Abstract
A major obstacle to successful smoking cessation is the prospect of weight gain. Despite a clear relationship between cigarette smoking and body weight, surprisingly little is known about the physiological and molecular mechanism by which nicotine affects energy homeostasis and food-motivated behaviors. Here we use loss-of-function mouse models to demonstrate that 2 nicotinic acetylcholine receptor (nAChR) subunits encoded by the CHRNA5-CHRNA3-CHRNB4 gene cluster, α5 and β4, exhibit divergent roles in food reward. We also reveal that β4-containing nAChRs are essential for the weight-lowering effects of nicotine in diet-induced obese mice. Finally, our data support the notion of crosstalk between incretin biology and nAChR signaling, as we demonstrate that the glycemic benefits of glucagon-like peptide-1 receptor activation partially relies on β4-containing nAChRs. Together, these data encourage further research into the role of cholinergic neurotransmission in regulating food reward and the translational pursuit of site-directed targeting of β4-containing nAChRs for treatment of metabolic disease.
Keywords: nicotinic receptor, nAChR, body weight, metabolism, reward, nicotine
Successful management of obesity and its metabolic comorbidities necessitates a deeper understanding of biological mechanisms that regulate body weight and the pathological mechanisms underlying excessive gain of body fat. Epidemiologic studies imply a strong relationship between cigarette smoking and body weight (1). Smokers weigh less than nonsmokers of the same age and sex (2,3), and smoking cessation has been linked to increased food intake, decreased metabolic rate, and weight gain (4–6). Nicotine is regarded as the primary molecular effector of smoking on energy balance, and animal studies have demonstrated that nicotine modulates body weight through combined actions on energy expenditure and hedonic and homeostatic feeding (7–10).
Nicotine signals via nicotinic acetylcholine receptors (nAChRs), a family of ligand-gated ion channels that are expressed in both the central and peripheral nervous systems and also in non-neuronal cells (11–13). In 2011, a landmark discovery revealed that the β4 nAChR subunit in arcuate nucleus (ARC) pro-opiomelanocortin (POMC) neurons is important for the effects of nicotine on short-term food intake (14). β4 is located on chromosome 15 in a gene cluster with α3 and α5, and the 3 subunits co-assemble to form functional receptors (15,16). Interestingly, the α5-α3-β4 (CHRNA5-CHRNA3-CHRNB4) gene cluster has been linked to smoking heaviness, nicotine dependence (17–21), and higher body mass index (BMI) (22,23). Animal studies have implicated all individual subunits of the α5-α3-β4 gene cluster in regulating nicotine consumption (titration of reward and aversion) (17,18,24). However, despite a clear implication of α5 and β4 in nicotine sensitivity, the role of these subunits in food reward has received very little attention.
Here, we employ loss-of-function mouse models to investigate the role of nAChR subunits α5 and β4 in body weight homeostasis and food reward. Moreover, we set out to clarify if nAChRs containing α5 or β4 subunits are essential for the weight-lowering effects of nicotine. Finally, the fact that signaling crosstalk between the glucagon-like peptide-1 receptor (GLP-1R) and nAChRs is important for nicotine sensitivity (25) prompted us to scrutinize if α5 or β4 is important for the benefits of GLP-1R agonists on weight loss and glucose metabolism.
Materials and Methods
Animal Experiments
Animals
Global α5 knockout (KO) and wild-type (WT) mice on C57BL6 background were generated as previously described (26). Global β4 KO and WT mice on a C57BL6J background were generated as previously described (27). Male mice were double or single housed at all times in a temperature- and humidity-controlled environment (22°C, 33-35% humidity) and on a 12-hour light–dark cycle. Mice had ad libitum access to standard chow diet (Altromin #1310, Lage, Germany) and tap water. For diet-induced obesity (DIO), mice were given ad libitum access to a high-fat, high-sucrose (HFHS) diet (D12331; Research Diets, New Brunswick, USA). All animal experiments were approved by the Danish Animal Experimentation Inspectorate (license: 2018-15-0201-01457) and performed according to institutional guidelines.
Food reward
Male α5 KO/WT (n = 7) and β4 KO/WT (n = 9) mice were kept on chow diet and single or double housed. To assess food reward, they were given a single HFHS diet pellet/animal for 1 hour daily 3-4 hours prior to the onset of the dark cycle. The pellet was placed in the cage bottom and was weighed before and after to determine the amount of HFHS diet consumed by each mouse. The experiment was conducted over 8 days: 3 days on, 2 days off, and 3 days on.
Sucrose preference test
Sucrose preference was measured using a 2-bottle free-choice test. For habituation, α5 KO/WT (n = 12) and β4 KO/WT (n = 8) mice were housed individually for 4 days in cages containing 2 water bottles. The bottles consisted of a 50-mL centrifugal tube with a metal drinking nipple. Following habituation, 1 of the water bottles was replaced with a bottle containing sucrose water. The volumes of water and sucrose solution along with body weight and food intake were measured daily. On consecutive days, the concentration of sucrose (Sigma Aldrich, St. Louis, USA) was increased from 0.5% to 3% to 10% to 30%. Mice had unlimited access to both bottles at all times and, to control for potential side-preferences, the positions of the bottles were changed daily.
