Abstract
Aim
The present study aimed to determine the folic acid supplement (FAS) effects on serum homocysteine and sortilin levels, glycemic indices, and lipid profile in type II diabetic patients.
Method
A double-blind randomized controlled clinical trial have been performed on 100 patients with T2DM randomly divided into two groups that received either placebo or folic acid 5 mg/d for 12 weeks.
Results
FAS caused a significant decrease in homocysteine and sortilin serum levels (28.2% and 33.7%, P < 0.0001, respectively). After 3 months of intervention, 8.7% decrease in fasting blood glucose (P = 0.0005), 8.2% in HbA1c (P = 0.0002), 13.7% in serum insulin (P < 0.0001) and 21.7% in insulin resistance (P < 0.0001) were found in the folic acid group, however no significant difference was observed in the placebo group. Serum hs-CRP level showed significant positive associations with sortilin (r = 0.237, P = 0.018), homocysteine (r = 0.308, P = 0.002) and fasting blood glucose (r = 0.342, P = 0.000). There were no significant changes in lipid profile in both groups after 12 weeks.
Conclusion
FAS might be beneficial for reducing homocysteine and sortilin levels, enhancing glycemic control, and improved insulin resistance in patients with T2DM.
Subject terms: Endocrine system and metabolic diseases, Lipids
Introduction
Diabetes mellitus is one of the extremely common chronic ailments in nearly all countries and major health care problem worldwide [1].
Multiple risk factors have been recognized as potential contributors for cardiovascular disease (CVD) in diabetic patients including hyperglycemia, elevated glycated hemoglobin (HbA1c) concentrations, hyperinsulinemia, hypertension, obesity, hypercholesterolemia, hypertriglyceridemia, and smoking [2].
In addition to these factors, it is known that elevated homocysteine (Hcy) levels can be associated with cardiovascular disease and increased atherosclerosis risk, peripheral vascular disease, ischemic heart disease, and stroke [3–5].
In diabetic patients, hyperhomocysteinemia is correlated with insulin resistance [6], dyslipidemia, and poor control of the disease [7, 8]. Hyperhomocysteinemia results in the worsening of T2DM by induction of reversible β-islet cell dysfunction and insulin secretion inhibition [9]. Levels of circulating Hcy are raised in patients with T2DM in contrast to non-diabetic individuals [10].
Folic acid is an essential factor in determining plasma Hcy concentrations [11, 12]. Therefore, folic acid supplementation (FAS) lowers the Hcy level by increasing the 5-methyltetrahydrofolate intracellular pool, which may improve the overall management of diabetic patients and may decrease cardiovascular events [3, 10]. Hcy level may be a useful predictor of end-organ damage, including cardiac, carotid, and renal diseases, in newly diagnosed T2DM patients [13].
Insulin resistance, β-cell dysfunction, poor glucose tolerance, and mitochondrial dysfunction have all been linked to oxidative stress [14]. Because of its antioxidant capabilities, the folic acid treatment improved the glycemic profile significantly [15]. Inflammation is linked to the development of insulin resistance. Proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and IL-6 have been shown to affect insulin signaling, which can lead to insulin resistance [16]. FAS has also been shown to reduce NF-kB activity, which inhibits the proinflammatory process [17].
Chronic inflammatory biomarkers, like C-reactive protein (CRP) levels, have been linked with the existence and extent of the metabolic syndrome [18], preclinical atherosclerosis, and the development of atherosclerosis [19]. Furthermore, it has been reported that high sensitivity C-reactive protein (hs-CRP) is recognized as the predictor of a cardiovascular event [20].
Sortilin is mainly found within cells in sites such as the cell membrane and systemic circulation, and it is also found in the trans-Golgi network [21, 22]. It plays a key role in many biological processes including glucose and lipid metabolism, and atherosclerosis which are risk factors for CVD [23, 24].
Sortilin has been linked with circulating low-density lipoprotein cholesterol (LDL-C) levels and with the risk of developing atherosclerosis [25–27]. Sortilin also appears to be a key factor in hepatic and muscular response to insulin, suggesting that it could be a link between insulin resistance and hypercholesterolemia. A study demonstrates that sortilin correlates with the incidence of major adverse cardiovascular events in T2DM [22].
Lipid profiles show the potential markers that can be used in predicting glycemic control in patients with T2DM [28].
This study aimed to examine the effect of oral FAS for 12 weeks on levels of serum Hcy and sortilin, glycemic control, lipid profile and insulin resistance in patients with T2DM. The primary outcome was the glycemic control [fasting blood glucose, glycated hemoglobin, and homeostasis model assessment-insulin resistance (HOMA-IR)]. The secondary outcome was the changes in serum levels of Homocysteine, sortilin, and lipid profile.
Patients and method
Study design
This was a randomized controlled double-blind clinical trial that was performed on 100 patients with T2DM aged between 45 and 75 years, who attended the Damanhour National Medical Institute, Egypt during the period starting from April 2020 to February 2021. The study protocol was approved by the Research Ethics Committee of the Faculty of Pharmacy, Damanhour University (Ref No. 122PP48) and adhered to the principles of the Declaration of Helsinki regarding the ethical principles for research involving human subjects. Written informed consent was obtained from all the patients prior to participation in the study.
Patients who visited the outpatient clinic of Internal Medicine and Diabetes Department were evaluated for their eligibility in the present study. Inclusion criteria were patients with a confirmed diagnosis of T2DM at the time of admission under metformin treatment (1500 mg daily for more than six months), other medications, and diet therapy continued unchanged during the study period.
