ABSTRACT
Background
Randomized controlled trials have demonstrated the benefits of sodium–glucose cotransporter 2 inhibitors (SGLT2is) in people with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD). However, real-world data on CKD progression and the development of end-stage kidney disease (ESKD) remains scarce. Our aim was to study renal outcomes of people with diabetic kidney disease (DKD) using SGLT2is in a highly prevalent DKD population.
Methods
Between 2016 and 2019 we recruited T2DM patients in the renal and diabetic clinics in a regional hospital in Singapore. Patients prescribed SGLT2is were compared with those on standard anti-diabetic and renoprotective treatment. The outcome measures were CKD progression [a ≥25% decrease from baseline and worsening of estimated glomerular filtration rate (eGFR) categories according to the Kidney Disease: Improving Global Outcomes guidelines] and ESKD (eGFR <15 mL/min/1.73 m2).
Results
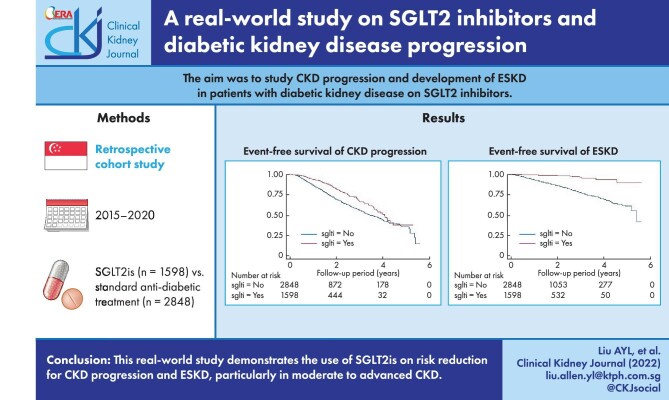
We analysed a total of 4446 subjects; 1598 were on SGLT2is. There was a significant reduction in CKD progression {hazard ratio [HR] 0.60 [95% confidence interval (CI) 0.49–0.74]} with SGLT2is. The HR for eGFR ≥45 mL/min/1.73 m2 and 15–44 mL/min/1.73 m2 was 0.60 (95% CI 0.47–0.76) and 0.43 (95% CI 0.23–0.66), respectively. There was also a reduction in risk for developing ESKD for the entire cohort [HR 0.33 (95% CI 0.17–0.65)] and eGFR 15–44 mL/min/1.73 m2 [HR 0.24 (95% CI 0.09–0.66)]. Compared with canagliflozin and dapagliflozin, empagliflozin showed a sustained risk reduction of renal outcomes across CKD stages 1–4.
Conclusions
This real-world study demonstrates the benefits of SGLT2is on CKD progression and ESKD. The effect is more pronounced in moderate to advanced CKD patients.
Keywords: CKD progression, diabetic kidney disease, diabetes mellitus, ESKD, real-world study, SGLT2is
Graphical Abstract
Graphical Abstract.
INTRODUCTION
There is an increasing prevalence of diabetic kidney disease (DKD) worldwide. DKD vastly accelerates the prevalence of end-stage kidney disease (ESKD) [1]. According to a local study from Singapore, 53% of people with type 2 diabetes mellitus (T2DM) have concomitant chronic kidney disease (CKD) and 66% of incident ESKD patients have pre-existing DKD [2]. Despite optimal diabetic and blood pressure control with the administration of renin–angiotensin system (RAS) blockers, the heightened risks of kidney disease progression, cardiovascular morbidity and mortality persist. The publication of the clinical benefit of using the sodium–glucose cotransporter 2 inhibitor (SGLT2i), empagliflozin to improve renal and cardiovascular outcomes in DKD provided a supplemental approach and possibly an additive benefit to RAS blockade [3]. The haemodynamic and natriuresis-related pharmacodynamic response to SGLT2is reduces intra glomerular pressure, exerting antihypertensive and anti-albuminuric properties [4]. With their renal function–dependent glycosuric and anti-hyperglycaemic effect, SGLT2is have been consistently demonstrated to have renoprotective effects in major cardiovascular outcome trials [5–8]. The current recommendations from most of these large randomized controlled trials (RCTs) conferred the benefits of SGLT2 inhibition to DKD with an estimated glomerular filtration (eGFR) ≥45 mL/min/1.73 m2 [3, 6, 7, 9, 10]. However, its haemodynamic, anti-proteinuric and anti-metabolic effects may still be carried on despite reducing eGFR [11]. Given the pleiotropic effects (anti-inflammatory, anti-oxidative and anti-fibrotic) on the heart [12] and liver [13], in addition to the kidneys [14, 15], the benefits of SGLT2is may extend to more advanced CKD. For example, in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, patients with an eGFR decrease to <30 mL/min/1.73 m2 were allowed to continue canagliflozin until dialysis or transplantation [16]. Therefore the benefit was generally believed to persist even to stage 4 CKD [17]. Renal outcome trials such as the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) [18] and the Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) [19] included patients with an eGFR of 25 and 20 mL/min/1.73 m2, respectively, providing further insights that the renoprotective effects of SGLT2is can be extended to patients with advanced CKD.
Nevertheless, the intriguing results from RCTs in which participants are recruited with specific inclusion criteria and monitored with a predefined protocol may not be generalizable in real-world clinical settings [20]. Moreover, most RCTs do not include participants from the Southeast Asian region, where the incidence and prevalence of ESKD due to DKD are among the highest in the world [21]. Currently, there remains a deficiency in knowledge on whether the initiation of SGLT2 inhibition in patients with moderate to advanced CKD in real-world clinical practice could protect DKD patients from CKD progression. Barriers that prohibit the prescription of SGLT2is include perceived ineffective glucose-lowering action with diminished eGFR and concern over a decrease in eGFR in the initial phase of treatment. With a solid impetus for controlling DKD progression despite the lack of standard guidelines on managing moderate to advanced CKD, clinicians worldwide have been actively pursuing an extension of current prescription criteria by treating DKD patients with SGLT2is with advanced CKD status (eGFR 15–44 mL/min/1.73 m2). Since early 2017 we have been prescribing SGLT2is for DKD patients with an eGFR >20 mL/min/1.73 m2 for reno protection and glycaemic control. This study aims to evaluate the renal outcomes of DKD patients with or without SGLT2is in a multi-ethnic Asian population. We hypothesized that SGLT2i use would improve renal outcomes despite initiation of treatment at advanced CKD status.
