Abstract
Background
Home-monitoring of spirometry has the potential to improve care for patients with a motor neuron disease (MND) by enabling early detection of respiratory dysfunction and reducing travel burden. Our aim was to evaluate the validity and feasibility of home-monitoring vital capacity (VC) in patients with MND.
Methods
We included 33 patients with amyotrophic lateral sclerosis, progressive muscular atrophy or primary lateral sclerosis who completed a 12-week home-monitoring protocol, consisting of 4-weekly unsupervised home assessments of VC and a functional rating scale. At baseline, during a home visit, patients/caregivers were trained in performing a VC test, and the investigator performed a supervised VC test, which was repeated at final follow-up during a second home visit. Validity of the unsupervised VC tests was evaluated by the differences between supervised and unsupervised VC tests, and through Bland–Altman 95% limits-of-agreement. Feasibility was assessed by means of a survey of user-experiences.
Results
The 95% limits-of-agreement were [− 14.3; 11.7] %predicted VC, and 88% of unsupervised VC tests fell within 10%predicted of supervised VC. 88% of patients experienced VC testing as easy and not burdensome, however, 15% patients did not think their VC test was performed as well as in the clinic. 94% of patients would like home-monitoring of VC in MND care.
Discussion
Unsupervised VC testing at home, with prior face-to-face training, is a valid and time-efficient method for the remote monitoring of respiratory function, and well-accepted by patients with MND and their caregivers.
Keywords: Motor neuron disease, Amyotrophic lateral sclerosis, Respiratory function, Vital capacity, Remote monitoring, Validity
Introduction
In patients with a motor neuron disease (MND), respiratory failure is the main cause of death [1, 2]. When patients show signs or symptoms of respiratory dysfunction, as described in clinical guidelines, non-invasive ventilation (NIV) is recommended [3–5]. Studies have shown that the use of NIV prolongs survival and improves quality of life [6–8]. Regular monitoring of respiratory function is essential to ensure timely detection of respiratory dysfunction so that NIV can be initiated [3–5]. In current MND healthcare, respiratory function is monitored during regular visits to a multidisciplinary clinic. Two drawbacks of this type of monitoring are that clinic visits can be time consuming and burdensome for patients with MND, and that patients have to visit the clinic irrespective of whether there is a decrease in respiratory function [9, 10]. This suggests that current respiratory monitoring may be insufficiently tailored to the needs of patients.
A potential solution is the home-monitoring of respiratory function through the use of telehealth. This approach allows for more frequent assessments, higher continuity of monitoring, especially when patients are not able to visit the clinic, and easy communication between patients and the multidisciplinary care team [11–16]. The use of telehealth may help to detect respiratory function decline early, and schedule clinic visits and initiate clinical interventions on time. One method of home-monitoring is the assessment of patient-reported symptoms of dyspnea, which was found to be useful for screening whether patients with MND had reduced vital capacity (VC) [17]. However, a drawback of dependence on self-reported dyspnea/orthopnea is that patients with low VC but without symptoms will not be identified (false negative rate = 14%). For this reason, combining patient-reported symptoms of dyspnea with home-based VC testing may reduce false negative findings and improve the home-monitoring of respiratory function.
The VC test has prognostic value in patients with MND [18, 19], and is easy to perform with a handheld spirometer, which is affordable and widely available. For these reasons, the VC test is suitable and relevant for home-monitoring; however, in MND care, its application for home-monitoring is still lacking [20]. Recently, a study showed that during COVID-19, it was feasible to perform home-based VC testing with supervision via video and that it was well-received by patients with MND in a healthcare setting [21]. However, one trial showed that when patients performed VC tests at home without supervision, the remote VC measurement was significantly higher than the usual in-clinic VC measurement and compliance was suboptimal [22]. These findings show the potential of home-monitoring of VC, but also indicate that more evidence is needed to support its implementation.
The aim of the present study is, therefore, to evaluate the validity and feasibility of unsupervised home-monitoring of VC in patients with MND.
Methods
Study design and population
This prospective cohort study aimed to include patients with MND, who were 18 years old or over and had access to a smartphone or tablet. Different diagnoses of MND were involved: amyotrophic lateral sclerosis (ALS), progressive muscular atrophy (PMA) and primary lateral sclerosis (PLS). The exclusion criteria were the use of non-invasive ventilation during the daytime, tracheostomy, or the inability to perform a VC test with or without caregiver assistance. Ethics approval from the Medical Ethics Committee of the University Medical Center Utrecht was obtained prior to the start of the study and patients gave their informed consent before participating.