The patients having the following circumstances were excluded from this study: thyroid dysfunction, hepatic failure, renal disorders, cardiovascular disease, gastrointestinal disorders, folate antagonist medication usage (i.e., methotrexate), cigarette smoking (those who smoke greater than or equal to 25 cigarettes per day), pregnancy, and chronic alcohol consumption. The patients who had insulin therapy, consumed folic acid, or treated with statins within the last 3 months were also excluded from the study.
The patients were randomly allocated using computer-generated random sequence in (1:1 ratio) to enroll either in the folic acid group, 50 patients received FAS for 12 weeks (5 mg daily); or in the placebo group, 50 patients received placebo. The sequence was given to an external data manager with no involvement in the study procedures and concealed on a password-protected computer. Once the patient has consented to participate, he assigns the treatment group using the sequentially numbered, opaque, sealed envelope (SNOSE) technique. Both groups were matched for age, weight, glycemic control indicators, and medication use.
The participants were advised to continue their physical activity, routine healthy dietary intake, and medication during the study period.
Adherence to FAS was determined by questioning the participants about the average number of tablets used per week throughout the study period.
Adherence to FAS: Respondents who consumed at least five tablets per week throughout their study duration were classified as adherent, while those who took less than five tablets per week were non-adherent and excluded from the study.
Blood sampling
Patients attended the hospital in the morning following an overnight fast for 8–12 h. Patients were permitted to sit comfortably for 10 min, and the patient’s height, weight, and blood pressure were assessed. Then venous blood samples (∼10 ml) were collected in ordinary and EDTA vacuum tubes, then centrifuged at 4000 rpm for 10 min at 4 °C.
After blood collection, the samples were instantly cooled, and the serum and the plasma were removed within 1 h and aliquoted into separate tubes, and stored at −80°C until analysis.
Anthropometric evaluation
Anthropometric measurements, age (years), weight (kilograms), and height (centimeters) of the selected patients were evaluated while patients were barefoot and wearing informal dresses. The blood pressure of the patients in the sitting position was measured, following a 15-minute resting period. The following equation was used to calculate body mass index (BMI): weight (kg)/square meter of height (m2) [29].
Biochemical assays
Plasma glucose was assessed by the glucose oxidase method. The ion exchange method using kits obtained from Stanbio Laboratory Company (USA) was used to measure HbA1c%. Serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were determined calorimetrically using kits acquired from Elitech Diagnostics Company (France). LDL-C was calculated by the Friedewald formula [30]. Fasting plasma insulin was analysed using Enzyme-Linked Immunosorbent Assay (ELISA) kit (DRG International, Inc., USA). Homeostasis model assessment-insulin resistance (HOMA-IR) was computed according to the following formula: fasting glucose (mg/dl) X fasting insulin (µIU/ml)/405.
hs-CRP was assayed in serum by nephelometric procedure (Dade Behring, BNII, Marburg, Germany).
Circulating serum sortilin and homocysteine were measured by ELISA kits (Cosmo Bio, Carlsbad, CA, USA). The intra and inter-assay coefficients of variation for homocysteine and sortilin, were <10% and <12%, respectively. The sensitivity was estimated to be 0.2 μmol/L-15 μmol/L for homocysteine and 30 pg/ml-2000 pg/ml for sortilin. The serum levels were measured twice, and the results were averaged for each patient.
Adverse effects
Participants were also followed-up by telephone calls and through direct meetings during the study to assess their adherence and report any drugs related adverse effects using an adverse effect questionnaire. The adverse effects were also collected from the patients’ laboratory data and patient sheets.
Statistical analyses
The sample size was calculated using G*Power software version 3.1.9.7 (Institut für Experimentelle Psychologie, Heinrich Heine Universität, Dfüsseldorf, Germany). It was estimated that a total sample size of 100 patients would have a power of 97% to detect a medium to large effect size of 0.80 in the measured parameters. Data were tested for normality by Kolmogorov–Smirnov test. Chi–squared or Fisher’s exact test (2-sided) was used to compare qualitative data which were described as numbers and percentages. Continuous data were expressed as means ± SD and paired t-test was used to determine differences in mean anthropometric and clinical characteristics before and after FAS. Independent sample t-test was used to compare between groups. Pearson’s correlation test was accomplished to evaluate the relationship between changes in Hcy, sortilin, and other variables after supplementation. Receiver-operating characteristic (ROC) area under the curve (AUC) analysis was used to evaluate the sensitivity of the measured variables among T2D patients. Furthermore, multiple regression model was used to determine whether there was any association between HOMA-IR with Hcy, hs-CRP, and sortilin. All statistical analyses were performed using the SPSS for Windows ver. 23.0 software. The reported adverse events in both groups were expressed as numbers and percentage. The level of significance set for the study was P < 0.05.
Results
At first, 105 patients participated in this study after verifying the inclusion and exclusion criteria as previously stated for patients’ selection. Although, 5 patients were excluded from the study throughout 3-month treatment period for the following purposes: two patients were non-adherent to folic acid administration and 3 patients were non-adherent to their medications. Finally, 100 patients were continued in this study (Fig. 1).
Fig. 1.
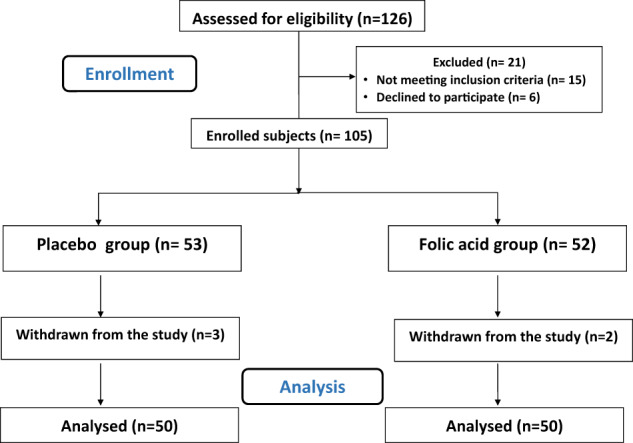
CONSORT flowchart of the study.
