ABSTRACT
Background
Angiopoietin-2 (Ang-2) plays a pivotal role in pathological vascular remodeling and angiogenesis. Both vascular mechanisms are active in patients with end-stage renal disease (ESRD) and may contribute to the high mortality in these patients. The aim of this multicenter prospective cohort study was to investigate baseline serum Ang-2 concentrations in ESRD patients on hemodialysis (HD) for their ability to predict all-cause mortality.
Methods
We conducted a prospective cohort study in 340 stable HD patients from different chronic dialysis centers in Berlin, Germany. The primary endpoint was all-cause mortality during a 5-year follow-up period. Blood samples and clinical data were collected at baseline. Serum Ang-2 was measured with a validated enzyme-linked immunosorbent assay (Biomedica, Vienna, Austria).
Results
A total of 313 HD patients (206 men and 107 women) were finally included in the study. Receiver operating characteristic (ROC) analysis of Ang-2 concentrations yielded an area under the curve (AUC) of 0.65 (P < 0.0001) for predicting all-cause mortality in the entire study population and was used to determine the optimal cut-off (111.0 pmol/L) for all-cause mortality. Kaplan–Meier survival analysis indicated that male but not female end-stage kidney disease patients on HD with higher Ang-2 concentrations had a significantly lower survival (log-rank test, P < 0.0001 and P = 0.380 for male and female patients, respectively). Multivariable Cox regression analyses adjusted for age, comorbidity, smoking, dialysis vintage, serum creatinine, hemoglobin, C-reactive protein, serum albumin, intact parathyroid hormone (iPTH), low-density lipoprotein (LDL) and Kt/V likewise indicated that elevated Ang-2 concentrations are associated with all-cause mortality in male {hazard ratio [HR] 3.294 [95% confidence interval (CI) 1.768–6.138]; P = 0.0002} but not in female end-stage kidney disease patients on HD [HR 1.084 (95% CI 0.476–2.467); P = 0.847].
Conclusion
Ang-2 at baseline is independently associated with all-cause mortality in male ESRD patients on HD.
Keywords: angiopoietin-2, all-cause mortality, hemodialysis patients, sex-dependent impact
KEY LEARNING POINTS.
What is already known about this subject?
Angiopoietins (Angs) are vascular growth factors involved in angiogenesis and vasculogenesis.
Angiopoietin-1 (Ang-1) binds to Tie tyrosine kinase 2 (Tie2) receptors and induces Tie2 phosphorylation–mediated anti-inflammatory signals to the endothelium, thereby promoting endothelial cell survival and stabilizing endothelial and vascular structure, whereas angiopoietin-2 (Ang-2) competes with Ang-1 to bind to the Tie2 receptor, thereby harming angiogenesis and vasculogenesis.
In the general population, elevated Ang-2 concentrations are associated with a higher risk of all-cause and cardiovascular mortality.
What this study adds?
Kaplan–Meier survival analysis indicated that male but not female end-stage kidney disease patients on hemodialysis (HD) with higher Ang-2 concentrations had a significantly lower survival (log-rank test, P < 0.0001 and P = 0.380 for males and females, respectively).
Multivariable Cox regression analyses adjusted for age, comorbidity, smoking, dialysis vintage, creatinine, hemoglobin, C-reactive protein, albumin, intact parathyroid hormone, low-density lipoprotein and Kt/V likewise indicated that elevated Ang-2 concentrations are associated with all-cause mortality in male {hazard ratio [HR] 3.294 [95% confidence interval (CI) 1.768–6.138]; P = 0.0002} but not in female end-stage renal disease (ESRD) patients on HD (P = 0.847).
What impact this may have on practice or policy?
The very strong sex dependency of the association of Ang-2 with all-cause mortality may stimulate further clinical and basic science studies.
Ang-2 is a small bioactive molecule and hence not just an all-cause mortally biomarker, but rather likely a vascular powerful hormone contributing to the pathophysiology of vascular damage in male ESRD patients.
Our study after independent confirmation might stimulate the development of Ang-2 antagonists to reduce all-cause mortality in ESRD patients.
INTRODUCTION
Angiogenesis is the process of forming new vessels on top of preexisting ones. It is involved not only in physiological conditions, but also in many pathological conditions with endothelial dysfunction/microinflammation features such as tumor metastasis and atherosclerotic plaque formation [1]. Angiopoietins (Angs) are vascular growth factors of ∼70 kDa that are involved in angiogenesis and vasculogenesis and function through the Tie tyrosine kinase receptors. Ang-1 and Ang-2 regulate endothelial cell survival, angiogenesis and maturation through opposing functions. Ang-1 binds to the Tie2 receptor and induces Tie2 phosphorylation to provide an anti-inflammatory signal to the endothelium, thereby promoting endothelial cell survival and stabilizing endothelial and vascular structure, whereas Ang-2 competes with Ang-1 to bind to the Tie2 receptor and therefore destabilizes the vessel and degrades the basal lamina [2–4].
Accumulating evidence has demonstrated that angiopoietins participate in cardiovascular burden. Elevated Ang-2 levels have been associated with traditional risk factors for cardiovascular diseases (CVDs), such as blood pressure, smoking, lipid levels and the metabolic syndrome [5, 6]. In the general population, elevated Ang-2 concentrations are associated with a higher risk of all-cause and cardiovascular mortality [7]. The kidney is a highly vascularized organ, characterized by a remarkable diversity of endothelial cells (ECs). The renal endothelium is both a target and a driver of kidney and systemic cardiovascular complications [8]. The Ang–Tie2 system has been shown to play a major role in injury induced by chronic kidney disease (CKD) and dialysis [9–11]. In CKS Stages 3–5 patients, high Ang-2 levels have been positively associated with systemic markers/mediators of inflammation [9, 10, 12, 13], abnormal cardiac structure [14] and major cardiac adverse events [15]. In patients with end-stage renal disease (ESRD), circulating Ang-2 levels have been found to be increased in patients treated with dialysis, although the mechanism is unknown. Furthermore, David et al. [9] suggested that Ang-2 might be a mediator and not just simply a biomarker of CVD events and thus accounts for accelerated atherosclerosis. They suggested that dialysis treatment is associated with severe disequilibrium of the Angs that probably confers permanent devastating activation of the endothelial layer.