Setting and procedure
This study was conducted by the University Medical Center in Utrecht, the Netherlands, in collaboration with the Revant Center for Rehabilitation in Breda. Both centers have a multidisciplinary care team, coordinated by a physician. All study activities were performed at the patients’ homes, meaning that patients could participate in the present study without having to visit a multidisciplinary clinic. Patients who, between August 2020 and February 2021, received MND care from the multidisciplinary care teams were invited by the treating physician to participate. Most patients had access to the telehealth service ALS Home-monitoring and Coaching as part of their usual care. This telehealth service included the mobile ALS app for self-monitoring and messaging, which facilitated remote monitoring and communication between the patient and the multidisciplinary care team. A full description of ALS Home-monitoring and Coaching is available in a previous publication [12].
Study assessments
Respiratory function was assessed by making three attempts to perform the vital capacity (VC) test in upright position, using a low-cost (ca. €100,-) handheld spirometer with Bluetooth connection to a mobile app (Spirobank Smart®, Medical International Research, Italy). The VC tests were performed with a full-face mask (Fig. 1) to enable testing in patients with bulbar impairment [23]. Patients recorded the time, date and VC test scores on a paper form, and also sent the VC test scores digitally to their multidisciplinary care team via the ALS app or by email, which allowed members of the multidisciplinary ALS care team to monitor respiratory function. The revised ALS functional rating scale (ALSFRS-R) was used to assess functional impairment [17, 24], and was self-monitored monthly as part of ALS Home-monitoring and Coaching. Patients who did not use telehealth completed the ALSFRS-R on paper at every follow-up. We created a survey to evaluate user-experiences of patients and caregivers who assisted with VC testing; see Tables 2 and 3 for the items of the survey. Items were scored on a 5-point Likert scale: the extent to which patients/caregivers considered aspects of VC testing to be difficult (answer options: Very easy–Very difficult), or the extent to which they agreed with a statement on VC testing (answer options: Totally agree–Totally disagree).
Fig. 1.
Performing a vital capacity test with a full-face mask. Left: A hammer grip around the tube. Right: Holding the mask with the tube placed between the fingers
Table 2.
User-experiences of patients
| Item | (Very) Easy n (%) | Neutral n (%) | (Very) Difficult n (%) | N* |
|---|---|---|---|---|
| Placing the mask on my face was | 23 (82.1) | 4 (14.3) | 1 (3.6) | 28 |
| Handling the spirometer was | 26 (92.8) | 1 (3.6) | 1 (3.6) | 28 |
| Starting a VC test in the app was | 30 (96.8) | 1 (3.2) | 0 (0) | 31 |
| Performing a VC test was | 29 (87.9) | 3 (9.1) | 1 (3) | 33 |
| Judging whether the test was performed correctly was | 26 (78.8) | 3 (9.7) | 3 (9.7) | 32 |
| Item | (Totally) Agree n (%) | Neutral n (%) | (Totally) Disagree n (%) | N* |
|---|---|---|---|---|
| The spirometer is user-friendly | 31 (93.9) | 1 (3) | 1 (3) | 33 |
| The spirometry app was user-friendly | 30 (90.9) | 3 (9.1) | 0 (0) | 33 |
| The spirometer is appropriate for home-monitoring of respiratory function | 30 (90.9) | 3 (9.1) | 0 (0) | 33 |
| I would like to monitor my respiratory function from home for care purposes | 30 (93.8) | 2 (6.3) | 0 (0) | 32 |
| I know how to perform a VC test | 33 (100) | 0 (0) | 0 (0) | 33 |
| I believe that my VC test at home is performed just as well as a usual VC test in the clinic | 24 (72.8) | 4 (12.1) | 5 (15.1) | 33 |
| I am unsure about performing the VC test correctly in the absence of a healthcare professional | 2 (6.5) | 4 (12.9) | 25 (80.6) | 31 |
| Performing VC tests at home is burdensome | 2 (6.3) | 2 (6.3) | 28 (87.5) | 32 |
VC vital capacity
*Missing data are due to patients answering “not applicable/ no opinion”
Table 3.
User-experiences of caregivers
| Item | (Very) Easy | Neutral | (Very) Difficult |
|---|---|---|---|
| Placing the mask on his/her face was | 8/9 | 1/9 | 1/9 |
| Handling the spirometer was | 8/9 | 0/9 | 1/9 |
| Starting a VC test in the app was | 8/8 | 0/8 | 0/8 |
| Performing a VC test was | 7/8 | 1/8 | 0/8 |
| Judging whether the test was performed correctly was | 7/9 | 2/9 | 0/9 |
| Item | (Totally) Agree | Neutral | (Totally) Disagree |
|---|---|---|---|
| The spirometer is user-friendly | 8/9 | 1/9 | 0/9 |
| The spirometry app is user-friendly | 7/8 | 1/8 | 0/8 |
| The spirometer is appropriate for home-monitoring of respiratory function | 8/9 | 1/9 | 0/9 |
| I would like to monitor my respiratory function from home for care purposes | 8/9 | 0/9 | 1/9 |
| I know how to (help) perform a VC test | 8/9 | 1/9 | 0/9 |
| I believe that my VC test at home is performed just as well as a usual VC test in the clinic | 5/9 | 1/9 | 3/9 |
| I am unsure about performing the VC test correctly in the absence of a healthcare professional | 1/9 | 1/9 | 7/9 |
| Helping to perform VC tests at home is burdensome | 0/9 | 2/9 | 7/9 |
Missing data are due to caregivers answering “not applicable/ no opinion”, VC vital capacity
Baseline protocol
At baseline (T0), the supervised VC test and ALSFRS-R were completed during a home visit. The investigator helped patients to install the mobile app on their smartphone, after which the supervised VC was performed. VC tests were either performed forcefully (FVC) or slowly (SVC), depending on which method was most effective/suitable for the patient [19]. Patients (and their caregivers) were instructed on how to perform the VC test independently, and practiced VC testing. If required, the investigator gave tips on how to improve the way the VC test was performed. When proper technique was observed (e.g. correct placement of mask, maximal in and exhalation, upright body position), the investigator left the room, and the patient performed an unsupervised VC test, to ensure that patients were able to do this without supervision. Unsupervised VC tests that were performed during the baseline home visit, were not included in the analysis.
