Abstract
Our previous study demonstrated that purple rice bran extract (PRBE) could inhibit diethylnitrosamine (DEN)-induced hepatocarcinogenesis. Protocatechuic acid (PCA) is the major phenolic acid contained in the PRBE. Therefore, this study aimed to determine whether PCA is an anticarcinogenic compound in purple rice extract. Rats were intraperitoneally injected with DEN to induce glutathione S-transferase placental form (GST-P)-positive foci. Rats were fed with PRBE at 500 mg kg−1 body weight or PCA at 4 mg kg−1 body weight for 5 and 15 weeks. PCA administration attenuated DEN-induced hepatic GST-P positive foci to a degree similar to PRBE. The molecular mechanisms of PCA in the initiation stage were correlated with reduced activity of cytochrome P450 reductase and induction of glutathione S-transferase. In addition, PCA also downregulated the expression of TNF-α and IL-1β genes in rat liver. These genes are associated with the inhibition of inflammation. In the promotion stage, PCA suppressed cell proliferation correlated with the downregulation of Cyclin D1 expression. Moreover, it also induced apoptosis, indicated by increased expression of P53 and Bad genes, and decreased the expression of the anti-apoptotic Bcl-xl in DEN-initiated rats. These findings suggest that PCA is an active compound in the anticarcinogenic action of purple rice bran.
Subject terms: Biochemistry, Cancer
Introduction
Globally, liver cancer is the third leading cause of cancer death1. The major risk factors are associated with hepatitis B and hepatitis C virus infection, fatty liver disease, alcohol consumption, and aflatoxin B1. There are several treatment options to cure cancer, such as surgical resection, liver transplantation, chemotherapy, immunotherapy, and oncolytic virus therapy2. However, the survival rate of most patients with liver cancer is low due to late diagnosis, drug resistance, and cancer recurrence, resulting in a poor prognosis3.
There is now an increasing number of scientific reports to support the use of foods and food components as cancer therapeutic candidates. Food-derived bioactive compounds could prevent all stages of carcinogenesis via diverse pathways, such as antioxidant and detoxifying enzyme systems, cell proliferation and death, cell cycle, and the inflammatory response4,5. For example, allicin, the main organic allyl sulfur compound in garlic, can inhibit the growth of cholangiocarcinoma tumor in a HuCCT-1 xenograft nude mouse model6. Apigenin, a flavonoid found in many fruits and vegetables, can attenuate the growth of human chondrosarcoma tumor in a nude mouse model by decreasing cell proliferation and increasing cell apoptosis7. Vanillic acid, a phenolic acid found in many plant species such as rice, rye, and almond skin, can prevent rat hepatocarcinogenesis induced by diethylnitrosamine and 1,2-dimethylhydrazine8. Ellagic acid, a phenolic acid found in berries, nuts, and pomegranates, can suppress cell proliferation and induce apoptosis in MCF-7 and MDA-MB-231 breast cancer cells9.
Rice (Oryza sativa L.) is an important source of nutrition, especially in Asia. Rice bran, the outer layer of the rice grain, contains phytochemical compounds which possess several types of biological activity such as antioxidant, anti-inflammatory, hepatoprotective, and anticancer activity10–13. Our previous study demonstrated that purple rice bran extract has higher anticancer activity against diethylnitrosamine-induced rat hepatocarcinogenesis than white rice bran. Based on chemical analysis, we found that protocatechuic acid is a major phenolic acid in purple rice extract14. Therefore, this study aimed to investigate whether protocatechuic acid is an anticarcinogenic compound in purple rice extract. In addition, the inhibitory mechanism of protocatechuic acid against diethylnitrosamine-induced rat hepatocarcinogenesis was also evaluated.
Materials and methods
Chemicals
Diethylnitrosamine (DEN) and 3, 3’-diaminobenzidine tetrahydrochloride hydrate were obtained from Sigma-Aldrich (St. Louis, MO, USA). ApopTag Peroxidase in situ kit was purchased from Merck (Darmstadt, Germany). Rabbit polyclonal antibody to rat glutathione S-transferase placental form (GST-P) was purchased from MBL (Nagoya, Japan). Protocatechuic acid (PCA) was purchased from Alfa-Aesar (Karlsruhe, Germany). Vectastain ABC kit was purchased from Vector Laboratories, Inc. (Burlingame, CA, USA). EnVision Doublestain system was purchased from Dako (Glostrup, Denmark). All other chemicals were analytical grade.
Purple rice bran extraction
Purple rice (Oryza sativa L. var. indica) cv. Kum Doi Saket bran was obtained from the Faculty of Agriculture, Chiang Mai University, Thailand, having been cultivated from August to November 2016. A voucher specimen, Rawiwan-001, has been deposited in the archives of the Faculty of Pharmacy (Herbarium number: CMU; 023,252), Chiang Mai University, Thailand. Briefly, the purple rice bran was twice defatted by hexane and then extracted twice with absolute methanol for 48 h at room temperature. After filtration, the solution was evaporated and freeze-dried to obtain the purple rice bran extract (PRBE). The extract was then kept at − 20 °C until further use.
Animals
Male Wistar rats (4 weeks old) were purchased from the National Laboratory Animal Center, Mahidol University, Salaya, Nakorn-Prathom, Thailand. Rats were housed in groups of three per cage under a 12 h light/dark cycle and 50–60% humidity at 23 ± 2 °C in the Animal House, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Feed and tap water were provided ad libitum. All procedures were approved by The Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University (Protocol No. 20/2560). All methods were performed in accordance with the relevant guidelines and regulations. This study follows the recommendation in the ARRIVE guidelines.
