ABSTRACT
Objectives
To assess mortality and cardiovascular and renal outcomes among patients with chronic kidney disease (CKD) (primary objective), with a particular focus on heart failure (HF) risk following diagnosis of CKD (secondary objective) in Spain.
Methods
We conducted an observational study comprising cross-sectional and longitudinal retrospective analyses using secondary data from electronic health records. For the primary objective, adults with prevalent CKD [estimated glomerular filtration rate (eGFR) <60 or ≥60 mL/min/1.73 m2 with a urine albumin:creatinine ratio (UACR) ≥30 mg/g at the index date (1 January 2017)] were included. For the secondary objective, adults with incident CKD in 2017 were enrolled.
Results
In the prevalent population, 46 786 patients with CKD without HF [75.8 ± 14.4 years, eGFR 51.4 ± 10.1 mL/min/1.73 m2; 75.1% on renin–angiotensin system inhibitors (RASis)] and 8391 with CKD and HF (79.4 ± 10.9 years, eGFR 46.4 ± 9.8 mL/min/1.73 m2) were included. In the prevalent population, the risk of all-cause death {hazard ratio [HR] 1.107 [95% confidence interval (CI) 1.064–1.153]}, HF hospitalization [HR 1.439 (95% CI 1.387–1.493)] and UACR progression [HR 1.323 (95% CI 1.182–1.481)] was greater in those patients with CKD and HF versus CKD only. For the incident population, 1594 patients with CKD without HF and 727 with CKD and HF were included. Within 24 months from the CKD diagnosis (with/without HF at baseline), 6.5% of patients developed their first HF hospitalization. Although 60.7% were taking RASis, only 3.4% were at maximal doses and among diabetics, 1.3% were taking sodium-glucose cotransporter-2 inhibitors.
Conclusions
The presence of HF among CKD patients markedly increases the risk of outcomes. CKD patients have a high risk of HF, which could be partially related to insufficient treatment.
Keywords: cardiovascular, chronic kidney disease, death, renal, SGLT2 inhibitors
INTRODUCTION
Chronic kidney disease (CKD) is very common in clinical practice, accounting for ∼850 million persons with this condition around the world [1, 2]. Importantly, CKD substantially increases the risk of cardiovascular and all-cause death as well as the risk of developing cardiovascular and renal complications, including heart failure (HF), ischaemic heart disease and end-stage renal disease (ESRD) [2, 3]. In fact, CKD is expected to become the second leading cause of death before the end of the century in Spain [4]. Of note, the risk of adverse outcomes increases with renal function impairment and the presence of albuminuria progress [5]. In addition, CKD is associated with substantial healthcare costs, with cardiovascular hospitalizations being the main determinant [6, 7].
Although it is expected that the prevalence of CKD will rise in the coming years due to the ageing of the population as well as the increase in the prevalence of some related conditions such as hypertension, diabetes and chronic cardiovascular disease [1, 2], some authors have suggested that the use of cardioprotective and nephroprotective agents, such as renin–angiotensin system inhibitors (RASis) could decrease these numbers [8–11]. In addition, it has been demonstrated that the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitors with proven renal benefits might change the prognosis of patients with CKD. In this line, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that in patients with type 2 diabetes mellitus (T2D), CKD and albuminuria, treatment with canagliflozin co-administered with RAS blockade significantly reduced the risk of kidney failure and cardiovascular events [12]. Furthermore, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial demonstrated that in patients with CKD and albuminuria, dapagliflozin reduced the risk of renal and cardiovascular complications, including cardiovascular mortality and HF hospitalizations, as well as the risk of all-cause mortality, regardless of the presence of T2D [13]. More recently, the Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial showed that in patients with T2D and CKD, with or without albuminuria, sotagliflozin reduced the risk of the composite of cardiovascular death, HF hospitalizations and urgent visits for HF compared with placebo, but with a higher risk of side effects [14].
As a result, it is important to determine the impact of cardiovascular and nephroprotective treatments on outcomes in patients with CKD. Unfortunately, current data are scarce [1–3] and more information is needed. The objective of this study was to assess all-cause mortality and cardiovascular and renal outcomes among patients with CKD (primary objective), with a particular focus on the risk of developing HF following diagnosis of CKD (secondary objective) in Spain.
MATERIALS AND METHODS
This was an observational cohort study comprising cross-sectional and longitudinal retrospective analyses using secondary data captured in electronic health records from seven Spanish regions, using the BIG-PAC database. The BIG-PAC database includes anonymized and dissociated data from 1.7 million non-selected persons from primary health centres and referral hospitals within the Spanish national health system, including information from routine practice, without requiring manual input. Previous studies have demonstrated the representativeness of the Spanish population’s clinical profile and management in the BIG-PAC database [15]. The database was approved by the Investigation Ethics Committee of Consorci Sanitari from Terrassa. No informed consent was provided, as this was a secondary data study and data were fully anonymized and dissociated from patients.
For the primary objective of the study, patients ≥18 years of age and with prevalent CKD, defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 by Chronic Kidney Disease Epidemiology Collaboration equation or ≥60 mL/min/1.73 m2 with a urine albumin:creatinine ratio (UACR) ≥30 mg/g at the index date (1 January 2017) (prevalent population) were included. For the secondary objective, patients ≥18 years of age with incident CKD, defined as at least one diagnosis of CKD or meeting the above eGFR or UACR definitions in 2017 were included (incident population). The index date was the first CKD diagnosis date in 2017. In both populations, at the index date, CKD stages were classified as follows: stage 1: eGFR ≥90 mL/min/1.73 m2 and UACR ≥30 mg/g or International Classification of Diseases, Tenth Revision (ICD-10) code N18.1; stage 2 (mild): eGFR 60–89 mL/min/1.73 m2 and UACR ≥30 mg/g or ICD-10 code N18.2; stage 3a (mild–moderate): eGFR 45–59 mL/min/1.73 m2 or ICD-10 code N18.3; stage 3b (moderate–severe): eGFR 30–44 mL/min/1.73 m2 or ICD-10 code N18.3; stage 4 (severe): eGFR 15–29 mL/min/1.73 m2 or ICD-10 code N18.4; stage 5 (kidney failure): eGFR <15 mL/min/1.73 m2 or ICD-10 code N18.1 or dialysis or ICD-10 code Z49 or Z99.2; CKD unspecified: no eGFR data available and ICD-10 code N18.9. In addition, to be included in the study, patients should have been registered on the database at least 12 months before the initiation of the study period, should have been included in the prescription programme (with verified records of daily doses, interdose intervals and treatment duration for each drug and at least two prescriptions during the follow-up period) and also should be traceable through certified regular follow-up (at least two recorded consultations in the electronic records). In contrast, patients who had moved or were located outside the included healthcare areas or were permanent elderly care home residents were excluded from the study.
