Abstract
Recent studies have shown that living and heat-killed cells of the rhizobacterium Rhizobium etli strain G12 induce in potato roots systemic resistance to infection by the potato cyst nematode Globodera pallida. To better understand the mechanisms of induced resistance, we focused on identifying the inducing agent. Since heat-stable bacterial surface carbohydrates such as exopolysaccharides (EPS) and lipopolysaccharides (LPS) are essential for recognition in the symbiotic interaction between Rhizobium and legumes, their role in the R. etli-potato interaction was studied. EPS and LPS were extracted from bacterial cultures, applied to potato roots, and tested for activity as an inducer of plant resistance to the plant-parasitic nematode. Whereas EPS did not affect G. pallida infection, LPS reduced nematode infection significantly in concentrations as low as 1 and 0.1 mg ml−1. Split-root experiments, guaranteeing a spatial separation of inducing agent and challenging pathogen, showed that soil treatments of one half of the root system with LPS resulted in a highly significant (up to 37%) systemic induced reduction of G. pallida infection of potato roots in the other half. The results clearly showed that LPS of R. etli G12 act as the inducing agent of systemic resistance in potato roots.
Antagonistic rhizobacteria have been repeatedly shown to be promising microorganisms for the biological control of plant-parasitic nematodes. In a screening program, 16 bacterial isolates out of 179 isolated from root and cysts caused a significant (>25%) reduction in Globodera pallida penetration of potato roots (27). A 68% reduction of sugar beet cyst nematode root invasion was obtained by application of the rhizobacterium Pseudomonas fluorescens P523 to beet seeds (23). Studies on a number of plant-microbe interactions showed that such antagonistic rhizobacteria can function directly by competition and antibiosis (3) but also indirectly by inducing systemic resistance in the plant toward soil-borne pathogens (9, 17, 36). However, bacterial compounds which induce plant defense mechanisms are highly variable. Enhanced defense by Pseudomonas aeruginosa strain 7NSK2 in bean toward the pathogenic fungus Botrytis cinerea was initiated by bacterial salicylic acid (5). The siderophore pyoverdin of P. fluorescens strain CHAO was involved in systemically induced suppression of tobacco necrosis virus in tobacco (8). In tomato and soybean leaves, lipopolysaccharides (LPS) of incompatible pseudomonads induced resistance against challenge inoculations by compatible bacteria (20). Induced systemic resistance in carnation to Fusarium wilt was triggered by heat-killed cells and purified LPS, extracted from the outer membrane of P. fluorescens strain WCS417r (36).
Previous work demonstrated that living and heat-killed cells of Rhizobium etli G12 induced in potato roots systemic resistance against G. pallida infection (9, 11). The results of these studies suggested that heat-stable surface structures of R. etli G12 may be the inducing factors. Surface carbohydrates of Rhizobium consist mainly of exopolysaccharides (EPS) as additional capsular or slimy layers around the bacterial cell and LPS, which are an integral part of the outer membrane of the cell. Surface carbohydrates play an important role during the recognition process in the symbiotic interaction between Rhizobium and legumes (6, 16). Furthermore, some authors proposed that degradation of rhizobial polysaccharides is involved in the regulation of the plant response (19).
The objective of this investigation was to extract EPS and LPS from the rhizobacterium R. etli G12 and to determine whether these carbohydrates act as inducers of systemic resistance in potato roots to G. pallida infection.
MATERIALS AND METHODS
Potato cultivar.
The potato cultivar Hansa, susceptible to G. pallida, was used in all experiments. Potato tubers were pregerminated at room temperature in the dark for 4 weeks, and sprouts approximately 2 cm in length with adjacent tuber tissue were cut and used for bioassays.
Bacterial inoculum.
R. etli G12 was originally isolated from the rhizosphere of potatoes and was repeatedly shown to suppress early root infection by the potato cyst nematode G. pallida (9, 28; M. Reitz, S. Hoffmann-Hergarten, J. Hallmann, and R. A. Sikora, submitted for publication). The bacterium was initially identified as Agrobacterium radiobacter but in 1998 was renamed Rhizobium etli. The bacteria were cultured in liquid King's medium B (KMB; pH 5.8) (13) on a rotary shaker for 36 h at 24°C. The bacterial suspension was centrifuged at 4°C for 20 min at 4,600 × g (Haereus Varifuge RF), and the bacterial cells in the pellet were resuspended in sterile one-fourth-concentrated Ringer solution (Merck). The optical cell density at 560 nm (OD560) was adjusted with a spectral photometer to 2.0, which corresponded to cell numbers of R. etli G12 of approximately 1.2 × 1010 CFU ml−1.