Experimental protocol
Our previous experiment indicated that PRBE can inhibit the DEN-induced early stage of rat hepatocarcinogenesis14. Therefore, to find out the active compounds contained in the extract, PCA, the most abundant phenolic compound in the extract, was selected for this study. The concentration of 4 mg kg−1 body weight (BW) of PCA was equivalent to PCA content found in 500 mg kg−1 BW of PRBE.
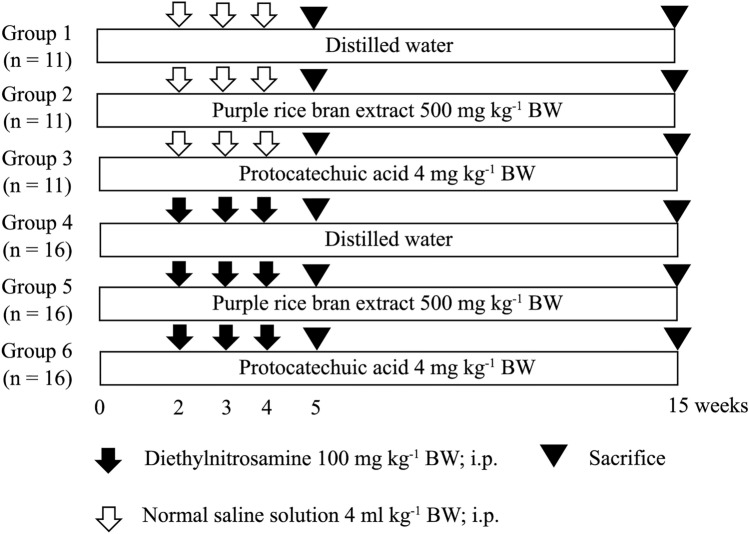
To determine the chemopreventive effect of PRBE and PCA on DEN-induced rat hepatocarcinogenesis, male Wistar rats (4 weeks old, 90–110 g) were divided into six groups. Rats in groups 1–3 were injected intraperitoneally with normal saline, while groups 4–6 were injected with 100 mg kg−1 BW of DEN in weeks 2, 3, and 4 of an experiment to initiate hepatocarcinogenesis. Group 1 was a negative control, while group 4 was a positive control. Rats in group 2 were fed with 500 mg kg−1 BW of PRBE via intragastric gavage feeding, whereas group 3 was fed with 4 mg kg−1 BW of PCA, to study their carcinogenicity. Two weeks before DEN injection, rats in group 5 were fed with 500 mg kg−1 BW of PRBE, while group 6 orally received PCA at a concentration of 4 mg kg−1 BW, to evaluate its anticancer activity. All rats were dosed once daily for 5 days per week. In week 5 of the experiment, six rats per group were sacrificed to determine the effect of PRBE and PCA on the initiation stage of hepatocarcinogenesis. The remaining rats were sacrificed in week 15 of an experiment to evaluate the effect of PRBE and PCA on the promotion stage of hepatocarcinogenesis. Body weight, water, and food intake were measured twice a week throughout the experiment. The weights of major organs including liver, kidney, and spleen were measured to evaluate the toxic effect of test compounds for the 5- and 15-week protocols. The treatment protocol is shown in Fig. 1.
Figure 1.
Experimental protocol for testing the anticarcinogenic effect of purple rice bran extract and protocatechuic acid.
Determination of glutathione S-transferase placental form positive foci in rat liver (5- and 15-week protocols)
To investigate the effect of PRBE and PCA on the formation of hepatic preneoplastic lesions, the GST-P positive foci were stained by immunohistochemistry according to Thumvijit et al.15. Briefly, liver sections were deparaffinized with xylene and dehydrated with ethanol. Endogenous peroxidase activity and non-specific protein bindings were blocked with H2O2 and nonfat milk, respectively. The slides were treated with an anti-rabbit GST-P antibody (MBL, Japan) diluted 1:1000. Then, they were incubated with a secondary antibody conjugated to an avidin–biotin peroxidase complex (Vector Laboratories, Inc., USA). The GST-P positive cells were detected using 3,3′-diaminobenzidine as a substrate. The number and area of GST-P-positive foci larger than 0.15 mm2 and 0.2 mm2 were measured in the 5- and 15-week experiments, respectively, using the Leica Application Suite (LAS) Interactive Measurement program (Leica Microsystems, Germany).
Evaluation of some phase I and II xenobiotic-metabolizing enzymes (5-week protocol)
To study the effect of PCA on the initiation stage of rat hepatocarcinogenesis, the activity and expression of some phase I and II xenobiotic-metabolizing enzymes in rat livers were investigated. The liver cytosolic and microsomal fractions were prepared according to Punvittayagul et al. (2011) and the protein concentration was determined by the Lowry method. The activity of cytochrome P450 reductase (CPR), UDP-glucuronyltransferase (UGT), and GST was determined according to our previous report16.
The expression of cytochrome P450 2E1 was determined by Western blot analysis using polyclonal anti-rat CYP2E1 antibody (Thermo Fisher Scientific, USA) at 1:12,000 dilution. The protocol was performed according to Punvittayagul et al. (2011)16. Protein detection was performed using HRP-conjugated secondary antibodies (Abcam, UK) and an enhanced chemiluminescent kit (Thermo Fisher Scientific, USA). The intensity of each band was evaluated with the Image J program, which was normalized against protein disulfide isomerase (PDI, Cell Signaling Technology, USA).