In the prevalent population, all baseline characteristics, including biodemographic data (age, sex), physical examination data (blood pressure, body mass index), laboratory data [haemoglobin A1c (HbA1c), eGFR, UACR, serum potassium, left ventricular ejection fraction], comorbidities [stroke, myocardial infarction, peripheral artery disease, atrial fibrillation, HF with reduced ejection fraction (left ventricular ejection fraction ≤40%) and HF with preserved ejection fraction (left ventricular ejection fraction >40%), CKD stages, T2D, hyperkalaemia (defined as serum potassium >5.5 mmol/L)] and medications (antihypertensives, antidiabetic, statins, digoxin, anticoagulants and antiplatelets) were described in relation to the index date (1 January 2017). Diagnostic codes are presented in Supplementary data, Table S1. Maximal doses of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) were defined as ‘maximal labelled daily doses’. In the incident population, all baseline characteristics, including comorbidities and medications were described in relation to the index date, defined as the first CKD diagnosis date in 2017. Data were presented according to diabetes status and CKD stage.
With regard to the primary objective (prevalent population), for all-cause mortality, patient follow-up began on the index date (1 January 2017) and continued until the death date or censored at the earliest of the end of enrolment for the latest available linked data or observational study period end date (i.e. 31 December 2019). For other outcomes, patient follow-up began on the index date and continued until the specified cardiorenal event (hospitalization for HF, CKD, or acute kidney failure or albuminuria transitions measured by UACR) occurred or was censored at the earliest of the end of enrolment for the latest available linked data, death date or observational study period end date (i.e. 31 December 2019). Within each event category, except for albuminuria transitions, patients were censored after the first event for the category but not for events from other categories. CKD outcome referred to the first hospitalization secondary to CKD after the index date. For albuminuria transitions, all events for each patient were considered without censoring. Therefore the same patient could be listed in several categories. For the secondary objective of the study, patients were followed for 24 months from CKD diagnosis (by diagnostic code or laboratory criteria) in 2017.
Statistical analysis
Categorical variables were described by their absolute (n) and relative frequencies (%) and continuous variables by the mean and standard deviation. Event rates were calculated as the number of new cases from the index date in the 24 months of follow-up divided by the total time at risk of the event. Event rates were presented as events and events per 100 patient-years for all-cause death, HF, CKD and albuminuria. Time to first hospitalization due to the event was analysed descriptively. Follow-up was censored at the observation period or study end unless an event occurred. The corresponding adjusted hazard ratios (HRs) and 95% confidence intervals (CIa) to estimate the risk of outcomes in the prevalent population after 3 years of follow-up were calculated. In contrast, the pathway to developing HF in patients with CKD and its types was evaluated for 24 months from the index. Categorical variables were compared with the chi-squared test or the Fisher's exact test when appropriate. When two means were compared, the Student's t-test was used. A level of statistical significance of 0.05 was applied in all the statistical tests. The data were analysed using SPSS version 25.0 (IBM, Armonk, NY, USA).
RESULTS
For the prevalent population, a total of 46 786 patients with CKD without HF and 8391 patients with CKD and HF were included (Supplementary data, Figure S1). The clinical characteristics and treatments in the prevalent CKD population at baseline are described in Table 1. Among patients with CKD without HF, the mean age was 75.8 ± 14.4 years, 52.4% were men, mean eGFR was 51.4 ± 10.1 mL/min/1.73 m2, 47.5% of patients had T2D and 12.5% had previous myocardial infarction. Regarding treatments, 89.5% were taking antihypertensive drugs, particularly RASis (75.1%). Among patients with T2D, the most common antidiabetic drugs prescribed were metformin (54.5%) and dipeptidyl peptidase 4 (DPP4) inhibitors (31.5%). When compared with the CKD population, those patients with CKD and HF were older and had higher systolic blood pressure, HbA1c and UACR. In contrast, eGFR was lower in this population. In addition, all comorbidities, such as T2D, stroke, myocardial infarction, peripheral artery disease and atrial fibrillation, were more frequent in those patients with CKD and HF.
Table 1.
Clinical characteristics and treatments in the prevalent CKD population at the index date
| Characteristics | Only CKD [n = 46.786 (84.8%)] | HF and CKD [n = 8.391 (15.2%)] | P-value (HF and CKD versus CKD) |
|---|---|---|---|
| Biodemographic data | |||
| Age (years) | 75.8 ± 14.4 | 79.4 ± 10.9 | <0.001 |
| Gender (male), n (%) | 24 493 (52.4) | 4 237 (50.5) | <0.001 |
| Physical examination | |||
| Systolic blood pressure (mmHg) | 131.3 ± 19.2 | 133.9 ± 20.5 | <0.001 |
| Diastolic blood pressure (mmHg) | 84.6 ± 7.3 | 83.7 ± 6.9 | <0.001 |
| BMI (kg/m2) BMI >30 kg/m2, n (%) |
28.2 ± 4.9 10 941 (23.4) |
28.9 ± 5.2 2013 (24.0) |
<0.001 0.332 |
