Abstract
The efficacy of cloning a recombinant mycotoxin antibody in plants was tested using Arabidopsis as a model. An antizearalenone single-chain Fv (scFv) DNA fragment was first cloned in the newly constructed phage display vector (pEY.5) and then recloned in the plant transformation vector pKYLX71::35S2. After transformation, constructs of antizearalenone scFv were introduced into immature Arabidopsis seeds via Agrobacterium tumefaciens mediation by vacuum infiltration. Only plants transformed with the construct containing a PR-1b signal peptide sequence produced transgenic offspring. The antizearalenone scFv “plantibody” from these transgenic plants bound zearalenone with a high affinity (50% inhibitory concentration, 11.2 ng/ml) that was comparable to that of bacterially produced scFv antibody and the parent monoclonal antibody (MAb). By electron microscopic immunogold labeling, the presence of antizearalenone scFv was detected mainly in the cytoplasm and only occasionally outside the cell. Like bacterially produced scFv antibody, antizearalenone scFv plantibody exhibited greater sensitivity to methanol destabilization than did the parent MAb. The sensitivity of antizearalenone scFv plantibody to acidic disassociation was similar to the sensitivities of bacterially produced scFv antibody and MAb. Expression of specific plantibodies in crops might be useful for neutralizing mycotoxins in animal feeds and for reducing mycotoxin-associated plant diseases.
Zearalenone [6-(10-hydroxy-6-oxo-trans-1-undecenyl)-β-resorcylic acid lactone] is a mycotoxin produced by members of the genus Fusarium after infection of corn and small grains (14, 24, 25). When fed to animals, the compound causes hyperestrogenism with symptoms such as enlargement of the uterus and nipples, vulvar swelling, vaginal prolapse, and infertility (16, 23). In the last 10 years, the expression of specific antibodies or antibody fragments in plants has attracted great interest (6, 15, 21, 30, 36, 37), and it has shown some potential in improving plant resistance against pathogens (33) and in altering plant metabolic pathways (1, 27). Recently, we developed a single-chain Fv (scFv) antibody with high affinity for zearalenone (38). To explore the possibility of using “plantibodies” to neutralize mycotoxin through passive immunization of animals in their feed, as a first step we used the newly cloned antizearalenone scFv DNA fragment to transform Arabidopsis plants. In this report, we demonstrate that expression of the antizearalenone scFv gene in transgenic Arabidopsis plants leads to the accumulation of a soluble scFv plantibody with high affinity for the mycotoxin zearalenone.
MATERIALS AND METHODS
General.
All chemicals and solvents were reagent grade or better. Chemicals were purchased from Sigma Chemical Company (St. Louis, Mo.) unless otherwise noted. All DNA manipulations, if not described, were carried out by standard procedures (28).
Construction of scFv cloning vector.
scFv, a single-chain fragment of the antibody variable region antigen-binding protein, is composed of an immunoglobulin heavy-chain variable domain (VH) and a light-chain variable domain (Vκ or Vλ) joined together by a flexible peptide linker. The assembly of the antibody sequence from VH, linker, and Vκ DNA fragments by PCR is one of the most problematic steps in scFv cloning (19). The assembly often results in undetectable amplificates or undefined DNA amplified products of various sizes. A new phage display vector was constructed to facilitate cloning and chain shuffling (intermixing of heavy and light chains) and to increase the efficiency of scFv assembly from VH and Vκ cDNA fragments. A 52-bp SfiI/NotI fragment in pCANTAB5E (Pharmacia Biotech, Piscataway, N.J.) was replaced with a DNA fragment encoding the (Gly4Ser)3 linker peptide and with new restriction sites which rarely exist in V gene regions. To test the efficacy of the new vector for scFv antibody cloning, an antizearalenone scFv DNA fragment was cloned and expressed by using this new vector in Escherichia coli.
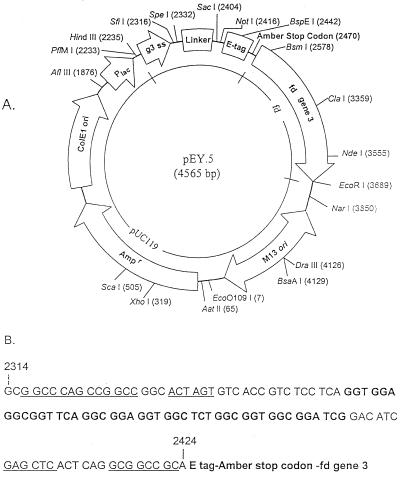
A DNA fragment (Pharmacia Biotech) that encoded a short peptide containing the structurally flexible peptide linker (Gly4Ser)3 was amplified by PCR with Taq DNA polymerase. The sense primer for amplifying the short peptide by PCR (5′-TCTATGCGGCCCAGCCGGCCGGCACTAGTGTCACCGTC-3′) contained the SfiI and SpeI restriction sites (underlined). The antisense primer (5′-AGCACCTGCGGCCGCCTGAGTGAGCTCGATGTCCGATCC-3′) had NotI and SacI sites (underlined). After electrophoresis in 1.5% low-melting-point agarose gel (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), the amplified fragment was excised from the gel and purified with the QIAEXII gel extraction kit (Qiagen, Chatsworth, Calif.). The purified DNA fragment was then digested with SfiI and NotI and ligated into pCANTAB5E (Pharmacia Biotech) digested with the same enzymes, creating pEY.5 (Fig. 1).