MATERIALS AND METHODS
This was a retrospective cohort study. The observational period was between January 2015 and December 2020. We recruited patients with T2DM [identified by the International Classification of Diseases, Tenth Revision or haemoglobin A1c (HbA1c) ≥6.5%], age ≥21 years with active follow-up in the Khoo Teck Puat Hospital (KTPH; a 600-bed acute care hospital with secondary- and tertiary-level care) specialist renal clinic and Admiralty Medical Centre (AdMC) Diabetes Mellitus centre and DKD clinic. Patients with known ESKD status were excluded. Referral sources for the KTPH renal clinic were primary care clinicians in both private and public sectors in the northern territory of Singapore. Criteria for referral of DKD patients to the renal clinic were changes in eGFR >10 mL/min/1.73 m2, measurements of urine albumin:creatinine ratio (uACR) >100 mg/mmol on two separate occasions and stage 3–4 CKD [i.e. eGFR 15–59 mL/min/1.73 m2 according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines for the evaluation of CKD] [22]. The inclusion criteria for referral to the DKD clinic were diabetes mellitus (DM) with persistent macro-albuminuria (uACR >50 mg/mmol) with preserved eGFR (≥60 mL/min) or stage 3–4 CKD. Exclusion criteria for referral to the DKD clinic were comorbidities that preclude renal retardation, such as terminal malignancies; severely limited life expectancy due to advanced organ failure; inability to intensify risk factor control due to psychosocial issues or resource constraints, cognitive impairment or psychiatric illness and stage 5 CKD, where the patient is already on renal replacement therapy (RRT) [23]. Patients followed up in the AdMC DM clinic were DM patients that require standard care for complications related to DM, with or without CKD. The treatment group in our study was T2DM patients who were prescribed one of the following SGLT2is for at least 3 months: canagliflozin (Invokana, Johnson and Johnson Pte, Singapore), empagliflozin (Jardiance, Boehringer Ingelheim Pte, Singapore) and dapagliflozin (Forxiga, AstraZeneca Pte, Singapore). Principal physicians decided on the choice and dosage of SGLT2is based on clinical indications and contraindications. In general, patients with eGFR <45 mL/min/1.73 m2 are given a lower dose of SGLT2is (∼25–50% less than the recommended dose). The date of treatment initiation was defined by the first filing or an SGLT2i prescription in iPharm (the KTPH outpatient pharmacy system). For patients not taking SGLT2is during the study period, the date of treatment initiation was defined by the first filing of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) prescription or the date of referral, whichever was available. The outcomes of this study were ≥25% decrease from baseline and worsening of eGFR categories according to the KDIGO guidelines [24] and ESKD (sustained eGFR <15 mL/min/1.73 m2 for 4 weeks).
De-identified data on demographics and medications were obtained from electronic medical records (Sunrise Enterprise and iPharm with assistance from Integrated Health Information Systems). Spot urine for ACR and blood samples for serum creatinine and HbA1c were collected and measured at the hospital laboratory accredited by the College of American Pathologists. Serum creatinine was quantitated using an enzymatic colourimeter test (Cobas c501; Roche Diagnostics, Mannheim, Germany). HbA1c was determined using a Tina-quant Haemoglobin A1c Gen.3 (Roche Diagnostics). Urinary albumin was determined using an immunoturbidimetric assay (Roche Diagnostics). The Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate eGFR [25].
For analytic purposes, the following additional exclusion criteria were applied to both groups: fewer than two eGFR readings and ˂3 months follow-up.
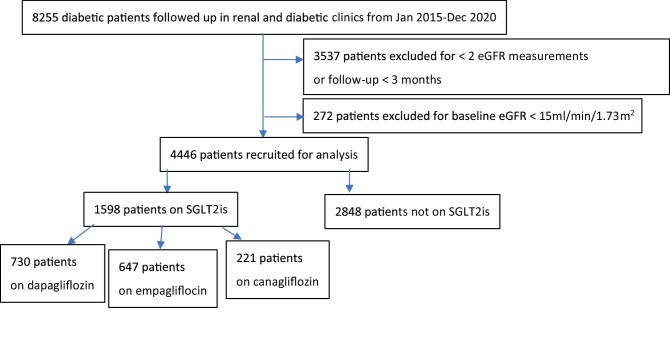
Previous studies revealed a composite outcome of a sustained 40% reduction in the decline of the eGFR, the need for RRT or death from renal causes [7]. The incidence over a 2-year follow-up period for renal-related composite outcomes was 1.89% versus 4.14%. To achieve a power of 80% and an α of 0.05, we needed 611 patients on SGLT2is (case) and 1833 patients in all stages of CKD without SGLT2is (control). Overall, we recruited 1598 DKD patients for whom SGLT2is were initiated and 2848 patients as controls (Figure 1).
FIGURE 1:
Patient selection.
Ethics approval was obtained from the National Healthcare Group Domain Specific Review Board (DSRB) and the study was conducted according to the Declaration of Helsinki. The DSRB waived written informed consent.
Statistical analysis
Categorical variables were expressed as frequency (percentages) and continuous variables as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Comparison of the baseline characteristics using SGLT2is was performed using the chi-squared test for categorical variables and the Student's t-test or Wilcoxon rank sum test for continuous variables, where appropriate. The Kaplan–Meier method was used to assess the time to event. Multivariate Cox proportional hazards models were constructed to establish the associations with CKD progression and ESKD, adjusting for age, gender and ethnicity in model 1 and age, gender, ethnicity, HbA1c, baseline eGFR, natural log-transformed ACR and use of RAS antagonists in model 2. Covariates with P < .1 during univariate analyses or those with established risk factors for CKD progression and ESKD were selected for adjustment to multivariate Cox proportional hazards models [26]. Statistical analysis was performed using Stata version 14.0 (StataCorp, College Station, TX, USA). All statistical tests were two-sided. The results were considered statistically significant for P < .05.
