Abstract
As one of the prevalent posttranscriptional modifications of RNA, N7-methylguanosine (m7G) plays essential roles in RNA processing, metabolism, and function, mainly regulated by the methyltransferase-like 1 (METTL1) and WD repeat domain 4 (WDR4) complex. Emerging evidence suggests that the METTL1/WDR4 complex promoted or inhibited the processes of many tumors, including head and neck, lung, liver, colon, bladder cancer, and teratoma, dependent on close m7G methylation modification of tRNA or microRNA (miRNA). Therefore, METTL1 and m7G modification can be used as biomarkers or potential intervention targets, providing new possibilities for early diagnosis and treatment of tumors. This review will mainly focus on the mechanisms of METTL1/WDR4 via m7G in tumorigenesis and the corresponding detection methods.
Keywords: N7-methylguanosine, METTL1, WDR4, tumor, tRNA
Graphical abstract

The METTL1/WDR4 complex regulates N7-methylguanosine (m7G) essentially in RNA fate, jointly promoting or inhibiting the processes of many tumors, which offers new insight into the mechanisms during tumorigenesis. The m7G modifications of tRNA and miRNA by METTL1/WDR4 will provide novel biomarkers or intervention targets for the early diagnosis and treatment of tumors.
Introduction
RNA modifications affect all of the RNA processes, including splicing, stability, and localization.1 Currently, there are more than 160 kinds of distinct RNA modifications,2 of which N7-methylguanosine (m7G) has existed in various species. m7G is positively charged and produced by the addition of a methyl group at position N7 of ribo-guanosine,3 which is the most ubiquitous mRNA cap modification4,5 and is also present in internal mRNA, microRNA (miRNA), tRNA and rRNA.6, 7, 8, 9 As one of the most prevalent posttranscriptional modifications of RNA, m7G functions in regulating gene expression and has critical roles in RNA processing, metabolism, and function,10 mainly modified by the methyltransferase-like 1 (METTL1) and WD repeat domain 4 (WDR4) complex.11
Emerging evidence has recently suggested that the METTL1/WDR4 complex regulates the initiation and progression of various tumors, tightly dependent on m7G methylation modification, which may be the new candidate target for the prevention and treatment of tumors.12 METTL1 and m7G can be used as biomarkers or potential intervention targets, providing new possibilities for early diagnosis and treatment of tumors. This review will focus on the detailed mechanisms of METTL1/WDR4 regulation, the association with m7G in tumors, and the corresponding detection methods.
m7G modification, the METTL1/WDR4 complex, and other methyltransferases
m7G cap modification and its function
m7G cap modifications have existed in nearly all eukaryotic cells and viral mRNAs.5 It is installed at the 5′ cap during transcription initiation, forming the first co-transcriptional modification of RNA polymerase II transcribed RNA.13 Capping of the 5′ end for nascent RNA is presented in eukaryotic cells and most viruses with three sequentially catalytic steps, including removal of the gamma phosphate by RNA triphosphatase, the addition of GMP from GTP by RNA guanylyl-transferase via a phosphoramide-linked GMP-enzyme intermediate, and N7 methylation of the added GMP by RNA cap methyltransferase.14 And then, three types of cap structures have been formed at different levels of methylation, containing caps 0, I, and II separately. The RNA cap methyltransferase adds a methyl group to N7 amine of the guanosine cap to form m7GPPPN as a cap 0 structure.15 m7GPPPN is methylated on the ribose 2′-hydroxyl (2′-O) of the first nucleoside by RNA 2′-O-ribose methyltransferase to produce m7GPPPNm as cap I. Further methylation of cap I at the ribose 2′-O position of the second nucleoside results in the formation of m7G-PPPNmNm as cap II.16 The RNA cap methyltransferase in mammalians is known as RNA guanine-7 methyltransferase (RNMT).