EPS and LPS extraction.
For EPS extraction, R. etli G12 was cultured on 15 agar plates with KMB at 24°C. After 3 days, the growth was scraped from the agar plates, suspended in a 500-ml sterile 0.9% NaCl solution containing 5 mM EDTA, and thoroughly stirred. After centrifugation at 4,600 × g for 20 min at 4°C, the supernatant containing loose and bound EPS was sterile filtered (pore size, 0.2 μm) to remove any remaining bacterial cells. The EPS solution was dialyzed (12,000 Da; Serva) against demineralized water for 4 days and lyophilized.
LPS of R. etli G12 were extracted from cells grown in 10 liters of KMB broth in three 3.5-liter plastic fermentors amended with 25 ppm of antifoam solution (Dow Corning antifoam emulsion; Boehringer Ingelheim). After 36 h of fermentation, the cells were harvested by centrifugation at 4°C for 15 min at 4,600 × g and washed three times with 0.5 M NaCl containing 5 mM EDTA to remove loose and bound EPS. The bacterial pellet was then lyophilized (5.5 mg [dry weight]), suspended in 50 ml of buffer L (50 mM sodium phosphate buffer [pH 7.0], 5 mM EDTA, 0.05% sodium azide), and digested by hen egg white lysozyme (6 mg g−1 [dry weight], 50,000 U; Sigma) at 4°C for 16 h (12). The bacterial extract was then treated with DNase (0.3 mg g−1 [dry weight], 2,000 U mg−1 [solid]; Boehringer) and RNase (0.3 mg g−1 [dry weight], 37 U mg−1 [solid]; Sigma) at 37°C for 30 min. Remaining protein was digested overnight by incubation with proteinase K (0.3 mg g−1 [dry weight], 15 U mg−1 [solid]; Sigma) followed by incubation for 10 min at 60°C to denature the protein. Finally, LPS was purified by the hot phenol-water method (40). The aqueous LPS solution was dialyzed (12,000 Da; Serva) for 4 days against demineralized water to remove traces of phenol and again lyophilized. Two stock solutions of EPS and LPS were prepared: 10 mg ml of demineralized water−1 (for analysis) and 1 mg ml of sterile one-fourth-concentrated Ringer solution−1 (for bioassays).
LPS analysis.
LPS patterns were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14). A 7.5-μl aliquot taken from each sample (10 mg/ml) was applied per slot. The polyacrylamide concentration was 12.5% in the separation gel and 6% in the stacking gel. LPS were stained by the silver-staining procedure (34).
The concentration of 2-keto-3-deoxyoctanate (KDO), a characteristic sugar component of the core region of bacterial LPS, was determined after hydrolysis (38). Aliquots of 5, 10, 20, and 30 μl of the sample (10 mg ml−1) were adjusted to 50 μl with demineralized water and hydrolyzed in acetate buffer (pH 4.4) for 2 h at 100°C. A 0.5 mM KDO (0 to 30 μl) solution was used as a standard.
Protein contents were determined by the Bio-Rad protein assay (catalogue no. 500-0007) (2). Bovine serum albumin (0 to 0.5 mg/ml) was used as a standard.
Nematode inoculum.
The potato cyst nematode G. pallida was originally isolated from a field population near Bonn and was multiplied continually on potato roots (9). Cysts for experiments were extracted from soil by the wet sieve decanting technique (1). The nematode inoculum was prepared by placing 25 cysts in a pouch made of 100-μm-mesh gauze sandwiched in a slide frame (26, 33). The total inoculum was 1,500 eggs and juveniles per 100 g of soil, which represents the economic threshold level on potato.
Bioassays. (i) Root dipping.