Baseline and anthropometric characteristics of the participants in both groups
The folic acid group showed no significant difference in baseline characteristics compared to the placebo group (P > 0.05). This study included 45 males. Participants’ mean age was 54.46 ± 7.81 vs 52.86 ± 9.46 years in the placebo and folic acid groups, respectively. The mean duration of diabetic status was 7.14 ± 3.76 vs 7.82 ± 3.94 years in the placebo and folic acid groups, respectively. Associated diseases recorded for the participants were HTN (54% vs 46%) and hyperlipidemia (28% vs 24%) in the placebo and folic acid groups, respectively. Among the participants, 34% were smokers in the placebo group whereas 30% were smokers in the folic acid group with in-significant differences between both groups. Commonly co-administered medications were ACEIs (38% vs 46%), ARBs (34% vs 42%), Beta-blockers (14% vs 6%) and CCB (20% vs 22%) in placebo and folic acid group, respectively. Both groups demonstrated no statistically significant change in demographic data, medical history, and medication history (Table 1).
Table 1.
General characteristics of the study participants at baseline.
| Variables | Placebo group (n = 50) | Folic acid group (n = 50) | P-value |
|---|---|---|---|
| Male, n (%) | 23 (46) | 22 (44) | 0.841 |
| Age (years) | 54.46 ± 7.81 | 52.86 ± 9.46 | 0.36 |
| Duration of diabetes (years) | 7.14 ± 3.76 | 7.82 ± 3.94 | 0.38 |
| Hypertension, n (%) | 27 (54) | 23 (46) | 0.424 |
| Hyperlipidemia, n (%) | 14 (28) | 12 (24) | 0.648 |
| Smoker, n (%) | 17 (34) | 15 (30) | 0.668 |
| ACEIs use, n (%) | 19 (38) | 23 (46) | 0.418 |
| ARB use, n (%) | 17 (34) | 21 (42) | 0.410 |
| BB use, n (%) | 7 (14) | 3 (6) | 0.182 |
| CCB use, n (%) | 10 (20) | 11 (22) | 0.806 |
| OHA Co-treatment with Metformin | |||
| Glimepiride, n (%) | 20 (50) | 25 (50) | 0.315 |
| Glibenclamide, n (%) | 18 (36) | 15 (30) | 0.523 |
| Vildagliptin, n (%) | 12 (24) | 10 (20) | 0.629 |
Data presented as number (percentage) or mean ± SD.
ACEIs Angiotensin-converting enzyme inhibitors, ARBs Angiotensin Receptor Blockers, BB Beta-blockers, CCB Calcium channel blocker, OHA Oral hypoglycemic agents.
Data analysed using independent sample t-test or Chi-square as appropriate. Statistically significant between groups at P < 0.05.
Glycemic control, HOMA-IR, and body mass index
The mean BMI of the participants not changed in studied groups after folic acid supplementation (P > 0.05). FAS for 3 months reduced fasting blood glucose (FBG) (P = 0.0005), HbA1c (P = 0.0002), insulin level (P < 0.0001) and HOMA-IR (P < 0.0001). After receiving FAS for 3 months, placebo group showed no statistically significant differences in FBG, HbA1c, insulin level, and HOMA-IR (P > 0.05). After 3 months of intervention, a significant change was found between both groups regarding FBG (P = 0.001), HbA1c (P = 0.003), and HOMA-IR (P = 0.004). On the other hand, insulin levels didn’t change significantly in both groups (Table 2).
Table 2.
Comparison of biochemical parameters at baseline and after folic acid supplementation between two study groups.
| Variables | Placebo group (n = 50) | Folic acid group (n = 50) | *P-value | |
|---|---|---|---|---|
| BMI (kg/m2) | Baseline | 27.69 ± 2.64 | 28.34 ± 3.11 | 0.265 |
| After 3 months | 27.44 ± 3.01 | 27.78 ± 3.30 | 0.584 | |
| ‡P-value | 0.06 | 0.35 | ||
| FBG (mg/dl) | Baseline | 120.30 ± 17.63 | 117.86 ± 16.77 | 0.48 |
| After 3 months | 116.58 ± 14.13 | 107.60 ± 10.90 | 0.001 | |
| ‡P-value | 0.108 | 0.0005 | ||
| HbA1c (%) | Baseline | 7.56 ± 0.76 | 7.57 ± 0.79 | 0.965 |
| After 3 months | 7.41 ± 0.85 | 6.95 ± 0.62 | 0.003 | |
| ‡P-value | 0.09 | 0.0002 | ||
| Insulin (µIU/ml) | Baseline | 18.98 ± 5.47 | 19.68 ± 5.06 | 0.238 |
| After 3 months | 18.45 ± 5.44 | 16.98 ± 4.68 | 0.151 | |
| ‡P-value | 0.927 | <0.0001 | ||
| HOMA-IR | Baseline | 5.50 ± 1.97 | 5.70 ± 1.58 | 0.590 |