The m7G cap is necessarily required for efficient pre-mRNA splicing,17 recruiting, and binding to the nuclear cap-binding complex (CBC) consisting of CBP80 and CBP20 and then co-transcribing together with a CBP80/20 heterodimer, which orchestrates processes such as spliceosome assembly, 3′ end processing, RNA export, miRNA biogenesis, and nonsense-mediated decay.18 Moreover, once exported into the cytoplasm, m7G-capped mRNAs recruit a second m7G CBC, composed of eukaryotic translation initiation factors such as eukaryotic translation initiation factor 4E (eIF4E), for the steady-state rounds of translation.15 Besides, m7G is not only restricted to the caps of mRNAs but also occurred internally in mRNAs. METTL1/WDR4 mediates m7G methylation to install in the inner mRNAs at the 5′ UTR region and in AG-rich contexts.8, 9, 10 Thus, the translation efficiency of internal m7G-modified mRNAs is enhanced compared with that of unmodified ones.10
Besides, m7G also has modified sites in miRNA at G-rich regions, modulated by RNA methyltransferase of the METTL1/WDR4 complex.19 m7G methylation affects non-canonical base pairing in primary miRNA (pri-miRNA), changing the stability of the secondary structure G-quadruplex. The m7G modification destabilizes G-quadruplexes, promoting the procession efficiency of the pri-miRNA transcript into pre-miRNA and matured miRNA.19
Meanwhile, m7G widely occurs in the tRNA variable loop of eubacteria, eukaryotes, and a few archaea,20,21 most of which are frequently located at position 46 in its variable region, forming a tertiary base pair with C13-G22 in the three-dimensional core to stabilize the structure of tRNA.20,22 The m7G tRNA modification is modulated by the METTL1/WDR4 complex in mammalians, essentially, for proper expression under normal growth conditions.23 In addition, the m7G tRNA modification also regulates the translation process of mRNA and ribosome biogenesis.11,24
METTL1/WDR4 complex and other methyltransferases associated with m7G
METTL1 serves as the m7G catalytic enzyme, while WDR4 plays a stabilizing role in the complex,9,24,25 which are required for the introduction of the m7G at position 46 of tRNA and the appropriate translation.26 METTL1 mapping to 12q1326 is transcribed in a large variety of organs and tissues and regulated by protein kinase B (Akt) and ribosomal S6 kinase (RSK) under growth-factor stimulation,27 playing important roles in self-renewal and differentiation of embryonic stem cells and cancer.24,28, 29, 30 WDR4 is a candidate gene for some of the Down syndrome phenotypes with mental retardation, located at human chromosome 21q22.3,31 serving as an important supporting role of METTL1 to be essential for m7G modification on tRNA. The depletion of WDR4 would obviously decrease METTL1 expression, suggesting that WDR4 was indispensable for maintaining normal METTL1 protein levels and the function of the METTL1/WDR4 complex.32 The mutation in WDR4 would cause a distinct form of microcephalic primordial dwarfism characterized by facial dysmorphism, brain malformation, and severe encephalopathy with seizures, of which the potential mechanisms might relate to the reduced level of tRNA m7G modification.33, 34, 35, 36 The reduction of m7G tRNA levels by depletion of METTL1 or WDR4 affected tRNA function, increasing ribosomes pausing at m7G tRNA-dependent codons and declining the expression of genes associated with a wide range of biological functions. Those genes were selectively enriched in abnormality of forebrain morphology, cerebrum, and skull size, which was consistent with microcephalic primordial dwarfism described in WDR4-mutated patients.24 Moreover, m7G tRNA was involved in human stem cell renewal and differentiation by affecting the translation of the cell-cycle genes and multipotent transcription factor translation in the same codon-dependent manner.24,29 Therefore, m7G tRNA modification is widespread in affecting proper expression, essentially for normal biological functions in mammalian cells.
m7G is conserved in 18S rRNA at G1639 of human eukaryotic cells and mediated by metastasis-related methyltransferase 1 (MERM1), which is also identified as Williams-Beuren syndrome chromosome region 22 (WBSCR22). The methyltransferase WBSCR22 partners with its metabolic stabilizer tRNA methyltransferase activator subunit 11-2 (TRMT112) and has two important functions in the biogenesis of small ribosomal subunits in human cells: efficient processing of nuclear 18S rRNA precursors and nuclear export of pre-40S ribosomal subunits. Ribosome biogenesis requires the presence of the WBSCR22/TRMT112 complex rather than its m7G-modifying catalytic activity, but the function of the 18S rRNA m7G methylation in ribosome biogenesis and translation needs to be understood further.37,38
Therefore, m7G methyltransferases have contained RNMT, METTL1/WDR4, and WBSCR22/TRMT122 in humans so far. RNMT mediated m7G cap modification and increased mRNA stability. METTL1/WDR4 controlled interior mRNAs, miRNAs, and tRNA m7G modifications to regulate mRNA translation, while WBSCR22/TRMT122 regulated rRNA m7G modification to conduct a potential effect on ribosomal biogenesis.
Different RNA modifications by specific m7G methyltransferases should be related to their conserved domains. RNMT, METTL1, and WBSCR22 contain the conserved domain of the S-adenosyl-L-methionine (AdoMet) methyltransferase, which plays a major role in the methylation reaction, but their binding motifs were slightly different. The AdoMet binding motifs are VL(D/E)LGCGKG on RNMT, DIGCGYGGLLVELSPLFPDTLILGLEIR on METTL1, and MAGRALELLYLPENKPCYLLDIGCG on WBSCR22.4,26,39 Besides, different methyltransferases also have their own special structures and functions. RNMT has an N-terminal domain, negatively regulating the methyltransferase activity and mediating recruitment to transcription initiation sites, which is necessary for transcript expression, translation, and cell proliferation.40 Meanwhile, the RNMT-activating mini protein, as an activating subunit of RNMT, stabilizes the structure and ensures optimal positioning of the RNMT lobe and its adjacent α-helix hinge in the active sites.41 The RNA substrates of METTL1/WDR4 could have clues from the yeast homologs Trm8/Trm82. The major recognition sites of Trm8/Trm82 were the D- and T-stem structures of tRNA, and the Py48 sequence in the variable region was required for efficient methylation.42 In terms of WBSCR22, there has been no idea to identify their binding sites or substrates on precursor ribosomes at present, but m7G synthesis was a late event that occurred specifically in small subunits, whereas WBSCR22/TRMT112 association with pre-ribosomes was an early step on nascent nucleolar transcripts.38
Thus, the differences among these enzymes mentioned above have constituted different domains and specific binding sites, supporting m7G methyltransferases to recognize special RNA substrates in the corresponding biological stages. Different kinds of m7G methyltransferases participate in protein synthesis via m7G modification, as shown in Figure 1.
