ABSTRACT
Background
The nonsteroidal mineralocorticoid receptor antagonist finerenone and the sodium–glucose cotransporter-2 inhibitor (SGLT-2i) canagliflozin reduce cardiorenal risk in albuminuric patients with chronic kidney disease (CKD) and type 2 diabetes (T2D). At first glance, the results of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) (ClinicalTrials.gov, NCT02540993) and Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) appear disparate. In FIDELIO-DKD, the primary endpoint had an 18% [95% confidence interval (CI) 7–27] relative risk reduction; in CREDENCE, the primary endpoint had a 30% (95% CI 18–41) relative risk reduction. Unlike CREDENCE, the FIDELIO-DKD trial included patients with high albuminuria but excluded patients with symptomatic heart failure with reduced ejection fraction. The primary endpoint in the FIDELIO-DKD trial was kidney specific and included a sustained decline in the estimated glomerular filtration rate (eGFR) of ≥40% from baseline. In contrast, the primary endpoint in the CREDENCE trial included a sustained decline in eGFR of ≥57% from baseline and cardiovascular (CV) death. This post hoc exploratory analysis investigated how differences in trial design—inclusion/exclusion criteria and definition of primary outcomes—influenced observed treatment effects.
Methods
Patients from FIDELIO-DKD who met the CKD inclusion criteria of the CREDENCE study (urine albumin: creatinine ratio >300–5000 mg/g and an eGFR of 30–<90 mL/min/1.73 m2 at screening) were included in this analysis. The primary endpoint was a cardiorenal composite (CV death, kidney failure, eGFR decrease of ≥57% sustained for ≥4 weeks or renal death). Patients with symptomatic heart failure with reduced ejection fraction were excluded from FIDELIO-DKD. Therefore, in a sensitivity analysis, we further adjusted for the baseline prevalence of heart failure.
Results
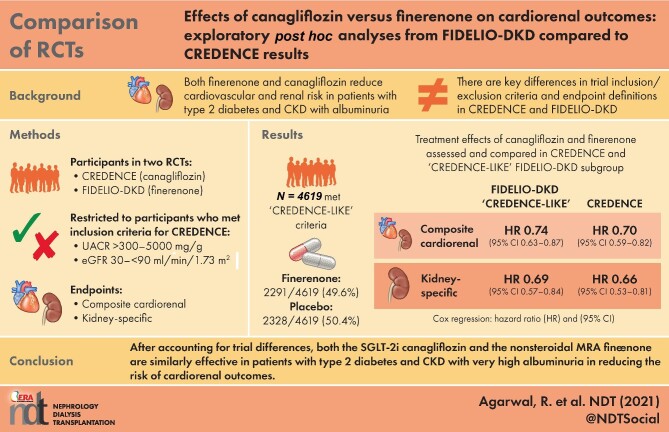
Of 4619/5674 (81.4%) patients who met the subgroup inclusion criteria, 49.6% were treated with finerenone and 50.4% received placebo. The rate of the cardiorenal composite endpoint was 43.9/1000 patient-years with finerenone compared with 59.5/1000 patient-years with placebo. The relative risk was significantly reduced by 26% with finerenone versus placebo [hazard ratio (HR) 0.74 (95% CI 0.63–0.87)]. In CREDENCE, the rate of the cardiorenal composite endpoint was 43.2/1000 patient-years with canagliflozin compared with 61.2/1000 patient-years with placebo; a 30% risk reduction was observed with canagliflozin [HR 0.70 (95% CI 0.59–0.82)].
Conclusions
This analysis highlights the pitfalls of direct comparisons between trials. When key differences in trial design are considered, FIDELIO-DKD and CREDENCE demonstrate cardiorenal benefits of a similar magnitude.
Keywords: canagliflozin, cardiorenal, CREDENCE, FIDELIO-DKD, finerenone
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Both the novel nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone and the sodium–glucose cotransporter-2 inhibitor (SGLT-2i) canagliflozin have been shown to benefit cardiovascular and kidney organ protection in albuminuric patients with type 2 diabetes (T2D) and chronic kidney disease (CKD).
At first glance, the efficacy of finerenone in Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) appears inferior to the efficacy of canagliflozin in Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE).
The primary endpoint had an 18% [95% confidence interval (CI) 7–27] relative risk reduction in FIDELIO-DKD; the primary endpoint had a 30% (95% CI 18–41) relative risk reduction in CREDENCE.
What this study adds?
There are risks in directly comparing two trials even when conducted in the same disease state.
When key differences in trial inclusion/exclusion criteria and endpoint definitions are accounted for, both FIDELIO-DKD and CREDENCE demonstrate cardiorenal benefits of a similar magnitude.
What impact this may have on practice or policy?
Both the SGLT-2i canagliflozin and the nonsteroidal MRA finerenone are similarly effective in patients with T2D and CKD and very high albuminuria in reducing the risk of cardiorenal outcomes.