In vivo pharmacology
To study the role of nAChR α5 and β4 subunits in nicotine-induced weight loss, nicotine was subcutaneously injected daily for 14 days. Male DIO α5 KO/WT (n = 7) and β4 KO/WT (n = 11) mice with an average body weight of ~50 g, received subcutaneous injections of 2 mg/kg body weight nicotine ((–)-nicotine ditartrate, Santa Cruz Biotechnology, Dallas, USA; injection volume: 5 µL/g body weight) or vehicle (isotonic saline, Amgros I/S, Copenhagen, Denmark; injection volume: 5 µL/g body weight). Injections were performed 2 hours prior to the onset of the dark cycle. At time of injection, food intake and body weight were measured. On the final day of the study, an intraperitoneal glucose tolerance test (ipGTT) was performed (see “Intraperitoneal glucose tolerance test”).
For liraglutide pharmacology, male DIO α5 KO/WT (n = 5) and β4 KO/WT (n = 6) mice with an average body weight of ~52 g received subcutaneous injections of 10 nmol/kg body weight liraglutide (Novo Nordisk, Copenhagen, Denmark; injection volume: 5 µL/g body weight) or vehicle (isotonic saline, Amgros I/S, Copenhagen, Denmark; injection volume: 5 µL/g body weight) daily for 7 days. Compounds were administered 2 hours prior to the onset of the dark cycle. At time of injection, body weight and food intake were measured. On the final day of the study, an ipGTT was performed (see “Intraperitoneal glucose tolerance test”).
Intraperitoneal glucose tolerance test
Shortly after entering the light phase, mice were moved to a quiet procedure room, where they were fasted for 6 hours. After 6 hours of fasting, the test commenced with an intraperitoneal injection of 1.5 g/kg body weight glucose (D-(+)-Glucose, Sigma Aldrich, St. Louis, USA) dissolved in isotonic saline (Amgros I/S, Copenhagen, Denmark). Blood glucose levels were determined at times 0 (prior to injection), 15, 30, 60, and 120 minutes after injection, using a handheld glucometer (Abbott GmbH & Co. KG, Wiesbaden, Germany).
Gene Expression Analysis (qPCR)
Hypothalamus and striatum were dissected, immediately frozen on dry ice, and stored at –80°C. Tissue was homogenized in a Trizolreagent (QIAzol Lysis Reagent, Qiagen, Hilden, Germany) using a 5-mm stainless-steel bead (Qiagen, Hilden, Germany) and a TissueLyser (Qiagen, Hilden, Germany) at 50 hZ for 3 minutes, followed by 5 minutes of incubation at room temperature. Chloroform (200 µL) (Sigma-Aldrich, St. Louis, USA) was added and tubes were shaken vigorously for 15 seconds and incubated for 2 minutes at room temperature. The samples were then centrifuged for 15 minutes at 12 000g at 4°C. The aqueous phase was mixed 1:1 with 70% ethanol. The samples were further processed using RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany) following the instructions provided by the manufacturer. RNA content was measured using a NanoDrop 2000 (Thermo Fisher, Waltham, USA). A 500-ng bolus of RNA was converted into cDNA by mixing FS buffer and DTT (Thermo Fisher, Waltham, USA) with random primers (Sigma-Aldrich, St. Louis, USA) and incubated for 3 minutes at 70°C. Then, dNTPs, RNase out, and Superscript III (Thermo Fisher, Waltham, USA) were added, and samples were placed in a thermal cycler for 5 minutes at 25°C, 60 minutes at 50°C, and 15 minutes at 70°C. cDNA was diluted 1:20 and kept at −20°C until further processing. Quantitative polymerase chain reaction (PCR) was performed using PrecisionPLUS qPCR Master Mix containing SYBR green (Primer Design, Camberly, UK). cDNA, primers (Table 1 and Table S1 (28)), and PrecisionPLUS qPCR Master Mix were mixed in a 384-well plate and incubated in a LightCycler (LightCycler 480 II, Roche, Basel, Switzerland) using 2 minutes of preincubation at 95°C followed by 45 cycles of 60 seconds at 60°C. Melting curve analysis was performed by stepwise increasing the temperature from 60 to 95°C (Table 1).
Table 1.
Primers for qPCR
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| CHRNA5 | CGTCCGCGAGGTTGTTGAAG | AGCTGCTTGACTGCTCACTAAG |
| CHRNA3 | GCCAAAGAGATTCAAGATGATTGG | TCTGGGGCTATTGAGAAAGTGC |
| CHRNB4 | ATCAGAGTGTCATCGAGGACTG | CACTAGGCTGCTCATATCATCC |
| CHRNB2 | TGACCAGAGTGTGAGGGAGG | AGCTGCAAATGAGAGACCTCAC |
| CHRNA4 | GACTTCTCGGTGAAGGAGGAC | GGAAGATGTGGGTGACTGACG |
| CHRNA7 | CCTAAGTGGACCAGGATCATTC | ATGTAGAGCAGGTTGCCATTGC |
| Rpl13a | GGA GGG GCA GGT TCT GGT AT | TGT TGA TGC CTT CAC AGC GT |
Statistical Analyses
The data were analyzed using a paired 2-tailed t test, an unpaired 2-tailed t test, or a 2-way analysis of variance (ANOVA) with Bonferroni post hoc test, as described in the figure legends. P < .05 was set as the criterion for statistical significance. All data are expressed as mean ± SEM. Analyses were performed using Prism version 9 (GraphPad, San Diego, USA).