| After 3 months | 5.30 ± 1.68 | 4.46 ± 1.09 | 0.004 | |
| ‡P-value | 0.131 | <0.0001 | ||
| TG mg/dl | Baseline | 142.22 ± 15.24 | 148.81 ± 31.34 | 0.185 |
| After 3 months | 131.06 ± 15.89 | 127.92 ± 16.87 | 0.340 | |
| ‡P-value | 0.0005 | <0.0001 | ||
| TC mg/dl | Baseline | 175.40 ± 22.44 | 177.98 ± 24.19 | 0.582 |
| After 3 months | 159.66 ± 20.25 | 159.66 ± 20.25 | 0.354 | |
| ‡P-value | <0.001 | <0.0001 | ||
| LDL-C mg/dl | Baseline | 112.65 ± 23.64 | 113.60 ± 26.89 | 0.851 |
| After 3 months | 95.56 ± 18.12 | 99.42 ± 20.73 | 0.324 | |
| ‡P-value | <0.001 | <0.001 | ||
| HDL-C mg/dl | Baseline | 34.31 ± 2.38 | 34.62 ± 2.41 | 0.521 |
| After 3 months | 34.35 ± 2.37 | 34.65 ± 2.43 | 0.532 | |
| ‡P-value | 0.568 | 0.48 | ||
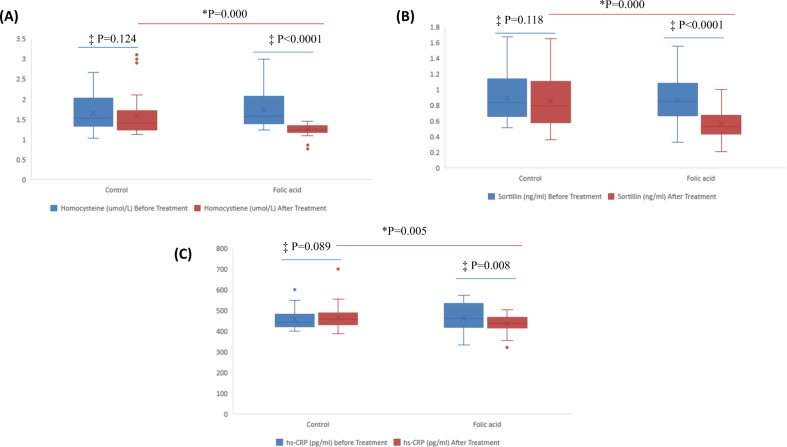
| Homocysteine (μmol/L) | Baseline | 1.66 ± 0.40 | 1.74 ± 0.44 | 0.293 |
| After 3 months | 1.57 ± 0.45 | 1.25 ± 0.14 | 0.000 | |
| ‡P-value | 0.124 | <0.0001 | ||
| Sortillin (ng/ml) | Baseline | 0.89 ± 0.28 | 0.86 ± 0.25 | 0.559 |
| After 3 months | 0.85 ± 0.35 | 0.57 ± 0.19 | 0.000 | |
| ‡P-value | 0.118 | <0.0001 | ||
| hs-CRP (pg/ml) | Baseline | 458.30 ± 50.19 | 464.69 ± 65.21 | 0.584 |
| After 3 months | 464.73 ± 52.92 | 438.05 ± 39.04 | 0.005 | |
| ‡P-value | 0.089 | 0.008 |
Data presented as mean ± SD.
BMI body mass index, FBG fasting blood glucose, HbA1c glycated hemoglobin, TG triglyceride, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, hs-CRP high-sensitivity C-reactive protein.
‡Paired sample t-test statistically significant within groups at P < 0.05.
*Independent sample t-test statistically significant between groups at P < 0.05.
Lipid profile
After intervention for 3 months, placebo group didn’t show statistically significant differences in TG, TC, LDL-C, and HDL-C levels compared with folic acid group (P > 0.05) (Table 2).
Homocysteine, sortilin, and hs-CRP
Following FAS for 3 months, serum Hcy levels decreased by 28.2% from 1.74 ± 0.44 μmol/L to 1.25 ± 0.14 μmol/L (P < 0.0001). Further, serum sortilin level significantly reduced by 33.7% from 0.86 ± 0.25 ng/ml to 0.57 ± 0.19 ng/ml (P < 0.0001). hs-CRP also reduced from 464.69 ± 65.21 to 438.05 ± 39.04 (P = 0.008) (Table 2). On the other hand, theses parameters didn’t change significantly in placebo group (Fig. 2).
Fig. 2. Changes in the measured variables (A) Homocysteine (B) Sortilin (C) hs-CRP.
‡Paired sample t-test statistically significant within groups at P< 0.05. *Independent sample t-test statistically significant between groups at P < 0.05.
Relationship between FBG, Homocysteine, sortilin, and hs-CRP
A positive correlation was found between sortilin with Hcy (r = 0.493, P = 0.000) and hs-CRP (r = 0.237, P = 0.018). Further, there was a positive association between Hcy with hs-CRP (r = 0.308, P = 0.002). A positive association was found between FBG with hs-CRP (r = 0.342, P = 0.000) (Table 3).
Table 3.
Association between sortilin, homocysteine, hs-CRP, and FBG after folic acid supplementation.
| Homocystiene (μmol/L) | hs-CRP (pg/ml) | FBG | ||
|---|---|---|---|---|
| Sortillin (ng/ml) | r | 0.493** | 0.237* | 0.169 |
| P | 0.000 | 0.018 | 0.093 | |
| Homocystiene (μmol/L) | r | 0.308** | −0.076 | |
| P | 0.002 | 0.451 | ||
| hs-CRP (pg/ml) | r | 0.342** | ||
| P | 0.000 | |||
FBG: fasting blood glucose; hs-CRP, high-sensitivity C-reactive protein.
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
Association between HOMA-IR with Hcy, sortilin, hs-CRP, and insulin levels via multivariate regression analysis
Multiple linear regression analysis was conducted to find whether there was an independent correlation between HOMA-IR with Hcy, sortilin, hs-CRP, and insulin levels. The analysis results demonstrated that HOMA-IR levels were inversely and independently correlated with Hcy, whereas HOMA-IR were positively related to sortilin, hs-CRP and insulin levels (Table 4).
Table 4.