| Laboratory data | |||
| HbA1c (%) <7%, n (%) 7–<8%, n (%) 8–<9%, n (%) ≥9%, n (%) |
7.0 ± 1.9 24 256 (51.8) 7733 (16.5) 4056 (8.7) 3439 (7.4) |
7.7 ± 2.0 4256 (50.7) 1680 (20.0) 867 (10.3) 769 (9.2) |
<0.001 0.177 <0.001 <0.001 <0.001 |
| eGFR (mL/min/1.73 m2) | 51.4 ± 10.1 | 46.4 ± 9.8 | <0.001 |
| UACR (mg/g) | 329.1 ± 145.3 | 361.2 ± 148.5 | <0.001 |
| Median (25th–75th percentile) | 276.4 (159.7–384.9) | 280.0 (130.3–444.1) | |
| <30 mg/g (stage 1), n (%) | 255 (0.5) | 366 (4.4) | <0.001 |
| 30–300 mg/g (stage 2), n (%) | 29 234 (62.5) | 4077 (48.6) | <0.001 |
| >300 mg/g (stage 3), n (%) | 17 297 (37.0) | 3948 (47.1) | <0.001 |
| Serum potassium (mmol/L) | 5.2 ± 1.5 | 5.7 ± 1.6 | <0.001 |
| Left ventricular ejection fraction (%) | – | 43.4 ± 10.1 | – |
| Comorbidities, n (%) | |||
| CVD | 14 578 (31.2) | 5180 (61.7) | <0.001 |
| Stroke | 3480 (7.4) | 1030 (12.3) | <0.001 |
| Myocardial infarction | 5861 (12.5) | 2154 (25.7) | <0.001 |
| PAD | 2303 (4.9) | 564 (6.7) | <0.001 |
| Atrial fibrillation | 5630 (12.0) | 2970 (35.4) | <0.001 |
| HF | – | 8391 (100) | – |
| HFrEF | – | 4465 (53.2) | – |
| HFpEF | – | 3926 (46.8) | – |
| CKD | 46 786 (100) | 8391 (100) | 0.908 |
| Stage 1 | 1370 (2.9) | 977 (11.6) | <0.001 |
| Stage 2 | 8403 (18.0) | 1584 (18.9) | 0.192 |
| Stage 3a | 15 578 (33.3) | 1753 (20.9) | <0.001 |
| Stage 3b | 12 266 (26.2) | 1961 (23.4) | <0.001 |
| Stage 4 | 3389 (7.2) | 1127 (13.4) | <0.001 |
| Stage 5 | 2433 (5.2) | 296 (3.5) | <0.001 |
| T2D | 22 229 (47.5) | 5034 (60.0) | <0.001 |
| Hyperkalaemia | 15 252 (32.6) | 3104 (37.0) | <0.001 |
| Medications, n (%) | |||
| CVD risk treatment | 38 116 (81.5) | 8391 (100) | - |
| Antihypertensives | 41 869 (89.5) | 7960 (94.9) | <0.001 |
| ACEi | 15 754 (33.7) | 2716 (32.4) | <0.001 |
| ARB | 19 377 (41.4) | 3548 (42.3) | 0.246 |
| ARNI | 0 | 743 (8.9) | <0.001 |
| Beta-blocker | 22 854 (48.8) | 5998 (71.5) | <0.001 |
| Loop diuretic | 22 499 (48.1) | 5978 (71.2) | <0.001 |
| Aldosterone antagonist | 3133 (6.7) | 2781 (33.1) | <0.001 |
| Calcium channel blocker | 2536 (5.4) | 658 (7.8) | <0.001 |
| Thiazide diuretic | 15 018 (32.1) | 3037 (36.2) | <0.001 |
| Antidiabetics | 17 456 (37.3) | 3571 (42.6) | <0.001 |
| Metformin | 12 806 (27.4) | 2021 (24.1) | <0.001 |
| Sulfonylurea | 3799 (8.1) | 969 (11.5) | <0.001 |
| DPP4 inhibitor | 5025 (10.7) | 962 (11.5) | 0.285 |
| SGLT2 inhibitor | 1700 (3.6) | 333 (4.0) | 0.75 |
| GLP-1 receptor agonist | 1108 (2.4) | 221 (2.6) | 0.786 |
| Metiglinides | 1401 (3.0) | 375 (4.5) | <0.001 |
| Thiazolidinediones | 115 (0.2) | 27 (0.3) | 0.962 |
| Acarbose | 98 (0.2) | 20 (0.2) | 0.711 |
| Insulin | 5194 (11.1) | 1255 (15.0) | <0.001 |
| Statins | 20 714 (44.3) | 5327 (63.5) | <0.001 |
| Digoxin | 378 (0.8) | 524 (6.2) | <0.001 |
| Warfarin/acenocoumarola | 4176 (8.9) | 1887 (22.5) | <0.001 |
| Low-dose aspirin | 11 245 (24.0) | 2518 (30.0) | <0.001 |
| Receptor P2Y12 antagonist | 3459 (7.4) | 880 (10.5) | <0.001 |
Values presented as mean ± standard deviation unless stated otherwise. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibition; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; PAD, peripheral artery disease; SBP, systolic blood pressure; hyperkalaemia, serum potassium >5.5 mmol/L.
aUse of direct oral anticoagulants was not collected.
In Table 2 and in Supplementary data, Figures S2–S10, the risk of outcomes [all-cause mortality and first hospitalization for cardiorenal events (HF, CKD, acute kidney failure) or albuminuria transitions during follow-up] between CKD compared with CKD and HF patients in the prevalent population after 3 years of follow-up is reported. The risk of all-cause death, hospitalization for HF and UACR progression from 30–300 to >300 mg/g was greater in those patients with CKD and HF compared with CKD-only patients.
Table 2.
Risk of outcomesa between CKD versus CKD and HF patients in the prevalent population after 3 years of follow-up
| Group | Endpoint | Follow-up (median, days) | Events, n | % | Event rates per 100 patient-years | HRb (CKD and HF versus CKD) |
95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| CKD and HF | All-cause death | 428 | 3132 | 37.3 | 17.1 | 1.107 | 1.064–1.153 | <.001 |
| CKD | 506 | 10 701 | 22.9 | 10.1 | ||||
| CKD and HF | Heart failure | 447 | 3994 | 47.6 | 21.7 | 1.439 | 1.387–1.493 | <.001 |
| CKD | 541 | 8293 | 17.7 | 7.1 | ||||
| CKD and HF | CKD | 545 | 2097 | 25.0 | 10.2 | 1.019 | 0.964–1.078 | .505 |
| CKD | 408 | 4114 | 8.8 | 3.6 | ||||
| CKD and HF | UACR Progression: <30 to 30–300 mg/g | 504 | 43 | 0.5 | 0.2 | 1.300 | 0.961–1.761 | .089 |
| CKD | 551 | 1865 | 4.0 | 1.6 | ||||
| CKD and HF | UACR Progression: 30–300 to >300 mg/g | 490 | 1158 | 13.8 | 5.5 | 1.323 | 1.182–1.481 | <.001 |
| CKD | 558 | 451 | 1.0 | 0.4 | ||||
| CKD and HF | UACR Regression: ≥30 to <30 mg/g | 522 | 85 | 1.0 | 0.4 | 1.147 | 0.854–1.538 | .363 |
| CKD | 601 | 109 | 0.2 | 0.1 | ||||
| CKD and HF | UACR Regression: ≥300 to <300 mg/g | 493 | 40 | 0.5 | 0.2 | 1.166 | 0.789–1.721 | .441 |
| CKD | 552 | 80 | 0.2 | 0.1 | ||||
| CKD and HF | Acute kidney failure (ICD-10 code N17) | 592 | 164 | 2.0 | 0.7 | 1.082 | 0.784–1.493 | .633 |
| CKD | 532 | 686 | 1.5 | 0.6 |
aAll-cause mortality and first hospitalization for cardiorenal events (HF, CKD, acute kidney failure) or albuminuria transitions during follow-up.
bHR was adjusted according to age, sex, eGFR and the number of associated clinical conditions.