Figure 1.
Diverse m7G sites and bio-effects mediated by RNA methyltransferases
The m7G modification presents in 5′ cap of mRNA, 5′ UTR, and AG-rich regions of internal mRNA, G-rich regions of miRNA, position 46 of tRNA, and G1639 of 18s rRNA in humans. The bio-effects are mediated by different RNA methyltransferases, including RNMT, the METTL1/WDR4 complex, and the WBSCR22/TRMT112 complex, acting on mRNA stability, translation, and ribosomal biogenesis.
Overall, m7G has critical roles in RNA processing, metabolism, and function, whose abnormal changes cause cellular pathological features. The METTL1/WDR4 complex regulates the modification abundance of m7G, affecting the occurrence and progression of diseases, including tumors.
Roles of METTL1/WDR4 via m7G in tumors
Subsequently, we focused on the one of the m7G methyltransferases, METTL1/WDR4 complex, which is significantly associated with the initiation, progression, and prognosis of tumors relying on the changes in the m7G modification level.
Head and neck cancer
Otorhinolaryngology, oral, maxillofacial, and neck cancer belong to head and neck cancer. Head and neck squamous cell carcinoma was the most common malignancy in the head and neck, developed from the mucosal epithelium in the oral cavity, pharynx, and larynx.43 The upregulation of the METTL1/WDR4 complex would increase m7G modification of tRNA, activating the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin complex (mTORC) signaling pathway in head and neck squamous cell carcinoma and the regulatory associated protein of mTOR complex 1 (RPTOR)/unc-51 like autophagy activating kinase 1 (ULK1)/autophagy axis in esophageal squamous cell carcinoma via a codon-dependent manner decoded by m7G-tRNA, contributing to tumorigenesis.44,45
Nasopharyngeal carcinoma had a high incidence in East and Southeast Asia, and terminal patients had a poorer prognosis.46 High-level METTL1 selectively promoted mRNA levels depending on m7G-tRNA codons and activated the Wnt/β-catenin pathway and increased the cyclin D1 level in nasopharyngeal carcinoma, promoting the proliferation and migration of cancer cells.47
Therefore, the METTL1/WDR4 complex can increase the m7G modification level of tRNA via a codon-dependent manner decoded by m7G tRNA to promote the translation of cyclin and associated oncogenes, accelerating the occurrence and development of head and neck cancer.
Lung cancer
As one of the most common malignant tumors, lung cancer had an overexpressed level of METTL1 and WDR4 to promote the proliferation, migration, and invasion capacities of cancer cells.32,48 Just like head and neck squamous cell carcinoma (HNSCC), the METTL1/WDR4 complex played a carcinogenic role via a codon-dependent manner decoded by m7G tRNA, selectively promoting the translation of oncogenes and modulating the processes of cell-cycle-related mRNAs including cyclin D3 and cyclin E1 in lung cancer.32
However, there was a study that found that the level of m7G modification had not been dramatically affected in tRNAs after reducing the level of METTL1.19 It pointed out that METTL1 inhibited the progression of lung cancer. The oncogene high-mobility group AT-hook 2 (HMGA2) promoted epithelial-to-mesenchymal transition and accelerated cancer progression with poor survival of cancer.49,50 The pri-let-7e miRNA was directly methylated by the METTL1/WDR4 complex, promoting the processes of its transcript into precursor pre-let-7e miRNA and matured let-7e miRNA, which regulated HMGA2 negatively and then inhibited the proliferation of A549 cells.
Liver cancer
Hepatocellular carcinoma (HCC), one of the most common malignant tumors, is one of the leading causes of cancer deaths worldwide,51 with upregulated METTL1 and WDR4. METTL1/WDR4-mediated m7G tRNA modification could raise the translation of cyclin A2, epidermal growth factor receptor (EGFR), and vascular endothelial growth factor A (VEGFA), which were decoded by m7G tRNA in a codon-dependent manner and then activated Akt and mitogen-activated protein kinase (MAPK) in EFGR and VEGFA signaling pathways, promoting cell proliferation and migration of HCC.52
Intrahepatic cholangiocarcinoma (ICC) currently accounts for 10%–20% of primary hepatic tumors.53 m7G tRNA modification and the METTL1/WDR4 complex were significantly upregulated in ICC and associated with a poor prognosis. m7G tRNA modification could selectively promote the translation of oncogenic transcripts, including EGFR, VEGFA, and MAPK signaling pathways and the corresponding downstream targets, as well as cell-cycle-related mRNAs of cyclin A2, cyclin D2, cyclin-dependent kinases 6 (CDK6), CDK8, and EFGR via a codon-dependent manner decoded by m7G tRNA.54
METTL1 and WDR4 promote tumor cell growth by stimulating the translation of mRNAs related to the cell cycle and activating oncogenic signaling pathways such as EGFR in HCC and ICC. Therefore, the mechanisms of METTL1/WDR4 promoting tumorigenesis in HCC and ICC were similar. METTL1/WDR4-complex-mediated m7G tRNA modification selectively promotes the translation of oncogenic transcripts and the relative genes via a codon-dependent manner decoded by m7G tRNA, affecting the occurrence and development of liver tumors.