Follow-up protocol
The total follow-up period was 12 weeks, with 4-weekly unsupervised assessments. One day after the home visit (T1), patients completed their baseline unsupervised VC tests. At 4 weeks (T2), 8 weeks (T3) and 12 weeks (T4) after baseline, patients completed unsupervised VC tests and the ALSFRS-R. The investigator sent a reminder on the days of follow-up either via text-message or e-mail, depending on patient preference. At T4 the investigator visited the patient’s home at least 1 h after patients had completed their unsupervised VC tests. During this final home visit, a supervised VC test was performed and patients (and their caregivers) were asked to fill in the survey on user-experiences and to indicate the average duration of their VC testing sessions.
Analyses
The highest VC test score, out of three attempts, at each time-point was converted to a percentage of the predicted (%predicted) VC, using height, age, and ethnicity (reference values used from Global Lung Function Initiative 2012) [25, 26]. We used the unsupervised test at T1 as baseline, since the unsupervised VC tests performed at T0 may have been affected by the prior supervised VC tests. Validity of unsupervised VC testing was assessed through the Bland–Altman 95% limits-of-agreement and Lin’s concordance correlation coefficient (CCC) between the supervised VC at T0 and the unsupervised VC at T1, and between the supervised and unsupervised VC at T4. Based on clinical experience, we considered a maximal difference of 10%predicted between supervised and unsupervised VC as an acceptable limit of agreement, since this will allow healthcare professionals to determine a trend of VC over time when the VC is monitored at 4-weekly intervals. Additionally, the coefficient of variation of supervised VC testing in patients with MND was already 6.3%predicted in a previous study [27]. A paired t-test was conducted to assess the change in supervised and unsupervised VC between T0 and T4, and whether there was a systematic difference between supervised and unsupervised VC. Furthermore, we evaluated whether the agreement between supervised and unsupervised VC was different after 12 weeks of home-monitoring compared to baseline. To obtain insight into the variation in unsupervised VC testing over time, we used linear regression to determine the average slope over the 12 week period for each individual patient, and we calculated the standard error (SE) which indicates to what extent the VC values deviate from the linear regression line. We then ranked patients from lowest to highest SE and created a subgroup for each quartile (25%) of patients. These subgroups were used to create 4 separate plots for the longitudinal unsupervised VC data of individual patients to facilitate interpretation of the data. Furthermore, in the Bland–Altman plots, the subgroups are indicated for each data point (i.e. patient), to indicate whether greater variability showed larger differences between unsupervised and supervised VC. An alpha of < 0.05 was considered to be statistically significant. Feasibility of unsupervised home-based VC testing was determined through the adherence to the 4-weekly VC protocol, time cost of VC testing and user-experiences. Unsupervised VC testing was considered feasible when ≥ 75% of all unsupervised VC tests had been carried out, and each testing session completed within 20 min. An item of the user-experience survey was considered feasible when ≥ 75% of patients answered ‘(totally) agree’ on positive statements, ‘(totally) disagree’ on negative statements, and ‘(very) easy’ on difficulty statements.
Results
We included 33 patients with MND, with an average age of 60.5 years, 79% of whom were male. 76% were diagnosed with ALS, 15% with PMA and 9% with PLS, and 78.8% had spinal onset. At baseline, three patients were on nightly NIV, and one patient started with nightly NIV during the study period. Most patients (88%) used telehealth as part of their usual care. All baseline patient characteristics are presented in Table 1. Nine patients were assisted with VC testing by a partner (N = 4), family member (N = 3) or a home nurse (N = 2). The mean change over the 12-week period for the ALSFRS-R total score was − 2.1 points.
Table 1.