FIG. 1.
Map of phage display vector pEY.5. (A) Restriction map with unique restriction sites marked. (B) Nucleotide sequence of linker (boldface) and the VH and Vκ cloning sites (underlined) in pEY.5.
Antizearalenone scFv antibody cloning in pEY.5.
VH and Vκ DNA fragments were reamplified from previously cloned antizearalenone scFv phagemid DNA (pQY1.5) (38) using the following restriction site-containing primers. The primers used for VH DNA reamplification were VHFORY (5′-TGAGGAGACGGTGACACTAGTGCCTTGGC-3′) and VHBACKY (5′-ATGACTCGCGGCCCAGCCGGCCATGGCCSAGGTSMARCTGCAGSAGTCWGG-3′), where S = C or G, M = A or C, R = A or G, and W = A or T. The primers used for Vκ DNA reamplification were VKBACKY (5′-TCGGACATCGAGCTCACTCAGTCTCC-3′) and VKFORY (5′-GAGTCATTCTGCGGCCGCCCGTTTBAKYTCCARCTTKGTSCC-3′), where B = T or C or G, K = T or G, and Y = C or T. The reamplified VH DNA fragments were digested with SfiI and SpeI and ligated into pEY.5 digested with the same enzymes. The ligated products were then digested with SacI and NotI and ligated with reamplified Vκ DNA fragments that had been digested with the same enzymes, yielding pEY.5HL. pEY.5HL phagemid DNA was introduced into E. coli TG1 for the production of recombinant phages in the presence of helper phage M13KO7 and into E. coli HB2151 for soluble scFv antibody production, as described previously (38). The soluble scFv antibody from E. coli cultures was characterized with an indirect enzyme-linked immunosorbent assay (ELISA) (38).
Construction of plant scFv expression plasmids.
The antizearalenone scFv DNA fragment was amplified from a zearalenone-binding-positive clone, pEY.5HL3, by PCR using Pfu DNA polymerase (Stratagene, La Jolla, Calif.) with a sense primer (5′-TATCCGCGGTATGGCCCAGGTGAAACTGC-3′) containing an SstII site and an antisense primer (5′-CCCTCTAGACCAGACGTTAGTAAATGAA-3′) containing an XbaI site. The amplified DNA fragment was isolated from a low-melting-point agarose gel, purified with QIAEXII, digested with XbaI, and ligated into HincII-XbaI-digested pUC19, yielding pQY2. For expression without a signal peptide, a HindIII-XbaI fragment from pQY2 was ligated directly into the HindIII-XbaI sites of pKYLX71::35S2 (22), creating pQY4.1-1. For expression with a plant protein signal sequence, a 110-bp HindIII-SstII fragment containing the tobacco pathogenesis-related protein PR-1b signal peptide (SP) sequence from pSIG3 (a clone with the PR-1b SP sequence [12] ligated into the EcoRV site of Stratagene's pBluescript SK) was ligated into the HindIII-SstII sites of pQY2, generating pQY3. Finally, a HindIII-XbaI fragment containing the SP-scFv fusion fragment from pQY3 was ligated into the HindIII-XbaI sites of pKYLX71::35S2 (22), creating pQY4.4-1. Both pQY4.1-1 and pQY4.4-1 were introduced from E. coli DH5α into Agrobacterium tumefaciens strain GV3850 (39) by triparental mating (11).
Plant transformation.
Arabidopsis thaliana ecotype Columbia (GLABROUS1) plants were transformed using a vacuum infiltration protocol similar to the one described by Bechtold et al. (3). Mature seeds from these infiltrated plants were germinated and selected on plant nutrient agar plates (3) supplemented with 50 μg of kanamycin/ml. Kanamycin-resistant seedlings were transferred into soil pots, and the crude protein from leaves of 2-month-old kanamycin-resistant plants was tested for scFv antibody binding activity with ELISA as described below.
Expression of scFv plantibody and ELISA analysis.
In order to coat microtiter plate wells with zearalenone for the indirect ELISA, a zearalenone-ovalbumin (ZEN-OVA) conjugate was made as described previously (38). Immulon-4 microtiter wells (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with 1 μg of ZEN-OVA conjugate/well in 100 μl of 0.05 M carbonate-bicarbonate coating buffer (pH 9.4) at 4°C overnight. The wells were washed and blocked as described previously (38). Two or three leaves from 2-month-old transgenic plants were cut and ground with a pestle in a 1.5-ml microcentrifuge tube on ice. After centrifugation at 13,800 × g in a microcentrifuge for 10 min at 4°C, the sap (supernatant) was collected. Fifty microliters of the leaf sap was diluted 1:1 (vol/vol) with 2% nonfat dry milk in phosphate-buffered saline (PBS) and added to each well, followed by incubation at 37°C for 1 h. After washing six times with 320 μl of PBS–0.1% Tween 20 per well, 100 μl of mouse anti-E tag antibody per well (1 μg/ml) was added, followed by addition of goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase conjugate (diluted 1:2,000 in 2% nonfat dry milk in PBS). Finally, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma Chemical Company) was added per well and the mixture was incubated at 37°C for 15 min. The reaction was stopped by adding 100 μl of 10% H2SO4 per well. Absorbances at 450 nm were determined with a microtiter plant reader (Molecular Device Corporation, Menlo Park, Calif.).
