Abstract
The B.1.617.2 (Delta) variant of SARS-CoV-2 emerged in India in October of 2020 and spread widely to over 145 countries, comprising over 99% of genome sequence-confirmed virus in COVID-19 cases of the United States (US) by September 2021. The rise in COVID-19 cases due to the Delta variant coincided with a return to in-person school attendance, straining COVID-19 mitigation plans implemented by educational institutions. Some plans required sick students to self-isolate off-campus, resulting in an unintended consequence: exposure of co-inhabitants of dwellings used by the sick person during isolation. We assessed air and surface samples collected from the bedroom of a self-isolating university student with mild COVID-19 for the presence of SARS-CoV-2. That virus’ RNA was detected by real-time reverse-transcription quantitative polymerase chain reaction (rRT-qPCR) in air samples from both an isolation bedroom and a distal, non-isolation room of the same dwelling. SARS-CoV-2 was detected and viable virus was isolated in cell cultures from aerosol samples as well as from the surface of a mobile phone. Genomic sequencing revealed that the virus was a Delta variant SARS-CoV-2 strain. Taken together, the results of this work confirm the presence of viable SARS-CoV-2 within a residential living space of a person with COVID-19 and show potential for transportation of virus-laden aerosols beyond a designated isolation suite to other areas of a single-family home.
Keywords: rRT-qPCR, Air sampling, Virus culture, Infectious
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 6.2 million deaths and over 503 million known cases worldwide through April 19, 2022 (WHO, 2022). The pandemic has strained medical resources in the US (Blumenthal et al., 2021; French et al., 2021; Parsons Leigh et al., 2021) and elsewhere (Bastos et al., 2021; Hallal & Victora, 2021) and has transformed public interactions by altering the frequency at and mechanisms by which people communicate and socialize (Philpot et al., 2021). In December of 2020, administration of vaccines against SARS-CoV-2 were initiated at a limited scale within healthcare settings and subsequently broadened with an increase of vaccine supplies. It has been estimated that 66% of the global population has received at least one dose of a vaccination series and that the rate stands at 77% within the US as of April 19, 2022 (Holder, 2022). Injected COVID-19 vaccines have provided protection against severe disease and have reduced the hospitalization and death rates of those vaccinated relative to those unvaccinated (Scobie et al., 2021; Tenforde et al., 2021). However, breakthrough events have demonstrated that vaccine protection is imperfect (Brown et al., 2021; Chau et al., 2021; Klompas, 2021) and studies have shown that protection against SARS-CoV-2 wanes after 6 or so months past vaccine series completion (Levine-Tiefenbrun et al., 2021; Thomas et al., 2021). This circumstance may correlate with the previous predominance of the Delta variant of SARS-CoV-2 (Pango genetic lineage B.1.617.2), which as of the week ending on November 20, 2021, accounted for 99.9% of genotyped SARS-CoV-2 infections within the U.S. (CDC, 2021d). The Delta variant carries spike protein substitutions at T19R, V70F, T95I, G142D, E156del, F157del, R158G, A222V, W258L, K417N, L452R, T478K, D614G, P681R, D950N, and has been linked to increased transmissibility of the virus, reduced effectiveness of vaccine-induced neutralizing antibodies, and reduced neutralization by some monoclonal antibody treatments (CDC, 2021c). Chia et al. (2021) showed decreased duration and severity of illness for symptomatic vaccinated persons compared to symptomatic unvaccinated persons infected with Delta variant SARS-CoV-2, but their assessment showed similar levels of viral load in both vaccinated and unvaccinated patients, indicating potential for similar post-vaccination transmission potential, despite the beneficial self-protective impact. Li et al. (2021) reported that a Delta variant outbreak resulted in an average decrease in time from exposure to positive rRT-qPCR test of two days and an average increase in viral loads determined by rRT-qPCR of 1260 times beyond values seen during 2020. Relative to the original SARS-CoV-2 strains, there have been increased risks of hospitalization, ICU admission, and death among COVID-19 patients infected with the Delta variant, and increased risk as well within those categories compared to earlier variants (Fisman & Tuite, 2021). Similarly, Allen et al. (2021) found that the odds of household transmission increased by 70% when the emitting source was infected with the Delta variant of SARS-CoV-2 compared to the Alpha variant.
The stability of SARS-CoV-2 on environmental surfaces and in the air has been explored, revealing that the virus can persist for hours to days outside of a human host (Fears et al., 2020; Kasloff et al., 2021; Liu et al., 2021; Pastorino et al., 2020; van Doremalen et al., 2020). According to the CDC, the main route of transmission occurs through inhalation of virus-laden particles (CDC, 2021b). However, that route of transmission has been challenging to study largely because the collection of airborne SARS-CoV-2 particles is technically difficult, as it is for other viruses (Coleman et al., 2021; Lednicky et al., 2020a,b; Nannu Shankar et al., 2022; Pan et al., 2019).
Despite uncertainty regarding the transmissibility of SARS-CoV-2 variants and the severity of the illnesses they cause, the widespread availability of vaccines in the US has driven the evolution of public health policies away from the prevention of infections by SARS-CoV-2 and toward less-restrictive pre-pandemic guidelines. As policymakers weigh how to combat the COVID-19 pandemic, knowledge and beliefs about the severity of illness caused by SARS-CoV-2 variants, and their transmissibility, have influenced decisions by administrators amid fluctuating community case rates. Those decisions have resulted in a patchwork of procedures across the US, particularly within schools during the autumn semester of 2021 (California Department of Public Health, 2021; Texas Education Agency, 2021). At the collegiate level, policies enacted at some institutions contained requirements for students with positive SARS-CoV-2 test results to isolate off-campus when on-campus isolation space was unavailable. That guidance created subsequent challenges when students normally living on-campus had to move off-campus to self-isolate: (a) While relocating, infectious individuals can expose others in cars, buses, or other forms of transportation, and (b) During self-isolation, those individuals might expose others who live in the same dwelling to infectious virus. Ultimately, policies requiring relocation for self-isolation may facilitate the spread of SARS-CoV-2 to populations which otherwise would not have interacted with those infected individuals.
Analyses of epidemiological outbreak data (Qian et al., 2020) and targeted sampling of quarantined households to determine secondary infection rates (Grijalva et al., 2020) have shown that transmission of SARS-CoV-2 within homes is high and results in more than three times more secondary infections than occurs in other types of venues. Those analyses add to the mounting body of evidence that time spent in residential environments is positively correlated with an increasing likelihood of virus transmission by infectious individuals to others within the residence. SARS-CoV-2 RNA has been detected on residential surface samples by rRT-qPCR (Döhla et al., 2020; Maestre et al., 2021; Nannu Shankar et al., 2022). Similarly, the presence of SARS-CoV-2 in aerosols collected at distances of ∼0.3–2.2 m from sick persons within residential isolation rooms was reported by Nannu Shankar et al. (2022). However, SARS-CoV-2 could not be isolated in cell cultures during that aerosol study for various reasons, including out-competition by a faster-growing human adenovirus B that was a co-infecting pathogen in one of the people afflicted with COVID-19, and thus it was not possible to infer that infectious virus was present in the environmental samples. Though it was not successfully isolated, the study nevertheless was informative regarding the distribution of airborne particle associated with the transport of SARS-CoV-2 as a three-stage National Institute for Occupational Safety and Health (NIOSH) bioaerosol sampler (Model BC-251) with dual cyclone stages and a final filter stage allowed aerosol particle size fractionation (Cao et al., 2010), revealing that SARS-CoV-2 was primarily within particles with average diameters <4.4 μm (Nannu Shankar et al., 2022). That finding is significant because particles < about 4 μm can travel deeper into the lungs, creating potential for greater risk of infection of the cells of the lower respiratory tract, leading to pneumonia (Pan et al., 2019). Whereas cyclones and filters can inactivate viruses during the collection process, resulting in difficulties isolating them in cell cultures, condensation growth tube (CGT) air samplers can collect airborne viruses in a manner that maintains their viability (“infectivity”) (Lednicky et al., 2020a,b), but those CGTs do not size-fractionate particle sizes. It is important to determine (a) the dimension of airborne particles that contain virions, and (b) whether the virions within the airborne particles are viable for proper inhalation risk assessments. As mentioned above, particles <4 μm in diameter can travel to the lower lungs, and only viable virus particles can cause disease. Therefore, multiple air sampler types were used in our investigation, both to improve the chances of maintaining virus viability during the collection process and to discern the distribution of particle sizes containing SARS-CoV-2.