Sensitivity analysis
We reran the statistical models using the same outcome measures by defining CKD as a uACR ≥3 mg/mmol or eGFR <60 mL/min/1.73 m2. We also incorporated the analysis by categorizing albuminuria according to the KDIGO guidelines (uACR <3 mg/mmol, 3–30 mg/mmol and >30 mg/mmol). Further, we analysed the effects of SGLT2is on patients with or without RAS blockers.
RESULTS
The duration of follow-up was up to 5.8 years [median 1.4 (IQR 0.8–2.1)]. There were 1461 (32.9%), 1175 (26.4%), 584 (13.1%), 642 (14.4%) and 584 (13.1%) patients with a baseline eGFR >90, 60–90, 45–<60, 30–<45 and <30 mL/min/1.73 m2, respectively, in our cohort. Among patients who received SGLT2is, 764 (47.8%), 479 (30%), 215 (13.4%), 120 (7.5%) and 20 (1.3%) initiated treatment at a baseline eGFR >90, 60–90, 45–<60, 30–<45 and <30 mL/min/1.73 m2, respectively. Baseline characteristics of individuals with and without SGLT2is are shown in Table 1. Supplementary data, Tables S1 and S2 further present the baseline characteristics with an eGFR ≥45 mL/min/1.73 m2 and 15–44 mL/min/1.73 m2, respectively. Both groups had comparable uACRs, while patients with SGLT2is tended to be younger, more likely to receive RAS blockers and diuretics and have higher baseline eGFR and HbA1c.
Table 1.
Baseline characteristics of patients with and without SGLT2is
| Characteristics | Total | SGLT2is | No SGLT2is | P-value |
|---|---|---|---|---|
| Patients, n (%) | 4446 (100) | 1598 (35.9) | 2848 (64.1) | |
| Age (years), mean ± SD | 60.6 ± 13.5 | 56.0 ± 12.0 | 63.1 ± 13.5 | <.0001 |
| Gender (male), n (%) | 2328 (52.4) | 884 (55.3) | 1445 (50.7) | .056 |
| Race, n (%) | <.0001 | |||
| Chinese | 2565 (57.7) | 884 (55.3) | 1681 (59.0) | |
| Malay | 865 (19.5) | 341 (21.3) | 523 (18.4) | |
| Indian | 638 (14.4) | 259 (16.2) | 379 (13.3) | |
| Other | 378 (8.5) | 113 (7.1) | 265 (9.3) | |
| ACEi, n (%) | 1066 (24) | 516 (32.3) | 1082 (38.0) | <.0001 |
| ARB, n (%) | 1788 (40.2) | 814 (50.9) | 784 (27.5) | <.0001 |