Multiple linear regression assessment of associated parameters with HOMA-IR in the studied participants (Adjusted R2 = 0.852).
| Model | Unstandardized coefficients | Standardized coefficients | t | P-value | 95.0% Confidence interval for B | ||
|---|---|---|---|---|---|---|---|
| β | Std. error | β | Lower Bound | Upper Bound | |||
| (Constant) | −1.259 | 0.602 | −2.090 | 0.039 | −2.455 | −0.063 | |
| Sortillin (ng/ml) After Treatment | 0.447 | 0.213 | 0.095 | 2.096 | 0.039 | 0.024 | 0.871 |
| Homocystiene (µmol/L) After Treatment | −0.521 | 0.180 | −0.133 | −2.903 | 0.005 | −0.878 | −0.165 |
| Insulin-A (µIU/ml) After Treatment | 0.266 | 0.011 | 0.922 | 23.305 | 0.000 | 0.243 | 0.288 |
| hs-CRP (pg/ml) After Treatment | 0.004 | 0.001 | 0.134 | 3.237 | 0.002 | 0.002 | 0.007 |
Dependent variable: HOMA-IR after multiple linear regression analysis was used. β: Unstandardized regression coefficient.
CI confidence interval, HOMA-IR homeostasis model assessment of insulin resistance, hs-CRP high-sensitivity C-reactive protein.
*P < 0.05 was significant.
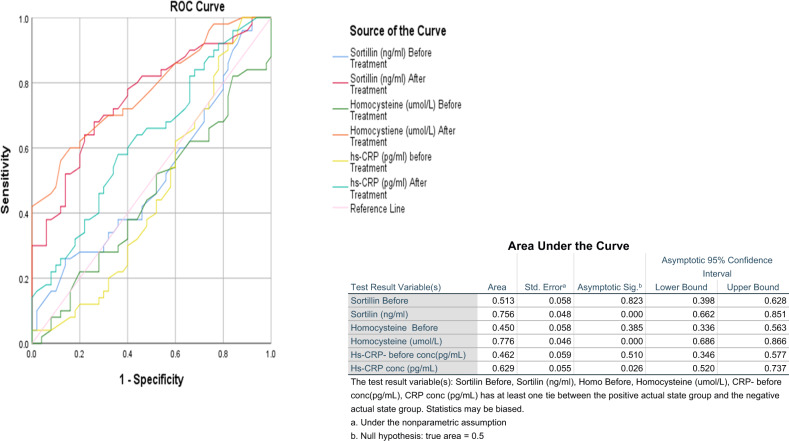
Area under ROC curve of different measured parameters in the studied groups
Figure 3 shows ROC-AUC of biomarkers among diabetic patients in the both groups before and after intervention duration. Hcy was the most sensitive (AUC = 0.776, P = 0.000) followed by sortilin (AUC = 0.756, P = 0.000) whereas hs-CRP level was the lowest AUC (0.629, P = 0.026) after 3 months of intervention.
Fig. 3.
Area under ROC curve of the determined parameters.
Reported adverse events
The most prevalent side effects were nausea, loss of appetite (14% vs 8%), bloating, gas, stomach pain (12% vs 2%), unpleasant taste (10% vs 2%), and confusion (6% vs 0%) in folic acid and placebo group respectively with insignificant difference between both groups as shown in Table 5.
Table 5.
Reported adverse events in both groups.
| Placebo group (n = 50) | Folic acid group (n = 50) | P-value | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Nausea, loss of appetite | 4 | 8 | 7 | 14 | 0.338 |
| Bloating, gas, and stomach pain | 1 | 2 | 6 | 12 | 0.050 |
| Unpleasant taste | 1 | 2 | 5 | 10 | 0.092 |
| Confusion | 0 | 0 | 3 | 6 | 0.079 |
Data are presented as number and percent.
Data were analyzed by Chi–square test.
Significance level was set at P < 0.05.
Discussion
In the present study, the folic acid impacts as co-treatment in diabetic patients on circulating levels of Hcy, sortilin, indices of glycemic control, and insulin resistance and lipid profile were investigated.
Our results revealed that treatment with folic acid (5 mg/d) for 12 weeks in diabetic patients receiving metformin treatment significantly improved serum levels of FBG, HbA1c, HOMA-IR, Hcy, sortilin, and hs-CRP.
Several studies indicated that blood Hcy levels are inversely correlated to plasma levels of folic acid [31, 32].
Our results showed that FAS reduced Hcy level by 28.2%, which correspond with other research’s findings; although, the percentage drop varies [33–35]. These discrepancies could be explained by changes in treatment duration and folic acid dose [7].
There is a substantial evidence that FAS may improve insulin resistance [36]. Our findings estimated that FAS decreased the level of insulin and HOMA-IR, which is in accordance with previous studies. A study confirmed that plasma insulin level and HOMA-IR were significantly decreased in overweight individuals after treatment with folic acid (2.5 mg/d) for 3 months [37]. Another study by Gargari et al. showed that FAS (5 mg/d) for eight weeks in obese patients with T2DM improved glycemic control and insulin resistance [10].
Several mechanisms have been proposed for the involvement of FAS and Hcy reduction in the pathogenesis of insulin resistance and T2DM. A potential mechanism is that Hcy may block insulin-stimulated tyrosine phosphorylation of insulin receptor β-subunit and its substrates, resulting in suppression of glycogen production [38]. Subsequently resulting in insulin resistance and hyperglycemia. Another suggested explanation is that folic acid improves endothelial dysfunction induced by high Hcy by preventing nitric oxide synthase dysfunction, which may be beneficial to glycomtabolism [7]. Folic acid has also been reported to diminish Hcy, which in turn reduces oxidative stress and systemic inflammation, which can disrupt insulin signaling and diminish release of insulin from pancreatic β cell [39].