Thus the aim of this study was to evaluate whether Ang-2 is associated with all-cause mortality in patients with ESRD on hemodialysis (HD).
MATERIALS AND METHODS
Study population
We conducted a prospective cohort study in 340 stable HD patients from different chronic dialysis centers in Berlin, Germany. The patients were followed up for 5 years. The study was approved by the local ethics committee and informed consent was obtained from all participants. Patients with any malignancy or active infections, pregnant or unwilling to take part were excluded from the study. All patients were routinely dialyzed at least three times a week for 4–5 h each time, using standard bicarbonate dialysis with biocompatible membranes. Dialysate flow rates were 500 mL/min and blood flow rates were 250–300 mL/min. All patients had a functioning permanent access. All-cause mortality was documented during the 5-year follow-up period. Patients who received a transplant during the follow-up period were censored at the time of transplantation.
Clinical data and serum parameters
The following patient characteristics were obtained: age, sex, weight, height, underlying renal disease, dialysis vintage, systolic and diastolic blood pressure (BP), presence of diabetes, hypertension, smoking or coronary heart disease and medications (use of angiotensin-converting enzyme inhibitors, beta-blockers, calcium channel blockers or erythropoietin). Blood samples were collected before one HD session at study entry. Serum albumin, cholesterol, triglycerides, urea, creatinine, calcium, potassium and phosphate were assessed in the clinical laboratory using standardized methods. Ang-2 concentrations were analyzed using a sandwich enzyme immunoassay [human angiopoietin-2 enzyme-linked immunosorbent assay (ELISA), BI-ANG2, Biomedica, Vienna, Austria] according to the instructions of the manufacturer (https://www.bmgrp.com/wp-content/uploads/2019/11/BI-ANG2-Angiopoietin-2-ELISA-Validation-Data-191128.pdf). The limit of detection of the kit was 3.7 pmol/L, and the average intra- and interassay coefficients of variation were ≤8 and ≤6, respectively.
Statistical analysis
Descriptive variables are shown as median [interquartile range (IQR)] or number (percentage). Comparisons were assessed by Mann–Whitney U test or Kruskal–Wallis test, as appropriate. The cut-off value for all-cause mortality of baseline Ang-2 concentrations was obtained with receiver operating characteristic (ROC) curve analysis, with the value maximized by the Youden index. Cumulative survival curves were generated using the Kaplan–Meier method by optimal prediction value and differences were evaluated with a log-rank test. Multivariable-adjusted survival analysis was performed using a proportional hazards regression model. Model A was adjusted for age, comorbidities (diabetes, hypertension and CVD) and smoking; model B was adjusted for serum creatinine, hemoglobin, C-reactive protein (CRP), serum albumin, ferritin, transferrin, intact parathyroid hormone (iPTH), serum calcium, serum phosphorus, low-density lipoprotein (LDL) cholesterol and Kt/V (a number used to quantify HD and peritoneal dialysis treatment adequacy/quality, where K is the dialyzer clearance of urea, t is the dialysis time and V is the volume of distribution of urea, approximately equal to the patient's total body water); model C was adjusted for all of the above risk factors (model A + model B) plus ultrafiltration volume. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. Furthermore, a series of multivariable Cox regression analyses was performed in these three models with three ROC-derived cut-off values: ROC-based cut-off for the entire study population (111.00 pmol/L), ROC-based cut-off for male participants (99.05 pmol/L) and ROC-based cut-off for female participants (85.85 pmol/L), as well as the median of male and female study participants (91.20 pmol/L). Statistical significance was defined as P < 0.05. All analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA) and Prism 8 (GraphPad Software, San Diego, CA, USA).