Results
nAChR Subunit Distribution in Hypothalamus and Striatum
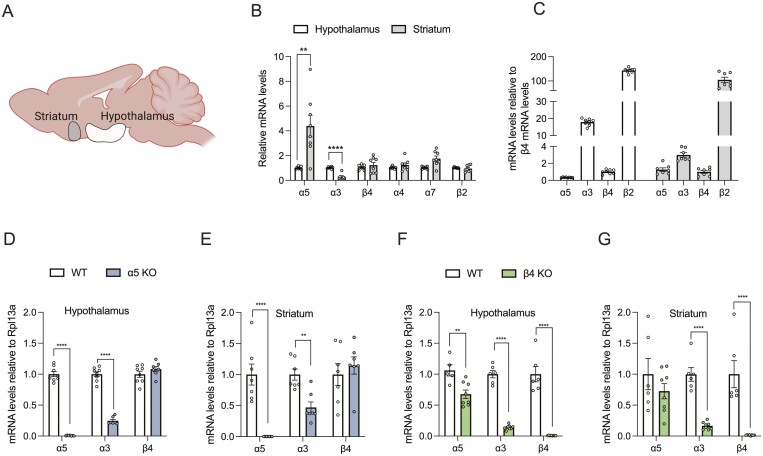
To delineate the physiological and pharmacological properties of β4 and α5 subunits in homeostatic and hedonic feeding, we profiled β4 and α5 mRNA expression in the hypothalamus and striatum and compared the relative expression levels with other major central nervous system nAChR subunits (Fig. 1A). We found that the β4, α4, α7, and β2 subunits are expressed at similar levels in the hypothalamus and striatum (Fig. 1B), but that α5 mRNA is significantly higher in the striatum relative to the hypothalamus, whereas the opposite is the case for α3 mRNA. Next, we wanted to compare the relative distribution of the same subunits within each region. In agreement with previous work (29), we found that β2 is the most abundant subunit in both regions (Fig. 1C). Of note, the α3 subunit is abundantly expressed in the hypothalamus. Finally, we wanted to evaluate the relative mRNA levels of the α5-α3-β4 gene cluster subunits in the 2 global KO mouse models (Fig. 1D-1G). As expected, these analyses confirmed complete ablation α5 mRNA in the α5 KO model and complete ablation of β4 mRNA in the β4 KO model. Notably, we demonstrate that KO of either α5 or β4 subunits results in the partial KO of the α3 subunit in both the hypothalamus and striatum (Fig. 1D-1G), and KO of the β4 subunit suppresses α5 mRNA levels in the hypothalamus (Fig. 1F). Despite the substantially reduced levels of α3 mRNA in both α5 and β4 KO models, this may be sufficient for α3-dependent nAChR signaling (30).
Figure 1.
nAChR subunit distribution in the hypothalamus and striatum. (A) Illustration of the 2 brain regions in focus: hypothalamus and striatum. (B) mRNA levels of nAChR subunits α5, α3, β4, α4, α7, β2 relative to Rpl13a in hypothalamus and striatum. (C) mRNA levels of nAChR subunits α5, α3, β4, and β2 relative to Rpl13a normalized to β4 mRNA in hypothalamus and striatum. (D) Levels of α5, α3, and β4 mRNA relative to Rpl13a in WT and α5 KO mice in hypothalamus. () Levels of α5, α3, and β4 mRNA relative to Rpl13a in WT and α5 KO mice in striatum. (F) Levels of α5, α3, and β4 mRNA relative to Rpl13a in WT and β4 KO mice in hypothalamus. (G) Levels of α5, α3, and β4 mRNA relative to Rpl13a in WT and β4 KO mice in striatum. Data analyzed by paired t-test (B) and unpaired t-test (D-G). Data presented as mean ± SEM. *P < .05, **P < .01, ****P < .0001.