| Diuretics, n (%) | 1143 (25.7) | 235 (14.7) | 908 (31.9) | <.0001 |
| eGFR at baseline (mL/min/1.73 m2), mean ± SD | 69.9 ± 31.7 | 83.9 ± 26.4 | 62.0 ± 31.8 | <.0001 |
| uACR at baseline (mg/mmol), median (IQR) | 6.8 (1.5–43.5) | 7.2 (1.4–51.5) | 5.9 (1.5–37.2) | .281 |
| HbA1c at baseline (%), mean ± SD | 8.3 ± 1.7 | 8.8 ± 1.5 | 8.1 ± 1.7 | <.0001 |
| CKD stages, n (%) | <.0001 | |||
| eGFR ≥45 mL/min/1.73 m2 | 3220 (724) | 1458 (91.2) | 1762 (61.9) | |
| eGFR 15–44 mL/min/1.73 m2 | 1226 (27.6) | 140 (8.8) | 1086 (38.1) |
ACEi, angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Dapagliflozin, empagliflozin and canagliflozin were prescribed in 730, 647 and 221 patients, respectively (Figure 1). The average daily dose was 6.1 mg for dapagliflozin (10 mg recommended dose), 17.8 mg for empagliflozin (25 mg recommended dose) and 225 mg for canagliflozin (300 mg recommended dose). More patients with an eGFR of 15–44 mL/min/1.73 m2 were prescribed empagliflozin (15%) than dapagliflozin (2.8%) and canagliflozin (10%) (see Supplementary data, Table S3 for baseline characteristics by different SGLT2is).
Effect on SGLT2is on CKD progression
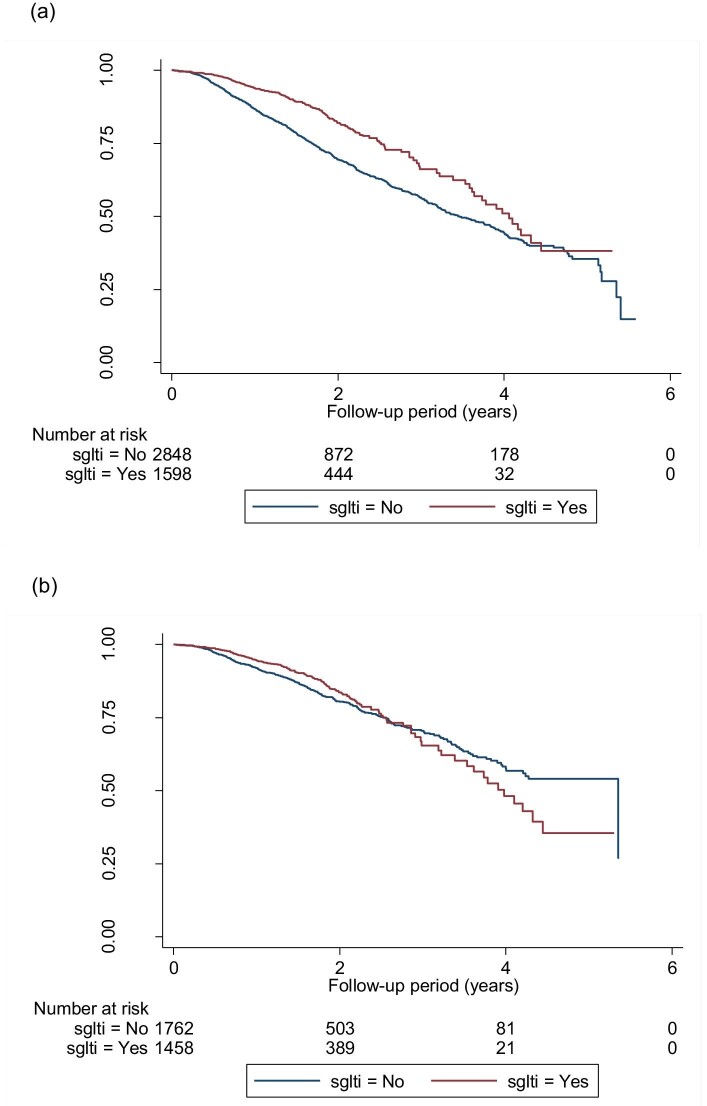
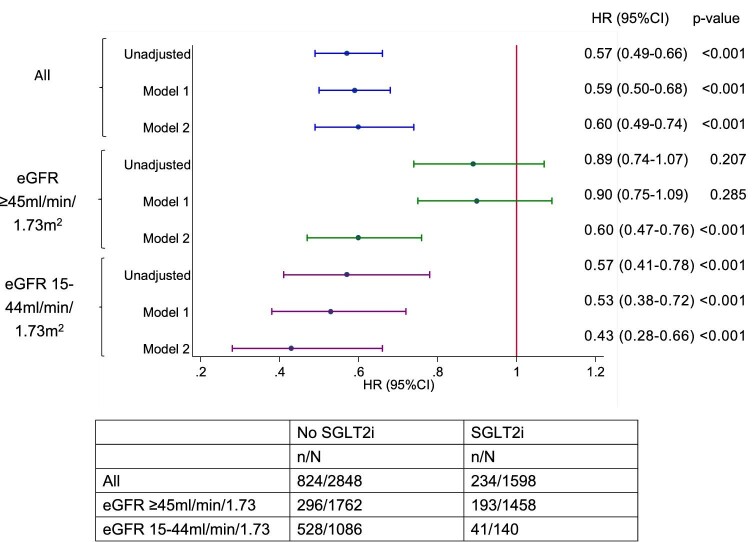
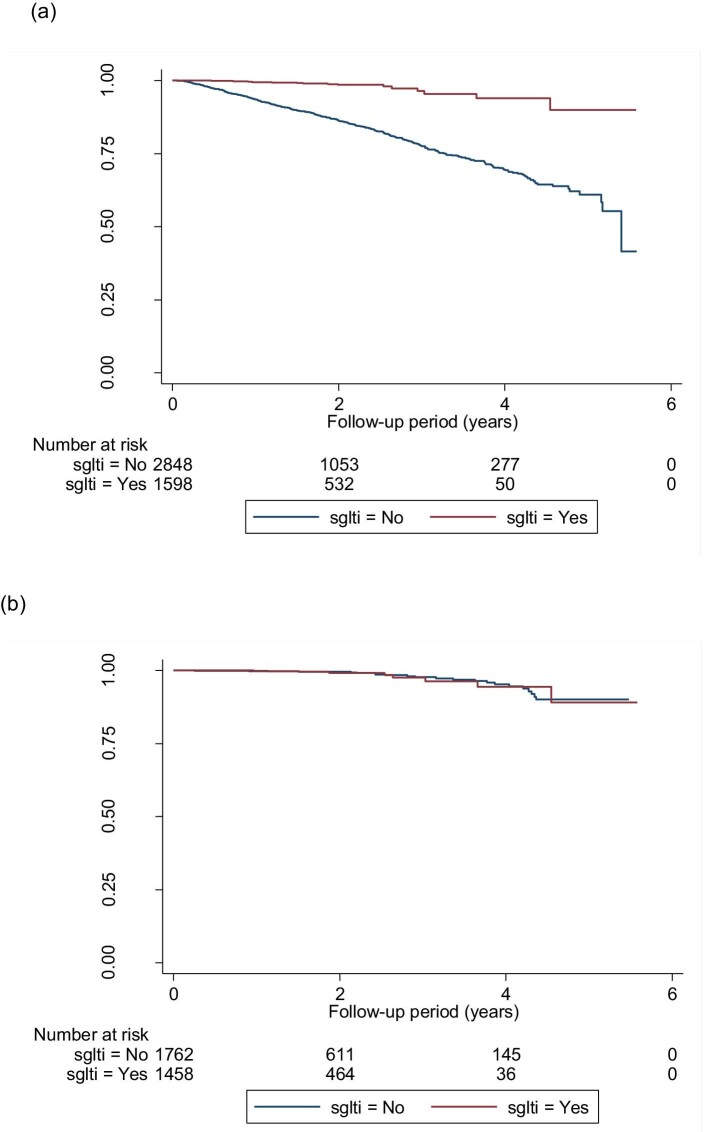
A total of 1058 (23.8%) patients had CKD progression and worsening of eGFR categories according to KDIGO guidelines. Figure 2a–c shows the Kaplan–Meier survival curves for CKD progression for all eGFR categories, eGFR ≥45 mL/min/1.73 m2 and eGFR 15–44 mL/min/1.73 m2 in both groups. Figure 3 shows the funnel plot for the hazard ratio (HR) of different models after the adjustment of variables. In model 2 (the fully adjusted model), Cox regression revealed a significant reduction in CKD progression {HR 0.60 [95% confidence interval (CI) 0.49–0.74]; P < .001} in the SGLT2i group. The HR for an eGFR ≥45 mL/min/1.73 m2 and eGFR 15–44 mL/min/1.73 m2 was 0.60 (95% CI 0.47–0.76; P < .001) and 0.43 (95% CI 0.23–0.66; P < .001), respectively.