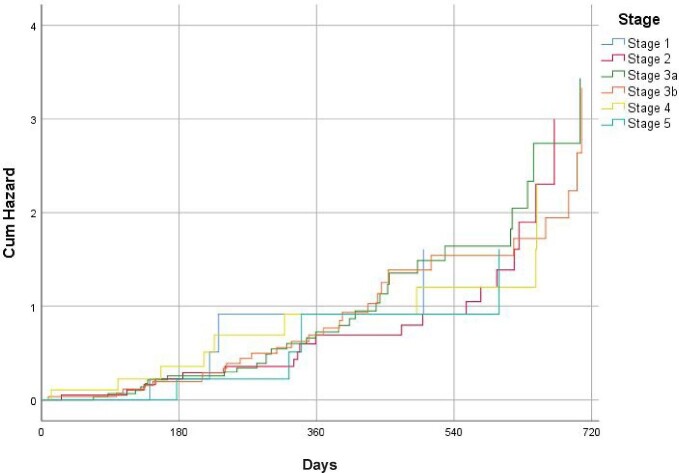
For the incident population, a total of 2321 patients with CKD (1594 without HF and 727 patients with CKD and HF) were included (Supplementary data, Figure S1). Clinical characteristics and treatments in the incident CKD population at baseline are presented in Table 3 and Supplementary data, Table S2. Overall, the mean age was 64.9 ± 23.4 years, 52.4% were men, mean eGFR was 60.6 ± 20.5 mL/min/1.73 m2 and mean UACR was 317.7 ± 168.4 mg/g. Although 60.7% of patients were taking RASis, only 3.4% of them were taking them at maximal doses. The use of RASis according to UACR and blood pressure levels according to treatment with RASis are reported in Supplementary data, Tables S3 and S4, respectively. Among diabetics, only 2.8% of patients were taking SGLT2 inhibitors and 2.5% were taking glucagon-like peptide-1 (GLP-1) receptor agonists. Age increased as renal function worsened, as well as comorbidities. Within 24 months from CKD diagnosis (with/without HF at baseline), 6.5% of patients developed their first HF hospitalization, regardless of renal function (Figure 1 and Supplementary data, Table S5). Among patients with CKD without HF at the index date, all-cause death, myocardial infarction, hospitalization for HF and stroke rates were 4.9, 3.4, 3.2 and 2.9 per 100 patient-years, respectively, after 24 months of follow-up (Table 4). Among patients with CKD with HF, all-cause death, myocardial infarction, hospitalization for HF and stroke rates were 5.0, 3.6, 3.2 and 3.0 per 100 patient-years, respectively, after 24 months of follow-up (Table 5). In both patients with and without HF, outcomes rates increased and the time to the first event decreased as renal function worsened (Tables 4 and 5).
Table 3.
Clinical characteristics and treatments in the incident CKD population at baseline in the overall population and according to T2D and HF status
| T2D status | HF status | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Non-T2D [n = 1216 (52.40%)] | T2D [n = 1105 (47.60%)] | P-value (T2D versus no T2D) | Non-HF [n = 1594 (68.7%)] | HF [n = 727 (31.3%)] | P-value (HF versus no HF) | Total [N = 2321 (100%)] |
| Biodemographic data | |||||||
| Age (years) | 65.0 ± 23.4 | 64.8 ± 23.5 | 0.837 | 63.8 ± 23.5 | 66.3 ± 23.2 | <0.001 | 64.9 ± 23.4 |
| Gender (male), n (%) | 614 (50.5) | 603 (54.6) | <0.001 | 832 (52.2) | 385 (53.0) | 0.720 | 1217 (52.4) |
| Physical examination | |||||||
| Systolic blood pressure (mmHg) | 129.8 ± 20.7 | 131.8 ± 19.3 | 0.016 | 129.7 ± 20.1 | 131.2 ± 19.6 | 0.093 | 130.8 ± 19.7 |
| Diastolic blood pressure (mmHg) | 83.9 ± 7 | 84.5 ± 7 | 0.039 | 84.2 ± 7.0 | 84.6 ± 7.1 | 0.204 | 84.3 ± 7 |
| BMI (kg/m2) | 29 ± 5 | 27.7 ± 5.1 | 0.001 | 27.8 ± 5.2 | 29.7 ± 5.2 | <0.001 | 28.7 ± 5 |
| Laboratory data | |||||||
| HbA1c (%) <7%, n (%) 7–<8%, n (%) 8–<9%, n (%) ≥9%, n (%) |
6.2 ± 1.8 834 (68.6) 10 (0.8) 3 (0.2) 2 (0.2) |
7.7 ± 1.8 401 (36.3) 344 (31.1) 215 (19.5) 145 (13.1) |
<0.001 <0.001 <0.001 <0.001 <0.001 |
6.8 ± 1.7 852 (53.5) 239 (15.0) 140 (8.8) 97 (6.1) |
7.0 ± 1.7 383 (52.7) 115 (15.8) 78 (10.7) 50 (6.9) |
0.001 0.720 0.619 0146 0.437 |
6.9 ± 1.7 1235 (53.2) 354 (15.3) 218 (9.4) 147 (6.3) |
| eGFR (mL/min/1.73 m2) | 60.7 ± 18.9 | 60.5 ± 20.2 | 0.436 | 60.6 ± 20.5 | |||
| UACR (mg/g) | 298.1 ± 155 | 381.9 ± 198.6 | <0.001 | 305.4 ± 169.2 | 330.1 ± 167.5 | 0.001 | 317.7 ± 168.4 |
| UACR (mg/g), median (IQR) | 261 (148.3–372.4) | 295.3 (142.3–438.6) | 255.6 (153.8–376.5) | ||||
| <30 mg/g (stage 1), n (%) | 7 (0.6) | 2 (0.2) | 0.971 | 6 (0.4) | 3 (0.4) | 0.999 | 9 (0.4) |
| 30–300 mg/g (stage 2), n (%) | 838 (68.9) | 585 (52.9) | <0.001 | 976 (58.3) | 447 (61.5) | 0.146 | 1423 (61.3) |
| >300 mg/g (stage 3), n (%) | 371 (30.5) | 518 (46.9) | <0.001 | 612 (35.7) | 277 (38.1) | 0.265 | 889 (38.3) |
| Serum potassium, (mmol/L) | 5.2 ± 1.5 | 5.3 ± 1.5 | 0.532 | 4.8 ± 1.7 | 5.3 ± 1.8 | <0.001 | 5.0 ± 1.4 |
| Left ventricular ejection fraction (%) | 44.1 ± 12.8 | 42.6 ± 12.4 | – | 43.5 ± 12.2 | – | 43.5 ± 12.2 | |
| Comorbidities, n (%) | |||||||
| CVD | 159 (13.1) | 220 (19.9) | <0.001 | 178 (11.2) | 201 (27.6) | <0.001 | 379 (16.3) |
| Stroke | 95 (7.8) | 111 (10.0) | 0.054 | 91 (5.7) | 115 (15.8) | <0.001 | 206 (8.9) |
