Abstract
Granulocyte colony-stimulating factor (G-CSF) has been widely used to mobilize bone marrow hematopoietic stem/progenitor cells for transplantation in the treatment of hematological malignancies for decades. Additionally, G-CSF is also accepted as an essential mediator in immune regulation, leading to reduced graft-versus-host disease following transplantation. Despite the important clinical roles of G-CSF, a comprehensive, unbiased, and high-resolution survey into the cellular and molecular ecosystem of the human G-CSF-primed bone marrow (G-BM) is lacking so far. Here, we employed single-cell RNA sequencing to profile hematopoietic cells in human bone marrow from two healthy donors before and after 5-day G-CSF administration. Through unbiased bioinformatics analysis, our data systematically showed the alterations in the transcriptional landscape of hematopoietic cells in G-BM, and revealed that G-CSF-induced myeloid-biased differentiation initiated from the stage of lymphoid-primed multipotent progenitors. We also illustrated the cellular and molecular basis of hyporesponsiveness of T cells and natural killer (NK) cells caused by G-CSF stimulation, including the potential direct mechanisms and indirect regulations mediated by ligand–receptor interactions. Taken together, our data extend the understanding of lymphomyeloid divergence and potential mechanisms involved in hyporesponsiveness of T and NK cells in human G-BM, which might provide basis for optimization of stem cell transplantation in hematological malignancy treatment.
Subject terms: Haematopoietic stem cells, Immunology
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative approach available for hematological malignancies1, but its broad application has been limited by the availability of human leukocyte antigen (HLA)-matched donors and severe graft-versus-host disease (GVHD). In the past ten years, unmanipulated haploidentical blood and marrow transplantation that combines granulocyte colony-stimulating factor (G-CSF)-primed bone marrow (G-BM) and G-CSF-mobilized peripheral blood stem cells (G-PBSC) without in vitro T-cell depletion, has been proved to be a reliable protocol with a low risk of GVHD2–6.
As a commonly used mobilizer, G-CSF is known to increase the numbers of hematopoietic stem/progenitor cells (HSPCs) harvested within the bone marrow (BM) and peripheral blood (PB), and lead to enhanced myeloid hematopoiesis and granulopoiesis7,8. However, it remains controversial about the mechanisms by which G-CSF regulates hematopoiesis9. Bernitz et al.10 found that G-CSF treatment could increase the number of myeloid-biased CD41+ hematopoietic stem cells (HSCs) in mouse BM. Recently, Xie et al.11 showed that G-CSF directly acted on lymphoid-biased HSCs to increase their divisions and maintain their repopulating activity after transplantation using functional experiments at the single-cell level in mice. In addition, due to technical limitations, our understanding of the effect of G-CSF on hematopoietic differentiation in human BM in vivo is scant.
Moreover, G-CSF mobilization has been demonstrated to reduce GVHD with preservation of the graft-versus-leukemia (GVL) effect12–14. A growing body of studies demonstrated that G-CSF could attenuate the reactivity of T and natural killer (NK) cells through inducing T helper 2 (Th2) cell polarization15, promoting the generation of regulatory T (Treg) cells16, tolerogenic dendritic cells (DCs)17,18 and possibly myeloid-derived suppressor cells (MDSCs)19–21. Intriguingly, compared with G-PBSC transplantation, G-BM transplantation resulted in attenuated acute GVHD and comparable engraftment22, indicating that G-BM was different from G-CSF-mobilized peripheral blood (G-PB) in terms of immunomodulatory properties. However, the systematical landscape of immune cells and underlying mechanisms of immunoregulation in G-BM have not yet been well established9,17,23.
Herein, using single-cell RNA sequencing (scRNA-seq) and unbiased bioinformatics analysis, we comprehensively characterized the cellular and molecular alterations in the BM from healthy donors upon G-CSF administration and revealed the potential mechanisms of hematopoiesis changes and immune hyporesponsiveness in T and NK cells.
Results
Single-cell transcriptomics of hematopoietic cells in human BM upon G-CSF treatment
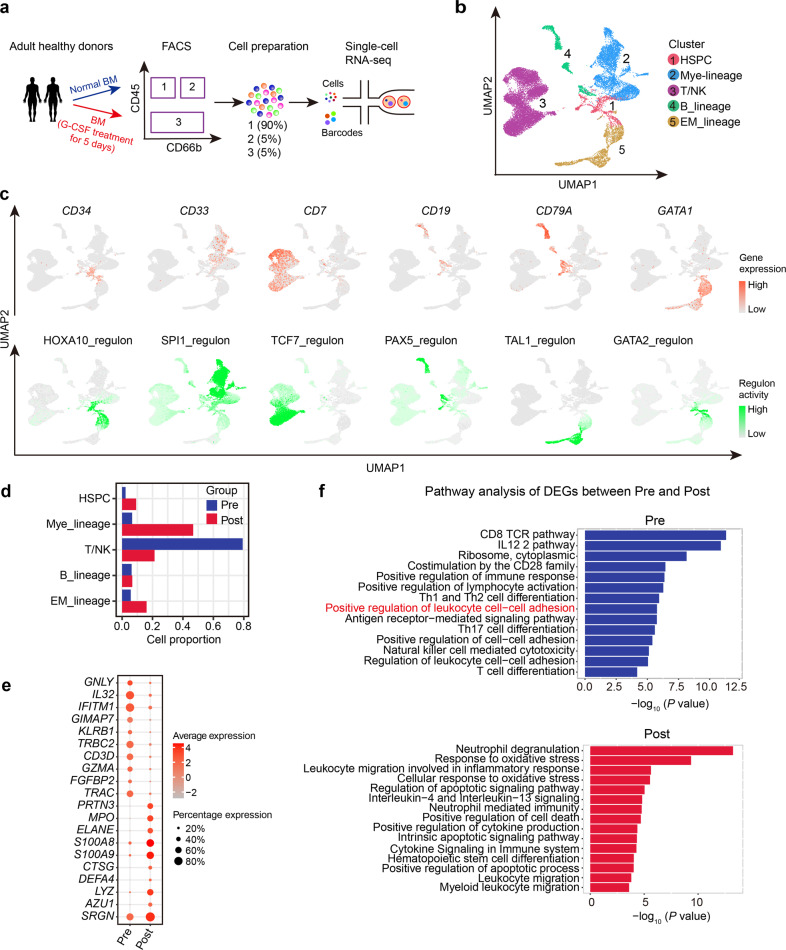
To generate a single-cell transcriptome map of cells in G-BM, we collected BM samples from two healthy donors before and after 5-day G-CSF treatment, and used fluorescence-activated cell sorting (FACS) to isolate different cell lineages and mixed them in a certain ratio for subsequent scRNA-seq (Fig. 1a; Supplementary Fig. S1a). After stringent quality control, a total of 21,005 cells were obtained from four BM samples of two donors and further classified into five cell lineages (Fig. 1b; Supplementary Fig. S1b–d). Based on the expression of canonical marker genes and lineage-associated transcriptional factors (Fig. 1c), we defined them as HSPC (CD34; HOXA10 regulon), myeloid lineage (CD33; SPI1 regulon), T/NK lineage (CD7; TCF7 regulon), B_lineage (CD19, CD79A; PAX5 regulon) and erythroid/megakaryocyte lineage (EM_lineage) (GATA1; TAL1 and GATA2 regulon). As expected, G-CSF treatment caused an increase in percentages of HSPCs and myeloid lineage, while a decrease in percentages of T/NK lineage (Fig. 1d), indicating myeloid-biased hematopoiesis in G-BM. This finding was also supported by increased expression of myeloid hematopoiesis-related genes, such as LYZ, S100A8, and S100A9, and decreased expression of T cell differentiation-related genes CD3D, TRAC, and IFITM1 (Fig. 1e, f; Supplementary Table S1). Besides, samples from two donors were highly reproducible for cell composition and overall changes in percentage of each cell lineage (Supplementary Fig. S1d, e).