INTRODUCTION
Patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) are at an increased risk of cardiovascular (CV) events and CKD progression [1]. Although there has been a hiatus of treatment advances for CKD in T2D since the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial and the Irbesartan Diabetic Nephropathy Trial (IDNT) published two decades ago [2, 3], in recent years both finerenone and sodium–glucose cotransporter-2 inhibitors (SGLT-2is) have shown benefits for CV and kidney organ protection in this albuminuric patient population [1, 4–6].
Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) investigated the selective, nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone in patients with CKD and T2D and demonstrated a significant reduction in the risk of kidney and CV outcomes compared with placebo [1]. Two SGLT-2i kidney outcome trials [Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD)] have reported positive cardiorenal outcomes for patients with CKD and T2D and also in patients with CKD without T2D in DAPA-CKD [4, 6]. In the CREDENCE, DAPA-CKD and FIDELIO-DKD trials, the relative risks of the primary outcomes were reduced by 30% [95% confidence interval (CI) 18–41], 39% (95% CI 28–49) and 18% (95% CI 7–27), respectively [1, 4, 5]. At first glance, finerenone may be perceived to have a smaller benefit on kidney outcomes compared with the SGLT-2is canagliflozin and dapagliflozin [7]. However, because of significant differences in the trial designs, primary endpoints [e.g. sustained change from baseline in estimated glomerular filtration rate (eGFR) of ≥40%, ≥50% and ≥57% in FIDELIO-DKD, DAPA-CKD and CREDENCE, respectively] and patient populations, unadjusted comparisons among the trials are prone to biases [1, 4–6].
The objective of this analysis was to facilitate a more nuanced comparison of the treatment effect of finerenone with that of canagliflozin by adjusting for key differences in trial design. These include the use of a cardiorenal composite endpoint equivalent to that used in the CREDENCE trial, restricting the analysis to a subgroup of patients from FIDELIO-DKD who met the CKD inclusion criteria of CREDENCE and, lastly, an adjustment for differences in baseline heart failure (HF) incidence. We included the adjustment for HF incidence because prior studies have shown that HF in patients with CKD can affect cardiorenal outcomes and that these patients benefit from treatment with an SGLT-2i or an MRA [8–12]. Similar comparisons cannot be made between FIDELIO-DKD and DAPA-CKD because DAPA-CKD included patients without diabetes who were not included in FIDELIO-DKD and the primary endpoint in DAPA-CKD was a ≥50% reduction in eGFR, which was not an adjudicated event in FIDELIO-DKD [1, 6].
MATERIALS AND METHODS
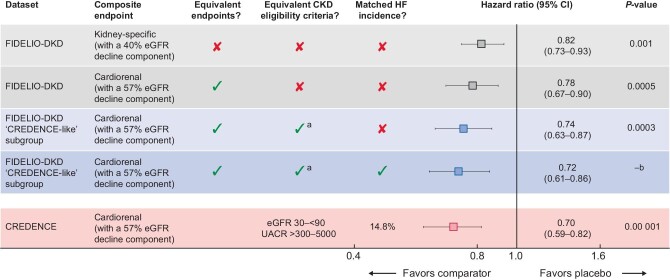
There were several important differences between the study designs of the FIDELIO-DKD and CREDENCE trials (Supplementary data, Figure S1). First, a kidney-specific composite endpoint (time to kidney failure, sustained ≥40% decrease in eGFR from baseline or renal death) was the primary endpoint in FIDELIO-DKD, whereas a cardiorenal composite endpoint [end-stage kidney disease (ESKD), ≥57% decrease in eGFR or death from renal and CV causes] was used in CREDENCE. Second, the inclusion criteria for eGFR in the FIDELIO-DKD trial was 25–<75 mL/min/1.73 m2 versus 30–<90 mL/min/1.73 m2 in CREDENCE [4]. Third, the urine albumin: creatinine ratio (UACR) levels were 30–5000 mg/g in FIDELIO-DKD compared with >300–5000 mg/g in CREDENCE. Fourth, FIDELIO-DKD excluded patients with symptomatic HF with reduced ejection fraction, whereas CREDENCE excluded patients with HF only if they were treated with an MRA (in CREDENCE, MRA use was an exclusion criterion, although postbaseline use was permitted if deemed medically necessary).
The present analysis reports outcomes and safety data in a ‘CREDENCE-like’ subgroup of patients from FIDELIO-DKD who had a UACR >300–5000 mg/g and eGFR 30–<75 mL/min/1.73 m2 at screening (patients with an eGFR >75 mL/min/1.73 m2 were not eligible for FIDELIO-DKD) and accounts for differences in the baseline incidence of HF.
Study design and patient population
The design of the phase 3, randomized, double-blind, placebo-controlled, multicenter FIDELIO-DKD study and the results of the primary analysis (including the clinical study protocol) have been published previously [1, 13]. The trial was performed in accordance with the principles of the Declaration of Helsinki and was approved by the competent authorities and ethics committees at each trial site. All participants provided written informed consent. Briefly, adults (≥18 years of age) with CKD and T2D who were receiving optimized renin-angiotensin system (RAS) inhibitor therapy, with serum potassium ≤4.8 mmol/L and without symptomatic HF with reduced ejection fraction (New York Heart Association Class II–IV) were eligible. The FIDELIO-DKD study comprised a run-in period (during which RAS inhibitor therapy was optimized for 4–16 weeks), a screening period and a double-blind treatment period. Patients were randomized 1:1 to receive oral finerenone or matching placebo once daily. The starting dose of finerenone was 20 mg in participants with an eGFR ≥60 mL/min/1.73 m2 and 10 mg in participants with an eGFR of 25–<60 mL/min/1.73 m2, increasing to 20 mg after 1 month if serum potassium remained ≤4.8 mmol/L and eGFR was stable. Finerenone downtitration from 20 mg to 10 mg due to safety concerns was permitted at any time.
