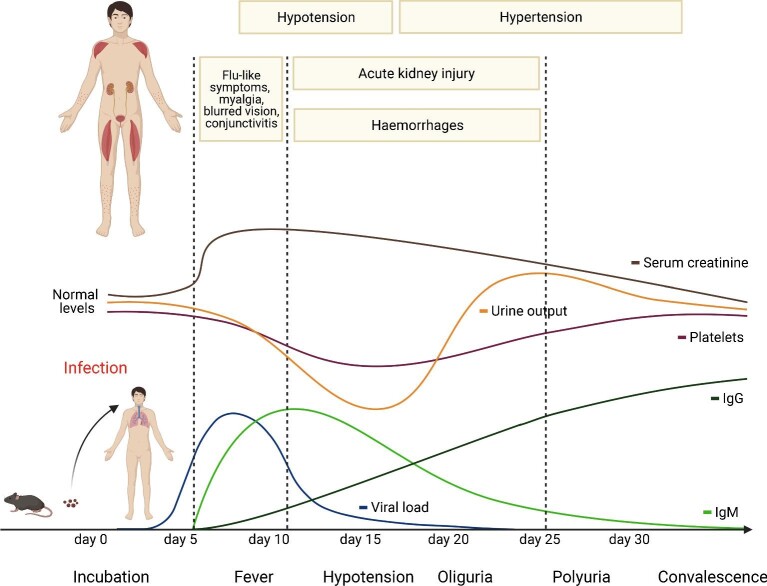
The typical disease course in HFRS can be divided into five distinct phases: fever, hypotension, oliguria, polyuria and convalescence. After human infection, viral load peaks after 5–10 days and prodromal symptoms such as flu-like symptoms, myalgia, backache, abdominal pain and blurred vision occur. In parallel, platelets as well as urine output and kidney function decrease, leading to the hallmark triad of AKI, hypotension and hemorrhages in HFRS. With the onset of clinical symptoms, antibodies increase, leading to viral clearance and convalescence. Source: Figure created with Biorender.com and figure concept adapted from Avsic-Zupanc et al. [3].
Due to the intrarenal inflammatory processes, swelling of the kidneys is a hallmark of hantavirus-caused AKI, which is typically accompanied by—sometimes very severe—abdominal pain or backache. Proteinuria, sometimes in the nephrotic range, is also a typical finding in hantavirus-caused AKI and reflects the involvement of podocytes. The magnitude of proteinuria is associated with disease severity [178–180]. Renal replacement therapy (RRT) is needed in ∼5% of PUUV and 30% of both DOBV and HTNV infections, and half of HFRS deaths occur in this oliguric phase [3, 181–185]. Approximately 10% of DOBV- and HTNV-infected patients require mechanical ventilation due to the development of acute respiratory distress syndrome (ARDS) caused by acute progressive noncardiac pulmonary edema, whereas mechanical ventilation is rarely needed in PUUV-caused disease [168, 172, 186–188]. With the onset of the polyuric stage, kidney function gradually improves and urinary output may be increased for several weeks (Figure 5). Finally, convalescence, which is characterized by full recovery of kidney function, is usually prolonged and takes up to 3–6 months [189]. Of note, the risk for acute myocardial infarction, stroke and venous thromboembolisms is significantly elevated in this phase as a consequence of enhanced coagulation and inflammation after hantavirus disease [129, 130]. Although kidney function is usually completely restored, chronic kidney disease (CKD) and hypertension have been reported as rare long-term sequelae—robust data on the frequency and relative risk of CKD and RRT in HFRS (especially in DOBV- and HTNV-infected patients) are missing (Table 2) [172, 190–192].
PUUV-caused nephropathia epidemica is a mild form of HFRS that is the most common hantavirus disease in Europe. The typical disease stages are often not clearly distinguishable, as most patients suffer from less severe kidney failure that rarely requires RRT and hemorrhagic manifestations are usually mild [177, 181, 182]. PUUV-caused HFRS is often misdiagnosed due to its rather uncharacteristic clinical phenotype that resembles a febrile disease with abdominal pain [3, 193]. Different from other hantaviruses, SEOV is characterized by accompanying liver dysfunction during HFRS [194].
Children have a similar clinical presentation of HFRS as adults; however, the disease course is usually milder, with abdominal manifestations of hantavirus infection being more frequent [195, 196].
HCPS
The clinical phenotype of HCPS is characterized by ARDS, with case fatality rates of 30–50% [15, 197]. However, disease severity ranges from mild hypoxemia to fatal respiratory failure with precapillary cardiogenic shock [198].
The prodromal phase observed in HCPS is similar to HFRS and includes unspecific flu-like symptoms, including fever, chills, myalgia, nausea and vomiting. The disease course is characterized by progress to a cardiopulmonary stage with tachycardia, arrhythmia, shortness of breath and cough. Due to the vascular leakage in lung capillaries, patients rapidly develop noncardiac pulmonary edemas, oxygenation impairment and hypotension. Additionally, pulmonary infiltrates and pleural effusion may be detected. Patients with severe HCPS frequently require mechanical ventilation; often the disease course is further complicated by cardiogenic shock, lactic acidosis and hemoconcentration [3]. Therefore patients often die within hours after hospitalization when entering the fulminant cardiopulmonary phase [3]. When pulmonary edema resolves, patients develop polyuria and full recovery is achieved only after months [199].
With regard to kidney involvement in HCPS, proteinuria is frequently seen and is associated with mortality [200–202]. Moreover, AKI occurs in ∼15–20% of HCPS patients and RRT is needed in ∼10% of all HCPS patients [200, 203, 204]. The pathogenesis of kidney involvement in HCPS is currently unclear, as—due to the profound thrombocytopenia and DIC in the critically ill HCPS patient cohort—kidney biopsy samples are not available. A recent but small Brazilian autopsy study reported marked acute tubular necrosis. This notwithstanding, more pathological characterization of kidney phenotype in HCPS is needed [205]. Long-term kidney outcome in HCPS has been scarcely examined, mainly due to the low incidence and high mortality of the disease (Table 2). A small prospective study from the USA reported kidney sequelae after HCPS consisting of proteinuria and development of CKD in half of the reported cases [206].
Hantavirus disease—an interconnected syndrome beyond HFRS and HCPS
Renal and pulmonary diseases are often assigned to either Old World or New World hantaviruses in a dichotomous manner. However, HFRS and HCPS are defined and named after symptoms that also may be partly absent or incomplete. Recent advances in characterizing and understanding pathogenesis, organ tropism and clinical phenotype have confirmed common features of HFRS and HCPS and have therefore led to the perception of an interconnected disease [200, 207]. To this end, various reports indicate a large overlap of renal and pulmonary impairment in Old World and New World hantaviruses [168, 200, 206, 208–210]. For example, frequent development of ARDS is observed in HFRS caused by DOBV and HTNV [186–188, 211]. In PUUV infections, there are case reports that the human lung may be the primarily organ system affected, with PUUV being additionally detected in bronchoalveolar lavage (BAL) fluid [168, 208, 209, 212–215]. In HCPS-assigned SNV and ANDV, RRT is often needed for acute treatment [198, 203, 216]. In order to refine our knowledge on epidemiologic and clinical aspects of hantavirus disease and to determine disease progression beyond the dichotomous denominations of HFRS and HCPS—i.e., to define diagnostic criteria from large cohorts rather than case reports—a novel platform, the Hantavirus Registry (NCT04323904), was recently developed [217].
Diagnosis
The diagnosis of hantavirus-associated diseases is based on clinical findings, local epidemiology and laboratory methods. Hallmark laboratory findings of hantavirus infection are thrombocytopenia, leukocytosis, hemoconcentration, elevated serum creatinine levels, hematuria and proteinuria. Further laboratory markers depend on the other preferentially targeted organs [2].
Hantavirus-specific immunoglobulin M (IgM) and IgG antibodies are usually present in patients at the onset of symptoms. In contrast to IgG antibodies that persist lifelong, IgM titers are detectable in the acute phase of infection and decline over a period of 2–6 months (Figure 5) (3, 218–220). Therefore an IgM capture test is combined with an IgG test to detect acute infection. In Europe, hantavirus-specific diagnostic strategies should at least cover DOBV and PUUV. The serodiagnosis of hantavirus infection can be performed by enzyme-linked immunosorbent assay (ELISA) or immunoblot, predominantly detecting antibodies against N proteins [3, 221, 222]. ELISA is used in most commercially available serologic tests and employs recombinant protein antigens taken from DOBV, HTNV, PUUV, SEOV, SNV and ANDV (Table 4) [223]. The sensitivity and specificity for ELISA-based serodiagnosis are both ∼95% [223–225]. Also, immunoblots for DOBV, HTNV, PUUV and SEOV are commercially available and the reported sensitivity and specificity are 96% and 100%, respectively [223, 226]. Immunofluorescence assays (IFAs) examine the reaction of patient serum samples to uninfected or hantavirus-infected cells. IFAs have been most widely used in Europe and facilitate the detection of DOBV/HTNV and PUUV. The reported sensitivity and specificity are 98% and 91%, respectively [227]. Of note, results obtained from ELISAs should be confirmed by independent tests and in Europe both immunoblot and IFA are used for confirmation. Rapid immunochromatographic IgM antibody tests allow for point-of-care diagnosis but have not yet entered widespread use; they are also limited in sensitivity [228, 229].