RESULTS
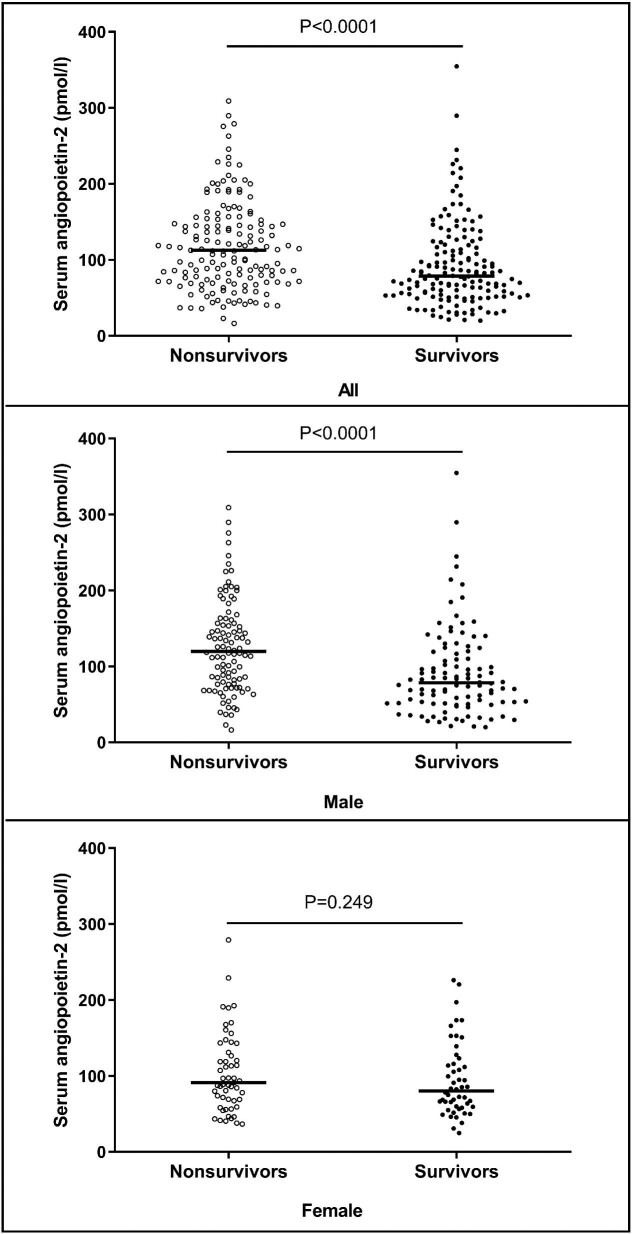
Initially, 340 HD patients were included in the study, but 27 patients were excluded due to limited sample volume for Ang-2 measurements. Finally, 313 patients were included in statistical analysis: 206 male patients and 107 female patients. The median age was 66 years (IQR 56–75), the median time since the initiation of dialysis (dialysis vintage) was 243 days (IQR 31–1172) and the median dialysis dose (Kt/V) was 1.2 (IQR 1.1–1.3). During the 5-year follow-up, 157 patients (50.2%) died and 41 patients (13.1%) underwent kidney transplantation. The median baseline serum Ang-2 concentration was 91.2 pmol/L (IQR 63.4–140.7). Patients with an Ang-2 concentration above the median value at baseline were on average older; had longer dialysis vintage; had higher levels of ferritin, CRP, serum creatinine and potassium and had lower levels of transferrin and LDL than the patients with Ang-2 concentration below the median (Supplementary data, Table S1). The Ang-2 concentration was significantly higher in patients who died during follow-up than in those who did not die [112.5 pmol/L (95% CI 72.0–153.8) versus 78.6 (53.3–117.2); P < 0.0001]. The median serum Ang-2 was also significantly higher in the male nonsurvivors than the male survivors [119.8 (IQR 77.0–161.6) versus 78.4 (51.5–118.5); P < 0.0001], but not in female survivors and female nonsurvivors [91.2 (IQR 67.2–142.9) versus 80.2 (59.0–117.7); P = 0.249] (Figure 1).
Figure 1:
Plots of serum Ang-2 concentrations. Median serum Ang-2 was significantly higher in the nonsurvivors than the survivors using Mann–Whitney U test [112.50 (IQR 71.95–153.80) versus 78.55 (53.33–117.20); P< 0.0001]. Median serum Ang-2 was significantly higher in the male nonsurvivors than the male survivors using Mann–Whitney U test [119.80 (IQR 76.98–161.60) versus 78.40 (51.45–118.50); P< 0.0001]. No significant difference of median serum Ang-2 was found between female nonsurvivors and female survivors using Mann–Whitney U test [91.20 (IQR 67.20–142.90) versus 80.20 (59.00–117.70); P = 0.249].
Table 1 summarizes the baseline clinical and biochemical variables for patients by sex. The cause of CKD was hypertensive nephropathy in 107 cases (34.2%), diabetic nephropathy in 97 cases (31.0%), glomerulonephritis in 25 cases (8.5%), polycystic kidney disease in 9 cases (2.9%) and other or unknown in 75 cases (24.0%). Males comprised 65.8% of the cohort (206 males, 107 females). Females were less likely to be diabetics and smokers than males and had higher high-density lipoprotein cholesterol, total cholesterol and dialysis doses. Males had higher CRP and serum creatinine than females. However, there were neither sex differences nor differences among the different groups according to the underlying renal diseases, such as hypertensive renal diseases or diabetic nephropathy, in baseline Ang-2 concentrations (Figure 2).
Table 1.
