Abstract
Iceland was one of six European countries with an adjusted incidence of kidney replacement therapy (KRT) in 2018 lower than 100 per million persons (pmp), along with Estonia, Montenegro, Russia, Serbia and Finland. It was also one of 10 countries with an adjusted KRT prevalence <900 pmp. Furthermore, the prevalence of chronic kidney disease (CKD) in Iceland is up to 2.44-fold lower and the death rate from CKD up to 3.44-fold lower than in other countries with a low incidence of KRT, suggesting that the low KRT incidence actually reflects a low need for KRT rather than low uptake or availability of KRT. This identifies Iceland as a benchmark for countries trying to reduce KRT incidence. Iceland also represents one of the best genetically characterized populations in the world, facilitating studies on the influence of the genetic background versus environment and lifestyle on CKD. This issue of CKJ reports the incidence and risk factors for CKD in Icelandic adults. Diabetes, acute kidney injury, hypertension, cardiovascular disease, chronic lung disease, malignancy and major psychiatric illness were associated with an increased risk of incident CKD, as were obesity and sleep apnea in women. However, in 75% of incident CKD cases, CKD was first detected in category G3 or higher, emphasizing the need for new tools that allow an earlier diagnosis of CKD that precedes the loss of >50% of the functioning kidney mass and/or wider use of albuminuria as a screening tool. The European Society of Cardiology just recommended assessing albuminuria for routine cardiovascular risk workups for all.
Keywords: albuminuria, benchmarking, chronic kidney disease, epidemiology, incidence, kidney replacement therapy, prevalence
Chronic kidney disease (CKD) is one of the fastest growing causes of death worldwide, predicted to become the fifth global cause of death by 2040 and the second cause of death before the end of the century in countries with long life expectancy [1–3]. The global struggle against CKD should focus not only on countries, regions or communities with very high incidence of CKD, the so-called CKD hotspots [4], but also on countries with the lowest incidence of both CKD and kidney replacement therapy (KRT) that may be used as CKD benchmarks. CKD hotspots will advance our understanding of drivers of CKD and, in the process, identify actionable risk factors, as recently exemplified by Aguascalientes in Mexico [5, 6], that will provide insight into genetic or environmental drivers of CKD and CKD progression. In contrast, CKD benchmarks should be studied for the genetic background of the population, environmental and lifestyle factors and access to healthcare, among other factors, that may contribute to a low incidence of CKD. A frequent barrier in the identification of both CKD hotspots and CKD benchmarks is the scarcity of data on the prevalence of CKD in national or regionally representative populations and the even lower availability of data on the incidence of CKD. Data are more complete for KRT. However, the incidence and prevalence of KRT is influenced by multiple factors beyond the incidence and prevalence of CKD [7, 8]. These additional factors include the estimated glomerular filtration rate (eGFR) at which KRT is initiated in different countries, incentives to enroll or reject patients for KRT, patient attitudes toward KRT and the availability of resources to offer KRT to all in need, and for KRT prevalence, mortality in incident KRT patients and access to kidney transplantation. Additionally, the cause of KRT may have become blurred in patients with long-standing CKD or simplified based on the presence/absence of hypertension, diabetes, kidney biopsy or cysts in imaging [9, 10]. In this issue of CKJ, Jonsson et al. [11] report both the incidence and risk factors for CKD in the Icelandic adult population in one of the most nationally representative studies to date.