In this opportunistic investigation, we sought to assess the potential for SARS-CoV-2 exposure in a residential setting. Environmental sampling occurred after a volunteer college student relocated to an off-campus self-isolation site in accordance with a policy requiring self-isolation away from campus after a positive SARS-CoV-2 test result.
2. Materials and methods
This work was IRB exempt as it did not involve human clinical trials or the collection of information that could reveal the identity of the volunteer.
2.1. Student volunteer and sampling site
A 19-year-old college student, non-vaccinated against COVID-19 and living in on-campus apartment-style housing, began experiencing symptoms of SARS-CoV-2 infection in the evening of August 31, 2021. A nasopharyngeal (NP) specimen was collected the following morning at a campus clinic and submitted for diagnostic rRT-qPCR tests, and the results of the rRT-qPCR tests were made available later the same day, confirming the student harbored a SARS-CoV-2 infection. Thereafter, the student departed the school dormitory in the afternoon on September 01, 2021 and drove along with a parent over 570 miles (917 km) to a single-family home of a family friend for self-isolation. Relocation concluded early on September 02, 2021, and samplings took place the following day (approximately three days after onset of the student's COVID-19 symptoms). The student's self-reported symptoms were body aches, congestion, fatigue, and a mild cough. The volunteer remained symptomatic during environmental samplings but noted their symptoms began abating September 02, 2021. The student and homeowner conveyed information about this situation by telephone during the relocation, and both consented to have environmental samples collected within the isolation suite and elsewhere in the home.
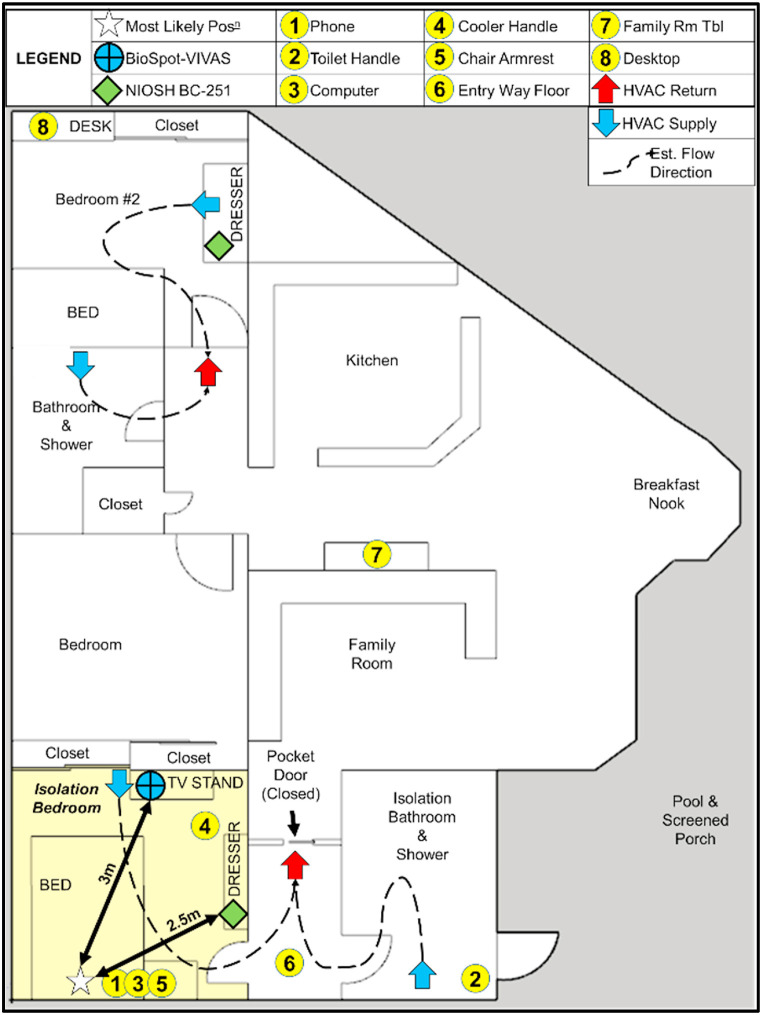
The test site consisted of an en suite bedroom (3.8 m x 3.7 m), connected to the main body of a single-family home through a short hallway. The doorway linking the isolation suite to the rest of the home remained closed throughout the isolation period and during all samplings. Air flow from the residential air conditioning unit was intermittent, and room temperatures ranged from 25.9 to 27.1 °C. Samples were collected both within the isolation suite and in the main body of the home. Sampling locations outside of the isolation suite were selected to investigate potential virus transport through the air handling system shared with the isolation suite. The sampling region, sample points, heating, ventilation, and air conditioning (HVAC) supply outlets, and HVAC return inlets near the sampling points are shown in Fig. 1 .
Fig. 1.
Sampling site layout depicting sampling points and estimated flow direction when the intermittent HVAC system is running. This sketch represents the larger sampling environment and the region of the home serviced by the same air handling unit. A second air handler serviced the remaining portion of the home.
2.2. Air samplers
A BioSpot Viable Virus Aerosol Sampler (BioSpot-VIVAS) (Model 300, Aerosol Devices Inc, US) and two NIOSH bioaerosol samplers (Model BC-251) were used to perform air samplings (Fig. 2 ).
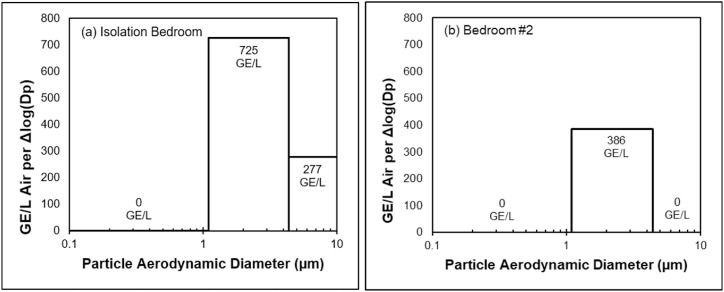
Fig. 2.
Estimated SARS-CoV-2 GE per L of air per change in particle diameter [Δlog(Dp)] versus particle diameter fractionalization within (a) the volunteer isolation bedroom and (b) non-isolation bedroom. Bins depicted represent particle size fractions collected by the NIOSH bioaerosol sampler. Bins with no detected virus are listed with concentrations of 0 GE/L.