Assessment of proliferating cell nuclear antigen and apoptotic hepatocytes by double-staining immunohistochemistry (15-week protocol)
To evaluate the effect of PRBE and PCA on cell proliferation and apoptosis, double-staining procedures were carried out using an EnVision Doublestain system from Dako (Hamburg, Germany). Liver sections were stained with anti-PCNA antibody (Biolegend, USA) at 1 : 2000 dilution and anti-GST-P antibody (MBL, Japan) at 1 : 1000 dilution, following the manufacturer’s instructions. The number of PCNA-positive cells was counted under a light microscope as described in our previous study8.
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was used to identify apoptotic hepatocytes. A double-labeling assay for TUNEL and GST-P was carried out using an ApopTag Peroxidase in situ kit (Merck, Germany) and EnVision Doublestain system (Dako, Denmark) according to Thumvijit et al. (2014)15. The number of apoptotic labeled cells was recorded as in our previous reports8.
Gene expression analysis by real-time polymerase chain reaction (5- and 15-week protocols)
The effect of PCA on the mRNA levels of genes involved in the initiation and promotion stages of hepatocarcinogenesis was determined by real-time polymerase chain reaction (RT-PCR). The liver tissue samples were homogenized in PureZOL™ RNA Isolation Reagent (Bio-Rad, USA), following the manufacturer’s procedure. cDNAs were synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, USA), according to the supplier’s protocol. Quantitative RT-PCR was carried out using specific primers (Integrated DNA Technologies, Inc., Singapore) as listed in Table 1. All PCR reactions were performed using a SensiFast™ SYBR Lo-ROX Kit (Bioline, France) and were carried out under the following conditions: initial denaturation at 95 °C for 1 min followed by 40 cycles of 15 s at 95 °C, 15 s at 56–60 °C and 10 s at 72 °C. Differences in gene expression between groups were calculated by the 2−ΔΔCT method, which was normalized against β-actin and expressed as relative expression compared with the control.
Table 1.
Primer lists for the real-time polymerase chain reaction.
| Gene | 5′-3′ Primer sequence | References |
|---|---|---|
| TNF-α |
Forward: 5′-AAA TGG CCC TCT CAT CAG TCC-3′ Reverse: 5′-TCT GCT TGG TGG TTT GCT ACG AC-3′ |
14 |
| IL-1β |
Forward: 5′-CAC CTC TCA AGC AGA GCA CAG-3′ Reverse: 5′-GGG TTC CAT GGT GAA GTC AAC-3′ |
14 |
| Cyclin D1 |
Forward: 5′-GTC GAG AAG AGA AAG CTC TG-3′ Reverse: 5′-TTA AAA GCC TCC TGT GTG AA-3′ |
17 |
| P53 |
Forward: 5′-CTT CGA GAT GTT CCG AGA GC-3′ Reverse: 5′-CTT CGG GTA GCT GGA GTG AG-3′ |
18 |
| Bad |
Forward: 5′-GGA GCA TCG TTC AGC AGC AG-3′ Reverse: 5′-CCA TCC CTT CAT CTT CCT CAG TC-3′ |
19 |
| Bcl-xl |
Forward: 5′-AGG CTG GCG ATG AGT TTG AA-3′ Reverse: 5′-TGA AAC GCT CCT GGC CTT TC-3′ |
20 |
| β-Actin |
Forward: 5′-ACA GGA TGC AGA AGG AGA TTA C-3′ Reverse: 5′-AGA GTG AGG CCA GGA TAG A-3′ |
14 |
Statistical analysis
Statistical comparison between groups was conducted using a one-way analysis of variance and the least significant difference (LSD) method was used to determine significantly different groups. Statistical significance was accepted as p ≤ 0.05.
Results
Anticarcinogenic effect of purple rice bran extract and protocatechuic acid against diethylnitrosamine-induced rat hepatocarcinogenesis
After 5 weeks of the experiment, final body weight was significantly reduced in DEN-initiated rats compared to the control group. This result was directly related to the amount of food and water consumed. This result indicates that the DEN treatment was toxic to the rats. However, treatment with PRBE and PCA slightly improved the body weight when compared with the positive control group. By the end of week 15 of the experiment, there was no significant difference in body weight or food or water consumption among groups. There was no considerable alteration in the body weight or food or water consumption of rats administered PRBE and PCA alone for 5 and 15 weeks (data not shown). These findings indicate that PRBE and PCA had no adverse effect on the growth of the rats.
One week after the last DEN injection (5-week protocol), the liver weight of DEN-treated rats decreased significantly compared to the negative control animals. Remarkably, PRBE and PCA treatment significantly improved liver weight in DEN-initiated rats. However, there were no significant intergroup differences in the relative weights of the kidney and spleen. At the time point of 15 weeks, there was no significant difference in kidney, spleen, or liver weights between groups. The results are presented in Table 2. These findings indicated that PRBE and PCA did not present any toxicity when administered to rats.
Table 2.
Relative organ weight of rats with DEN-induced hepatocarcinogenesis treated with purple rice bran extract and protocatechuic acid.
| Treatment | Initiation (5-week protocol) | Promotion (15-week protocol) | ||||
|---|---|---|---|---|---|---|
| Relative organ weight | Relative organ weight | |||||
| Liver | Spleen | Kidney | Liver | Spleen | Kidney | |
| NSS | 4.02 ± 0.21 | 0.66 ± 0.03 | 0.23 ± 0.04 | 3.16 ± 0.23 | 0.23 ± 0.04 | 0.51 ± 0.02 |
| NSS + PRBE 500 mg kg−1 BW | 4.12 ± 0.18 | 0.68 ± 0.04 | 0.20 ± 0.02 | 2.94 ± 0.25 | 0.17 ± 0.03 | 0.49 ± 0.04 |
| NSS + PCA 4 mg kg−1 BW | 4.32 ± 0.05 | 0.68 ± 0.03 | 0.24 ± 0.03 | 3.12 ± 0.19 | 0.18 ± 0.01 | 0.47 ± 0.03 |
| DEN | 3.08 ± 0.14* | 0.66 ± 0.03 | 0.28 ± 0.04 | 3.06 ± 0.22 | 0.19 ± 0.02 | 0.53 ± 0.04 |
| DEN + PRBE 500 mg kg−1 BW | 3.36 ± 0.11# | 0.68 ± 0.03 | 0.26 ± 0.03 | 3.12 ± 0.26 | 0.19 ± 0.02 | 0.53 ± 0.02 |
| DEN + PCA 4 mg kg−1 BW | 3.50 ± 0.18# | 0.70 ± 0.03 | 0.29 ± 0.03 | 2.92 ± 0.10 | 0.19 ± 0.01 | 0.51 ± 0.02 |
Values are expressed as mean ± SD.