Baseline patient characteristics
| Characteristic | Patients (N = 33) |
|---|---|
| Gender (male), n (%) | 26 (78.8) |
| Age (years), mean(SD) | 60.5 (13.2) |
| Diagnosis, n (%) | |
| ALS | 25 (75.8) |
| PMA | 5 (15.2) |
| PLS | 3 (9.1) |
| Site of onset, n (%) | |
| Bulbar | 7 (21.2) |
| Spinal | 26 (78.8) |
| Nightly NIV, n (%) | 3 (12.1) |
| Gastrostomy, n (%) | 2 (6.1) |
| Telehealth use, n (%) | 29 (87.8) |
| Respiratory function (% of predicted VC), mean (SD) | 78.4 (25.6) |
| Disease duration from first symptoms (months), median (IQR) | 35.6 (17.2–52.2) |
| ALSFRS-R, mean (SD) | 35.9 (7.3) |
| ALSFRS-R (respiratory domain), mean (SD) | 11.0 (1.3) |
ALS amyotrophic lateral sclerosis, PMA progressive muscular atrophy, PLS primary lateral sclerosis, NIV non-invasive ventilation, VC vital capacity, SD standard deviation, IQR interquartile range, MND motor neuron disease, ALSFRS-R revised ALS functional rating scale
Validity of unsupervised VC testing
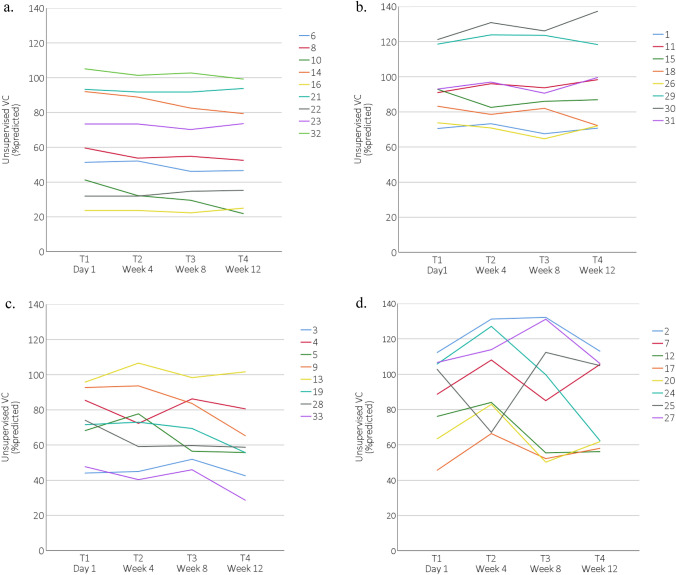
The 95% limits-of-agreement and the mean difference were [− 15.1; 15.4] and 0.12%predicted (p = 0.928) at baseline, respectively, and [− 14.3; 11.7] and –1.33%predicted (p = 0.259) at final follow-up, respectively (Fig. 2). The difference between unsupervised and supervised VC was smaller than 10%predicted in 28 of 33 (85%) patients at baseline and in 29 of 33 (88%) patients at final follow-up. The median absolute difference between supervised and unsupervised VC at baseline and final follow-up were 2.6 (IQR = 1.3–7.8) and 4.1 (IQR = 1.6–5.8) %predicted, respectively. Lin’s CCC was excellent at baseline (0.953), as well as at final follow-up (0.971) (Fig. 3). Between baseline and final follow-up both the supervised VC (Mean = − 3.31, p = 0.045) and unsupervised VC (-4.77, p = 0.036) decreased significantly. We also compared the change in supervised and unsupervised VC between baseline and final follow-up, which showed a good correlation (ρ = 0.74, p < 0.001). The plots of individual unsupervised VC data can be found in Fig. 4, where the range of SE was 0.36–0.96%predicted for the first quartile of patients, 1.02–2.16%predicted for the second quartile of patients, 2.28–3.98%predicted for the third quartile of patients, and 4.56–10.47%predicted for the fourth quartile of patients.
Fig. 2.
Bland–Altman plots. VC = vital capacity, Dashed line = 95% limits of agreement. The 4 quartile groups are based on the variability of the unsupervised VC scores over time, where 1st quartile = lowest variability and 4th quartile = highest variability. a. At baseline, b. at final follow-up
Fig. 3.
Scatterplot of unsupervised vs supervised vital capacity. VC vital capacity. Dashed line = line of identity. a At baseline, Lin's CCC = 0.953, b at final follow-up, Lin's CCC = 0.971
Fig. 4.