Our results showed that FAS has resulted in significant reduction in FBG and HbA1c levels, which are in consistence with a meta-analysis [40] that reported a link between a decrease in Hcy level and a decrease in fasting glucose and HbA1c levels, indicating the role of Hcy as an intermediary. The findings might indicate that a significant reduction in Hcy is required for improvement of glycemic control, which might be accomplished efficiently by better compliance and/or increase of FAS dose.
In consistence with our results, Chiarelli et al. revealed an elevated plasma Hcy level in adolescents and young adults with type 1 DM and microvascular consequences; they also reported a positive link between plasma Hcy level and HbA1c [41].
Inconsistence with our results, other studies showed that FAS didn’t decrease HbA1c significantly in T2DM patients [10, 42].
Hyperhomocysteinemia tends to be a greater (1.9-fold) predictor for death in patients with T2DM than in nondiabetic individuals [43]; a meta-analysis demonstrated that FAS can diminish Hcy in patients without cardiovascular disease history. FAS could reduce the risk of cardiovascular events in T2DM patients, particularly as a primary preventative strategy [3].
Our results showed a significant decrease in hs-CRP level in diabetic patients after FAS, which are in consistence with a study [37] that estimated reduction of some inflammatory mediators levels as hs-CRP regardless of weight change after FAS, implying that FAS could have a therapeutic effect in the atherogenesis and cardiovascular diseases prevention.
In another study, patients with acute ischemic stroke who received a combination of 5 mg of folic acid with vitamin B complex for 2 weeks showed significant reduction in hs-CRP levels [44].
According to a meta-analysis, T2DM patients had a greater decrease in hs-CRP levels following FAS compared with healthy controls. T2DM patients are thought to have greater or equivalent levels of inflammatory biomarkers compared to healthy individuals [45].
Folic acid supplementation reduces hs-CRP concentration through several mechanisms. The reduction in Hcy levels following FAS has been linked to a reduction in oxidative stress [46]. Furthermore, the reduction in insulin resistance after FAS may result in reduced synthesis of inflammatory factors [47]. Other researchers, on the other hand, claim that FAS has no effect on serum level of CRP [48].
Our results revealed that there were no significant changes in levels of TC, TG, LDL-C, and HDL-C following FAS. In agreement with a pervious study by Tabrizi et. al., 2018 which concluded that Folate supplementation did not affect blood pressures and lipid profiles among patients with metabolic diseases [49].
Gargari et. al., found no significant differences in lipid profile following 8 weeks of FAS (5 mg/d) in obese males with T2DM, which is consistent with earlier studies [7]. Similarly, Mangoni et al. reported that FAS with 5 mg/d for 4 weeks did not induce significant differences in levels of TC, TG, LDL-C, and HDL-C in T2DM patients [42].
Our results were incompatible with a trial on post-menopausal Korean females with T2DM that found a decrease in level of LDL-C along with decrease in LDL-C/HDL-C and TC/HDL-C ratios after FAS with 800 μg/d for 8 weeks [9].
An experimental animal study demonstrated a correlation between higher levels of Hcy as well as reduced HDL-C and elevated levels of TC and non-HDL-C [50]. Elevated levels of Hcy have also been demonstrated to decrease the enzymatic activities of two HDL- metabolizing enzymes [51]. Increased Hcy levels were shown to increase LDL receptors expression and LDL-C transport [52]. As a result, improved lipid profile caused by FAS-induced decline in serum levels of Hcy.
Sortilin has been linked to many pathological processes including inflammation and calcification of arterial wall, insulin resistance, and disrupted lipoprotein metabolism [24, 53].
The present study showed that levels of hs-CRP were significantly greater at baseline assessment of studied patients. Furthermore, among the studied patients, the levels of hs-CRP and sortilin had a substantial positive correlation. Similarly, plasma sortilin has demonstrated significant association with hs-CRP, which has been found to be formed in vascular smooth muscle cells, in patients with coronary artery disease whose platelets were suppressed by aspirin treatment [25]. In another study, newly diagnosed T2DM patients had higher levels of hs-CRP than controls, despite no correlation between levels of hs-CRP and sortilin [23]. Therefore, hs-CRP is a promising predictor for atherosclerotic cardiovascular disorders in T2DM patients [54].
Conflicting data were reported in clinical trials regarding the association between sortilin and lipids.
To our knowledge, this is the first study to investigate the association between sortilin and lipid profile in Egyptian patients with T2DM. In the current study, we found no link between sortilin and lipid profile. Consistently with the present results, Oh et. al., reported insignificant correlation between levels of sortilin and lipid profile [21].
Inconsistently, Ogawa et al. showed that there was a significant association between sortilin levels and LDL-C as well as TC [25]. In addition, it was shown in a preclinical trial that in sortilin-deficient mice, the lipoproteins production from the liver and hypercholesterolemia were decreased [55]. Likewise, mice lacking macrophage sortilin illustrated decreased atherosclerotic plaques due to LDL uptake reduction [56]. Contrary, Demir et al. reported a negative correlation between sortilin levels and TC, LDL-C and TG, while levels of sortilin were positively linked with HDL-C in newly diagnosed T2DM patients suggesting that sortilin may contribute to dyslipidemia in T2DM. Another study showed that hepatic sortilin decreases hepatic apolipoprotein B production while enhancing LDL-C degradation [57].
This study has several limitations: including small sample size, short period of follow-up, and the lack of intention to treat analysis. Further large-scale studies are needed, and further extension of this work is warranted.