Endpoints
The primary endpoint of interest for this FIDELIO-DKD ‘CREDENCE-like’ subgroup analysis was a composite cardiorenal endpoint of time to first occurrence of CV death, kidney failure [defined as ESKD (the initiation of long-term dialysis for ≥90 days or kidney transplantation) or an eGFR <15 mL/min/1.73 m2 sustained for ≥4 weeks], an eGFR decrease of ≥57% sustained for ≥4 weeks or renal death. Other endpoints included a kidney-specific composite endpoint of time to onset of kidney failure, a sustained decrease of eGFR ≥57% or renal death, a composite CV endpoint of CV death or hospitalization for HF, CV death, death from any cause and a change in UACR from baseline over time. Urinalysis was performed centrally during the run-in and screening visits as well as at baseline, month 4, month 12 and every 12 months thereafter. Central laboratory values, including serum potassium and serum creatinine, were obtained at all study visits.
Statistical analyses
A stratified log-rank test was used to analyze the time-to-event superiority of finerenone versus placebo. The individual components of the cardiorenal endpoints were analyzed in post hoc exploratory analyses from patient-level data in the FIDELIO-DKD trial. Analysis included descriptive statistics and a statistical test for significance. Treatment effects for time-to-event endpoints are expressed as hazard ratios (HRs) with corresponding 95% CIs from a stratified Cox regression model. Events were counted from randomization to the end-of-study visit; participants without an event were censored at the time of their last contact when complete information on all components of the endpoint under investigation were available. Subgroup analyses for the primary and secondary efficacy variables included descriptive statistics and a statistical test for interaction. On-treatment analysis refers to an analysis of events that occurred within 30 days of the last study drug intake.
To investigate the effect of finerenone in a population with more patients with HF at baseline, in a sensitivity analysis we inflated the baseline HF population. The population of patients was constructed by drawing 100 random bootstrap samples with replacements containing the desired inflated number of patients with HF from the existing baseline HF population. The remaining population was randomly sampled in a similar fashion from the patients without HF at baseline. The 100 randomly bootstrapped sampled data sets were analyzed using stratified Cox proportional hazards regression and estimates combined using standard bootstrap combination assumptions.
The change in UACR was analyzed using a repeated measures mixed-model approach that incorporated additional variables to account for effects on UACR. Assuming an unstructured covariance matrix, the model was adjusted for the treatment group, stratification factors (region, albuminuria category at screening and eGFR category at screening), visit, interaction between treatment group and visit, log-transformed baseline value and interaction between the log-transformed baseline value and visit.
RESULTS
Patients
Between September 2015 and June 2018, 13 911 patients from 48 countries were screened for eligibility to participate in FIDELIO-DKD 5674 of whom were randomized and included in the full analysis set [1]. A total of 4619/5674 patients in FIDELIO-DKD met the ‘CREDENCE-like’ criteria (with a UACR >300–5000 mg/g and an eGFR of 30–<75 mL/min/1.73 m2 at screening) and were included in this analysis. Comparison of the baseline demographics from the FIDELIO-DKD subgroup and the CREDENCE group found they were broadly similar with regard to the duration of diabetes, median UACR, percentage of patients who were Black/African American or White and RAS inhibitor use (Table 1). Notable differences in the FIDELIO-DKD ‘CREDENCE-like’ subgroup compared with the CREDENCE group included slightly older patients, with lower systolic blood pressure, lower glycated hemoglobin, lower mean eGFR levels (including fewer patients with eGFR ≥ 60 mL/min/1.73 m2), fewer patients with a history of HF, more frequent use of CV medications and a greater proportion of Asian patients. Of the 4619 (81.4%) patients in the FIDELIO-DKD subgroup included in this analysis, 2291 (49.6%) were treated with finerenone and 2328 (50.4%) were treated with placebo, and baseline characteristics were balanced between treatment groups (Supplementary data, Table S1). The median follow-up was 2.6 years. A total of 1273 (27.6%) patients prematurely discontinued treatment in the study and the number of discontinuations was similar between treatment arms. Of the 4608 patients assessed, mean treatment adherence was high at 92.0%.
Table 1.
