ABSTRACT
Background
The coronavirus disease (COVID) pandemic has resulted in a major disruption in healthcare that has affected several medical and surgical specialties. European and American Vascular Societies have proposed deferring the creation of an elective vascular access (VA) [autologous or prosthetic arteriovenous fistula (AVF) or arteriovenous graft (AVG)] in incident patients on haemodialysis (HD) in the era of the COVID pandemic. The aim of this study is to examine the impact of the COVID pandemic on VA creation and the central venous catheter (CVC)-related hospitalizations and complications in HD patients dialyzed in 16 Spanish HD units of three different regions.
Methods
We compared retrospectively two periods of time: the pre-COVID (1 January 2019–11 March 2020) and the COVID era (12 March 2020–30 June 2021) in all HD patients (prevalent and incident) dialyzed in our 16 HD centres. The variables analysed were type of VA (CVC, AVF and AVG) created, percentage of CVC in incident and prevalent HD patients, CVC-related hospitalizations and complications (infection, extrusion, disfunction, catheter removal) and percentage of CVC HD sessions that did not reach the goal of Kt (>45) as a marker of HD adequacy.
Results
A total of 1791 VAs for HD were created and 905 patients started HD during the study period. Patients who underwent vascular access surgery during the COVID period compared with pre-COVID period were significantly younger, with a significant decrease in surgical activity to create AVFs and AVGs in older HD patients (>75 and >85 years of age). There was a significant increase in CVC placement (from 59.7% to 69.5%; P < 0.001) from the pre-COVID to the COVID period. During the COVID pandemic, a significantly higher number of patients started HD through a CVC (80.3% versus 69.1%; P < 0.001). The percentage of CVC in prevalent HD patients has not decreased in the 19 months since the start of the pandemic [414 CVC/1058 prevalent patients (39.4%)]. No significant changes were detected in CVC-related hospitalizations between the pre-COVID and COVID periods. In the COVID period, a significant increase in catheter replacement and the percentage of HD session that did not reach the HD dose objective (Kt > 45) was observed.
Conclusions
COVID has presented a public health system crisis that has influenced VA for HD, with an increase in CVCs relative to AVFs. A decrease in HD sessions that did not reach the HD dose objective was observed in the COVID period compared with a pre-COVID period.
Keywords: catheter, chronic haemodialysis, COVID, SARS-CoV-2, vascular access
Graphical Abstract
Graphical Abstract.
INTRODUCTION
A new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced pneumonia appeared in late November 2019 in Wuhan, China and was named coronavirus 2019 disease (COVID-19) by the World Health Organization (WHO) on 11 February, 2020 [1]. The world is experiencing its third major epidemic of coronavirus infections [2]. Haemodialysis (HD) patients have a high risk of severe COVID, with an incidence of 16.7% and a mortality rate of 30.8% during the first pandemic wave in Spain [3]. The COVID pandemic has resulted in a major disruption in the healthcare system that has affected several medical and surgical specialties [4, 5]. In an effort to address the burden of COVID disease and the demand for healthcare resources, elective procedures were deferred or suspended [6]. European and American vascular societies in the era of the COVID pandemic have recommended classifying operations into urgent, emergent and elective based on the nature of their pathology [7, 8]. Based on this, they proposed to defer the creation of an elective vascular access (VA), either an autologous or prosthetic arteriovenous fistula (AVF) or arteriovenous graft (AVG) for incident patients on HD, and revision for VA malfunction/steal in prevalent patients on HD. The potential benefit of early permanent VA has had to be weighed against the downside of potentially exposing patients to the virus and consuming valuable resources, including operating rooms, staff and personal protection equipment. However, most nephrologists believe that the procedures that guarantee AVFs or AVGs for HD patients should not be postponed [9]. No data have been published on the real effect that this measure has had on the proportion of central venous catheters (CVCs) in HD patients. The aim of this study was to examine the impact of the COVID pandemic on VA creation and the CVC-related hospitalizations and complications in HD patients dialyzed in our HD units in several Spanish regions during the pandemic.