Divergent Roles of α5 and β4 Subunits in Food Reward
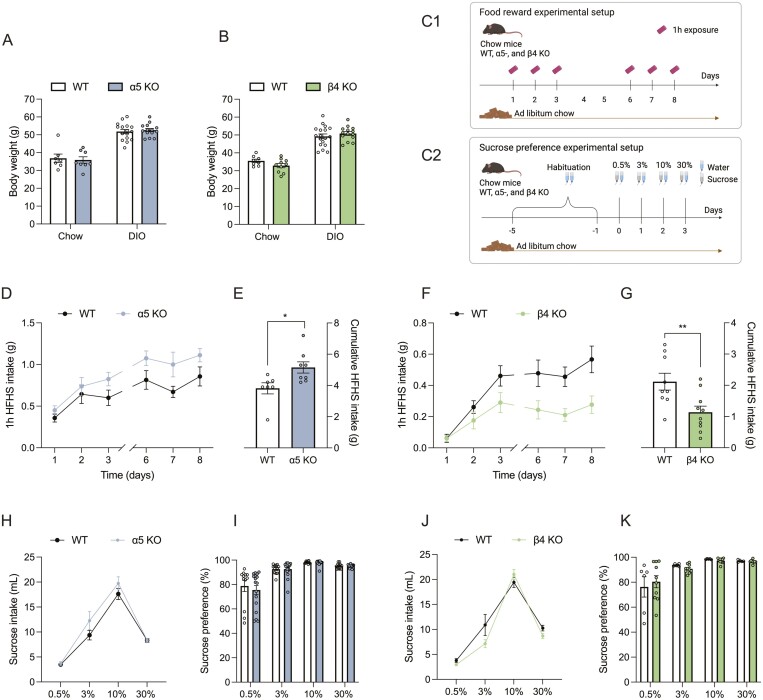
To understand the physiological implication of α5 and β4 subunits on energy homeostasis, we measured body weight of α5 KO and WT and β4 KO and their respective WT control mice, maintained on a chow diet or following 25 weeks on a HFHS diet. We found that ablation of either the α5 or β4 subunit did not elicit perturbations in long-term weight homeostasis (Fig. 2A and 2B), which might relate to compensatory mechanisms in the KO strains. Additionally, gene expression analyses of inguinal white adipose tissue, brown adipose tissue, and hypothalamus from both KO strains reveal similar expression of genes related to thermogenesis (inguinal white adipose tissue and brown adipose tissue) and appetite regulation (hypothalamus) in both KO mouse strains relative to their respective WT controls (Fig. S1 (31)). To gauge if α5 and β4 subunits are involved in food reward and palatable food intake, we established a simple experimental paradigm. Animals maintained on a chow diet were given 1-hour access to a single pellet of HFHS diet for 3 consecutive days. Subsequently, mice had 2 days of wash-out with no HFHS diet exposure, before being re-introduced to the HFHS pellet paradigm for 3 additional days (Fig. 2C1). Whereas the initial intake might relate to palatability and novelty, intake at later stages of the experiment is likely governed by the motivational value of the HFHS pellet. For the first 2 days, α5 KO and WT mice had a comparable intake of the HFHS pellet, but, from day 3 and onward, the α5 KO mice had a greater intake of HFHS diet, leading to a significantly higher cumulative HFHS diet intake by the end of the experiment (P = .0208, Fig. 2D and 2E). In contrast, β4 KO mice displayed a lower daily intake of the HFHS reward and plateaued at ~0.3 g consumed during the 1-hour exposure as opposed to ~0.6 g for the WT mice (and as opposed to >1 g for α5 KO mice). Thus, β4 KO mice had a significantly lower cumulative intake relative to WT mice in this food reward paradigm (P = .0084, Fig. 2F and 2G).
Figure 2.
Divergent roles of α5 and β4 in food reward. (A) Body weight of WT and α5 KO on chow and HFHS diet. (B) Body weight of WT and β4 KO on chow and HFHS diet. (C) Schematic illustration of experimental setups for food reward and sucrose preference. (D) Daily intake of HFHS diet for WT and α5 KO mice. (E) Cumulative HFHS diet intake for WT and α5 KO mice. (F) Daily intake of HFHS diet for WT and β4 KO mice. (G) Cumulative HFHS diet intake for WT and β4 KO mice. (H) Daily intake of sucrose water for WT and α5 KO mice. (I) Sucrose preference in %. Sucrose intake divided by total intake of sucrose and water for WT and α5 KO. (J) Daily intake of sucrose water for WT and β4 KO mice. (K) Sucrose preference in %. Sucrose intake divided by total intake of sucrose and water for WT and β4 KO. Data presented as mean ± SEM. Data analyzed by unpaired t-test. *P < .05, **P < .01.
Given the divergent roles of the α5 and β4 subunits in mixed high fat, high sugar reward, we employed 2-bottle free-choice paradigm to directly evaluate the sweet preference component of this behavior. For this, an uptitrated sucrose preference test was employed (Fig. 2C2). These experiments revealed similar preference for sucrose between α5 KO, β4 KO, and WT mice (Fig. 2H-2K). Together, these data reveal that both α5 and β4 subunits are implicated in food reward, but whereas loss of β4 decreases food reward, loss of α5 has the opposite effect. Further, the body weight data imply that any motivational aspects consequential to genetic ablation of either α5 or β4 subunits are accounted for homeostatically over time.