Baseline characteristics for the FIDELIO-DKD ‘CREDENCE-like’ subgroupa and CREDENCE
| Characteristics | FIDELIO-DKD ‘CREDENCE-like’ subgroup total (N = 4619) | CREDENCE total (N = 4401) |
|---|---|---|
| Age (years), mean ± SD | 65.3 ± 9.2 | 63.2 ± 9.2 |
| Female, n (%) | 1335 (28.9) | 1494 (33.9) |
| Race, n (%) | ||
| White | 2920 (63.2) | 2931 (66.6) |
| Black/African American | 208 (4.5) | 224 (5.1) |
| Asian | 1171 (25.4) | 877 (19.9) |
| Systolic blood pressure (mmHg), mean ± SD | 138.2 ± 14.1 | 140.0 ± 15.6 |
| Diastolic blood pressure (mmHg), mean ± SD | 76.2 ± 9.5 | 78.3 ± 9.4 |
| BMI (kg/m2), mean ± SD | 31.1 ± 6.0 | 31.3 ± 6.2 |
| Duration of diabetes (years), mean ± SD | 16.1 ± 8.6 | 15.8 ± 8.6 |
| HbA1c (%), mean ± SD | 7.68 ± 1.36 | 8.3 ± 1.3 |
| Serum potassium (mmol/L), mean ± SD | 4.36 ± 0.46 | N/A |
| eGFR (mL/min/1.73 m2), n (%) | 46.5 ± 12.0 | 56.2 ± 18.2 |
| <15 | 0 (0) | 2 (<0.1) |
| ≥15–<30 | 222 (4.8) | 172 (3.9) |
| ≥30–<45 | 2051 (44.4) | 1191 (27.1) |
| ≥45–<60 | 1715 (37.1) | 1266 (28.8) |
| ≥60–<90 | 621 (13.4) | 1558 (35.4) |
| ≥90 | 10 (0.2) | 211 (4.8) |
| UACR (mg/g), median (IQR) | 917 (512–1696) | 927 (463–1833) |
| <30, n (%) | 9 (0.2) | 31 (0.7) |
| 30–≤300, n (%) | 293 (6.3) | 496 (11.3) |
| >300–≤3000, n (%) | 3951 (85.5) | 3371 (76.6) |
| >3000, n (%) | 366 (7.9) | 503 (11.4) |
| History of CV disease, n (%) | ||
| Coronary artery disease | 1398 (30.3) | 1313 (29.8) |
| Cerebrovascular disease | 555 (12.0) | 700 (15.9) |
| Peripheral artery disease | 741 (16.0) | 1046 (23.8) |
| Heart failure | 350 (7.6) | 652 (14.8) |
| Current smoker, n (%) | 695 (15.0) | 639 (14.5) |
| Medication use at baseline, n (%) | ||
| RAAS inhibitor | 4609 (99.8) | 4395 (99.9) |
| Beta-blocker | 2393 (51.8) | 1770 (40.2) |
| Diuretic | 2535 (54.9) | 2057 (46.7) |
| Statin | 3432 (74.3) | 3036 (69.0) |
| Antithrombotic | 2889 (62.4) | 2624 (59.6) |
| Glucose-lowering therapies, n (%) | ||
| Insulin and analogs | 2901 (62.8) | 2884 (65.5) |
| Metformin | 2211 (47.9) | 2545 (57.8) |
| Sulfonylurea | 1101 (23.8) | 1268 (28.8) |
| DPP-4 inhibitor | 1216 (26.3) | 751 (17.1) |
| GLP-1RA | 329 (7.1) | 183 (4.2) |
| SGLT-2i | 231 (5.0) | 2202 (50.0) |
| Alpha glucosidase inhibitor | 269 (5.8) | 139 (3.2) |
| Thiazolidinedione | 184 (4.0) | 136 (3.1) |
UACR >300 mg/g and eGFR >30 mL/min/1.73 m2 at baseline.
DPP-4, dipeptidyl peptidase-4; GLP-1RA, glucagon-like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; RAAS, renin–angiotensin–aldosterone system.
Thirty-five (0.8%) of the patients in the CREDENCE trial were on steroidal MRAs at baseline.
Efficacy
Cardiorenal composite endpoint
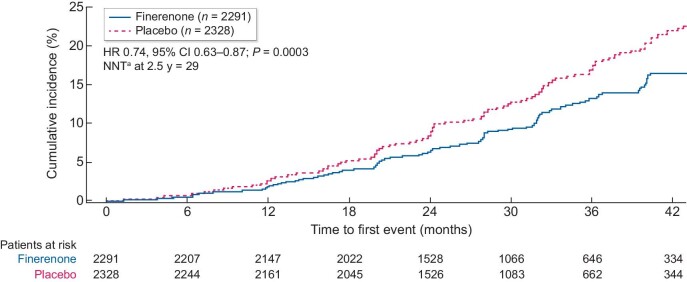
In the FIDELIO-DKD ‘CREDENCE-like’ subgroup (with UACR >300–5000 mg/g and an eGFR of 30–<75 mL/min/1.73 m2 at baseline), the risk of the cardiorenal composite endpoint of time to first occurrence of CV death, kidney failure, eGFR decrease of ≥57% sustained for ≥4 weeks or renal death was significantly reduced by 26% with finerenone versus placebo [HR 0.74 (95% CI 0.63–0.87); P = 0.0003; Figures 1 and 2]. In the overall FIDELIO-DKD population (N = 5674, i.e. before selecting patients that meet the ‘CREDENCE-like’ inclusion criteria), the risk of the same cardiorenal composite endpoint was 22% lower with finerenone versus placebo [HR 0.78 (95% CI 0.67–0.90); P = 0.0005].