The BioSpot-VIVAS operates on the same principle as the Viable Virus Aerosol Sampler (VIVAS) (Lednicky et al., 2016; Pan et al., 2017). Briefly, airborne particles enter the BioSpot-VIVAS through the inlet port on the top of the device. The device diverts that single inlet airstream into eight parallel condensation growth tubes. Particles collect water on their surfaces and enlarge as they move downward within the tubes, gently impacting into 1.5 mL of liquid collection medium within a 35 mm x 10 mm petri dish (catalog no. 150318, ThermoFisher Scientific, US).
The BC-251 has been described previously (Cao et al., 2010; Lindsley et al., 2006; Nannu Shankar et al., 2022). Briefly, BC-251 consists of three stages, a first-stage cyclone with 15 mL tube (Falcon conical centrifuge tubes, catalog no. 14-959-53A, Fisher Scientific, US), a second-stage cyclone with 1.5 mL Eppendorf tube (Microcentrifuge tubes, Fisher scientific, catalog no. 02-681-373) and a third-stage polytetrafluoroethylene (PTFE) filter (37 mm, 2 μm pore, catalog no. 225–1709, SKC Inc., US). The air inlet port on the BC-251 is situated on the front face of the sampler housing.
Further description of the air samplers is included in the supplementary material section of this manuscript.
2.3. Dual-function liquid collection medium
We produced the liquid collection medium that was used in the air samplers and that also served as the virus transport medium (VTM) for swab samples. It contained sucrose that functioned as a cryopreservation agent, brain-heart infusion (BHI) broth and bovine serum albumin (BSA) Fraction V as sources of proteins to help stabilize SARS-CoV-2 virions, and its formula is given in this manuscript's supplementary material section. Following verification that the VTM was sterile, a little more than 1.5 mL of VTM was aseptically transferred from stock supply into sterile 15 mL conical polypropylene centrifuge tubes (Falcon brand, catalog no. 14-959-53A, Fisher Scientific, US) within a biosafety cabinet (BSC). At the testing site, 1.5 mL of VTM from those tubes was transferred into the BioSpot-VIVAS′ petri dishes to serve as sample collection media. 15 mL centrifuge tubes containing 1.5 mL of VTM for immersion and transport of surface swab samples were prepared in the same manner.
2.4. Preparation of BC-251 samplers
The filters and centrifuge tubes mentioned in section 2.2 above served as collection tubes in the BC-251 samplers and were installed into the sampler housing within a BSC in a BSL2 laboratory. The two BC-251 samplers with connected filter and tube stages were then inserted into inside plastic bags and sealed with tape for transport to the testing sites.
2.5. Collection of air samples
Air samplers were set at fixed points in rooms with sampling inlets ∼1.5 m from the ground, a height that approximates the distance from the ground to the nose of the most likely seated (bedside chair) and supine (bed) positions of the volunteer. Air sampling with a BC-251 and a BioSpot-VIVAS occurred on September 03, 2021 (Day 3 following onset of symptoms). The BioSpot-VIVAS was positioned 3 m from the center of the bed headboard, and it was elevated by placing it atop a television stand. The BC-251 was situated on a dresser near the bedroom entry, 2.5 m from the center of the bed headboard. Inlet heights and distances of the air samplers were selected to focus sampling on virions transported in aerosols, rather than those in large particles settling out of the air close to the individual.
BC-251 samplers were connected to Airchek sampler pumps (Model 224-PCXR4, SKC Inc., US) and operated at 3 LPM for 3 h for two samplings. Likewise, the BioSpot-VIVAS was operated for 3 h at 8 LPM for two samplings. Swab samples were collected after the start of the air sampler run times and were immersed in 1.5 mL of VTM within centrifuge tubes. After the completion of each sampling, the BC-251 sampler was placed into a polypropylene bag with tube and filter stages still attached, and the bag was folded shut and sealed with tape. Each petri dish from the BioSpot-VIVAS was sealed with parafilm (Fisher Scientific catalog no. 13-374-12, Bemis Company Inc, US) and placed into an empty pipet tip box (SureOne™ Aerosol Barrier Pipette Tips, catalog no. 02-707-404, Fisher Scientific, US) for transportation. All samples were stored in a cooler containing dry ice, to freeze the samples in-transit to help retain virus viability during the 3-h return drive. Samples were stored in a −80 °C freezer at UF until rRT-qPCR and virology analyses were completed later.
2.6. Surface sampling
Surfaces of frequently touched items reported by the volunteer and assessed by the research team were swabbed with sterile nylon swabs (Nasal Flocked Swab, part no. 96000, Miraclean Technology Co, Ltd., China). The surfaces chosen for sampling were a mobile phone (∼80 cm2), laptop computer touch pad (∼60 cm2), toilet handle (∼20 cm2), chair arm (∼230 cm2), entry way floor (∼230 cm2), and a food container's lid handle (∼80 cm2). Outside of the room, two additional surfaces were swabbed to assess whether SARS-CoV-2 was transported elsewhere in the home and deposited on those surfaces (Fig. 1). Those samples were taken at a table (∼230 cm2) and a second bedroom desk (∼230 cm2). A 6 in. × 6 in. (15.2 cm × 15.2 cm) template, precut from an 8.5 in. × 11 in. sheet of laminated paper, was used to collect samples from surfaces large enough to accommodate it. Surfaces with sampling areas smaller than the template were measured on-site using a LASER measure (GLM165-40, Robert Bosch GmbH, Gerlingen, Germany). Surface samples were collected by swabbing in two directions over the sampling surface, with the direction of the second pass perpendicular to the first.
2.7. Personal protective equipment for air sampling
Nitrile gloves (manufacturer number 52818, Kimberly-Clark, Irving, TX, US) and an N95 mask (Model 1870+, 3M, Saint Paul, MN, US) were worn while operating and manipulating devices and samples. Gloves were changed between uses and were disposed as biohazard waste. Interactions with the volunteer were minimized and direct contact was avoided. The outer surfaces of all equipment used within the volunteer's room during the sampling event were disinfected with 70% ethanol following the packaging of the collected samples.
2.8. Sample processing
Samples were initially processed in a BSL-2+ laboratory. In work performed in a BSC, 1.5 mL and 0.5 mL of VTM were added to the stage 1 and stage 2 tubes of the BC-251 samplers, respectively, after the tubes were removed from the sampler housing, and the tubes were set aside at room temperature for 30 min to allow material captured on the inside surfaces of the tubes to rehydrate. The third-stage filter was immersed in 5 mL of VTM in a 50 mL tube and set aside at room temperature for 30 min to allow material captured on the filter to rehydrate. Third-stage filters and their immersion fluids were then transferred into a sterile 100 × 15 mm plastic petri dish (Fisher Scientific, Cat. No. S33580A), and matter still trapped on the filters was scraped off with flocked nylon swabs (Copan Diagnostics, Inc., Murrieta, CA, US) pre-wetted with VTM, as it had been used as the recovery solution. The process used for recovering virus from the third-stage filter was based on side-by-side tests performed with laboratory-generated aerosols on the recovery of enveloped virus on PTFE filters (Lednicky & Loeb, 2013; Lednicky, unpublished results). Enhanced recovery of enveloped viruses (influenza A, human coronavirus OC43, and respiratory syncytial virus A) was observed when filters were pre-soaked and then scraped, and this because both PTFE and the enveloped viruses that were tested are hydrophobic; scraping may thus assist in dislodging the virus off the filter surface. Swabs were twirled against the inner surface of the petri dish to extrude the material collected by the swabs into the recovery solution. All fluids were concentrated by centrifugation in Amicon Ultra-15 centrifugal filter units with Ultracel-100 membranes that had a molecular mass cutoff of 100 kDa (Millipore, Bedford, MA) at 4000×g for 12 min. The concentrates were thereafter adjusted to 400 μL by addition of VTM, then aseptically transferred to sterile plastic cryotubes with silicone gaskets (Fisher Scientific, cat. No. 10-500-26), and the tubes transported in a Styrofoam container with ice packs to a BSL2-enhanced laboratory at the UF Emerging Pathogens Institute, where they were stored in a locked −80 °C freezer until further analysis.