Table 4.
Comparison of frequently used commercially available diagnostic approaches for hantavirus disease
| Diagnostic test | Antigen type/hantavirus detected | Sensitivity (%) | Specificity (%) | Comments | References |
|---|---|---|---|---|---|
| ELISA | DOBVHTNVPUUVSEOVANDVSNV | 95–97 | 94–99 | Combination of IgG test and IgM capture test is recommended | [224, 225] |
| IFA | DOBVHTNVPUUV | 98 | 91 | Used as a confirmation test in Europe | [227] |
| Immunoblot assay | DOBVHTNVPUUVSEOV | 96 | 100 | Used as a confirmation test in Europe | [226] |
| Rapid immunochromatographic IgM antibody tests | DOBVHTNVPUUVSNV | 80–93 | 96 | Point-of-care test | [228, 229] |
| (Real-time)-PCR | Facilitates sequencing of the viral genome and the detection of novel hantaviruses | 92–98 | 80–98 | Time to test positivity <24 h Only useful in early viremic stage of infection | [265, 266] |
The hantavirus genome can be detected by both traditional and real-time polymerase chain reaction (PCR)-based diagnostics in blood, saliva, BAL fluids and tissue samples. Of note, PCR-based diagnostics facilitate postmortem diagnosis and organ involvement. The viral load detected in blood samples at the onset of infection may even predict disease severity in both HFRS and HCPS; however, the viremic phase is limited to the very early stage of the disease (Figure 5) [230–233]. Another drawback of PCR-based diagnostics is the possibility of obtaining false-negative results in low-viremic infections often seen in, for example, PUUV or in patients tested late in disease [2]. Moreover, PCR-based diagnostics are prone to cross-contamination. Major advantages of molecular-based diagnostics are rapid test results obtained within 24 h, especially important in critically ill patients, and the ability to sequence the viral genome for phylogenetic analysis [223].
Prevention
Exposure to infected rodents is the main risk factor for hantavirus-associated diseases and, due to a lack of targeted antiviral therapy, preventive measures such as rodent control and avoiding contact with potentially infected areas are recommended to minimize risk of transmission [234–236]. A history of possible exposure to rodent excreta can be obtained in ∼50% of cases. Thus, identifying typical scenarios (e.g., sweeping out areas that are likely inhabited by rodents) is helpful but can certainly not be a prerequisite for making the diagnosis. Especially in endemic areas, hantavirus diseases should be suspected based on the clinical picture and laboratory findings rather than a history of exposure [236].
Vaccinations still remain controversial: in Korea, Hantavax is in use, a vaccine derived from formalin-inactivated HTNV-infected suckling mouse brain; however, frequent booster doses are mandatory for achieving immunity [237, 238]. Several formalin-inactivated vaccines have been used in China with unknown protective efficacies [238, 239]. No vaccines are currently approved in Europe or the USA. DNA vaccines for hantaviruses that may facilitate construction of multivalent vaccines and long-lasting immunity are being tested in clinical trials [240, 241]. Based on the recent experiences in the COVID-19 pandemic, RNA vaccines may actually be a very promising strategy to extend to hantaviruses.
Therapy
Currently treatment of both HFRS and HCPS is mainly supportive, as there is no specific therapy available [242]. For close monitoring, patients with a severe disease course should be admitted to the intensive care unit to maintain euvolemia and electrolyte balance. Especially in anuric patients, the volume status should be closely monitored to avoid extensive fluid retention and pulmonary edema due to leaky capillaries. RRT is needed in 4–6% of the hospital-treated patients in PUUV, in 10–20% in DOBV and up to 40–50% in HTNV infections [170, 179, 182, 183, 193, 243–245]. In the case of severe thrombocytopenia and major bleeding events due to DIC, platelet transfusion and/or fresh frozen plasma may be necessary to improve coagulation and control the bleeding event [3, 246]. Of note, there are hints that the extent of thrombocytopenia at the onset of infection predicts AKI severity as well as the clinical course in HFRS patients [170, 247]. In HCPS patients, supportive therapy consists of oxygen supplementation, mechanical ventilation when necessary, inotropic support and maintenance of euvolemia [199]. Additionally, extracorporeal membrane oxygenation improves outcome in patients with refractory shock and ARDS [248, 249].
Currently there is no targeted antiviral therapy available in Europe or the USA. Ribavirin has shown some antiviral effects against hantaviruses in in vitro models of HTNV-caused HFRS, since it is inhibiting viral replication through targeting the RdRp [250, 251]. In humans, ribavirin has been tested in a double-blind, placebo-controlled clinical trial in China, suggesting improved outcome and reduced severity of renal insufficiency when administered within 5 days after the onset of symptoms in HTNV-caused HFRS (Table 5) [252, 253]. In contrast, a randomized, open-label Russian trial revealed that ribavirin-associated side effects such as rash, sinus bradycardia, hyperbilirubinemia and anemia were significantly increased, while showing no protective effects in PUUV-infected patients [254]. Of note, disease severity in PUUV-caused HFRS is—in contrast to HTNV —not associated with the viral load, which may be a reasonable explanation for these different outcomes (Table 5) [255–257]. Ribavirin has also been examined in HCPS patients in a prospective, double-blind, placebo-controlled trial revealing no beneficial effects. Currently there is no recommendation to use ribavirin in HFRS or HCPS [258, 259].
Table 5.
Comparison of clinical trials examining ribavirin in hantavirus disease
| Characteristics | Huggins et al. [252] | Malinin et al. [254] | Mertz et al. [258] |
|---|---|---|---|
| Disease entity | HFRS | HFRS | HCPS |
| Study period | 1985–1987 | 2004–2005 | 1996—2001 |
| Trial design | Prospective, double-blind, placebo-controlled trial | Prospective, open-label, phase II study | Prospective, double-blind, placebo-controlled trial |
| Number of patients | 293 | 73 | 36 |
| Dose of ribavirin | Loading dose of 33 mg/kg, followed by a dose of 16 mg/kg every 6 h for the first 4 days and 8 mg/kg every 8 h for the subsequent 3 days | Loading dose of 33 mg/kg, followed by a dose of 16 mg/kg every 6 h for the first 4 days and 8 mg/kg every 8 h for the subsequent 3 days | Loading dose of 33 mg/kg, followed by a dose of 16 mg/kg every 6 h for the first 4 days and 8 mg/kg every 8 h for the subsequent 3 days |
| Timing of ribavirin with respect to onset of infection | 4 days (lengthened to 6 days in 1986) after onset of symptoms | 4 days after onset of symptoms | Not specified; however, patients had to be in the prodromal or cardiopulmonary stage |
| Hantaviruses involved | HTNV (confirmed in 82.6% by ELISA) | PUUV | SNV (confirmed in 63.9% by ELISA) |
| Primary endpoint | Reduction in mortality, occurrence of oliguria and hemorrhages | Change in viral load | Survival at day 28 after study entry |
| Results for primary endpoint | 7-fold reduction of mortality in the ribavirin group (P = .01)3.7 reduction of oliguria in the ribavirin group (P = .01) | Insufficient efficacy of ribavirin | No difference in survival between the ribavirin and placebo group |
| Adverse events | Drug-related anemia in all male study subjects (males accounted for 75% of study subjects). Females showed similar trends that were less dramatic due to sex-related differences in hematocrit | Low hemoglobin levels in 95%, hyperbilirubinemia in 81%, sinus bradycardia in 43% and rash in 19% of the ribavirin-treated patients | No significant differences in the frequency of adverse events; however, there was trend toward a higher rate in anemia in the ribavirin group |
| Inclusion criteria | Age ≥14 years Fever duration ≤4 days (lengthened to 6 days in 1986) Clinical diagnosis of HFRS including fever and proteinuria History making exposure to infection likelyOR Findings consistent with early HFRS Hantaviral IgM antibodies No other evidence for an alternative diagnosis | Age 18–65 years Suspected diagnosis of HFRS within 4 days of onset of disease SOFA score = 1 | Age ≥12 years Suspected or serologically confirmed acute hantavirus disease in the prodromal of cardiopulmonary stage |
| Exclusion criteria | Advanced renal failure manifested by oliguria or uremia Pregnancy or breast feeding Known intolerance to ribavirin Moribund on presentation or life expectancy <48 h Pre-existing non-HFRS life-threatening condition | Known intolerance to ribavirin Pregnancy or breast feeding NYHA cardiac function ≥2 History of severe chronic pulmonary or kidney disease History of autoimmune hepatitis Serum aminotransferase levels greater than two times the upper limit of normal Hemoglobin level <12 g/dL | Pregnancy or breast-feeding A likely diagnosis other than HCPS Immunocompromised status Receipt of systemic corticosteroids within 30 days prior to enrolment A mean arterial pressure of <60 mmHg for 2 h despite optimal medical management A cardiac index <2.1 L/min/m2 Arterial oxygen pressure <65 mmHg in intubated subjects receiving 100% oxygen The presence of unilateral pulmonary infiltrates that did not become bilateral within 24 h |
| Comments | Study showed efficacy in reducing case fatality and oliguria in HTNV-infected patients | Study revealed insufficient efficacy and safety of intravenous ribavirin in PUUV-infected patients. Severity of PUUV-caused HFRS is not associated with viral load, in contrast to HTNV, explaining the different outcomes observed [255–257] | Premature termination of the study due to the slow rate of accrual of subjects and the findings of futility analysis |
NYHA: New York Hearth Association; SOFA: Sepsis-related Organ Failure Assessment score.
