ABSTRACT
Background
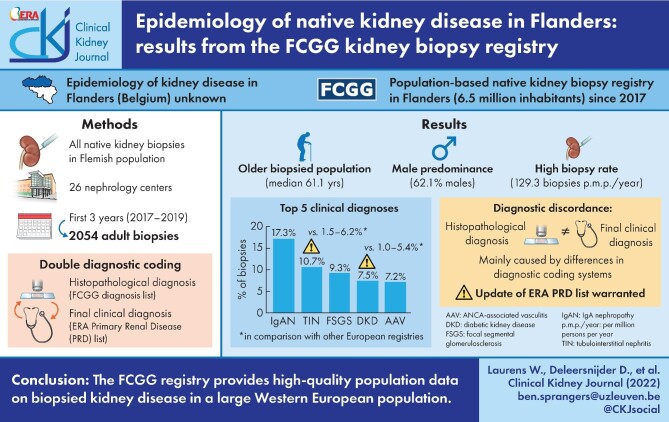
The Flemish Collaborative Glomerulonephritis Group (FCGG) registry is the first population-based native kidney biopsy registry in Flanders, Belgium. In this first analysis, we report on patient demographics, frequency distribution and incidence rate of biopsied kidney disease in adults in Flanders.
Methods
From January 2017 to December 2019, a total of 2054 adult first native kidney biopsies were included. A ‘double diagnostic coding’ strategy was used, in which every biopsy sample received a histopathological and final clinical diagnosis. Frequency distribution and incidence rate of both diagnoses were reported and compared with other European registries.
Results
The median age at biopsy was 61.1 years (interquartile range, 46.1–71.7); male patients were more prevalent (62.1%) and biopsy incidence rate was 129.3 per million persons per year. Immunoglobulin A nephropathy was the most frequently diagnosed kidney disease (355 biopsies, 17.3% of total) with a similar frequency as in previously published European registries. The frequency of tubulointerstitial nephritis (220 biopsies, 10.7%) and diabetic kidney disease (154 biopsies, 7.5%) was remarkably higher, which may be attributed to changes in disease incidence as well as biopsy practices. Discordances between histopathological and final clinical diagnoses were noted and indicate areas for improvement in diagnostic coding systems.
Conclusions
The FCGG registry, with its ‘double diagnostic coding’ strategy, provides useful population-based epidemiological data on a large Western European population and allows subgroup selection for future research.
Keywords: biopsy, epidemiology, incidence, frequency, native kidney, observational, pathology, registry
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Kidney histology remains the gold standard for the diagnosis and classification of many kidney diseases [1, 2]. Kidney biopsy registries provide valuable data that describe the epidemiology of kidney diseases, ideally in a well-defined geographical area [3]. Additionally, these data can help in the design of and recruitment for clinical trials to develop new preventive and therapeutic strategies. However, kidney biopsy registries are also prone to pitfalls that may bias observations. First, single-centre registries may suffer from referral bias and population-based multicentre registries are therefore preferred. On the other hand, granular data are much more easily collected in a single-centre setting. Second, the lack of a uniform coding system for registration of diagnoses hampers comparison between studies [4]. Third, most registries solely report the histopathological diagnosis, while the final clinical nephrological diagnosis, which integrates both clinical and histopathological information, is rarely included.
As the epidemiology of kidney disease in Flanders (Dutch-speaking part of Belgium) was unknown, the Flemish Collaborative Glomerulonephritis Group (FCGG) initiated a population-based kidney biopsy registry in the region of Flanders in 2017, which aims to include all native kidney biopsies performed in its population of approximately 6.5 million inhabitants. The registry implemented a ‘double diagnostic coding’ strategy, in which both a histopathological and final clinical diagnosis are recorded for each biopsy. In this first analysis, we report on patient demographics, frequency distribution and incidence rate of biopsied kidney disease in Flanders from 2017 to 2019 and compare them with previously published European kidney biopsy registries.
MATERIALS AND METHODS
Ethics
This study was conducted in accordance with all applicable regulatory requirements, the principles of the declaration of Helsinki and Good Clinical Practice Guidelines. The study was approved by the Ethical Committee of the University Hospitals Leuven (study reference S59182) and local committees of all participating centres. All patients or their legal representatives provided written informed consent prior to inclusion in the registry.
Organization and participating centres
All 24 nephrology departments in Flanders and two departments in Brussels participated in the registry. Four centres were university hospitals (university hospitals of Antwerp, Brussels, Ghent and Leuven); the remaining centres were general hospitals. Biopsies were analysed by 19 (nephro)pathologists from 10 Flemish pathology departments and one department in Brussels.
Patient eligibility and biopsy inclusion
From 1 January 2017 to 31 December 2019, 2419 adult and paediatric native kidney biopsies were identified from the registry, to which additional selection criteria were applied (Figure 1). First, only official Flemish inhabitants (based on ZIP codes) were retained. Next, repeat biopsies in the time frame of 2017–19 were excluded unless the result of the first biopsy was not diagnostic and the patient underwent a second biopsy procedure within the following 4 months, in which case the first biopsy was excluded, and the repeat biopsy included instead. Finally, paediatric patients (<18 years old) were excluded (124 biopsies), resulting in 2054 adult kidney biopsies available for analysis.
Figure 1:
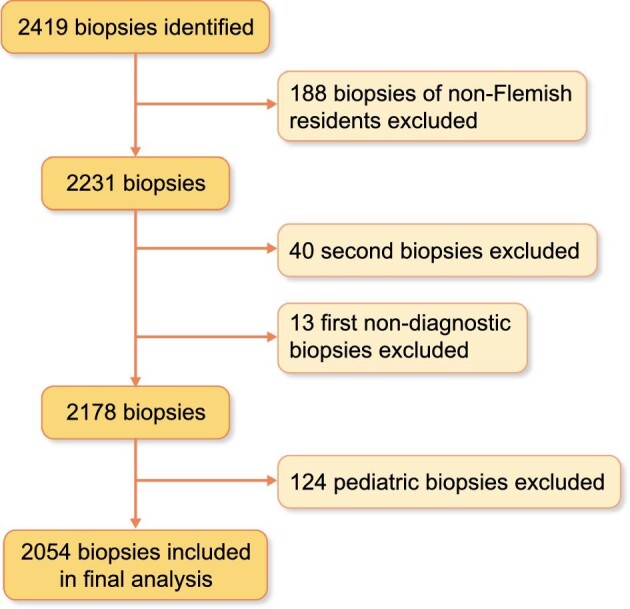
Flowchart of kidney biopsy selection for final analysis.
Data collection
Patient demographics and clinical data were provided by the treating nephrologist through a standardized data form. Histopathological data were provided by the (nephro)pathologist in a standardized report; two nephropathologists examined 58.7% of all biopsies. After biopsy results were available, the treating nephrologist provided the final clinical diagnosis.