DEN: diethylnitrosamine, NSS: normal saline solution, PCA: protocatechuic acid, PRBE: purple rice bran extract.
*Significantly different from the negative control group, p < 0.05.
#Significantly different from the positive control group, p < 0.05.
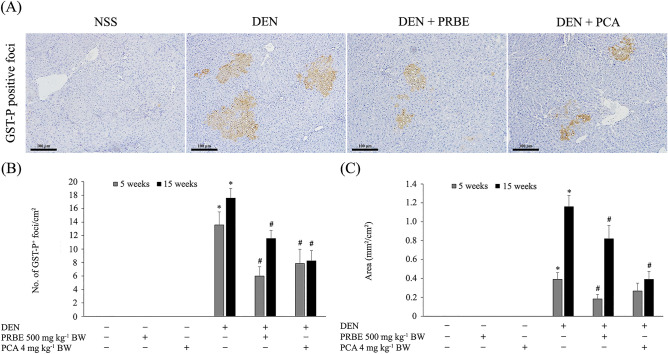
Figure 2 illustrates the effect of PRBE and PCA on the number and area of GST-P-positive foci. By the end of weeks 5 and 15 of the experiments, no GST-P formation was detected in the groups given PRBE and PCA alone, suggesting a lack of carcinogenicity. A significant increase in the number and area of GST-P-positive foci was observed in the DEN-treated group compared to their control group. Interestingly, PRBE and PCA treatments in DEN-induced rats resulted in a significant reduction of GST-P-positive foci compared to the group given DEN alone. These results indicate that PRBE and PCA had preventive effects against hepatocarcinogenesis caused by DEN treatment. Comparatively, the levels of anticarcinogenic activity of PCA and PRBE were similar to each other.
Figure 2.
Effect of purple rice bran extract and protocatechuic acid on the number and area of hepatic GST-P-positive foci. (A) Immunohistochemical staining of GST-P-positive foci (brown) (20 ×); (B) number of GST-P+ foci/cm2; (C) area (mm2/cm2). Values are expressed as mean ± SEM. *Significantly different from the negative control group, p < 0.05. #Significantly different from the positive control group, p < 0.05. DEN: diethylnitrosamine, PCA: protocatechuic acid, PRBE: purple rice bran extract.
Effect of protocatechuic acid on some phase I and phase II xenobiotic-metabolizing enzymes
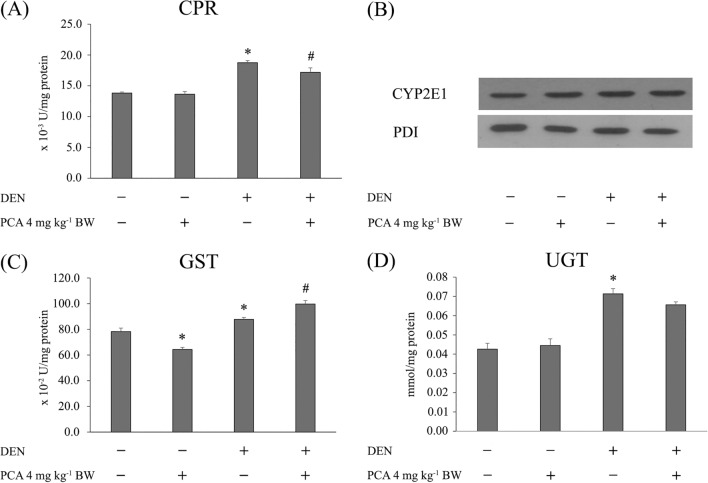
Protection against carcinogen-induced carcinogenesis can be accomplished by phase I inhibition and phase II induction. Figure 3 illustrates the activity and expression of some phase I and phase II xenobiotic-metabolizing enzymes in rat liver. Administration of DEN significantly increased the activity of CPR, an electron donor, when compared with the control. Remarkably, supplementation of the DEN-treated rats with PCA significantly decreased CPR activity, whereas treatment with PCA alone did not result in any significant variation from the negative control group (Fig. 3A). In addition, PCA treatment did not lead to any alteration in the expression of CYP2E1, a key enzyme in DEN metabolism (Fig. 3B). The activity of UGT and GST was significantly enhanced in DEN-administered rats. PCA-treated rats displayed a significant increase in GST activity as compared to DEN-treated rats but did not show any effect on UGT activity. However, treatment with PCA alone decreased GST activity (Fig. 3C and D). These results indicate that PCA acts as a dual-acting agent by reducing phase I enzyme activity and inducing phase II enzyme activity, thereby promoting detoxification and excretion.
Figure 3.