2.9. Real-time reverse transcription quantitative polymerase chain reaction (rRT-qPCR)
The Centers for Disease Control and Prevention N2 gene target (CDC, 2020) was used for SARS-CoV-2 detection and quantification by rRT-qPCR, as previously detailed (Rainey et al., 2022). The quantity of SARS-CoV-2 genomes per sample was extrapolated based on an 8-point standard curve of 1:5 dilutions ranging from 100,000 to 1.28 CDC N2 genomic copies/μL as detailed in Rainey et al. (2022).
2.10. Cell lines for SARS-CoV-2 isolation
Two cell lines obtained from the American Type Culture Collection (ATCC) were used for SARS-CoV-2 isolation attempts: LLC-MK2 (Rhesus monkey kidney cells, catalog no. ATCC CCL-7), and Vero E6 cells (African green monkey kidney cells, catalog no. ATCC CRL-1586) (Lednicky et al., 2020a,b). The cells were propagated in cell culture medium comprised of aDMEM (advanced Dulbecco's modified essential medium, Invitrogen, Carlsbad, CA) supplemented with 10% low antibody, heat-inactivated, gamma-irradiated fetal bovine serum (FBS, Hyclone, GE Healthcare Life Sciences, Pittsburgh, PA), l-alanine, l-glutamine dipeptide supplement (GlutaMAX, Invitrogen), and 50 μg/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL neomycin (PSN antibiotics, Invitrogen) with incubation at 37 °C in 5% CO2.
2.11. SARS-CoV-2 isolation and plaque assays
All work on SARS-CoV-2 was performed in a BSL3 laboratory by a trained analyst who wore appropriate personal protection equipment that included a chemically impervious Tyvek suit, gloves, and used a full-head covering powered-air purifying respirator.
2.11.1. SARS-CoV-2 isolation in cultured cells
LLC-MK2 and Vero E6 cells grown as monolayers in a T-25 flask (growing surface 25 cm2) were inoculated when they were at 80% confluency (Lednicky et al., 2020a,b). Briefly, 60 μL aliquots of the concentrated air sampler collection media were filtered through sterile 0.45-μm pore size PVDV syringe-tip filters to remove bacterial and fungal cells and spores. Next, the spent cell culture medium was removed from each flask and replaced with 1 mL of cell culture medium, and the cells thereafter inoculated with 50 μL of filtrate. When virus-induced cytopathic effects (CPE) were evident (approximately 4 days post-inoculation), the presence of SARS-CoV-2 was determined by rRT-qPCR.
2.11.2. SARS-CoV-2 plaque assays
Plaque assays were performed in Vero E6 cells (essentially as described in Ragan et al. (2020). Briefly, newly confluent Vero E6 cells in 6-well tissue culture plates were inoculated with 0.2 mL of 10-fold serial dilutions of the complete cell growth medium, and the virus allowed to bind to the cells for 1 h at 37 °C, with rocking of the plates performed manually every 15 min. After virus adsorption, the cells were washed with serum-free DMEM and the wells subsequently overlaid with 3 mL/well of primary overlay consisting of 1.6% w/v agarose (Invitrogen Corp.) mixed 1:1 with 2X EMEM (Lonza, Walkersville, MD) containing antibiotics, sodium pyruvate, Glutamax, antibiotics, and 10% FBS. After they had solidified, the plates were inverted and incubated for 3 days, then overlaid with 2 mL of secondary overlay at day 5 post-infection. The cells were fixed with 10% buffered formalin, followed by the removal of the overlay, and then stained with 0.2% crystal violet to visualize plaques.
2.12. Sequencing of virus genomes
Sanger sequencing based on a gene-walking approach with tiled primers was attempted for three samples (A, B, and C) to obtain the consensus virus sequence, as described in Lednicky et al., 2020a,b and Rainey et al. (2022).
3. Results
SARS-CoV-2 RNA was detected by rRT-qPCR in nucleic acids extracted and purified from one surface swab sample and five air samples. Among eight collected surface samples, only the swab taken from the front and back of the volunteer's mobile phone resulted in detection of SARS-CoV-2 (Table 1 ).
Table 1.
Environmental surface sampling. Results reflect sampling conducted on September 03, 2021. Sample locations are depicted within Fig. 1.
| Location | Surface Area Swabbed [cm2] | rRT-qPCR Cq Value |
SARS-CoV-2 Conc. [Total GE/Sample] |
SARS-CoV-2 Conc. [Total GE/cm2 Surface] |
Estimated Viable SARS-CoV-2 Conc. [PFU/cm2 Surface] |
||
|---|---|---|---|---|---|---|---|
| Direct from Specimens | LLC MK2 8 dpi |
Vero E6 8 dpi |
|||||
| Cell Phone | 80 | 30.29 | 34.20 | 33.71 | 1.46E+06 | 1.82E+04 | 1.00E+03 |
| Toilet Handle | 20 | NEG | NEG | NEG | |||
| Computer Trackpad & Hand Rest | 60 | NEG | NEG | NEG | |||
| Food Cooler Lid Exterior | 80 | NEG | NEG | NEG | |||
| Chair Arm Rest | 230 | NEG | NEG | NEG | |||
| Entryway Floor | 230 | NEG | NEG | NEG | |||
| Family Room Table | 230 | NEG | NEG | NEG | |||
| Bedroom #2 Desktop | 230 | NEG | NEG | NEG | |||
| Positive Template (SARS-COV-2 RNA) | 30.65 | 2.03E+04 | |||||
| No Template (Negative) | NEG | ||||||
Virus was not captured on either final filter stage of the BC-251 samplers (<1.1 μm) but was collected in greatest amounts (Table 2 ) as measured through rRT-qPCR on both second stages (1.1 μm–4.4 μm), ranging from 233 to 437 GE/L of air. The device in which viral RNA (vRNA) was collected in the first stage was positioned inside the isolation area.
Table 2.
Environmental air sampling. Results reflect sampling conducted on September 03, 2021.