Pregerminated potato tubers were placed in a plastic box containing heat-sterilized sand and were incubated in a climatic chamber at 21°C and a photoperiod of 16 h. After 5 days, the plantlets were removed and the root system of each plant was thoroughly rinsed with tap water; the tubers were then dipped for 2 min in a 20-ml suspension of either R. etli (OD560 = 2.0), EPS (1 mg ml−1), or LPS (0.1 or 1 mg ml−1). Sterile one-fourth-concentrated Ringer solution (Merck) was used as a control. Treated potato plants were transferred individually into plastic pots (8 cm in diameter) filled with a heat-sterilized mixture of sand and field soil (1:1) and were grown under the same conditions as described above. Two days after bacterial colonization, the plants were inoculated with nematodes by inserting one slide frame containing the 25 cysts into the soil about 2 cm from the root. Each treatment was performed in three independent experiments with nine replicates per treatment.
(ii) Split-root system.
The split-root (three-pot) system allows inoculation of the bacterium and cyst nematode at separate locations on the root system (9). Three plastic pots (diameter of 8 cm) were filled with a 1:1 mixture of sterilized sand and field soil. Pregerminated potato tubers were planted in the upper pot to allow the root system to grow through the two openings in the bottom of the upper pot and to spread to the lower two pots. After 3 weeks, plants were inoculated with living bacterial cells or with the LPS solution by pipetting 2.5 ml of each suspension into the soil of one side of the split-root system. One-fourth-concentrated Ringer solution (Merck) served as control treatment. After 2 days, the other side of the split-root system was inoculated with nematodes by inserting one slide frame filled with 25 cysts into the soil. Each treatment was replicated eight times in two independent experiments.
Nematode penetration was determined 16 days after inoculation. Potato roots were rinsed with tap water to remove soil, blotted on tissue paper to remove excess water, and boiled in 0.1% lactic acid fuchsin (7). After homogenization in an Ultra-Turrax (IKA-Werk), the juveniles were counted under a stereomicroscope.
Data were analyzed for significance by analysis of variance and Duncan's multiple-range test, using the software Statgraphics Plus.
RESULTS
Analysis of EPS and LPS.
Analysis of LPS patterns by SDS-PAGE confirmed the presence of LPS constructs in the LPS extract (Fig. 1). The LPS of R. etli G12 separated into five to six bands, the lowest of which represented truncated LPS consisting of lipid A-core region; the upper bands represented complete LPS molecules with O antigens of different sizes. The LPS clearly possessed characteristic patterns of Rhizobium spp. (4, 24). In the EPS extract of Rhizobium etli G12, typical LPS bands were not detected.
FIG. 1.

Pattern of EPS and LPS extracts from R. etli G12 after SDS-PAGE and silver staining. Each lane was loaded with a 7.5-μl aliquot of the sample (10 mg ml−1).
The test for KDO verified the presence of the core region in the LPS of R. etli G12 at concentrations of 15 nmol of KDO mg of LPS−1. The lack of KDO in the EPS extract excluded any contamination by LPS. The protein contents of the EPS and LPS extracts were low, reaching 0.25 and 0.38%, respectively. These data demonstrated that the silver-staining method had specifically stained polysaccharide bands and not proteins.
Bioassays.
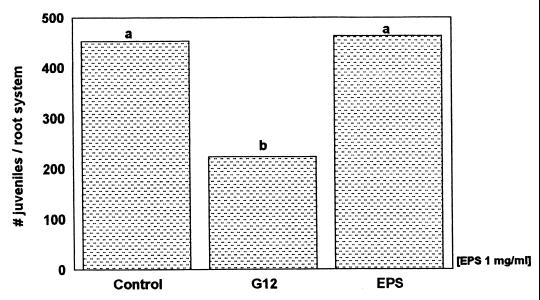
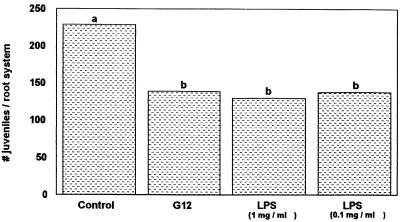
Our experiments confirmed the induction of systemic resistance in potato roots against nematode infection by pretreatment with R. etli G12 (Fig. 2 and 4). The EPS of R. etli G12 showed no effect on G. pallida infection when applied as a root dip (Fig. 2). Treatment with the LPS extract (1 mg ml−1), however, resulted in a significant (up to 44%) decrease in G. pallida infection of potato roots (Fig. 3). Even the lowest LPS concentration (0.1 mg ml−1) reduced nematode infection by 40%.