| Myocardial infarction | 122 (10.0) | 162 (14.7) | <0.001 | 136 (8.5) | 148 (20.4) | <0.001 | 284 (12.2) |
| PAD | 43 (3.5) | 56 (5.1) | 0.081 | 64 (4.0) | 35 (4.8) | 0.376 | 99 (4.3) |
| Atrial Fibrillation | 164 (13.5) | 151 (13.7) | 0.97 | 151 (9.5) | 164 (22.6) | <0.001 | 315 (13.6) |
| Heart failure | 354 (29.1) | 373 (33.8) | <0.001 | 0 | 727 (100) | – | 727 (31.2) |
| HFrEF | 180 (14.8) | 192 (17.4) | <0.001 | 0 | 372 (51.2) | – | 372 (16.0) |
| HFpEF | 174 (14.3) | 181 (16.4) | 0.23 | 0 | 355 (48.8) | – | 355 (15.3) |
| T2D | 48 (3.9) | 1105 (100) | – | 783 (49.1) | 370 (50.9) | 0.421 | 1153 (49.7) |
| Hyperkalaemia (potassium >5.5 mmol/L) | 318 (26.6) | 299 (27.1) | 0.786 | 420 (26.3) | 197 (27.1) | 0.686 | 617 (26.6) |
| Medications, n (%) | |||||||
| Antihypertensive medication | 725 (59.6) | 827 (74.8) | <0.001 | 973 (61.0) | 579 (79.6) | <0.001 | 1552 (66.9) |
| RASi | 633 (52.1) | 775 (70.1) | <0.001 | 920 (57.7) | 488 (67.1) | <0.001 | 1408 (60.7) |
| ACEi | 311 (25.6) | 294 (26.6) | 0.309 | 383 (24.0) | 222 (30.5) | 0.002 | 605 (26.1) |
| ACEi at maximal dose | 15 (1.2) | 23 (2.1) | 0.623 | 24 (1.5) | 14 (1.9) | 0.367 | 38 (1.6) |
| ARB | 347 (28.5) | 516 (46.7) | <0.001 | 564 (35.4) | 299 (41.1) | 0008 | 863 (37.2) |
| ARB at maximal dose | 18 (1.5) | 23 (2.1) | 0.818 | 26 (1.6) | 15 (2.1) | 0.395 | 41 (1.8) |
| Aldosterone antagonist | 49 (4.0) | 67 (6.1) | 0.265 | 77 (4.8) | 39 (5.4) | 0.538 | 116 (5.0) |
| Direct renin inhibitor | 3 (0.2) | 1 (0.1) | 0.84 | 3 (0.2) | 1 (0.1) | 0.586 | 4 (0.2) |
| ARNI | 51 (4.2) | 67 (6.1) | 0.059 | 78 (4.9) | 40 (5.5) | 0.542 | 118 (5.1) |
| Beta-blocker | 342 (28.1) | 363 (32.9) | <0.001 | 456 (28.6) | 249 (34.3) | 0.001 | 705 (30.4) |
| Diuretics | 376 (30.9) | 397 (35.9) | <0.001 | 484 (30.4) | 289 (39.8) | 0.001 | 773 (33.3) |
| Thiazide diuretic | 257 (21.1) | 239 (21.6) | 0.769 | 332 (20.8) | 164 (22.6) | 0.224 | 496 (21.4) |
| Loop diuretic | 311 (25.6) | 365 (33.0) | <0.001 | 433 (27.2) | 243 (33.4) | 0.002 | 676 (29.1) |
| Potassium-sparing diuretic | 57 (4.7) | 68 (6.2) | 0.18 | 79 (5.0) | 46 (6.3) | 0.199 | 125 (5.4) |
| Calcium channel blocker | 301 (24.8) | 312 (28.2) | <0.001 | 384 (24.1) | 229 (31.5) | <0.001 | 613 (26.4) |
| Dihydropyridines | 289 (23.8) | 262 (23.7) | 0.963 | 345 (21.6) | 206 (28.3) | <0.001 | 551 (23.7) |
| Non-dihydropyridines | 22 (1.8) | 26 (2.4) | 0.982 | 30 (1.9) | 18 (2.5) | 0.348 | 48 (2.1) |
| Antidiabetics | 11 (0.9) | 957 (86.6) | <0.001 | 607 (38.1) | 361 (49.7) | <0.001 | 968 (41.7) |
| Metformin | 0 | 602 (54.5) | – | 385 (24.2) | 217 (29.8) | 0.004 | 602 (25.9) |
| Sulfonylurea | 0 | 108 (9.8) | – | 69 (4.3) | 39 (5.4) | 0.243 | 108 (4.7) |
| DPP4 inhibitor | 0 | 348 (31.5) | – | 225 (14.1) | 123 (16.9) | 0.079 | 348 (15.0) |
| SGLT2 inhibitor | 0 | 31 (2.8) | – | 20 (1.3) | 11 (1.5) | 0.699 | 31 (1.3) |
| GLP-1 receptor agonist | 0 | 28 (2.5) | – | 18 (1.1) | 10 (1.4) | 0.537 | 28 (1.2) |
| Metiglinides | 0 | 123 (11.1) | – | 81 (5.1) | 42 (5.8) | 0.486 | 123 (5.3) |
| Glitazones | 0 | 14 (1.3) | – | 9 (0.6) | 5 (0.7) | 0.778 | 14 (0.6) |
| Acarbose | 0 | 19 (1.7) | – | 12 (0.8) | 7 (1.0) | 0.629 | 19 (0.8) |
| Insulin | 11 (0.9) | 184 (16.7) | <0.001 | 123 (7.7) | 72 (9.9) | 0.076 | 195 (8.4) |
| Statins | 506 (41.6) | 491 (44.4) | <0.001 | 625 (39.2) | 372 (512) | <0.001 | 997 (43.0) |
| Warfarin/acenocoumarola | 131 (10.8) | 121 (11.0) | 0.992 | 161 (10.1) | 91 (12.5) | 0.085 | 252 (10.9) |
| Low-dose aspirin | 237 (19.5) | 274 (24.8) | <0.001 | 334 (21.0) | 177 (24.3) | 0.075 | 511 (22.0) |
| Receptor P2Y12 antagonist | 45 (3.7) | 75 (6.8) | <0.001 | 76 (4.8) | 44 (6.1) | 0.191 | 120 (5.2) |
Values presented as mean ± standard deviation unless stated otherwise. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor and neprilysin inhibition; BMI, body mass index; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; PAD, peripheral artery disease; SBP, systolic blood pressure; hyperkalaemia, serum potassium >5.5 mmol/L.
aUse of direct oral anticoagulants was not collected
FIGURE 1:
Kaplan–Meier survival curves for patients with/without HF at baseline who developed first HF hospitalization within 24 months from CKD diagnosis.