Baseline clinical and biochemical characteristics of HD patients by sex
| Characteristics | All (N = 313) | Male (n = 206) | Female (n = 107) | P-value |
|---|---|---|---|---|
| Age (years) | 66.00 (56.00–75.00) | 66.00 (56.00–74.00) | 67.00 (57.00–76.00) | 0.617 |
| Primary kidney diseases, n (%) | 0.722 | |||
| Hypertensive nephropathy | 107 (34.2) | 76 (36.9) | 31 (29.0) | |
| Diabetic nephropathy | 97 (31.0) | 57 (27.7) | 40 (37.4) | |
| Glomerulonephritis | 25 (8.0) | 13 (6.3) | 12 (11.2) | |
| Polycystic kidney disease | 9 (2.9) | 7 (3.4) | 2 (1.9) | |
| Other or unknown cause | 75 (24.0) | 53 (25.7) | 22 (20.6) | |
| Diabetes mellitus, n (%) | 117 (37.4) | 66 (32.0) | 51 (47.7) | 0.007 |
| Hypertension, n (%) | 246 (78.6) | 160 (77.7) | 86 (80.4) | 0.580 |
| CHD, n (%) | 148 (47.3) | 99 (48.1) | 49 (45.8) | 0.704 |
| Smoker, n (%) | 97 (31.0) | 75 (36.4) | 22 (20.6) | 0.004 |
| Body mass index (kg/m2) | 24.53 (22.01–27.55) | 24.91 (22.25–27.35) | 24.12 (21.60–28.51) | 0.306 |
| Dialysis vintage (days) | 243 (31–1172) | 271 (31–1347) | 227 (31–919) | 0.180 |
| Medication, n (%) | ||||
| RAAS inhibitors | 82 (26.2) | 54 (26.2) | 28 (26.2) | 0.993 |
| Beta-blockers | 186 (59.4) | 116 (56.3) | 70 (65.4) | 0.120 |
| Calcium channel blockers | 98 (31.3) | 65 (31.6) | 33 (30.8) | 0.897 |
| Erythropoietin | 158 (50.5) | 104 (50.5) | 54 (50.5) | 0.998 |
| Hemoglobin (mg/dL) | 10.20 (9.05–11.45) | 10.10 (9.00–11.20) | 10.20 (9.15–11.60) | 0.593 |
| Ferritin (ng/ml) | 516.50 (243.50–1074.00) | 513.00 (239.00–1150.50) | 520.00 (243.00–914.00) | 0.762 |
| Transferrin (μg/ml) | 138.00 (107.00–173.00) | 138.00 (104.00–172.00) | 142.00 (116.25–177.00) | 0.286 |
| Serum albumin (g/L) | 3.30 (2.90–3.70) | 3.30 (2.90–3.70) | 3.20 (2.80–3.60) | 0.565 |
| LDL (mg/dL) | 92.70 (72.35–120.08) | 92.70 (70.40–114.23) | 103.00 (75.78–128.60) | 0.101 |
| HDL (mg/dL) | 39.80 (32.00–50.20) | 36.45 (30.90–46.32) | 43.80 (36.43–56.68) | <0.001 |
| Total cholesterol (mg/dL) | 150.60 (127.40–187.00) | 146.70 (119.70–182.25) | 166.00 (134.15–204.00) | 0.020 |
| Triglycerides (mmol/L) | 159.30 (109.50–247.80) | 159.30 (106.20–247.80) | 162.80 (112.40–247.80) | 0.708 |
| CRP (mg/dL) | 2.60 (1.00–5.00) | 3.10 (1.15–5.95) | 1.40 (0.67–3.33) | <0.001 |
| Urea (mg/dL) | 195.52 (147.31–268.57) | 205.09 (145.94–279.53) | 183.18 (150.67–242.39) | 0.110 |
| Serum creatinine (mg/dL) | 6.66 (4.25–8.39) | 6.90 (4.56–8.71) | 5.74 (3.76–7.46) | 0.004 |
| Serum potassium (mmol/L) | 4.70 (4.10–5.26) | 4.80 (4.10–5.30) | 4.60 (4.00–5.20) | 0.186 |
| Serum calcium (mmol/L) | 2.22 (2.09–2.40) | 2.25 (2.08–2.40) | 2.20 (2.10–2.44) | 0.774 |
| Serum phosphorus (mg/dL) | 1.61 (1.20–2.10) | 1.63 (1.21–2.10) | 1.59 (1.17–2.01) | 0.485 |
| iPTH (ng/L) | 50.18 (18.96–129.60) | 47.71 (18.96–131.88) | 52.79 (18.85–127.40) | 0.769 |
| Dialysis dose (Kt/V) | 1.19 (1.07–1.33) | 1.16 (1.06–1.31) | 1.24 (1.11–1.44) | 0.017 |
| Ang-2 (pmol/L) | 91.20 (63.35–140.65) | 94.30 (64.48–143.78) | 86.30 (59.30–127.70) | 0.352 |
Values are presented as median (IQR) unless stated otherwise. Between groups (male versus female), comparisons were made using a nonparametric Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables. Body mass index was calculated as weight in kilograms divided by height in meters squared. CHD, coronary heart disease; RAAS, renin–angiotensin–aldosterone system.
Figure 2:
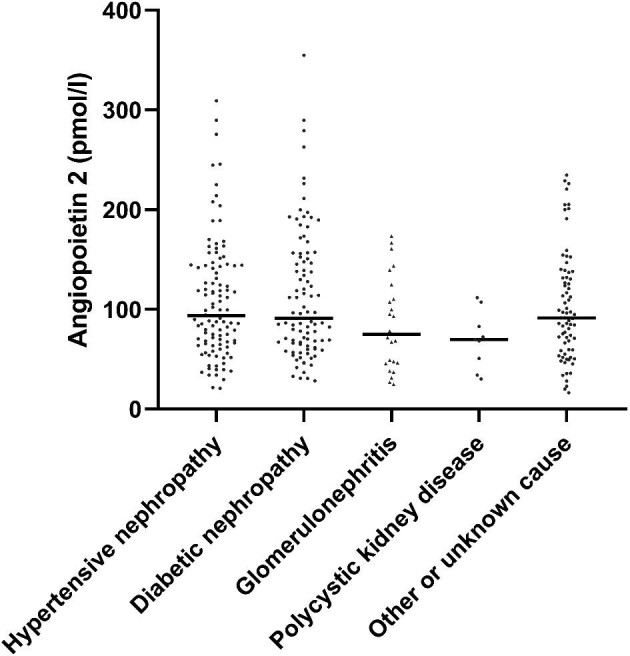
Plots of serum Ang-2 concentrations according to the underlying renal diseases. Lines indicate the median value of the Ang-2 concentration in each group.
The optimal cut-off value for baseline serum Ang-2 to predict all-cause mortality was 111.0 pmol/L (AUC 0.65, P < 0.001; sensitivity 0.522, specificity 0.727, Youden index value 0.250) based on the ROC analysis (Supplementary data, Figure S1).