Effect of purple rice bran extract and protocatechuic acid on some phase I and phase II xenobiotic-metabolizing enzymes (5-week protocol). (A) CPR: NADPH: cytochrome P450 reductase activity; (B) Western blotting of cytochrome P450 2E1 (CYP2E1); (C) GST: glutathione-S-transferase activity; (D) UGT: UDP-glucuronyltransferase. Values are expressed as mean ± SEM. *Significantly different from the negative control group, p < 0.05. #Significantly different from the positive control group, p < 0.05. DEN: diethylnitrosamine, PCA: protocatechuic acid, PRBE: purple rice bran extract.
The alteration of cell proliferation and apoptosis of purple rice bran extract and protocatechuic acid in rats
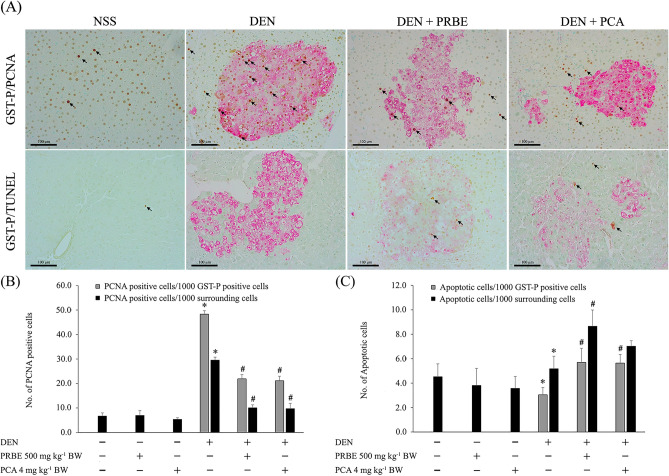
Figure 4 shows the effect of PRBE and PCA on cell proliferation and apoptosis in rat livers. Double immunohistochemical staining of GST-P/PCNA and GST-P/apoptotic cells is shown in Fig. 4A. PCNA is a nuclear protein, widely used as a standard marker of cell proliferation, which was found to be significantly increased in DEN-treated rats. Administration of PCA at the tested dose level significantly decreased the number of PCNA-positive cells in both GST-P-positive foci and the surrounding area in DEN-initiated rats. In addition, PCA significantly increased apoptosis in GST-P-positive foci of DEN-induced rats, but not in the surrounding area (Fig. 4B and C). These results produced by PCA were almost comparable with those for PRBE. These findings indicate that PCA suppresses the development of liver cancer by reducing cell proliferation and inducing cell apoptosis in DEN-initiated rats.
Figure 4.
Effect of purple rice bran extract and protocatechuic acid on cell proliferation and apoptosis (15-week protocol). Arrowheads indicate stained hepatocytes. (A) Representative double immunohistochemical staining of GST-P (red)/PCNA (brown) and GST-P (red)/apoptosis (TUNEL) (black) in the liver of control and experimental animals (20 ×); (B) number of PCNA-labeled cells per 1000 hepatocytes; (C) number of apoptotic cells per 1000 hepatocytes. Values are expressed as mean ± SEM. *Significantly different from the negative control group, p < 0.05. #Significantly different from the positive control group, p < 0.05. DEN: diethylnitrosamine, PCA: protocatechuic acid, PRBE: purple rice bran extract.
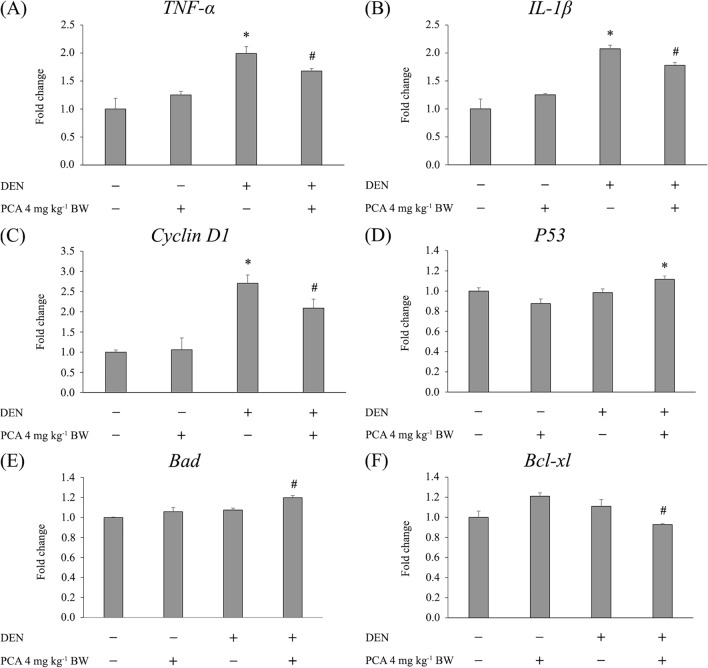
Response of protocatechuic acid to pro-inflammatory, cell proliferation, and apoptosis-related gene expression in diethylnitrosamine-initiated rats
The effect of PCA on genes associated with inflammation, cell proliferation, and apoptosis is presented in Fig. 5. After 5 weeks of the experiment (initiation stage), DEN-treated animals demonstrated significant upregulation of TNF-α and IL-1β mRNA in the liver compared to control animals. Oral administration of PCA significantly downregulated the expression of TNF-α and IL-1β mRNA in DEN-initiated rats when compared to the group given DEN alone (Fig. 5A and B). After 15 weeks of the experiment (promotion stage), a significant increase in the mRNA expression of Cyclin D1, a key regulator of G1/S phase transition, was observed in the DEN-treated group compared to the negative control group. Interestingly, supplementation with PCA significantly decreased the expression of Cyclin D1 when compared to the DEN-treated group (Fig. 5C). Furthermore, administration of PCA to DEN-treated rats significantly increased the expression of P53 and Bad (pro-apoptotic genes) accompanied by a decrease of Bcl-xl (anti-apoptotic gene) expression (Fig. 5D–F). In comparison, the control and PCA-alone groups presented similar results. Our findings demonstrate that PCA has potent anti-inflammatory action against the DEN-induced initiation stage of hepatocarcinogenesis by reducing the expression of the pro-inflammatory cytokines TNF-α and IL-1β. In the promotion stage, the anti-cell proliferative effect has been linked to the decrease of Cyclin D1. It also efficiently triggered apoptosis by induction of P53 and the pro-apoptotic gene Bad, accompanied by reduction of the anti-apoptotic gene Bcl-xl.