Fig. 1. Transcriptomic landscape of hematopoietic cells in human G-BM.
a Workflow showing the processing of paired unstimulated BM and G-CSF-primed BM (G-BM) samples from healthy donors for scRNA-seq. b Uniform manifold approximation and projection (UMAP) visualization of main lineages in human BM based on single-cell transcriptomes. Each dot represents a single cell; colors indicate cell clusters with numbered labels. c UMAP visualization of the expression of lineage-specific marker genes (top) and activity of canonical cell type-specific regulons (bottom) in identified cell lineages. d Bar plot showing the changes in the percentage of each cell lineage upon G-CSF treatment. Pre, representing samples before G-CSF treatment; Post, representing samples after G-CSF treatment. e Dot plot showing average expression of top 10 DEGs for hematopoietic cells in unstimulated BM and G-BM and the percentage of cells expressing the genes. DEGs were detected using function FindAllMarkers in Seurat (Wilcoxon-rank-sum test, with P value adjusted for multiple testing using Benjamini–Hochberg correction). f Bar plots from the Metascape analysis showing the major enriched terms of DEGs before (top) and after (bottom) G-CSF administration. The length of each bar represents –log10(P value).
Notably, hematopoietic cells in G-BM exhibited higher expression of protease genes such as CTSG and ELANE (Supplementary Fig. S1f)24. Gene Ontology (GO) term enrichment analysis also presented reduced cell adhesion (Fig. 1f), suggesting that HSPC mobilization in G-BM could be mediated by disruption of cell adhesion and enhanced proteolytic activity as reported in previous studies25,26.
The myeloid-biased hematopoietic differentiation in G-BM
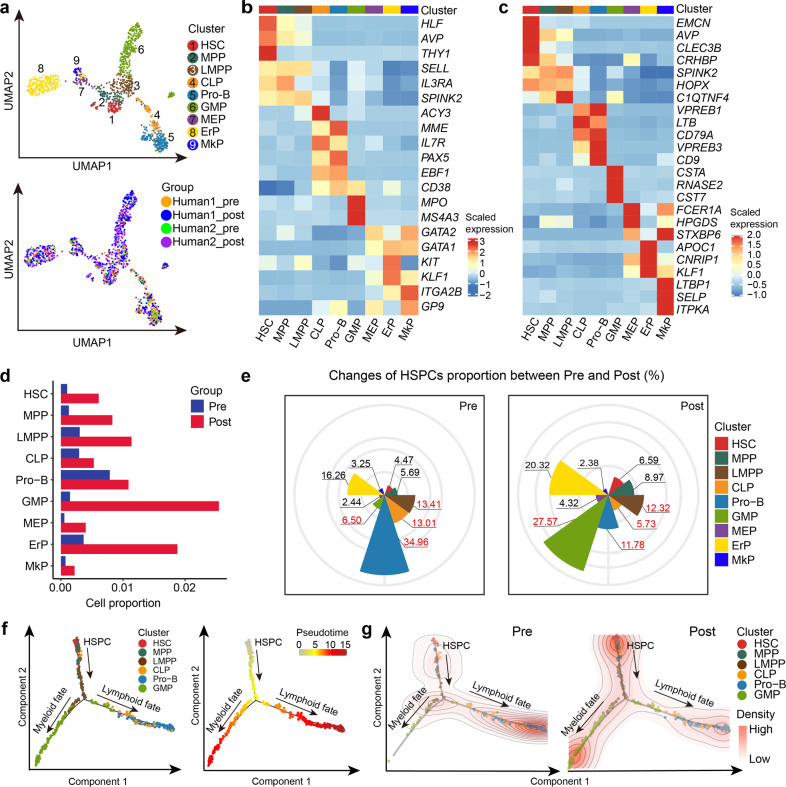
We re-clustered the identified HSPCs into nine subpopulations based on the expression of cell type-associated genes, namely HSC, multipotent hematopoietic progenitor (MPP), lymphoid-primed multipotent progenitor (LMPP), common lymphoid progenitor (CLP), progenitor B cell (Pro-B), granulocyte-monocyte progenitor (GMP), megakaryocyte-erythrocyte progenitor (MEP), erythrocyte progenitor (ErP), megakaryocyte progenitor (MkP) (Fig. 2a–c; Supplementary Table S2), all of which underwent an increase in cell percentages upon G-CSF stimulation (Fig. 2d). Interestingly, the mobilized HSC showed a lower proportion in the G1 phase and a higher proportion in the G2M phase, as well as a significantly increased proliferation score (Supplementary Fig. S1g, h), indicating the proliferative status of HSCs in human G-BM, which is consistent with results in mice10,27. We further calculated the relative cell proportion within HSPCs. An obviously increased percentage was observed in GMP, which contrasted with the reduced percentage of lymphoid progenitors CLP and Pro-B, suggesting a myeloid-biased differentiation in G-BM (Fig. 2e). However, as the progenitor of CLP and GMP28, LMPP showed no obvious change in percentage (Fig. 2e), indicating that LMPP was the earliest target progenitor of G-CSF inducing myeloid-biased hematopoiesis in G-BM. Trajectory inference also showed similar myeloid-biased hematopoiesis bifurcated from LMPP in G-BM (Fig. 2f, g).
Fig. 2. The effect of G-CSF on hematopoietic differentiation in human BM.
a UMAP visualization of subclusters in HSPCs. Each dot represents a single cell; colors indicate clusters (top) and sources of samples (bottom). HSC hematopoietic stem cell; MPP multipotential progenitor; LMPP lymphoid-primed multipotent progenitor; CLP common lymphocyte progenitor; Pro-B progenitor B cell; GMP granulocyte-monocyte progenitor; MEP megakaryocyte/erythroid progenitor; ErP erythroid progenitor; MkP megakaryocyte progenitor. b Heatmap displaying scaled expression of canonical cell type-associated genes for clusters in a. Color scale corresponds to z-scored, log-transformed mean gene-expression counts for each cell state. c Heatmap showing scaled expression of top 3 DEGs for clusters in a. Color scale corresponds to z-scored, log-transformed mean gene-expression counts for each cell state. Detailed DEGs can be found in Supplementary Table S2. d Bar plot showing the changes in the percentage of subclusters of HSPCs upon G-CSF treatment. e Cell proportion of each subcluster within HSPCs derived from BM grafts before (left) and after (right) G-CSF administration. f Monocle prediction of HSPC developmental trajectory with cluster information (left) and pseudotime (right) mapped. g Density plots displaying the distribution of different hematopoietic progenitor cells in trajectories before (left) and after (right) G-CSF administration. These plots were based on the 2D kernel density estimation. The density of lines and intensity of colors are proportional to cell density in each state.