Unsupervised vital capacity over time per individual patient. VC = vital capacity. Patients were ranked from low to high variability, based on the standard error (SE) of the unsupervised VC scores over time and split into four quartiles (i.e. 25% of patients in each group). a) patients in the first quartile (SE range = 0.36–0.96 %predicted), b) patients in the second quartile (SE range = 1.02–2.16 %predicted), c) patients in the third quartile (SE range = 2.28–3.98 %predicted), and d) patients in the fourth quartile (SE range = 4.56–10.47 %predicted)
Feasibility of home-monitoring
All 33 participants completed 100% of their VC assessments, 32 (97%) within 20 min, and 29 (88%) within 15 min. Patients reported that the spirometer and spirometry app were user-friendly, and that unsupervised VC testing was considered to be easy and not burdensome (Table 2). Most patients (30, 93.8%) would like their respiratory function from home for care purposes. Even patients with limited hand function were able to handle the spirometer and independently perform a VC test, as 29% (7/24) of patients who were not assisted by a caregiver had an ALSFRS-R fine motor score of ≤ 6. This was due to the fact that the face mask, which was attached to the mouthpiece of the spirometer, made it easier to hold the spirometer. Three patients experienced difficulties with determining whether a VC test was performed correctly and two patients felt insecure about their VC test performance in the absence of a healthcare professional. Furthermore, five patients did not think that the unsupervised VC tests were performed as well as supervised tests in the clinic.
Based on the comments reported by patients during unsupervised VC testing, there were some difficulties that affected VC test performance: excessive mucus in throat (patient 4, Fig. 4c at T2), physical fatigue (patient 17, Fig. 4d at T1), pain in stomach caused by a gastrostomy tube (patient 24, Fig. 4d at T4), not being able to concentrate during testing (patient 26, Fig. 4b at T3), or physical discomfort due to an uncomfortable body position in wheelchair (patient 33, Fig. 4c at T4).
Most caregivers who assisted with VC testing reported that the spirometer (n = 7) and mobile app (n = 8) were user-friendly, and that helping with VC testing was easy (n = 7) and not burdensome (n = 7) (Table 3). The majority of caregivers believed they were able to (help) perform a VC test correctly (n = 8), and judge whether a VC test had been performed correctly (n = 7). Some of the caregivers (n = 3) did not think that they performed the unsupervised VC as well as a healthcare professional in a clinic.
Discussion
The present study showed that unsupervised home-monitoring of VC, after one face-to-face training, was a valid method for the remote monitoring of respiratory function in patients with MND. Furthermore, the 4-weekly home-monitoring of VC without supervision was feasible, since adherence was excellent, and most patients and caregivers experienced VC testing as easy and not burdensome. Lastly, patients and caregivers were motivated to continue with home-monitoring of VC in MND healthcare.
Our results on the validity and feasibility of unsupervised VC testing at home are promising and show that this can be a time-efficient method in MND care for both patients and healthcare professionals for remotely monitoring respiratory function. We provided insight into the variation in unsupervised VC testing over time, which showed that most patients had a stable trend of VC during the 12-week period. However, the course of the unsupervised VC of some patients were highly variable over time, and generally showed larger differences with the supervised VC, indicating that these patients may require additional supervision during home-monitoring, e.g. through video.
We found that there was no systematic difference between unsupervised and supervised VC, but at final follow-up we observed that supervised VC test scores were more likely to be higher than the unsupervised VC test scores, when compared to baseline. This may indicate that the performance of the unsupervised VC test decreases over time in some patients. This finding is in contrast to previous studies, which reported that remote VC assessments were systematically higher than usual in-clinic VC assessments [22, 28]. An explanation for this finding, is that in the present study all VC tests were performed at patients’ homes, including the supervised tests. This limited the factors that may have negatively affected VC test performance, such as the burden of travelling and visiting a clinic.
We found that all patients adhered to the 4-weekly monitoring protocol, and that this frequency was acceptable. This corresponds to findings of a recent study, in which most patients reported that the highest acceptable frequency for remote respiratory assessments was monthly [21]. In the present study, facilitating factors for adherence to VC testing at home were that the spirometer and app were user-friendly, and VC testing was easy, not burdensome and not time consuming. A previous study reported suboptimal adherence with a weekly VC protocol, mainly due to connection problems and patients forgetting to complete measurements [22]. During our study we were fortunate that the spirometer and app only rarely malfunctioned, which resulted in re-doing a VC test, but never prevented patients from testing. Furthermore, the problem of forgetting a VC test was tackled by sending a reminder at each follow-up. Another facilitator for adherence was the fact that home-monitoring of VC was part of an existing telehealth service and that VC test results were monitored by the multidisciplinary care team. Patients are likely to be more motivated to complete assessments at home, when they know healthcare professionals are monitoring their data closely and will provide feedback when necessary [29].
During unsupervised home-monitoring there were several factors, unrelated to respiratory muscle weakness, which hindered optimal VC test performance, such as pain, physical fatigue or loss of concentration. This suggests that it is important that patients provide comments on their physical and psychological well-being at time of VC testing, to help healthcare professionals interpret VC scores remotely. Moreover, some patients and caregivers experienced difficulties with determining whether a VC test was performed correctly, and felt insecure about proper VC test performance without supervision. These patients may prefer access to online instruction-videos [30] or require video-supervision during home-monitoring, which has been shown to be well-accepted by patients with MND [21, 28]. A disadvantage of video-supervised monitoring, is that it takes healthcare professionals considerably more time, compared to unsupervised monitoring. Interestingly, one study reported that only a few patients felt they were able to perform a VC test at home without video-supervision, which contrasts with our study sample, where the majority believed they were able to perform a VC test at home without supervision. A reason for this discrepancy may be that patients in the present study were trained in unsupervised VC, and that most patients already had experience with telehealth and remote monitoring.