MATERIALS AND METHODS
Patients and variables
A retrospective study of the pre- versus post-COVID pandemic was performed on all HD patients from our 16 centres in three geographical regions of Spain [Madrid (9 centres, 848 patients); Galicia (3 centres, 169 patients) and Castilla-León (4 centres, 119 patients)] from 1 January 2019 to 31 June 2021. All dialysis patients (incident and prevalent) in these 16 centres were included.
The period of the study included two similar time periods: pre-COVID period (1 January 2019–11 March 2020; 15 months) and COVID period [12 March 2020 (the date of announcement of the coronavirus pandemic by the WHO)–30 June 2021; 15 months). To determine the ability to reverse the situation in the medium term, we analysed the percentage of CVCs in prevalent HD patients in the last period after the pandemic (1 July–30 October 2021).
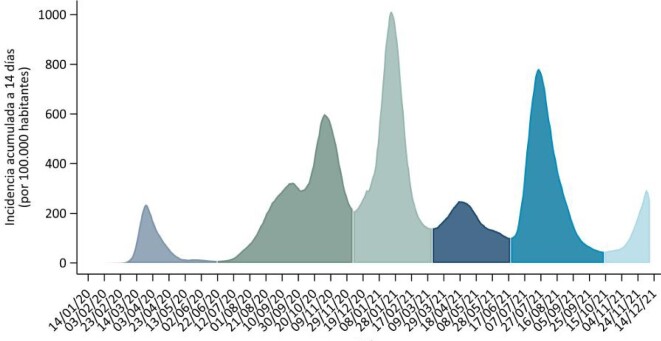
In the evolution of the COVID pandemic in Spain, five periods (waves) are indicated (Figure 1) [10]:
FIGURE 1:
Waves of the COVID pandemic in Spain.
First period: From the start of the pandemic until 21 June 2020, the date on which the state of alarm in Spain ended once the first epidemic wave of COVID ended.
Second period: From 22 June to 6 December 2020, the inflection points of the 14-day cumulative incidence (AI) of COVID cases, between the second and third epidemic period.
Third period: From 7 December 2020 to 14 March 2021, the inflection points of the AI to 14 days of COVID cases, between the third and the fourth epidemic period.
Fourth period: From 15 March to 19 June 2021, the inflection points of the AI to 14 days of COVID cases, between the fourth and the fifth epidemic period.
Fifth period: From 20 June to 13 October 2021, the inflection points of the AI to 14 days of COVID cases, between the fifth and the sixth epidemic period [10].
A wave was defined both as a rapid exponential increase and a rapid decrease in cases.
We analysed the type of consecutive VAs created (CVC, AVF, AVG), AVF location [radiocephalic AVF/ brachiocephalic AVF), VA in prevalent and incident HD patients, patient demographics (age, sex) and comorbidities (diabetes mellitus (DM)], urgent versus scheduled entry on hemodialysis, CVC-related hospitalizations, CVC-related complications (infection, disfunction, CVC removal, extrusion) and hemodialysis adequacy [percentage of patients who reach the Kt goal (Kt > 45)].
Statistical analysis
The demographic and baseline characteristics of the study populations were compared using the Pearson’s chi-squared test for categorical variables, Student’s t-test (parametric) or Mann–Whitney U test (non-parametric) for continuous variables, or one-way analysis of variance for multiple comparisons. Normality of distribution was assessed by the Kolmogorov–Smirnov test. Continuous variables were reported as mean (standard deviation) or median (interquartile range), as appropriate. Categorical variables were reported as number (percentage). All P-values were two-sided. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS Statistics version 20 (IBM, Armonk, NY, USA).
RESULTS
Vascular access creation in the pre-COVID and COVID period
We included 1791 VAs for HD created in 1136 prevalent and incident patients between 1 January 2019 and 31 June 2021 divided into two periods: 930 (51.9%) during the pre-COVID period and 861 VA (48.1%) during the COVID pandemic.