Sub-clustering of T/NK lineage in BM before and after G-CSF administration
To further assess the effect of G-CSF on immune cells, we analyzed the T and NK cell populations. Through unsupervised clustering analysis, a total of 16 subpopulations were identified, including six subtypes of CD4+ T cells, five subtypes of CD8+ T cells, two subtypes of NK cells, gamma delta T (γδ T) cells, type 1 innate lymphoid cell (ILC1), and type 3 innate lymphoid cell (ILC3)-like cells (Fig. 3a–c; Supplementary Table S3). Among CD4+ T cells, we defined three subtypes of naïve CD4+ T cells (CD4_naïve1, CD4_naïve2 and CD4_naïve3) and Treg, as well as two effector CD4+ T cell subtypes, namely CD4_effector_CCL5 and CD4_effector_CCR6. CD4_effector_CCL5 showed higher expression of T helper 1 (Th1)-related genes (TBX21 and IFNG), while CD4_effector_CCR6 was characterized by higher expression of genes commonly expressed in T helper 17 (Th17) cells (CCR6, FUT7, and LTB). CD8+ T cells included naïve CD8+ T cells (CD8A+CCR7+) and 4 effector CD8+ T cell subsets (CD8A+CCR7–) exhibiting distinct signatures, namely CD8_effector_CD69, CD8_effector_GZMH, CD8_effector_IFIT3 and CD8_effector_RORC. In detail, CD8_effector_CD69 and CD8_effector_GZMH had higher expression of cytotoxicity-associated genes GZMK and GZMH, respectively. Intriguingly, GZMK (highly expressed by CD8_effector_CD69 and CD8_effector_RORC) and ZNF683 (highly expressed by CD8_effector_GZMH) were reported to be highly expressed by CD8+ T cells in a possible “pre-exhausted” state29,30. CD8_effector_IFIT3 was characterized by the expression of genes involved in the type I interferon response (IFIT3, MX1, and RSAD2)31 and related receptors (IFNAR1 and IFNAR2) (Fig. 3b, c; Supplementary Fig. S2a), suggesting a role involved in type I interferon response. CD8_effector_RORC with higher expression of RORC and CXCR6, might be related to IL17-producing CD8+ T (Tc17) cells (Fig. 3b, c). Tc17 cells were reported as proinflammatory, plastic pathogenic CD8+ T cells that induced GVHD without antileukemic effects32. As expected, the percentages of T and NK subpopulations decreased in G-BM (Fig. 3d), which might result from the dilution caused by increased myeloid cells (Fig. 1d). Besides, T and NK cells exhibited higher expression of genes related to migration (Fig. 3e; Supplementary Fig. S2b), which might also contribute to the decrease in the percentage of T/NK lineage.
Fig. 3. Sub-clustering of T/NK lineage in human BM before and after G-CSF mobilization.
a t-distributed Stochastic Neighbor Embedding (t-SNE) visualization of 16 subclusters of the T/NK lineage pooled from human BM across two donors. Treg, regulatory T cell; γδT, gamma-delta T cells. b Identification of subclusters of T/NK lineage based on the expression of key cell type-associated marker genes. c Heatmap showing scaled expression of top 3 DEGs of the subclusters in T/NK cells. Detailed DEGs can be found in Supplementary Table S4. d Bar plot displaying the changes in the percentage of various subclusters of T/NK cells upon G-CSF administration. e Violin plots showing the changes in expression of genes related to leukocyte migration upon G-CSF administration. Representative upregulated DEGs enriched in leukocyte migration (GO: 0050900) are displayed. ****P value < 0.0001.
Transcriptomic changes of the T/NK lineage in G-BM and the potential direct mechanisms of immunomodulatory effect
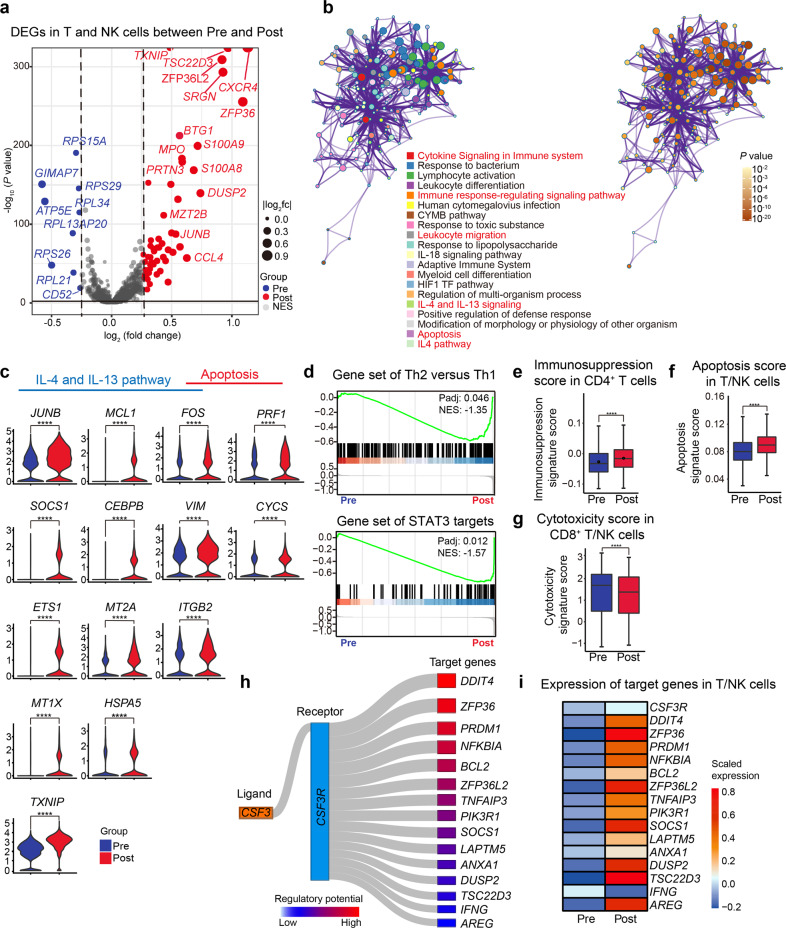
We next elucidated the effects of G-CSF on transcriptomic profiling of T and NK cells in G-BM. In total, the overall differentially expressed gene (DEG) analysis showed upregulated expression of genes related to immune modulation (DUSP233 and TSC22D334) (Fig. 4a, b; Supplementary Table S4), Th2 developmental pathways (Interleukin (IL)-4 and IL-13 signaling pathway)35, and apoptosis (Fig. 4b, c). Gene Set Enrichment Analysis (GSEA) also showed the enrichment of Th2-related signatures in CD4+ T cells (Fig. 4d; Supplementary Table S5), which was in line with the previous evidence36. Besides, CD4+ T cells also displayed a higher enrichment of signal transducer and activator of transcription 3 (STAT3) target genes in G-BM (Fig. 4d), which was required for the Th2 development37. In addition, gene scoring analysis showed a significantly elevated immunosuppression score in CD4+ T cells (Fig. 4e), and a higher apoptosis score in T/NK cells (Fig. 4f), which further suggests the immunosuppressive features induced by G-CSF.