| Location | Sampler Typea | Flow Rate [LPM] | Time [min] | Vol. of Air [L] | Dist. [m] | rRT-qPCR Cq Value |
SARS-CoV-2 Conc. [Total GE/Sample] |
SARS-CoV-2 Conc. [Total GE/L Air] |
Est. Viable SARS-CoV-2 Conc.[PFU/L of Air] |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct from Specimens | LLC MK2 8 dpi |
Vero E6 8 dpi |
|||||||||
| Bedroom #2 | BC-251 Stage 1 |
3 | 180 | 540 | NEG | NEG | NEG | ||||
| Bedroom #2 | BC-251 Stage 2 |
3 | 180 | 540 | 33.86 | NEG | NEG | 1.26 E+05 |
2.33 E+02 |
No plaques | |
| Bedroom #2 | BC-251 Stage 3 |
3 | 180 | 540 | NEG | NEG | NEG | ||||
| Volunteer Bedroom | BC-251 Stage 1 |
3 | 180 | 540 | 2.5 | 35.23 | NEG | NEG | 5.34 E+04 |
9.89 E+01 |
No plaques |
| Volunteer Bedroom | BC-251 Stage 2 |
3 | 180 | 540 | 2.5 | 32.85 | NEG | NEG | 2.36 E+05 |
4.37 E+02 |
No plaques |
| Volunteer Bedroom | BC-251 Stage 3 |
3 | 180 | 540 | 2.5 | NEG | NEG | NEG | |||
| Volunteer Bedroom | BioSpot-VIVAS #1 | 8 | 180 | 1440 | 3 | 29.22 | 11.46 | 11.18 | 2.27 E+06 |
1.58 E+03 |
292 |
| Volunteer Bedroom | BioSpot-VIVAS #1 | 8 | 180 | 1440 | 3 | 30.47 | 11.55 | 11.64 | 1.04 E+06 |
7.23 E+02 |
132 |
| Clean Air | BioSpot-VIVAS #1 | 8 | 30 | 240 | NEG | NEG | NEG | ||||
| Positive Template (SARS-COV-2 RNA) | 30.65 | 2.03 E+04 |
|||||||||
| No Template (Negative) | NEG | ||||||||||
Particle size ranges for the BC-251 sampler are: >4.4 μm (Stage 1), 1.1–4.4 μm (Stage 2), and <1.1 μm (Stage 3).
Viable virus was subsequently cultured from the mobile phone swab sample (Table 1) and from two air samples that had been collected using the BioSpot-VIVAS (Table 2). The concentrations of viable virus were 132 and 292 PFU/L of air. No viable virus was isolated from material collected using the NIOSH samplers.
A complete consensus SARS-CoV-2 genomic sequence was obtained for all three virus isolates, and the sequences were identical. The genomic sequence was deposited in GenBank as SARS-CoV-2/Environment/USA/UF-41/2021, under GenBank accession number OL622105.1. The nucleotide sequence of this virus matches (perfectly) SARS-CoV-2/human/USA/TN-CDC-STM-000723021/2021, which was collected August 17, 2021 (GenBank accession number OL536032), whereas our samples were collected September 3, 2021, suggesting possible introduction of the virus by a visitor from the state of Tennessee. Compared to those of the reference strain of SARS-CoV-2, the deduced amino acid sequences of the virus proteins of our isolate contain the following amino acid substitutions: Spike (T19R, G142D, 156G, F157del, R158del, L452R, T478K, I598V, D614G, P681R, D950N, T95I, I1232S), M (I82T), N (D63G, R203M, G215C, D377Y), NS3 (S26L), NS7a (V82A,T120I), NS7b (T40I), NSP3 (A488S, H563Y, P1228L, P1469S), NSP4 (V167L, T492I), NSP6 (L37F, T77A), NSP12 (P323L, L372I, G671S), NSP13 (P77L, A296S), NSP14 (A394V). The virus sequence places it in GISAID clade GK, and in Pango Lineage: B.1.617.2 (Pango v.3.1.16 2021-11-18), Delta (B.1.617.2-like) (Scorpio). It is thus a SARS-CoV-2 variant of concern (VOC) of the type GK/478K.V1 (B.1.617.2+AY.x) that was first detected in India.
4. Discussion
The CDC announced that SARS-CoV-2 can be transmitted by aerosols through inhalation (CDC, 2021b), and public health reliance on six-feet physical distancing zones as a primary prevention method has lessened with growing knowledge of how virus spreads person-to-person. Yet, references to the original six-feet physical distancing zone persist in some public health guidelines (CDC, 2021a, 2022), a distance which does not negate exposure risk due to transmission by aerosols. Transport of viable SARS-CoV-2 in aerosols was reported in a hospital (Lednicky et al., 2020a,b) and demonstrated under laboratory conditions (Hawks et al., 2021; Schuit et al., 2021). Other attempts to collect virus in air samples and isolate the virus in cell cultures have been made in residential settings, wherein virus was either not collected (Döhla et al., 2020) or not isolated in cell cultures from material containing SARS-CoV-2 RNA, indicating that the virus either was truly not viable or had been inactivated through the collection process (Lednicky et al., 2021; Nannu Shankar et al., 2022; Santarpia et al., 2020). Our results show that viable SARS-CoV-2 virions can be transported by air currents to distances relatively far away from an emitting source (i.e., a person with an active COVID-19 respiratory tract infection). With sampler locations beyond a six-feet physical distancing protection zone (CDC, 2022), our work indicates that, as with other SARS-CoV-2 variants, the Delta variant can also be present in aerosols.
Of particular interest was the detection of SARS-CoV-2 in an area of the home far separated from the isolation suite. It is well known that human activities within a residence can impact particle concentrations in the immediate area and within other rooms within the residence (Balasubramanian & Sheng, 2007). Assessment of SARS-CoV-2 concentrations based on rRT-qPCR tests of material in swab and filter samples (Maestre et al., 2021) as well as by mathematical modeling (Pease et al., 2021) have indicated that SARS-CoV-2 transport within residences is plausible. Though we cannot conclude from this investigation how SARS-CoV-2 arrived in the non-isolation room, and we detected only non-viable SARS-CoV-2 by rRT-qPCR, our detection of vRNA in an air sample in a room far-removed from the isolation suite builds support for potential inhalation risks throughout domestic isolation sites. The detection of SARS-CoV-2 within a residential air sample collected outside of a designated self-isolation space also supports the need for further research into residential transport and viability of SARS-CoV-2 and other infectious agents.
Successful collection and conservation of airborne viruses has been demonstrated in several studies through the use of CGT collection devices like the BioSpot-VIVAS (Lednicky et al., 2016, 2020a,b; Nannu Shankar et al., 2022), which collect particles by gentle impaction onto a liquid collection medium. The use of devices like gelatin filters (Santarpia et al., 2020) and cyclones (Döhla et al., 2020) during environmental air sampling has not led to successful isolation of viable SARS-CoV-2 in cell cultures. Though an array of air samplers such as PTFE filters, impingers, cascade impactors, and cyclones have been successfully used for the collection of viable virus in aerosols, the proportion of the collected virus that remains viable typically is minimal (Ratnesar-Shumate et al., 2021). CGT devices are able to conserve viable virus with greater success due to two advantages: (a) minimal damage to the virus, and (b) prevention of deleterious desiccation of the virus particles. During the same sampling periods and at nearly the same distance from the volunteer in this work, the BioSpot-VIVAS consistently collected and conserved virus viability, whereas virus collected using the BC-251 was non-infectious. The results of our work reinforce the growing body of evidence in that CGT devices are suitable devices for exploring potential exposure risks to aerosolized viruses (Hawks et al., 2021).
Past assessment of the BC-251 with influenza A virus (Influenza A/WS/33, H1N1, VR-825, ATCC Lot Nos. 58023547 and 58772128) resulted in much higher recovery in the second cyclone stage than in the first cyclone stage (Cao et al., 2010). Similarly, SARS-CoV-2 collection primarily in the second fractionalized range of the BC-251 samples in this work mirrored results from a previous residential study by this research group (Nannu Shankar et al., 2022). The presence of virus in particles <4.4 μm is important because this indicates that inhaled virus, if viable, has potential to travel to the lower respiratory tract and infect cells there. From the BioSpot-VIVAS samples, we saw that the concentration of viable virus in air was about 10-fold higher than that measured in a previous study of a person infected with a variant of SARS-CoV-2 with high nucleotide sequence identity to the original isolate (Lednicky et al., 2020a,b). Taken together, the size range, concentration, and demonstrated viability of SARS-CoV-2 collected in air samples during this investigation indicate potential for exposure to airborne virus within a residential environment with a self-isolating COVID-19 patient. The existence of such exposure potential indicates that co-occupants of residences also housing self-isolating individuals should consider using respiratory protection to reduce SARS-CoV-2 exposure.