Table 4.
Event rates per 100 patient-years for CKD patients diagnosed in 2017 without HF at baseline and followed for 24 months
| Events | Stage 1 (n = 95) | Stage 2 (n = 335) | Stage 3a (n = 522) | Stage 3b (n = 402) | Stage 4 (n = 116) | Stage 5 (n = 49) | Unspecified (n = 75) | Total CKD (N = 1594) |
|---|---|---|---|---|---|---|---|---|
| All-cause death, n (event rate) Time to first event (days) |
7 (3.9) 516.0 |
26 (4.2) 482.2 |
44 (4.5) 450.7 |
36 (4.9) 405.6 |
11 (5.3) 369.1 |
5 (5.7) 343.3 |
6 (4.0) 484.2 |
135 (4.9) 429.2 |
| Heart failure, n (event rate) Time to first event (days) |
5 (2.6) 317.5 |
18 (2.8) 288.7 |
29 (3.0) 269.8 |
24 (3.3) 248.2 |
8 (3.5) 223.4 |
3 (3.8) 203.3 |
4 (2.6) 309.1 |
91 (3.2) 287.0 |
| CKD, n (event rate) Time to first event (days) |
3 (1.8) 479.1 |
12 (2.0) 447.7 |
20 (2.1) 426.4 |
18 (2.3) 396.6 |
5 (2.5) 356.9 |
3 (2.8) 324.8 |
3 (2.0) 463.4 |
64 (2.6) 394.8 |
| Myocardial infarction, n (event rate) Time to first event (days) |
5 (2.6) 423.3 |
17 (2.8) 403.1 |
28 (2.9) 366.5 |
23 (3.2) 337.1 |
7 (3.4) 316.9 |
3 (3.7) 288.4 |
4 (2.6) 396.7 |
87 (3.4) 336.2 |
| Stroke, n (event rate) Time to first event (days) |
4 (2.1) 390.7 |
14 (2.3) 368.6 |
24 (2.5) 338.2 |
21 (2.8) 314.5 |
7 (3.0) 292.5 |
3 (3.2) 272.0 |
3 (2.1) 364.5 |
76 (2.9) 363.6 |
| PAD, n (event rate) Time to first event (days) |
2 (1.1) 359.9 |
8 (1.2) 342.8 |
13 (1.3) 320.4 |
10 (1.4) 304.3 |
3 (1.6) 277.0 |
2 (1.7) 249.3 |
2 (1.2) 358.4 |
40 (1.3) 299.4 |
| Albuminuria (UACR ≥30 mg/g), n (event rate) Time to first event (days) |
2 (1.0) 521.5 |
7 (1.1) 492.0 |
11 (1.2) 455.6 |
10 (1.3) 410.0 |
3 (1.4) 389.5 |
1 (1.4) 350.6 |
1 (1.1) 496.6 |
35 (1.3) 429.8 |
| Albuminuria (UACR ≥300 mg/g), n (event rate) Time to first event (days) |
1 (0.5) 562.2 |
3 (0.5) 525.4 |
6 (0.6) 491.0 |
5 (0.6) 446.8 |
1 (0.6) 411.1 |
1 (0.7) 386.4 |
1 (0.5) 516.6 |
18 (0.7) 446.4 |
PAD, peripheral artery disease.
Table 5.
Event rates per 100 patient-years for CKD patients diagnosed in 2017 with HF at baseline and followed for 24 months
|
Events |
Stage 1 (n = 23) | Stage 2 (n = 98) | Stage 3a (n = 242) | Stage 3b (n = 194) | Stage 4 (n = 66) | Stage 5 (n = 36) | Unspecified (n = 68) | Total CKD (N = 727) | P-value (Total CKD + HF versus total CKD without HF) |
Total CKD with and without HF in 2017 (N = 2321) |
|---|---|---|---|---|---|---|---|---|---|---|
| All-cause death, n (event rate) Time to first event (days) |
2 (4.2) 500.5 |
9 (4.7) 477.4 |
22 (4.9) 428.2 |
18 (5.2) 373.2 |
6 (5.4) 339.6 |
4 (5.8) 315.8 |
5 (4.3) 440.6 |
66 (5.0) 424.9 |
0.889 0.647 |
201 (5.0) 423.6 |
| Heart failure, n (event rate) Time to first event (days) |
1 (2.6) 304.8 |
5 (3.0) 282.9 |
14 (3.2) 256.3 |
13 (3.6) 223.4 |
5 (3.9) 221.1 |
3 (4.1) 189.0 |
3 (2.7) 296.8 |
44 (3.2) 278.4 |
0.999 0.078 |
135 (3.2) 272.9 |
| CKD, n (event rate) Time to first event (days) |
1 (1.9) 445.5 |
4 (2.1) 411.9 |
9 (2.1) 396.6 |
9 (2.5) 384.7 |
3 (2.7) 353.3 |
2 (2.9) 308.5 |
3 (2.1) 421.7 |
31 (2.7) 386.9 |
0.772 0.356 |
95 (2.5) 396.3 |
| Myocardial infarction, n (event rate) Time to first event (days) |
1 (2.7) 419.0 |
5 (2.9) 383.0 |
14 (3.0) 362.8 |
12 (3.4) 330.4 |
4 (3.7) 307.4 |
3 (4.0) 268.2 |
3 (2.6) 380.8 |
42 (3.6) 319.4 |
0.671 0.013 |
129 (3.6) 327.6 |
| Stroke, n (event rate) Time to first event (days) |
1 (2.1) 351.6 |
4 (2.5) 335.4 |
12 (2.7) 304.3 |
10 (2.9) 289.3 |
4 (3.3) 266.2 |
2 (3.2) 266.6 |
3 (2.1) 360.8 |
36 (3.0) 341.8 |
0.858 0.060 |
112 (3.0) 374.6 |
| PAD, n (event rate) Time to first event (days) |
1 (1.2) 338.3 |
2 (1.2) 325.6 |
7 (1.4) 307.5 |
5 (1.5) 289.1 |
2 (1.7) 257.6 |
1 (1.8) 236.8 |
2 (1.3) 329.7 |
20 (1.4) 272.5 |
0.893 <0.001 |
60 (1.3) 285.2 |
| Albuminuria (UACR ≥30 mg/g), n (event rate) Time to first event (days) |
0 (1.0) 479.8 |
2 (1.1) 457.6 |
5 (1.2) 423.7 |
5 (1.5) 405.9 |
2 (1.4) 373.9 |
1 (1.5) 326.0 |
1 (1.1) 466.8 |
16 (1.3) 395.4 |
0.894 <0.001 |
51 (1.3) 423.2 |
| Albuminuria (UACR ≥300 mg/g), n (event rate) Time to first event (days) |
0 528.5 |
1 (0.6) 488.6 |
3 (0.6) 456.7 |
2 (0.6) 433.4 |
1 (0.7) 370.0 |
0 371.0 |
1 (0.5) 480.4 |
8 (0.7) 401.8 |
0.999 <0.001 |
26 (0.7) 449.8 |
PAD, peripheral artery disease.