Histopathological diagnosis
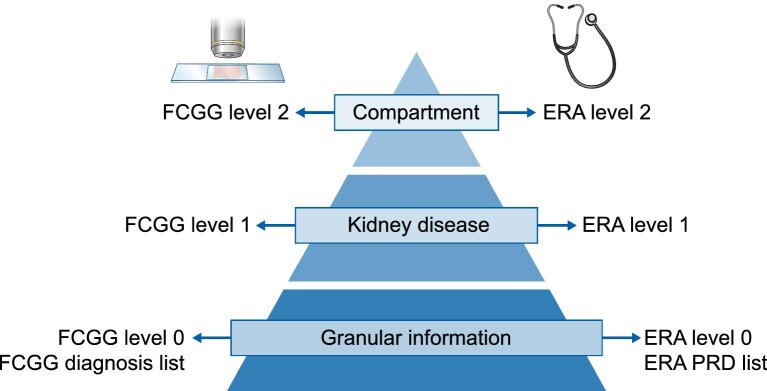
The primary histopathological diagnosis was coded by the (nephro)pathologist reading the biopsy according to a proprietary coding system created for this registry (https://www.nbvn.be/blog/organisatie/fcgg-in-english). The histopathological diagnosis list (FCGG level 0) was categorized at two levels with decreasing detail (Figure 2, Supplementary data, Table S1). At the first level (FCGG level 1), different aetiologies, classes or manifestations of the same disease were pooled together to define 39 individual kidney diseases. At the second level (FCGG level 2), five categories were defined according to the most frequently affected kidney tissue compartment: ‘Glomerular’, ‘Tubulointerstitial’, ‘Vascular’, ‘All/any compartment(s)’ and ‘No kidney disease/No diagnosis/Tumour’.
Figure 2:
Categorization of histopathological and final clinical coding systems in the FCGG registry.
Final clinical diagnosis
The final clinical diagnosis was coded by the treating nephrologist according to the European renal association-European dialysis and transplant association (ERA-EDTA) Primary Renal Disease (PRD) coding system [5]. The ERA-EDTA PRD list (ERA level 0) was categorized at two levels with decreasing detail, analogous to the histopathological diagnosis list (Figure 2, Supplementary data, Table S2). At the first level (ERA level 1), 46 individual kidney diseases were defined. At the second level (ERA level 2), six categories were defined: ‘Glomerular’, ‘Tubulointerstitial’, ‘Vascular’, ‘All/any compartment(s)’, ‘No kidney disease/Tumour’ and ‘Postrenal’.
Diagnostic concordance between histopathological and final clinical diagnosis
When the histopathological and final clinical diagnosis of a biopsy were the same (on FCGG level 1 and ERA level 1, respectively), diagnoses were considered concordant. When both diagnoses differed, they were considered discordant.
Statistical analysis
Numerical variables were described using median and interquartile range (IQR) as they did not fit the normal distribution, and were calculated with GraphPad Prism version 9.1.1 for Mac OS, GraphPad Software (www.graphpad.com). Kidney disease frequencies were shown as absolute numbers and percentages of total biopsies. Incidence rates were calculated using the sum of case biopsies in 2017, 2018 and 2019 in the numerator and the person-year follow-up from 2017 to 2019 in the denominator, and reported as biopsies per million paersons per year (p.m.p./year).
RESULTS
Patient demographics and kidney biopsy rate
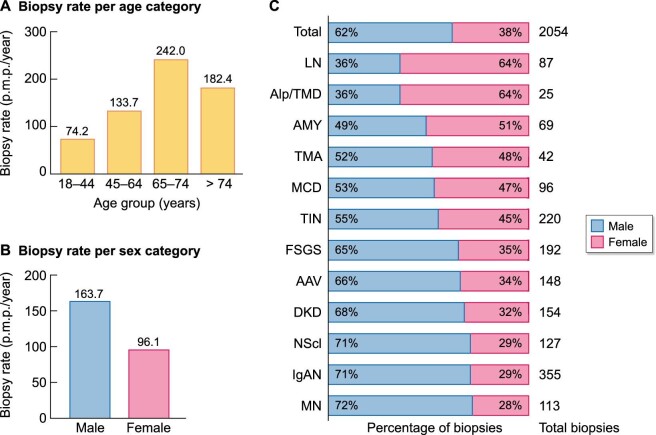
From January 2017 to December 2019, 2054 adult native kidney biopsies were included for analysis. The incidence rate of Flemish adults undergoing first kidney biopsy was 129.3 p.m.p./year. The median age at biopsy was 61.1 years (IQR 46.1–71.7) and biopsy rate was higher in the elderly (>74 years) when compared with younger adults (<65 years, Figure 3A). Males were more often biopsied and predominant (62.1% males, Figure 3B and C), although patients with a final clinical diagnosis of lupus nephritis (LN), Alport syndrome/thin basement membrane disease (Alport/TMD) and amyloidosis were more often female (64.4%, 64.0% and 50.7% females, respectively, Figure 3C).
Figure 3:
Demographics and biopsy rate of biopsied adult patients. (A) Biopsy rate according to age category in Flemish adult patients from 2017 to 2019. (B) Biopsy rate according to sex category in Flemish adult patients from 2017 to 2019. (C) Sex distribution in adult patients, shown for the total number of biopsies and for individual kidney diseases (ERA level 1, male proportion in blue, female proportion in red). AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; Alp/TMD: Alport syndrome and thin basement membrane disease; AMY: amyloidosis; DKD: diabetic kidney disease; FSGS: focal segmental glomerulosclerosis; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; MN: membranous nephropathy; NScl: nephrosclerosis; TIN: tubulointerstitial nephritis; TMA: thrombotic microangiopathy.
Frequency and incidence rate of biopsied kidney diseases
Every biopsy sample received a histopathological and final clinical diagnosis (Tables 1 and 2, detailed data shown in Supplementary data, Tables S3 and S4). The majority of patients had a clinical diagnosis of glomerular disease (1152 biopsies, 56.1%, Figure 4). Remaining diagnoses consisted of tubulointerstitial disease (293 biopsies, 14.3%), vascular disease (186 biopsies, 9.1%), disease that affects all or any kidney tissue compartment(s) (408 biopsies, 19.9%), no kidney disease or incidental diagnosis of malignancy (11 biopsies, 0.5%) and postrenal disease (4 biopsies, 0.2%, Figure 4). Overall, the top five most frequent diagnoses were immunoglobulin A nephropathy (IgAN), tubulointerstitial nephritis (TIN), focal segmental glomerulosclerosis (FSGS), diabetic kidney disease (DKD) and antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis/pauci-immune glomerulonephritis (AAV, Figure 5).
Table 1.