Fig. 4. Hyporesponsiveness of T and NK cells induced by G-CSF treatment.
a Volcano plot showing DEGs of T/NK lineage between unstimulated BM and G-BM. DEGs were detected by differential expression analysis (two-sided Student’s t-test). Each dot represents a single gene; genes with log2(fold change) (logFC) < −0.25 and logFC > 0.25 were selected and colored in blue and red, respectively. b Metascape network enrichment analysis of DEGs in T/NK cells in G-BM, with top 20 enriched terms and corresponding P values displayed at the bottom. Nodes were colored to reflect the memberships of enriched terms (left) and statistical P values (right). c Violin plots displaying the expression of key genes related to the IL-4 and IL-13 pathway, as well as apoptosis in T/NK cells after G-CSF treatment. Representative DEGs enriched in the IL-4 and IL-13 pathway and apoptosis in b are displayed. d GSEA plots of the enrichment of the Th2 versus Th1 signatures (top) and STAT3 targets (bottom) in CD4+ T cells in G-BM compared with unstimulated BM. P values adjusted with Benjamini–Hochberg correction and normalized enrichment score (NES) are displayed. e Box plot showing immunosuppression scores before and after G-CSF administration in CD4+ T cells. P values were calculated using the Wilcoxon-rank-sum test. ****P value < 0.0001. f Box plot showing the scores of the positive regulation of apoptosis before and after G-CSF administration in T/NK cells. ****P value < 0.0001. g Box plot showing the cytotoxicity scores before and after G-CSF administration in CD8+ T cells and NK cells. ****P value < 0.0001. h Sankey plot showing representative predicted target genes of G-CSF via binding with G-CSFR. Target genes with significantly changed expression are shown. Both the width of connecting lines and the color intensity of boxes represent the regulatory potential. i Heatmap showing the expression of CSF3R and target genes as presented in h in T/NK lineage.
Upon G-CSF administration, T and NK cells in G-BM unanimously exhibited higher expression of genes related to immunosuppression such as SOCS138, TSC22D3, ZFP3639, and DUSP2 (Supplementary Figs. S2c–h and S3, Table S6). Besides, CD8+ T and NK cells exhibited a lower cytotoxicity activity in G-BM (Fig. 4g).
Furthermore, based on ligand–target relationship analysis in NicheNet40, the expression of the above upregulated genes related to anti-inflammation (ZFP3639 and NFKBIA41) and immunosuppression was predicted to be regulated directly by G-CSF binding with G-CSF receptor (G-CSFR, encoded by CSF3R) in T/NK cells40, along with slightly higher expression of CSF3R following G-CSF stimulation (Fig. 4h, i). G-CSF treatment also reduced the expression of IFNG (encoding IFN-γ) in G-BM as previously reported42,43, which could result from potential direct regulation by binding of G-CSF with G-CSFR (Fig. 4h, i).
The effect on myeloid cells of G-CSF in human BM
We further identified subclusters in the myeloid lineage. Myeloid cells contained subclusters such as CD14+ monocytes, CD16+ monocytes, intermediate monocytes (CD14+FCGR3A+), plasmacytoid dendritic cells (pDCs), conventional dendritic cells (cDCs), neutrophils and two relatively naïve subclusters, named pre-monocyte and pre-neutrophil (Fig. 5a, b; Supplementary Fig. S4a, b and Table S7). With no surprise, the percentage of myeloid lineage cells increased significantly (Fig. 5c), with higher expression of neutrophil degranulation genes (AZU1 and MPO) and lower expression of lymphocyte activation genes (LST1 and HLA-DRA) (Fig. 5d). As the most potent antigen-presenting cells with the ability to coordinate tolerance and immunity among myeloid cells44, DCs exhibited higher expression of genes associated with anti-inflammation (GRN45 and ZFP36) and negative regulation of immune response (TSC22D3 and SOCS1) in G-BM (Supplementary Fig. S4c, d and Table S8). It has been described that SOCS1 highly expressed in DCs could induce T-cell hyporesponsiveness46,47. In addition, downregulated pathways in CD14+ monocytes included antigen processing and presentation, CD8 TCR downstream pathway (HLA-DRA and IER2), and proinflammatory IL-12 pathway (ZFP36L1 and JUN) (Fig. 5e, f). CD16+ monocytes showed higher expression of genes related to IL-4 signaling pathway (NFKBIA and DDIT4) (Fig. 5g–i), which is critical in anti-inflammation and Th2 cell differentiation48,49. Monocytic-MDSCs (M-MDSCs) have been identified as immature myeloid cells with a remarkable ability to suppress T-cell responses both in antigen-specific and -nonspecific manners associated with the production of nitric oxide and cytokines50,51. In our data, all monocyte subclusters exhibited stronger M-MDSC features as indicated by gene scoring analysis using reported MDSC gene signatures (Fig. 5j; Supplementary Table S5)52, indicating that G-CSF might modulate M-MDSC generation. Collectively, myeloid cells underwent significant changes in transcriptional features upon G-CSF stimulation, which might contribute to the regulation of T-cell response in multiple ways.
Fig. 5. Effect of G-CSF on myeloid cells.
a UMAP visualization of nine subclusters of the myeloid lineage across two donors. b Dot plot showing the average expression of cell type-associated marker genes in myeloid subclusters and percentage of cells expressing these genes. c Bar plot displaying changes in the percentage of myeloid subclusters before and after G-CSF treatment. d Volcano plot showing the DEGs of myeloid cells in G-BM compared with unstimulated BM. DEGs were detected by differential expression analysis (two-sided Student’s t-test). Each dot represents a single gene. Genes with logFC < −0.25 and logFC > 0.25 were selected and colored in blue and red, respectively. e Heatmap showing scaled expression of top 15 DEGs in CD14+ monocytes before and after G-CSF treatment. f Metascape bar graph showing functional enrichment of downregulated genes in CD14+ monocytes after G-CSF treatment. Enrichment terms sorted by −log10(P value) were displayed by bars with a discrete color scale representing statistical significance. The top 20 enriched terms are displayed. g Heatmap showing scaled expression of top DEGs in CD16+ monocytes after G-CSF treatment. h Metascape bar graph showing functional enrichment of upregulated genes in CD16+ monocytes after G-CSF treatment. Enrichment terms sorted by −log10(P value) were displayed by bars with a discrete color scale representing statistical significance. The top 20 enriched terms are shown. i Dot plot showing average expression of genes related to IL-4 signaling pathway in CD16+ monocytes before and after G-CSF treatment and the percentage of cells expressing these genes. Representative upregulated DEGs enriched in the IL-4 signaling pathway in h are displayed. j Violin plot showing scores of the M-MDSC signatures before and after G-CSF administration in monocyte subclusters. AddModuleScore function in Seurat R package was used to calculate the average expression with default settings. *P value < 0.05; ****P value < 0.0001.