For the competitive indirect (CI) ELISA, 50 μl of free zearalenone/well at various concentrations (0, 1, 10, 100, and 1,000 ng/ml in PBS containing 1% methanol) was added to ZEN-OVA-coated wells as described previously (38), and then 50 μl of diluted (1:49) sap from the transgenic Arabidopsis plants was added per well. After 1 h of incubation, the bound plantibodies were detected as described above. For comparison, a CI-ELISA was performed simultaneously with bacterial antizearalenone scFv expressed as a soluble protein in E. coli and diluted (1:9).
Methanol effects on binding activity were measured for the monoclonal antibody (MAb), bacterial scFv antibody, and scFv plantibody (diluted 10-fold in 10% nonfat milk-PBS) using the CI-ELISA as described above (38). The effect of pH on these antibodies was also tested. The antibodies were diluted (1:5 [vol/vol]) in buffer solutions of pH 2.3 and pH 6.3 (adjusted with 0.05 M citric acid and 0.05 M Na2HPO4) and incubated at 4°C overnight. A portion of the pH 2.3-treated sample was adjusted back to pH 6.3 and incubated at room temperature for 1 h. To wells coated with a zearalenone-keyhole limpet hemocyanin conjugate (1 μg/well), 100 μl of pH-treated antibody sample was added per well. Bound antibodies were measured as described above.
Protein analysis.
Proteins in leaf sap from independent transgenic plants were separated in a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel. Two identical gels were run at the same time. After electrophoresis, one gel was stained with Coomassie brilliant blue G-250. The proteins in the other gel were transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.). Mouse anti-E tag antibody, goat anti-mouse IgG-alkaline phosphatase conjugate (Sigma Chemical Company), and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Pierce, Rockford, Ill.) were used to detect plantibodies in a procedure similar to that previously described (38).
scFv plantibody cytolocation with immunogold labeling.
Segments (approximately 0.5 by 1.0 mm) of leaves from 2-month-old transgenic Arabidopsis plants were fixed by vacuum infiltration with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 h at room temperature. After three washes in the above buffer, the samples were postfixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer at room temperature for 2 h followed by dehydration with a graded acetone series. The samples were then embedded by infiltration with Spurtol resin (31; H. Kushida, Letter, J. Electron Microsc. 23:197, 1974). Ultrathin sections were cut and supported on Formvar-coated grids.
The grids containing ultrathin sections were pretreated by incubation in a saturated aqueous solution of sodium metaperiodate as described by Bendayan and Zollinger (4). After a thorough washing in distilled water, the pretreated grids were blocked for 1 h at room temperature in 20 mM Tris-buffered saline (TBS, pH 7.5) containing 0.05% Tween 20, 1% (wt/vol) bovine serum albumin, and 0.01% NaN3. The samples on the grids were then incubated with the primary antibody (mouse anti-E tag antibody) diluted in the blocking buffer for 2 h at room temperature. After four 5-min washings with TBS, the grids were incubated with goat anti-mouse IgG-gold conjugate (10-nm gold particles) (1:20 dilution) for 2 h at room temperature. After four 5-min washings with TBS and two 5-min washings in distilled water, the grids were poststained with 1% uranyl acetate for 30 min, rinsed in distilled water, and then stained with Hanaichi's lead solution (13) for 5 min. The grids were then rinsed in 20 mM NaOH and distilled water and examined with a CM10 transmission electron microscope (Philips, Amsterdam, The Netherlands). Immunogold labeling of sections of nontransformed Arabidopsis thaliana ecotype Columbia was performed as a control.
RESULTS
Cloning of antizearalenone scFv in pEY.5.
To determine the feasibility of using the new cloning vector pEY.5 for scFv antibody expression, the DNA fragments of VH and Vκ from pQY1.5 (38) were reamplified and then recloned into pEY.5 as described in the Materials and Methods section. In E. coli HB2151, one new clone (pEY.5HL3) produced soluble scFv antibody with high affinity for zearalenone (Fig. 2). The concentration of free zearalenone required for 50% inhibition of antizearalenone scFv binding to ZEN-OVA in the microtiter wells, 15.5 ng/ml, was similar to the results previously described for soluble scFv expressed by pQY1.5 (38). Because VH or Vκ alone was cloned in pEY.5 in frame with E tag and g3p, this vector is also suitable for selecting single-domain Fv antibodies and for chain shuffling. However, neither VH nor Vκ alone from pQY1.5 (38) showed any zearalenone binding activity when they were cloned into pEY.5 separately (data not shown), indicating that both VH and Vκ were required for the scFv binding to zearalenone. It was notable that E. coli HB2151/pEY.5HL3 grew much slower than HB2151/pEY.5, suggesting that functional antizearalenone scFv expression affected the growth of the E. coli host cells.
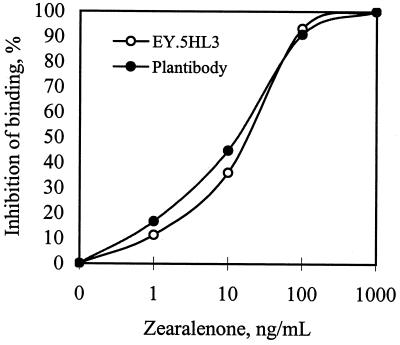
FIG. 2.