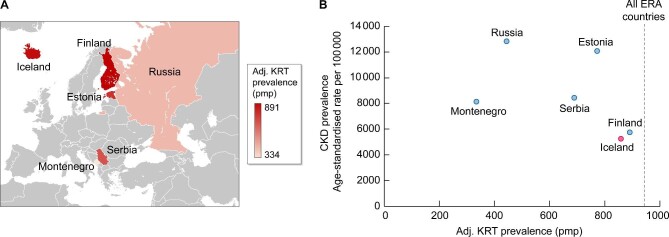
Iceland is one of six European countries with an adjusted incidence of KRT in 2018 <100 per million persons (pmp), together with Estonia, Montenegro, Russia, Serbia and Finland [12]. It is also one of the 10 countries with an adjusted prevalence of KRT <900 pmp. However, the prevalence of CKD in Iceland is up to 2.44-fold lower and the death rate from CKD up to 3.44-fold lower than in other countries with a low incidence of KRT [13–15] (Table 1 and Fig. 1], suggesting that the low incidence of KRT actually reflects a low need of KRT rather than low uptake or availability of KRT. In this regard, the six countries with the lowest incidence of KRT in the ERA can be divided in two groups, according to the CKD prevalence and death rate (Table 1]. Thus Estonia, Montenegro, Russia and Serbia have a higher CKD prevalence and death rate, despite the low incidence of KRT, while Finland and Iceland, which represent the countries with the highest gross domestic product (GDP), have a lower CKD prevalence and death rate. This identifies Iceland as a CKD benchmark for countries trying to reduce the incidence of KRT. On top of that, Iceland represents one of the best genetically characterized populations in the world [16], paving the way for studies on the influence of genetic background on CKD and its progression, as well as on the genetics–environment interaction.
Table 1.
Countries with the lowest incidence of KRT in 2018 according to the ERA Registry
| CKD prevalence | Country | Adjusted incidence of KRT (pmp) | Adjusted prevalence of KRT (pmp) | Median incident KRT age (years) | Median prevalent KRT age (years) | Incident KRT DM (pmp) | Prevalent KRT DM (pmp) | Age-standardized CKD prevalence pmp, 2017 | Age-standardized CKD deaths pmp, 2017 | Life expectancy at birth (years) | GDP per capita (US$ PPP; IMF 2021) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher | Estonia | 76 | 772 | 58.5 | 59 | 15 | 133 | 120 580 | 89 | 78 | 39 729 |
| Montenegro | 78 | 334 | 60.1 | 59.5 | 16 | 45 | 81 180 | 131 | 75.9 | 21 355 | |
| Serbia | 82 | 690 | 63.6 | 62.4 | 20 | 129 | 84 210 | 148 | 75.7 | 20 545 | |
| Russia | 87 | 443 | 61 | 59 | 14 | 59 | 128 320 | 51 | 73 | 29 485 | |
| Lower | Finland | 89 | 891 | 64.1 | 62.1 | 29 | 233 | 57 610 | 40 | 81.9 | 51 867 |
| Iceland | 96 | 859 | 59.6 | 57.5 | 14 | 85 | 52 350 | 43 | 84.1 | 58 151 |
DM, diabetes mellitus; IMF, International Monetary Fund; PPP, purchasing power parity.
Sources: references 11–15.
Figure 1:
ERA countries with the lowest adjusted KRT incidence (<100 pmp) in 2018. (A) Geographical localization. Colour-coding is for adjusted KRT prevalence. KRT prevalence was chosen as it is more stable than incidence for countries with a small number of inhabitants and KRT patients. Source: Kramer et al. [12]. (B) Relationship between adjusted CKD prevalence in 2017 and adjusted KRT prevalence in 2018 among ERA countries with the lowest KRT incidence in 2018. Note that higher CKD prevalence is not associated with higher KRT prevalence. In this regard, Iceland and Finland both had a low prevalence of CKD and a low incidence and prevalence of KRT and may be considered benchmark countries for achieving low CKD prevalence and low need of KRT. Data from Jonsson et al. [11] and GBD Chronic Kidney Disease Collaboration [13]. Vertical discontinuous line represents the KRT prevalence in all ERA countries combined.