The β4 nAChR Subunit Is Indispensable for Nicotine-induced Weight Loss
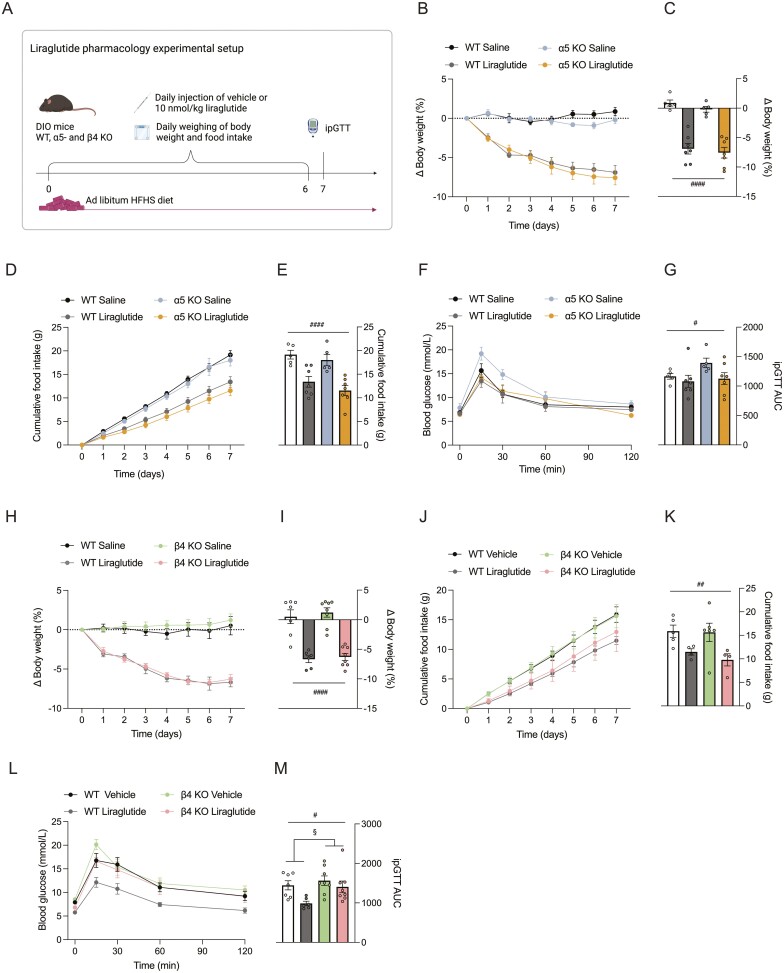
Studies with viral-mediated ablation of the hypothalamic β4 subunit demonstrated that this subunit is essential for the acute effects of nicotine on food intake (14,32). However, whether β4 is exclusively responsible for this pharmacological effect, whether other nAChR subunits are implicated, or whether there is subunit redundancy, is less clear. Similarly, it is unknown if the β4 subunit is important for the long-term effect of nicotine on energy balance. To address these questions, we exposed α5 KO, β4 KO, and WT mice to a 2-week nicotine pharmacology treatment study. In agreement with previous studies, we used 2 mg/kg nicotine administered daily to DIO mice maintained on a HFHS diet (33). For the α5 KO strain, we observed a similar weight loss in response to nicotine for both KO and WT mice (Fig. 3B and 3C, main effect of nicotine; P = .0005). The nicotine-induced weight loss was not accompanied by significant changes in cumulative food intake, and nicotine treatment did not influence glucose tolerance (Fig. 3D-3G). In contrast, nicotine treatment of β4 KO mice did not lower body weight in contrast to the ~6% weight loss observed in the WT mice (Fig. 3H-3I). For the β4 subunit study, nicotine, at least in part, mediated the weight loss in the WT mice via reduced food intake (Fig. 3J-3K, P < .0001). Finally, following the 2-week treatment study, a glucose tolerance test revealed that the β4 subunit KO had a slightly impaired glucose tolerance, but this was independent of nicotine treatment (Fig. 3L-3KM).
Figure 3.
The β4 nAChR subunit is responsible for nicotine-induced weight loss. (A) Schematic illustration of experimental setup for nicotine pharmacology. (B-G) Data from α5 KO and WT mice. H-M. Data from β4 KO and WT mice. (B) Percentage change in body weight in response to 2 mg/kg nicotine or vehicle (saline) for α5 KO and WT mice. (C) Percentage change in body weight at day 14 for α5 KO and WT mice. (D) Cumulative HFHS diet intake through experiment for α5 KO and WT mice. (E) Cumulative HFHS diet intake at day 14 for α5 KO and WT mice. (F) Blood glucose during ipGTT on day 14 of experiment for α5 KO and WT mice. (G) Area under curve (AUC) calculated from individual blood glucose traces after the ipGTT for α5 KO and WT mice. (H) Percentage change in body weight in response to 2 mg/kg nicotine or vehicle (saline) for β4 KO and WT mice. (I) Percentage change in body weight at day 14 for β4 KO and WT mice. (J) Cumulative HFHS diet intake through experiment for β4 KO and WT mice. (K) Cumulative HFHS diet intake at day 14 for β4 KO and WT mice. (L) ipGTT at day 14 of experiment for β4 KO and WT mice. (M) AUC calculated from individual blood glucose traces after the ipGTT for β4 KO and WT mice. Data presented as mean ± SEM. Data analyzed by 2-way ANOVA with Bonferroni post hoc test. Main effect of treatment: ###P < .001. Main effect of genotype: §§§§P < .0001. Post hoc effect: **P < .01, ***P < .001.
These findings clearly demonstrate that the β4 subunit, but not the α5 subunit, is required for nicotine-induced weight loss, and loss of the β4 subunit leads to perturbed glucose metabolism in the context of a HFHS diet. Thus, our data further contribute to the specific role of β4-nAChRs as a relevant pharmacological target for weight loss.