There was a significant increase in CVC placement (from 59.7 to 69.5%) and consequently a significant decrease in AVF creation (from 34.5 to 26%) during the pandemic time period (P < 0.001) compared with the pre-COVID period (Table 1, Figure 2). No changes in the distribution of AVF radiocephalic AVF/brachiocephalic AVF were observed between the pre-COVID (57.8/58.9%) and COVID period (42.1/41.1%) (P = 0.670).
Table 1.
Type of vascular access for HD created in the pre-COVID and COVID period and CVC prevalence in incident and prevalent HD patients
| Type of access | Pre-COVID period | COVID period | P-value |
|---|---|---|---|
| Consecutive VA created, n (%) |
930 VA |
861 VA |
<0.001 |
| CVC | 555 (59.7) | 598 (69.5) | |
| AVF | 321 (34.5) | 224 (26) | |
| AVG | 54 (5.8) | 39 (4.5) | |
| VA in incident HD patients, n (%) |
447 patients |
458 patients |
<0.001 |
| CVC | 309 (69.1) | 368 (80.3) | |
| AVF | 131 (29.3) | 81 (17.7) | |
| AVG | 7 (1.9) | 9 (2) | |
| VA in prevalent HD patients, n (%) |
1083 patients |
1343 patients |
<0.001 |
| CVC | 372 (34.4) | 494 (36.83) | |
| AVF | 663 (61.2) | 804 (59.8) | |
| AVG | 48 (4.4) | 45 (3.3) |
FIGURE 2:
Vascular access created in pre-COVID and COVID period (P < 0.001).
In all, 14 centres suspended or delayed AV creation and 2 maintained AV surgical activity. The centres that maintained surgical activity, as expected, obtained similar results in the percentage of catheters (60.5% versus 62%) and the number of AVFs created (32% versus 29.1%) in the pre-COVID and COVID period (P = 0.733), while the rest decreased AVF creation (30.5% versus 24%) and increased CVC placement (59.5% versus 72.2%) (P < 0.001).
Figure 3 shows the evolution of the type of vascular access for HD according to different waves of COVID. As of the first quarter of 2020, when the COVID pandemic began, an increase in the percentage of patients who started HD with catheters and a decrease in the number of AVFs created was observed. A change in the trend of performance of VA was observed in the second and fifth waves, as we recorded a reduction of the difference between CVC and AVF, which denotes a discreet recovery of surgical activity, that was more accentuated in the fifth wave.
FIGURE 3:
Evolution of the type of VA for HD according to different waves of COVID (P < 0.001).
Vascular access in prevalent and incident patients in the pre-COVID and COVID period
A significantly higher number of incident patients started HD through a CVC (80.3% versus 69.1%) (Table 1) and the prevalence of CVC increased significantly in the COVID period (Table 1) (34.4% versus 36.8%) (P < 0.001). The percentage of CVC in prevalent HD patients did not decrease until 19 months after the start of the pandemic [414 CVC/1058 prevalent patients (39.4%)].
Patient demographics and comorbidities in the pre-COVID and COVID period
The demographic parameters and comorbidities of patients who underwent VA surgery are shown in Table 2. Patients who underwent VA surgery during the COVID period compared with those who did not were significantly younger (65.7±14.5 versus 67.9±14.6 years; P = 0.002). No significant differences were found in relation to sex or the presence of DM.
Table 2.
Patients' demographics according to date of VA creation (pre-COVID versus COVID period)
| Characteristics | Pre-COVID | COVID | Total | P-value |
|---|---|---|---|---|
| N (%) | 614 (54) | 628 (55.2) | 1136 (100) | |
| Age (years), mean ± SD | 67.9 ± 14.5 | 65.9 ± 14.6 | 66.9 ± 14.6 | 0.003 |
| Age >75 years, n (%) | 339 (36.1) | 261 (29.7) | 600 (33) | 0.004 |
| Age >85 years, n (%) | 77 (8.2) | 39 (4.4) | 116 (6.4) | <0.001 |
| Sex, n (%) | 0.160 | |||
| Male | 611 (65) | 598 (68.1) | 1209 (66.4) | |
| Female | 329 (35) | 280 (31.9) | 609 (33.5) | |
| DM, n (%) | 0.722 | |||
| Yes | 235 (27.6) | 221 (28.4) | 456 (28) | 0.722 |
| No | 616 (72.4) | 557 (71.6) | 1173 (72) |
Values in bold are statistically significant.