Primary histopathological diagnoses of adult kidney biopsies in Flanders
| Primary histopathological diagnosis | N | % | Incidence rate (p.m.p./year) |
|---|---|---|---|
| Glomerular | 1119 | 54.5 | 70.4 |
| IgA nephropathy | 357 | 17.4 | 22.5 |
| FSGS | 185 | 9.0 | 11.6 |
| ANCA-associated vasculitis and pauci-immune glomerulonephritis | 150 | 7.3 | 9.4 |
| Membranous nephropathy | 113 | 5.5 | 7.1 |
| Lupus nephritis | 85 | 4.1 | 5.3 |
| MCD | 69 | 3.4 | 4.3 |
| Glomerulopathy, NOS | 45 | 2.2 | 2.8 |
| Infection-related immune-complex GN | 38 | 1.9 | 2.4 |
| Alport syndrome/thin membrane disease | 29 | 1.4 | 1.8 |
| Anti-GBM nephritis | 11 | 0.5 | 0.7 |
| C3 glomerulopathy | 10 | 0.5 | 0.6 |
| Cryoglobulinemic GN | 8 | 0.4 | 0.5 |
| Monoclonal immunoglobulin-associated glomerulopathy | 8 | 0.4 | 0.5 |
| FSGS/MCD | 7 | 0.3 | 0.4 |
| Nonamyloid deposition glomerulopathy | 4 | 0.2 | 0.3 |
| Tubulointerstitial | 376 | 18.3 | 23.7 |
| Tubulointerstitial nephritis | 187 | 9.1 | 11.8 |
| ATN | 137 | 6.7 | 8.6 |
| Tubulointerstitial pathology, NOS | 18 | 0.9 | 1.1 |
| Monoclonal immunoglobulin-associated tubular disease | 17 | 0.8 | 1.1 |
| Crystal/cylinder deposition | 16 | 0.8 | 1.0 |
| Chronic pyelonephritis | 1 | <0.1 | 0.1 |
| Vascular | 173 | 8.4 | 10.9 |
| Nephrosclerosis | 119 | 5.8 | 7.5 |
| Thrombotic microangiopathy | 48 | 2.3 | 3.0 |
| Cholesterol emboli | 4 | 0.2 | 0.3 |
| Vasculitis without glomerulonephritis | 1 | <0.1 | 0.1 |
| Kidney infarction | 1 | <0.1 | 0.1 |
| All/any compartment(s) | 248 | 12.1 | 15.6 |
| Diabetic kidney disease | 138 | 6.7 | 8.7 |
| Amyloidosis | 69 | 3.4 | 4.3 |
| Nonamyloid monoclonal deposition disease | 17 | 0.8 | 1.1 |
| End-stage kidney disease | 7 | 0.3 | 0.4 |
| Medication-induced nephropathy | 7 | 0.3 | 0.4 |
| Idiopathic nodular glomerulosclerosis | 5 | 0.2 | 0.3 |
| Congenital/hereditary syndromes | 4 | 0.2 | 0.3 |
| Storage disease | 1 | <0.1 | 0.1 |
| No kidney disease/no diagnosis/tumour | 138 | 6.7 | 8.7 |
| No diagnosis | 101 | 4.9 | 6.4 |
| Normal | 33 | 1.6 | 2.1 |
| Tumour | 4 | 0.2 | 0.3 |
| Total | 2054 | 100.0 | 129.3 |
The primary histopathological diagnoses (FCGG level 1) are categorized per kidney tissue compartment (FCGG level 2). The bold values are the 4 main categories and bold numbers represent the sums of the values of the different subcategories listed below.
Anti-GBM nephritis: anti-glomerular basement membrane nephritis; FSGS/MCD: focal segmental glomerulosclerosis or minimal change disease, subdivision not possible; NOS: not otherwise specified; GN: glomerulonephritis.
Table 2.
Final clinical diagnoses of adult kidney biopsies in Flanders
| Final clinical diagnosis | N | % | Incidence rate (p.m.p./year) |
|---|---|---|---|
| Glomerular | 1152 | 56.1 | 72.5 |
| IgA nephropathy | 355 | 17.3 | 22.3 |
| FSGS | 192 | 9.3 | 12.1 |
| ANCA-associated vasculitis and pauci-immune glomerulonephritis | 148 | 7.2 | 9.3 |
| Membranous nephropathy | 113 | 5.5 | 7.1 |
| MCD | 96 | 4.7 | 6.0 |
| Lupus nephritis | 87 | 4.2 | 5.5 |
| Glomerulopathy, NOS | 76 | 3.7 | 4.8 |
| Alport syndrome/thin membrane disease | 25 | 1.2 | 1.6 |
| Membranoproliferative GN | 15 | 0.7 | 0.9 |
| Infection-related immune-complex GN | 13 | 0.6 | 0.8 |
| Anti-GBM nephritis | 10 | 0.5 | 0.6 |
| Cryoglobulinemia | 8 | 0.4 | 0.5 |
| Nephrotic syndrome, no histology | 6 | 0.3 | 0.4 |
| Immunotactoid/fibrillary nephropathy | 6 | 0.3 | 0.4 |
| Hematuria and proteinuria, no histology | 2 | 0.1 | 0.1 |
| Tubulointerstitial | 293 | 14.3 | 18.4 |
| Tubulointerstitial nephritis | 220 | 10.7 | 13.8 |
| ATN | 33 | 1.6 | 2.1 |
| Monoclonal immunoglobulin-associated tubular disease | 20 | 1.0 | 1.3 |
| Crystal/cylinder deposition | 15 | 0.7 | 0.9 |
| Medication-induced nephropathy | 3 | 0.1 | 0.2 |
| Acute pyelonephritis | 2 | 0.1 | 0.1 |
| Vascular | 186 | 9.1 | 11.7 |
| Nephrosclerosis | 127 | 6.2 | 8.0 |
| Thrombotic microangiopathy | 42 | 2.0 | 2.6 |
| Non-AAV vasculitis | 11 | 0.5 | 0.7 |
| Cholesterol emboli | 4 | 0.2 | 0.3 |
| Vascular, NOS | 1 | <0.1 | 0.1 |
| Sickle cell nephropathy | 1 | <0.1 | 0.1 |
| All/any compartment(s) | 408 | 19.9 | 25.7 |
| Diabetic kidney disease | 154 | 7.5 | 9.7 |
| AKI/CKD, NOS | 129 | 6.3 | 8.1 |
| Amyloidosis | 69 | 3.4 | 4.3 |
| Nonamyloid monoclonal deposition disease | 22 | 1.1 | 1.4 |
| Medication-induced nephropathy | 17 | 0.8 | 1.1 |
| Isolated proteinuria or hematuria, no histology | 11 | 0.5 | 0.7 |
| Congenital/hereditary syndromes | 5 | 0.2 | 0.3 |
| Iatrogenic | 1 | <0.1 | 0.1 |
| No kidney disease/tumour | 11 | 0.5 | 0.7 |
| Normal | 7 | 0.3 | 0.4 |
| Tumour | 4 | 0.2 | 0.3 |
| Postrenal | 4 | 0.2 | 0.3 |
| Retroperitoneal fibrosis | 2 | 0.1 | 0.1 |
| Acquired obstructive uropathy/nephropathy | 2 | 0.1 | 0.1 |
| Total | 2054 | 100.0 | 129.3 |
The final clinical diagnoses (ERA level 1) are categorized per kidney tissue compartment (ERA level 2). The bold values are the 4 main categories and bold numbers represent the sums of the values of the different subcategories listed below.
non-AAV vasculitis: non-ANCA-associated vasculitis; AKI/CKD, NOS: non-specific diagnoses of acute kidney injury or chronic kidney disease; Anti-GBM nephritis: anti-glomerular basement membrane nephritis; GN: glomerulonephritis; NOS: not otherwise specified.