FIGURE 2:
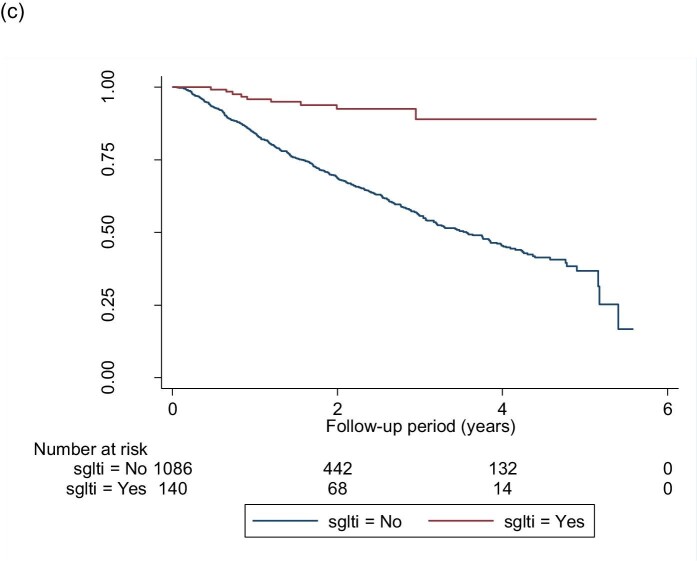
Kaplan–Meier survival curves for the event-free survival of CKD progression (defined by α ≥25% decrease in eGFR from baseline and worsening of eGFR categories according to KDIGO guidelines) in (a) the whole cohort, (b) patients with eGFR ≥45 mL/min/1.73 m2 and (c) patients with eGFR 15–44mL/min/1.73 m2. (a) Log-rank test statistics = 58.64, P < .001; (b) log-rank test statistics = 1.59, P = .207; (c) log-rank test statistics = 12.59, P = < .001.
FIGURE 3:
Funnel plot of the HR for CKD progression based on eGFR categories with different adjustment models. Model 1 adjusted for age, gender and ethnicity. Model 2 adjusted for age, gender, ethnicity, HbA1c, baseline eGFR, natural log-transformed uACR and use of RAS antagonists.
Our sensitivity analyses revealed a significant reduction in CKD regression with worsening albuminuria (uACR >3 mg/mmol) was incorporated into the CKD definition (Supplementary data, Table S4 for baseline characteristics). The HR for patients with and without CKD was 0.58 (95% CI 0.46–0.72; P < .001) and 0.60 (95%CI 0.38–0.96; P = .033), respectively (Supplementary data, Figures S1 and S9). The reduction in CKD progression was also shown in our cohort by categorizing albuminuria according to the KDIGO guidelines (Supplementary data, Table S5 for baseline characteristics). The HR for a uACR <3 mg/mmol, 3–30 mg/mmol an >30 mg/mmol was 0.56 (95% CI 0.39–0.81; P = .002), 0.62 (95% CI 0.46–0.84; P = .002) and 0.55 (95% CI 0.36–0.85; P = .008), respectively (Supplementary data, Figures S2 and S10). We further analysed the use of RAS blockers together with SGLT2is and the effects on CKD progression (Supplementary data, Table S6 for baseline characteristics). The HR for patients with and without the use of RAS blockers was 0.59 (95% CI 0.47–0.74; P < .001) and 0.59 (95% CI 0.37–0.93, P = .024), respectively (Supplementary data, Figures S3 and S11). Supplementary data, Figure S4a–c shows the event-free survival curve for CKD progression for all CKD stages, eGFR ≥45 mL/min/1.73 m2 and eGFR 15–44 mL/min/1.73 m2 for patients on different SGLT2is, respectively. In the fully adjusted model, all three SGLT2is significantly reduced the risk of CKD progression across all eGFR categories [dapagliflozin: HR 0.56 (95% CI 0.42–0.75), P < .001; empagliflozin: HR 0.70 (95% CI 0.55–0.89), P = .003; canagliflozin: HR 0.44 (95% CI 0.28–0.68), P < .001] (Supplementary data, Figure S12) and in eGFR ≥45 mL/min/1.73 m2 [dapagliflozin: HR 0.57 (95% CI 0.42–0.78), P < .001; empagliflozin: HR 0.69 (95% CI 0.51–0.91), P = .011; canagliflozin: HR 0.43 (95% CI 0.25–0.73), P = .002]. Only empagliflozin and canagliflozin showed a reduction in risk of CKD progression for patients with eGFR 15–44 mL/min/1.73 m2 [empagliflozin: HR 0.49 (95% CI 0.29–0.81), P = .006; canagliflozin: HR 0.34 (95% CI 0.15–0.75), P = .008] in this subgroup analysis.
Effect on SGLT2is on development of ESKD
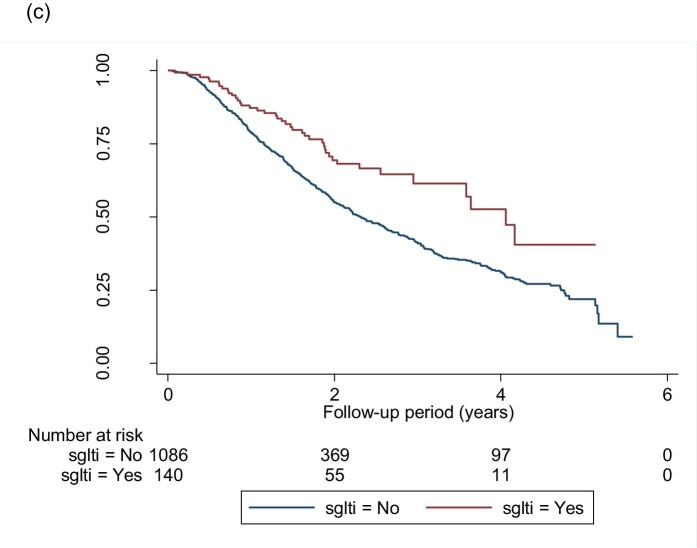
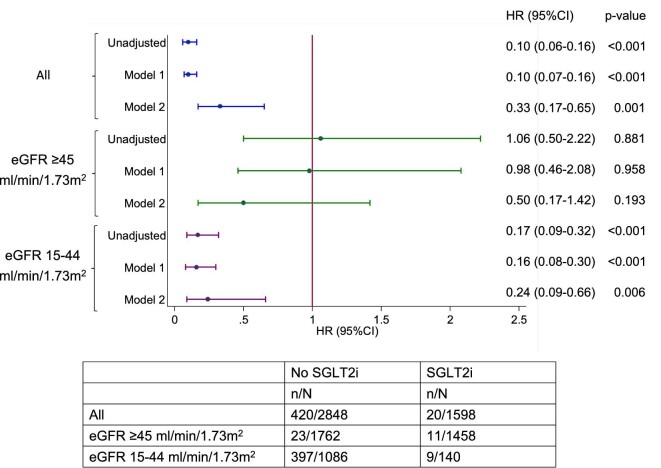
A total of 440 (9.9%) patients reached ESKD (sustained eGFR <15 mL/min/1.73 m2) during the study period, 20 (4.6%) of which received SGLT2i treatment. Figure 4a–c shows the Kaplan–Meier survival curves of ESKD. Figure 5 shows the funnel plot of the HR of different models after adjusting variables. In the fully adjusted model, the use of SGLT2i was associated with a lower hazard of ESKD in the entire cohort [HR 0.33 (95% CI 0.17–0.65), P = .001], with eGFR ≥45 mL/min/1.73 m2 [HR 0.50 (95% CI 0.17–1.42), P = .193] and with eGFR 15–44 mL/min/1.73 m2 [HR 0.24 (95% CI 0.09–0.66), P = .006].