Sensitivity and specificity of antizearalenone scFv antibodies measured by CI-ELISA using ZEN-OVA-coated wells. Bound soluble scFv antibody was detected by mouse anti-E tag antibody followed by horseradish-peroxidase-conjugated goat anti-mouse IgG. EY.5HL3 was produced in E. coli and plantibody was produced in Arabidopsis.
Expression of antizearalenone scFv plantibody.
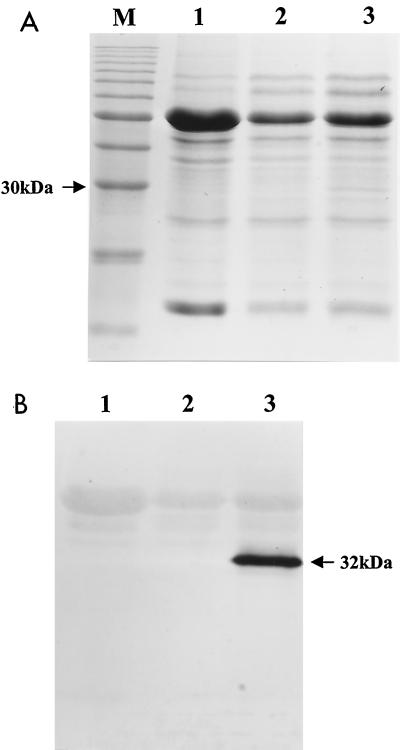
The antizearalenone scFv DNA fragment from pEY.5HL3 was recloned into a plant transformation vector to generate clones with and without the PR-1b SP sequence, pQY4.1-1 and pQY4.4-1, respectively (Fig. 3). About 1% of 20,000 seeds from plants transformed with GV3850/pQY4.4-1 were kanamycin resistant, whereas plants transformed with GV3850/pQY4.1-1 produced no kanamycin-resistant seedlings from approximately 20,000 seeds. In the putative transgenic (kanamycin-resistant) plants transformed with GV3850/pQY4.4-1, approximately 20% of the tested plants produced functional antibody for zearalenone as determined by ELISA. Western analysis showed that GV3850/pQY4.4-1-transformed Arabidopsis plants produced a 32-kDa scFv plantibody protein (Fig. 4) that was similar in size to the scFv antibody produced in E. coli (38). The specificity and sensitivity of the plantibody to zearalenone was determined by CI-ELISA (Fig. 2). The plantibody had a 50% inhibitory concentration of 11.2 ng/ml for zearalenone, showing an affinity for zearalenone similar to that of the scFv antibody produced by bacteria and the MAb from hybridoma cell cultures (38).
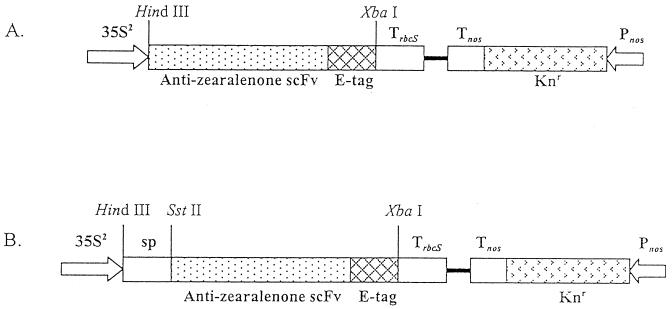
FIG. 3.
The transgene in plant transformation constructs pQY4.1-1 (A) and pQY4.4-1 (B). 35S2, CaMv 35S promoter with a duplicated enhancer; sp, plant pathogenicity-related protein PR-1b SP sequence; Trbcs and Tnos, transcription termination sequences for rbcS and nos, respectively; Pnos, nos promoter; Knr, kanamycin resistance gene.
FIG. 4.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of antizearalenone scFv plantibody expression. (A) An SDS-PAGE gel was stained with Coomassie brilliant blue G-250. (B) Proteins in a duplicate SDS-PAGE gel were detected by anti-E tag antibody. Lanes 1, soluble proteins of Arabidopsis from untransformed plants; lanes 2, vector pKYLX71::35S2-transformed plants; lanes 3, pQY4.4-1-transformed plants; lane M, protein molecular size marker with a 10-kDa ladder.
Crude leaf protein was extracted from 15 to 20 T1 generation plants grown from seed from each of the transformed parent plants and tested with ELISA for zearalenone binding. The number of T1 plants producing antizearalenone scFv varied. However, in the T2 generation derived only from plants expressing functional antizearalenone scFv, most of the plants produced antizearalenone scFv plantibody. This suggested that the antizearalenone scFv antibody gene was stably integrated into the genome of Arabidopsis.
Effects of methanol and low pH on plantibody.