Figure 4:
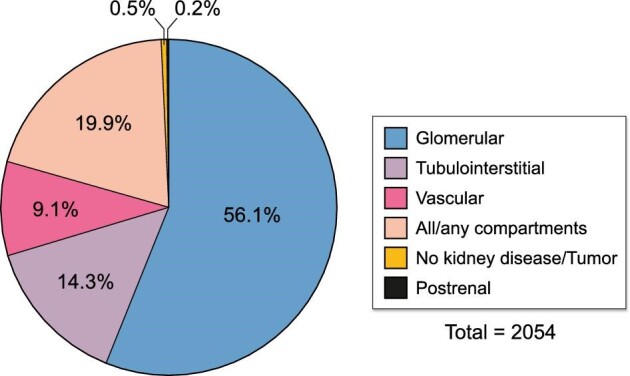
Final clinical diagnoses of biopsied adult patients per kidney tissue compartment. Final clinical diagnoses are categorized according to the most frequently affected kidney tissue compartment (ERA level 2).
Figure 5:
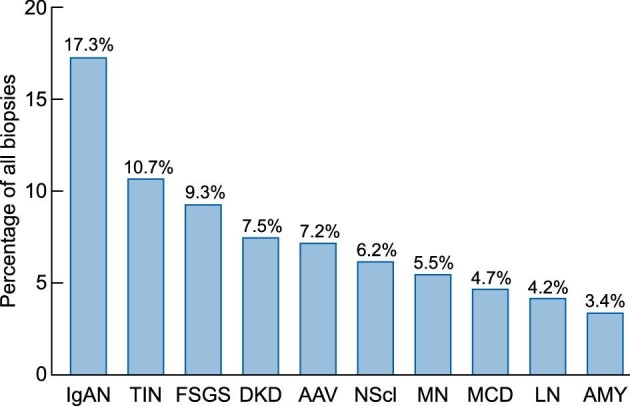
Most frequently biopsied kidney diseases in Flanders. The 10 most frequent final clinical diagnoses are shown (ERA level 1). Frequencies were calculated relative to the total number of adult biopsies (N = 2054). The nonspecific clinical categories ‘AKI/CKD, NOS’ (6.3%) and ‘glomerulopathy, NOS’ (3.7%) were omitted from the chart. AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AMY: amyloidosis; DKD: diabetic kidney disease; FSGS: focal segmental glomerulosclerosis; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; MN: membranous nephropathy; NScl: nephrosclerosis; TIN: tubulointerstitial nephritis.
IgAN (including IgA vasculitis) was the most frequently diagnosed glomerular disease (355 biopsies, 17.3% of total patients, incidence rate of 22.3 p.m.p./year, Table 2). Other primary glomerular diseases included FSGS (192 biopsies, 9.3%, 12.1 p.m.p./year), membranous nephropathy (MN, 113 biopsies, 5.5%, 7.1 p.m.p./year) and minimal change disease (MCD, 96 biopsies, 4.7%, 6.0 p.m.p./year). Membranoproliferative glomerulonephritis (MPGN) was infrequently diagnosed (15 biopsies, 0.7%, 0.9 p.m.p./year). AAV and LN were the most frequent secondary glomerular diseases (7.2% and 4.2%, 9.3 and 5.5 p.m.p./year, respectively). Alport/TMD was diagnosed in 25 biopsies (1.2%, 1.6 p.m.p./year). Less prevalent glomerular diseases included infection-related immune-complex glomerulonephritis (GN), anti-glomerular basement membrane (GBM) nephritis, cryoglobulin-associated GN and immunotactoid/fibrillary glomerulopathy (Table 2).
TIN covered 75% of all diagnoses with tubulointerstitial disease (220 biopsies, 10.7% of total patients, incidence rate of 13.8 p.m.p./year, Table 2). Acute tubular necrosis (ATN) pooled all diagnoses of haemodynamic-mediated ATN (acute kidney injury due to hypovolemia, sepsis or circulatory failure, Supplementary data, Table S4) and was much less frequent (33 biopsies, 1.6%, 2.1 p.m.p./year). Other rare tubulointerstitial diseases included monoclonal immunoglobulin-associated tubular disease, tubular crystal/cylinder deposition disease, medication-induced nephropathy (i.e. secondary to cisplatin or lithium) and acute pyelonephritis (Table 2).
Nephrosclerosis was diagnosed in approximately two-thirds of patients in the category of vascular disease (127 biopsies, 6.2% of total patients, incidence rate of 8.0 p.m.p./year), followed by thrombotic microangiopathy (TMA, 42 biopsies, 2.0%, 2.6 p.m.p./year). Remaining infrequent diagnoses included non-AAV vasculitis, cholesterol emboli and sickle cell nephropathy (Table 2).
In the category of diseases that affect all or any kidney compartment(s), DKD was most frequent (154 biopsies, 7.5% of total patients, incidence rate of 9.7 p.m.p./year), followed by amyloidosis (69 biopsies, 3.4%, 4.3 p.m.p./year). Less frequent diagnoses included nonamyloid monoclonal deposition disease, medication-induced nephropathy (i.e. nonspecific nephrotoxicity or due to calcineurin inhibitors or analgesic drugs) and various congenital disorders. Nonspecific diagnoses of acute or chronic kidney failure were pooled under ‘AKI/CKD, NOS’ (Table 2).
In seven patients (0.3% of total), the clinician concluded that kidney disease was absent (Table 2). An incidental malignancy was found in four patients (0.2%). Biopsied postrenal disease was very rare (four biopsies, 0.2%).
Diagnostic concordance between histopathological and clinical diagnoses
In most glomerular diseases (IgAN, FSGS, MCD, MN, AAV, LN and Alport/TMD), the concordance between histopathological and final clinical diagnoses of a biopsy was high (Supplementary data, Table S5). The histopathological diagnosis was withheld as the clinical diagnosis in 79.3–89.9% of biopsies. Vice versa, clinical diagnoses were made with corresponding histopathological diagnoses in 64.6–92.0% of patients. Discordances were mainly seen in biopsies with a histopathological diagnosis of C3 glomerulopathy (C3GP) or infection-related immune complex GN, as the ERA-EDTA PRD list does not provide corresponding diagnostic coding terms. In TIN and ATN, the diagnostic concordance differed considerably. In TIN, concordance was high (histopathological diagnosis withheld as clinical diagnosis in 86.6% of biopsies and clinical diagnosis based on similar histopathological diagnosis in 73.6%). However, in biopsies with a histopathological diagnosis of ATN, corresponding clinical diagnoses were much more diverse and only 16.1% of biopsies received a clinical diagnosis consistent with ATN secondary to hypovolemia, sepsis or circulatory failure, while other clinical diagnoses were mainly nonspecific diagnoses of kidney injury (Supplementary data, Table S5). In vascular diseases (nephrosclerosis and TMA), the histopathological diagnosis was withheld as the clinical diagnosis in 66.4–68.8% of biopsies and a clinical diagnosis was based on a similar histopathological diagnosis in 62.2–78.6% of patients. In both DKD and amyloidosis, concordance was very high (histopathological diagnosis withheld as clinical diagnosis in 95.7% of biopsies and clinical diagnosis based on similar histopathological diagnosis in 85.7–95.7%). Finally, in 30 out of 33 patients (90.9%) with normal kidney histology, the clinician deemed that kidney disease was present, most frequently MCD (13 patients, Supplementary data, Table S5).