Apart from ribavirin, methylprednisolone has been tested in a randomized controlled clinical trial in ADNV-infected patients, failed to provide any significant benefit to patients (Table 6) [204]. Novel potential therapeutic strategies that were tested in noncontrolled clinical trials include murine and human-derived neutralizing antibodies that block viral entry. In contrast to murine-derived neutralizing antibodies, for which efficacy is unknown, human-derived neutralizing antibodies resulted in a 50% reduction of case fatalities in ANDV infections (Table 6) [260–262]. In addition, bradykinin type 2 receptor antagonists have been tested in PUUV-infected patients and may be promising candidates [107, 111, 112, 263]. However, standardized, large-scale clinical trials examining both efficacy and safety are lacking [238].
Table 6.
Summary of potential antiviral approaches for hantavirus disease tested in humans
| Drug | Known /putative target | Purpose | Virus | Number of patients | Outcome/ comments | References |
|---|---|---|---|---|---|---|
| Murine monoclonal antibodies | Gc/Gn | Blocking viral entry | n.a. | 22 | Safety in a phase I trial examined, proof of efficacy lacking | [261] |
| Human immune plasma | Gc / Gn | Blocking viral entry | ANDV | 32 | Safe and efficient with a 50% reduction in case fatalities in an uncontrolled clinical trial | [267] |
| Icatibant | Bradykinin receptor 2 | Improving vascular function | PUUV | Case reports | Clinical trials apart from case reports lacking | [110–112] |
| Methylprednisolone | Immunotherapy | Rebuilding immune homeostasis | ANDV | 66 | No beneficial effect reported | [204] |
| Ribavirin | RdRp | Inhibiting viral replication | ANDV, HTNV, PUUV | 547 | Efficient in HTNV, inefficient and unsafe in PUUV and ANDV (see Table 5) | [252, 254, 258, 259] |
Gc: C-glycoprotein; Gn: N-glycoprotein; n.a.: not applicable.
CONCLUSION
Hantavirus-associated diseases are emerging zoonotic infections with increasing incidence rates in Europe. The distribution of hantaviruses is influenced by climate change and disturbed rodent habitats and new hantavirus species with unknown pathogenicity are encountered in these reservoirs. Recently discovered human-to-human transmission by superspreading events may further cause hantaviral spread and make a pandemic a possible scenario. Therefore further research in hantavirus pathogenesis, diagnosis antiviral and preventive measures, including vaccine development, remain mandatory.
Contributor Information
Felix C Koehler, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; CECAD, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Veronica Di Cristanziano, Institute of Virology, University of Cologne, Faculty of Medicine and University Hospital of Cologne, Cologne, Germany.
Martin R Späth, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; CECAD, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
K Johanna R Hoyer-Allo, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; CECAD, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Manuel Wanken, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Roman-Ulrich Müller, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; CECAD, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Volker Burst, Department II of Internal Medicine and Center for Molecular Medicine Cologne, University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
AUTHORS’ CONTRIBUTIONS
F.C.K., R.-U.M. and V.B. wrote the manuscript. V.D.C., M.R.S., K.J.R.H.-A. and M.W. revised the manuscript.
CONFLICT OF INTEREST STATEMENT
F.C.K. has received a grant from the Maria-Pesch Stiftung, Cologne, Germany for setup and maintenance of the Hantavirus Registry. All other authors have nothing to disclose.
REFERENCES
- 1. Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010; 23: 412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kruger DH, Figueiredo LT, Song JWet al. Hantaviruses—globally emerging pathogens. J Clin Virol 2015; 64: 128–136 [DOI] [PubMed] [Google Scholar]
- 3. Avsic-Zupanc T, Saksida A, Korva M. Hantavirus infections. Clin Microbiol Infect 2019; 21S: e6–e16 [DOI] [PubMed] [Google Scholar]
- 4. Heyman P, Ceianu CS, Christova Iet al. A five-year perspective on the situation of haemorrhagic fever with renal syndrome and status of the hantavirus reservoirs in Europe, 2005–2010. Euro Surveill 2011; 16: 19961. [DOI] [PubMed] [Google Scholar]
- 5. Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis 1978; 137: 298–308 [DOI] [PubMed] [Google Scholar]
- 6. Brummer-Korvenkontio M, Vaheri A, Hovi Tet al. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis 1980; 141: 131–134 [DOI] [PubMed] [Google Scholar]
- 7. Avsic-Zupanc T, Xiao SY, Stojanovic Ret al. Characterization of Dobrava virus: a hantavirus from Slovenia, Yugoslavia. J Med Virol 1992; 38: 132–137 [DOI] [PubMed] [Google Scholar]
- 8. Gligic A, Dimkovic N, Xiao SYet al. Belgrade virus: a new hantavirus causing severe hemorrhagic fever with renal syndrome in Yugoslavia. J Infect Dis 1992; 166: 113–120 [DOI] [PubMed] [Google Scholar]
- 9. Taller AM, Xiao SY, Godec MSet al. Belgrade virus, a cause of hemorrhagic fever with renal syndrome in the Balkans, is closely related to Dobrava virus of field mice. J Infect Dis 1993; 168: 750–753 [DOI] [PubMed] [Google Scholar]
- 10. Markotić A, LeDuc JW, Hlaca Det al. Hantaviruses are likely threat to NATO forces in Bosnia and Herzegovina and Croatia. Nat Med 1996; 2: 269–270 [DOI] [PubMed] [Google Scholar]
- 11. Bugert JJ, Welzel TM, Zeier Met al. Hantavirus infection—haemorrhagic fever in the Balkans—potential nephrological hazards in the Kosovo war. Nephrol Dial Transplant 1999; 14: 1843–1844 [DOI] [PubMed] [Google Scholar]
- 12. Makary P, Kanerva M, Ollgren Jet al. Disease burden of Puumala virus infections, 1995–2008. Epidemiol Infect 2010; 138: 1484–1492 [DOI] [PubMed] [Google Scholar]
- 13. Hjertqvist M, Klein SL, Ahlm Cet al. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 2010; 16: 1584–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heyman P, Vaheri A, Lundkvist Aet al. Hantavirus infections in Europe: from virus carriers to a major public-health problem. Expert Rev Anti Infect Ther 2009; 7: 205–217 [DOI] [PubMed] [Google Scholar]
- 15. Duchin JS, Koster FT, Peters CJet al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med 1994; 330: 949–955 [DOI] [PubMed] [Google Scholar]
- 16. Hjelle B, Jenison S, Torrez-Martinez Net al. A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relationships to known hantaviruses. J Virol 1994; 68: 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nichol ST, Spiropoulou CF, Morzunov Set al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 1993; 262: 914–917 [DOI] [PubMed] [Google Scholar]
- 18. Martínez VP, Di Paola N, Alonso DOet al. “Super-spreaders” and person-to-person transmission of Andes virus in Argentina. N Engl J Med 2020; 383: 2230–2241 [DOI] [PubMed] [Google Scholar]
- 19. Wells RM, Sosa Estani S, Yadon ZEet al. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg Infect Dis 1997; 3: 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padula PJ, Edelstein A, Miguel SDet al. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 1998; 241: 323–330 [DOI] [PubMed] [Google Scholar]
- 21. Pizarro E, Navarrete M, Mendez Cet al. Immunocytochemical and ultrastructural evidence supporting that Andes hantavirus (ANDV) is transmitted person-to-person through the respiratory and/or salivary pathways. Front Microbiol 2019; 10: 2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis 1997; 3: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaheri A, Strandin T, Hepojoki Jet al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 2013; 11: 539–550 [DOI] [PubMed] [Google Scholar]
- 24. Klempa B. Hantaviruses and climate change. Clin Microbiol Infect 2009; 15: 518–523 [DOI] [PubMed] [Google Scholar]
- 25. Zeimes CB, Quoilin S, Henttonen Het al. Landscape and regional environmental analysis of the spatial distribution of hantavirus human cases in Europe. Front Public Health 2015; 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guterres A, de Lemos ERS. Hantaviruses and a neglected environmental determinant. One Health 2018; 5: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koch DE, Mohler RL, Goodin DG. Stratifying land use/land cover for spatial analysis of disease ecology and risk: an example using object-based classification techniques. Geospat Health 2007; 2: 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen A, Cameron S, Liu Qet al. Transmission of haemorrhagic fever with renal syndrome in China and the role of climate factors: a review. Int J Infect Dis 2015; 33: 212–218 [DOI] [PubMed] [Google Scholar]
- 29. Prist PR, Uriarte M, Fernandes Ket al. Climate change and sugarcane expansion increase Hantavirus infection risk. PLoS Negl Trop Dis 2017; 11: e0005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roda Gracia J, Schumann B, Seidler A. Climate variability and the occurrence of human Puumala hantavirus infections in Europe: a systematic review. Zoonoses Public Health 2015; 62: 465–478 [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Xue FZ, Wang JZet al. Association of haemorrhagic fever with renal syndrome and weather factors in Junan County, China: a case-crossover study. Epidemiol Infect 2013; 141: 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prevedello JA, Dickman CR, Vieira MVet al. Population responses of small mammals to food supply and predators: a global meta-analysis. J Anim Ecol 2013; 82: 927–936 [DOI] [PubMed] [Google Scholar]