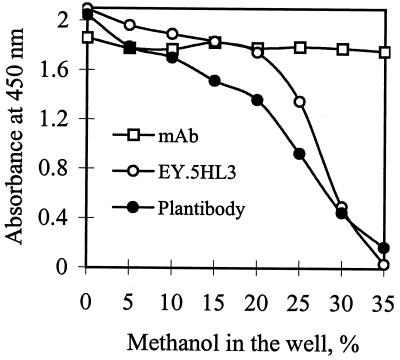
ELISAs used to detect zearalenone in grain all require the use of methanol-water (70:30) to extract zearalenone from the tissue sample (38). Because the extraction liquid comes into contact with antibody on the surface of the microtiter plate, the stability of the antibody in the presence of methanol is important. As shown previously, antizearalenone scFv antibody from bacteria exhibited greater sensitivity to methanol destabilization than the parent MAb (38). To test the effects of posttranslational modifications on the stability of scFv antibodies, antizearalenone scFv plantibody was compared with scFv antibody produced in E. coli and the parental MAb for stability in methanol and in an acidic environment. The scFv plantibody from pQY4.4-1-transformed Arabidopsis plants was as sensitive to methanol destabilization as the soluble scFv expressed in E. coli (Fig. 5), whereas the parent MAb exhibited tolerance to much higher concentrations of methanol. In the low-pH environment (pH 2.3), all three antibodies (MAb, bacterial scFv antibody, and scFv plantibody) lost binding activity to zearalenone (Table 1). When the pH was adjusted back to neutral (pH 6.3), the binding activity was recovered.
FIG. 5.
Effect of methanol on binding of antizearalenone MAb and bacterial scFv antibody (EY.5HL3) or plant scFv antibody (plantibody) to immobilized zearalenone-keyhole limpet hemocyanin conjugate.
TABLE 1.
Effect of pH environment on antibody binding activitya
| Antizearalenone antibody | Relative binding activity (%)b
|
||
|---|---|---|---|
| pH 6.3 | pH 2.3 | pH 2.3 then pH 6.3 | |
| MAb | 100 | 0 | 99 |
| Bacterial scFv | 100 | 0 | 98 |
| scFv plantibody | 100 | 0 | 89 |
Antibodies were diluted (1:5 [vol/vol]) in pH 2.3 or pH 6.3 buffer solutions (adjusted with 0.05 M citric acid or 0.05 M Na2HPO4) and incubated at 4°C overnight. A portion of the pH 2.3-treated sample was adjusted back to pH 6.3 and incubated at room temperature for 1 h.
Binding activity at pH 6.3 was set at 100%.
Localization of antizearalenone scFv plantibody.
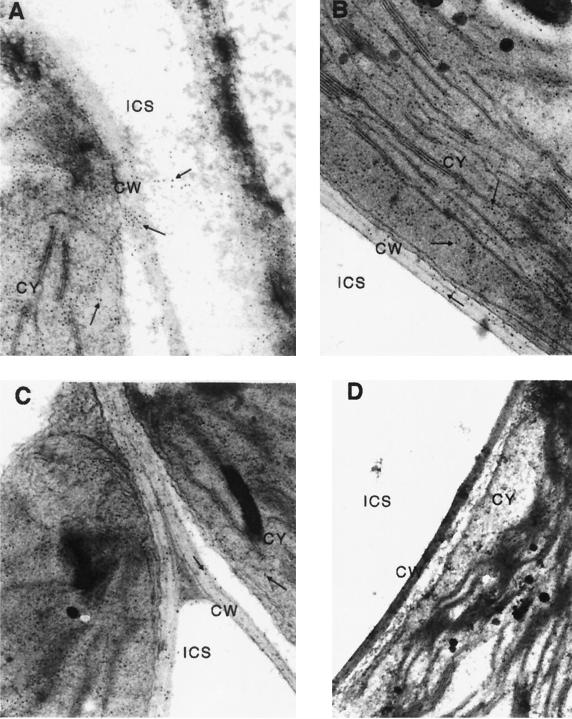
Transgenic Arabidopsis plants expressing antizearalenone scFv antibody were processed and labeled for electron microscopic immunolocalization as described in Materials and Methods. Gold particle deposition indicated that scFv antibody was distributed evenly in electron-dense areas such as the chloroplast, cytoplasm, nucleus, and endoplasmic reticulum, suggesting that some expressed plantibody remained inside the plant cells (Fig. 6A and B and Table 2). The immunogold labeling density in the electron-thin areas, such as the intercellular spaces and vacuoles, was dramatically lower than that in the electron-dense areas (Fig. 6A and Table 2). Incubation of the nontransformed plant sections with the antizearalenone scFv antibody and the gold-labeled anti-IgG antibodies also resulted in discernible gold labeling (Fig. 6C). This finding suggested that antizearalenone scFv antibody was binding to Arabidopsis cell components. In the nontransformed control plant samples, very little immunogold labeling was due to nonspecific binding (Fig. 6D and Table 2).
FIG. 6.
Cytolocation of antizearalenone scFv plantibody by immunogold labeling and transmission electron microscopy. Antizearalenone scFv plantibodies from leaf sections of plants expressing plantibody (A and B) and from nontransformed control plants (D) were detected by mouse anti-E tag antibody followed by goat anti-mouse IgG-gold conjugate. (C) Leaf sections from nontransformed control plants were preincubated with antizearalenone scFv antibody and then incubated with mouse anti-E tag antibody followed by goat anti-mouse IgG-gold conjugate. CW, cell wall; CY, cytoplasm; ICS, intercellular space. Arrows indicate gold labeling. Magnification, ×39,000.
TABLE 2.