Kaplan–Meier survival analysis indicated that patients in the higher Ang-2 concentration group ( 111.0 pmol/L) had a significantly lower survival rate (log-rank test, P < 0.0001), with similar results for male patients (Figure 3).
111.0 pmol/L) had a significantly lower survival rate (log-rank test, P < 0.0001), with similar results for male patients (Figure 3).
Figure 3:
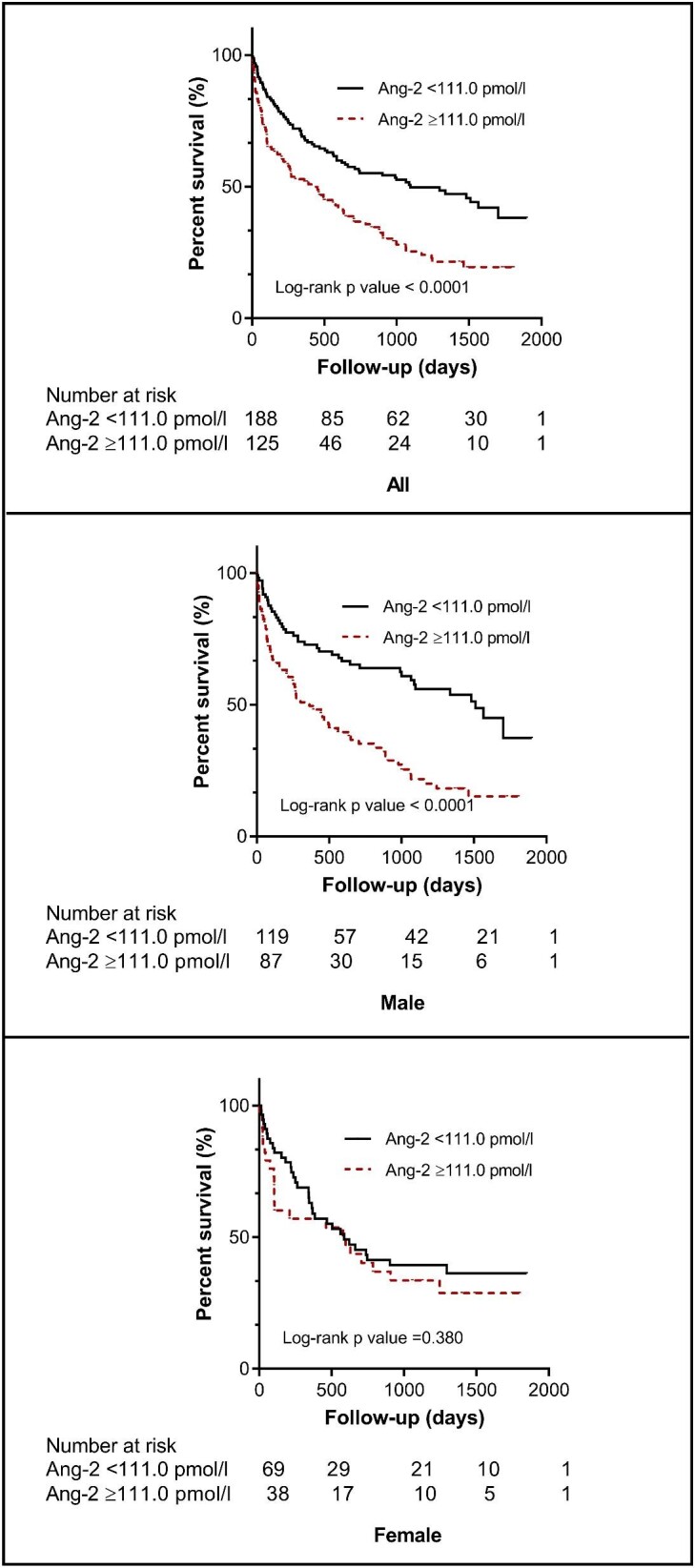
Kaplan–Meier survival curves for all-cause mortality. Patients were divided according to optimal cut-off values of Ang-2 concentrations.
Next we performed univariate and multivariable Cox regression analyses. Binary Ang-2 was divided according to the optimal cut-off value (ROC-based cut-off for the entire study population 111.00 pmol/L). Univariate Cox regression analyses showed that both increasing Ang-2 and the binary upper half of Ang-2 were positively associated with all-cause mortality in both overall and male patients. Multivariable Cox regression analyses were then performed in three models (as described in the Materials and Methods section) (Table 2). Furthermore, multivariable Cox regression analysis was also performed in three models with ROC-derived cut-off values in sex subgroups (ROC-based cut-off for male participants 99.05 pmol/L, ROC-based cut-off for female participants 85.85 pmol/L) as well as the median of male and female study participants (91.20 pmol/L).
Table 2.