Urgent versus scheduled entry on HD in the pre-COVID and COVID period
A higher percentage of patients started HD urgently in the COVID period compared with the pre-COVID period (44.3% versus 34.2%; P = 0.030). No statistical differences were observed in patients referred from advanced chronic kidney disease (ACKD) consultation and crash landers between the two periods (Table 3).
Table 3.
Urgent versus scheduled entry on HD in the pre-COVID and COVID period
| Entry on HD | Pre-COVID [n = 447(49.4%)] | COVID [n = 458 (50.6%)] | Total [n = 905 (100%)] | P-value |
|---|---|---|---|---|
| Started urgent HD, n % | 153 (34.2) | 203 (44.3) | 356 (39.3) | 0.030 |
| ACKD consultation, n (%) | 52 (33.9) | 90 (44.3) | 142 (39.8) | 0.249 |
| Crash landers, n (%) | 101 (66) | 113 (55.6) | 214 (60.1) | |
| Started scheduled HD, n (%) | 294 (65.7) | 255 (55.6) | 549 (60.6) |
Values in bold are statistically significant.
Hospitalizations and complications from vascular access
Several complications (bloodstream infection, extrusion and disfunction), including lower dialysis efficacy, were associated with the use of CVCs (Table 4). No significant changes were detected in CVC-related hospitalizations and complications between the pre-COVID and COVID periods (Table 4). During the COVID period, a significant increase in catheter replacement and the percentage of HD sessions that did not reach the Kt objective (Kt > 45) were observed (Table 4).
Table 4.
Complications related to VA in the pre-COVID and COVID period
| Complications | Pre-COVID period (n = 1083) | COVID period (n = 1343) | P-value |
|---|---|---|---|
| Hospitalizations and rate of hospitalization (hospitalization/patient/year), n/N (%) |
928/1083 (0.85) |
930/1343 (0.69) |
0.152 |
| Hospitalizations related to vascular access | 98/1083 (0.0009) | 84/1343 (0.0007) | |
| Other hospitalizations | 830/1083 (0.76) | 846/1343 (0.62) | |
| Incidences during HD related to use of catheter/number of prevalent catheters, n/N (%) | |||
| Bloodstream infection | 26/372 (6.9) | 32/494 (6.4) | 0.683 |
| Extrusion | 5/372 (1.5) | 7/494 (1.4) | 0.458 |
| Disfunction | 16/372 (43) | 20/494 (40) | 0.254 |
| Catheter removal | 16/372 (4.3) | 30/494 (6) | 0.034 |
| Percentage of HD sessions with Kt > 45, n/N (%) | 116622/153404 (76) | 141789/190201 (74.5) | <0.001 |
| HD sessions with Kt > 45, n/N (%) | |||
| CVC | 33277/51041 (65.2) | 40563/65009 (62.4) | <0.001 |
| AVF | 15616/88924 (82.4) | 19970/110626 (81.9) | 0.004 |
| AVG | 3073/12450 (75.3) | 3711/13623 (72.8) | <0.001 |
DISCUSSION
The most relevant finding during the COVID pandemic was the change in the type of VA performed. A significant increase in the placement of CVCs and a decrease in the creation of autologous AVs and/or prostheses was observed during the COVID period compared with the pre-COVID period. This suggests that CVCs were the chosen VA in patients who required the initiation of dialysis during the COVID pandemic.
The decrease in the creation of autologous fistulas and prostheses was motivated by the suspension or delay of any scheduled surgical activity and the consideration of fistula surgery as a non-urgent surgical activity [7, 8]. In our study, 14 centres suspended or delayed AV creation and only 2 maintained AV surgical activity. Centres that suspended VA surgery during the pandemic showed worse results and a significant increase in the percentage of CVCs compared with centres that did not suspend surgical activity. During the COVID period, performing elective procedures such as VA creation for HD was reduced or ceased [11, 12]. Furthermore, the change in nephrology activity from face-to-face outpatient visits to virtual ones [13] also probably contributed to the use of CVCs due to the transient loss of close follow-up of some patients and the need to use CVCs for urgent admission to HD. In our study, a greater number of patients started HD urgently.