Intensity of gold labeling of antizearalenone scFv associated with different components of plant cells in transgenic plants expressing antizearalenone scFv and in nontransformed healthy plantsa
| Cell component | No. of gold particles/μm2 (mean ± SE)
|
|
|---|---|---|
| Transgenic plant | Healthy plant | |
| Chloroplast | 189 ± 3.1 | 3.6 ± 0.55 |
| Nucleus | 187 ± 3.6 | 4.6 ± 0.70 |
| Cytoplasm | 191 ± 2.5 | 2.6 ± 0.80 |
| Vacuole | 1.9 ± 0.56 | 2.3 ± 0.61 |
| Rough endoplasmic reticulum | 194 ± 3.0 | 3.2 ± 0.85 |
| Cell wall | 172 ± 2.0 | 2.4 ± 0.89 |
| Intercellular space | 23 ± 2.1 | 2.8 ± 0.7 |
ScFv was detected using mouse anti-E tag antibody followed by goat anti-mouse IgG-gold conjugate. Untransformed plant leaf sections were treated with antizearalenone scFv followed by goat anti-mouse IgG-gold conjugate.
DISCUSSION
In this investigation, an scFv plantibody with high affinity for zearalenone was produced from stable transgenic Arabidopsis plants. To our knowledge, this is the first report of an antimycotoxin antibody gene expressed in plants. Like other scFv plantibodies (5), this antizearalenone scFv plantibody had antigen-binding properties similar to those of bacterially produced scFv antibody.
The antizearalenone scFv DNA fragment was cloned in a newly constructed vector, pEY.5. This new vector maintained the advantageous features of the original vector, pCANTAB5E—an inducible lac promoter, a short peptide linker (Gly4Ser)3, an E tag marker, and an amber stop codon that allows switching between expression of a membrane-anchored scFv-g3p fusion and expression of a soluble scFv—simply by changing the expression host from E. coli strain TG1 to strain HB2151. The new vector also allowed the direct cloning of VH and Vκ with the introduction of two rare-cutting restriction enzyme sites (SpeI and SacI). In the Kabat Database of Sequences of Proteins of Immunological Interest (http://immuno.bme.nwu.edu), the SpeI recognition sequence was found in less than 1% of 4,701 known murine VH chains and 1% of 2,492 known murine Vκ chains. SacI has a slightly higher cutting frequency but accounts for only 4.5 and 2.3% of known VH and Vκ chains, respectively. Therefore, the majority of variable region cDNAs would be free from internal cutting. We tested the feasibility of this new vector by cloning and expressing an antizearalenone scFv in E. coli. By utilizing the SfiI/SpeI or SacI/NotI sites, this new vector could also facilitate chain shuffling, which may be useful for cloning compatible variable chains and improving antigen-binding ability (18).
For plant transformation, two constructs of the antizearalenone scFv were made by fusion with or without the N-terminal SP from the tobacco pathogenesis-related protein PR-1b, which has been used to secrete foreign proteins into the plant apoplast (9, 20). The PR-1b signal sequence was included with the expectation that it would target the fusion protein to the endoplasmic reticulum, where the SP would be cleaved and the scFv protein would be transported to the apoplast via the default pathway (7). Plants transformed with this signal-sequence-containing construct produced many transformants expressing scFv plantibody with high affinity for zearalenone. However, electron microscopic immunolocalization indicated the presence of the scFv plantibody mainly in the cytoplasm (Fig. 6 and Table 2). Only a low level of labeling signals was found in the intercellular space and was limited to those parts of the intercellular space that were filled with electron-dense material. The labeling signals in the cytoplasm might have reflected binding activity of antizearalenone scFv to some plant cell components, since immunogold labeling showed that antizearalenone scFv antibody binds to nontransformed Arabidopsis cell components in the cytoplasm (Fig. 6C). Although some antibodies or antibody fragments that have been fused with plant signal sequences have been confirmed by immunogold electron microscopy as being secreted through the cell wall into intercellular spaces, despite limitations in cell wall porosity (8), other antibodies were retained in the endoplasmic reticulum compartment or in the cytoplasm (1, 10, 29). The construct without the signal sequence failed to generate any transgenic plants in this study. One explanation might be that the high accumulation of antizearalenone scFv in the plant cytoplasm and the sequential binding to plant cell components were toxic to the host plant cell.
As previously reported, antizearalenone scFv antibody produced from bacteria had greater sensitivity to methanol destabilization than its parent MAb. One possible explanation may be the differences between prokaryotes and eukaryotes in posttranslational protein modifications, especially glycosylation. N-linked glycosylation of proteins in plants is very similar to the glycosylation process in mammals (17, 32). Possible plant posttranslational changes in addition to glycosylation include fatty acylation, phosphorylation, sulfation, and modifications that change the pI of the protein (17). The comparison between antibodies produced in bacteria and plants may help elucidate the role of posttranslational modifications in stabilizing the antibodies. Unfortunately, it was not possible to test the hypothesis that differences between antizearalenone scFv antibody and its parent MAb in methanol sensitivity reflect structural differences derived from posttranslational modifications, because the antizearalenone scFv lacks the canonical Asn-X-Ser/Thr signal sequence for glycosylation. The small differences observed between plantibody and bacterial scFv (Fig. 5 and Table 1) may be attributable to the use of crude scFv preparations rather than purified scFv. Also, the CI-ELISA was not optimized for the crude scFv preparations.
Some mycotoxins not only cause toxic effects in animals and humans but also serve as virulence factors of the producing fungi for causing plant diseases. The mycotoxin zearalenone has been reported to cause certain phytotoxicities (26, 34, 35). The expression of antizearalenone antibody in plants might be useful in reducing the severity of plant diseases associated with this mycotoxin.