DISCUSSION
This study describes the epidemiology of all adult native kidney biopsies in Flanders from 2017 to 2019, using a ‘double diagnostic coding’ strategy. The overall biopsy rate was high and elderly patients were more frequently biopsied than young adults. Although more than half of all patients were diagnosed with glomerular disease, TIN was the second most frequent diagnosis after IgAN. Discordances between histopathological and final clinical diagnoses differed considerably between kidney diseases.
In Flanders, the median age of biopsied adults was rather old (61.1 years) when compared with other recent Western European registries that cover adult biopsies (44.5–55.6 years in time frame 1994–2011, Table 3) [6–8]. A temporal trend is observed in European registries, in which the age at kidney biopsy is gradually increasing, supporting this observation [9–12]. Male patients were predominant (62.1%), which is in line with previous European data [6, 8, 10, 13, 14] (54–62% males, Table 3), although studies from Romania [15] and Serbia [16, 17] found a more equal sex distribution (50–52% males). The percentage of male patients with LN in Flanders was unusually high (36% males) when compared with previous studies (9–24% males) [7, 12, 14, 18, 19], warranting further research. The biopsy rate in Flemish adults (129.3 p.m.p./year) is among the highest in national and regional European registries and resembles data from Scotland (126 biopsies p.m.p./year, Supplementary data, Table S6) [8]. Biopsy rates are not only influenced by disease incidence, but also confounded by many additional factors [3]. The high socioeconomic status of the Flemish population, resembling neighboring Western European countries, might contribute to these high biopsy rates [3, 11, 17]. A temporal trend is also observed in European studies toward higher biopsy rates in more recent time periods [7, 14, 16, 17]. Finally, biopsy rate was calculated in the adult population, while some other European studies included the paediatric population, yielding lower total biopsy rates (Supplementary data, Table S6) [7, 11, 13, 14, 20].
Table 3.
Frequency distribution of native kidney disease in large European kidney biopsy registries
| Author | Region Population | Timeframe Biopsies (N) | Age (years) M/F (%) | Freq. | IgAN (%) | FSGS (%) | MCD (%) | MN (%) | MPGN (%) | LN (%) | AAV (%) | AMY (%) | DKD (%) | TIN (%) | NScl (%) | Dx | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rivera et al. [13] 2002 | SpainNational40 million | 1994–19996525 biopsies (A)491 biopsies (P) | NA60/40 (A) | A | 14.6 | 9.7 | 6.6 | 10.3 | 4.5 | 8.8 | 7.8 | 4.3 | 1.0 | NA | 5.9 | Pr. | Spanish Renal Registry, 70.4% participation of centres in Spain |
| López-Gómez et al. [10] 2020 | SpainNational40 million | 1994–201925 440 biopsies (A)1231 biopsies (P) | 50 (A + P)60/40 (A) | A + P | 14.6 | 8.0 | 6.8 | 9.9 | 3.9 | 8.7 | 6.8 | 3.8 | 4.8 | NA | 5.3 | Pr. | Spanish Renal Registry |
| Schena et al. [20] 1997 | ItalyNational56.8 milliona | 1987–199313 835 biopsies (A + P) | NA65/35 (A + P) | A + P | 21.1 | 7.1 | 4.7 | 12.4 | 4.0 | 6.7 | 3.4 | 2.7 | NA | 4.0 | NA | ERA-EDTA PRD | Italian Registry of Renal Biopsies, 96% participation of centres in Italy |
| Gesualdo et al. [22] 2004 | ItalyNational56.9 millionb | 1996–200013 132 biopsies (A + P) | NANA | A + P | NA | NA | NA | NA | NA | NA | NA | NA | NA | ∼5.3 | NA | ERA-EDTA PRD | Italian Registry of Renal Biopsies |
| Zaza et al. [6] 2013 | ItalyRegionalc5 million | 1998–20104378 biopsies (A) | 50.4 (A)62/38 (A) | A | NA | NA | NA | NA | NA | NA | NA | NA | NA | ∼5.5 | NA | Pr. | ‘Triveneto’ Register of Renal Biopsies |
| Maixnerova et al. [7] 2015 | Czech RepublicNational10.3 million | 1994–20119051 biopsies (A)1421 biopsies (P) | 44.5 (A)58/42 (A) | A + P | 20.5 | 6.9 | 6.1 | 7.1 | 3.2 | 7.1 | 5.7 | 3.1 | 4.1 | 3.3 | 3.0 | Pr. | Czech Registry of Renal Biopsies |
| Perkowska-Ptasinska et al. [14] 2017 | PolandNational38.5 million | 2009–20147349 biopsies (A)2939 biopsies (P) | NA54/46 (A) | A | 20.0 | 15.0 | 5.5 | 11.2 | 4.6 | 8.4 | 5.5 | 4.5 | 3.7 | 1.9 | 0.7 | Pr. | Polish Registry of Renal Biopsies |
| Brazdziute et al. [12] 2015 | LithuaniaNational3.4 milliond | 1994–20123213 biopsies (A)427 biopsies (P) | 43.2 (A + P)58/42 (A + P) | A + P | 20.2 | 7.8 | 4.9 | 4.7 | 7.4 | 2.3 | NA | 6.6 | 1.2 | 6.1 | 2.7 | Pr. | No formal registry, 11 centres |
| McQuarrie et al. [8] 2009 | UKRegionale4.2 million | 2002–20062480 biopsies (A) | 55.6 (A)57/43 (A) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ERA-EDTA PRD | Scottish Renal Biopsy Registry |
| Wirta et al. [11] 2008 | FinlandRegionalf0.4 millionf0.8 millionf | 1980–20003310 biopsies (A + P) | 38–52g (A + P)59/41 (A + P) | A + P | 21.7 | 2.4 | 3.1 | 7.3 | 2.4 | NA | NA | NA | NA | 9.0h14.7h | NA | SNOMED | No formal registry, 6 centres |
| Covic et al. [15] 2006 | RomaniaRegionali6.2 million | 1995–2004606 biopsies (A) | 38.5 (A)52/48 (A) | A | 19.1 | 7.6 | 5.6 | 7.4 | 19.5 | 7.8 | 5.4 | 3.1 | NA | 1.5 | NA | Pr. | Two referral centres, >50% of all biopsies performed in Romania |
| Naumovic et al. [17] 2009 | Serbia1 centrej7.5 million | 1987–20061626 biopsies (A) | 39.1 (A)51/49 (A) | A | 7.7 | 12.1 | 4.8 | 12.6 | 6.7 | 10.1 | 2.3 | 1.4 | NA | 2.7 | 3.6 | Pr. | Single nephrology centre in Serbia |
| Brkovic et al. [16] 2018 | Serbia1 centrek7.2 million | 2007–2014665 biopsies (A) | 42 (A)50/50 (A) | A | 9.0 | 12.5 | 3.8 | 17.3 | 4.8 | 17.7 | 4.4 | 0.9% | NA | ∼2.1 | NA | Pr. | Single nephrology centre in Serbia |
| O'Shaughnessy et al. [24] 2018 | EuropeInternationalNA | 2006–2018l19 302 biopsies (A + P)m | 48 (A + P)56/44 (A + P) | A + P | 16.2 | 11.6 | 5.0 | 9.8 | 2.8 | 7.8 | 6.2 | 3.4 | 5.4 | 6.2 | 7.7 | Pr. | 13 centres in Europe and 1 in Saudi Arabia |
| FCGG registry | BelgiumRegional5.3 millionn | 2017–20192054 biopsies (A) | 61.1 (A)62/38 (A) | A | 17.3 | 9.3 | 4.7 | 5.5 | 0.7 | 4.2 | 7.2 | 3.4 | 7.5 | 10.7 | 6.2 | ERA-EDTA PRD | Flemish Collaborative Glomerulonephritis Group (FCGG) biopsy registry |
aNot mentioned; population of Italy in 1993.