FIGURE 4:
Kaplan–Meier survival curves for the event-free survival of ESKD in (a) the whole cohort, (b) patients with an eGFR ≥45 mL/min/1.73 m2 and (c) patients with an eGFR of 15–44 mL/min/1.73 m2. (a) Log-rank test statistics = 150.95, P < .001; (b) log-rank test statistics = 0.02, P = .881; (c) log-rank test statistics = 36.46, P < .001.
FIGURE 5:
Funnel plot of the HR for ESKD based on CKD categories with different adjustment models. Model 1 adjusted for age, gender and ethnicity. Model 2 adjusted for age, gender, ethnicity, HbA1c, baseline eGFR, natural log-transformed uACR and use of RAS antagonists.
Supplementary data, Figure S5 shows the Kaplan–Meier curves for event-free survival of ESKD and CKD status. In the fully adjusted model (Supplementary data, Figure S9) there was a significant reduction in the risk of ESKD in patients with CKD [HR 0.32 (95% CI 0.16–0.65), P = .002] but not in patients without [HR 2.59 (95% CI 0.11–63.36), P = .56]. When stratified by uACR status, patients receiving SGLT2is with a uACR 3–30 mg/mmol were associated with a significant reduction in the risk of ESKD [HR 0.27 (95% CI 0.10–0.77), P = .014] (Supplementary data, Figures S6 and S10). In the model adjusted for age, gender and ethnicity, the HR was 0.36 (95% CI 0.18–0.71, P = .003) and 0.10 (95% CI 0.02–0.39, P = .001) for RAS blocker users and non-users, respectively (Supplementary data, Figures S7 and S11).
In the fully adjusted model, only empagliflozin was shown to reduce the risk of ESKD in all eGFR categories [HR 0.12 (95% CI 0.03–0.49), P = .003] (see Supplementary data, Figure S12) and eGFR 15–44 mL/min/1.73 m2 [HR 0.10 (95% CI 0.02–0.71), P = .021. Supplementary data, Figure S8a–c shows the Kaplan–Meier curve for event-free survival of ESKD for all eGFR categories, eGFR ≥45 mL/min/1.73 m2 and eGFR 15–44 mL/min/1.73 m2 for patients on different SGLT2is.
DISCUSSION
Summary of findings
This study demonstrated sustained benefits to DKD patients on composite renal outcomes in patients receiving SGLT2is. Patients with advanced CKD (eGFR 15–44 mL/min/1.73 m2) with SGLT2is significantly reduced the risk of CKD progression and the development of ESKD. Moreover, SGLT2is attenuated the decline of eGFR over time for patients with an eGFR of 15–44 mL/min/1.73 m2. Compared with moderate to advanced CKD, the effects of SGLT2is in early CKD (eGFR ≥45 mL/min/1.73 m2) were less pronounced. Although all three SGLT2is reduced the risk of CKD progression, empagliflozin was more likely to exert sustained renoprotection from early to advanced CKD. Empagliflozin was also shown to minimize the risk of developing ESKD in our cohort, while there was inadequate power to conclude if other SGLT2is exerted similar effects. Note that the number of patients on empagliflozin among those with low eGFR in our cohort was >4-fold higher than the other two SGLT2is.
Our results from real-world practice revealed comparable risk reduction on CKD progression in RCTs with renal composite outcomes as primary [3, 16, 18] and secondary endpoints [9, 27, 28]. On the other hand, we found that empagliflozin had a lower risk of developing ESKD, contrary to the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG). Post hoc analysis did not show a significant risk reduction of ESKD as the renal composite outcome [HR 0.60 (95% CI 0.18–1.98)].
SGLT2is, CKD progression and ESKD
Meta-analyses demonstrated benefits on renal composite outcomes across different levels of eGFRs, although the proportional effects on reno protection might be attenuated with declining kidney function [29, 30]. However, there was a more significant reduction in the risk of CKD progression and ESKD in our patients with advanced CKD than in those at an earlier stage. We could argue that our study's number of hard events (ESKD) was small. However, the number of participants who developed ESKD in the EMPA-REG and the Canagliflozin Cardiovascular Assessment Study (CANVAS) were of comparable sizes (27 events in EMPA‐REG and 21 in CANVAS). Our cohort is from a region with one of the highest incidences of ESKD secondary to DKD. In this study, 9.9% of patients developed ESKD, compared with 0.37% in EMPA-REG and 0.2% in CANVAS. Therefore, the benefits of SGLT2is on renoprotection may be more profound in real-world practice when prescribed in a highly prevalent DKD population.