DISCUSSION
Our study showed in a large sample of patients representative of the Spanish population that both prevalent and incident patients with CKD are predominantly at stage 3, have many comorbidities and, with regard to cardio- and nephroprotective medications, there is much room for improvement. This undertreatment may consequently be translated into a higher risk of cardiovascular and renal complications.
Different studies performed in different clinical settings have reported the same clinical profile, with a high risk of developing cardiovascular complications [16–18]. Of note, our data were collected from the BIG-PAC database, which has been previously validated. In addition, data included in this database are representative of the Spanish population attended in primary health centres and referral hospitals within the Spanish national health system [15].
There is a bidirectional relationship between CKD and HF. The presence of one condition promotes the development of the other and vice versa [19]. Our study showed that patients with both conditions were older and had a worse clinical profile and poorer cardiovascular risk factor control rates, with more comorbidities, lower renal function and more albuminuria. As a result, these patients have a marked risk of developing outcomes during the follow-up. In fact, our data in the prevalent population showed that compared with CKD-only patients, the concomitance of both conditions substantially increased the risk of all-cause death, HF hospitalizations and UACR progression. In addition, outcomes increased as renal function worsened. This was more evident when CKD and HF occurred concomitantly. As a result, a comprehensive therapeutic approach is warranted to reduce the risk of cardiovascular and renal complications in this population.
Unfortunately, our data also indicated that a substantial number of patients with CKD were not taking those drugs that have been shown to be cardio- and nephroprotective in the CKD population. For instance, ∼25–40% of patients were not taking RASis and only a small proportion at maximal doses. This undertreatment was not justified by differences in UACR, renal function or blood pressure levels. In addition, among diabetics, the use of SGLT2 inhibitors was marginal. However, it should be taken into account that during the study period, treatment with SGLT2 inhibitors was not to be initiated to improve glycaemic control in patients with an eGFR <60 mL/min and was to be discontinued at a GFR persistently <45 mL/min, and also that penetration was low since the completion of pivotal clinical trials was close to or after index date [19–21]. For years, RASis have been considered a cornerstone in the management of patients with CKD and are recommended by clinical practice guidelines for the prevention of cardiovascular and renal complications [22, 23]. In this regard, more efforts are needed to increase the proportion of patients taking RASis [24]. In addition, it is important to achieve the maximal tolerated doses of RASis, as their beneficial effects are greater at maximal doses compared with lower doses [25]. However, our study showed that despite the fact that hyperkalaemia was reported in only a small proportion of patients, regardless of CKD stage, the great majority of patients were not taking RASis at maximal doses, leading to inappropriate use of these drugs and consequently to a higher risk of developing cardiovascular and renal complications. Moreover, the use of drugs that reduce potassium levels, such as patiromer and sodium zirconium cyclosilicate, could allow optimizing the use of RASis [26, 27].
In our study, after only 2 years from CKD diagnosis, 6.5% of patients developed HF. In addition, in the incident population, the risk of myocardial infarction and HF was equally high in patients with CKD. This means that the aim of the therapeutic approach in patients with CKD should not be limited to the prevention of atherosclerotic cardiovascular outcomes, but also HF and renal complications [22, 23]. Similarly, this has been observed in previous studies [28]. For instance, in a DAPA-CKD-like cohort of real-life patients, the number of adverse renal and cardiovascular outcomes was substantial [28]. In the DAPA-CKD trial, treatment with dapagliflozin was associated with significant reductions in the risk of major adverse kidney and cardiovascular events, including HF hospitalizations, as well as all-cause mortality in both diabetic and non-diabetic CKD patients. In addition, these beneficial effects were consistent across all eGFR and UACR stages [29–32]. Moreover, in the Dapagliflozin Effect on Cardiovascular Events--Thrombolysis in Myocardial Infarction 58 trial, dapagliflozin not only prevented and reduced the progression of renal disease among patients with T2D at high risk for cardiovascular events, but also HF hospitalizations in patients with normal and impaired renal function [19, 33]. Furthermore, a recent meta-analysis in patients with T2D and CKD showed that treatment with SGLT2 inhibitors was associated with a decreased risk of cardiovascular and renal events [34], indicating the protective effects of SGLT2 inhibitors across the cardiorenal continuum, even regardless of T2D status [35]. In fact, a recent study has shown that following the 2020 Kidney Disease: Improving Global Outcomes guidelines, more than one-third of patients with T2D in the USA should be treated with SGLT2 inhibitors, as all patients with CKD would obtain a cardiovascular benefit with such drugs [36]. Unfortunately, in our study, the use of SGLT2 inhibitors was marginal in patients with T2D, even in those patients with CKD and HF. As a result, greater use of these types of drugs would be desirable to reduce the cardiovascular and renal burden in patients with CKD.
Limitations
Due to the design of the study (observational and retrospective cohort study), without a control group, only indirect causality can be suggested that should be confirmed in specific studies. As this was a retrospective study, taking data from secondary healthcare resources, some relevant variables, such as UACR, could not be reported in all patients, leading to an underdiagnosis of CKD. However, the high number of patients included, with prevalent and incident CKD, as well as the robustness of the data, may provide an accurate picture of the current management and cardiovascular/renal risk in the Spanish population with CKD. On the other hand, although the ICD-10 code N-17 may underestimate AKI, this code was used, as it is widely used in electronic health records in Spain.