Colon cancer
Colon cancer is also a common malignant tumor of the human digestive tract with high morbidity and mortality, seriously degrading the quality of human life.55 let-7e miRNA regulated the expression of HMGA2 negatively and then inhibited the proliferation, migration, and invasion of colon cancer cells.56 In the meantime, the METTL1/WDR4 complex promoted the let-7e miRNA process in an m7G-dependent manner and overexpressed METTL1-decreased HMGA2 by upregulating let-7e miRNA via m7G, inhibiting the progression of colon cancer.19,57
Bladder cancer
Bladder cancer, a grave urogenital malignancy worldwide, was one of the most frequent malignancies in males of developed countries.58 Overexpression of METTL1 would mediate m7G tRNA modification, upregulating levels of EGFR and EGF-containing fibulin extracellular matrix protein 1 (EFEMP1) in an m7G tRNA codon-dependent manner to activate the EFGR pathway and promote bladder tumorigenesis.59
Teratoma
Downregulation of m7G tRNA methylation enhanced teratoma formation in vivo by promoting human induced pluripotent stem cell proliferation and angiogenesis in nude mice.29 Knockdown of Mettl1 decreased the mRNA translation of stem cell transcription factors recombinant octamer binding transcription factor 4 (Oct4), Nanog, and sex-determining region Y (Sox2) by downregulating m7G tRNA, thus increasing the differentiation, inhibiting the cell-cycle signaling pathway, and impairing self-renewal of human induced pluripotent stem cells, which enhanced teratoma formation in mice.24,29,60, 61, 62
The functions of METTL1/WDR4 in tumors via m7G methylation are shown in Table 1.
Table 1.
METTL1 regulated tumor progression by confirmed association with m7G
| Tumors | Role in tumors | m7G target | Mechanisms | Reference |
|---|---|---|---|---|
| Head and neck cancer | accelerator | tRNA | activated the PI3K/Akt/mTORC signaling pathway, RPTOR/ULK1/autophagy axis, and Wnt/β-catenin pathway, as well as promoted the cyclin D1 translation | 44,45 |
| Lung cancer | accelerator | tRNA | promoted translation of cell-cycle genes including cyclin D3 and cyclin E1 | 32 |
| suppressor | miRNA | promoted transcription of let-7e miRNA and inhibited expression of HMGA2 | 19 | |
| Liver cancer | accelerator | tRNA | promoted the translation of cell-cycle genes including cyclin A2, cyclin D2, CDK6, and CDK8 as well as activated EGFR, VEGFA, and MAPK signaling pathways | 52,54 |
| Colon cancer | suppressor | miRNA | promoted transcription of let-7e miRNA and inhibited expression of HMGA2 | 56 |
| Bladder cancer | accelerator | tRNA | promoted the translation of EGFR and EFEP1 and activated EGFR pathway | 59 |
| Teratoma | suppressor | tRNA | promoted translation of pluripotency genes, including Oct4, Nanog, and Sox2, as well as activated cell-cycle signaling pathway | 29 |
Detection methods of m7G modification
To test the m7G modification level, the most common methods currently include quantitative detection and high-throughput sequencing. The quantitative detection method could get the overall m7G level of RNA, including high-performance liquid chromatography and coupling of liquid chromatography to mass spectrometry,8,63 while the high-throughput sequencing method could identify the exact m7G sites based on antibodies or chemicals.
Methylated RNA immunoprecipitation sequencing (MeRIP-seq) was used for the whole-transcriptome analysis of m7G by immunoprecipitation of the specific antibody.8 With a high resolution of m7G localization on fragmented RNA, individual-nucleotide-resolution cross-linking and immunoprecipitation with sequencing (miCLIP-seq) was adopted to detect m7G at the single-base resolution induced by an antibody cross-linked to ultraviolet.10
However, these antibody-based m7G immunoprecipitation sequencing techniques would have the issue of false positives because of inevitable non-specific binding.64 So chemical-based sequencing technologies relying on borohydride reduction were applied, including AlkAniline-seq,65,66 m7G mutational profiling sequencing (m7G-MaP-seq),67 and tRNA reduction and cleavage sequencing (TRAC-seq),68 which not only could identify m7G modification at the single-base resolution but also were more specific than the antibody-based sequencing technique.65,67, 68, 69 Moreover, chemical-based sequencing technologies have higher specificity and better resolution power than those that are antibody based.
Conclusion
RNA methylation contributes to revealing the underlying mechanisms of many aspects of tumors, involving initiation, development, invasion, infiltration, and so on. m7G methylation is a double-edged sword to tumors, needing appropriate level boundaries. The excessive m7G modification of certain genes leads to the acceleration of tumor development, whereas deficient m7G modification might also accelerate tumor progression. METTL1/WDR4-complex-mediated m7G acts on different RNA targets, affecting the processes of tumorigenesis.
METTL1/WDR4-complex-mediated m7G tRNA methylation selectively promotes the translation of certain cyclin and oncogenic transcripts and its downstream pathway-related mRNAs, regulating cell proliferation and apoptosis with affluent homologous codons of m7G tRNAs correspondingly.70 The upregulation of m7G abundance would cause the reduction of ribosome pausing and the elimination of ribosome collision-mediated translation inhibition.71 m7G modification targets are Arg-TCT tRNAs responsible for decoding AGA codons, promoting the stabilization and increasing the translation of mRNAs of enriched AGA, including cell-cycle-related genes.72
However, abnormal m7G modification will bring about different tumor outcomes to promote the formation and progression of head and neck, and liver as well as bladder cancer while inhibiting teratoma by METTL1/WDR4-mediated m7G tRNA changes. Differentially targeted cells might also be one of the intrinsic reasons. For head and neck, liver, and bladder cancer, the epigenetic mutations occur in somatic cells, while it was reported in human induced pluripotent stem cells to occur in teratoma with higher pluripotency levels.