Döhla et al. (2020) detected SARS-CoV-2 RNA in 4 of 119 surface swabs taken in various homes housing COVID-19 patients in isolation or persons in quarantine due to SARS-CoV-2 exposure. Santarpia et al. (2020) collected swabs from two isolation and nine quarantine hospital rooms, detecting SARS-CoV-2 RNA in 70.6% of personal item samples and 75.0% of room items. Nannu Shankar et al. (2022) detected SARS-CoV-2 within a swab from the mobile phone of an individual self-isolating with COVID-19 at home. Each of these studies was unable to confirm the presence of viable virus, despite detecting virus presence by RT-PCR. In general, studies suggest that exposure from fomite transportation to mucus membranes is possible but does not exist as the primary exposure pathway for SARS-CoV-2 (Meyerowitz et al., 2021). Among the environmental surfaces tested in our study, viable virus was only detected on the mobile phone. While it is possible that virus collected from the phone could have been deposited by hand-to-phone transfer, non-detection of viable virus and/or viral RNA by RT-PCR in all other high-touch surface samples indicates that viable virus detection on the phone is consistent with the notion that virus-laden large respiratory droplets deposit close to the emitting source.
After over two years of focused response to SARS-CoV-2, community mitigation measures intended to reduce the impact of the virus on various populations have varied widely. In the circumstance covered here, a collegiate institution provided isolation housing for students living on-campus who tested positive for SARS-CoV-2, but, when no vacancy remained, students were required to isolate off-campus. The policy sought to protect the on-campus community from spread within non-isolation housing areas, but it did so at the expense of off-campus communities, resulting in the movement of a strain of Delta variant SARS-CoV-2 across multiple states and creating potential for broader regional propagation. In this case, policy necessitated co-occupancy of an automobile for many hours of driving. Past work has isolated SARS-CoV-2 from an automobile cab driven by an infectious individual (Lednicky et al., 2021), demonstrating that exposures during transit create increased risk of transmission and indicating that the movement of sick individuals to areas beyond the community where they contracted SARS-CoV-2 should be minimized. An epidemiological investigation was beyond the scope of our work, though we noted that the volunteer wore an N95 respirator for the duration of travel; neither the passenger who traveled with the volunteer nor the people living at the house developed signs of COVID-19.
Given that SARS-CoV-2 can be detected 1–3 days before symptom onset and that viral loads are greatest close to the date of symptom onset, tapering off over a period of about a week in most cases (WHO, 2020; Young et al., 2020), sampling as we did on day three following mild symptom onset may have occurred at a time-point after peak viral shedding (Cevik et al., 2020). While this work does not investigate timeframes most likely to result in peak viral aerosol and virus concentrations on fomites, it does demonstrate potential for viable SARS-CoV-2 collection from both air and surfaces several days after symptom onset, even with presentation of only mild signs of COVID-19.
A limitation of this study is that the opportunistic nature of the event and the required travel distance resulted in a low quantity of data. Uncertainty also exists about air flow associated with HVAC systems at the isolation site. The house that had been surveyed has two independent forced-air HVAC systems that are intermittently cycled according to temperature set points. Therefore, airflow was not consistent, and determination of dominant airflow pathways during sampling was not conducted. The distance between the sampling site and sample storage freezer at the laboratory may have affected sample integrity, resulting in inactivation of some of the collected virus. Ideally, sampling sites should be close to cold storage locations to minimize any such losses. The BC-251 enabled only partial size fractionation of aerosol particles. A particle counter used in conjunction with air samplers would have provided better fidelity regarding physical particle distributions collected by the samplers. Placement of a second BioSpot-VIVAS in Bedroom #2, collocated with the BC-251, would have improved the quality of data related to aerosol collection outside of the isolation environment. However, resource limitations prevented this as the research team had access to only a single BioSpot-VIVAS. Relative humidity (RH) measurements would have provided better understanding of how sampling conditions might have affected virus transport in the air and virus viability, as it is known that both viability and transport of viruses can change across RH ranges (Yang & Marr, 2012). Indoor RH in Florida is expected to typically range between 30% and 60% (Withers & Jeff Sonne, 2014). That range of intermediate RH levels has been shown by bacteriophage assessment (Lin & Marr, 2020) and analysis of reported RH levels in literature assessing SARS-CoV-2 (Ahlawat et al., 2020) as one which can result in variable impacts on the viability and airborne transportation of virus. Alternatively, modeling of SARS-CoV-2 biological decay rates (Aganovic et al., 2021) has indicated that minimal impact on virus viability occurs from 40% to 60% RH. To improve understanding of SARS-CoV-2 aerosol transportation in residences, more expansive studies should collect air samples from a variety of dwelling types housing individuals known to be infected with SARS-CoV-2 with annotation of environmental factors including temperature, RH, and air exchange rates.
5. Conclusions
In this investigation, viable Delta variant SARS-CoV-2 was detected in air collected by air samplers positioned ∼3 m from an infected individual experiencing mild symptoms as well as through surface sampling of a device (mobile phone) that was in frequent proximity to the individual's exhaled breath. Results suggested potential for virus transmission through recirculated air flow or opening of doors. Our investigation supports a possibility of SARS-CoV-2 transport through aerosols and situation-dependent transport through fomites within a single-family home, and we therefore make the following recommendations to reduce virus exposure risk within such settings: (1) when an individual in a home becomes ill with a respiratory virus, that person should make use of an isolation area whenever possible and endeavor to wear a mask to reduce risk of recirculating infectious virus to other areas of the home, (2) even outside of an isolation area, persons at increased health risk within a residence housing an ill individual should take precautions to protect themselves from respiratory pathogens by wearing of a suitable face mask, such as an N95 mask, (3) policy-makers should carefully assess policy decisions so as to not induce unintended second-order effects which require infectious individuals to relocate away from the community where they became ill to another community where they may spread infectious illnesses to new communities.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by National Institutes of Health (Grants No. R44ES030649 and R01AI158868) and tuition for William B. Vass paid through the US Army Medical Service Corps Long-Term Health Education and Training fellowship. The authors thank Dr. William G. Lindsley of the National Institute for Occupational Safety and Health for the free loan of NIOSH BC-251 samplers; Dr. David Kaplan, University of Florida, for providing access to the state vehicle; Dr. Katherine Deliz Quiñones, University of Florida, for Quiñones s to her laboratory's biosafety hood; and Aerosol Devices, Inc., for reach-back maintenance support for the BioSpot-VIVAS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaerosci.2022.106038.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aganovic A., Bi Y., Cao G., Drangsholt F., Kurnitski J., Wargocki P. Building and Environment; 2021. Estimating the impact of indoor relative humidity on SARS-CoV-2 airborne transmission risk using a new modification of the Wells-Riley model. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat A., Wiedensohler A., Mishra S.K. An overview on the role of relative humidity in airborne transmission of sars-cov-2 in indoor environments. Aerosol and Air Quality Research. 2020;20(9):1856–1861. doi: 10.4209/aaqr.2020.06.0302. [DOI] [Google Scholar]
- Allen H., Vusirikala A., Flannagan J., Twohig K.A., Zaidi A., Chudasama D., Lamagni T., Groves N., Turner C., Rawlinson C., Lopez-Bernal J., Harris R., Charlett A., Dabrera G., Kall M., The Covid-19 Genomics UK (COG-UK Consortium) Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): National case-control study. The Lancet Regional Health - Europe. 2021 doi: 10.1016/j.lanepe.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R., Sheng S.L. Characteristics of indoor aerosols in residential homes in urban locations: A case study in Singapore. Journal of the Air and Waste Management Association. 2007;57(8):981–990. doi: 10.3155/1047-3289.57.8.981. [DOI] [PubMed] [Google Scholar]
- Bastos L.S., Ranzani O.T., Souza T.M.L., Hamacher S., Bozza F.A. Vol. 9. Lancet Publishing Group; 2021. COVID-19 hospital admissions: Brazil's first and second waves compared. (The lancet respiratory medicine). Issue 8, e82–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D., Fowler E.J., Abrams M., Collins S.R. Covid-19 implications for the health care system. New England Journal of Medicine. 2021;383(15) doi: 10.1056/NDJMsb2021088. [DOI] [PubMed] [Google Scholar]
- Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Sabo R.T., Hall N., Foreman A., Schubert P.L., Gallagher G.R., Fink T., Madoff L.C., Gabriel S.B., MacInnis B., Park D.J., Siddle K.J., Harik V., Arvidson D.…Scott Laney A. Morbidity and mortality weekly report outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings-barnstable county, Massachusetts, July 2021. 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/large-events/ [DOI] [PMC free article] [PubMed]
- California Department of Public Health COVID-19 public health guidance for K-12 schools in California, 2021-22 school year. 2021, October 20. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/K-12-Guidance-2021-22-School-Year.aspx K-12 Guidance 2021-22 School Year.