Sex and DM did not influence the impact of COVID on VA. In patients who underwent VA surgery during the COVID period, a significant decrease in surgical activity was seen in older HD patients (>75 and >85 years of age) was observed in COVID compared with the pre-COVID period. Higher mortality by COVID in older HD patients [14] could have contributed to the lower age of incident patients in the COVID period.
Quality results of VA have been affected during the COVID period. A significantly higher percentage of incident and prevalent patients were dialyzed through a CVC, far from the objective proposed by Spanish guidelines (<25% for CVC and >75% for AVF) [16]. The AVF offering was already suboptimal prior to the pandemic in our patients [17], but the situation worsened substantially afterward.
The importance of a functioning VA for kidney patients with HD is a widely accepted fact and is endorsed not only by the Spanish Clinical Guide for Vascular Access for Haemodialysis [18], but also by several worldwide guidelines [19, 20].
The clinical guidelines establish that AVF and AVG (when the former is not possible) are the VAs of choice over CVC for several reasons [21]. Having a catheter at some point in their life means a lower survival for that individual compared with those who did not [22]. This could have a detrimental impact on dialysis patients’ outcomes in the near future and needs to be addressed urgently. Keeping the use of CVCs to a minimum, with the goal of creating the; right access, in the right patient, at the right time, for the right reasons; is mandatory [20, 23]. In our study, any significant increase in CVC-related hospitalizations and complications during the 15 months of follow-up was observed. Hygienic precautions for preventing viral transmission have been reinforced and probably the greater implementation of hygienic precautions in the dialysis setting during the COVID pandemic is behind improvement of the problem of CVC-related bloodstream infections [24].
Nevertheless, a secondary effect of the increase in CVC use is the lower delivered HD dose, which can determine worse clinical outcomes in the near future [25, 26].
One of the difficulties that we found in this post-COVID era is the need to solve all the delayed interventions and the poor follow-up of chronic patients. It is clear that in the most acute phases of the pandemic, the suspension of surgery made sense (it was necessary to convert the resuscitation units in the intensive care unit), however, VA surgery can be performed on an outpatient basis, outside of the hospital, and does not require admission, general anaesthesia or resuscitation or a respirator. The high proportion of CVCs makes it compulsory to establish strategies to reverse this situation. These strategies may require the pooling of resources and management of both public and private capacities as well as assessing the problem from a regional geographic perspective of providing health services for patients with ACKD, not just in a group or in a hospital.
VA interventions should be performed in an outpatient setting rather than a hospital setting due to the lower risk of exposure. The intervention allows patients to receive uninterrupted dialysis and alleviates new interventions and unnecessary hospital admissions [27, 28].
Our study has some limitations, such as its retrospective design. Nevertheless, we did not exclude any patient and we obtained information from our structured health medical record with a prospective collection of all data related to VA. Our results may not be representative of the situation in all health systems or countries, but this is the first analysis specifically aimed at describing the negative impact of COVID on VA management and warning on it.
In conclusion, COVID has presented a public health crisis that has influenced VA for HD with an increase in CVCs with respect to AVFs. Not preserving VA surgery during the COVID pandemic will have a significant impact for a long period of time. As can be seen in our results, the percentage of catheters did not decrease until 19 months after the start of the pandemic. Because AVFs can take ≥6 months to mature, it is foreseeable that it will take a long time to reverse the situation even after the crisis has abated.
The assumption that VA is a planned elective procedure is the problem.
Collaborative formulas between nephrologists and vascular surgeons, as well as between the public and private sectors, are necessary to ensure the adequate provision of VA for HD, of the best quality and with the greatest safety at this time.