FIGURE 1:
Analysis of the composite cardiorenal endpoint in the FIDELIO-DKD ‘CREDENCE-like’ subgroup.b
aBased on an absolute risk reduction of 3.4%. bUACR >300 mg/g and eGFR >30 mL/min/1.73 m2 at baseline. NNT, number needed to treat.
FIGURE 2:
Importance of trial design when comparing cardiorenal outcomes—effects of different composite endpoints and patient characteristics. aPatients with an eGFR >75 mL/min/1.73 m2 were ineligible for FIDELIO-DKD. bP-value unavailable as inflation analysis.
Among patients who did not meet the ‘CREDENCE-like’ inclusion criteria, the composite cardiorenal ‘CREDENCE-like’ outcome occurred in 99 of 542 patients who were assigned to finerenone and 104 of 513 patients who were assigned to placebo [HR 0.86 (95% CI 0.65–1.13)]. In contrast, among patients who met the ‘CREDENCE-like’ inclusion criteria, the composite cardiorenal outcome occurred in 241 of 2291 patients who were assigned to finerenone and 330 of 2328 patients who were assigned to placebo [HR 0.74 (95% CI 0.63–0.87)]. The test of heterogeneity was not significant (P = 0.37).
In the FIDELIO-DKD ‘CREDENCE-like’ subgroup, adjustment of the baseline HF incidence from 7.6% to 14.8% of the overall population resulted in a 28% lower risk of the cardiorenal composite endpoint with finerenone versus placebo [HR 0.72 (95% CI 0.61–0.86); Figure 2]. For comparison, in the CREDENCE study, canagliflozin reduced the risk of the cardiorenal composite endpoint by 30% compared with placebo [HR 0.70 (95% CI 0.59–0.82); P = 0.00 001; Figure 2] [4].
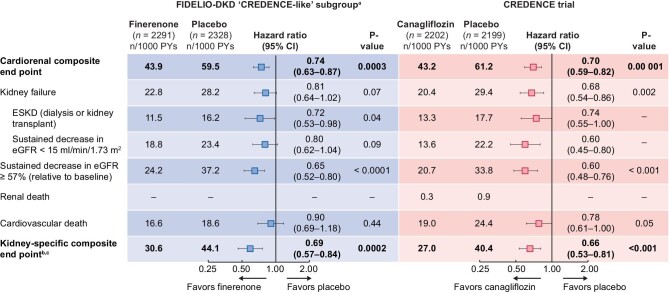
The incidences of each of the components of the cardiorenal composite endpoint were lower with finerenone than placebo (Figure 3). Notably, the risk of ESKD was 28% lower with finerenone versus placebo [HR 0.72 (95% CI 0.53–0.98); P = 0.04]. Overall, the relative risk of the cardiorenal composite endpoint in the placebo groups of the FIDELIO-DKD subgroup and CREDENCE study were similar, with event rates of 59.5 and 61.2 per 1000 patient-years, as were incidences of eGFR <15 mL/min/1.73 m2 (23.4 versus 22.2 per 1000 patient-years) and ESKD (16.2 versus 17.7 per 1000 patient-years) [4]. However, whereas the incidence rate of sustained eGFR decline ≥57% from baseline was larger in the placebo arm of the FIDELIO-DKD subgroup than the CREDENCE population (37.2 versus 33.8 patients with event per 1000 patient-years), the CV death rate was lower with placebo in FIDELIO-DKD than in CREDENCE (18.6 versus 24.4 patients with event per 1000 patient-years) [4].
FIGURE 3:
Analysis of key endpoints in the FIDELIO-DKD ‘CREDENCE-like’ subgroup and the CREDENCE trials.b
aFull analysis set restricted to patients with UACR >300 mg/g and eGFR >30 mL/min/1.73 m2. bTime to onset of kidney failure, sustained decrease of eGFR ≥57% or renal death for FIDELIO-DKD. cESKD, doubling of serum creatinine or renal death for CREDENCE. PY, patient-years.
The efficacy of finerenone compared with placebo in the FIDELIO-DKD ‘CREDENCE-like’ population was consistent for the cardiorenal composite endpoint for several key subgroups (Supplementary data, Figure S2) and in an ‘on-treatment’ sensitivity analysis; details are included in the Supplementary data.
Kidney-specific endpoints
A kidney-specific composite endpoint of kidney failure of time to onset of kidney failure, a sustained decrease of eGFR ≥57% or renal death was significantly improved by 31% with finerenone versus placebo in the subgroup of ‘CREDENCE-like’ patients [HR 0.69 (95% CI 0.57–0.84); P = 0.0002; Figure 3]. In the CREDENCE study, canagliflozin reduced the risk of kidney composite endpoint of ESKD, doubling of the creatinine level (equivalent to a sustained decrease of eGFR ≥57%) or renal death by 34% compared with placebo [HR 0.66 (95% CI 0.53–0.81); P < 0.001; Figure 3] [4].