The potential indirect mechanisms of G-CSF inducing lower reactivity of T and NK cells
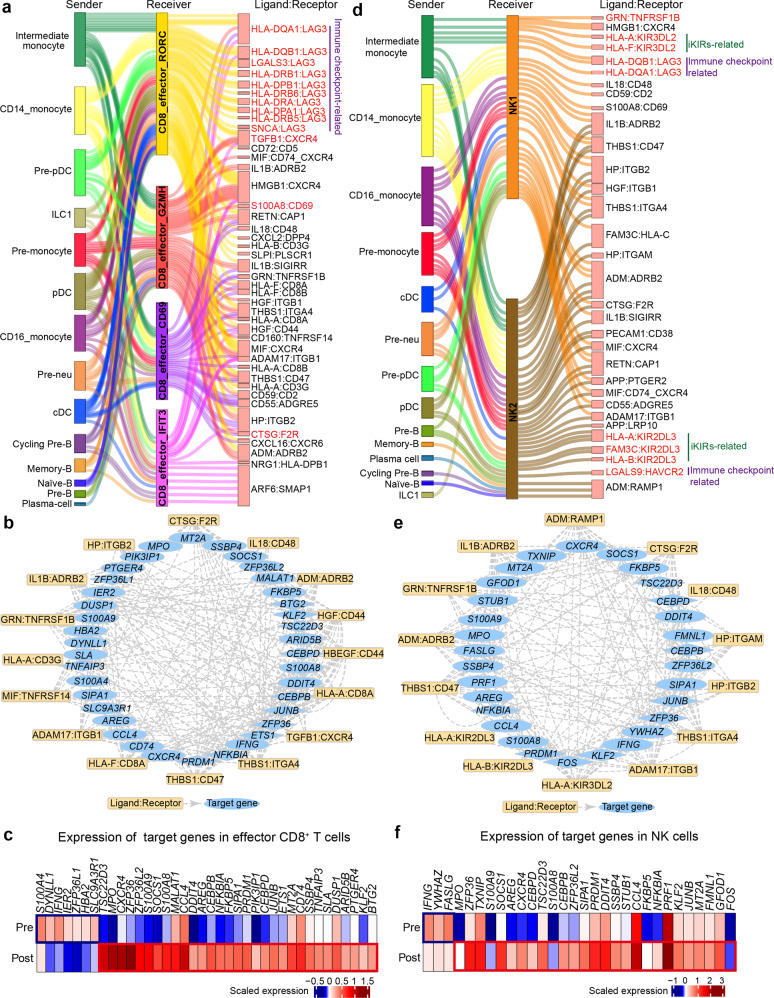
It is generally assumed that T and NK cells can be affected by G-CSF indirectly42,53. Thus, we further explored altered cell–cell communication between T/NK cells and other cells including monocytes, DCs, Treg, ILC1, and B subclusters (Supplementary Fig. S4e, f) in G-BM. Cell–cell ligand–receptor interaction analysis showed extensive communication between T/NK lineage and various potential sender cells, especially monocytes and DCs (Supplementary Fig. S5a). We then performed differential intercellular communication analysis of T and NK cells with their potential sender cells using scDiffCom54. In mobilized effector CD8+ T cells, upregulated ligand–receptor interactions contained pairs such as HLA-DQA1:LAG3 and LGALS3:LAG3 (Fig. 6a; Supplementary Table S9), which involve the immune checkpoint receptor gene LAG3 with ability to regulate immune response55. In addition, upregulated interactions also contained ligand–receptor pairs potentially related to immunomodulation such as CTSG:F2R and TGFB1:CXCR4 (Fig. 6a)56,57. Moreover, these upregulated interactions can regulate the increased expression of downstream target genes related to immunosuppression and anti-inflammation (TSC22D3, AREG58 and NFKB1A41), further supporting their roles in immune regulation (Fig. 6b, c). Remarkably, another upregulated ligand–receptor pair S100A8:CD69, which was considered as an immunomodulation-related interaction, was enhanced in naïve CD4+ T cells, effector CD4+ T cells and certain effector CD8+ T cells (CD8_effector_RORC, CD8_effector_GZMH, and CD8_effector_CD69) with their sender cells (CD14+ monocytes, pre-monocytes, and intermediate monocytes) in G-BM (Fig. 6a; Supplementary Fig. S5b–g)59.
Fig. 6. Indirect modulation of G-CSF on T and NK cells.
a Sankey plot showing the upregulated intercellular communication between the potential sender cells and effector CD8+ T cells upon G-CSF administration. Differential intercellular communication analysis was performed using R package scDiffCom. Ligand–receptor pairs with adjusted P value < 0.05 and fold change > 1.5 are displayed. b Plot showing regulation of top predicted target genes in effector CD8+ T cells mediated by upregulated ligand–receptor pairs between effector CD8+ T cells and potential sender cells. Transcription factors in target genes are represented as diamonds. The ligand–receptor–target relationship was obtained from the database containing ligand–receptor interactions and receptor–transcription factor interactions in R package scMLnet, and database including ligand–receptor interactions and ligand–target links in R package NicheNet. c Heatmap showing the average expression of the downstream target genes in b in effector CD8+ T cells before and after G-CSF administration. d Sankey plot showing the upregulated intercellular communication between the potential sender cells and NK cells upon G-CSF administration. e Plot showing the regulation of top predicted target genes in NK cells mediated by upregulated ligand–receptor pairs between NK cells and potential sender cells. f Heatmap showing the average expression of the target genes in e in NK cells before and after G-CSF administration.
In mobilized NK cells, genes of upregulated ligand–receptor pairs were enriched in immunosuppressive pathways such as those associated with reduced NK cell-mediated cytotoxicity and negative regulation of immune effector process (Fig. 6d; Supplementary Fig. S6a, b and Table S9). These upregulated interactions in mobilized NK cells can further regulate similar downstream target genes as those in CD8+ T cells (Fig. 6e, f). In detail, the elevated ligand–receptor pairs included the interaction between type I MHC molecules and inhibitory killer cell immunoglobulin-like receptors (iKIRs) KIR2DL3, KIR3DL2 (HLA-A:KIR2DL3, HLA-A:KIR3DL2) (Fig. 6d), which were reported to play essential roles in NK cell activity60. In addition, it was worth noting that G-CSF increased GRN:TNFRSF1B interactions between NK cells and their sender cells (cDCs and pDCs) (Fig. 6d; Supplementary Fig. S6c–g). Interaction of PGRN (encoded by GRN, an antagonist of TNFα signaling) and TNFR2 (encoded by TNFRSF1B) has been reported to mediate anti-inflammation process in multiple inflammatory conditions61,62. Collectively, we comprehensively explored the potential indirect mechanisms by which G-CSF regulated immune response of T and NK cells via cell-to-cell communication.
Discussion
G-CSF is a hematopoietic growth factor that induces granulopoiesis and HSPC mobilization. Our data showed a significantly increased percentage of myeloid cells, as well as that of HSPCs, especially GMP. A previous study also reported acceleration of GMP formation upon the release of G-CSF in mouse BM probably by triggering the Irf8 and β-catenin progenitor self-renewal network in GMP7. However, the specific stages in which G-CSF induces enhancement of myelopoiesis and the underlying molecular mechanisms remain unclear, especially in humans. Combining the significantly changed percentages of GMP and lymphoid progenitors as well as the trajectory inference for hematopoiesis, LMPP was speculated as the earliest target progenitor of G-CSF inducing myeloid-biased hematopoiesis in human BM.
Besides, we further characterized the immunologic alterations in immune cells upon G-CSF administration. Consistent with the previous studies18,63,64, our data showed that CD4+ T cells tended to be Th2 polarized, with the downregulation of proinflammatory pathway-related cytokines (e.g., IFN-γ). T and NK cells in G-BM had stronger apoptosis properties. It has been reported that G-CSF enhanced the apoptosis of splenic T cells to inhibit inflammation and potentially induce immune tolerance in mice65. We also observed that CD8+ T and NK cells had lower cytotoxicity scores, which further indicated hyporesponsiveness of T and NK cells in G-BM.