Conclusion
FAS might be beneficial for reducing Hcy and sortilin levels, improving glycemic control and insulin resistance in T2DM patients. As a result, FAS could have a possible contribution in the primary prevention of cardiovascular events in diabetic patients. Homocysteine and sortilin could be used as therapeutic targets for T2DM treatment and could aid clinicians for identification of cardiovascular risk in diabetic patients.
Acknowledgements
The authors would like to thank all of the patients who participate in this study and Damanhour National Medical Institute for collecting patient data.
Author contributions
Noha M. El-Khodary and Rehab H. Werida contributed to conception, methodology, data curation, analysis, writing and reviewing the manuscript. Hossam Dabees contributed to eligibility assessment, participants selection and enrolment. All of the authors reviewed and approved the final copy of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data that support the findings of this study are available upon reasonable request.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang T, Asimi S, Lou D, Li D. Plasma phospholipid polyunsaturated fatty acids and homocysteine in Chinese type 2 diabetes patients. Asia Pac J Clin Nutr. 2012;21:394. [PubMed] [Google Scholar]
- 2.Chokrungvaranon N, Deer J, Reaven PD. Intensive glycemic control and cardiovascular disease: are there patients who may benefit? Postgrad Med. 2011;123:114–23. doi: 10.3810/pgm.2011.11.2501. [DOI] [PubMed] [Google Scholar]
- 3.Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pr. 2012;98:151–8. doi: 10.1016/j.diabres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768. doi: 10.1161/JAHA.116.003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werida RH, Omran A, El-Khodary NM. Sortilin and Homocysteine as Potential Biomarkers for Coronary Artery Diseases. Int J Gen Med. 2021;14:6167. doi: 10.2147/IJGM.S324889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001;24:1403–10. doi: 10.2337/diacare.24.8.1403. [DOI] [PubMed] [Google Scholar]
- 7.Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pr. 2011;94:33–38. doi: 10.1016/j.diabres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ndrepepa G, Kastrati A, Braun S, Koch W, Kölling K, Mehilli J, et al. Circulating homocysteine levels in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18:66–73. doi: 10.1016/j.numecd.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Vijayakumar A, Kim E, Kim H, Choi YJ, Huh KB, Chang N. Effects of folic acid supplementation on serum homocysteine levels, lipid profiles, and vascular parameters in post-menopausal Korean women with type 2 diabetes mellitus. Nutr Res Pr. 2017;11:327–33. doi: 10.4162/nrp.2017.11.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghamohammadi V, Gargari BP, Aliasgharzadeh A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2011;30:210–5. doi: 10.1080/07315724.2011.10719962. [DOI] [PubMed] [Google Scholar]
- 11.Ponziani FR, Cazzato IA, Danese S, Fagiuoli S, Gionchetti P, Annicchiarico BE, et al. Folate in gastrointestinal health and disease. Eur Rev Med Pharm Sci. 2012;16:376–85. [PubMed] [Google Scholar]
- 12.Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications. 2007;21:118–23. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Kundi H, Kiziltunc E, Ates I, Cetin M, Barca AN, Ozkayar N, et al. Association between plasma homocysteine levels and end-organ damage in newly diagnosed type 2 diabetes mellitus patients. Endocr Res. 2017;42:36–41. doi: 10.3109/07435800.2016.1171235. [DOI] [PubMed] [Google Scholar]
- 14.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30:1390–9. doi: 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 16.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. J Parenter Enter Nutr. 2008;32:638–44. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagherieh M, Kheirollahi A, Zamani-Garmsiri F, Emamgholipour S, Meshkani R. Folic acid ameliorates palmitate-induced inflammation through decreasing homocysteine and inhibiting NF-κB pathway in HepG2 cells. Arch Physiol Biochem. 2021;17:1–8. doi: 10.1080/13813455.2021.1878539. [DOI] [PubMed] [Google Scholar]
- 18.Timpson NJ, Lawlor DA, Harbord RM, Gaunt TR, Day INM, Palmer LJ, et al. C-reactive protein and its role in metabolic syndrome: mendelian randomisation study. Lancet. 2005;366:1954–9. doi: 10.1016/S0140-6736(05)67786-0. [DOI] [PubMed] [Google Scholar]
- 19.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ESG, Kastelein JJP. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–91. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 20.Seo SM, Baek SH, Jeon HK, Kang SM, Kim DS, Kim WS, et al. Correlations between the level of high-sensitivity C-reactive protein and cardiovascular risk factors in Korean adults with cardiovascular disease or diabetes mellitus: the CALLISTO study. J Atheroscler Thromb. 2013;20:616–22. doi: 10.5551/jat.16089. [DOI] [PubMed] [Google Scholar]
- 21.Oh TJ, Ahn CH, Kim BR, Kim KM, Moon JH, Lim S, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovascular Diabetol. 2017;16:92. doi: 10.1186/s12933-017-0568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biscetti F, Bonadia N, Santini F, Angelini F, Nardella E, Pitocco D, et al. Sortilin levels are associated with peripheral arterial disease in type 2 diabetic subjects. Cardiovascular Diabetol. 2019;18:5. doi: 10.1186/s12933-019-0805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demir İ, Akan OY, Guler A, Bozkaya G, Aslanipour B, Calan M. Relation of decreased circulating sortilin levels with unfavorable metabolic profiles in subjects with newly diagnosed Type 2 diabetes mellitus. Am J Med Sci. 2020;359:8–16. doi: 10.1016/j.amjms.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Goettsch C, Kjolby M, Aikawa E. Sortilin and its multiple roles in cardiovascular and metabolic diseases. Arterioscler Thromb Vasc Biol. 2018;38:19–25. doi: 10.1161/ATVBAHA.117.310292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa K, Ueno T, Iwasaki T, Kujiraoka T, Ishihara M, Kunimoto S, et al. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–5. doi: 10.1016/j.atherosclerosis.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–8. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artha IMJR, Bhargah A, Dharmawan NK, Pande UW, Triyana KA, Mahariski PA, et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc Health Risk Manag. 2019;15:149. doi: 10.2147/VHRM.S209830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prochaska, J, Gellman M, Turner J. Encyclopedia of behavioral medicine. New York: Springer; 2013;1–136.