- Cao G., Blachere F.M., Lindsley W.G., Noti J.D., Beezhold D.H. Development of a methodology to detect viable airborne virus using personal aerosol samplers. 2010. www.epa.gov/ord [DOI] [PMC free article] [PubMed]
- CDC What to do if you are sick. 2021. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html
- CDC Variant proportions. 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- CDC . 2020, May 29. 2019-Novel Coronavirus (2019-ncov) Real-time rrt-pcr Panel Primers and Probes.https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf [Google Scholar]
- CDC SARS-CoV-2 transmission. CDC COVID-19 Scientific Briefs. 2021 https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html [Google Scholar]
- CDC . 2021. SARS-CoV-2 Variant Classifications and Definitions.https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html [Google Scholar]
- CDC . 2022, February 25. How to Protect Yourself & Others.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html [Google Scholar]
- Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- Chau N.V.V., Ngoc N.M., Nguyet L.A., Quang V.M., Ny N.T.H., Khoa D.B., Phong N.T., Toan L.M., Hong N.T.T., Tuyen N.T.K., Phat V.V., Nhu L.N.T., Truc N.H.T., That B.T.T., Thao H.P., Thao T.N.P., Vuong V.T., Tam T.T.T., Tai N.T.…Tan L. van. An observational study of breakthrough SARS-CoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Ong S.W.X., Chiew C.J., Ang L.W., Chavatte J.-M., Mak T.-M., Cui L., Kalimuddin S., Chia W.N., Tan C.W., Chai L.Y.A., Tan S.Y., Zheng S., Lin R.T.P., Wang L., Leo Y.-S., Lee V.J., Lye D.C., Young B.E. 2021 Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections. medRxiv. 2021 doi: 10.1101/2021.07.28.21261295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K.K., Jie Wen Tay D., sen Tan K., Wei Xiang Ong S., The Son T., Hui Koh M., Qing Chin Y., Nasir H., Minn Mak T., Jang Hann Chu J., Milton D.K., K Chow V.T., Anantharajah Tambyah P., Chen M., Kwok Wai T. Viral load of SARS-CoV-2 in respiratory aerosols emitted by COVID-19 patients while breathing, talking, and singing. 2021. [DOI] [PMC free article] [PubMed]
- Döhla M., Wilbring G., Schulte B., Kümmerer B.M., Diegmann C., Sib E., Richter E., Haag A., Engelhart S., Eis-Hübinger A.M., Exner M., Streeck H., Schmithausen R.M. SARS-CoV-2 in environmental samples of quarantined households. medRxiv. 2020 doi: 10.1101/2020.05.28.20114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.S., Mirchandani D., Plante J.A., Aguilar P.v., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerging Infectious Diseases. 2020;26(9):2168–2171. doi: 10.3201/eid2609.201806. https://wwwnc.cdc.gov/eid/article/26/9/20-1806_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman D.N., Tuite A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: A retrospective cohort study in Ontario, Canada. Canadian Medical Association Journal. 2021;193(42):E1619–E1625. doi: 10.1503/cmaj.211248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G., Hulse M., Nguyen D., Sobotka K., Webster K., Corman J., Aboagye-Nyame B., Dion M., Johnson M., Zalinger B., Ewing M. Vol. 167. Elsevier Ireland Ltd; 2021. Impact of hospital strain on excess deaths during the COVID-19 pandemic - United States, July 2020-July 2021.https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7046a5-H.pdf (Critical reviews in oncology/hematology). [Google Scholar]
- Grijalva C.G., Rolfes M.A., Zhu Y., Mclean H.Q., Hanson K.E., Belongia, Edward A., Halasa N.B., Kim A., Reed C., Fry A.M., Keipp Talbot H. 2020. Morbidity and Mortality Weekly Report Transmission of SARS-COV-2 Infections in Households-Tennessee and Wisconsin. April-September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal P.C., Victora C.G. Vol. 27. Nature Research; 2021. Overcoming Brazil's monumental COVID-19 failure: An urgent call to action; p. 933. (Nature medicine). Issue 6. [DOI] [PubMed] [Google Scholar]
- Hawks S.A., Prussin A.J., Kuchinsky S.C., Pan J., Marr L.C., Duggal N.K. Infectious SARS-CoV-2 is emitted in aerosol particles. mBio. 2021 doi: 10.1128/mbio.02527-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. The New York Times; 2022, April 19. Tracking coronavirus vaccinations Around the world.https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html [Google Scholar]
- Kasloff S.B., Leung A., Strong J.E., Funk D., Cutts T. Stability of SARS-CoV-2 on critical personal protective equipment. Scientific Reports. 2021;11(1) doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA. 2021;326(20) doi: 10.1001/jama.2021.19063. 2018. [DOI] [PubMed] [Google Scholar]
- Lednicky, J. (n.d.). Unpublished results.