APPENDIX
Renal Foundation’s Iñigo Álvarez de Toledo work team:
Dolores Arenas, Mdarenas@friat.es
Blanca Miranda, bmiranda@friat.es
David Hernán, dhernan@friat.es
Fabiola Dapena, fdapena@friat.es
Los llanos II, Getafe (Madrid)
Angel Mendez, Amendez@friat.es
Mariano Acuña, macuña@friat.es
Daniel Gaitán, dgaitan@friat.es.
Elena Guerrero, eguerrero@friat.es
Los llanos I, Móstoles (Madrid)
Karina Furaz, kfuraz@friat.es
Jose Carlos de la flor, jcdelaflor@friat.es
Alfredo Cordón, acordon@friat.es
Nardeth Benavides, bbenavides@friat.es
Alicia González, agonzalez@friat.es
Los lauros, Majadahonda (Madrid)
Ana Botella, abotella@friat.es
Javier Naranjo, jnaranjo@friat.es
Felipe Zalamea, fzalamea@friat.es
Paula Manso, pmanso@friat.es
Os Carballos II, Porriño (Vigo)
Mara Lisbet Cabana, mlcabana@friat.es
Laura Beato, lbeato@friat.es
Marina Burgos, mburgos@friat.es
Os Carballos I, Vigo
Delfina Yetman, dyetman@friat.es
Jeanette Fernández, jfernandez@friat.es
Jose Sobrado, jsobrado@friat.es
Damián Carneiro, dcarneiro@friat.es
Cynthia Caramés, ccarames@friat.es
El Castañar (Béjar) y las Encinas (Ciudad Rodrigo) Salamanca
Marc Handel, mhandel@friat.es
Miguel Terleira, mterleira@friat.es
Maria Luz Sánchez, lsanchez@friat.es
Los Pinos, Medina del campo (Valladolid)
Margarita Delgado Cerón, mdelgadoc@friat.es
Jose Herruzo, jherruzo@friat.es
Javier Barbeito, jbarbeito@friat.es
Los Olmos, Segovia
Maria Melissa Vasquez, mmvasquez@friat.es
Marta San Juan, msanjuan@fria.es
Santa Engracia (Madrid)
Luis Nieto, lnieto@fria.es
Valeria Sainz, vsainz@friat.es
Ramiro Cazar, rxcazar@hotmail.com
Jesús Hernández, jhernandez@friat.es
Jose Guerrero Carrillo, jguerrero@friat.es
Teixedal; Lalin (Pontevedra)
Isabel Martinez, mimartinez@friat.es
Araceli Rossignoli, arossignoli@friat.es
Hospital Universitario de Villalba (Madrid)
Rosa Sanchez Hernández rosa, shernandez@hgvillalba.es
Rocío Zamora
Laura Rodriguez-Osorio, lrodriguezos@quironsalud.es
Cristina Ledesma, cledesma@friat.es
Hospital Universitario Infanta Elena. Valdemoro (Madrid)
Alicia García Pérez, agarciape@quironsalud.es
Raquel Esteras Rubio, raquel.esteras@quironsalud.es
Ignacio Sanz Garayzábal, ignacio.sanzg@quironsalud.es
Adriana Iglesias, aiglesias@friat.es
Hospital Universitario Rey Juan Carlos (Madrid)
Maria Soledad Pizarro-Sanchez, maria.pizarro@fjd.es
Lola Piña, lpiña@friat.es
Saul Enrique Pampa Saíco, saul.pampa@hospitalreyjuan-carlos.es
Marisol Poma Tapia, marisol.poma@hospitalreyjuancarlos.es
Simona Alexandru, Salexandru@quironsalud.es
Maria Lopez Picasso, maria.lopez@hospitalreyjuancarlos.es
Fundación Jiménez Diaz (Madrid)
Emilio Gonzalez-Parra, EGParra@quironsalud.es; egonza-lezpa@senefro.org
Monica Pereira, mpereira@friat.es
Centros Orense (Galicia)
Concepción Ferreira Feijoo, cpereira@friat.es
Contributor Information
María Dolores Arenas Jimenez, Department of Nephrology Friat, Madrid, Spain.
Angel Méndez, Department of Nephrology, Los Ilanos II Centre, Getafe, Madrid, Spain.