bNot mentioned; population of Italy in 1998.
cNorth-Eastern region of Italy.
dPopulation of Lithuania in 2003.
e8/9 Scottish regions (82.4% of Scottish population).
fWestern Finland, one university hospital (UH, 0.4 million) + five central hospitals (CH, 0.8 million).
g38 years in 1976, 52 years in 2000.
h9% at CH, 14.7% at UH.
iNorth-Eastern and Western Romania.
jCovers ∼70% of all biopsies in Serbia.
kCovers ∼70% of all biopsies in Serbia.
lExact time frame not mentioned.
mInclusion of paediatric biopsies not explicitly mentioned in study.
nAdult population.
A: adult; AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AMY: amyloidosis; DKD: diabetic kidney disease; Dx: column showing the coding practice/list used in the study or registry; Freq.: column showing age category in which disease frequency was calculated (adult and/or paediatric biopsies); FSGS: focal segmental glomerulosclerosis; GN: glomerulonephritis; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; M/F: male/female distribution; MN: membranous nephropathy; MPGN: membranoproliferative glomerulonephritis; NA: not mentioned or not applicable; NScl: nephrosclerosis; P: paediatric; Pr.: proprietary coding list; TIN: tubulointerstitial nephritis.
Comparing disease frequency and incidence between kidney biopsy registries is complicated by a number of factors. First, registries do not always report both disease frequency and incidence rates. Second, disease frequencies are often calculated in variably defined subgroups instead of total biopsies. Third, diagnostic terms used for registration of kidney diseases differ considerably. Previous literature reviews have used older diagnostic terms and disease frequencies were not always calculated relative to the total number of biopsies [3, 21]. We therefore reviewed the incidence rate and frequency distribution of native kidney disease in 16 studies that cover the region of Europe (including Scotland, Table 3, Supplementary data, Table S6) [6, 7, 16, 17, 20, 22–24, 8–15]. Some disease frequencies were recalculated relative to the total number of adult biopsies [13, 15–17, 24] or the total number of adult and paediatric biopsies when data on the adult subgroup were limited [11, 12, 20]. All studies covered a reference population of >1 million inhabitants and were organized on the international [24], national [7, 9, 10, 12–14, 20, 22], regional [6, 8, 11, 15, 23] or single-centre level [16, 17].
The frequency of IgAN, FSGS and MCD in Flanders resembled most other European studies (Table 3). Interestingly, we observed relatively less MN cases (5.5% versus 4.7–17.3% [12, 16] in other European registries). Further research will need to clarify whether this represents a real difference in disease incidence or whether it already reflects a decrease in kidney biopsy procedures in patients with a serological diagnosis of antiphospholipase A2-receptor (anti-PLA2R) antibody positive MN. MPGN was very infrequently diagnosed in the FCGG registry (0.7%), partially because chronic infections that cause MPGN in Europe have decreased in the last decades [23], but more importantly because MPGN is a histopathological pattern and many patients received aetiological diagnoses instead. The frequency of LN in Flanders was quite low [4.2% versus 6.7–8.8% [13, 20] in European registries (excluding outlying values in Lithuania [12] and Serbia [16, 17])], while the frequency of AAV was rather high (7.2% versus 2.3–7.8% [13, 17] in European registries, Table 3). This observation resembled data from the Scottish registry, which noted a similar overall biopsy rate, an equally low incidence rate of LN (3.0–5.0 versus 5.5 p.m.p./year in Flanders) and even higher rate of AAV (14.0–16.0 versus 9.3 p.m.p./year in Flanders) [8]. The high frequency of TIN in Flanders is striking (10.7% versus 1.5–6.2% [15, 24] in most European registries), which was only surpassed by data from Finland (9.0–14.7%) [11], where the Puumala hantavirus is endemic. The rising incidence of TIN in Europe [25] and the increased use of immune checkpoint inhibitors (ICI) that potentially cause ICI-related acute TIN [26, 27] may partially explain this observation, although we hypothesize that kidney biopsies are currently more frequently performed to confirm a suspected diagnosis of TIN. We also observed a remarkably higher frequency of biopsies with DKD (7.5% versus 1.0–5.4% [13, 24] in European registries). Flemish DKD patients had less severe symptoms [49.7% had nephrotic-range proteinuria, 20.9% had nephrotic syndrome (NS)] when compared with patients from the Spanish registry [28] (50.0% of DKD patients with NS) and the Polish registry [14] (76.4% of DKD patients with nephrotic-range proteinuria). This suggests that, currently in Flanders, the threshold to perform a kidney biopsy in patients with diabetes mellitus appears to be lower and patients are biopsied earlier in the disease course.
In this study, we found two main causes for the observed discrepancy between what the (nephro)pathologist withholds as the primary diagnosis of kidney disease versus the final clinical diagnosis made by the nephrologist. First, the histopathological diagnosis was not always withheld as clinically relevant and the clinical diagnosis was not solely based on pathology results. For example, in some patients, the nephrologist made a clinical diagnosis of TIN, while the primary histopathological diagnosis may have been an unrelated disease, or TIN was only considered to be a secondary histopathological diagnosis. In such examples, the clinical course and biochemical parameters may have enabled the clinician to confidently make a diagnosis despite discordant or less conclusive biopsy results. This stresses the importance of clinicopathological correlation between pathologist and nephrologist. Second, some discordances were attributed to differences in the FCGG histopathological coding system and the ERA-EDTA PRD clinical coding system. The ERA-EDTA PRD list does not contain updated and well-categorized coding terms for newer disease entities, such as C3GP and monoclonal immunoglobulin-associated kidney diseases. Furthermore, the list does not provide a general diagnostic term for ATN, but neither does it differentiate between ATN secondary to sepsis, haemodynamic injury/ischaemia or nephrotoxins, which explains the various and often nonspecific corresponding final clinical diagnoses in our study. The ERA-EDTA PRD coding system was initially designed to be applied to end-stage kidney disease registries and its application to a kidney biopsy registry therefore has inherent limitations. Future updates or alternative coding systems may allow more detailed diagnostic coding.