- Lednicky J., Lauzardo M., Alam M.M., Elbadry M., Stephenson C., Gibson J.C., Morris J.G. Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. International Journal of Infectious Diseases. 2021;108:212–216. doi: 10.1016/j.ijid.2021.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Nannu Shankar S., Mohamed K., Eiguren-Fernandez A., Stephenson C.J., Alam M.M., Elbadry M.A., Loeb J.C., Subramaniam K., Waltzek T.B., Cherabuddi K., Morris J.G., Wu C.Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. International Journal of Infectious Diseases. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Loeb J.C. Detection and isolation of airborne influenza A H3N2 virus using a sioutas personal cascade impactor sampler. Influenza Res. Treat. 2013:1–8. doi: 10.1155/2013/656825. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Nannu Shankar S., Elbadry M.A., Gibson J.C., Alam M.M., Stephenson C.J., Eiguren-Fernandez A., Glenn Morris J., Mavian C.N., Salemi M., Clugston J.R., Wu C.Y. Collection of SARS-CoV-2 virus from the air of a clinic within a university student health care center and analyses of the viral genomic sequence. Aerosol and Air Quality Research. 2020;20(6):1167–1171. doi: 10.4209/aaqr.2020.05.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J., Pan M., Loeb J., Hsieh H., Eiguren-Fernandez A., Hering S., Fan Z.H., Wu C.Y. Vol. 50. Taylor and Francis Inc; 2016. Highly efficient collection of infectious pandemic influenza H1N1 virus (2009) through laminar-flow water based condensation; pp. i–iv. (Aerosol science and Technology). Issue 7. [DOI] [Google Scholar]
- Levine-Tiefenbrun M., Yelin I., Alapi H., Katz R., Herzel E., Kuint J., Chodick G., Gazit S., Patalon T., Kishony R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nature Medicine. 2021 doi: 10.1038/s41591-021-01575-4. [DOI] [PubMed] [Google Scholar]
- Li B., Deng A., Li K., Hu Y., Li Z., Liu Z., Guo Q., Zou L., Zhang H., Zhang M., Ouyang F., Su J., Su W., Xu J., Lin H., Sun J., Peng J., Jiang H., Zhou P., He J. Viral infection and transmission in a large, well-traced outbreak caused by the 1 SARS-CoV-2 Delta variant 2 3. medRxiv. 2021 doi: 10.1101/2021.07.07.21260122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Schmechel D., Chen B.T. A two-stage cyclone using microcentrifuge tubes for personal bioaerosol sampling. Journal of Environmental Monitoring. 2006;8(11):1136–1142. doi: 10.1039/b609083d. [DOI] [PubMed] [Google Scholar]
- Lin K., Marr L.C. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environmental Science and Technology. 2020;54(2):1024–1032. doi: 10.1021/acs.est.9b04959. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li T., Deng Y., Liu S., Zhang D., Li H., Wang X., Jia L., Han J., Bei Z., Li L., Li J. Vol. 107. W.B. Saunders Ltd; 2021. Stability of SARS-CoV-2 on environmental surfaces and in human excreta; pp. 105–107. (Journal of hospital infection). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre J.P., Jarma D., Yu J.R.F., Siegel J.A., Horner S.D., Kinney K.A. Distribution of SARS-CoV-2 RNA signal in a home with COVID-19 positive occupants. Science of the Total Environment. 2021;778 doi: 10.1016/j.scitotenv.2021.146201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Annals of Internal Medicine. 2021;174(1):69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannu Shankar S., Witanachchi C.T., Morea A.F., Lednicky J.A., Loeb J.C., Alam M.M., Fan Z.H., Eiguren-Fernandez A., Wu C.Y. SARS-CoV-2 in residential rooms of two self-isolating persons with COVID-19. Journal of Aerosol Science. 2022;159 doi: 10.1016/j.jaerosci.2021.105870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Lednicky J.A., Wu C.Y. Journal of applied microbiology (Vol. Vol. 127, 6, pp. 1596–1611). Blackwell Publishing Ltd. 2019. Collection, particle sizing and detection of airborne viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons Leigh J., Kemp L.G., de Grood C., Brundin-Mather R., Stelfox H.T., Ng-Kamstra J.S., Fiest K.M. A qualitative study of physician perceptions and experiences of caring for critically ill patients in the context of resource strain during the first wave of the COVID-19 pandemic. BMC Health Services Research. 2021;21(1) doi: 10.1186/s12913-021-06393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Emerging infectious diseases (Vol. 26, Issue 9). NLM (Medline) 2020. Prolonged infectivity of SARS-CoV-2 in fomites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L.F., Wang N., Salsbury T.I., Underhill R.M., Flaherty J.E., Vlachokostas A., Kulkarni G., James D.P. Vol. 197. Building and Environment; 2021. (Investigation of potential aerosol transmission and infectivity of SARS-CoV-2 through central ventilation systems). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot L.M., Ramar P., Roellinger D.L., Barry B.A., Sharma P., Ebbert J.O. Changes in social relationships during an initial “stay-at-home” phase of the COVID-19 pandemic: A longitudinal survey study in the U.S. Social Science & Medicine. 2021;274 doi: 10.1016/j.socscimed.2021.113779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Miao T., Liu L., Zheng X., Luo D., Li Y. Indoor transmission of SARS-CoV-2. Indoor Air. 2020;31(3):639–645. doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Ragan I., Hartson L., Pidcoke H., Bowen R., Goodrich R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey A.L., Loeb J.C., Robinson S.E., Lednicky J.A., McPherson J., Colson S., Allen M., Coker E.S., Sabo-Attwood T., Maurelli A.T., Bisesi J.H. Wastewater surveillance for SARS-CoV-2 in a small coastal community: Effects of tourism on viral presence and variant identification among low prevalence populations. Environmental Research. 2022;208 doi: 10.1016/j.envres.2021.112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Bohannon K., Williams G., Holland B., Krause M., Green B., Freeburger D., Dabisch P. Comparison of the performance of aerosol sampling devices for measuring infectious SARS-CoV-2 aerosols. Aerosol Science and Technology. 2021;55(8):975–986. doi: 10.1080/02786826.2021.1910137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., Lawler J.v., Reid S.P., Lowe J.J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Biryukov J., Beck K., Yolitz J., Bohannon J., Weaver W., Miller D., Holland B., Krause M., Freeburger D., Williams G., Wood S., Graham A., Rosovitz M.J., Bazinet A., Phillips A., Lovett S., Garcia K., Abbott E., Dabisch P. The stability of an isolate of the SARS-CoV-2 B.1.1.7 lineage in aerosols is similar to three earlier isolates. The Journal of Infectious Diseases. 2021 doi: 10.1093/infdis/jiab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie H.M., Johnson A.G., Suthar A.B., Severson R., Alden N.B., Balter S., Bertolino D., Blythe D., Brady S., Cadwell B., Cheng I., Davidson S., Delgadillo J., Devinney K., Duchin J., Duwell, Monique Fisher R., Fleischauer A., Grant A., Silk B.J. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status — 13 U.S. Jurisdictions. 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7037e1.htm April 4–July 17, 2021. [DOI] [PMC free article] [PubMed]
- Tenforde M.W., Self W.H., Adams K., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., Mohr N.M., Zepeski A., Shapiro N.I., Gibbs K.W., Files D.C., Hager D.N., Shehu A., Prekker M.E., Erickson H.L., Influenza and Other Viruses in the Acutely Ill (IVY) Network Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021 doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texas Education Agency . 2021, September 17. Coronavirus (COVID-19) Support and Guidance.https://tea.texas.gov/texas-schools/health-safety-discipline/covid/coronavirus-covid-19-support-and-guidance [Google Scholar]
- Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.v., Cooper D., Frenck R.W., Jansen K.U. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. New England Journal of Medicine. 2021;385(19):1761–1773. doi: 10.1056/nejmoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Scientific Brief; 2020, July 9. Transmission of SARS-CoV-2: Implications for infection prevention precautions.https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions [Google Scholar]
- WHO . 2022, April 19. World Health Organization (WHO) Coronavirus (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- Withers C.R., Jeff Sonne J. Final report assessment of energy efficient methods of indoor humidity control for Florida building commission research. 2014. http://publications.energyresearch.ucf.edu/wp-content/uploads/2018/06/FSEC-CR-1976-14.pdf
- Yang W., Marr L.C. Mechanisms by which ambient humidity may affect viruses in aerosols. Applied and Environmental Microbiology. 2012;78(19):6781–6788. doi: 10.1128/AEM.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I.C.…Lye D.C. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA, the Journal of the American Medical Association. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.