Glycemic Benefits of Liraglutide Implicates β4-nAChRs
GLP-1R agonists have emerged as potent agents for treatment of type 2 diabetes and obesity. Interestingly, central GLP-1 signaling modulates nicotine intake via the habenular–interpeduncular pathway (25). Given that the habenular–interpeduncular pathway is particularly enriched with α5- and β4-nAChRs (34,35) and site-specific loss- and gain-of-function models have demonstrated key roles for both receptor subunits in governing nicotine consumption (17,18), neural crosstalk between GLP-1R and nAChR signaling might be relevant to this neurobiology. To evaluate this, we exposed α5 KO, β4 KO, and WT mice to a pharmacological treatment study with the long-acting GLP-1R agonist liraglutide (36). The experiment was conducted over 7 days with daily injections of either liraglutide or vehicle followed by an ipGTT on day 7 (Fig. 4A). We demonstrate that liraglutide decreases body weight and food intake to a similar degree in both WT mice and α5 KO mice (Fig. 4B-4G), and in WT mice and β4 KO mice (Fig. 4H-4K), but the antidiabetic effect of liraglutide is diminished in β4 KO mice (Fig. 4L-4M).
Figure 4.
Glycemic benefits of GLP-1R agonism implicates β4-containing nAChRs. (A) Schematic illustration of experimental setup for liraglutide pharmacology. (B-G) Data from α5 KO and WT mice. H-M. Data from β4 KO and WT mice. (B) Percentage change in body weight in response to 10 nmol/kg liraglutide or vehicle (saline) for α5 KO and WT mice. (C) Percentage change in body weight at day 7 for α5 KO and WT mice. (D) Cumulative HFHS diet intake through experiment for α5 KO and WT mice. (E) Cumulative HFHS diet intake at day 14 for α5 KO and WT mice. (F) Blood glucose during ipGTT at day 14 of experiment for α5 KO and WT mice. (G) AUC calculated from individual blood glucose traces after the ipGTT for α5 KO and WT mice. (H) Percentage change in body weight in response to 10 nmol/kg liraglutide or vehicle (saline) for β4 KO and WT mice. (I) Percentage change in body weight at day 7 for β4 KO and WT mice. (J) Cumulative HFHS diet intake through experiment for β4 KO and WT mice. (K) Cumulative HFHS diet intake at day 14 for β4 KO and WT mice. (L) Blood glucose during ipGTT at day 14 of experiment for β4 KO and WT mice. (M) AUC calculated from individual blood glucose traces after the ipGTT for β4 KO and WT mice. Data presented as mean ± SEM. Data analyzed by 2-way ANOVA with Bonferroni post hoc test. Main effect of treatment: #P < .05, ##P < .01, ####P < .0001. Main effect of genotype: §P < .05.
Discussion
Despite ~1 billion tobacco users globally (37), remarkably little is known about the physiological and molecular mechanism by which nicotine affects energy homeostasis. In the brain, nicotine signals through pentameric nAChRs comprising 7 different α subunits and 3 different β subunits. Here, we evaluated the role of the α5 and β4 subunits from the α5-α3-β4 (CHRNA5-CHRNA3-CHRNB4) gene cluster in the physiological regulation of energy homeostasis and food reward and in response to nicotine and GLP-1 weight-lowering pharmacology. We show that both subunits are abundantly expressed in the hypothalamus and striatum and that they have opposing roles in food reward. Moreover, we demonstrate that nAChRs containing β4 subunits are indispensable for the effects of nicotine on food intake and weight loss and that they are important for the benefits of GLP-1R agonism on glucose metabolism. In agreement with previous work (50), these findings suggest that ligands selective for β4-nAChRs hold promise for targeting the inherent benefits of nicotinic receptor activity on energy metabolism.
The rewarding effects of nicotine are often ascribed to nAChRs containing β2 subunits in the ventral tegmental area (38–40). High doses of nicotine also result in co-activation of nAChRs containing α5, α3, and β4 subunits in the medial habenula that, through projections to the interpeduncular nucleus, promote nicotine avoidance (17,19,41,42). Loss-of-function models have confirmed key roles for both α5 and β4 subunits in governing nicotine sensitivity (43). At low doses, β4 KO mice administer less nicotine than WT mice (18), whereas α5 KO mice are reported to have reduced nicotine sensitivity and thus self-administer more nicotine than WT mice at high concentrations of nicotine (17,44,45). Here, we find that β4 KO mice consume less HFHS diet than WT mice in a food reward paradigm. This observation agrees with recent work showing diminished food reward in β4 KO mice using an operant conditioning task for palatable food reward (18). Speaking against a robust role for β4 in food reward and palatable food intake, we failed to demonstrate a sucrose-drinking phenotype. Conversely, and in agreement with a role for the α5 subunit in providing an inhibitory reward signal, we find that α5 KO mice overconsume the rewarding HFHS diet relative to WT mice. Further, our data agree with recent work showing increased food seeking relapse in rats with a CHRNA5 gene variant (46). Together, this points to shared neurobiological pathways of nicotine and food reward titration. Importantly, the observed genotype-specific food motivated behavior is abrogated in the context of chronic ad libitum access to HFHS diet as both α5 KO mice and β4 KO mice develop a diet-induced obese phenotype similar to WT mice.