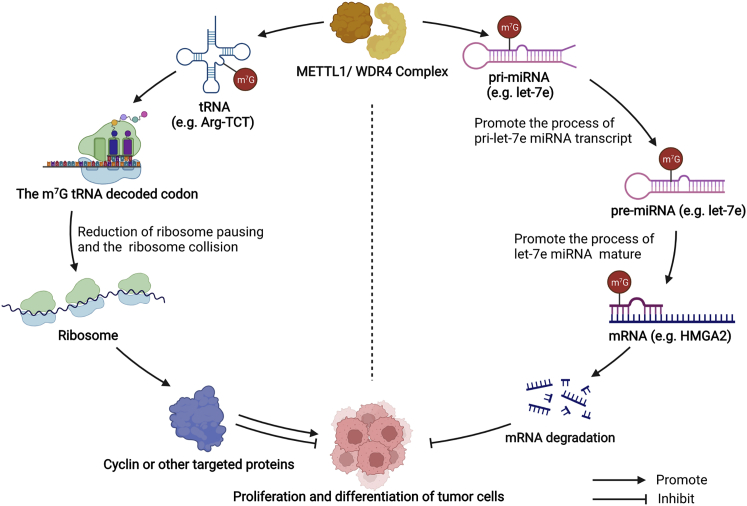
m7G miRNA methylation shows the role of the suppressor in tumors. m7G methylation promotes the process of pri-miRNA transcript into pre-miRNA and accelerates the maturation efficiency of miRNA, inhibiting the expression of targeted genes in colon and lung cancers. The roles of m7G modification modulated by the METTL1/WDR4 complex in tumorigenesis are concluded in Figure 2.
Figure 2.
Mechanisms of METTL1/WDR4-m7G modification affecting tumorigenesis
METTL1/WDR4-complex-mediated m7G regulates the processes of the tumor by targeting tRNA or miRNA. The m7G-modified tRNA causes the reduction of ribosome pausing and the elimination of ribosome collision-mediated translation inhibition, selectively promoting the translation of certain cell-cycle regulatory mRNAs, which are enriched in corresponding m7G-tRNA cognate codons, regulating the proliferation and differentiation of tumor cells. m7G modifies pri-miRNA directly to mature miRNA efficiency, inhibits the expression of oncogene, and then inhibits the proliferation and differentiation of tumor cells.
Perspectives and challenges
The critical role of METTL1 and m7G RNA methylation in tumor initiation and progression provides new possibilities for early diagnosis and treatment. A remarkable upregulation of METTL1 or m7G might suggest the tumor progression in head and neck, liver, or bladder cancer, but downregulation would connect with teratoma or colon cancer. Cells need normal expressions of METTL1 and m7G, and once they are imbalanced, the associated tumors would more likely occur and proceed.
Additionally, METTL1-mediated m7G is also crucial for tumor chemoresistance. The regulators or inhibitors of m7G methylation may have prospects for tumor treatment. Cisplatin was an ordinary chemotherapeutic drug for colon cancer treatment clinically, but its continuous chemotherapy would induce the drug resistance of cancer cells, and overexpressed METTL1 could increase the chemosensitivity of colon cancer cells to cisplatin by regulating the miR-149-3p/S100A4/p53 axis.73 Besides, 5-fluorouracil (5-FU) was a pyrimidine analog, most widely used as a chemotherapeutic agent for varieties of solid cancers. Interfering with m7G tRNA methylation by knocking down METTL1 in HeLa cells could potentiate the sensitivity to 5-FU, providing a new idea to improve 5-FU chemotherapy effects on cancer.74
However, there are still numerous challenges in the field of m7G RNA methylation research. The molecular mechanism is bidirectional of METTL1/WDR4-complex-mediated m7G during tumor progression. Especially in lung cancer, m7G-tRNA promoted progression, while m7G-miRNA showed the opposite effect, indicating different m7G modification targets had differential results sometimes.
Furthermore, whether m7G methylation exists in internal mRNA and miRNA or not still needs to be studied further because of controversial results from different current detection methods. Some studies reported no evidence for internal m7G modifications presented in other small RNAs and mRNAs using chemical-based sequencing technologies,65,67,69 while other research showed the m7G methylation was identified to exist in internal mRNA and miRNA using mostly antibody-based sequencing technologies.8,10,19 Additionally, another study using the chemical-based technique identified m7G existing in miRNAs, but it doubted the observation of m7G-dependent enrichment because of the sequencing protocol rather than special pull-down.19,69 Thus, more reliable methods are eagerly needed to prove the exact existence of m7G modification in internal mRNA and miRNA, with good repeatability and verification.
Besides, the links between m7G RNA modification and other tumors are still waiting to be discovered and verified apart from the mentioned tumors above. Some studies revealed that METTL1 contributed to the initiation and progression of gastric cancer and glioma with poor prognosis, offering a good foundation for relations to the m7G methylation.75, 76, 77 The associations between the METTL1/WDR4 complex and m7G modification in tumors need to be explored further to obtain more interesting discoveries in the future.