Figure 5.
Effect of protocatechuic acid on mRNA levels of genes involved in the initiation and promotion stages of hepatocarcinogenesis (5- and 15-week protocols). Data were normalized to the β-actin mRNA level and are expressed as the fold difference versus the negative control group. (A) TNF-α; (B) IL-1β; (C) Cyclin D1; (D) P53; (E) Bad; (F) Bcl-xl. Values are expressed as mean ± SEM. *Significantly different from the negative control group, p < 0.05. #Significantly different from the positive control group, p < 0.05. DEN: diethylnitrosamine, PCA: protocatechuic acid.
Discussion
Food is not only a source of energy but also contains several bioactive compounds that have been shown to possess health-promoting properties4,5. Rice is the chief food for more than half of the world’s population. Our recent experiment demonstrated the effectiveness of PRBE against DEN-induced rat hepatocarcinogenesis14. In this study, PCA, the most abundant phenolic acid in PRBE, could inhibit DEN-induced formation of GST-P positive foci similarly to PRBE. Previous reports have shown the cancer protective activities of PCA, such as prevention of carcinogen biotransformation, detoxification of xenobiotics, anti-inflammation, reduction of cell proliferation, and induction of apoptosis in several types of cancers21–23. Yip et al.24 have demonstrated that PCA could induce cell death in HepG2 hepatocellular carcinoma cells. It also inhibits rat hepatocarcinogenesis induced by DEN25. In addition, nanoparticles containing protocatechuic acid suppressed liver tumors in DEN- and phenobarbital-induced mice26. Although the anticarcinogenic effects of PCA on DEN-initiated liver cancer have been reported, they lacked the relevant amount of PCA in food. This study first showed that PCA at the dose of 4 mg kg−1 body weight attenuated DEN-induced early stages of rat hepatocarcinogenesis with the efficacy as PRBE. It might be indicated that PCA is an anticarcinogenic compound of PRBE. In addition, these findings contribute to understanding the underlying molecular mechanisms by which PCA ameliorates DEN-induced hepatocarcinogenesis.
The biotransformation of DEN by the cytochrome P450–dependent pathway produces active metabolites, which are primarily responsible for the initiation of hepatocarcinogenesis27. However, the electrophilic intermediates can be eliminated by making conjugates with hydrophilic molecules such as glutathione and glucuronic acid28. Previous investigation has shown that PCA reduced the activity and expression of CYP1A1/2 and CYP2E1 but induced the activity of GST and NQO1 in 3-methylcholantren-induced rats29. Recently, our report has shown that PRBE does not affect the activity of phase I and phase II enzymes14. Interestingly, the decreased activity of CPR accompanied by increased activity of GST observed in the present study provides evidence for the preventive action of PCA in DEN-induced rat hepatocarcinogenesis. Our results demonstrate that PCA acts as a potential dual-acting agent by inhibiting phase I enzymes and activating phase II enzymes. Other than that, inflammation is a critical factor contributing to the development of cancer. Interleukin 1 (IL-1) and tumor necrosis factor α (TNF-α) are considered key molecules for cancer progression30. The increased levels of IL-1β and TNF-α presented in this study provide evidence that inflammation was induced in DEN-treated rats. Importantly, PCA can downregulate the expression of IL-1β and TNF-α genes in DEN-initiated rats, presenting the same effect as PRBE14. This result is supported by a previous report that PCA suppresses the production of IL-1β and TNF-α in LPS-stimulated RAW 264.7 cells and also inhibits COX-2 expression in carrageenan-induced inflammation in mice31. These findings revealed that PCA inhibits liver inflammation, resulting in the suppression of the development of hepatocarcinogenesis.
The progression of cancer is related to an increase in the proportion of proliferating cells. One common approach to studying cell proliferation activity is immunohistochemical staining of PCNA, which is a marker of cell transformation32. DEN-initiated rats showed an increased number of PCNA, indicating hyperproliferative activity. However, PCA treatment in DEN-treated rats decreased the level of PCNA in both hepatic GST-P-positive foci and the surrounding area. This effect revealed that PCA attenuates DEN-induced hepatocarcinogenesis by suppressing cell proliferation. This positive result is consistent with our previous study in which PRBE inhibited cell proliferation in DEN-treated rats14. Therefore, it may be assumed that PCA acts as a potent inhibitor of cancer cell proliferation in PRBE. Overexpression of cyclin D1 has been linked to the development of cancers because it is an essential regulator that activates the transition from G1 to S phase33,34. DEN treatment upregulated the expression of Cyclin D1, whereas PCA downregulated the expression of the Cyclin D1 gene in DEN-administered rats. This finding demonstrates that PCA suppresses cell proliferation via downregulation of Cyclin D1 in DEN-induced rat hepatocarcinogenesis.