- 33. Monchatre-Leroy E, Crespin L, Boué Fet al. Spatial and temporal epidemiology of nephropathia epidemica incidence and hantavirus seroprevalence in rodent hosts: identification of the main environmental factors in Europe. Transbound Emerg Dis 2017; 64: 1210–1228 [DOI] [PubMed] [Google Scholar]
- 34. Luis AD, Douglass RJ, Mills JNet al. The effect of seasonality, density and climate on the population dynamics of Montana deer mice, important reservoir hosts for Sin Nombre hantavirus. J Anim Ecol 2010; 79: 462–470 [DOI] [PubMed] [Google Scholar]
- 35. Fang LQ, Wang XJ, Liang Set al. Spatiotemporal trends and climatic factors of hemorrhagic fever with renal syndrome epidemic in Shandong Province, China. PLoS Negl Trop Dis 2010; 4: e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borg MA, Bi P. The impact of climate change on kidney health. Nat Rev Nephrol 2021; 17: 294–295 [DOI] [PubMed] [Google Scholar]
- 37. Xiao H, Tian HY, Cazelles Bet al. Atmospheric moisture variability and transmission of hemorrhagic fever with renal syndrome in Changsha City, Mainland China, 1991–2010. PLoS Negl Trop Dis 2013; 7: e2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou Y, Hu J, Wang ZXet al. Genetic characterization of hantaviruses isolated from Guizhou, China: evidence for spillover and reassortment in nature. J Med Virol 2008; 80: 1033–1041 [DOI] [PubMed] [Google Scholar]
- 39. Reusken C, Heyman P. Factors driving hantavirus emergence in Europe. Curr Opin Virol 2013; 3: 92–99 [DOI] [PubMed] [Google Scholar]
- 40. Clement J, Underwood P, Ward Det al. Hantavirus outbreak during military manoeuvres in Germany. Lancet 1996; 347: 336. [DOI] [PubMed] [Google Scholar]
- 41. Vapalahti K, Paunio M, Brummer-Korvenkontio Met al. Puumala virus infections in Finland: increased occupational risk for farmers. Am J Epidemiol 1999; 149: 1142–1151 [DOI] [PubMed] [Google Scholar]
- 42. Watson DC, Sargianou M, Papa Aet al. Epidemiology of hantavirus infections in humans: a comprehensive, global overview. Crit Rev Microbiol 2014; 40: 261–272 [DOI] [PubMed] [Google Scholar]
- 43. Vapalahti O, Mustonen J, Lundkvist Aet al. Hantavirus infections in Europe. Lancet Infect Dis 2003; 3: 653–661 [DOI] [PubMed] [Google Scholar]
- 44. Hofmann J, Meisel H, Klempa Bet al. Hantavirus outbreak, Germany, 2007. Emerg Infect Dis 2008; 14: 850–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ettinger J, Hofmann J, Enders Met al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg Infect Dis 2012; 18: 1461–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Avsic-Zupanc T, Petrovec M, Furlan Pet al. Hemorrhagic fever with renal syndrome in the Dolenjska region of Slovenia—a 10-year survey. Clin Infect Dis 1999; 28: 860–865 [DOI] [PubMed] [Google Scholar]
- 47. Antoniadis A, Stylianakis A, Papa Aet al. Direct genetic detection of Dobrava virus in Greek and Albanian patients with hemorrhagic fever with renal syndrome. J Infect Dis 1996; 174: 407–410 [DOI] [PubMed] [Google Scholar]
- 48. Klempa B, Radosa L, Kruger DH. The broad spectrum of hantaviruses and their hosts in Central Europe. Acta Virol 2013; 57: 130–137 [DOI] [PubMed] [Google Scholar]
- 49. Lee SH, No JS, Kim WKet al. Molecular epidemiology and genetic diversity of orthohantaviruses in small mammals in Western Poland. Am J Trop Med Hyg 2020; 103: 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Faber M, Krüger DH, Auste Bet al. Molecular and epidemiological characteristics of human Puumala and Dobrava–Belgrade hantavirus infections, Germany, 2001 to 2017. Euro Surveill 2019; 24: 1800675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dzagurova TK, Klempa B, Tkachenko EAet al. Molecular diagnostics of hemorrhagic fever with renal syndrome during a Dobrava virus infection outbreak in the European part of Russia. J Clin Microbiol 2009; 47: 4029–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hofmann J, Kramer S, Herrlinger KRet al. Tula virus as causative agent of hantavirus disease in immunocompetent person, Germany. Emerg Infect Dis 2021; 27: 1234–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klempa B, Stanko M, Labuda Met al. Central European Dobrava hantavirus isolate from a striped field mouse (Apodemus agrarius). J Clin Microbiol 2005; 43: 2756–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sjölander KB, Golovljova I, Vasilenko Vet al. Serological divergence of Dobrava and Saaremaa hantaviruses: evidence for two distinct serotypes. Epidemiol Infect 2002; 128: 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Klempa B, Meisel H, Rath Set al. Occurrence of renal and pulmonary syndrome in a region of northeast Germany where Tula hantavirus circulates. J Clin Microbiol 2003; 41: 4894–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baek LJ, Kariwa H, Lokugamage Ket al. Soochong virus: an antigenically and genetically distinct hantavirus isolated from Apodemus peninsulae in Korea. J Med Virol 2006; 78: 290–297 [DOI] [PubMed] [Google Scholar]
- 57. Jiang JF, Zhang WY, Wu XMet al. Soochong virus and Amur virus might be the same entities of hantavirus. J Med Virol 2007; 79: 1792–1795 [DOI] [PubMed] [Google Scholar]
- 58. Zhang YZ, Zou Y, Fu ZFet al. Hantavirus infections in humans and animals, China. Emerg Infect Dis 2010; 16: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee SH, Kim WK, Park Ket al. Genetic diversity and phylogeography of Jeju orthohantavirus (Hantaviridae) in the Republic of Korea. Virology 2020; 543: 13–19 [DOI] [PubMed] [Google Scholar]
- 60. Lee SH, Kim WK, No JSet al. Dynamic circulation and genetic exchange of a shrew-borne hantavirus, Imjin virus, in the Republic of Korea. Sci Rep 2017; 7: 44369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee PW, Gibbs CJ Jr, Gajdusek DCet al. Identification of epidemic haemorrhagic fever with renal syndrome in China with Korean haemorrhagic fever. Lancet 1980; 1: 1025–1026 [DOI] [PubMed] [Google Scholar]
- 62. Knust B, Brown S, de St Maurice Aet al. Seoul virus infection and spread in US home-based ratteries-rat and human testing results from a multistate outbreak investigation. J Infect Dis 2020; 222: 1311–1319 [DOI] [PubMed] [Google Scholar]
- 63. Reynes JM, Carli D, Bour JBet al. Seoul virus infection in humans, France, 2014–2016. Emerg Infect Dis 2017; 23: 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee HW, van der Groen G. Hemorrhagic fever with renal syndrome. Prog Med Virol 1989; 36: 62–102 [PubMed] [Google Scholar]
- 65. Calderón G, Pini N, Bolpe Jet al. Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emerg Infect Dis 1999; 5: 792–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khan A, Khan M, Ullah Set al. Hantavirus: the next pandemic we are waiting for? Interdiscip Sci 2021; 13: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Klempa B, Fichet-Calvet E, Lecompte Eet al. Hantavirus in African wood mouse, Guinea. Emerg Infect Dis 2006; 12: 838–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Klempa B, Koulemou K, Auste Bet al. Seroepidemiological study reveals regional co-occurrence of Lassa- and Hantavirus antibodies in Upper Guinea, West Africa. Trop Med Int Health 2013; 18: 366–371 [DOI] [PubMed] [Google Scholar]
- 69. Weiss S, Witkowski PT, Auste Bet al. Hantavirus in bat, Sierra Leone. Emerg Infect Dis 2012; 18: 159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sumibcay L, Kadjo B, Gu SHet al. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Côte d'Ivoire. Virol J 2012; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmaljohn CS, Hasty SE, Dalrymple JMet al. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science 1985; 227: 1041–1044 [DOI] [PubMed] [Google Scholar]
- 72. Schmaljohn CS, Dalrymple JM. Analysis of Hantaan virus RNA: evidence for a new genus of bunyaviridae. Virology 1983; 131: 482–491 [DOI] [PubMed] [Google Scholar]
- 73. Hewlett MJ, Pettersson RF, Baltimore D. Circular forms of Uukuniemi virion RNA: an electron microscopic study. J Virol 1977; 21: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kallio ER, Klingstrom J, Gustafsson Eet al. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J Gen Virol 2006; 87: 2127–2134 [DOI] [PubMed] [Google Scholar]
- 75. Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost 2009; 102: 1030–1041 [DOI] [PubMed] [Google Scholar]
- 76. Mackow ER, Gavrilovskaya IN. Cellular receptors and hantavirus pathogenesis. Curr Top Microbiol Immunol 2001; 256: 91–115 [DOI] [PubMed] [Google Scholar]
- 77. Raftery MJ, Kraus AA, Ulrich Ret al. Hantavirus infection of dendritic cells. J Virol 2002; 76: 10724–10733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zaki SR, Greer PW, Coffield LMet al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol 1995; 146: 552–579 [PMC free article] [PubMed] [Google Scholar]