Clinical implications
Our findings indicate that a single face-to-face training session prior to VC testing at home was sufficient for most patients to learn how to perform a VC test independently. In clinical practice, patients could be trained in VC testing during a visit to a multidisciplinary clinic or at home. Starting home-monitoring of VC shortly after diagnosis is most beneficial, as insight into the rate of disease progression can guide the timing of clinical interventions. When patients show noticeable or unexpected changes in their unsupervised VC during home-monitoring, a face-to-face or video consultation may be scheduled to determine whether a change in VC was caused by respiratory muscle weakness, or other factors, such as pain/discomfort, illness, fatigue or performing the VC test incorrectly. Support during VC testing at home could be improved by including MND-specific prompts, and written and visual feedback (e.g. flow-volume curve) in the mobile spirometry app.
Home-monitoring of VC could be combined with patient-reported symptoms of dyspnea, to provide healthcare professionals with more insight into the patient’s respiratory function and reduce false negative findings. When home-monitoring data indicates the presence of respiratory dysfunction, based on VC, symptoms or both, patients should be referred to a multidisciplinary clinic for further examination. An advantage of this approach is that the frequency and timing of clinic visits will be tailored to the rate of disease progression and needs of individual patients. In turn, this may result in earlier detection of a respiratory function decline, and more timely referral to a pulmonologist or initiation of NIV, compared to the usual 3 monthly in-clinic care. This study contributes to the recently published Road Map, which was created to facilitate the wide-scale adoption of digital technology and remote monitoring in MND, as it provides evidence on how to measure respiratory function in patients with MND [31].
Strengths and limitations
A strength of the present study is that home-monitoring of VC was part of an existing telehealth service, which facilitated home-monitoring and communication, and optimized adherence. A limitation is the fact that the majority of patients in our cohort were male and relatively young, which reduces the generalizability of our results. Future studies could assess long-term home-monitoring of VC, and determine to what extent the course of unsupervised VC over time corresponds to disease progression, and how it relates to decision-making in MND care. We assessed the upright VC in the present study, despite studies showing that in some patients the upright FVC may remain stable even when respiratory insufficiency is already present [32–35]. Based on existing literature, the maximal inspiratory pressure (MIP), sniff nasal inspiratory pressure (SNIP) or supine VC may be more sensitive in detecting respiratory muscle weakness [9, 18, 27–29]. However, due to the lack of low-cost respiratory pressure meters, home-monitoring of MIP and SNIP will be much more costly. Furthermore, the supine VC test can be challenging and burdensome to perform for patients with gross motor disability, as it requires transfer to a flat surface. As a result, more patients may require assistance from a caregiver, which increases caregiver burden and may reduce adherence. However, future studies could evaluate whether other pulmonary function tests, besides the upright VC, are valid and feasible for home-monitoring in patients with MND.
Conclusion
Unsupervised VC testing at home, with prior face-to-face training and reminders during follow-up, is a valid and feasible method for the remote monitoring of respiratory function in MND care, and well-received by patients and their caregivers.
Acknowledgements
We would like to thank all patients who participated in this study.
Author contributions
JH contributed to designing the study, data analysis, data interpretation, main author of manuscript. JB contributed to data analysis, interpretation of data, revision of manuscript. EP contributed to interpretation of data, revision of manuscript. LHvdB contributed to interpretation of data, revision of manuscript. JMAV-M contributed to interpretation of data, revision of manuscript. AB contributed to designing the study, data analysis, interpretation of data, revision of manuscript.
Funding
This study was funded by the Netherlands ALS Foundation.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval was provided by the Medical Ethical Testing Committee of the University Medical Center Utrecht (No. 20-158); this study was, therefore, performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All individuals who participated in this study were properly informed and signed an informed consent form prior to study participation.
Consent for publication
All contributing authors have given their consent for this manuscript to be submitted, and declare that the submitted work has not been published before, and that the work is not under consideration for publication elsewhere.