Moreover, m7G also has a crucial role in vasculogenesis, possibly with the occurrence of hemangioma.29,78 A critical angiogenic factor for angiogenesis, VEGF was highly expressed in hemangioma, contributing to proliferation and abnormal angiogenesis of vascular endothelial cells because METTL1 promoted its m7G modification by upregulating the mRNA translation.79,80
Therefore, METTL1 and m7G have dual roles of tumor promotion and suppression. METTL1 would act as an oncogene in cancers of the head and neck and liver as well as bladder but as a tumor suppressor in colon cancer and teratoma, which may also induce the formation of other types of cancers or other diseases via specific m7G regulation accordingly. The clinical application of m7G in human tumors needs to be assessed further for targeted therapy and precise intervention in future studies.
Acknowledgments
This work was funded by the Natural Science Foundation of Guangdong Province of China (2021A1515011220), the Administration of Traditional Chinese Medicine of Guangdong Province of China (20211008), Top Young Talents of Guangdong Hundreds of Millions of Projects (87316004), Outstanding Young Talent of Double Hundred Talents Plan in Jinan University, and the National Natural Science Foundation of China (81473014).
Author contributions
W.C. finished the manuscript, A.G. updated the literature search and revision, H.L. reviewed and edited the revision, and W.Z. completed critical revisions and proofread the manuscript. All authors have read and approved the final manuscript.
Declaration of interests
The authors declare that they do not have any conflicts of interest related to this study. This manuscript has been read and approved by all the authors and has not been submitted to or is not under consideration for publication elsewhere.
Contributor Information
Hui Lin, Email: linhui@gdph.org.cn.
Wenjuan Zhang, Email: zwj2080@126.com.
References
- 1.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadumuri R.V., Janga S.C. Epitranscriptomic code and its alterations in human disease. Trends. Mol. Med. 2018;24:886–903. doi: 10.1016/j.molmed.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Chen J., Ma J., Liu H. HN-CNN: a heterogeneous network based on convolutional neural network for m(7) G site disease association prediction. Front. Genet. 2021;12:655284. doi: 10.3389/fgene.2021.655284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowling V.H. Regulation of mRNA cap methylation. Biochem. J. 2009;425:295–302. doi: 10.1042/bj20091352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuichi Y. Discovery of m(7)G-cap in eukaryotic mRNAs. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015;91:394–409. doi: 10.2183/pjab.91.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauss D.H., Grüter F., Sprinzl M. Compilation of tRNA sequences. Nucleic. Acids. Res. 1979;6:419. doi: 10.1093/nar/6.1.419-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motorin Y., Helm M. RNA nucleotide methylation. Wiley Interdiscip. Rev. RNA. 2011;2:611–631. doi: 10.1002/wrna.79. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L.S., Liu C., Ma H., Dai Q., Sun H.L., Luo G., Zhang Z., Zhang L., Hu L., Dong X., He C. Transcriptome-wide mapping of internal N(7)-methylguanosine methylome in mammalian mRNA. Mol. Cell. 2019;74:1304–1316.e8. doi: 10.1016/j.molcel.2019.03.036. e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulias K., Greer E.L. Put the pedal to the METTL1: adding internal m7G increases mRNA translation efficiency and augments miRNA processing. Mol. Cell. 2019;74:1105–1107. doi: 10.1016/j.molcel.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Malbec L., Zhang T., Chen Y.S., Zhang Y., Sun B.F., Shi B.Y., Zhao Y.L., Yang Y., Yang Y.G. Dynamic methylome of internal mRNA N(7)-methylguanosine and its regulatory role in translation. Cell. Res. 2019;29:927–941. doi: 10.1038/s41422-019-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandrov A., Martzen M.R., Phizicky E.M. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8 doi: 10.1017/s1355838202024019. S1355838202024019-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbieri I., Kouzarides T. Role of RNA modifications in cancer. Nat. Rev. Cancer. 2020;20:303–322. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 13.Moteki S., Price D. Functional coupling of capping and transcription of mRNA. Mol. Cell. 2002;10:599–609. doi: 10.1016/s1097-2765(02)00660-3. [DOI] [PubMed] [Google Scholar]
- 14.Chu C., Das K., Tyminski J.R., Bauman J.D., Guan R., Qiu W., Montelione G.T., Arnold E., Shatkin A.J. Structure of the guanylyltransferase domain of human mRNA capping enzyme. Proc. Natl. Acad. Sci. U S A. 2011;108:10104–10108. doi: 10.1073/pnas.1106610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan A., Robb G.B., Chan S.H. mRNA capping: biological functions and applications. Nucleic. Acids. Res. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh A., Lima C.D. Enzymology of RNA cap synthesis. Wiley. Interdiscip. Rev. RNA. 2010;1:152–172. doi: 10.1002/wrna.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fresco L.D., Buratowski S. Conditional mutants of the yeast mRNA capping enzyme show that the cap enhances, but is not required for, mRNA splicing. RNA. 1996;2:584–596. https://rnajournal.cshlp.org/content/2/6/584.long [PMC free article] [PubMed] [Google Scholar]