The consistency of results was found across other RCTs designed for renal composite outcomes as primary or secondary endpoints. In the CREDENCE trial, 30% of the cohort had an eGFR of 30–45 mL/min/1.73 m2. Canagliflozin reduced the renal-specific composite endpoints {ESKD, doubling of serum creatinine, or renal death [HR 0.71 (95% CI 0.53–0.94)] but not ESKD alone [HR 0.76 (95% CI 0.56–1.01]} [16]. In the DAPA-CKD trial, 59.1% of participants in the dapagliflozin arm had an eGFR of 15–44 mL/min/1.73 m2. The HR for composite primary outcomes (a sustained decline in the eGFR of ≥50%, ESKD, or death from renal or cardiovascular causes) for this group was 0.49 (95% CI 0.34–0.69) [18]. Further analysis on CKD stage 4 for the DAPA-CKD cohort, although not adequately powered, showed a statistically non-significant reduction in renal composite outcomes {≥50% sustained decline in eGFR, ESKD or death from kidney disease [HR 0.71 (95% CI 0.49–1.02)]} [31]. A recent meta-analysis including 98 284 patients showed 25 and 38 fewer per 1000 patients in 5 years for high (with CKD) and very high risk (with CKD and cardiovascular disease) groups in the development of ESKD [32]. In response to the findings from the CREDENCE and DAPA-CKD trials, canagliflozin and dapagliflozin were approved for prescription to CKD patients with an eGFR ≥30 mL/min/1.73 m2. With the upcoming completion of the EMPA-KIDNEY study in 2022–23, there will be a paradigm shift in treatment strategy for advanced CKD and DKD management. There will also be a foreseeable increase in prescription rates across different clinical care settings in the coming decade.
Prescribing SGLT2is in CKD patients
In our cohort, 35.9% of patients across all eGFR categories were prescribed SGLT2is. We had 140 (8.0%) patients who initiated SGLT2is at an eGFR of 15–44 mL/min/1.73 m2. There has been a general increasing trend of prescribing SGLT2is since they were approved by the US Food and Drug Administration in the mid-2010s. In one study on the prescription of anti-diabetic drugs in Austria between 2012 and 2018, SGLT2i use increased from 3.7 to 11.7% [33]. However, prescriptions for moderate to advanced CKD remained limited. Only 0.2% of patients with an eGFR ≤45 mL/min/1.73 m2 in the primary care settings of the UK were given SGLT inhibitors [34]. Early reports on the use of SGLT2is in advanced CKD raised concerns of safety issues, mainly when patients are dehydrated [35, 36]. With its renal function–dependent anti-hyperglycaemic effect, more focus was on weight reduction and blood pressure control and the glucose-lowering effect lessened with lower eGFR [11, 37]. We did not present safety and adverse events data, as only information from outpatient clinics was collected. The DAPA-CKD revealed a similar safety profile between treatment and control groups [18]. Therefore, further pursuing the use of SGLT2is in patients with moderate to advanced CKD is necessary, with benefits outweighing the risks.
Evidence of real-world studies on renal outcomes
The first real-world study on SGLT2is using renal composite endpoints as the outcome was published in 2019 [38]. Kidney outcomes associated with using SGLT2is in real-world clinical practice (CVD-REAL 3) was studied in a multinational study with outcome measures defined by the rate of eGFR decline, 50% eGFR decline or ESKD. With 35 561 episodes of treatment with SGLT2is (57% of which used dapagliflozin), there was an eGFR decrease per year of 1.53 mL/min/1.73 m2 (95% CI 1.34–1.72) and an HR of 0.49 (95% CI 0.35–0.67) for a 50% eGFR decrease or ESKD. The mean eGFR of patients in this observational study was 90.7 mL/min/1.73 m2. Another study used the Scandinavian registry (2013–18) to compare SGLT2is versus dipeptidyl peptidase-4 inhibitors on serious renal events (RRT, death from renal causes and hospital admission for renal events) [39]. There was a reduction in these events with patients using SGLT2is [2.6 events per 1000 person-years versus 6.2 events per 1000 person-years; HR 0.42 (95% CI 0.34–0.53)]. Notably, the baseline eGFR was not mentioned in this study. Both studies used retrospective data until 2018, before the results of the CREDENCE and DAPA-CKD trials were published, so patients with advanced CKD were unlikely to be recruited in their cohort. Therefore our real-world study is the first to include patients with moderate to advanced CKD to evaluate the risk of CKD progression and the development of ESKD in a highly prevalent DKD population. Moreover, this study provides a more comprehensive view of how SGLT2is could alleviate the renal disease burden in the community. It also offers crucial information to policymakers on implementing public health measures and reimbursements on using SGLT2is from tertiary to primary care.
Limitations and caveats
This study has several limitations. First, this was a single-centre study on a multi-ethnic Asian T2DM population. We should interpret the results with caution, particularly their generalizability to other ethnic groups. Second, the doses of SGLT2is prescribed to patients varied across the nephrology and endocrinology teams. In general, for patients with lower eGFR, the doses prescribed were 25–50% lower than the recommended dose from RCTs, primarily due to concerns with adverse events. Despite reducing doses, the benefits accrued from using SGLT2is remained strong. Third, we could not avoid indication and time-related biases where those patients prescribed SGLT2is tend to have better predefined outcomes [40, 41]. The systematic differences in baseline characteristics between treatment-exposed and unexposed groups departed from what we usually observed in RCTs. We do not know if patients adhered to the assigned treatment or received medications from other healthcare institutions, leading to misclassification of drug exposure [42]. Essentially we commenced the prescription of SGLT2is in early 2016. This study cohort thus encompasses all patients followed up in our clinics, minimizing selection bias. Fourth, we could not completely rule out unmeasured or unknown confounding factors with the intrinsic fallacy of design in observational studies. Fifth, information on adverse events relating to SGLT2i use in this cohort was not collected. Nonetheless, routine practice of the clinics prescribing SGLT2is is to review patients within 2–4 weeks of SGLT2i initiation to detect any adverse events and determine continuation or discontinuation of treatment.
CONCLUSIONS
This real-world study demonstrates the benefits of SGLT2is on CKD progression and ESKD in a population with a high prevalence of DKD. The findings of risk reduction on these renal composite outcomes from RCTs are shown in this study to be extrapolated into clinical practice, particularly in moderate to advanced CKD.
Supplementary Material
ACKNOWLEDGEMENTS
The first and corresponding author is supported by the Singapore Ministry of Health's National Medical Research Council under its Clinician Investigator Salary Support Program.
Contributor Information
Allen Yan Lun Liu, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Serena Low, Clinical Research Unit, Khoo Teck Puat Hospital, Singapore.