- 79. Hägele S, Müller A, Nusshag Cet al. Motility of human renal cells is disturbed by infection with pathogenic hantaviruses. BMC Infect Dis 2018; 18: 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gavrilovskaya IN, Shepley M, Shaw Ret al. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci USA 1998; 95: 7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gavrilovskaya IN, Brown EJ, Ginsberg MHet al. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J Virol 1999; 73: 3951–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dieterle ME, Solà-Riera C, Ye Cet al. Genetic depletion studies inform receptor usage by virulent hantaviruses in human endothelial cells. Elife 2021; 10: e69708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mittler E, Dieterle ME, Kleinfelter LMet al. Hantavirus entry: perspectives and recent advances. Adv Virus Res 2019; 104: 185–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Klempa B, Koivogui L, Sylla Oet al. Serological evidence of human hantavirus infections in Guinea, West Africa. J Infect Dis 2010; 201: 1031–1034 [DOI] [PubMed] [Google Scholar]
- 85. Martínez-Valdebenito C, Angulo J, Le Corre Net al. A single-nucleotide polymorphism of αVβ3 integrin is associated with the Andes virus infection susceptibility. Viruses 2019; 11: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen XP, Xiong HR, Zhu Net al. Lack of association between integrin αvβ3 gene polymorphisms and hemorrhagic fever with renal syndrome in Han Chinese from Hubei, China. Virol Sin 2017; 32: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jangra RK, Herbert AS, Li Ret al. Protocadherin-1 is essential for cell entry by New World hantaviruses. Nature 2018; 563: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jin M, Park J, Lee Set al. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 2002; 294: 60–69 [DOI] [PubMed] [Google Scholar]
- 89. Ramanathan HN, Jonsson CB. New and Old World hantaviruses differentially utilize host cytoskeletal components during their life cycles. Virology 2008; 374: 138–150. [DOI] [PubMed] [Google Scholar]
- 90. Hepojoki J, Strandin T, Lankinen Het al. Hantavirus structure—molecular interactions behind the scene. J Gen Virol 2012; 93: 1631–1644 [DOI] [PubMed] [Google Scholar]
- 91. Hussein IT, Haseeb A, Haque Aet al. Recent advances in hantavirus molecular biology and disease. Adv Appl Microbiol 2011; 74: 35–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ravkov EV, Compans RW. Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region. J Virol 2001; 75: 1808–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rowe RK, Suszko JW, Pekosz A. Roles for the recycling endosome, Rab8, and Rab11 in hantavirus release from epithelial cells. Virology 2008; 382: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Noack D, Goeijenbier M, Reusken Cet al. Orthohantavirus pathogenesis and cell tropism. Front Cell Infect Microbiol 2020; 10: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kanerva M, Mustonen J, Vaheri A. Pathogenesis of puumala and other hantavirus infections. Rev Med Virol 1998; 8: 67–86 [DOI] [PubMed] [Google Scholar]
- 96. Mustonen J, Outinen T, Laine Oet al. Kidney disease in Puumala hantavirus infection. Infect Dis (Lond) 2017; 49: 321–332 [DOI] [PubMed] [Google Scholar]
- 97. Mustonen J, Mäkelä S, Outinen Tet al. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antiviral Res 2013; 100: 589–604 [DOI] [PubMed] [Google Scholar]
- 98. Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J Virol 2010; 84: 11227–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gavrilovskaya I, Gorbunova E, Koster Fet al. Elevated VEGF levels in pulmonary edema fluid and PBMCs from patients with acute hantavirus pulmonary syndrome. Adv Virol 2012; 2012: 674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fosse JH, Haraldsen G, Falk Ket al. Endothelial cells in emerging viral infections. Front Cardiovasc Med 2021; 8: 619690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mackow ER, Dalrymple NA, Cimica Vet al. Hantavirus interferon regulation and virulence determinants. Virus Res 2014; 187: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem 2000; 275: 39867–39873 [DOI] [PubMed] [Google Scholar]
- 103. Byzova TV, Goldman CK, Pampori Net al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 2000; 6: 851–860 [PubMed] [Google Scholar]
- 104. Reynolds LE, Wyder L, Lively JCet al. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med 2002; 8: 27–34 [DOI] [PubMed] [Google Scholar]
- 105. Wang W, Zhang Y, Li Yet al. Dysregulation of the β3 integrin-VEGFR2 complex in Hantaan virus-directed hyperpermeability upon treatment with VEGF. Arch Virol 2012; 157: 1051–1061 [DOI] [PubMed] [Google Scholar]
- 106. Pal E, Korva M, Resman Rus Ket al. Relationship between circulating vascular endothelial growth factor and its soluble receptor in patients with hemorrhagic fever with renal syndrome. Emerg Microbes Infect 2018; 7: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bird BH, Shrivastava-Ranjan P, Dodd KAet al. Effect of Vandetanib on Andes virus survival in the hamster model of hantavirus pulmonary syndrome. Antiviral Res 2016; 132: 66–69 [DOI] [PubMed] [Google Scholar]
- 108. Grande E, Kreissl MC, Filetti Set al. Vandetanib in advanced medullary thyroid cancer: review of adverse event management strategies. Adv Ther 2013; 30: 945–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Taylor SL, Wahl-Jensen V, Copeland AMet al. Endothelial cell permeability during hantavirus infection involves factor XII-dependent increased activation of the kallikrein–kinin system. PLoS Pathog 2013; 9: e1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vaheri A, Strandin T, Jääskeläinen AJet al. Pathophysiology of a severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Antiviral Res 2014; 111: 23–25 [DOI] [PubMed] [Google Scholar]
- 111. Antonen J, Leppänen I, Tenhunen Jet al. A severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Scand J Infect Dis 2013; 45: 494–496 [DOI] [PubMed] [Google Scholar]
- 112. Laine O, Leppänen I, Koskela Set al. Severe Puumala virus infection in a patient with a lymphoproliferative disease treated with icatibant. Infect Dis (Lond) 2015; 47: 107–111 [DOI] [PubMed] [Google Scholar]
- 113. Marsac D, García S, Fournet Aet al. Infection of human monocyte-derived dendritic cells by ANDES Hantavirus enhances pro-inflammatory state, the secretion of active MMP-9 and indirectly enhances endothelial permeability. Virol J 2011; 8: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Niikura M, Maeda A, Ikegami Tet al. Modification of endothelial cell functions by Hantaan virus infection: prolonged hyper-permeability induced by TNF-alpha of hantaan virus-infected endothelial cell monolayers. Arch Virol 2004; 149: 1279–1292 [DOI] [PubMed] [Google Scholar]
- 115. Mäkelä S, Mustonen J, Ala-Houhala Iet al. Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am J Kidney Dis 2004; 43: 809–816 [DOI] [PubMed] [Google Scholar]
- 116. Linderholm M, Ahlm C, Settergren Bet al. Elevated plasma levels of tumor necrosis factor (TNF)-α, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis 1996; 173: 38–43 [DOI] [PubMed] [Google Scholar]
- 117. Linderholm M, Groeneveld PH, Tärnvik A. Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome—relation to arterial hypotension and tumor necrosis factor. Infection 1996; 24: 337–340 [DOI] [PubMed] [Google Scholar]
- 118. Khaiboullina SF, Levis S, Morzunov SPet al. Serum cytokine profiles differentiating hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Front Immunol 2017; 8: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Morzunov SP, Khaiboullina SF, St Jeor Set al. Multiplex analysis of serum cytokines in humans with hantavirus pulmonary syndrome. Front Immunol 2015; 6: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Varga Z, Flammer AJ, Steiger Pet al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ackermann M, Verleden SE, Kuehnel Met al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jessie K, Fong MY, Devi Set al. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 2004; 189: 1411–1418 [DOI] [PubMed] [Google Scholar]
- 123. Martines RB, Ng DL, Greer PWet al. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 2015; 235: 153–174 [DOI] [PubMed] [Google Scholar]
- 124. Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J Virol 2010; 84: 4832–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Raymond T, Gorbunova E, Gavrilovskaya INet al. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent αvβ3 integrin conformers. Proc Natl Acad Sci USA 2005; 102: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Laine O, Mäkelä S, Mustonen Jet al. Platelet ligands and ADAMTS13 during Puumala hantavirus infection and associated thrombocytopenia. Blood Coagul Fibrinolysis 2011; 22: 468–472 [DOI] [PubMed] [Google Scholar]
- 127. Laine O, Mäkelä S, Mustonen Jet al. Enhanced thrombin formation and fibrinolysis during acute Puumala hantavirus infection. Thromb Res 2010; 126: 154–158 [DOI] [PubMed] [Google Scholar]
- 128. Sundberg E, Hultdin J, Nilsson Set al. Evidence of disseminated intravascular coagulation in a hemorrhagic fever with renal syndrome-scoring models and severe illness. PLoS One 2011; 6: e21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Connolly-Andersen AM, Whitaker H, Klingström Jet al. Risk of venous thromboembolism following hemorrhagic fever with renal syndrome: a self-controlled case series study. Clin Infect Dis 2018; 66: 268–273 [DOI] [PubMed] [Google Scholar]