Cox regression analyses of serum Ang-2 levels predicting all-cause mortality
| All (N = 313) | Male (n = 206) | Female (n = 107) | ||||
|---|---|---|---|---|---|---|
| Analyses | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Univariate Cox regression | ||||||
| Continuous Ang-2 | 1.002 (1.001–1.004) | 0.005 | 1.002 (1.001–1.004) | 0.009 | 1.003 (0.998–1.008) | 0.221 |
| Binary Ang-2 | 1.934 (1.412–2.649) | <0.0001 | 2.475 (1.660–3.689) | <0.0001 | 1.273 (0.742–2.185) | 0.381 |
| Multivariable Cox regression | ||||||
| Model A | 1.756 (1.274–2.419) | 0.001 | 2.467 (1.624–3.747) | <0.0001 | 0.885 (0.503–1.555) | 0.670 |
| Model B | 2.613 (1.698–4.023) | <0.0001 | 2.937 (1.660–5.196) | 0.0002 | 1.990 (0.946–4.186) | 0.070 |
| Model C | 2.245 (1.443–3.493) | 0.0003 | 3.294 (1.768–6.138) | 0.0002 | 1.084 (0.476–2.467) | 0.847 |
Binary Ang-2 was divided according to optimal cut-off values of Ang-2 concentrations (111.0 pmol/L). Multivariable Cox regression analyses were performed in three models. Model A was adjusted for age, comorbidity and smoking; model B was adjusted for dialysis vintage, serum creatinine, hemoglobin, CRP, serum albumin, ferritin, transferrin, iPTH, serum calcium, serum phosphorus, LDL and Kt/V and model C was adjusted for the above risk factors (model A + model B) plus ultrafiltration volume.
Results were consistent in all models and revealed a significant predictive effect of Ang-2 on all-cause mortality in the whole cohort as well as in the male population, but not in the female population (Supplementary data, Table S2).
DISCUSSION
We assessed the association of circulating Ang-2 at baseline with all-cause mortality during a follow-up of 5 years in a multicenter HD cohort of patients with ESRD. Baseline serum Ang-2 concentrations showed a consistent positive association with all-cause mortality and this association was only seen in male HD patients.
Ang-2 is a secreted glycoprotein, synthesized mainly by endothelial cells but also by other cell types, that plays a critical role in vascular development. It mediates its effect via inhibition of Ang-1-mediated phosphorylation of Tie2. Ang-1 binds to Tie2 receptor and induces Tie2 phosphorylation to provide an anti-inflammatory signal to the endothelium, therefore Ang-2 release leads to inflammation and is associated with a range of pathological conditions [2–4]. In observational studies, circulating Ang-2 levels are elevated in a variety of diseases known for their common characteristics of endothelial dysfunction and/or vascular inflammation, such as diabetes mellitus [16], cardiovascular diseases [14, 17], systemic lupus erythematosus [18] and systemic inflammatory response syndrome (SIRS) [19–22]. Moreover, Ang-2 levels were positively associated with cardiovascular disease risk factors, including metabolic syndrome [5]. In CKD patients, Ang-2 levels are associated with systemic markers/mediators of inflammation. In addition, circulating Ang-2 increases with the progression of CKD, which is predictive of mortality and correlates with the severity of vascular disease in dialysis patients [9, 10, 13]. Previous studies have found that glomerular Ang-2 is upregulated in preclinical models of glomerulonephritis [23] and podocyte-specific expression of Ang-2 causes proteinuria [24], suggesting that the mechanism linking endothelial dysfunction, albuminuria and CVD is that of endothelial dysfunction, leading to increased vascular permeability and glomerular albumin leakage [25]. ESRD patients on HD have a higher incidence of CVD. In addition to conventional cardiovascular risk factors such as age, sex and comorbidities, risk factors such as chronic inflammation and endothelial dysfunction highlight the impact of damage to the vascular endothelium in this complex pathophysiology. Endothelial dysfunction is associated with a higher incidence of CVD, which may be one of the main causes of the high morbidity and mortality in ESRD patients on HD. In the present study, we found a robust and consistent association between serum Ang-2 and all‐cause mortality even after adjusting for multiple risk factors, i.e., age, comorbidities, smoking, dialysis vintage, serum creatinine, hemoglobin, CRP, serum albumin, ferritin, transferrin, iPTH, serum calcium, serum phosphorus, LDL cholesterol, Kt/V and ultrafiltration volume.
To our knowledge, the present study is the first to demonstrate a sex-dependent association between elevated Ang-2 concentrations and all-cause mortality. This finding is based on several independent statistical approaches. Kaplan–Meier survival analysis showed that male patients in the higher Ang-2 concentration groups (≥111.0 pmol/L) had a significantly lower survival rate (log-rank test, P < 0.0001), but not female patients. Multivariable Cox regression models revealed that Ang-2 has a sex-dependent impact on all-cause mortality in patients with ESRD on HD. Sexual dimorphism has been reported for serum Ang-2 levels in a relatively small case–control study of obese versus nonobese individuals [26] and in the general population [5]. These sex-related differences have also been observed in other endothelium-derived growth factors, such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor [27]. However, some reported higher levels in males [28], whereas some observed increased levels in females [27, 29]. The mechanisms for the sex-related differences in circulating growth factors are not fully understood. Previous experimental and clinical evidence indicates that at least a part of cardiovascular benefits of 17β-estradiol can be attributed to the direct effect of the ovarian sex steroid hormone on vascular endothelial cells [30]. Furthermore, experimental studies suggest that sex hormones influence vascular growth factor expression [31]. Tsuzuki et al. [32] showed that sex hormones are involved in the regulation of Ang-1, Ang-2 and VEGF messenger RNA and protein production in human endometrial stromal cells. Teubner et al. [33] demonstrated a proangiogenic effect of testosterone, which may be due to stimulation of Ang-2 and transforming growth factor α expression [33]. A potential explanation for our sex-specific finding is that in ESRD patients on HD, males may be more sensitive than females to Ang-2, which is involved in the pathogenesis of vascular inflammation, endothelial dysfunction and atherosclerosis and therefore shows a significant association with all-cause mortality.
Our study, after independent confirmation, might stimulate the development of Ang-2 antagonists to reduce all-cause mortality in ESRD patients. Ang-2 is a small molecule acting as a paracrine endothelial hormone with a preside molecular target, thus being most likely not just a mortality biomarker, but rather a player in the pathogenesis of vascular damage in male ESRD patients. Given the very strong association with mortality in male ESRD patients, our study might stimulate the development of Ang-2 antagonists.