In the FIDELIO-DKD ‘CREDENCE-like’ subgroup, the efficacy of finerenone compared with placebo was consistent for the kidney-specific composite endpoints for several subgroups (Figure 3) and in an ‘on-treatment’ sensitivity analysis; details are included in the Supplementary data. Improvements in albuminuria were similar between the two trials (see the Supplementary data).
Other efficacy endpoints
Other endpoints, including composite CV endpoint of time to first occurrence of CV death or hospitalization for HF and death from any cause, trended toward favoring finerenone but did not meet statistical significance [HR 0.86 (95% CI 0.71–1.05) and HR 0.88 (95% CI 0.72–1.08), respectively].
Safety
The overall numbers of adverse events and serious adverse events were similar with finerenone versus placebo (Supplementary data, Table S2). Consistent with the mechanism of action of finerenone, hyperkalemia events were increased in patients treated with finerenone versus placebo (15.3% versus 7.6%). In CREDENCE, hyperkalemia events were less frequent in patients treated with canagliflozin versus placebo (6.9% versus 8.2%) [4].
The incidences of acute kidney injury (AKI) in the two trials were similar and were not altered by finerenone or canagliflozin.
In the FIDELIO-DKD trial with ‘CREDENCE-like’ inclusion criteria, those assigned to finerenone had 104 AKI events (4.5%) reported in 2288 patients (21.4 events per 1000 patient-years), compared with those assigned to placebo who had 106 AKI events (4.6%) in 2320 patients (21.2 events per 1000 patient-years). By comparison, in the CREDENCE trial, those assigned to canagliflozin had 86 AKI events (3.9%) reported in 2200 patients (16.9 events per 1000 patient-years) compared with those assigned to placebo who had 98 AKI events (4.5%) in 2197 patients (20.0 events per 1000 patient-years) [4].
The incidences of amputation, fracture and diabetic ketoacidosis were balanced and low in both the finerenone and placebo treatment groups (Supplementary data, Table S2).
DISCUSSION
Our study demonstrates the central importance of considering trial eligibility criteria and endpoint definitions on the effect size of therapies approved for the management of CKD in T2D. Three progressive adjustments—inclusion criteria, endpoint and key exclusion criteria—provided effect size estimates that were strikingly similar between the trials. The joint consideration of the CREDENCE trial inclusion criteria (UACR >300–5000 mg/g and eGFR >30–<90 mL/min/1.73 m2 at screening) and CREDENCE-like endpoint (the cardiorenal composite endpoint of time to first occurrence of CV death, kidney failure, eGFR decrease of ≥57% sustained for ≥4 weeks or renal death) demonstrated that compared with placebo, finerenone demonstrated clinical efficacy. Furthermore, the relative risk reduction with finerenone was larger in this subgroup population than in the overall patient population.
In the present FIDELIO-DKD ‘CREDENCE-like’ subgroup, there was a 26% reduction in the risk of the cardiorenal composite endpoint with finerenone compared with placebo. In CREDENCE, a 30% risk reduction in the same composite endpoint was observed with canagliflozin compared with placebo. Because FIDELIO-DKD excluded patients with symptomatic HF with reduced ejection fraction, whereas CREDENCE excluded patients with HF treated with an MRA, the proportion of patients with HF was lower in the FIDELIO-DKD subgroup studied here than in CREDENCE (7.6% versus 14.8%) [1, 4]. This imbalance is noteworthy because patients with HF are at much greater risk of experiencing CV death [14]; indeed, we saw a larger CV death event rate in the placebo arm of this FIDELIO-DKD ‘CREDENCE-like’ subgroup than in CREDENCE (18.6 versus 24.4 patients with an event per 1000 patient-years, respectively). Therefore we adjusted the proportion of patients with a history of HF; after this modification, the risk of the cardiorenal composite endpoint was reduced by 28% with finerenone versus placebo in the FIDELIO-DKD subgroup studied here. The results of this analysis highlight the pitfalls of direct comparisons between trials with differing patient eligibility criteria and how subtle differences in inclusion eligibility criteria, especially HF, can lead to meaningful variations in outcomes.
Comparisons between the FIDELIO-DKD ‘CREDENCE-like’ subgroup studied here and the CREDENCE population reveal differences in patient baseline characteristics (Supplementary data, Table S1), which are important to be aware of. Because FIDELIO-DKD did not include patients with an eGFR ≥ 75 mL/min/1.73 m2, patients in the FIDELIO-DKD subgroup had a lower mean eGFR (and fewer patients had a baseline eGFR ≥60 mL/min/1.73 m2) than in CREDENCE. Moreover, compared with CREDENCE, the FIDELIO-DKD ‘CREDENCE-like’ subgroup included fewer patients with nephrotic-range severely elevated albuminuria. Together, these differences mean that the FIDELIO-DKD subgroup represents a more advanced CKD stage, with less albuminuria, so their kidney damage may potentially be more difficult to repair. The length of the period allowed for optimization of medical therapy was different between the trials, which may, in part, account for lower systolic blood pressure (138 mmHg versus 140 mmHg) and glycated hemoglobin levels (7.7% versus 8.3%) between the FIDELIO-DKD subgroup and the CREDENCE population, as well as more frequent use of CV medications in the FIDELIO-DKD subgroup. Moreover, FIDELIO-DKD allowed combination therapy with finerenone and an SGLT-2i (with 4.6% of patients receiving an SGLT-2i at baseline), whereas combination therapy with an SGLT-2i and a steroidal MRA was limited in CREDENCE (as baseline MRA use was prohibited). Furthermore, in FIDELIO-DKD, the protocol allowed for treatment interruptions and the discontinuation rate was greater than in CREDENCE. Therefore, on-treatment analysis is very important because the real comparison effect is somewhere between the intention-to-treat and on-treatment analyses. In the future, matched indirect comparisons might be a suitable tool to further reduce the trial differences. However, such an analysis can also introduce bias.