- 130. Connolly-Andersen AM, Hammargren E, Whitaker Het al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: a self-controlled case series study. Circulation 2014; 129: 1295–1302 [DOI] [PubMed] [Google Scholar]
- 131. Schmedes CM, Grover SP, Hisada YMet al. Circulating extracellular vesicle tissue factor activity during orthohantavirus infection is associated with intravascular coagulation. J Infect Dis 2020; 222: 1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tatsumi K, Hisada Y, Connolly AFet al. Patients with severe orthohantavirus cardiopulmonary syndrome due to Sin Nombre virus infection have increased circulating extracellular vesicle tissue factor and an activated coagulation system. Thromb Res 2019; 179: 31–33 [DOI] [PubMed] [Google Scholar]
- 133. Connolly-Andersen AM, Sundberg E, Ahlm Cet al. Increased thrombopoiesis and platelet activation in hantavirus-infected patients. J Infect Dis 2015; 212: 1061–1069 [DOI] [PubMed] [Google Scholar]
- 134. Kim S, Kang ET, Kim YGet al. Localization of Hantaan viral envelope glycoproteins by monoclonal antibodies in renal tissues from patients with Korean hemorrhagic fever H. Am J Clin Pathol 1993; 100: 398–403 [DOI] [PubMed] [Google Scholar]
- 135. Krautkrämer E, Grouls S, Stein Net al. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J Virol 2011; 85: 9811–9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Krautkramer E, Zeier M, Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int 2013; 83: 23–27 [DOI] [PubMed] [Google Scholar]
- 137. Terajima M, Hayasaka D, Maeda Ket al. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: Do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol Lett 2007; 113: 117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Temonen M, Mustonen J, Helin Het al. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol 1996; 78: 47–55 [DOI] [PubMed] [Google Scholar]
- 139. Matthys V, Mackow ER. Hantavirus regulation of type I interferon responses. Adv Virol 2012; 2012: 524024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schönrich G, Rang A, Lütteke Net al. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol Rev 2008; 225: 163–189 [DOI] [PubMed] [Google Scholar]
- 141. Strandin T, Mäkelä S, Mustonen Jet al. Neutrophil activation in acute hemorrhagic fever with renal syndrome is mediated by hantavirus-infected microvascular endothelial cells. Front Immunol 2018; 9: 2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sadeghi M, Eckerle I, Daniel Vet al. Cytokine expression during early and late phase of acute Puumala hantavirus infection. BMC Immunol 2011; 12: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Outinen TK, Mäkelä SM, Ala-Houhala IOet al. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect Dis 2010; 10: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Saksida A, Wraber B, Avšič-Županc T. Serum levels of inflammatory and regulatory cytokines in patients with hemorrhagic fever with renal syndrome. BMC Infect Dis 2011; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Maleki KT, García M, Iglesias Aet al. Serum markers associated with severity and outcome of hantavirus pulmonary syndrome. J Infect Dis 2019; 219: 1832–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Vernet MA, Reynard S, Fizet Aet al. Clinical, virological, and biological parameters associated with outcomes of Ebola virus infection in Macenta, Guinea. JCI Insight 2017; 2: e88864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ergonul O, Tuncbilek S, Baykam Net al. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean–Congo hemorrhagic fever. J Infect Dis 2006; 193: 941–944 [DOI] [PubMed] [Google Scholar]
- 148. Juffrie M, Meer GM, Hack CEet al. Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. Am J Trop Med Hyg 2001; 65: 70–75 [DOI] [PubMed] [Google Scholar]
- 149. Maude SL, Barrett D, Teachey DTet al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014; 20: 119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Teachey DT, Lacey SF, Shaw PAet al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016; 6: 664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen LB, Yang WS. Abnormalities of T cell immunoregulation in hemorrhagic fever with renal syndrome. J Infect Dis 1990; 161: 1016–1019 [DOI] [PubMed] [Google Scholar]
- 152. Tuuminen T, Kekäläinen E, Mäkelä Set al. Human CD8+ T cell memory generation in Puumala hantavirus infection occurs after the acute phase and is associated with boosting of EBV-specific CD8+ memory T cells. J Immunol 2007; 179: 1988–1995 [DOI] [PubMed] [Google Scholar]
- 153. Mustonen J, Partanen J, Kanerva Met al. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int 1996; 49: 217–221 [DOI] [PubMed] [Google Scholar]
- 154. Wang ML, Lai JH, Zhu Yet al. Genetic susceptibility to haemorrhagic fever with renal syndrome caused by Hantaan virus in Chinese Han population. Int J Immunogenet 2009; 36: 227–229 [DOI] [PubMed] [Google Scholar]
- 155. Mäkelä S, Mustonen J, Ala-Houhala Iet al. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-α(–308) G/A polymorphism. J Infect Dis 2002; 186: 843–846 [DOI] [PubMed] [Google Scholar]
- 156. Kilpatrick ED, Terajima M, Koster FTet al. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol 2004; 172: 3297–3304 [DOI] [PubMed] [Google Scholar]
- 157. Plyusnin A, Hörling J, Kanerva Met al. Puumala hantavirus genome in patients with nephropathia epidemica: correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J Clin Microbiol 1997; 35: 1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mustonen J, Partanen J, Kanerva Met al. Association of HLA B27 with benign clinical course of nephropathia epidemica caused by Puumala hantavirus. Scand J Immunol 1998; 47: 277–279 [DOI] [PubMed] [Google Scholar]
- 159. Ma Y, Yuan B, Yi Jet al. The genetic polymorphisms of HLA are strongly correlated with the disease severity after Hantaan virus infection in the Chinese Han population. Clin Dev Immunol 2012; 2012: 308237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Charbonnel N, Pagès M, Sironen Tet al. Immunogenetic factors affecting susceptibility of humans and rodents to hantaviruses and the clinical course of hantaviral disease in humans. Viruses 2014; 6: 2214–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ferrer CP, Vial CP, Ferrés GMet al. Genetic susceptibility to Andes Hantavirus: association between severity of disease and HLA alíeles in Chilean patients. Rev Chilena Infectol 2007; 24: 351–359 [DOI] [PubMed] [Google Scholar]
- 162. Manigold T, Mori A, Graumann Ret al. Highly differentiated, resting Gn-specific memory CD8+ T cells persist years after infection by Andes hantavirus. PLoS Pathog 2010; 6: e1000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Korva M, Saksida A, Kunilo Set al. HLA-associated hemorrhagic fever with renal syndrome disease progression in Slovenian patients. Clin Vaccine Immunol 2011; 18: 1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Golden JW, Hammerbeck CD, Mucker EMet al. Animal models for the study of rodent-borne hemorrhagic fever viruses: arenaviruses and hantaviruses. Biomed Res Int 2015; 2015: 793257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hooper JW, Larsen T, Custer DMet al. A lethal disease model for hantavirus pulmonary syndrome. Virology 2001; 289: 6–14 [DOI] [PubMed] [Google Scholar]
- 166. Brocato RL, Hammerbeck CD, Bell TMet al. A lethal disease model for hantavirus pulmonary syndrome in immunosuppressed Syrian hamsters infected with Sin Nombre virus. J Virol 2014; 88: 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Safronetz D, Zivcec M, Lacasse Ret al. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog 2011; 7: e1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Rasmuson J, Andersson C, Norrman Eet al. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur J Clin Microbiol Infect Dis 2011; 30: 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Turčinov D, Puljiz I, Markotić Aet al. Clinical and laboratory findings in patients with oliguric and non-oliguric hantavirus haemorrhagic fever with renal syndrome: an analysis of 128 patients. Clin Microbiol Infect 2013; 19: 674–679 [DOI] [PubMed] [Google Scholar]
- 170. Wang M, Wang J, Wang Tet al. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS One 2013; 8: e53236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pal E, Korva M, Resman Rus Ket al. Sequential assessment of clinical and laboratory parameters in patients with hemorrhagic fever with renal syndrome. PLoS One 2018; 13: e0197661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Latus J, Schwab M, Tacconelli Eet al. Clinical course and long-term outcome of hantavirus-associated nephropathia epidemica, Germany. Emerg Infect Dis 2015; 21: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang X, Chen HY, Zhu LYet al. Comparison of Hantaan and Seoul viral infections among patients with hemorrhagic fever with renal syndrome (HFRS) in Heilongjiang, China. Scand J Infect Dis 2011; 43: 632–641 [DOI] [PubMed] [Google Scholar]
- 174. Outinen TK, Mantula P, Laine OKet al. Haematuria is a marker for the severity of acute kidney injury but does not associate with thrombocytopenia in acute Puumala hantavirus infection. Infect Dis (Lond) 2017; 49: 840–846 [DOI] [PubMed] [Google Scholar]
- 175. Mustonen J, Helin H, Pietilä Ket al. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin Nephrol 1994; 41: 121–126 [PubMed] [Google Scholar]