References
- 1.Burkhardt C, Neuwirth C, Sommacal A, Andersen PM, Weber M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS ONE. 2017;12(5):1–12. doi: 10.1371/journal.pone.0177555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corcia P, Pradat PF, Salachas F, Bruneteau G, le Forestier N, Seilhean D, et al. Causes of death in a post-mortem series of ALS patients. Amyotroph Lateral Scler. 2008;9(1):59–62. doi: 10.1080/17482960701656940. [DOI] [PubMed] [Google Scholar]
- 3.Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS)—revised report of an EFNS task force. Eur J Neurol. 2012;19:360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 4.NICE. Motor neurone disease: assessment and Motor neurone disease: assessment and management management NICE guideline [Internet]. 2016 [cited 2019 Aug 30]. Available from: www.nice.org.uk/guidance/ng42
- 5.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew FD, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review) Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 7.Vrijsen B, Buyse B, Belge C, Robberecht W, Van Damme P, Decramer M, et al. Noninvasive ventilation improves sleep in amyotrophic lateral sclerosis: a prospective polysomnographic study. J Clin Sleep Med. 2015;11(5):559–566. doi: 10.5664/jcsm.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustfa N, Walsh E, Bryant V, Lyall RA, Addington-Hall J, Goldstein LH, et al. The effect of noninvasive ventilation on ALS patients and their caregivers. Neurology. 2006;66(8):1211–1217. doi: 10.1212/01.wnl.0000208957.88534.11. [DOI] [PubMed] [Google Scholar]
- 9.Stephens HE, Young J, Felgoise SH, Simmons Z. A qualitative study of multidisciplinary ALS clinic use in the United States. Amyotroph Lateral Scler Front Degener. 2016;17(1–2):55–61. doi: 10.3109/21678421.2015.1069851. [DOI] [PubMed] [Google Scholar]
- 10.Schellenberg KL, Hansen G. Patient perspectives on transitioning to amyotrophic lateral sclerosis multidisciplinary clinics. J Multidiscip Healthc. 2018;11:519–524. doi: 10.2147/JMDH.S177563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobson E, Baird W, Bradburn M, Cooper C, Mawson S, Quinn A, et al. Process evaluation and exploration of telehealth in motor neuron disease in a UK specialist centre. BMJ Open. 2019;9(10):e028526. doi: 10.1136/bmjopen-2018-028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helleman J, Van Eenennaam R, Kruitwagen ET, Kruithof WJ, Slappendel MJ, Van Den Berg LH, et al. Telehealth as part of specialized ALS care: feasibility and user experiences with “ALS home-monitoring and coaching”. Amyotroph Lateral Scler Front Degener. 2020;21(3–4):183–192. doi: 10.1080/21678421.2020.1718712. [DOI] [PubMed] [Google Scholar]
- 13.De Marchi F, Cantello R, Ambrosini S, Mazzini L, Sarnelli MF, De Marchi I, et al. Telemedicine and technological devices for amyotrophic lateral sclerosis in the era of COVID-19. Neurol Sci. 2020;41(6):1365–1367. doi: 10.1007/s10072-020-04457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capozzo R, Zoccolella S, Musio M, Barone R, Accogli M, Logroscino G. Telemedicine is a useful tool to deliver care to patients with Amyotrophic Lateral Sclerosis during COVID-19 pandemic: results from Southern Italy. Amyotroph Lateral Scler Front Degener. 2020;21(7–8):542–548. doi: 10.1080/21678421.2020.1773502. [DOI] [PubMed] [Google Scholar]
- 15.Selkirk SM, Washington MO, McClellan F, Flynn B, Seton JM, Strozewski R. Delivering tertiary centre specialty care to ALS patients via telemedicine: a retrospective cohort analysis. Amyotroph Lateral Scler Front Degener. 2017;18(5–6):324–332. doi: 10.1080/21678421.2017.1313867. [DOI] [PubMed] [Google Scholar]
- 16.Van De Rijn M, Paganoni S, Levine-Weinberg M, Campbell K, Swartz Ellrodt A, Estrada J, et al. Experience with telemedicine in a multi-disciplinary ALS clinic. Amyotroph Lateral Scler Front Degener. 2018;19(1–2):143–148. doi: 10.1080/21678421.2017.1392577. [DOI] [PubMed] [Google Scholar]
- 17.Helleman J, Kruitwagen-van Reenen ET, Bakers J, Kruithof WJ, van Groenestijn AC, Jaspers Focks RJH, et al. Using patient-reported symptoms of dyspnea for screening reduced respiratory function in patients with motor neuron diseases. J Neurol. 2020 doi: 10.1007/s00415-020-10003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann F, Henderson RD, Morrison SC, Brown M, Hutchinson N, Douglas JA, et al. Use of respiratory function tests to predict survival in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11(1–2):194–202. doi: 10.3109/17482960902991773. [DOI] [PubMed] [Google Scholar]
- 19.Calvo A, Vasta R, Moglia C, Matteoni E, Canosa A, Mattei A, et al. Prognostic role of slow vital capacity in amyotrophic lateral sclerosis. J Neurol. 2020;267(6):1615–1621. doi: 10.1007/s00415-020-09751-1. [DOI] [PubMed] [Google Scholar]