CONCLUSIONS
In Spain, patients with CKD are predominantly at stage 3 and have many comorbidities and there is a marked underuse of cardio- and nephroprotective medications. Patients with CKD have a substantial risk of developing HF, as well as atherosclerotic cardiovascular outcomes, renal progression disease and all-cause mortality. Therefore it is expected that greater use of guideline-recommended therapy could translate into a reduction of cardiovascular and renal burden in patients with CKD.
FUNDING
This study was fully funded by AstraZeneca.
Supplementary Material
Contributor Information
Roberto Alcázar, University Hospital Infanta Leonor, Madrid, Spain.
Carlos Escobar, University Hospital La Paz, Madrid, Spain.
Beatriz Palacios, AstraZeneca, Madrid, Spain.
Unai Aranda, AstraZeneca, Madrid, Spain.
Luis Varela, AstraZeneca, Madrid, Spain.
Margarita Capel, AstraZeneca, Madrid, Spain.
Antoni Sicras, Health Economics and Outcomes Research, Atrys Health, Barcelona, Spain.
Aram Sicras, Health Economics and Outcomes Research, Atrys Health, Barcelona, Spain.
Antonio Hormigo, Primary Care Center Salud Puerta Blanca, Malaga, Spain.
Nicolás Manito, Hospital de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.
Manuel Botana, Hospital Universitario Lucus Augusti, Lugo, Spain.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 2. Webster AC, Nagler EV, Morton RLet al. Chronic kidney disease. Lancet 2017; 389: 1238–1252 [DOI] [PubMed] [Google Scholar]
- 3. Jha V, Garcia-Garcia G, Iseki Ket al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 4. Ortiz A, Sanchez-Niño MD, Crespo-Barrio Met al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia (Engl Ed) 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 5. Darlington O, Dickerson C, Evans Met al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: evidence from a systematic literature review. Adv Ther 2021; 38: 994–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim GJ, Liu YL, Low Set al. Medical costs associated with severity of chronic kidney disease in type 2 diabetes mellitus in Singapore. Ann Acad Med Singap 2020; 49: 731–741 [PubMed] [Google Scholar]
- 7. Escobar C, Palacios B, Aranda Uet al. Costs and healthcare utilisation of patients with chronic kidney disease in Spain. BMC Health Serv Res 2021; 21: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98(4 Suppl): S1–S115 [DOI] [PubMed] [Google Scholar]
- 9. Ruggenenti P, Perna A, Gherardi Get al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354: 359–364 [DOI] [PubMed] [Google Scholar]
- 10. Lewis EJ, Hunsicker LG, Clarke WRet al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 11. Escobar C, Aranda U, Palacios Bet al. Epidemiology, clinical profile, management, and two-year risk complications among patients with chronic kidney disease in Spain (Engl Ed). Nefrologia 2021; doi: 10.1016/j.nefro.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 12. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Szarek M, Pitt Bet al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021; 384: 129–139 [DOI] [PubMed] [Google Scholar]
- 15. Sicras-Mainar A, Sicras-Navarro A, Palacios Bet al. Epidemiology and treatment of heart failure in Spain: the HF-PATHWAYS study. Rev Esp Cardiol 2022; 75: 31–38 [DOI] [PubMed] [Google Scholar]
- 16. Simal F, Martín Escudero JC, Bellido Jet al. [Prevalence of mild to moderate chronic kidney disease in the general population of Spain. Hortega study]. Nefrologia 2004; 24: 329–332 [PubMed] [Google Scholar]
- 17. Otero A, de Francisco A, Gayoso Pet al. Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrologia 2010; 30: 78–86 [DOI] [PubMed] [Google Scholar]
- 18. Gorostidi M, Sánchez-Martínez M, Ruilope LMet al. Chronic kidney disease in Spain: prevalence and impact of accumulation of cardiovascular risk factors. Nefrologia 2018; 38: 606–615 [DOI] [PubMed] [Google Scholar]
- 19. Wiviott SD, Raz I, Bonaca MPet al. DECLARE–TIMI 58 investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Wanner C, Lachin JMet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 [DOI] [PubMed] [Google Scholar]
- 21. Neal B, Perkovic V, Mahaffey KWet al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SDet al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975 [DOI] [PubMed] [Google Scholar]
- 23. de Boer IH, Caramori ML, Chan JCNet al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int 2020; 98: 839–848 [DOI] [PubMed] [Google Scholar]
- 24. Epstein M, Reaven NL, Funk SEet al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(Suppl 11): S212–S220 [PubMed] [Google Scholar]
- 25. Blacklock CL, Hirst JA, Taylor KSet al. Evidence for a dose effect of renin-angiotensin system inhibition on progression of microalbuminuria in type 2 diabetes: a meta-analysis. Diabet Med 2011; 28: 1182–1187 [DOI] [PubMed] [Google Scholar]
- 26. Palmer BF, Carrero JJ, Clegg DJet al. Clinical management of hyperkalemia. Mayo Clin Proc 2021; 96: 744–762 [DOI] [PubMed] [Google Scholar]
- 27. Takkar C, Nassar T, Qunibi W. An evaluation of sodium zirconium cyclosilicate as a treatment option for hyperkalemia. Expert Opin Pharmacother 2021; 22: 19–28 [DOI] [PubMed] [Google Scholar]
- 28. Olufade T, Lamerato L, Sánchez JJGet al. Clinical outcomes and healthcare resource utilization in a real-world population reflecting the DAPA-CKD trial participants. Adv Ther 2021; 38: 1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler DC, Stefánsson BV, Jongs Net al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021; 9: 22–31 [DOI] [PubMed] [Google Scholar]
- 30. McMurray JJV, Wheeler DC, Stefánsson BVet al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation 2021; 143: 438–448 [DOI] [PubMed] [Google Scholar]
- 31. Heerspink HJL, Sjöström CD, Jongs Net al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J 2021; 42: 1216–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zelniker TA, Raz I, Mosenzon Oet al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol 2021; 6: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mosenzon O, Wiviott SD, Cahn Aet al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617 [DOI] [PubMed] [Google Scholar]
- 34. McGuire DK, Shih WJ, Cosentino Fet al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021; 6: 148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fontes-Carvalho R, Santos-Ferreira D, Raz Iet al. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J Prev Cardiol 2021; zwab034; doi: 10.1093/eurjpc/zwab034 [DOI] [PubMed] [Google Scholar]
- 36. Ciardullo S, Trevisan R, Perseghin G. Sodium-glucose transporter 2 inhibitors for renal and cardiovascular protection in US adults with type 2 diabetes: impact of the 2020 KDIGO clinical practice guidelines. Pharmacol Res 2021; 166: 105530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.