Ester Yeoh, Diabetes Centre, Admiralty Medical Centre, Kampung Admiralty, Singapore.
Eng Kuang Lim, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Claude Jeffrey Renaud, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Selene Tse Yen Teoh, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Grace Feng Ling Tan, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Chung Cheen Chai, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Bo Liu, Department of General Medicine, Khoo Teck Puat Hospital, Singapore.
Tavintharan Subramaniam, Diabetes Centre, Admiralty Medical Centre, Kampung Admiralty, Singapore.
Chee Fang Sum, Diabetes Centre, Admiralty Medical Centre, Kampung Admiralty, Singapore.
Su Chi Lim, Clinical Research Unit, Khoo Teck Puat Hospital, Singapore; Diabetes Centre, Admiralty Medical Centre, Kampung Admiralty, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease , 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Low SK, Sum CF, Yeoh LYet al. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Ann Acad Med Singap 2015; 44: 164–171 [PubMed] [Google Scholar]
- 3. Wanner C, Inzucchi SE, Lachin JMet al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 4. Giorgino F, Vora J, Fenici Pet al. Renoprotection with SGLT2 inhibitors in type 2 diabetes over a spectrum of cardiovascular and renal risk. Cardiovasc Diabetol 2020; 19: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JMet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MPet al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KWet al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 8. Cannon CP, Pratley R, Dagogo-Jack Set al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–1435 [DOI] [PubMed] [Google Scholar]
- 9. Kadowaki T, Nangaku M, Hantel Set al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME® trial. J Diabetes Investig 2019; 10: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larmour K, Levin A. Slowing progression in CKD: DAPA CKD and beyond. Clin J Am Soc Nephrol 2021; 16: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park HS, Jung YJ, Lee DYet al. Use of dapagliflozin in patients with advanced diabetic kidney disease. Kidney Res Clin Pract 2018; 37: 292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grubić Rotkvić P, Cigrovski Berković M, Bulj Net al. Sodium-glucose co-transporter 2 inhibitors' mechanisms of action in heart failure. World J Diabetes 2020; 11: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324 [DOI] [PubMed] [Google Scholar]
- 14. Yaribeygi H, Butler AE, Atkin SLet al. Sodium-glucose co-transporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J Cell Physiol 2018; 234: 223–230 [DOI] [PubMed] [Google Scholar]
- 15. Chewcharat A, Takkavatakarn K, Isaranuwatchai Set al. Pleiotropic effects of anti-diabetic agents on renal and cardiovascular outcomes: a meta-analysis of randomised controlled trials. Int Urol Nephrol 2020; 52: 1733–1745 [DOI] [PubMed] [Google Scholar]
- 16. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 17. Tonneijck L, Muskiet MHA, Smits MMet al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017; 28: 1023–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 19. Herrington WG, Preiss D, Haynes Ret al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shao SC, Lin YH, Chang KCet al. Sodium glucose co-transporter 2 inhibitors and cardiovascular event protections: how applicable are clinical trials and observational studies to real-world patients? BMJ Open Diabetes Res Care 2019; 7: e000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khoo CM, Deerochanawong C, Chan SPet al. Use of sodium-glucose co-transporter-2 inhibitors in Asian patients with type 2 diabetes and kidney disease: an Asian perspective and expert recommendations. Diabetes Obes Metab 2021; 23: 299–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830 [DOI] [PubMed] [Google Scholar]
- 23. Low S, Lim SC, Wang Jet al. Long-term outcomes of patients with type 2 diabetes attending a multidisciplinary diabetes kidney disease clinic. J Diabetes 2018; 10: 572–580 [DOI] [PubMed] [Google Scholar]
- 24. Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl 2013; 3: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Low S, Lim SC, Zhang Xet al. Development and validation of a predictive model for chronic kidney disease progression in type 2 diabetes mellitus based on a 13-year study in Singapore. Diabetes Res Clin Pract 2017; 123: 49–54 [DOI] [PubMed] [Google Scholar]
- 27. Jardine MJ, Zhou Z, Mahaffey KWet al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol 2020; 31: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mosenzon O, Wiviott SD, Cahn Aet al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617 [DOI] [PubMed] [Google Scholar]
- 29. Neuen BL, Young T, Heerspink HJLet al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7: 845–854 [DOI] [PubMed] [Google Scholar]
- 30. Zheng C, Lin M, Chen Yet al. Effects of sodium-glucose co-transporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomised controlled trials. Cardiovasc Diabetol 2021; 20: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chertow G, Vart P, Jongs Net al. Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol 2021; 32: 2352–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer SC, Tendal B, Mustafa RAet al. Sodium-glucose co-transporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021; 372: m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engler C, Leo M, Pfeifer Bet al. Long-term trends in the prescription of anti-diabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012–2018. BMJ Open Diabetes Res Care 2020; 8: e001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinton W, Feher MD, Munro Net al. Prescribing sodium-glucose co-transporter-2 inhibitors for type 2 diabetes in primary care: influence of renal function and heart failure diagnosis. Cardiovasc Diabetol 2021; 20: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heerspink HJ, Perkins BA, Fitchett DHet al. Sodium glucose cotransporter 2 inhibitor in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772 [DOI] [PubMed] [Google Scholar]
- 36. Yale JF, Bakris G, Cariou Bet al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohan DE, Fioretto P, Tang Wet al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heerspink HJL, Karasik A, Thuresson Met al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol 2020; 8: 27–35 [DOI] [PubMed] [Google Scholar]
- 39. Pasternak B, Wintzell V, Melbye Met al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. BMJ 2020; 369: m1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care 2018; 41: 6–10 [DOI] [PubMed] [Google Scholar]
- 41. Krogh J, Hjorthøj C, Kristensen SLet al. The effect of sodium-glucose transport protein 2 inhibitors on mortality and heart failure in randomised trials versus observational studies. Diabet Med 2021; 38: e14600. [DOI] [PubMed] [Google Scholar]
- 42. Hempenius M, Groenwold RHH, de Boer Aet al. Drug exposure misclassification in pharmacoepidemiology: sources and relative impact. Pharmacoepidemiol Drug Saf 2021; 30: 1703–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.