Karina Furaz, Department of Nephrology, Los Ilanos I Centre, Móstoles, Madrid, Spain.
Ana Botella, Department of Nephrology, Los Lauros Centre, Majadahonda, Madrid, Spain.
Delfina Yetman, Department of Nephrology, Os Carballos I Centre; Vigo, Madrid, Spain.
Ramiro Cazar, Department of Nephrology, Santa Engracia Centre, Madrid, Spain.
Mara Lisbet Cabana, Department of Nephrology, Os Carballos II Centre; Porriño, Madrid, Spain.
Marc Handel, Department of Nephrology, El Castañar (Béjar) y las Encinas (Ciudad Rodrigo) Centres Salamanca, Spain.
María Luz Sanchez, Department of Nephrology, El Castañar (Béjar) y las Encinas (Ciudad Rodrigo) Centres Salamanca, Spain.
Margarita Delgado, Department of Nephrology, Los Pinos Centre, Medina Del Campo, Valladolid, Spain.
Maria Melissa Vasquez, Department of Nephrology, Los Olmos Centre, Segovia, Spain.
Isabel Martinez, Department of Nephrology, Teixedal Centre; Lalin, Pontevedra, Spain.
Monica Pereira, Department of Nephrology, Hospital Fundación Jimenez Diaz, Madrid, Spain.
Emilio González-Parra, Department of Nephrology, Hospital Fundación Jimenez Diaz, Madrid, Spain.
Maria Soledad Pizarro-Sánchez, Department of Nephrology, Hospital Universitario Rey Juan Carlos, Madrid, Spain.
Ignacio Sanz Garayzabal, Department of Nephrology, Hospital Universitario Infanta Elena, Valdemoro, Madrid, Spain.
Laura Rodriguez-Osorio, Department of Nephrology, Hospital Universitario Villalba, Madrid, Spain.
José Portoles, Department of Nephrology, Hospital Universitario Puerta de Hierro, Madrid, Spain.
David Hernán, Department of Nephrology Friat, Madrid, Spain.
Blanca Miranda, Department of Nephrology Friat, Madrid, Spain.
Renal Foundation’s Iñigo Álvarez de Toledo work team:
Dolores Arenas, Blanca Miranda, David Hernán, Fabiola Dapena, Los llanos, II, Angel Mendez, Mariano Acuña, Daniel Gaitán, Elena Guerrero, Los llanos, I, Karina Furaz, Jose de la flor Carlos, Alfredo Cordón, Nardeth Benavides, Alicia González, Los Lauros, Ana Botella, Javier Naranjo, Felipe Zalamea, Paula Manso, Os Carballos, II, Mara Lisbet Cabana, Laura Beato, Marina Burgos, Os Carballos, I, Delfina Yetman, Jeanette Fernández, Jose Sobrado, Damián Carneiro, Cynthia Caramés, Marc Handel, Miguel Terleira, Maria Luz Sánchez, Los Pinos, Margarita Delgado Cerón, Jose Herruzo, Javier Barbeito, Los Olmos, Maria Melissa Vasquez, Marta San Juan, Santa Engracia, Luis Nieto, Ramiro Cazar, Jesús Hernández, Jose Guerrero Carrillo, Teixedal Lalin, Isabel Martinez, Araceli Rossignoli, Rosa Sanchez Hernández Rosa, Rocío Zamora, Laura Rodriguez-Osorio, Cristina Ledesma, Alicia García Pérez, Raquel Esteras Rubio, Ignacio Sanz Garayzábal, Adriana Iglesias, Maria Soledad Pizarro-Sanchez, Lola Piña, Saul Enrique Pampa Saíco, Marisol Poma Tapia, Simona Alexandru, Maria Lopez Picasso, Emilio Gonzalez-Parra, Monica Pereira, and Concepción Ferreira Feijoo
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91: 157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020; 395: 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alcázar-Arroyo R, Portolés J, López-Sánchez Pet al. Rapid decline of anti-SARS-CoV-2 antibodies in patients on haemodialysis: the COVID-FRIAT study. Clin Kidney J 2021; 14: 1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bromage DI, Cannatà A, Rind IAet al. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail 2020; 22: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Søreide K, Hallet J, Matthews JBet al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg 2020; 107: 1250–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basile C, Lomonte C, Combe Cet al. A call to optimize haemodialysis vascular access care in healthcare disrupted by COVID-19 pandemic. J Nephrol 2021; 34: 365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American College of Surgery . Vascular conditions by category, with tier class 2020. https://vascular.org/sites/default/files/Vascular%20surgery%20triage%20by%20Tier%20Class%203.24.20.pdf