It is notable that ablation of both α5 and β4 subunits leads to a comparable and concomitant reduction in α3 mRNA in the hypothalamus and striatum. As such, the respective KO models can be regarded as full KOs of the α5 and β4 subunits and partial knockdown of the α3 subunit. The remnant α3 mRNA expression, however, does not necessarily result in reduced levels of α3 protein expression. Importantly, the similar reduction in α3 mRNA between α5 and β4 KO mice, along with α5 mRNA reduction in β4 KO mouse hypothalamus, implies that the phenotypes observed in this study are not confounded by differential changes in redundant subunit expression. This underscores the exclusive role for β4-nAChRs in driving nicotine-induced weight loss. This finding substantiates previous work focusing on β4 knockdown in the ARC (14,32). α7- and β2-nAChRs have been implicated previously in homeostatic food intake regulation (47–49), and here we show a role for α5 and β4 subunits in food reward. However, in terms of pharmacological nicotine, the effects on weight loss appear to be exclusively governed by β4-nAChRs.
Central GLP-1 receptors are reported to act on habenular avoidance circuits to control nicotine intake (25), emphasizing neural crosstalk between nAChRs and GLP-1Rs. This encouraged us to evaluate if the full metabolic benefits of pharmacological GLP-1R agonism implicates functional α5- or β4-nAChRs. Whereas the liraglutide-induced weight loss was intact in both α5 KO and β4 KO mice, the effect of liraglutide on reversing diet-induced glucose intolerance was impaired in β4 KO mice. The implication of β4-nAChRs in glucose metabolism is supported by previous work demonstrating that pharmacological activation of α3β4-nAChRs improves peripheral insulin sensitivity and reverses diet-induced glucose intolerance in mice (33,50). Together, these findings encourage further investigations into nAChRs, and in particular β4-nAChRs, in physiological and pharmacological regulation of glucose metabolism. It should be of particular interest to investigate metabolic benefits of coordinated pharmacological agonism at both β4-nAChRs and GLP-1R as well as to evaluate if variations in the CHRNA5-CHRNA3-CHRNB4 gene region influence responsiveness to long-acting GLP-1R agonists in humans.
In summary, we report a divergent role of α5- and β4-nAChRs in food reward. More work is needed to decipher the mechanistic underpinnings by which the cholinergic system governs food motivated behaviors and how this integrates with canonical energy homeostatic signals. Smoking cessation leads to weight gain and short-term risk of type 2 diabetes. Importantly, the negative influence on metabolic parameters is surpassed by the benefits of quitting smoking through reduction of cardiovascular and all-cause mortality (51). The question is whether or not the metabolic benefits of nAChR targeting can be harnessed without compromising safety. The results presented here substantiate the importance of the β4 subunit in energy metabolism and encourage further work evaluating selective targeting of β4-nAChRs for treatment of metabolic disease.
Acknowledgments
We thank the members of the Clemmensen Group for valuable input.
Glossary
Abbreviations
- ANOVA
analysis of variance
- ARC
arcuate nucleus
- AUC
area under curve
- BMI
body mass index
- DIO
Diet induced obese
- GLP-1R
glucagon-like peptide-1 receptor
- HFHS
high-fat, high-sucrose
- ipGTT
intraperitoneal glucose tolerance test
- KO
knockout
- nAChR
nicotinic acetylcholine receptor
- POMC
pro-opiomelanocortin
- qPCR
quantitative polymerase chain reaction
- WT
wild type
Contributor Information
Alberte Wollesen Breum, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Sarah Falk, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Charlotte Sashi Aier Svendsen, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Trine Sand Nicolaisen, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; The August Krogh Section for Molecular Physiology, Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen, Copenhagen, Denmark.
Cecilie Vad Mathiesen, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Uwe Maskos, Institut Pasteur, Université de Paris, Integrative Neurobiology of Cholinergic Systems, CNRS UMR 3571, Paris, France.
Christoffer Clemmensen, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Funding
This work was supported by research grants from the Lundbeck Foundation (Fellowship R238-2016-2859) and the Novo Nordisk Foundation (grant number NNF17OC0026114). Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark, and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (grant number NNF18CC0034900).