- 18.Gonatopoulos-Pournatzis T., Cowling V.H. Cap-binding complex (CBC) Biochem. J. 2014;457:231–242. doi: 10.1042/bj20131214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandolfini L., Barbieri I., Bannister A.J., Hendrick A., Andrews B., Webster N., Murat P., Mach P., Brandi R., Robson S.C., et al. METTL1 promotes let-7 MicroRNA processing via m7G methylation. Mol. Cell. 2019;74:1278–1290.e9. doi: 10.1016/j.molcel.2019.03.040. e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic. Acids. Res. 2009;37 doi: 10.1093/nar/gkn772. D159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmonds C.G., Crain P.F., Gupta R., Hashizume T., Hocart C.H., Kowalak J.A., Pomerantz S.C., Stetter K.O., McCloskey J.A. Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria) J. Bacteriol. 1991;173:3138–3148. doi: 10.1128/jb.173.10.3138-3148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.H., Sussman J.L., Suddath F.L., Quigley G.J., McPherson A., Wang A.H.J., Seeman N.C., Rich A. The general structure of transfer RNA molecules. Proc. Natl. Acad. Sci. U S A. 1974;71:4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomikawa C. 7-Methylguanosine modifications in transfer RNA (tRNA) Int. J. Mol. Sci. 2018;19:4080. doi: 10.3390/ijms19124080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S., Liu Q., Lelyveld V.S., Choe J., Szostak J.W., Gregory R.I. Mettl1/Wdr4-Mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018;71:244–255. doi: 10.1016/j.molcel.2018.06.001. e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandrov A., Grayhack E.J., Phizicky E.M. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahr A., Hankeln T., Fiedler T., Hegemann J., Schmidt E.R. Molecular analysis of METTL1, a novel human methyltransferase-like gene with a high degree of phylogenetic conservation. Genomics. 1999;57:424–428. doi: 10.1006/geno.1999.5780. [DOI] [PubMed] [Google Scholar]
- 27.Cartlidge R.A., Knebel A., Peggie M., Alexandrov A., Phizicky E.M., Cohen P. The tRNA methylase METTL1 is phosphorylated and inactivated by PKB and RSK in vitro and in cells. EMBO. J. 2005;24:1696–1705. doi: 10.1038/sj.emboj.7600648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Q.-H., Zhang M.-F., Zeng J.-S., Luo R.-G., Wen Y., Chen J., Gan L.-G., Xiong J.-P. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J. Mol. Med. 2019;97:1535–1545. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y., Zhou Z., Ji W., Lin S., Wang M. METTL1-mediated m(7)G methylation maintains pluripotency in human stem cells and limits mesoderm differentiation and vascular development. Stem. Cell. Res. Ther. 2020;11:306. doi: 10.1186/s13287-020-01814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Na W., Fu L., Luu N., Shi Y.B. Direct activation of tRNA methyltransferase-like 1 (Mettl1) gene by thyroid hormone receptor implicates a role in adult intestinal stem cell development and proliferation during Xenopus tropicalis metamorphosis. Cell. Biosci. 2020;10:60. doi: 10.1186/s13578-020-00423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaud J., Kudoh J., Berry A., Bonne-Tamir B., Lalioti M.D., Rossier C., Shibuya K., Kawasaki K., Asakawa S., Minoshima S., et al. Isolation and characterization of a human chromosome 21q22.3 gene (WDR4) and its mouse homologue that code for a WD-repeat protein. Genomics. 2000;68:71–79. doi: 10.1006/geno.2000.6258. [DOI] [PubMed] [Google Scholar]
- 32.Ma J., Han H., Huang Y., Yang C., Zheng S., Cai T., Bi J., Huang X., Liu R., Huang L., et al. METTL1/WDR4-mediated m(7)G tRNA modifications and m(7)G codon usage promote mRNA translation and lung cancer progression. Mol. Ther. 2021;29:3422–3435. doi: 10.1016/j.ymthe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen R., Abdel-Salam G.M.H., Guy M.P., Alomar R., Abdel-Hamid M.S., Afifi H.H., Ismail S.I., Emam B.A., Phizicky E.M., Alkuraya F.S. Mutation in WDR4 impairs tRNA m(7)G46 methylation and causes a distinct form of microcephalic primordial dwarfism. Genome. Biol. 2015;16:210. doi: 10.1186/s13059-015-0779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trimouille A., Lasseaux E., Barat P., Deiller C., Drunat S., Rooryck C., Arveiler B., Lacombe D. Further delineation of the phenotype caused by biallelic variants in the WDR4 gene. Clin. Genet. 2018;93:374–377. doi: 10.1111/cge.13074. [DOI] [PubMed] [Google Scholar]