So far, several studies have shown that Ang-2 blockade alleviates pathological angiogenesis and hence might offer a novel therapeutic approach to treatment. Most data so far are coming from oncology. Preclinical studies indicate that blockade of Ang-2 with humanized monoclonal antibodies inhibited angiogenesis and tumor growth and induced vascular regression in multiple tumor models [34, 35]. In addition to compelling preclinical data, inhibition of Ang-2-Tie2 has been evaluated in numerous early clinical trials and demonstrated safety and potential efficacy for antitumor activity [36–39]. Furthermore, this antiangiogenic efficacy also produced encouraging results in preclinical studies in myocardial infarction [40] and nonalcoholic steatohepatitis [41] as well as in clinical trials in different retinal vascular disease models [42–45].
We acknowledge limitations of our study. First, this study is an observational epidemiologic study, which makes it difficult to draw conclusions on causality, and results should be validated in an independent cohort. The blood samples for this study were taken according to the approval of the ethical committee only at study entry, thus the Ang-2 concentrations were only measured once at study entry, hence it is impossible to investigate the association of mortality with the longitudinal profile of Ang-2 concentrations. In addition, we have no data on cardiovascular events or cardiovascular mortality and therefore cannot clarify whether this prognostic significance of Ang-2 is independent of underlying vascular disease or is associated with coronary atherosclerosis.
CONCLUSION
In conclusion, Ang-2 at baseline is independently associated with all-cause mortality in male ESRD patients on HD.
Supplementary Material
ACKNOWLEDGEMENTS
Biomedica Medizinprodukte provided the angiopoietin-2 ELISA (cat. no. BI-ANG2, Biomedica, Vienna, Austria). The China Scholarship Council supported C.C and X.C. Local ethics committees approved this study and informed consent was obtained from all study participants.
Contributor Information
Chang Chu, Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany; Department of Nephrology, Charité - Universitätsmedizin Berlin, Campus Mitte, Berlin, Germany.
Xin Chen, Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany; Department of Nephrology, Charité - Universitätsmedizin Berlin, Campus Mitte, Berlin, Germany.
Ahmed A Hasan, Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany.
Angelika Szakallova, Biomedica Slovakia s.r.o., Bratislava, Slovakia.
Bernhard K Krämer, Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany; European Center for Angioscience ECAS, Medical Faculty Mannheim of the University of Heidelberg, Mannheim, Germany.
Martin Tepel, Department of Nephrology, Charité - Universitätsmedizin Berlin, Campus Mitte, Berlin, Germany; Department of Nephrology, Odense University Hospital, Odense, Denmark.
Berthold Hocher, Fifth Department of Medicine (Nephrology/Endocrinology/Rheumatology), University Medical Centre Mannheim, University of Heidelberg, Mannheim, Germany; Institute of Medical Diagnostics, IMD Berlin-Potsdam, Berlin, Germany; Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, School of Medicine, Hunan Normal University, Changsha, China; Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha, China.
FUNDING
The authors declare no funding was received for this study.
AUTHORS’ CONTRIBUTIONS
B.H. contributed to the research idea and study design. A.S. and M.T. were responsible for data acquisition. C.C., X.C. and A.A.H. were responsible for data analysis. C.C. and B.H. were responsible for article drafting. B.H., B.K.K. and M.T. were responsible for supervision or mentorship. All authors took part in the interpretation of the results and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Ma Q, Reiter RJ, Chen Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020; 23: 91–104 [DOI] [PubMed] [Google Scholar]
- 2. Parikh SM. The angiopoietin-Tie2 signaling axis in systemic inflammation. J Am Soc Nephrol 2017; 28: 1973–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hakanpaa L, Sipila T, Leppanen VMet al. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat Commun 2015; 6: 5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He FF, Zhang D, Chen Qet al. Angiopoietin-Tie signaling in kidney diseases: an updated review. FEBS Lett 2019; 593: 2706–2715 [DOI] [PubMed] [Google Scholar]
- 5. Lieb W, Zachariah JP, Xanthakis Vet al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet 2010; 3: 300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anuradha S, Mohan V, Gokulakrishnan Ket al. Angiopoietin-2 levels in glucose intolerance, hypertension, and metabolic syndrome in Asian Indians (Chennai Urban Rural Epidemiology Study-74). Metabolism 2010; 59: 774–779 [DOI] [PubMed] [Google Scholar]
- 7. Lorbeer R, Baumeister SE, Dorr Met al. Circulating angiopoietin-2, its soluble receptor Tie-2, and mortality in the general population. Eur J Heart Fail 2013; 15: 1327–1334 [DOI] [PubMed] [Google Scholar]
- 8. Jourde-Chiche N, Fakhouri F, Dou Let al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol 2019; 15: 87–108 [DOI] [PubMed] [Google Scholar]
- 9. David S, Kumpers P, Hellpap Jet al. Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am J Kidney Dis 2009; 53: 770–778 [DOI] [PubMed] [Google Scholar]
- 10. David S, John SG, Jefferies HJet al. Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant 2012; 27: 1867–1872 [DOI] [PubMed] [Google Scholar]
- 11. Papayianni A, Alexopoulos E, Giamalis Pet al. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant 2002; 17: 435–441 [DOI] [PubMed] [Google Scholar]
- 12. Post S, Peeters W, Busser Eet al. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res 2008; 45: 244–250 [DOI] [PubMed] [Google Scholar]