In this issue of CKJ, Jonsson et al. [11] analyzed 2 120 147 serum creatinine measurements from 218 437 adults in Iceland in 2008–2016. The population of Iceland was ∼325 000 at the 90 time, thus the sample represents most of the adult Icelandic population. The baseline prevalence of CKD was 5.4%, 2- to 3-fold lower than reported for countries such as Spain [1]. Thereafter, 6.9% of those not having CKD at baseline developed incident CKD categories G1–G5 during the study. This corresponds to a mean annual age-standardized incidence of CKD of 671 per 100 000 (0.67%/year), being slightly lower for men than for women: 649 [95% confidence interval (CI) 630–668] and 694 (95% CI 674–714), respectively. However, it reached 4000 in men and 3000 in women per 100 000 in those >65 years of age. In this regard, the lifetime risk of developing CKD category G3–G5 in Iceland was estimated at 36% for women and 21% for men at the age of 45 years in a smaller study (∼19 000 participants) with follow-up to 2005 [17]. Lifetime risks decrease as persons get older. The data collected by Jonsson et al. [11] may help update these estimates and contribute to an updated European-wide estimation of lifetime risk of CKD, as there are already data for lifetime risk of KRT [18]. The European lifetime risk of KRT was estimated at 0.77% in 20-year-old women and 1.45% in 20-year-old men, although there was wide variability between countries, further illustrating the concept of CKD benchmarks. In this regard, for Finland, a country with similar KRT incidence and prevalence as Iceland, lifetime risk of KRT was estimated at 0.44% in 20-year-old women and 0.88% in 20-year-old men, well below corresponding figures of 1.20% and 2.00%, respectively, in Greece.
Jonsson et al. [11] identified diabetes, acute kidney injury (AKI), hypertension, cardiovascular disease, chronic lung disease, malignancy and major psychiatric illness as independent risk actors for incident CKD, and additionally, obesity and sleep apnea in women. Risk factors were also estimated for incident CKD categories G4–G5. Of interest, AKI conferred the highest independent risk for severe CKD for both men [hazard ratio 8.36 (95% CI 6.29–11.12)] and women [hazard ratio 2.73 (95% CI 2.55–2.93)]. This emphasizes the need to prevent AKI and the interaction between AKI and CKD, as CKD is a key risk factor for AKI [19]. In this regard, a key issue in patients with CKD is unawareness by their physicians of the diagnosis of CKD, despite laboratory values that allow the diagnosis. This was the case in ∼80% of patients with strict diagnostic criteria for CKD (i.e. using both eGFR thresholds and the 3-month time frame) in Stockholm, Sweden [20, 21]. Failure of physicians to annotate a diagnosis of CKD in patient charts was associated with a higher risk of prescribing a variety of nephrotoxic medications, which may likely increase the risk of AKI and CKD progression [20]. A similar underdiagnosis of CKD has been observed in China [22].
In contrast, Jonsson et al. [11] identified intermediate or severe frailty risk scores as associated with decreased risk of incident CKD, likely reflecting the limitations of using serum creatinine to estimate GFR and diagnose CKD in patients with low muscle mass [23]. This additionally implies that the incidence of CKD may have been underestimated.
Finland was also characterized by low prevalence of CKD and low prevalence of KRT and could potentially be another CKD benchmark [12, 13]. Both have a high life expectancy, meaning that the low risk of CKD and KRT is not easily explained by competing risks or early death: Iceland, at 84.1 years, and Finland, at 81.9 years, compare favorably with Greece, at 80.9 years, as an example of a country with high lifetime risk of KRT [15]. However, Iceland and Finland KRT patients differ in several aspects. Thus Icelandic patients on KRT are younger and have a roughly 50% lower incidence and 33% lower prevalence of diabetic kidney disease, pointing to differences in the cause of CKD. Some of these differences may depend on the low population and low incidence of KRT in Iceland that may be associated with higher variability in numbers. As an example, in the 2019 European Renal Association–European Dialysis and Transplant Association report, the age of incident KRT patients was similar in Finland and Iceland, but the differences in incident diabetic kidney disease persisted [24, 25]. So what are the main causes of KRT in Iceland? Focusing on prevalence, which may display less variability than incidence data, Finland has the highest prevalence of KRT due to type 1 diabetes in Europe, and diabetic kidney disease (DKD) is the most frequent cause of prevalent DKD, while Iceland has one of the lowest KRT prevalences due to DKD, and glomerulonephritis is the most frequent cause of prevalent KRT. In this regard, Jonsson et al. [11] do not provide information on cause. In the future, CKD benchmarks may be envisioned for different causes of CKD.