Apoptosis is an essential physiological process for cell homeostasis. The inhibition of apoptosis contributes to the pathogenesis of cancer35. Alteration of p53, a tumor suppressor gene, is one of the key regulators in cell cycle arrest and apoptosis. Normally, DNA-damaged cells undergo apoptosis which is a protective mechanism to prevent the development of cancer36. PCA administration increased the number of TUNEL-positive cells in the DEN-treated group. This finding was similar to that observed for the activity of PRBE. Additionally, treatment with PCA increased the level of P53, thereby potentially driving the mutated cells to apoptosis. A previous report demonstrated that the Bcl-2 antagonist of cell death (BAD)-mediated apoptotic pathway plays an important role in carcinogenesis. To initiate apoptosis, Bad protein dimerizes with Bcl-xL and Bcl-2, displacing Bax, leading to the induction of cell apoptosis37. Previous investigation has reported that Bad is transcriptionally upregulated by p53 and it can also form a Bad/p53 complex at the mitochondria to induce apoptosis38. In this study, the quantitative results showed that PCA induced programmed cell death in DEN-treated rats through upregulation of Bad (a pro-apoptotic gene) and downregulation of Bcl-xl (an anti-apoptotic gene). Taken together, these data suggest that PCA induces apoptosis via a mitochondria-mediated apoptotic pathway.
This study demonstrates for the first time that PCA (4 mg kg−1 BW) is an active compound in the anticarcinogenic action of purple rice bran against the formation of DEN-induced hepatic preneoplastic lesions in rats. The preventive mechanisms were associated with xenobiotic-metabolizing enzymes (decreased CPR and increased GST enzyme activity). Additionally, PCA also exerted anti-inflammatory activity (downregulating mRNA levels of the pro-inflammatory cytokines TNF-α and IL-1β). Moreover, it suppressed hepatic cell proliferation by decreasing Cyclin D1. PCA also induced hepatic apoptosis via upregulation of P53 and Bad and downregulation of Bcl-xl mRNA levels. Notably, the effective dose of PCA, 4 mg kg−1 BW, corresponds to a human-equivalent dose (HED) of 39 mg/day39, which is greater than the daily recommended dose of PCA in food, approximately 2–10 mg g−1 21. However, the cancer chemopreventive dose of PCA is only estimated within a critical period of carcinogen exposure. PCA is one of the active metabolites of dietary anthocyanins40; therefore, the regular consumption of anthocyanin-rich foods such as purple rice and other colored vegetables and fruits should be recommended. This knowledge might provide an important implication for the use of PCA as a potential anticancer agent in humans. Nevertheless, clinical trials testing this compound need to be performed.
Supplementary Information
Acknowledgements
This research project was supported by Fundamental Fund 2022, Chiang Mai University and the CMU Junior Research Fellowship Program. This work was partially supported by the Faculty of Veterinary Medicine, Chiang Mai University. Special acknowledgment is directed to the Faculty of Veterinary Medicine and Faculty of Medicine, Chiang Mai University for the necessary research works and instruments.
Author contributions
C.P. Conceptualization; Investigation; Methodology; Funding acquisition; writing—original draft. T.L. Investigation; Data curation. R.W. Supervision; Methodology; Writing—review & editing. All authors reviewed and approved the final version of the manuscript to be published.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14888-2.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Li Y, Zhou T, Zheng J, Li S, Li HB. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8:1–23. doi: 10.3390/nu8030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S, Srivastava SK. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019;20:1–17. doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Oliveira P, Otero P, Pereira AG, Chamorro F, Carpena M, Echave J, Fraga-Corral M, Simal-Gandara J, Prieto MA. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals. 2021;14:1–28. doi: 10.3390/ph14020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Zhu B, Zhao L, Liu Y, Zhao F, Feng J, Jin Y, Sun J, Geng R, Wei Y. Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-mediated STAT3 signaling in cholangiocarcinoma. Cell Physiol. Biochem. 2018;47:641–653. doi: 10.1159/000490019. [DOI] [PubMed] [Google Scholar]
- 7.Yan X-B, Xie T, Wang S-D, Wang Z, Li H-Y, Ye Z-M. Apigenin inhibits proliferation of human chondrosarcoma cells via cell cycle arrest and mitochondrial apoptosis induced by ROS generation-an in vitro and in vivo study. Int. J. Clin. Exp. Med. 2018;11:1615–1631. [Google Scholar]
- 8.Punvittayagul C, Chariyakornkul A, Jarukamjorn K, Wongpoomchai R. Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules. 2021;26:1–12. doi: 10.3390/molecules26092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousuf M, Shamsi A, Khan P, Shahbaaz M, AlAjmi MF, Hussain A, Hassan GM, Islam A, Rizwanul Haque QM, Hassan MI. Ellagic acid controls cell proliferation and induces apoptosis in breast cancer cells via inhibition of cyclin-dependent kinase 6. Int. J. Mol. Sci. 2020;21:1–18. doi: 10.3390/ijms21103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd El Fattah MA, Abdelhamid YA, Elyamany MF, Badary OA, Heikal OA. Rice bran extract protected against LPS-induced neuroinflammation in mice through targeting PPAR-γ nuclear receptor. Mol. Neurobiol. 2021;58:1504–1516. doi: 10.1007/s12035-020-02196-7. [DOI] [PubMed] [Google Scholar]