- 176. Gnemmi V, Verine J, Vrigneaud Let al. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavirus nephropathy. Hum Pathol 2015; 46: 827–835 [DOI] [PubMed] [Google Scholar]
- 177. Koskela S, Mäkelä S, Strandin Tet al. Coagulopathy in acute Puumala hantavirus infection. Viruses 2021; 13: 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Mantula PS, Outinen TK, Clement JPGet al. Glomerular proteinuria predicts the severity of acute kidney injury in Puumala hantavirus-induced tubulointerstitial nephritis. Nephron 2017; 136: 193–201 [DOI] [PubMed] [Google Scholar]
- 179. Meier M, Kramer J, Jabs WJet al. Proteinuria and the clinical course of Dobrava–Belgrade hantavirus infection. Nephron Extra 2018; 8: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Clement J, LeDuc JW, McElhinney LMet al. Clinical characteristics of ratborne Seoul hantavirus disease. Emerg Infect Dis 2019; 25: 387–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Outinen TK, Makela S, Clement Jet al. Community acquired severe acute kidney injury caused by hantavirus-induced hemorrhagic fever with renal syndrome has a favorable outcome. Nephron 2015; 130: 182–190 [DOI] [PubMed] [Google Scholar]
- 182. Braun N, Haap M, Overkamp Det al. Characterization and outcome following Puumala virus infection: a retrospective analysis of 75 cases. Nephrol Dial Transplant 2010; 25: 2997–3003 [DOI] [PubMed] [Google Scholar]
- 183. Hukić M, Valjevac A, Tulumovic Det al. Pathogenicity and virulence of the present hantaviruses in Bosnia and Herzegovina: the impact on renal function. Eur J Clin Microbiol Infect Dis 2011; 30: 381–385 [DOI] [PubMed] [Google Scholar]
- 184. Rista E, Pilaca A, Akshija Iet al. Hemorrhagic fever with renal syndrome in Albania. Focus on predictors of acute kidney injury in HFRS. J Clin Virol 2017; 91: 25–30 [DOI] [PubMed] [Google Scholar]
- 185. Jha V, Prasad N. CKD and infectious diseases in Asia Pacific: challenges and opportunities. Am J Kidney Dis 2016; 68: 148–160 [DOI] [PubMed] [Google Scholar]
- 186. Guo Q, Xu J, Shi Qet al. Acute pancreatitis associated with hemorrhagic fever with renal syndrome: a cohort study of 346 patients. BMC Infect Dis 2021; 21: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Du H, Li J, Jiang Wet al. Clinical study of critical patients with hemorrhagic fever with renal syndrome complicated by acute respiratory distress syndrome. PLoS One 2014; 9: e89740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kruger DH, Tkachenko EA, Morozov VGet al. Life-threatening sochi virus infections, Russia. Emerg Infect Dis 2015; 21: 2204–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kim GH, Han JS, Earm Jet al. Evaluation of renal tubular functions in convalescent phase of hemorrhagic fever with renal syndrome. Am J Nephrol 1998; 18: 123–130 [DOI] [PubMed] [Google Scholar]
- 190. Kleinknecht D, Rollin PE. Hypertension after hemorrhagic fever with renal syndrome. Nephron 1992; 61: 121. [DOI] [PubMed] [Google Scholar]
- 191. Miettinen MH, Makela SM, Ala-Houhala IOet al. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int 2006; 69: 2043–2048 [DOI] [PubMed] [Google Scholar]
- 192. George J, Patnaik M, Bakshi Eet al. Hantavirus seropositivity in Israeli patients with renal failure. Viral Immunol 1998; 11: 103–108 [DOI] [PubMed] [Google Scholar]
- 193. Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis 2000; 32: 125–132 [DOI] [PubMed] [Google Scholar]
- 194. Kim YS, Ahn C, Han JSet al. Hemorrhagic fever with renal syndrome caused by the Seoul virus. Nephron 1995; 71: 419–427 [DOI] [PubMed] [Google Scholar]
- 195. Mustonen J, Huttunen NP, Brummer-Korvenkontio Met al. Clinical picture of nephropathia epidemica in children. Acta Paediatr 1994; 83: 526–529 [DOI] [PubMed] [Google Scholar]
- 196. Ferrés M, Vial P. Hantavirus infection in children. Curr Opin Pediatr 2004; 16: 70–75 [DOI] [PubMed] [Google Scholar]
- 197. Hallin GW, Simpson SQ, Crowell REet al. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med 1996; 24: 252–258 [DOI] [PubMed] [Google Scholar]
- 198. Enria DA, Briggiler AM, Pini Net al. Clinical manifestations of New World hantaviruses. Curr Top Microbiol Immunol 2001; 256: 117–134 [DOI] [PubMed] [Google Scholar]
- 199. Jonsson CB, Hooper J, Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res 2008; 78: 162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Clement J, Maes P, Van Ranst M. Hemorrhagic fever with renal syndrome in the new, and hantavirus pulmonary syndrome in the old world: paradi(se)gm lost or regained? Virus Res 2014; 187: 55–58 [DOI] [PubMed] [Google Scholar]
- 201. Chand S, Thapa S, Kon Set al. Hantavirus infection with renal failure and proteinuria, Colorado, USA, 2019. Emerg Infect Dis 2020; 26: 383–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. López R, Espinoza M, Graf Jet al. Proteinuria in hantavirus cardiopulmonary syndrome: a frequent finding linked to mortality. Int J Infect Dis 2021: 466–468 [DOI] [PubMed] [Google Scholar]
- 203. Peters CJ, Khan AS. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin Infect Dis 2002; 34: 1224–1231 [DOI] [PubMed] [Google Scholar]
- 204. Vial PA, Valdivieso F, Ferres Met al. High-dose intravenous methylprednisolone for hantavirus cardiopulmonary syndrome in Chile: a double-blind, randomized controlled clinical trial. Clin Infect Dis 2013; 57: 943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Santos J, Adad SJ, Vergara MSet al. Clinical and anatomopathological aspects of patients with hantavirus cardiopulmonary syndrome in Uberaba, Minas Gerais, Brazil. Rev Inst Med Trop Sao Paulo 2019; 61: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pergam SA, Schmidt DW, Nofchissey RAet al. Potential renal sequelae in survivors of hantavirus cardiopulmonary syndrome. Am J Trop Med Hyg 2009; 80: 279–285 [PMC free article] [PubMed] [Google Scholar]
- 207. Clement J, Maes P, Lagrou Ket al. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis 2012; 31: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Vollmar P, Lubnow M, Simon Met al. Hantavirus cardiopulmonary syndrome due to Puumala virus in Germany. J Clin Virol 2016; 84: 42–47 [DOI] [PubMed] [Google Scholar]
- 209. Rasmuson J, Lindqvist P, Sorensen Ket al. Cardiopulmonary involvement in Puumala hantavirus infection. BMC Infect Dis 2013; 13: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hjelle B, Goade D, Torrez-Martinez Net al. Hantavirus pulmonary syndrome, renal insufficiency, and myositis associated with infection by Bayou hantavirus. Clin Infect Dis 1996; 23: 495–500 [DOI] [PubMed] [Google Scholar]
- 211. Dzagurova TK, Witkowski PT, Tkachenko EAet al. Isolation of sochi virus from a fatal case of hantavirus disease with fulminant clinical course. Clin Infect Dis 2012; 54: e1–4 [DOI] [PubMed] [Google Scholar]
- 212. Larbig R, Lehman C, Rottländer Det al. Systemic hantavirus-infection in a comatose HIV patient. Wien Med Wochenschr 2013; 163: 32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Lebecque O, Falticeanu A, Abraham Cet al. Early chest imaging in patients with Puumala hantavirus infection. J Belg Soc Radiol 2020; 104: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Clement J, Colson P, McKenna P. Hantavirus pulmonary syndrome in New England and Europe. N Engl J Med 1994; 331: 545–546. author reply 547-548 [PubMed] [Google Scholar]
- 215. Gizzi M, Delaere B, Weynand Bet al. Another case of “European hantavirus pulmonary syndrome” with severe lung, prior to kidney, involvement, and diagnosed by viral inclusions in lung macrophages. Eur J Clin Microbiol Infect Dis 2013; 32: 1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dara SI, Albright RC, Peters SG. Acute Sin Nombre hantavirus infection complicated by renal failure requiring hemodialysis. Mayo Clin Proc 2005; 80: 703–704 [DOI] [PubMed] [Google Scholar]
- 217. Koehler FC, Blomberg L, Brehm TTet al. Development and design of the hantavirus registry—HantaReg—for epidemiological studies, outbreaks and clinical studies on hantavirus disease. Clin Kidney J 2021; 14: 2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lundkvist A, Hörling J, Niklasson B. The humoral response to Puumala virus infection (nephropathia epidemica) investigated by viral protein specific immunoassays. Arch Virol 1993; 130: 121–130 [DOI] [PubMed] [Google Scholar]
- 219. Maes P, Keyaerts E, Clement Jet al. Detection of Puumala hantavirus antibody with ELISA using a recombinant truncated nucleocapsid protein expressed in Escherichia coli. Viral Immunol 2004; 17: 315–321 [DOI] [PubMed] [Google Scholar]
- 220. Padula PJ, Rossi CM, Valle MODet al. Development and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J Med Microbiol 2000; 49: 149–155 [DOI] [PubMed] [Google Scholar]
- 221. Krüger DH, Ulrich R, Lundkvist AA. Hantavirus infections and their prevention. Microbes Infect 2001; 3: 1129–1144 [DOI] [PubMed] [Google Scholar]
- 222. Meisel H, Wolbert A, Razanskiene Aet al. Development of novel immunoglobulin G (IgG), IgA, and IgM enzyme immunoassays based on recombinant Puumala and Dobrava hantavirus nucleocapsid proteins. Clin Vaccine Immunol 2006; 13: 1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mattar S, Guzmán C, Figueiredo LT. Diagnosis of hantavirus infection in humans. Expert Rev Anti Infect Ther 2015; 13: 939–946 [DOI] [PubMed] [Google Scholar]