- 8. https://www.facs.org/covid-19/clinical-guidance/elective-case/vascular-surgery (3 September 2021, date last accessed)
- 9. Franco RP, Costa CBS, Sousa CSet al. Hemodialysis vascular access maintenance in the Covid-19 pandemic: positioning paper from the Interventional Nephrology Committee of the Brazilian Society of Nephrology. J Bras Nefrol 2020; 42: 41–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Instituto de Salud Carlos III. Informe nº 108. Situación de COVID-19 en España. Informe COVID-19. 9 de diciembre de 2021. RENAVE. CNE. CNM (ISCIII). https://www.isciii.es (14 December 2021, date last accessed) [Google Scholar]
- 11. Ng JJ, Ho P, Dharmaraj RBet al. The global impact of COVID-19 on vascular surgical services. J Vasc Surg 2020; 71: 2182–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soler MJ, Macia Heras M, Ortiz Aet al. Impact of the COVID-19 pandemic on Spanish Nephrology Services. Nefrologia 2020; 40: 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basile C, Combe C, Pizzarelli Fet al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 2020; 35: 737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arenas MD, Crespo M, Pérez-Sáez MJet al. Clinical profiles in renal patients with COVID-19. J Clin Med 2020; 9: 2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín Cet al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia 2020; 40: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truong T, Dittmar M, Ghaffari Aet al. Policy and pandemic: the changing practice of nephrology during the coronavirus disease-2019 outbreak. Adv Chronic Kidney Dis 2020; 27: 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gruss E, Portolés J, Caro Pet al. Vascular access models cause heterogeneous results in the centres of one community. Nefrologia 2010; 30: 310–316 [DOI] [PubMed] [Google Scholar]
- 18. Ibeas J, Roca-Tey R, Vallespín Jet al. Spanish clinical guidelines on vascular access for haemodialysis. Nefrologia 2017; 37(Suppl 1): 1–191 [DOI] [PubMed] [Google Scholar]
- 19. Schmidli J, Widmer MK, Basile Cet al. Editor's choice – vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 757–818 [DOI] [PubMed] [Google Scholar]
- 20. Lok CE, Huber TS, Lee Tet al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75(4 Suppl 2): S1–S164 [DOI] [PubMed] [Google Scholar]
- 21. Ravani P, Palmer SC, Oliver MJet al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013; 24: 465–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Balas A, Lee T, Young CJet al. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J Am Soc Nephrol 2017; 28: 3679–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basile C, Lomonte C, Combe Cet al. A call to optimize haemodialysis vascular access care in healthcare disrupted by COVID-19 pandemic. J Nephrol 2021; 34: 365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heidempergher M, Sabiu G, Orani MAet al. Targeting COVID-19 prevention in hemodialysis facilities is associated with a drastic reduction in central venous catheter-related infections. J Nephrol 2021; 34: 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu H, Li X, Zeng Cet al. Analysis of different vascular accesses on dialysis quality and infection risk factors of hemodialysis patients. Evid Based Complement Alternat Med 2021; 2021: 4554417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Salahudeen AK, Fleischmann EH, Bower JD. Impact of lower delivered Kt/V on the survival of overweight patients on hemodialysis. Kidney Int 1999; 56: 2254–2259 [DOI] [PubMed] [Google Scholar]
- 27. Kirksey L, Droz NM, Vacharajani Tet al. COVID era “essential surgery” dialysis access management considerations. J Vasc Surg 2020; 72: 1845–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Søreide K, Hallet J, Matthews JBet al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg 2020; 107: 1250–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]