To explore the underlying mechanisms of these particular immunologic changes, we initially investigated the potential direct mechanism wherein G-CSF regulates T and NK cells by binding with G-CSFR. Our results showed that the expression of CSF3R was slightly higher upon G-CSF treatment as previously reported66. We further speculated that G-CSF might directly regulate the target genes related to the immune response such as SOCS1. Notably, our recent work, which has been posted on bioRxiv and is now under review elsewhere, has validated that the upregulated SOCS1 in T cells inhibited T cell proliferation in vitro, thus potentially contributing to immune tolerance38. Apart from the direct effect on T/NK cells, G-CSF was generally recognized to regulate T and NK cell function in an indirect manner mediated by interacting cells, especially monocytes and DCs67,68. Through cell-to-cell communication analysis, we found that intercellular interactions of T/NK cells with monocytes and DCs have undergone significant changes upon G-CSF administration. Some ligand–receptor interaction pairs were significantly upregulated, including S100A8:CD69 and GRN:TNFRSF1B. Previous studies have demonstrated that S100A8:CD69 interaction was required for the upregulation of STAT3 signaling pathway, thus promoting Treg differentiation and probably inhibiting Th17 differentiation59. The expression of Cd69 in Treg cells in mice was considered to be necessary to maintain immune tolerance, probably by affecting the secretion of factors such as IL-1069,70. Here, CD69 in CD8+ T cells also showed elevated expression and enhanced interaction with S100A8 from monocytes in G-BM, implying a presumed role in immunomodulation of CD8+ T cells. In addition, the binding of PGRN and TNFR2 (GRN:TNFRSF1B) was reported to exhibit anti-inflammatory properties by competitively interfering with TNF/TNFR-mediated inflammation62. Furthermore, experiments in mice indicated that PGRN could significantly protect Treg from a downregulation caused by TNFα, and regulate the function of effector T cells by inhibiting IFN-γ secretion45,61. Taken together, these ligand–receptor interactions may play complicated roles in cell-mediated regulation of immune response and inflammation in G-BM.
In summary, we provided a high-resolution transcriptome map of G-BM and deciphered the effects of G-CSF on lymphomyeloid divergence and immune hyporesponsiveness. Our results suggest that G-CSF-induced myeloid-biased differentiation initiates from the stage of LMPPs in human BM. Moreover, we delineated the potential cellular and molecular mechanisms of the immunosuppressive effect of G-CSF in human G-BM. G-CSF stimulation can upregulate the expression of genes related to immune hyporesponsiveness in T and NK cells via the direct binding with G-CSFR and diverse cell-to-cell communications. These findings will further provide implications for allo-HSCT and immune cell-based targeted therapy.
Materials and methods
Human samples
We recruited two healthy donors and treated them with recombinant G-CSF (filgrastim; Kirin Co., Ltd., Tokyo, Japan) at a dosage of 5 μg/kg body weight per day for 5 consecutive days. The BM samples were collected by aspiration on the fourth day of G-CSF treatment. The study was approved by the Ethics Committee of Peking University People’s Hospital and was conducted in accordance with The Declaration of Helsinki. Informed consents were obtained from all participants.
FACS
Human BM cells were isolated from healthy donors before and after in vivo 5-day G-CSF application by Ficoll density centrifugation. Erythrocytes were removed by incubating with RBC lysis buffer (BD, 555899). After neutralization, the remaining cells were collected by spinning at 350× g for 6 min and suspended in FACS buffer (1× PBS with 1% BSA) for subsequent staining. Cells were stained in sorting buffer with specific antibodies for 30 min at 4 °C. The following antibodies were used for the staining and sorting: anti-CD45 (BV421, BD, 563879), anti-CD66b (PE, BD, 561650), alongside the viability dye 7-amino-actinomycin D (7-AAD) PerCP-Cy5.5 (eBioscience, 00699350). Given the dramatic increase in neutrophils after G-CSF treatment, we pooled neutrophils and other cells together in a certain ratio for scRNA-seq to construct a complete single-cell transcriptional landscape of human G-BM. Through flow sorting, the final cells used for scRNA-seq contained CD45+CD66b– population (90%), CD45+CD66b+ population (5%), and CD45– population (5%). Cells were sorted using an Aria II Flow Cytometer (BD Bioscience). Pre-gating was first done for live cells based on a 7-AAD staining. Data were analyzed using BD FACSDIVA v8.0.1 and Flowjo (v10).
Single-cell RNA library preparation and sequencing
For 10× Genomics-based scRNA-seq, we implemented the Chromium Single Cell 3’ v2 libraries, under the guidance of the official instruction manual (https://support.10xgenomics.com/single-cell-gene-expression/library-prep/doc/technical-note-assay-scheme-and-configuration-of-chromium-single-cell-3-v2-libraries). Sequencing was performed on the Illumina Hiseq X Ten platform (Novogene, provided by Berry Genomics Corporation, Beijing, China).
scRNA-seq data processing
For scRNA-seq data, raw gene expression matrices were generated for each sample by the Cell Ranger (version 4.0.0) pipeline coupled with human reference hg19. The output gene expression matrices were analyzed by R software (version 3.6.3) with the Seurat package (version 3.2.2)71. Low-quality cells were removed if they met any of the following criteria: (1) < 1000 unique molecular identifiers (UMI); (2) < 300 genes; or (3) > 10% UMIs derived from the mitochondrial genome. Doublets were identified by the R package DoubletFinder72 with default settings. After the removal of the low-quality cells and doublets, a total of 21,005 cells and 26,937 genes were kept for further analyses. Then, UMI counts were normalized by the NormalizeData function and top 2000 features with high cell-to-cell variation were calculated using the FindVariableFeatures function. Next, the ScaleData function was conducted to scale and center features in the datasets and the RunPCA function was used with default parameters to reduce dimensionality. The R package Harmony (version 0.1.0)73 was used for batch correction to avoid the batch effect across samples. The data were used in the subsequent nonlinear dimensional reduction with the RunUMAP function and cluster analysis by the FindNeighbors and FindClusters functions. All details regarding the Seurat analyses performed in this work can be found in the website tutorial (https://satijalab.org/seurat/v3.0/pbmc3k_tutorial.html).
Cell type annotation and cluster marker identification
After the projection of all cells into two-dimensional spaces by UMAP, cells were clustered together according to common features. The annotation of cell clusters was identified by the expression of cell type-associated genes. SCENIC74 was also used to help the cell state identification based on key regulon activity. The regulon activity scores were calculated with AUC by the AUCell package. Signature genes of each cell cluster were identified with the FindAllMarkers function in Seurat. To further decode the hematopoietic landscape in G-BM, major cell types were further sub-clustered. Scaling, principal component analysis, and clustering were performed as described above. Additionally, all percentages of different cell types were calculated after removing neutrophils.
Differential gene expression and functional enrichment
DEGs were recognized using the FindAllMarkers function in Seurat with parameter ‘test.use = wilcox’ by default and the Benjamini–Hochberg method was used to estimate the false discovery rate (FDR). DEGs were filtered by fold change of > 1.25. DEGs with P value adjusted by Benjamini–Hochberg < 0.05 were considered significant. Enrichment analysis of DEGs was conducted using the Metascape webtool (https://metascape.org). GSEA was performed using the clusterProfiler package (v3.13.0)75.