In the DAPA-CKD trial, which included patients with and without T2D, a decline in eGFR of at least 50% from baseline was used in the primary cardiorenal composite endpoint, in addition to ESKD and renal or CV death. In the cohort of patients with T2D, a 36% risk reduction in the primary outcome was observed with dapagliflozin compared with placebo [HR 0.64 (95% CI 0.52–0.79)] [6]. We did not include DAPA-CKD as a comparator because a decline in eGFR of at least 50% from baseline was not a prespecified adjudicated outcome in the FIDELIO-DKD trial. Whereas the primary outcome points to a larger magnitude of risk reduction with dapagliflozin using a smaller threshold for eGFR decrease, the 95% CI largely overlaps with that observed for the primary outcome in the CREDENCE study. This might suggest that a decline of at least 50% in eGFR could be as sensitive as a doubling of serum creatinine. On the other hand, it might suggest that there was a larger treatment effect with dapagliflozin in the trial population studied. Given the equipoise, future trials should at least use consistent definitions of clinical endpoints.
There are several limitations of our analyses. Despite attempts to match the CREDENCE and FIDELIO-DKD populations and endpoints, it is impossible to account for all differences. A true comparison of canagliflozin and finerenone would require a head-to-head trial. Furthermore, our analysis was not prespecified; we did not have individual patient-level data from CREDENCE and we cannot account for differences in trial duration or adherence to study treatments between the two trials.
Nonetheless, our article highlights the risks of directly comparing two trials even when conducted in the same disease state; when key differences in trial design and endpoint definitions are accounted for, both FIDELIO-DKD and CREDENCE demonstrate cardiorenal benefits of a similar magnitude. With finerenone, canagliflozin and dapagliflozin demonstrating clinically meaningful improvements in cardiorenal outcomes in patients with advanced CKD and T2D on top of RAS blockade, the results from the FIDELIO-DKD, CREDENCE and DAPA-CKD trials suggest a more optimistic future for patients with CKD and T2D.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the patients who participated in this trial, the FIDELIO-DKD study investigators, the study centers who supported the trial and the study teams. Medical writing assistance was provided by Oyinkan Adesakin, of Chameleon Communications International, and was funded by Bayer AG.
Contributor Information
Rajiv Agarwal, Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN, USA.
Stefan D Anker, Department of Cardiology (CVK) and Berlin Institute of Health Center for Regenerative Therapies, German Centre for Cardiovascular Research Partner Site Berlin, Charité Universitätsmedizin, Berlin, Germany.
Gerasimos Filippatos, National and Kapodistrian University of Athens, School of Medicine, Department of Cardiology, Attikon University Hospital, Athens, Greece.
Bertram Pitt, Department of Medicine, University of Michigan School of Medicine, Ann Arbor, MI, USA.
Peter Rossing, Steno Diabetes Center Copenhagen, Gentofte, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Luis M Ruilope, Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, Madrid, Spain; CIBER-CV, Hospital Universitario 12 de Octubre, Madrid, Spain; Faculty of Sport Sciences, European University of Madrid, Madrid, Spain.
John Boletis, Faculty of Medicine, Laiko General Hospital, University of Athens, Greece.
Robert Toto, Department of Internal Medicine, UT Southwestern, Dallas, TX, USA.
Guillermo E Umpierrez, Division of Endocrinology, Metabolism, Emory University School of Medicine, Atlanta, GA, USA.
Christoph Wanner, Medizinische Klinik und Poliklinik 1, Schwerpunkt Nephrologie, Universitätsklinik Würzburg, Würzburg, Germany.
Takashi Wada, Department of Nephrology, Kanazawa University, Ishikawa, Japan.
Charlie Scott, Data Science and Analytics, Bayer PLC, Reading, UK.
Amer Joseph, Cardiology and Nephrology Clinical Development, Bayer AG, Berlin, Germany.
Ike Ogbaa, US Medical Affairs—Cardiovascular and Renal, Bayer U.S. LLC, Wayne, NJ, USA.
Luke Roberts, Study Medical Experts, Bayer PLC, Reading, UK.
Markus F Scheerer, Global Medical Affairs and Pharmacovigilance, Pharmaceuticals, Bayer AG, Berlin, Germany.
George L Bakris, Department of Medicine, University of Chicago Medicine, Chicago, IL, USA.