FIG. 2.
Penetration of G. pallida into potato roots pretreated with Ringer solution (control), R. etli G12, or its EPS, measured 16 days after nematode inoculation. Bars with different letters are significantly different at P ≤ 0.05 (Duncan's multiple range test, n = 9).
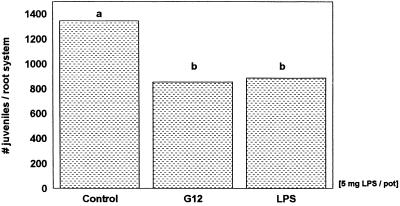
FIG. 4.
Penetration of G. pallida into the untreated half of potato roots growing in the split-root system, 16 days after the other root half was treated with Ringer solution (control), living bacteria (R. etli G12), or bacterial LPS. Bars with different letters are significantly different at P ≤ 0.05 (Duncan's multiple range test, n = 8).
FIG. 3.
Penetration of G. pallida into potato roots pretreated with Ringer solution (control), R. etli G12, or different concentrations of its LPS, measured 16 days after nematode inoculation. Bars with different letters are significantly different at P ≤ 0.01 (Duncan's multiple range test, n = 9).
To clarify whether the LPS of R. etli G12 act in potato roots as an inducer of systemic resistance to G. pallida, bacterial LPS and the parasite were applied spatially separated in a split-root system to prevent direct contact. Application of LPS (5 mg per pot) to one half of the split-root system caused a significant (37%) systemic reduction in nematode penetration in the other half of the split-root system (Fig. 4). Living cells of R. etli G12 applied in the same manner reduced nematode infection similarly, by 34%. The results indicate that surface components of R. etli G12 act in potato roots as an inducing factor of systemic resistance to G. pallida infection.
DISCUSSION
Previous studies demonstrated that specific rhizobacteria reduce plant infection by various parasitic nematodes (21, 23, 32). Recently, it was shown that the rhizobacterium R. etli G12 impaired infection by the potato cyst nematode G. pallida indirectly by inducing systemic resistance (9). Since the plant defense capacity in potato roots was enhanced by both living and heat-killed cells of this strain, it was concluded that heat-stable surface structures such as EPS and/or LPS act as inducing agents.
In this study, we demonstrated that the EPS of R. etli G12 did not affect plant defense reactions in potato roots to nematode infection. Also, the EPS of another Rhizobium strain did not affect the interaction with legumes (29). These authors demonstrated that EPS-negative mutants induced levels of nodulation similar to those induced by the wild-type strain. However, treatment of alfalfa with an EPS-negative mutant of Rhizobium meliloti resulted in an accumulation of phenolics and callose (22). It was suggested, therefore, that EPS function as a suppressor of plant defense reactions, enabling the bacterium to infect the plant and grow endophytically. Since R. etli G12-mediated induced resistance is also not associated with typical plant defense reactions such as enhanced activity of pathogenesis-related proteins or increased lignin content, even though the bacteria colonize the roots locally (30), EPS of R. etli G12 may also act as a suppressor of specific plant defense reactions. However, a role of EPS as an inducing factor leading to a systemic reduction in nematode infection throughout the root system can be ruled out.
We demonstrated that root dipping in the LPS solution of R. etli G12 reduced nematode infection significantly at concentrations as low as 1 and 0.1 mg ml−1. Furthermore, a split-root experiment confirmed that the bacterium-free LPS of R. etli G12 were in large part responsible for systemic induced resistance of potato to G. pallida infestation. Also, during colonization of potato roots by R. etli G12, the bacterial LPS may be the decisive resistance inducer, since it is known from studies of many gram-negative bacteria that LPS is released into the environment during bacterial growth (31, 39). Inducer activity has also been shown for the LPS of the rhizobacterium P. fluorescens strain WCS417r towards Fusarium wilt in carnation, radish, and Arabidopsis (15, 36, 37). Similar to the results in our experiments (30), the induced resistance state was not associated with enhanced production of pathogenesis-related proteins (10, 25). Interestingly, LPS mutants of WCS417r lacking the O-antigenic side chain of the LPS failed to protect radish from Fusarium wilt even though they colonized the root to the same extent as the wild-type strain (15). However, other authors showed that in Arabidopsis the O-antigen-negative mutant of WCS417r induced levels of protection similar to those induced by the wild-type strain (37). To identify the LPS component of R. etli G12 inducing systemic resistance of potato roots to G. pallida attack, the potential roles of the O antigen, core region, and lipid A of the rhizobial LPS are currently under investigation.