Our study has several advantages. First, we were able to report on population data and two experienced nephropathologists together examined approximately 60% of all biopsies, which reduces interobserver variability. Second, our ‘double diagnostic coding’ strategy enabled clinicopathological correlation, which uncovered some limitations in the ERA-EDTA PRD coding system and highlights the importance of more frequent updates and clinical feedback in such coding lists. Our study also has limitations. We only covered biopsy-proven kidney diseases and therefore may have underestimated the incidence of less frequently biopsied pathologies such as DKD or kidney diseases that nowadays can be alternatively diagnosed with serological markers, such as MN with circulating anti-PLA2R antibodies. Additionally, using the ERA-EDTA PRD coding system, our registry underestimated clinical diagnoses of C3GP, ATN and infection-related GN, which are more reliably estimated with the FCGG histopathological coding system.
In conclusion, the FCGG registry provides useful population-based epidemiological data on a large Western European population and allows subgroup selection for future observational, interventional and translational research.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all collaborating nephrologists in Flanders and Brussels and responsible persons at the data entry centres (Elsie De Man, Sabine Verhofstede and B.S.) for their participation in the FCGG registry.
The FCGG registry was initiated in collaboration with the Nederlandstalige Belgische Vereniging voor Nefrologie (NBVN), the organization that represents nephrologists in Flanders.
This study was approved by the Ethical Committee of the University Hospitals Leuven and local committees of all participating centres.
APPENDIX
‘FCGG reference nephrologists’ and collaborating pathologists of participating centres:
Reference nephrologist(centre)
An De Vriese(AZ Sint-Jan, Brugge),
Anja De Rycke(AZ Sint-Blasius, Dendermonde),
Anne-Marie Bogaert(AZ Glorieux, Ronse),
Annemie Woestenburg(AZ Voorkempen, Malle),
Bart Denys(Onze-Lieve-Vrouwziekenhuis, Aalst),
Bart Maes(AZ Delta, Roeselaere),
Domien Peeters(Sint-Trudo Ziekenhuis, Sint-Truiden),
Hilde Vanbelleghem(Jan Yperman ziekenhuis, Ypres),
Jan Donck(AZ Sint-Lucas, Ghent),
Johan Scharpé, Nele De Clippeleir(GZA, Antwerp),
Joris Vanparys (Kliniek Sint-Jan, Brussels),
Karen Meyvis(AZ Monica, Antwerp),
Kurt Vandepitte(Heilig-Hartziekenhuis, Lier),
Liza-Maria Reyns(AZ Sint-Lucas, Brugge),
Luc Verresen(Ziekenhuis Oost-Limburg, Genk),
Marc Decupere(AZ Groeninge, Kortrijk),
Mark Helbert(ZNA, Antwerp),
Miranda Zeegers(AZ Turnhout, Turnhout),
Nathalie Neirynck(AZ Nikolaas, Sint-Niklaas),
Pascale Bernaert(AZ Maria Middelares, Ghent),
Tom DejagereJessa (Ziekenhuis, Hasselt),
Wim Lemahieu(Imeldaziekenhuis, Bonheiden),
Ben Sprangers(University Hospitals Leuven, Leuven),
Lissa Pipeleers(University Hospital Brussels, Brussels),
Rachel Hellemans(Antwerp University Hospital, Antwerp),
Steven Van Laecke(Ghent University Hospital, Ghent),
Elena Levtchenko(Paediatric Nephrology Department, University Hospitals Leuven, Leuven),
Sevasti Karamaria(Paediatric Nephrology Department, Ghent University Hospital, Ghent),
Koen Van Hoeck, Dominique Trouet(Paediatric Nephrology Department, Antwerp University Hospital, Antwerp),
Reiner Mauel(Paediatric Nephrology Department, University Hospital Brussels, Brussels).
Pathologist (centre)
Amélie Dendooven (Antwerp University Hospital, Antwerp,
Ghent University Hospital, Ghent),
Anne Hoorens, Jo Van Dorpe, Marleen Praet (Ghent University Hospital, Ghent),
Caroline Geers (University Hospital Brussels, Brussels),
Evelyne Lerut, Priyanka Koshy, Tania Roskams (University Hospitals Leuven, Leuven),
Selda Aydin (Cliniques Universitaires Saint-Luc, Brussels),
Vasiliki Siozopoulou(Antwerp University Hospital, Antwerp),
Anne-Marie Schelfhout, Hendrik De Raeve (Onze-Lieve-Vrouwziekenhuis, Aalst),
Edwin Steenkiste, Francesca Dedeurwaerdere(AZ Delta, Roeselaere),
Ignace Dalle(AZ Sint-Lucas, Brugge),
Kristof Cokelaere, Stijn Deloose(Jan Yperman ziekenhuis, Ypres),
Pascale De Paepe(AZ Sint-Jan, Brugge),
Peter Van Eyken(Ziekenhuis Oost-Limburg, Genk).
Notes
†Members of the FCGG collaborative group are mentioned in the Appendix. These members are collaborators, unless also mentioned in the author list above, in which case they are authors.
Contributor Information
Wim Laurens, Department of Nephrology and Dialysis, AZ Nikolaas Hospital, Sint-Niklaas, Belgium; Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium.
Dries Deleersnijder, Department of Microbiology, Immunology and Transplantation, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Leuven, Belgium; Division of Nephrology, University Hospitals Leuven, Leuven, Belgium.
Amélie Dendooven, Division of Pathology, University Hospital Ghent, Ghent, Belgium; Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium.
Evelyne Lerut, Department of Imaging and Pathology, KU Leuven, Leuven, Belgium; Department of Pathology, University Hospitals Leuven, Leuven, Belgium.
An S De Vriese, Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Division of Nephrology and Infectious Diseases, AZ Sint-Jan, Brugge, Belgium.
Tom Dejagere, Department of Nephrology, Jessa Hospital, Hasselt, Belgium.
Mark Helbert, Department of Nephrology, ZNA Middelheim Hospital, Antwerp, Belgium.
Rachel Hellemans, Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium; Department of Nephrology, Antwerp University Hospital, Edegem, Belgium.
Priyanka Koshy, Department of Pathology, University Hospitals Leuven, Leuven, Belgium.
Bart Maes, Department of Nephrology, AZ Delta, Roeselare, Belgium.
Lissa Pipeleers, Department of Nephrology, University Hospital Brussels, Brussels, Belgium.
Amaryllis H Van Craenenbroeck, Division of Nephrology, University Hospitals Leuven, Leuven, Belgium; Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium.
Steven Van Laecke, Renal Division, Department of Internal Medicine, Ghent University Hospital, Ghent, Belgium.
Johan Vande Walle, Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Department of Pediatric Nephrology, Ghent University Hospital, Ghent, Belgium.
Marie M Coutteneye, Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium; Department of Nephrology, Antwerp University Hospital, Edegem, Belgium.