Antibodies have been used successfully as antidotes for some low-molecular-weight toxins, through passive immunization (2). However, large quantities of antibody are generally required for passive immunization in vivo. The prospect of harvesting antibody on an agricultural scale offers the potential for economical production of almost limitless amounts of antibody. The production of antibody in edible plants makes it possible to deliver large amounts of toxin-specific antibody through food. Feed-delivered antibody could theoretically bind zearalenone in the intestine, provided that the antibody can survive passage through the acidic stomach environment. When the antizearalenone antibody was subjected to low pH, the MAb and scFv antibodies from both bacteria and plants lost their binding ability. However, all three antibodies recovered binding activity when the pH was adjusted back to neutral (pH 6.3). This suggests that the pH effects on binding activity are not the result of irreversible denaturation. Thus, provided that these antibodies survive proteolysis in the gut, they could bind to zearalenone in the small intestine, where the pH is close to neutral. Whether the antizearalenone plantibody reported in this study will neutralize zearalenone in animal feed and thus reduce toxicity will be determined in the future.
The expression of mycotoxin-specific plantibody could have a role in the development of new or novel forms of plant resistance for some mycotoxins that are important for the initiation or development of plant diseases, especially if catalytic plantibodies that degrade mycotoxins could be developed and expressed in crops.
The most promising role for plantibodies is their potential for large-scale production in plants. Plants are relatively easy and inexpensive to produce, and the equipment already exists for harvesting and processing large volumes of plant material. The prospect of harvesting antibody or antibody fragments on an agricultural scale would mean that antibodies would be available extremely cheaply in almost limitless amounts. In addition, plants are capable of targeting proteins to a number of different tissues for long-term storage and distribution. Although there may be safety concerns about using plantibodies in therapy, antibodies produced in plants also could be used for in vitro diagnosis and for environmental detoxification.
ACKNOWLEDGMENTS
This work was supported by USDA-NRI grant no. 9702545, Michigan State University Agricultural Experiment Station, the MSU Crop and Food Bioprocessing Center, and the MSU National Food Safety and Toxicology Center.
We thank Jing Yuan for her help in plant transformation.
REFERENCES
- 1.Artsaenko O, Peisker M, zur Nieden U, Fiedler U, Weiler E W, Muntz K, Conrad U. Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J. 1995;8:745–750. doi: 10.1046/j.1365-313x.1995.08050745.x. [DOI] [PubMed] [Google Scholar]
- 2.Baud F J, Borron S W, Scherrmann J M, Bismuth C. A critical review of antidotal immunotherapy for low molecular weight toxins. Current antidotes and perspectives. Arch Toxicol Suppl. 1997;19:271–287. doi: 10.1007/978-3-642-60682-3_25. [DOI] [PubMed] [Google Scholar]
- 3.Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III. 1993;316:1194–1199. [Google Scholar]
- 4.Bendayan M, Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983;31:101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- 5.Bruyns A M, De Jaeger G, De Neve M, De Wilde C, Van Montagu M, Depicker A. Bacterial and plant-produced scFv proteins have similar antigen-binding properties. FEBS Lett. 1996;386:5–10. doi: 10.1016/0014-5793(96)00372-9. [DOI] [PubMed] [Google Scholar]
- 6.Conrad U, Fiedler U. Expression of engineered antibodies in plant cells. Plant Mol Biol. 1994;26:1023–1030. doi: 10.1007/BF00040685. [DOI] [PubMed] [Google Scholar]
- 7.Denecke J, Bottermann J, Deblaere R. Protein secretion in plant cells can occur via the default pathway. Plant Cell. 1990;2:51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wilde C, De Neve M, De Rycke R, Bruyns A M, De Jaeger G, Van Montagu M, Depicker A, Engler G. Intact antigen-binding MAK33 antibody and F-ab fragment accumulate in intercellular spaces of Arabidopsis thaliana. Plant Sci. 1996;114:233–241. [Google Scholar]
- 9.Dixon D C, Cutt J R, Klessig D F. Differential targeting of the tobacco PR-1 pathogenesis-related proteins to the extracellular space and vacuoles of crystal idioblasts. EMBO J. 1991;10:1317–1324. doi: 10.1002/j.1460-2075.1991.tb07650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Düring K, Hippe S, Kreuzaler F, Schell J. Synthesis and self-assembly of a functional monoclonal antibody in transgenic Nicotiana tabacum. Plant Mol Biol. 1990;15:281–293. doi: 10.1007/BF00036914. [DOI] [PubMed] [Google Scholar]
- 11.Figurski D, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalan S, Bauer D W, Alfano J R, Loniello A O, He S Y, Collmer A. Expression of the Pseudomonas syringae avirulence protein AvrB in plant cells alleviates its dependence on the hypersensitive response and pathogenicity (Hrp) secretion system in eliciting genotype-specific hypersensitive cell death. Plant Cell. 1996;8:1095–1105. doi: 10.1105/tpc.8.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanaichi T, Sato T, Iwamoto T, Malavasi-Yamashiro J, Hoshino M, Mizuno N. A stable lead by modification of Sato's method. J Electron Microsc. 1986;35:304–306. [PubMed] [Google Scholar]