ACKNOWLEDGMENTS
We thank Petra Müller for advice on EPS and LPS analysis.
We thank the Deutsche Forschungsgemeinschaft for funding this project.
REFERENCES
- 1.Ayoub S M. Plant nematology—an agricultural training aid. Sacramento, Calif: Nema Aid Publication; 1980. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Buchenauer H. Biological control of soil-borne diseases by rhizobacteria. Z Pflanzenkrankh Pflanzenschutz. 1998;105:329–348. [Google Scholar]
- 4.Carlson R W. The heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984;158:1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Meyer G, Höfte M. Induction of systemic resistance by the rhizobacterium Pseudomonas aeruginosa 7NSK2 is a salicylic acid dependent phenomenon in tobacco. IOBC Bull. 1998;21:117–122. [Google Scholar]
- 6.Denny T P. Involvement of bacterial polysaccharides in plant pathogenesis. Annu Rev Phytopathol. 1995;33:173–195. doi: 10.1146/annurev.py.33.090195.001133. [DOI] [PubMed] [Google Scholar]
- 7.Ferris J M. Crop loss prediction and modeling for management decisions. In: Zuckermann B M, Mai W F, Harrison M B, editors. Plant nematology—laboratory manual. Amherst, Mass: The University of Massachusetts Agricultural Experimental Station; 1985. pp. 27–33. [Google Scholar]
- 8.Hasky K, Sikora R A. Resistance against the potato cyst nematode Globodera pallida systemically induced by the rhizobacteria Agrobacterium radiobacter (G12) and Bacillus sphaericus (B43) Nematologica. 1995;41:306. [Google Scholar]
- 9.Hasky-Günther K, Hoffmann-Hergarten S, Sikora R A. Resistance against the potato cyst nematode Globodera pallida systemically induced by the rhizobacteria Agrobacterium radiobacter (G12) and Bacillus sphaericus (B43) Fundam Appl Nematol. 1998;21:511–517. [Google Scholar]
- 10.Hoffland E, Pieterse C M J, Bik L, van Pelt J A. Induced systemic resistance in radish is not associated with accumulation of pathogenesis related-proteins. Physiol Mol Plant Pathol. 1995;46:309–320. [Google Scholar]
- 11.Hoffmann-Hergarten S, Hasky-Günther K, Reitz M, Sikora R A. Induced systemic resistance by rhizobacteria toward the cyst nematode Globodera pallida on potato. In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Plant growth-promoting rhizobacteria—present status and future prospects. Sapporo, Japan: Nakanishi Printing; 1997. pp. 292–295. [Google Scholar]
- 12.Johnson K G, Perry M B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976;22:29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- 13.King E O, Warth M, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Leeman M, van Pelt J A, den Ouden F M, Heinsbroek M, Bakker P A H M, Schippers B. Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology. 1995;85:1021–1027. [Google Scholar]
- 16.Leigh J A, Coplin D L. Exopolysaccharides in plant-bacterial interactions. Annu Rev Microbiol. 1992;46:307–346. doi: 10.1146/annurev.mi.46.100192.001515. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Kloepper J, Tuzun S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology. 1995;85:695–698. [Google Scholar]
- 18.Maurhofer M, Hase C, Meuwly P, Métraux J P, Defago G. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHAO: influence of the gacA gene and pyoverdine production. Phytopathology. 1994;84:139–146. [Google Scholar]
- 19.Mellor R B, Collinge D B. A simple model based on known defense reactions is sufficient to explain most aspects of nodulation. J Exp Bot. 1995;46:1–18. [Google Scholar]
- 20.Müller P, Zähringer U, Rudolph K. Induced resistance by bacterial lipopolysaccharides (LPS) In: Mahadevan A, editor. Plant pathogenic bacteria. Proceedings of the 9th International Conference. Madras, India: Centre for Advanced Study in Botany, University of Madras; 1998. pp. 569–575. [Google Scholar]