Trajectory inference
To map hematopoietic differentiation, pseudotime analysis was performed with Monocle v2. For the pseudotime ordering analyses (before and after G-CSF administration), the gene expression matrix issued from the integrated samples was loaded in Monocle2 using the new CellData function. The filtered genes with mean expression ≥ 0.1 and an empirical dispersion at least twice as large as the fitted dispersion were set as ordering genes. To avoid cell cycle effects, cell cycle genes in GO: 0007049 were further excluded from ordering genes. Then, ‘DDRTree’ function was used for dimensionality reduction and ‘plot_cell_trajectory’ function for visualization.
Defining cell state scores and cell cycle analysis
AddModuleScore function in Seurat package was used to calculate the gene set scores. We used cell proliferation (GO: 0007049), leukocyte migration (GO: 0050900), positive regulation of apoptosis process (GO: 0043065), an immnuosuppression-related gene set (61 genes)76, 4 cytotoxicity-associated genes (GZMA, GZMB, KLRD1, and NKG7) and 829 highly expressed genes reported in M-MDSCs52 to define proliferation, migration, apoptosis, immunosuppression, cytotoxicity, and M-MDSC scores, respectively. For cell cycle analysis, CellCycleScoring function in Seurat package was used to predict the classification of each cell in either G1, S or G2M phase.
Cell–cell communication analysis
Ligand–receptor interactions were identified by CellPhoneDB77 with default parameters. Only ligand–receptor interaction pairs with P value < 0.05 returned by CellPhoneDB were considered significant. Scdiffcom54 was used to perform differential intercellular communication analysis before and after G-CSF administration. Up- or downregulated ligand–receptor pairs were then filtered with adjusted P value < 0.05 and fold change > 1.5. The regulatory effect of the differential communication on T and NK cells was further explored by predicting downstream target genes of curated ligand–receptor interactions. To speculate target genes of altered ligand–receptor pairs, we retrieved ligand–receptor–target relationship from publicly available resources: intercellular links (ligand–receptor interactions) and intracellular subnetworks (receptor–transcriptional factor pathways and ligand–target links) from NicheNet40 and scMLnet78 for further analysis. Only DEGs before and after G-CSF administration were included into analysis of target gene prediction. The igraph R package and Cytoscape (version 3.6.1) were used for visualization of the ligand–receptor–target relationship.
Statistical analysis
Wilcoxon-rank-sum test and Student’s t-test were used for comparisons of gene expression levels. We considered P value < 0.05 statistically significant. Analyses were conducted using R software (version 3.6.3).
Supplementary information
Acknowledgements
The authors thank all donors and their families who gave them permission to include samples and clinical data in this study. This study was supported by grants from the National Key R&D Program of China (2021YFA0805703, 2021YFA1100102, 2020YFA0112402, 2017YFA0103401, 2016YFA0100601, 2017YFA0104500), the National Natural Science Foundation of China (31930054, 32100646, 81890991, 31871173, 82170211, 32100698), Beijing Natural Science Foundation (5222035), the Beijing Nova Program (Z211100002121033), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S347), the State Key Laboratory of Proteomics (SKLP-K202003) and Research Grant of Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University (ZSYXM202101).
Author contributions
B.L., X.H., Y.C., and Y.G. designed the experiments; Z.B. and Z.X. performed the sample preparation and FACS with help from M.Z. and Y.N.; H.G. collected and prepared the samples; Y.G. and G.Y. conducted the data analysis with help from Y.L. and W.Y.; Y.G., Z.B., and M.Z. coordinated all aspects of the research; G.Y., Y.G., M.Z., Z.B., H.G., and Z.X. wrote the manuscript; and all authors read, revised and approved the final manuscript.
Data availability
The scRNA-seq data of our study have been deposited at GEO (NCBI) with accession number GSE193138.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Guoju You, Man Zhang, Zhilei Bian, Huidong Guo, Zhengyang Xu
Contributor Information
Yandong Gong, Email: yandgong@126.com.
Yingjun Chang, Email: rmcyj@bjmu.edu.cn.
Xiaojun Huang, Email: xjhrm@medmail.com.cn.
Bing Liu, Email: bingliu17@yahoo.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41421-022-00417-y.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Lu DP, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 3.Huang XJ, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38:291–297. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–3962. doi: 10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 5.Di Bartolomeo P, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849–857. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011;118:2609–2617. doi: 10.1182/blood-2011-02-339838. [DOI] [PubMed] [Google Scholar]
- 7.Hérault A, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017;544:53–58. doi: 10.1038/nature21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weston BR, Li L, Tyson JJ. Mathematical analysis of cytokine-induced differentiation of granulocyte-monocyte progenitor cells. Front. Immunol. 2018;9:2048. doi: 10.3389/fimmu.2018.02048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shier LR, et al. Differential effects of granulocyte colony-stimulating factor on marrow- and blood-derived hematopoietic and immune cell populations in healthy human donors. Biol. Blood Marrow Transpl. 2004;10:624–634. doi: 10.1016/j.bbmt.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Bernitz JM, Daniel MG, Fstkchyan YS, Moore K. Granulocyte colony-stimulating factor mobilizes dormant hematopoietic stem cells without proliferation in mice. Blood. 2017;129:1901–1912. doi: 10.1182/blood-2016-11-752923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie M, et al. Granulocyte colony-stimulating factor directly acts on mouse lymphoid-biased but not myeloid-biased hematopoietic stem cells. Haematologica. 2021;106:1647–1658. doi: 10.3324/haematol.2019.239251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan L, et al. Granulocyte colony-stimulating factor-mobilized allogeneic stem cell transplantation maintains graft-versus-leukemia effects through a perforin-dependent pathway while preventing graft-versus-host disease. Blood. 1999;93:4071–4078. doi: 10.1182/blood.V93.12.4071. [DOI] [PubMed] [Google Scholar]
- 13.Morris ES, MacDonald KP, Hill GR. Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood. 2006;107:3430–3435. doi: 10.1182/blood-2005-10-4299. [DOI] [PubMed] [Google Scholar]
- 14.Huang XJ, et al. Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant. 2009;44:309–316. doi: 10.1038/bmt.2009.26. [DOI] [PubMed] [Google Scholar]
- 15.Pan L, Delmonte J, Jr., Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. doi: 10.1182/blood.V86.12.4422.bloodjournal86124422. [DOI] [PubMed] [Google Scholar]
- 16.Wen Q, et al. G-CSF-induced macrophage polarization and mobilization may prevent acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:1419–1433. doi: 10.1038/s41409-019-0449-9. [DOI] [PubMed] [Google Scholar]
- 17.Jun HX, Jun CY, Yu ZX. In vivo induction of T-cell hyporesponsiveness and alteration of immunological cells of bone marrow grafts using granulocyte colony-stimulating factor. Haematologica. 2004;89:1517–1524. [PubMed] [Google Scholar]
- 18.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. doi: 10.1182/blood.V95.8.2484. [DOI] [PubMed] [Google Scholar]
- 19.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 20.Lv M, et al. Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2015;90:E9–E16. doi: 10.1002/ajh.23865. [DOI] [PubMed] [Google Scholar]
- 21.Tumino N, et al. PMN-MDSC are a new target to rescue graft-versus-leukemia activity of NK cells in haplo-HSC transplantation. Leukemia. 2020;34:932–937. doi: 10.1038/s41375-019-0585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton J, Hutchins C, Durrant S. Granulocyte–colony-stimulating factor (G-CSF)–primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF–mobilized peripheral blood stem cells. Blood. 2001;98:3186–3191. doi: 10.1182/blood.V98.12.3186. [DOI] [PubMed] [Google Scholar]
- 23.De Felice L, et al. Hematopoietic, mesenchymal, and immune cells are more enhanced in bone marrow than in peripheral blood from granulocyte colony-stimulating factor primed healthy donors. Biol. Blood Marrow Transplant. 2016;22:1758–1764. doi: 10.1016/j.bbmt.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 25.Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28:31–40. doi: 10.1016/j.blre.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.V98.5.1289. [DOI] [PubMed] [Google Scholar]
- 27.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Karamitros D, et al. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells. Nat. Immunol. 2018;19:85–97. doi: 10.1038/s41590-017-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 30.Galletti G, et al. Two subsets of stem-like CD8(+) memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 2020;21:1552–1562. doi: 10.1038/s41590-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gartlan KH, et al. Tc17 cells are a proinflammatory, plastic lineage of pathogenic CD8+ T cells that induce GVHD without antileukemic effects. Blood. 2015;126:1609–1620. doi: 10.1182/blood-2015-01-622662. [DOI] [PubMed] [Google Scholar]
- 33.Dan L, et al. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 2020;21:287–297. doi: 10.1038/s41590-019-0577-9. [DOI] [PubMed] [Google Scholar]
- 34.Yosef N, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang W, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/S1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 36.Jun HX, Jun CY, Yu ZX. A direct comparison of immunological characteristics of granulocyte colony-stimulating factor (G-CSF)-primed bone marrow grafts and G-CSF-mobilized peripheral blood grafts. Haematologica. 2005;90:715–716. [PubMed] [Google Scholar]
- 37.Stritesky GL, et al. The transcription factor STAT3 Is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, R. et al. Three-dimensional genome structure and chromatin accessibility reorganization during in vivo induction of human T cell tolerance. bioRxiv10.1101/2020.03.11.988253 (2020).