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
All data generated and analyzed are included in this article or in the data repositories; Figshare repository for supplementary Figure 1 and Supplementary Table 1, which are listed in References;
References
- 1. Audrain-Mcgovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90(1):164-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77(4):439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12(5):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med. 1986;314(2):79-82. [DOI] [PubMed] [Google Scholar]
- 5. Perkins K. Metabolic effects of cigarette smoking. J Appl Physiol. 1992;72(2):401-409 [DOI] [PubMed] [Google Scholar]
- 6. Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324(11):739-745. [DOI] [PubMed] [Google Scholar]
- 7. Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14(11):1270-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martínez De Morentin PB, Whittle AJ, Fernø J, et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes. 2012;61(4):807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz A, Bellissimo N. Nicotine and energy balance: a review examining the effect of nicotine on hormonal appetite regulation and energy expenditure. Appetite. 2021;164:105260. [DOI] [PubMed] [Google Scholar]
- 10. Grunberg N. The effects of nicotine and cigarette smoking on food consumption and taste preferences. Addict Behav. 1982;7(4):317-331. [DOI] [PubMed] [Google Scholar]
- 11. Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11(6):389-401. [DOI] [PubMed] [Google Scholar]
- 12. Jun H, Yu H, Gong J, et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med. 2018;24(6):814-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berrettini W, Yuan X, Tozzi F, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13(4):368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mineur YS, Abizaid A, Rao Y, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332(6035):1330-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lassi G, Taylor AE, Timpson NJ, et al. The CHRNA5-A3-B4 gene cluster and smoking: from discovery to therapeutics. Trends Neurosci. 2016;39:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scholze P, Koth G, Orr-Urtreger A, Huck S. Subunit composition of α5-containing nicotinic receptors in the rodent habenula. J Neurochem. 2012;121(4):551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Husson M, Harrington L, Tochon L, et al. b4-Nicotinic receptors are critically involved in reward-related behaviors and self-regulation of nicotine reinforcement. J Neurosci. 2020;40(17):3465-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen KP, Devito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M. A CHRNA5 Smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology. 2015;40(12):2813-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furberg H, Kim Y, Dackor J, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor AE, Morris RW, Fluharty ME, et al. Stratification by smoking status reveals an association of CHRNA5-A3-B4 genotype with body mass index in never Smokers. PLoS Genet. 2014;10(12):e1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Justice AE, Winkler TW, Cupples LA. Genome-wide meta-analysis of 241 258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat Commun. 2017;8(1):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elayouby KS, Ishikawa M, Dukes AJ, et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J Neurosci. 2021;41(8):1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuesta LM, Chen Z, Duncan A, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63(5):1059-1066. [DOI] [PubMed] [Google Scholar]
- 27. Xu W, Orr-Urtreger A, Nigro F, et al. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19(21):9298-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Breum AW, Falk S, Svendsen CSA, Nicolaisen TS, Maskos U, Clemmensen C. Supplementary Table 1. figshare. Dataset. Accessed March 03, 2022. 10.6084/m9.figshare.19698550.v2 [DOI] [PMC free article] [PubMed]
- 29. Zoli M, Le Novère N, Hill JA, Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous sy stems. J Neurosci. 1995;15(3 Pt 1):1912-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Novére N, Corringer PJ, Changeux JP.. The Diversity of Subunit Composition in nAChRs: Evolutionary Orgins, physiologic and Pharmacologic Consequences. 2002;53(4):447-456. [DOI] [PubMed] [Google Scholar]
- 31. Breum AW, Falk S, Svendsen CSA, Nicolaisen TS, Maskos U, Clemmensen C. Supplementary Figure 1. figshare. Figure. Accessed March 03, 2022. 10.6084/m9.figshare.19698427.v2 [DOI] [PMC free article] [PubMed]
- 32. Calarco CA, Li Z, Taylor SR, et al. Molecular and cellular characterization of nicotinic acetylcholine receptor subtypes in the arcuate nucleus of the mouse hypothalamus. Eur J Neurosci. 2018;48(1):1600-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clemmensen C, Jall S, Kleinert M, et al. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nat Commun. 2018;9(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eggan B, McCallum S. The medial habenula-interpeduncular nucleus pathway in nicotine sensitization: the role of α3β4 nicotinic acetylcholine receptors and substance P. Neurosci Nicotine. Academic Press 2019:251-258. 10.1016/B978-0-12-813035-3.00032-0 [DOI] [Google Scholar]
- 35. Grady SR, Moretti M, Zoli M, et al. Rodent habenulo–interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the α3β4 and α3β3β4 subtypes mediate acetylcholine release. J Neurosci. 2009;29(7):2272-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract. 2010;64(suppl 167):4-11. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization. WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products. 2021; WHO. https://www.who.int/publications/i/item/9789240032095 [Google Scholar]
- 38. Subramaniyan M, Dani JA. Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Ann N Y Acad Sci. 2015;1349(1):46-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173-177. [DOI] [PubMed] [Google Scholar]
- 41. Fowler CD, Tuesta L, Kenny PJ. Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl). 2013;229:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frahm S, Ślimak MA, Ferrarese L, et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70(3):522-535. [DOI] [PubMed] [Google Scholar]
- 43. Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76(Part B):533-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morel C, Fattore L, Pons S, et al. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry. 2014;19(8):930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bagdas D, Diester CM, Riley J, et al. Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology. 2019;157(Oct):107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Besson M, Forget B, Correia C, Blanco R, Maskos U. Profound alteration in reward processing due to a human polymorphism in CHRNA5: a role in alcohol dependence and feeding behavior. Neuropsychopharmacology. 2019;44(11):1906-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dezfuli G, Kellar K, Dretchen K, Tizabi Y, Sahibzada N, Gillis R. Evidence for the role of β2* nAChR desensitization in regulating body weight in obese mice. Neuropharmacology. 2016;110(Pt A):165-174. [DOI] [PubMed] [Google Scholar]
- 48. Marrero MB, Lucas R, Salet C, et al. An α7 Nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332(1):173-180. [DOI] [PubMed] [Google Scholar]
- 49. Somm E, Guérardel A, Maouche K, et al. Concomitant alpha7 and beta2 nicotinic AChR subunit deficiency leads to impaired energy homeostasis and increased physical activity in mice. Mol Genet Metab. 2014;112(1):64-72. [DOI] [PubMed] [Google Scholar]
- 50. Jall S, De Angelis M, Lundsgaard A, et al. Pharmacological targeting of 34 nicotinic receptors improves peripheral insulin sensitivity in mice with diet-induced obesity. Diabetologia. 2020;63:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu Y, Zong G, Liu G, et al. smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379(7):623-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed are included in this article or in the data repositories; Figshare repository for supplementary Figure 1 and Supplementary Table 1, which are listed in References;