Johan De Meester, Department of Nephrology and Dialysis, AZ Nikolaas Hospital, Sint-Niklaas, Belgium.
Ben Sprangers, Department of Microbiology, Immunology and Transplantation, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Leuven, Belgium; Division of Nephrology, University Hospitals Leuven, Leuven, Belgium.
The FCGG collaborative group:
An De Vriese, Anja De Rycke, Anne-Marie Bogaert, Annemie Woestenburg, Bart Denys, Bart Maes, Domien Peeters, Hilde Vanbelleghem, Jan Donck, Johan Scharpé, Nele De Clippeleir, Joris Vanparys, Karen Meyvis, Kurt Vandepitte, Liza-Maria Reyns, Luc Verresen, Marc Decupere, Mark Helbert, Miranda Zeegers, Nathalie Neirynck, Pascale Bernaert, Tom Dejagere, Wim Lemahieu, Ben Sprangers, Lissa Pipeleers, Rachel Hellemans, Steven Van Laecke, Elena Levtchenko, Sevasti Karamaria, Koen Van Hoeck, Dominique Trouet, Reiner Mauel, Amélie Dendooven, Anne Hoorens, Jo Van Dorpe, Marleen Praet, Caroline Geers, Evelyne Lerut, Priyanka Koshy, Tania Roskams, Selda Aydin, Vasiliki Siozopoulou, Anne-Marie Schelfhout, Hendrik De Raeve, Edwin Steenkiste, Francesca Dedeurwaerdere, Ignace Dalle, Kristof Cokelaere, Stijn Deloose, Pascale De Paepe, and Peter Van Eyken
FUNDING
D.D. is supported by a PhD Fellowship grant fundamental research from the Research Foundation Flanders (F.W.O., grant number 11L5622N). B.S. is a senior clinical investigator of The Research Foundation Flanders (F.W.O., grant number 1842919N). The FCGG registry is funded by the Nederlandstalige Belgische Vereniging voor Nefrologie (NBVN).
AUTHORS’ CONTRIBUTIONS
W.L., D.D., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.M.C., J.D.M. and B.S. were responsible for the conception, design and data acquisition of the study. W.L., D.D., J.D.M., A.D., A.H.V.C. and B.S. were responsible for analysis and interpretation of the data. W.L., D.D., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.C., J.D.M. and B.S. drafted the work and revised it critically for important intellectual content. W.L., D.D., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.C., J.D.M. and B.S. approved the submitted version of the manuscript. W.L., D.D., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.C., J.D.M. and B.S. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
B.S. is a member of the CKJ editorial board. The results presented in this article have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Hogan JJ, Mocanu M, Berns JS. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol 2016; 11: 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sethi S, Haas M, Markowitz GSet al. Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of Gn. J Am Soc Nephrol 2016; 27: 1278–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiorentino M, Bolignano D, Tesar Vet al. Renal biopsy in 2015—from epidemiology to evidence-based indications. Am J Nephrol 2016; 43: 1–19 [DOI] [PubMed] [Google Scholar]
- 4. Dendooven A, Peetermans H, Helbert Met al. Coding practice in national and regional kidney biopsy registries. BMC Nephrol 2021; 22: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venkat-Raman G, Tomson CRV, Gao Yet al. New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 2012; 27: 4414–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaza G, Bernich P, Lupo A. Incidence of primary glomerulonephritis in a large North-eastern Italian area: a 13-year renal biopsy study. Nephrol Dial Transplant 2013; 28: 367–372 [DOI] [PubMed] [Google Scholar]
- 7. Maixnerova D, Jancova E, Skibova Jet al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994–2011. J Nephrol 2015; 28: 39–49 [DOI] [PubMed] [Google Scholar]
- 8. McQuarrie EP, Mackinnon B, Young Bet al. Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant 2009; 24: 1524–1528 [DOI] [PubMed] [Google Scholar]
- 9. Heaf JG, Sørensen SS, Hansen A. Increased incidence and improved prognosis of glomerulonephritis: a national 30-year study. Clin Kidney J 2021; 14: 1594–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. López-Gómez JM, Rivera F. Spanish Registry of glomerulonephritis 2020 revisited: past, current data and new challenges. Nefrología 2020; 40: 371–383 [DOI] [PubMed] [Google Scholar]
- 11. Wirta O, Mustonen J, Helin Het al. Incidence of biopsy-proven glomerulonephritis. Nephrol Dial Transplant 2008; 23: 193–200 [DOI] [PubMed] [Google Scholar]
- 12. Brazdziute E, Miglinas M, Gruodyte Eet al. Nationwide renal biopsy data in Lithuania 1994–2012. Int Urol Nephrol 2015; 47: 655–662 [DOI] [PubMed] [Google Scholar]
- 13. Rivera F, López-Gómez JM, Pérez-García R.. Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 2002; 17: 1594–1602 [DOI] [PubMed] [Google Scholar]
- 14. Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz Met al. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 2017; 32: ii209–ii218 [DOI] [PubMed] [Google Scholar]
- 15. Covic A, Schiller A, Volovat Cet al. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 2006; 21: 419–424 [DOI] [PubMed] [Google Scholar]
- 16. Brkovic V, Milinkovic M, Kravljaca Met al. Does the pathohistological pattern of renal biopsy change during time? Pathol Res Pract 2018; 214: 1632–1637 [DOI] [PubMed] [Google Scholar]
- 17. Naumovic R, Pavlovic S, Stojkovic Det al. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant 2009; 24: 877–885 [DOI] [PubMed] [Google Scholar]
- 18. Heaf J, Løkkegaard H, Larsen S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 1999; 14: 1889–1897 [DOI] [PubMed] [Google Scholar]
- 19. Hanly JG, O'Keeffe AG, Su Let al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 1997; 12: 418–426 [DOI] [PubMed] [Google Scholar]
- 21. Woo KT, Chan CM, Lim Cet al. A global evolutionary trend of the frequency of primary glomerulonephritis over the past four decades. Kidney Dis 2019; 5: 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gesualdo L, Di Palma AM, Morrone LFet al. The Italian experience of the national registry of renal biopsies. Kidney Int 2004; 66: 890–894 [DOI] [PubMed] [Google Scholar]
- 23. Stratta P, Segoloni GP, Canavese Cet al. Incidence of biopsy-proven primary glomerulonephritis in an Italian Province. Am J Kidney Dis 1996; 27: 631–639 [DOI] [PubMed] [Google Scholar]
- 24. O'Shaughnessy MM, Hogan SL, Thompson BDet al. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant 2018; 33: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goicoechea M, Rivera F, Lopez-Gomez JM. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2013; 28: 112–115 [DOI] [PubMed] [Google Scholar]
- 26. Oleas D, Bolufer M, Agraz Iet al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-centre experience. Clin Kidney J 2021; 14: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta S, Short SAP, Sise MEet al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021; 9: e003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rivera F, López-Gómez JM, Pérez-García R. Clinicopathologic correlations of renal pathology in Spain. Kidney Int 2004; 66: 898–904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.