- 11.Ghasemzadeh A, Karbalaii MT, Jaafar HZE, Rahmat A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018;12:1–13. doi: 10.1186/s13065-017-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saji N, Francis N, Schwarz LJ, Blanchard CL, Santhakumar AB. The antioxidant and anti-inflammatory properties of rice bran phenolic extracts. Foods. 2020;9:1–12. doi: 10.3390/foods9060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao J, Zhang R, Wu Y, Wu C, Jia X, Dong L, Liu L, Chen Y, Bai Y, Zhang M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin-TLR4-NF-κB pathway. J. Agric. Food. Chem. 2020;68:1237–1247. doi: 10.1021/acs.jafc.9b04961. [DOI] [PubMed] [Google Scholar]
- 14.Dokkaew A, Punvittayagul C, Insuan O, Limtrakul Dejkriengkraikul P, Wongpoomchai R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules. 2019;24:1–12. doi: 10.3390/molecules24112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thumvijit T, Taya S, Punvittayagul C, Peerapornpisal Y, Wongpoomchai R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kutzing on diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian Pac. J. Cancer Prev. 2014;15:1611–1616. doi: 10.7314/APJCP.2014.15.4.1611. [DOI] [PubMed] [Google Scholar]
- 16.Punvittayagul C, Wongpoomchai R, Taya S, Pompimon W. Effect of pinocembrin isolated from Boesenbergia pandurata on xenobiotic-metabolizing enzymes in rat liver. Drug Metab. Lett. 2011;5:1–5. doi: 10.2174/187231211794455226. [DOI] [PubMed] [Google Scholar]
- 17.Khan M, Halagowder D, Devaraj S. Methylated chrysin induces coordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem. Biol. Interact. 2011;193:12–21. doi: 10.1016/j.cbi.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Lv N, Liu H, Gu C, Zhou X, Qin W, Chen AC, Chen L, Yang H, Chen X, Liu T, He F. Melatonin improves the resistance of oxidative stress-induced cellular senescence in osteoporotic bone marrow mesenchymal stem cells. Oxid. Med. Cell. Longev. 2022;2022:1–22. doi: 10.1155/2022/7420726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badr R, Hashemi M, Javadi G, Movafagh A, Mahdian R. Gene expression profiles of BAD and Bcl-xL in the CA1 region of the hippocampus following global ischemic/reperfusion and FK-506 administration. Iran Red. Crescent Med. J. 2015;17:1–5. doi: 10.5812/ircmj.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafari Anarkooli I, Sankian M, Ahmadpour S, Varasteh AR, Haghir H. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp. Diabetes Res. 2008;2008:1–6. doi: 10.1155/2008/638467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka T, Tanaka T, Tanaka M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011;3:27–33. doi: 10.1016/j.jecm.2010.12.005. [DOI] [Google Scholar]
- 22.Kakkar S, Bais S. A review on protocatechuic Acid and its pharmacological potential. ISRN pharmacol. 2014;2014:1–9. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Z, Guo Z, Wang Y, Lei J, Yu J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018;32:2256–2263. doi: 10.1002/ptr.6163. [DOI] [PubMed] [Google Scholar]
- 24.Yip EC, Chan AS, Pang H, Tam YK, Wong YH. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell. Biol. Toxicol. 2006;22:293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Kojima T, Kawamori T, Yoshimi N, Mori H. Chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis by a simple phenolic acid protocatechuic acid in rats. Cancer Res. 1993;53:2775–2779. [PubMed] [Google Scholar]
- 26.Gani SA, Muhammad SA, Kura AU, Barahuie F, Hussein MZ, Fakurazi S. Effect of protocatechuic acid-layered double hydroxide nanoparticles on diethylnitrosamine/phenobarbital-induced hepatocellular carcinoma in mice. PLoS ONE. 2019;14:1–24. doi: 10.1371/journal.pone.0217009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Il'nitskaya SI, Kaledin VI, Bogdanova LA, Morozkova TS, Kapustina VI, Perepechaeva ML, Grishanova AY. Stimulation of diethylnitrosamine metabolism reduces its general toxic and hepatocarcinogenic effects. Bull. Exp. Biol. Med. 2016;162:98–101. doi: 10.1007/s10517-016-3555-3. [DOI] [PubMed] [Google Scholar]
- 28.Kwak M-K, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajka-Kuzniak V, Szaefer H, Baer-Dubowska W. Modulation of 3-methylcholanthrene-induced rat hepatic and renal cytochrome P450 and phase II enzymes by plant phenols: Protocatechuic and tannic acids. Toxicol. Lett. 2004;152:117–126. doi: 10.1016/j.toxlet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakur Y, Segal S, Dinarello CA, Apte RN. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 31.Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010;10:959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Cardano M, Tribioli C, Prosperi E. Targeting proliferating cell nuclear antigen (PCNA) as an effective strategy to inhibit tumor cell proliferation. Curr. Cancer Drug. Targets. 2020;20:240–252. doi: 10.2174/1568009620666200115162814. [DOI] [PubMed] [Google Scholar]
- 33.Alao JP. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer. 2007;6:1–16. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R-A, Li Q-L, Li Z-S, Zheng P-J, Zhang H-Z, Huang X-F, Chi S-M, Yang A-G, Cui R. Apoptosis drives cancer cells proliferate and metastasize. J. Cell. Mol. Med. 2013;17:205–211. doi: 10.1111/j.1582-4934.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letai A. Apoptosis and cancer. Ann. Rev. Cancer Biol. 2017;1:275–294. doi: 10.1146/annurev-cancerbio-050216-121933. [DOI] [Google Scholar]
- 36.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016;6:1–15. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stickles XB, Marchion DC, Bicaku E, Al Sawah E, Abbasi F, Xiong Y, Zgheib NB, Boac BM, Orr BB, Judson PL, Berry A, Hakam A, Wenham RM, Apte SM, Berglund AE, Lancaster JM. BAD-mediated apoptotic pathway is associated with human cancer development. Int. J. Mol. Med. 2015;35:1081–1087. doi: 10.3892/ijmm.2015.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang P, Du W, Heese K, Wu M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol. Cell. Biol. 2006;26:9071–9082. doi: 10.1128/MCB.01025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.