- 13. David S, Kumpers P, Lukasz Aet al. Circulating angiopoietin-2 levels increase with progress of chronic kidney disease. Nephrol Dial Transplant 2010; 25: 2571–2576 [DOI] [PubMed] [Google Scholar]
- 14. Tsai YC, Lee CS, Chiu YWet al. Angiopoietin-2, angiopoietin-1 and subclinical cardiovascular disease in chronic kidney disease. Sci Rep 2016; 6: 39400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsai YC, Lee CS, Chiu YWet al. Angiopoietin-2 as a prognostic biomarker of major adverse cardiovascular events and all-cause mortality in chronic kidney disease. PLoS One 2015; 10: e0135181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005; 180: 113–118 [DOI] [PubMed] [Google Scholar]
- 17. Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor Tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 2004; 110: 2355–2360 [DOI] [PubMed] [Google Scholar]
- 18. Kumpers P, David S, Haubitz Met al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis 2009; 68: 1638–1643 [DOI] [PubMed] [Google Scholar]
- 19. Kumpers P, Lukasz A, David Set al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 2008; 12: R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parikh SM, Mammoto T, Schultz Aet al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006; 3: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. David S, Kumpers P, Lukasz Aet al. Circulating angiopoietin-2 in essential hypertension: relation to atherosclerosis, vascular inflammation, and treatment with olmesartan/pravastatin. J Hypertens 2009; 27: 1641–1647 [DOI] [PubMed] [Google Scholar]
- 22. Alves BE, Montalvao SA, Aranha FJet al. Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia. BMC Infect Dis 2010; 10: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan HT, Tipping PG, Li XZet al. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int 2002; 61: 2078–2089 [DOI] [PubMed] [Google Scholar]
- 24. Davis B, Dei Cas A, Long DAet al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 2007; 18: 2320–2329 [DOI] [PubMed] [Google Scholar]
- 25. Hillege HL, Fidler V, Diercks GFet al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782 [DOI] [PubMed] [Google Scholar]
- 26. Silha JV, Krsek M, Sucharda Pet al. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes 2005; 29: 1308–1314 [DOI] [PubMed] [Google Scholar]
- 27. Lieb W, Safa R, Benjamin EJet al. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J 2009; 30: 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura K, Hashiguchi T, Deguchi Tet al. Serum VEGF—as a prognostic factor of atherosclerosis. Atherosclerosis 2007; 194: 182–188 [DOI] [PubMed] [Google Scholar]
- 29. Malamitsi-Puchner A, Tziotis J, Tsonou Aet al. Changes in serum levels of vascular endothelial growth factor in males and females throughout life. J Soc Gynecol Investig 2000; 7: 309–312 [PubMed] [Google Scholar]
- 30. Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vasc Pharmacol 2002; 38: 89–98 [DOI] [PubMed] [Google Scholar]
- 31. Ye F, Florian M, Magder SAet al. Regulation of angiopoietin and Tie-2 receptor expression in non-reproductive tissues by estrogen. Steroids 2002; 67: 305–310 [DOI] [PubMed] [Google Scholar]
- 32. Tsuzuki T, Okada H, Cho Het al. Divergent regulation of angiopoietin-1, angiopoietin-2, and vascular endothelial growth factor by hypoxia and female sex steroids in human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol 2013; 168: 95–101 [DOI] [PubMed] [Google Scholar]
- 33. Teubner A, Muller K, Bartmann CPet al. Effects of an anabolic steroid (Durateston) on testicular angiogenesis in peripubertal stallions. Theriogenology 2015; 84: 323–332 [DOI] [PubMed] [Google Scholar]
- 34. Mazzieri R, Pucci F, Moi Det al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011; 19: 512–526 [DOI] [PubMed] [Google Scholar]
- 35. Gillen J, Richardson D, Moore K. Angiopoietin-1 and angiopoietin-2 inhibitors: clinical development. Curr Oncol Rep 2019; 21: 22. [DOI] [PubMed] [Google Scholar]
- 36. Vergote I, Oaknin A, Baurain JFet al. A phase 1b, open-label study of trebananib in combination with paclitaxel and carboplatin in patients with ovarian cancer receiving interval or primary debulking surgery. Eur J Cancer 2014; 50: 2408–2416 [DOI] [PubMed] [Google Scholar]
- 37. Monk BJ, Poveda A, Vergote Iet al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014; 15: 799–808 [DOI] [PubMed] [Google Scholar]
- 38. Monk BJ, Poveda A, Vergote Iet al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): long-term survival, impact of ascites, and progression-free survival-2. Gynecol Oncol 2016; 143: 27–34 [DOI] [PubMed] [Google Scholar]
- 39. Herbst RS, Hong D, Chap Let al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol 2009; 27: 3557–3565 [DOI] [PubMed] [Google Scholar]
- 40. Lee SJ, Lee CK, Kang Set al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J Clin Invest 2018; 128: 5018–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lefere S, Van de Velde F, Hoorens Aet al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology 2019; 69: 1087–1104 [DOI] [PubMed] [Google Scholar]
- 42. Khan M, Aziz AA, Shafi NAet al. Targeting angiopoietin in retinal vascular diseases: a literature review and summary of clinical trials involving faricimab. Cells 2020; 9: 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hussain RM, Neiweem AE, Kansara Vet al. Tie-2/angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs 2019; 28: 861–869 [DOI] [PubMed] [Google Scholar]
- 44. Sahni J, Patel SS, Dugel PUet al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology 2019; 126: 1155–1170 [DOI] [PubMed] [Google Scholar]
- 45. Khanani AM, Patel SS, Ferrone PJet al. Efficacy of every four monthly and quarterly dosing of faricimab versus ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol 2020; 138: 964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.