AUTHORS’ CONTRIBUTIONS
The FIDELIO-DKD trial was conducted and funded by Bayer AG. The sponsor participated in the study design, data collection, data analysis, data interpretation and approval of the manuscript. Analyses were conducted by the sponsor and all authors had access to and participated in the interpretation of the data. R.A. wrote the first draft of the report. All authors were involved in drafting and critically revising the report. All authors had access to study results and the first author assumes responsibility for the integrity and accuracy of the data reported. All authors reviewed and approved the final submitted version of the article.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are not currently available in a public repository.
CONFLICT OF INTEREST STATEMENT
R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals during the conduct of the study. He also reports personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius, Janssen, Relypsa, Sanofi and Vifor Pharma. He has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co and Reata and nonfinancial support from E.R. Squibb & Sons, Opko Pharmaceuticals and Otsuka America Pharmaceutical. He is a member of data safety monitoring committees for AstraZeneca and Ironwood Pharmaceuticals; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen and Relypsa; and a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim and Janssen. He has served as an associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate. He has received research grants from the National Institutes of Health and the US Veterans Administration. S.D.A. has received research support from Abbott Vascular and Vifor Pharma and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier and Vifor Pharma. G.F. reports lectures fees and/or that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier and Vifor Pharma. He is a senior consulting editor for JACC Heart Failure and has received research support from the European Union. B.P. reports consultant fees for Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida and Vifor/Relypsa and stock options for Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, Sarfez, scPharmaceuticals, SQ Innovation, Tricida and Vifor/Relypsa. He also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent number 9 931 412) and a provisional patent for histone acetylation–modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045 784). P.R. reports personal fees from Bayer during the conduct of the study. He has received research support and personal fees from AstraZeneca and Novo Nordisk and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Merck, Merck Sharp & Dohme, Mundipharma, Sanofi and Vifor. All fees are given to the Steno Diabetes Center Copenhagen. L.M.R. reports receipt of consultancy fees from Bayer. J.B. reports that he has no disclosures. R.T. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Medscape, Otsuka, Reata and Vifor. G.E.U. reports that he is a principal investigator in clinical studies sponsored by AstraZeneca, Dexcom and Novo Nordisk. C.W. has received honoraria for services to AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Merck Sharp & Dohme. T.W. reports personal fees from Astellas Pharma, AstraZeneca K.K., Bayer Yakuhin, Daiichi Sankyo, Eli Lilly Japan, Kissei Pharmaceutical, Kowa, Kyowa Kirin, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Sanwa Chemistry, Sanofi, Taisho Pharma and Takeda Pharmaceutical. He reports research support from Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Kissei Pharmaceutical, Kyowa Kirin, Mitsubishi Tanabe Pharma, MSD, Sanofi and Takeda Pharmaceutical. C.S. and L.R. are full-time employees of Bayer PLC, UK. A.J. and M.F.S. are full-time employees of Bayer AG, Germany. M.F.S. is also a shareholder in AstraZeneca, Bayer, Eli Lilly and Novo Nordisk. I.O. is a full-time employee of Bayer U.S. G.L.B. reports research funding paid to the University of Chicago Medicine from Bayer during the conduct of the study. He also reports research funding paid to the University of Chicago Medicine from Novo Nordisk and Vascular Dynamics. He acted as a consultant for and received personal fees from Alnylam Pharmaceuticals, Merck and Relypsa. He is an editor of the American Journal of Nephrology, Nephrology and Hypertension, section editor of UpToDate and an associate editor of Diabetes Care and Hypertension Research.
REFERENCES
- 1. Bakris GL, Agarwal R, Anker SDet al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219–2229 [DOI] [PubMed] [Google Scholar]
- 2. Lewis EJ, Hunsicker LG, Clarke WRet al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 3. Brenner BM, Cooper ME, de Zeeuw Det al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 5. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 6. Wheeler DC, Stefánsson BV, Jongs Net al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabet Endocrinol 2021; 9: 22–31 [DOI] [PubMed] [Google Scholar]
- 7. Sridhar VS, Liu H, Cherney DZI. Finerenone—a new frontier in renin-angiotensin-aldosterone system inhibition in diabetic kidney disease. Am J Kidney Dis 2021; 78: 309–311 [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJV, Solomon SD, Inzucchi SEet al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008 [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Anker SD, Butler Jet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424 [DOI] [PubMed] [Google Scholar]
- 10. Pitt B, Zannad F, Remme WJet al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717 [DOI] [PubMed] [Google Scholar]
- 11. Pitt B, Remme W, Zannad Fet al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321 [DOI] [PubMed] [Google Scholar]
- 12. Zannad F, McMurray JJ, Krum Het al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21 [DOI] [PubMed] [Google Scholar]
- 13. Filippatos G, Anker SD, Agarwal Ret al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021; 143: 540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. United States Renal Data System . USRDS Annual Data Report. Volume 1: Chronic kidney disease. Chapter 4: Cardiovascular disease in patients with CKD. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2020/chronic-kidney-disease/4-cardiovascular-disease-in-patients-with-ckd (11 November 2020, date last accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are not currently available in a public repository.