- 20.Helleman J, Kruitwagen ET, van den Berg LH, Visser-Meily JMA, Beelen A. The current use of telehealth in ALS care and the barriers to and facilitators of implementation: a systematic review. Amyotroph Lateral Scler Front Degener. 2020;21(3–4):167–182. doi: 10.1080/21678421.2019.1706581. [DOI] [PubMed] [Google Scholar]
- 21.Tattersall R, Carty S, Meldrum D, Hardiman O, Murray D. The patient’s perspective of remote respiratory assessments during the COVID-19 pandemic. Amyotroph Lateral Scler Front Degener. 2021 doi: 10.1080/21678421.2021.1920982. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Rudnicki SA, Lechtzin N, Cockroft B, Malik FI, Wolff AA, et al. Correlation between slow vital capacity measured in the home and in the clinic for patients with amyotrophic lateral sclerosis. Neurology. 2019;92(15 supplement):P4.4–002. [Google Scholar]
- 23.Pellegrino GM, Sferrazza Papa GF, Centanni S, Corbo M, Kvarnberg D, Tobin MJ, et al. Measuring vital capacity in amyotrophic lateral sclerosis: effects of interfaces and reproducibility. Respir Med. 2021 doi: 10.1016/j.rmed.2020.106277. [DOI] [PubMed] [Google Scholar]
- 24.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 25.van Eijk RPA, Bakers JNE, van Es MA, Eijkemans MJC, van den Berg LH. Implications of spirometric reference values for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener. 2019;20(7–8):473–80. doi: 10.1080/21678421.2019.1634736. [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza M, Gelinas DF, Moore DH, Miller RG. A comparison of maximal inspiratory pressure and forced vital capacity as potential criteria for initiating non-invasive ventilation in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2007;8(2):106–111. doi: 10.1080/17482960601030188. [DOI] [PubMed] [Google Scholar]
- 28.Geronimo A, Simmons Z. Evaluation of remote pulmonary function testing in motor neuron disease. Amyotroph Lateral Scler Front Degener. 2019;20(5–6):348–355. doi: 10.1080/21678421.2019.1587633. [DOI] [PubMed] [Google Scholar]
- 29.Simblett S, Greer B, Matcham F, Curtis H, Polhemus A, Ferrão J, et al. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res. 2018;20(7):e10480. doi: 10.2196/10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards C, Costello E, Cassidy N, Vick B, Russell AM. Use of the patientMpower app with home-based spirometry to monitor the symptoms and impact of fibrotic lung conditions: longitudinal observational study. JMIR mHealth uHealth. 2020;8(11):1–9. doi: 10.2196/16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Eijk RPA, Beelen A, Kruitwagen ET, Murray D, Radakovic R, Hobson E, et al. A road map for remote digital health technology for motor neuron disease. J Med Internet Res. 2021;23(9):e28766. doi: 10.2196/28766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson CE, Rosenfeld J, Moore DH, Bryan WW, Barohn RJ, Wrench M, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. J Neurol Sci. 2001;191:75–78. doi: 10.1016/S0022-510X(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 33.Elman LB, Siderowf AD, McCluskey LF. Nocturnal oximetry: utility in the respiratory management of amyotrophic lateral sclerosis. Am J Phys Med Rehabil. 2003;82(11):866–870. doi: 10.1097/01.PHM.0000091985.22659.30. [DOI] [PubMed] [Google Scholar]
- 34.Panchabhai TS, Mireles Cabodevila E, Pioro EP, Wang X, Han X, Aboussouan LS. Pattern of lung function decline in patients with amyotrophic lateral sclerosis: implications for timing of noninvasive ventilation. ERJ Open Res. 2019;5(3):00044–2019. doi: 10.1183/23120541.00044-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prell T, Ringer TM, Wullenkord K, Garrison P, Gunkel A, Stubendorff B, et al. Assessment of pulmonary function in amyotrophic lateral sclerosis: when can polygraphy help evaluate the need for non-invasive ventilation? J Neurol Neurosurg Psychiatry. 2016;87(9):1022–1026. doi: 10.1136/jnnp-2015-312185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt EP, Drachman DB, Wiener CM, Clawson L, Kimball R, Lechtzin N. Pulmonary predictors of survival in amyotrophic lateral sclerosis: use in clinical trial design. Muscle Nerve. 2006;33(1):127–132. doi: 10.1002/mus.20450. [DOI] [PubMed] [Google Scholar]
- 37.Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest. 2002;121:436–442. doi: 10.1378/chest.121.2.436. [DOI] [PubMed] [Google Scholar]
- 38.Carratú P, Cassano A, Gadaleta F, Tedone M, Dongiovanni S, Fanfulla F, et al. Association between low sniff nasal-inspiratory pressure (SNIP) and sleep disordered breathing in amyotrophic lateral sclerosis: preliminary results. Amyotroph Lateral Scler. 2011;12(6):458–463. doi: 10.3109/17482968.2011.593038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.