- 21.Neipp P W, Becker J O. Evaluation of biocontrol activity of rhizobacteria from Beta vulgaris against Heterodera schachtii. J Nematol. 1999;31:54–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Niehaus K, Kapp D, Pühler A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPSI)-deficient Rhizobium meliloti mutant. Planta. 1993;190:415–425. [Google Scholar]
- 23.Oostendorp M, Sikora R A. In-vitro interrelationships between rhizosphere bacteria and Heterodera schachtii. Rev Nematol. 1990;13:269–274. [Google Scholar]
- 24.Perotto S, Brewin N J, Kannenberg E L. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol Plant-Microbe Interact. 1994;7:99–112. [Google Scholar]
- 25.Pieterse C M J, van Wees S C M, Hoffland E, van Pelt J A, van Loon L C. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyrowolakis A, Schuster R-P, Sikora R A. The effect of cropping pattern and green manure on the antagonistic potential and the diversity of egg pathogenic fungi in fields with Heterodera schachtii infection. Nematology. 1999;1:165–171. [Google Scholar]
- 27.Racke J, Sikora R A. Isolation, formulation and antagonistic activity of rhizobacteria toward the potato cyst nematode Globodera pallida. Soil Biol Biochem. 1992;24:521–526. [Google Scholar]
- 28.Racke J, Sikora R A. Wirkung der pflanzengesundheitsfördernden Rhizobakterien Agrobacterium radiobacter und Bacillus sphaericus auf den Globodera pallida-Befall der Kartoffel und das Pflanzenwachstum. J Phytopathol. 1992;134:198–208. [Google Scholar]
- 29.Raleigh E, Signer E. Positive selection of nodulation-deficient Rhizobium phaseoli. J Bacteriol. 1982;151:83–88. doi: 10.1128/jb.151.1.83-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitz M. Biochemische und molekularbiologische Untersuchungen zur bakterieninduzierten systemischen Resistenz in Kartoffeln gegenüber dem Zystennematoden Globodera pallida. Dissertation. Bonn, Germany: Rheinische-Friedrich-Wilhelms-Universität; 1999. [Google Scholar]
- 31.Schröder I. Extrazellulärer Transport von Virulenzfaktoren—Bedeutung der Membranvesikel von Pseudomonas syringae Pathovarietäten bei der Pathogenese und Resistenzinduktion in Lycopersicon esculentum und Nicotiana tabacum. Dissertation. Germany: Georg-August-Universität, Göttingen; 2000. [Google Scholar]
- 32.Sikora R A. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annu Rev Phytopathol. 1992;30:245–270. [Google Scholar]
- 33.Sikora R A, Schuster R-P, Kiewnick S. Indexing biodiversity and antagonistic potential in agricultural soil of Madagascar. Med Fac Landbouww Univ Gent. 1994;59/2b:781–790. [Google Scholar]
- 34.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 35.van Peer R, Schippers B. Lipopolysaccharides of plant-growth promoting Pseudomonas sp. strain WCS417r induce resistance in carnation to Fusarium wilt. Neth J Plant Pathol. 1992;98:129–139. [Google Scholar]
- 36.van Peer R, Niewmann G J, Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology. 1991;81:728–734. [Google Scholar]
- 37.van Wees S C M, Pieterse C M J, Trijssenaar A, van't Westende Y A M, Hartog F, van Loon L C. Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol Plant Pathol. 1997;10:716–724. doi: 10.1094/MPMI.1997.10.6.716. [DOI] [PubMed] [Google Scholar]
- 38.Waravdekar V, Saslaw L D. A sensitive colorimetric method for estimation of 2-deoxy sugars with the use of the malonaldehydethiobarbituric acid reaction. J Biol Chem. 1959;234:1945–1950. [PubMed] [Google Scholar]
- 39.Wensink J, Wilholth B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem. 1981;116:331–335. doi: 10.1111/j.1432-1033.1981.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 40.Westphal O, Jann K. Bacterial lipopolysaccharides. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]