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 31.Ajabnoor M, AL-Ama MN, Banjar Z, Rafee AA, Sheweita SA. Homocysteine level and other biochemical parameters in cardiovascular disease patients with diabetes mellitus. Med Sci Monit. 2003;9:CR523–27. [PubMed] [Google Scholar]
- 32.Clarke R, Frost J, Sherliker P, Lewington S, Collins R, Brattstrom L, et al. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 33.Mashavi M, Hanah R, Boaz M, Gavish D, Matas Z, Fux A, et al. Effect of homocysteine-lowering therapy on arterial elasticity and metabolic parameters in metformin-treated diabetic patients. Atherosclerosis. 2008;199:362–7. doi: 10.1016/j.atherosclerosis.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Yegnanarayan R, Suryavanshi M, Singh M, Desai S. A comparative study of the glycemic control of various antidiabetic agents and the role of homocysteine in the therapy of type 2 diabetes mellitus. J Diabetes Complications. 2008;22:104–11. doi: 10.1016/j.jdiacomp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Van Ede AE, Laan RF, Blom HJ, Boers GH, Haagsma CJ, Thomas CM, et al. Homocysteine and folate status in methotrexate‐treated patients with rheumatoid arthritis. Rheumatology. 2002;41:658–65. doi: 10.1093/rheumatology/41.6.658. [DOI] [PubMed] [Google Scholar]
- 36.Büttner R, Wobser H, Wrede C, Schäffler A, Schölmerich J, Büchler C, et al. Supplementation of folic acid improves insulin resistance in the high fat fed rat. Exp Clin Endocrinol Diab. 2007;115:P02–060. doi: 10.1055/s-2007-972467. [DOI] [Google Scholar]
- 37.Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes. 2006;30:1197–202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 38.Najib S, Sanchez-Margalet V. Homocysteine thiolactone inhibits insulin signaling, and glutathione has a protective effect. J Mol Endocrinol. 2001;27:85–91. doi: 10.1677/jme.0.0270085. [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58:1921–8. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lind MV, Lauritzen L, Kristensen M, Ross AB, Eriksen JN. Effect of folate supplementation on insulin sensitivity and type 2 diabetes: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:29–42. doi: 10.1093/ajcn/nqy234. [DOI] [PubMed] [Google Scholar]
- 41.Chiarelli F, Pomilio M, Mohn A, Tumini S, Vanelli M, Morgese G, et al. Homocysteine levels during fasting and after methionine loading in adolescents with diabetic retinopathy and nephropathy. J Pediatr. 2000;137:386–92. doi: 10.1067/mpd.2000.108103. [DOI] [PubMed] [Google Scholar]
- 42.Mangoni AA, Sherwood RA, Asonganyi B, Swift CG, Thomas S, Jackson SH. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005;18:220–6. doi: 10.1016/j.amjhyper.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, et al. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation. 2000;101:1506–11. doi: 10.1161/01.CIR.101.13.1506. [DOI] [PubMed] [Google Scholar]
- 44.Ullegaddi R, Powers HJ, Gariballa SE. Antioxidant supplementation with or without B‐group vitamins after acute ischemic stroke: a randomized controlled trial. J Parenter Enter Nutr. 2006;30:108–14. doi: 10.1177/0148607106030002108. [DOI] [PubMed] [Google Scholar]
- 45.Fatahi S, Pezeshki M, Mousavi SM, Teymouri A, Rahmani J, Varkaneh HK, et al. Effects of folic acid supplementation on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2019;29:432–9. doi: 10.1016/j.numecd.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088–93. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Zhao G, Ford ES, Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among US adults without physician-diagnosed diabetes: NHANES, 2003–2006. Diabetes Care. 2010;33:344–7. doi: 10.2337/dc09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Title LM, Ur E, Giddens K, McQueen MJ, Nassar BA. Folic acid improves endothelial dysfunction in type 2 diabetes-an effect independent of homocysteine-lowering. Vasc Med. 2006;11:101–9. doi: 10.1191/1358863x06vm664oa. [DOI] [PubMed] [Google Scholar]
- 49.Tabrizi R, Lankarani KB, Akbari M, Naghibzadeh-Tahami A, Alizadeh H, Honarvar B, et al. The effects of folate supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr: Clin Res Rev. 2018;12:423–30. doi: 10.1016/j.dsx.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein AI Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99:598–606. doi: 10.1161/01.RES.0000242559.42077.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Namekata K, Enokido Y, Ishii I, Nagai Y, Harada T, Kimura H. Abnormal lipid metabolism in cystathionine β-synthase-deficient mice, an animal model for hyperhomocysteinemia. J Biol Chem. 2004;279:52961–9. doi: 10.1074/jbc.M406820200. [DOI] [PubMed] [Google Scholar]
- 52.Austin R, Werstuck G. Methods and compositions for inhibiting ER-stress induced cholesterol/triglyceride accumulation. Google Patents. 2008.
- 53.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–9. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa TA. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2006;29:329–33. doi: 10.2337/diacare.29.02.06.dc05-1700. [DOI] [PubMed] [Google Scholar]
- 55.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, et al. Sort1, encoded by the cardiovascular risk locus 1p13. 3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–23. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116:789–96. doi: 10.1161/CIRCRESAHA.116.305811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strong A, Ding Q, Edmondson AC, Millar JS, Sachs KV, Li X, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122:2807–16. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.