- 224. Ma C, Wang Z, Li Set al. Analysis of an outbreak of hemorrhagic fever with renal syndrome in college students in Xi'an, China. Viruses 2014; 6: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Goeijenbier M, Hartskeerl RA, Reimerink Jet al. The hanta hunting study: underdiagnosis of Puumala hantavirus infections in symptomatic non-travelling leptospirosis-suspected patients in the Netherlands, in 2010 and April to November 2011. Euro Surveill 2014; 19: 20878. [DOI] [PubMed] [Google Scholar]
- 226. Lederer S, Lattwein E, Hanke Met al. Indirect immunofluorescence assay for the simultaneous detection of antibodies against clinically important Old and New World hantaviruses. PLoS Negl Trop Dis 2013; 7: e2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Engler O, Klingstrom J, Aliyev Eet al. Seroprevalence of hantavirus infections in Switzerland in 2009: difficulties in determining prevalence in a country with low endemicity. Euro Surveill 2013; 18: 20660. [DOI] [PubMed] [Google Scholar]
- 228. Hjelle B, Jenison S, Torrez-Martinez Net al. Rapid and specific detection of Sin Nombre virus antibodies in patients with hantavirus pulmonary syndrome by a strip immunoblot assay suitable for field diagnosis. J Clin Microbiol 1997; 35: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hujakka H, Koistinen V, Kuronen Iet al. Diagnostic rapid tests for acute hantavirus infections: specific tests for Hantaan, Dobrava and Puumala viruses versus a hantavirus combination test. J Virol Methods 2003; 108: 117–122 [DOI] [PubMed] [Google Scholar]
- 230. Terajima M, Hendershot JD 3rd, Kariwa Het al. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J Infect Dis 1999; 180: 2030–2034 [DOI] [PubMed] [Google Scholar]
- 231. Evander M, Eriksson I, Pettersson Let al. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J Clin Microbiol 2007; 45: 2491–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Xiao R, Yang S, Koster Fet al. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J Infect Dis 2006; 194: 1403–1409 [DOI] [PubMed] [Google Scholar]
- 233. Saksida A, Duh D, Korva Met al. Dobrava virus RNA load in patients who have hemorrhagic fever with renal syndrome. J Infect Dis 2008; 197: 681–685 [DOI] [PubMed] [Google Scholar]
- 234. Armstrong LR, Zaki SR, Goldoft MJet al. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. J Infect Dis 1995; 172: 1166. [DOI] [PubMed] [Google Scholar]
- 235. Abu Sin M, Stark K, van Treeck Uet al. Risk factors for hantavirus infection in Germany, 2005. Emerg Infect Dis 2007; 13: 1364–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. de St Maurice A, Ervin E, Schumacher Met al. Exposure characteristics of hantavirus pulmonary syndrome patients, United States, 1993–2015. Emerg Infect Dis 2017; 23: 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Cho HW, Howard CR. Antibody responses in humans to an inactivated hantavirus vaccine (Hantavax). Vaccine 1999; 17: 2569–2575 [DOI] [PubMed] [Google Scholar]
- 238. Liu R, Ma H, Shu Jet al. Vaccines and therapeutics against hantaviruses. Front Microbiol 2019; 10: 2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Hooper JW, Li D. Vaccines against hantaviruses. Curr Top Microbiol Immunol 2001; 256: 171–191 [DOI] [PubMed] [Google Scholar]
- 240. Krüger DH, Schönrich G, Klempa B. Human pathogenic hantaviruses and prevention of infection. Hum Vaccin 2011; 7: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hooper J, Paolino KM, Mills Ket al. A phase 2a randomized, double-blind, dose-optimizing study to evaluate the immunogenicity and safety of a bivalent DNA vaccine for hemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Vaccines (Basel) 2020; 8: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Brocato RL, Hooper JW. Progress on the prevention and treatment of hantavirus disease. Viruses 2019; 11: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Outinen TK, Laine OK, Mäkelä Set al. Thrombocytopenia associates with the severity of inflammation and variables reflecting capillary leakage in Puumala hantavirus infection, an analysis of 546 Finnish patients. Infect Dis (Lond) 2016; 48: 682–687 [DOI] [PubMed] [Google Scholar]
- 244. Christova I, Pishmisheva M, Trifonova Iet al. Clinical aspects of hantavirus infections in Bulgaria. Wien Klin Wochenschr 2017; 129: 572–578 [DOI] [PubMed] [Google Scholar]
- 245. Han D, Liu Z, Han Qet al. Acute kidney injury in patients with hemorrhagic fever with renal syndrome caused by Hantaan virus: comparative evaluation by RIFLE and AKIN criteria. Vector Borne Zoonotic Dis 2011; 11: 723–730 [DOI] [PubMed] [Google Scholar]
- 246. Linderholm M, Elgh F. Clinical characteristics of hantavirus infections on the Eurasian continent. Curr Top Microbiol Immunol 2001; 256: 135–151 [DOI] [PubMed] [Google Scholar]
- 247. Fan X, Liu Z, Fu Set al. Platelet distribution width at first day of hospital admission in patients with hemorrhagic fever with renal syndrome caused by Hantaan virus may predict disease severity and critical patients’ survival. Dis Markers 2018; 2018: 9701619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wernly JA, Dietl CA, Tabe CEet al. Extracorporeal membrane oxygenation support improves survival of patients with hantavirus cardiopulmonary syndrome refractory to medical treatment. Eur J Cardiothorac Surg 2011; 40: 1334–1340 [DOI] [PubMed] [Google Scholar]
- 249. Chang B, Crowley M, Campen Met al. Hantavirus cardiopulmonary syndrome. Semin Respir Crit Care Med 2007; 28: 193–200 [DOI] [PubMed] [Google Scholar]
- 250. Huggins JW, Kim GR, Brand OMet al. Ribavirin therapy for Hantaan virus infection in suckling mice. J Infect Dis 1986; 153: 489–497 [DOI] [PubMed] [Google Scholar]
- 251. Chung DH, Västermark Å, Camp JVet al. The murine model for Hantaan virus-induced lethal disease shows two distinct paths in viral evolutionary trajectory with and without ribavirin treatment. J Virol 2013; 87: 10997–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Huggins JW, Hsiang CM, Cosgriff TMet al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis 1991; 164: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 253. Rusnak JM, Byrne WR, Chung KNet al. Experience with intravenous ribavirin in the treatment of hemorrhagic fever with renal syndrome in Korea. Antiviral Res 2009; 81: 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Malinin OV, Platonov AE.. Insufficient efficacy and safety of intravenous ribavirin in treatment of haemorrhagic fever with renal syndrome caused by Puumala virus. Infect Dis (Lond) 2017; 49: 514–520 [DOI] [PubMed] [Google Scholar]
- 255. Pettersson L, Thunberg T, Rocklöv Jet al. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients—implications for treatment. Clin Microbiol Infect 2014; 20: 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Korva M, Saksida A, Kejžar Net al. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin Microbiol Infect 2013; 19: E358–366 [DOI] [PubMed] [Google Scholar]
- 257. Yi J, Xu Z, Zhuang Ret al. Hantaan virus RNA load in patients having hemorrhagic fever with renal syndrome: correlation with disease severity. J Infect Dis 2013; 207: 1457–1461 [DOI] [PubMed] [Google Scholar]
- 258. Mertz GJ, Miedzinski L, Goade Det al. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin Infect Dis 2004; 39: 1307–1313 [DOI] [PubMed] [Google Scholar]
- 259. Chapman LE, Mertz GJ, Peters CJet al. Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antivir Ther 1999; 4: 211–219 [DOI] [PubMed] [Google Scholar]
- 260. de Carvalho Nicacio C, Lundkvist A, Sjölander KBet al. A neutralizing recombinant human antibody Fab fragment against Puumala hantavirus. J Med Virol 2000; 60: 446–454 [DOI] [PubMed] [Google Scholar]
- 261. Xu R, Yang XY, Yang DFet al. Phase I evaluation of the safety and pharmacokinetics of a single-dose intravenous injection of a murine monoclonal antibody against Hantaan virus in healthy volunteers. Antimicrob Agents Chemother 2009; 53: 5055–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Garrido JL, Prescott J, Calvo Met al. Two recombinant human monoclonal antibodies that protect against lethal Andes hantavirus infection in vivo. Sci Transl Med 2018; 10: eaat6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Gorbunova EE, Gavrilovskaya IN, Pepini Tet al. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J Virol 2011; 85: 2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Abudurexiti A, Adkins S, Alioto Det al. Taxonomy of the order Bunyavirales: update 2019. Arch Virol 2019; 164: 1949–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Ahn C, Cho JT, Lee JGet al. Detection of Hantaan and Seoul viruses by reverse transcriptase-polymerase chain reaction (RT-PCR) and restriction fragment length polymorphism (RFLP) in renal syndrome patients with hemorrhagic fever. Clin Nephrol 2000; 53: 79–89 [PubMed] [Google Scholar]
- 266. Aitichou M, Saleh SS, McElroy AKet al. Identification of Dobrava, Hantaan, Seoul, and Puumala viruses by one-step real-time RT-PCR. J Virol Methods 2005; 124: 21–26 [DOI] [PubMed] [Google Scholar]
- 267. Vial PA, Valdivieso F, Calvo Met al. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome caused by Andes virus. Antivir Ther 2015; 20: 377–386 [DOI] [PubMed] [Google Scholar]