- 39.Taylor GA, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/S1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 40.Browaeys R, Saelens W, Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 41.Jimi E, Strickland I, Voll RE, Long M, Ghosh S. Differential role of the transcription factor NF-kappaB in selection and survival of CD4+ and CD8+ thymocytes. Immunity. 2008;29:523–537. doi: 10.1016/j.immuni.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawa Y, et al. G-CSF reduces IFN-gamma and IL-4 production by T cells after allogeneic stimulation by indirectly modulating monocyte function. Bone Marrow Transplant. 2000;25:1035–1040. doi: 10.1038/sj.bmt.1702402. [DOI] [PubMed] [Google Scholar]
- 43.Sloand EM, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95:2269–2274. doi: 10.1182/blood.V95.7.2269. [DOI] [PubMed] [Google Scholar]
- 44.Iberg CA, Jones A, Hawiger D. Dendritic cells as inducers of peripheral tolerance. Trends Immunol. 2017;38:793–804. doi: 10.1016/j.it.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacol. Ther. 2012;133:124–132. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu H, et al. Dendritic cells transduced with SOCS1 gene exhibit regulatory DC properties and prolong allograft survival. Cell. Mol. Immunol. 2009;6:87–95. doi: 10.1038/cmi.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanya S, et al. Enhanced induction of HIV-specific cytotoxic T lymphocytes by dendritic cell-targeted delivery of SOCS-1 siRNA. Mol. Ther. 2010;18:2028–2037. doi: 10.1038/mt.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimoda K, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 49.Takenaka M, Yabuta A, Takahashi Y, Takakura Y. Interleukin-4-carrying small extracellular vesicles with a high potential as anti-inflammatory therapeutics based on modulation of macrophage function. Biomaterials. 2021;278:121160. doi: 10.1016/j.biomaterials.2021.121160. [DOI] [PubMed] [Google Scholar]
- 50.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alshetaiwi H, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 2020;5:44. doi: 10.1126/sciimmunol.aay6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelosi A, et al. NK cells and PMN-MDSCs in the graft from G-CSF mobilized haploidentical donors display distinct gene expression profiles from those of the non-mobilized counterpart. Front. Immunol. 2021;12:657329. doi: 10.3389/fimmu.2021.657329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lagger, C. et al. scAgeCom: a murine atlas of age-related changes in intercellular communication inferred with the package scDiffCom. bioRxiv10.1101/2021.08.13.456238 (2021).
- 55.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galindo-Albarrán AO, et al. CD8(+) T cells from human neonates are biased toward an innate immune response. Cell Rep. 2016;17:2151–2160. doi: 10.1016/j.celrep.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 57.Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CR, et al. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. 2015;29:5006–5017. doi: 10.1096/fj.15-273987. [DOI] [PubMed] [Google Scholar]
- 60.Gao F, Ye Y, Gao Y, Huang H, Zhao Y. Influence of KIR and NK cell reconstitution in the outcomes of hematopoietic stem cell transplantation. Front. Immunol. 2020;11:2022. doi: 10.3389/fimmu.2020.02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang W, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Siegel RM. Medicine. Progranulin resolves inflammation. Science. 2011;332:427–428. doi: 10.1126/science.1205992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sivakumaran M. Modulation of Th1/Th2 subsets by granulocyte-colony stimulating factor. Blood. 2001;97:333. doi: 10.1182/blood.V97.1.333. [DOI] [PubMed] [Google Scholar]
- 64.Boneberg EM, Hareng L, Gantner F, Wendel A, Hartung T. Human monocytes express functional receptors for granulocyte colony-stimulating factor that mediate suppression of monokines and interferon-gamma. Blood. 2000;95:270–276. doi: 10.1182/blood.V95.1.270. [DOI] [PubMed] [Google Scholar]
- 65.Peng W. G-CSF treatment promotes apoptosis of autoreactive T cells to restrict the inflammatory cascade and accelerate recovery in experimental allergic encephalomyelitis. Exp. Neurol. 2017;289:73–84. doi: 10.1016/j.expneurol.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Franzke A, et al. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734–739. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 67.Mielcarek M, Martin PJ, Torok-Storb B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 1997;89:1629–1634. doi: 10.1182/blood.V89.5.1629. [DOI] [PubMed] [Google Scholar]
- 68.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 69.Cortés JR, et al. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014;55:51–62. doi: 10.1016/j.jaut.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu L, et al. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018;9:905. doi: 10.1038/s41419-018-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGinnis CS, Murrow LM, Gartner ZJ. DoubletFinder: doublet detection in single-cell rna sequencing data using artificial nearest neighbors. Cell Syst. 2019;8:329–337.e4. doi: 10.1016/j.cels.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korsunsky I, et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aibar S, et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rouillard AD, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database. 2016;2016:baw100. doi: 10.1093/database/baw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 78.Cheng J, Zhang J, Wu Z, Sun X. Inferring microenvironmental regulation of gene expression from single-cell RNA sequencing data using scMLnet with an application to COVID-19. Brief. Bioinform. 2021;22:988–1005. doi: 10.1093/bib/bbaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The scRNA-seq data of our study have been deposited at GEO (NCBI) with accession number GSE193138.