Despite the potential role of Iceland as a CKD benchmark, there is still room for improvement. In 75% of incident CKD cases, CKD was first detected in category G3 or higher, emphasizing the need for new tools that allow an earlier diagnosis of CKD that precedes the loss of >50% of the functioning kidney mass and/or wider use of albuminuria as a screening tool. The European Society of Cardiology just recommended assessing albuminuria in routine cardiovascular risk workups for all [26]. In this regard, baseline albuminuria levels did not significantly influence the nephroprotection by sodium–glucose cotransport protein 2 inhibitors [27]. Thus any degree of pathological albuminuria found may benefit from intervention. Albuminuria assessment is currently restricted by many payers and healthcare systems to high-risk populations, which may limit its use in screening. As Jonsson et al. [11] have shown, the increased risk of CKD is not limited to diabetes and hypertension and delayed diagnosis of CKD continues to be an issue, as there are no kidney-regenerative therapies and current therapeutic approaches only aim at slowing CKD progression. Additionally, implementation of screening for albuminuria among high-risk populations may be suboptimal. On top of the limitations on albuminuria testing, a further greater issue is the lack of tools to identify most patients with earlier stages of CKD, for whom albuminuria may be in the normal range when eGFR is still >60 mL/min/1.73 m2. This has been termed the blind spot of CKD, i.e. kidney disease is ongoing, sometimes for decades, but eGFR and albuminuria levels are not yet diagnostic of CKD [28]. A classic example is autosomal dominant polycystic kidney disease, for which there is a tool, sonography, that may identify CKD decades earlier than albuminuria or eGFR. Tools such as improved imaging techniques (e.g. multiparametric magnetic resonance imaging) or urinary peptidomics are promising for the earlier detection of and intervention in CKD [29, 30].
In conclusion, the recent report by Jonsson et al. [11] helps to better characterize the epidemiology of CKD in one of the most interesting countries in Europe from the point of view of CKD benchmarking and to advance our understanding of CKD epidemiology as a driver of change in the management of CKD worldwide.
FUNDING
Funding was provided by FIS/Fondos FEDER (PI18/01 366, PI19/00 588, PI19/00 815, PI21/00 251, DTS18/00 032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00 064 and PERSTIGAN AC18/00 071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM, Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) and FEDER funds.
CONFLICT OF INTEREST STATEMENT
A.O. has received consultancy or speaker fees or travel support from Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma; is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes; and is the Editor-in-Chief of CKJ.