- 14.Hart L P, Braselton W E, Stebbins T C. Production of zearalenone and deoxynivalenol in commercial sweet corn. Plant Dis. 1982;66:1133–1135. [Google Scholar]
- 15.Hiatt A, Ma J K. Monoclonal antibody engineering in plants. FEBS Lett. 1992;307:71–75. doi: 10.1016/0014-5793(92)80904-u. [DOI] [PubMed] [Google Scholar]
- 16.Hidy P H, Baldwin R S, Greasham R L, Keith C L, McMullen J R. Zearalenone and some derivatives: production and biological activities. Adv Appl Microbiol. 1977;22:59–82. doi: 10.1016/s0065-2164(08)70160-6. [DOI] [PubMed] [Google Scholar]
- 17.Jones R L, Robinson D G. Protein secretion in plants. Tansley Rev. 1989;17:567–588. doi: 10.1111/j.1469-8137.1989.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang A S, Jones T M, Burton D R. Antibody redesign by chain shuffling from random combinatorial immunoglobulin libraries. Proc Natl Acad Sci USA. 1991;88:11120–11123. doi: 10.1073/pnas.88.24.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer K, Hock B. Recombinant single-chain antibodies against s-triazines. Food Agric Immunol. 1996;8:97–109. [Google Scholar]
- 20.Lund P, Dunsmuir P. A plant signal sequence enhances the secretion of bacterial ChiA in transgenic tobacco. Plant Mol Biol. 1992;18:47–53. doi: 10.1007/BF00018455. [DOI] [PubMed] [Google Scholar]
- 21.Ma J K C, Hein M B. Plant antibodies for immunotherapy. Plant Physiol. 1995;109:341–346. doi: 10.1104/pp.109.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiti I B, Murphy J F, Shaw J G, Hunt A G. Plants that express a potyvirus proteinase gene are resistant to virus infection. Proc Natl Acad Sci USA. 1993;90:6110–6114. doi: 10.1073/pnas.90.13.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirocha C J, Christensen C M. Oestrogenic mycotoxins synthesized by Fusarium. In: Purchase I F H, editor. Mycotoxins. Amsterdam, The Netherlands: Elsevier Scientific Publishing Co.; 1974. pp. 129–148. [Google Scholar]
- 24.Mirocha C J, Christensen C M, Nelson G H. F-2 (zearalenone) estrogenic mycotoxin from Fusarium. In: Kadis S, Ciegler A, Ayl S J, editors. Microbial toxins. Vol. 7. New York, N.Y: Academic Press; 1971. pp. 107–138. [Google Scholar]
- 25.Mirocha C J, Pathre S V, Christensen C M. Zearalenone. In: Rodricks J V, Hesseltine C W, Mehlman M A, editors. Mycotoxins in human and animal health. Forest Park, Ill: Pathotox Publishers, Inc.; 1977. pp. 345–364. [Google Scholar]
- 26.Nedelnik J. Phytotoxicity of some Fusarium spp. toxins to clover plants. Ochr Rostl. 1993;29:69–76. [Google Scholar]
- 27.Owen M, Gandecha A, Cockburn B, Whitelam G. Synthesis of a functional anti-phytochrome single-chain Fv protein in transgenic tobacco. Bio/Technology. 1992;10:790–794. doi: 10.1038/nbt0792-790. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Schouten A, Roosien J, de Boer J M, Wilmink A, Rosso M N, Bosch D, Stiekema W J, Gommers F J, Bakker J, Schots A. Improving scFv antibody expression levels in the plant cytosol. FEBS Lett. 1997;415:235–241. doi: 10.1016/s0014-5793(97)01129-0. [DOI] [PubMed] [Google Scholar]
- 30.Smith M D. Antibody production in plants. Biotechnol Adv. 1996;14:267–281. doi: 10.1016/0734-9750(96)00020-1. [DOI] [PubMed] [Google Scholar]
- 31.Spurr A R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 32.Sturm A, Kuik A V, Vliegenthart J F G, Crispeels M J. Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem. 1987;262:13392–13403. [PubMed] [Google Scholar]
- 33.Tavladoraki P, Benvenuto E, Trinca S, De M D, Cattaneo A, Galeffi P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature. 1993;366:469–472. doi: 10.1038/366469a0. [DOI] [PubMed] [Google Scholar]
- 34.Vianello A, Macri F. Effect of zearalenone (F-2) on pea (Pisum sativum cultivar Alaska) stem, maize (Zea mays cultivar DeKalb XL 342) root and rat liver mitochondria. Planta. 1981;153:443–446. doi: 10.1007/BF00394983. [DOI] [PubMed] [Google Scholar]
- 35.Wakulinski W. Phytotoxicity of the secondary metabolites of fungi causing wheat head fusariosis (head blight) Acta Physiol Plant. 1989;11:301–306. [Google Scholar]
- 36.Whitelam G C. The production of recombinant proteins in plants. J Sci Food Agric. 1995;68:1–9. [Google Scholar]
- 37.Whitelam G C, Cockburn W. Antibody expression in transgenic plants. Trends Plant Sci. 1996;1:268–272. [Google Scholar]
- 38.Yuan Q, Clarke J R, Zhou H-R, Linz J E, Pestka J J, Hart L P. Molecular cloning, expression, and characterization of a functional single-chain Fv antibody to the mycotoxin zearalenone. Appl Environ Microbiol. 1997;63:263–269. doi: 10.1128/aem.63.1.263-269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zambryski P, Joos H, Gentello C, Leemans J, Van Montagu M, Schell J. Ti-plasmid vector for the introduction of DNA into plant cells without altering their normal regeneration capacity. EMBO J. 1983;2:2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]