REFERENCES
- 1. Ortiz A, Asociación Información Enfermedades Renales Genéticas (AIRG-E), European Kidney Patients' Federation (EKPF), Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER), et al. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2022; 15: 372–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foreman KJ, Marquez N, Dolgert Aet al. . Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortiz A, Sanchez-Niño MD, Crespo-Barrio Met al. . The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 4. Martín-Cleary C, Ortiz A.. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin Kidney J 2014; 7: 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villalvazo P, Carriazo S, Martin-Cleary C, Ortiz A.. Aguascalientes: one of the hottest chronic kidney disease (CKD) hotspots in mexico and a CKD of unknown aetiology mystery to be solved. Clin Kidney J 2021; 14: 2285–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutierrez-Peña M, Zuñiga-Macias L, Marin-Garcia Ret al. . High prevalence of end-stage renal disease of unknown origin in aguascalientes mexico: role of the registry of chronic kidney disease and renal biopsy in its approach and future directions. Clin Kidney J 2021; 14: 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ortiz A. Burden, access and disparities in kidney disease: chronic kidney disease hotspots and progress one step at a time. Clin Kidney J 2019; 12: 157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sever MŞ, Jager KJ, Vanholder Ret al. . A roadmap for optimizing chronic kidney disease patient care and patient-oriented research in the eastern european nephrology community. Clin Kidney J 2021; 14: 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carriazo S, Vanessa Perez-Gomez M, Ortiz A. Hypertensive nephropathy: a major roadblock hindering the advance of precision nephrology. Clin Kidney J 2020; 13: 504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torra R, Furlano M, Ortiz Aet al. . Genetic kidney diseases as an underrecognized cause of chronic kidney disease: the key role of international registry reports. Clin Kidney J 2021; 14: 1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jonsson AJ, Lund SH, Eriksen BOet al. . Incidence and risk factors of chronic kidney disease: results of a nationwide study in iceland. Clin Kidney J 2022; 10.1093/ckj/sfac051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kramer A, Boenink R, Stel VSet al. . The ERA-EDTA registry annual report 2018: a summary. Clin Kidney J 2021; 14: 107–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wikipedia. List of countries by GDP (PPP) per capita . https://en.wikipedia.org/wiki/List_of_countries_by_GDP_(PPP)_per_capita(8 January 2022, date last accessed)
- 15. GBD 2019 Demographics Collaborators . Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1160–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wuttke M, Li Y, Li Met al. . A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 2019; 51: 957–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inker LA, Tighiouart H, Aspelund Tet al. . Lifetime risk of stage 3–5 CKD in a community-based sample in Iceland. Clin J Am Soc Nephrol 2015; 10: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Brand JAJG, Pippias M, Stel VSet al. . Lifetime risk of renal replacement therapy in europe: a population-based study using data from the ERA-EDTA registry. Nephrol Dial Transplant 2017; 32: 348–355 [DOI] [PubMed] [Google Scholar]
- 19. Martin-Cleary C, Molinero-Casares LM, Ortiz Aet al. . Development and internal validation of a prediction model for hospital-acquired acute kidney injury. Clin Kidney J 2021; 14: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bosi Alessandro, Xu Yunwen, Gasparini Alessandroet al. , Use of nephrotoxic medications in adults with chronic kidney disease in Swedish and US routine care. Clin Kidney J 2022; 15: 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carriazo S, Villalvazo P, Ortiz A.. More on the invisibility of chronic kidney disease and counting. Clin Kidney J 2022; 15: 388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang HY, Ding GH, Lin Het al. . Influence of doctors' perception on the diagnostic status of chronic kidney disease: results from 976 409 individuals with electronic health records in China. Clin Kidney J 2021; 14: 2428–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortiz A, Sanchez-Niño MD.. Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J 2019; 12: 110–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boenink R, Astley ME, Huijben JAet al. . The ERA registry annual report 2019: summary and age comparisons. Clin Kidney J 2022; 15: 452–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ERA-EDTA Registry Annual Report 2019. https://www.era-online.org/registry/AnnRep2019.pdf (10 January 2021, date last accessed)
- 26. Visseren FLJ, Mach F, Smulders YMet al. . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227–3337 [DOI] [PubMed] [Google Scholar]
- 27. Delanaye P, Wissing KM, Scheen AJ.. Sodium-glucose cotransporter 2 inhibitors: renal outcomes according to baseline albuminuria. Clin Kidney J 2021; 14: 2463–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez-Niño MD, Sanz AB, Ramos AMet al. . Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J 2017; 10: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez-Ortiz ME, Pontillo C, Rodríguez Met al. . Novel urinary biomarkers for improved prediction of progressive egfr loss in early chronic kidney disease stages and in high risk individuals without chronic kidney disease. Sci Rep 2018; 8: 15940; correction Sci Rep 2018; 8: 17822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makvandi K, Hockings PD, Jensen Get al. . Multiparametric magnetic resonance imaging allows non-invasive functional and structural evaluation of diabetic kidney disease. Clin Kidney J 2022; 10.1093/ckj/sfac054 [DOI] [PMC free article] [PubMed] [Google Scholar]