- 35.Filonava L., Torres A.G., de Pouplana L.R. A novel cause for primordial dwarfism revealed: defective tRNA modification. Genome. Biol. 2015;16:216. doi: 10.1186/s13059-015-0786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Gao Y., Yang L., Wu B., Dong X., Liu B., Lu Y., Zhou W., Wang H. Speech and language delay in a patient with WDR4 mutations. Eur. J. Med. Genet. 2018;61:468–472. doi: 10.1016/j.ejmg.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Haag S., Kretschmer J., Bohnsack M.T. WBSCR22/Merm1 is required for late nuclear pre-ribosomal RNA processing and mediates N7-methylation of G1639 in human 18S rRNA. RNA. 2015;21:180–187. doi: 10.1261/rna.047910.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zorbas C., Nicolas E., Wacheul L., Huvelle E., Heurgué-Hamard V., Lafontaine D.L.J. The human 18S rRNA base methyltransferases DIMT1L and WBSCR22-TRMT112 but not rRNA modification are required for ribosome biogenesis. Mol. Biol. Cell. 2015;26:2080–2095. doi: 10.1091/mbc.E15-02-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll A., Grzeschik K.H. Characterization of two novel genes, WBSCR20 and WBSCR22, deleted in Williams-Beuren syndrome. Cytogenet. Cell. Genet. 2001;95:20–27. doi: 10.1159/000057012. [DOI] [PubMed] [Google Scholar]
- 40.Aregger M., Cowling V.H. Human cap methyltransferase (RNMT) N-terminal non-catalytic domain mediates recruitment to transcription initiation sites. Biochem. J. 2013;455:67–73. doi: 10.1042/bj20130378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varshney D., Lombardi O., Schweikert G., Dunn S., Suska O., Cowling V.H. mRNA cap methyltransferase, RNMT-RAM, promotes RNA pol II-dependent transcription. Cell. Rep. 2018;23:1530–1542. doi: 10.1016/j.celrep.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto K., Toyooka T., Tomikawa C., Ochi A., Takano Y., Takayanagi N., Endo Y., Hori H. RNA recognition mechanism of eukaryote tRNA (m7G46) methyltransferase (Trm8-Trm82 complex) FEBS. Lett. 2007;581:1599–1604. doi: 10.1016/j.febslet.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J., Li K., Chen J., Wang X., Ling R., Cheng M., Chen Z., Chen F., He Q., Li S., et al. Aberrant translation regulated by METTL1/WDR4-mediated tRNA N7-methylguanosine modification drives head and neck squamous cell carcinoma progression. Cancer. Commun. 2022;42:223–244. doi: 10.1002/cac2.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han H., Yang C., Ma J., Zhang S., Zheng S., Ling R., Sun K., Guo S., Huang B., Liang Y., et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat. Commun. 2022;13:1478. doi: 10.1038/s41467-022-29125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su Z.Y., Siak P.Y., Leong C.O., Cheah S.C. Nasopharyngeal carcinoma and its microenvironment: past, current, and future perspectives. Front. Oncol. 2022;12:840467. doi: 10.3389/fonc.2022.840467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B., Jiang W., Huang Y., Zhang J., Yu P., Wu L., Peng H. N(7)-methylguanosine tRNA modification promotes tumorigenesis and chemoresistance through WNT/β-catenin pathway in nasopharyngeal carcinoma. Oncogene. 2022;41:2239–2253. doi: 10.1038/s41388-022-02250-9. [DOI] [PubMed] [Google Scholar]
- 48.Wang C., Wang W., Han X., Du L., Li A., Huang G. Methyltransferase-like 1 regulates lung adenocarcinoma A549 cell proliferation and autophagy via the AKT/mTORC1 signaling pathway. Oncol. Lett. 2021;21:330. doi: 10.3892/ol.2021.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Zhao Z., Xu C., Zhou Z., Zhu Z., You T. HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer. Lett. 2014;355:130–140. doi: 10.1016/j.canlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Xi X., Teng M., Zhang L., Xia L., Chen J., Cui Z. Retracted : MicroRNA 204 3p represses colon cancer cells proliferation, migration, and invasion by targeting HMGA2. J. Cell. Physiol. 2020;235:1330–1338. doi: 10.1002/jcp.29050. [DOI] [PubMed] [Google Scholar]
- 51.Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z., Zhu W., Zhu S., Sun K., Liao J., Liu H., Dai Z., Han H., Ren X., Yang Q., et al. METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clin. Transl. Med. 2021;11:e661. doi: 10.1002/ctm2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Job S., Rapoud D., Dos Santos A., Gonzalez P., Desterke C., Pascal G., Elarouci N., Ayadi M., Adam R., Azoulay D., et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai Z., Liu H., Liao J., Huang C., Ren X., Zhu W., Zhu S., Peng B., Li S., Lai J., et al. N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol. Cell. 2021;81:3339–3355.e8. doi: 10.1016/j.molcel.2021.07.003. e3338. [DOI] [PubMed] [Google Scholar]
- 55.Garza Treviño E.N., González P.D., Valencia Salgado C.I., Martinez Garza A. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer. Cell. Int. 2019;19:173. doi: 10.1186/s12935-019-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z., Liu J. MicroRNA-let-7 targets HMGA2 to regulate the proliferation, migration, and invasion of colon cancer cell HCT116. Evid. Based Complement Alternat Med. 2021;2021:1–9. doi: 10.1155/2021/2134942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Liu Y., Zhang Y., Chi Q., Wang Z., Sun B. RETRACTED: methyltransferase-like 1 (METTL1) served as a tumor suppressor in colon cancer by activating 7-methyguanosine (m7G) regulated let-7e miRNA/HMGA2 axis. Life. Sci. 2020;249:117480. doi: 10.1016/j.lfs.2020.117480. [DOI] [PubMed] [Google Scholar]
- 58.Paramanantham Y., Chung I., Bm Said N.A. The role of tumour microenvironment-driven miRNAs in the chemoresistance of muscle-invasive bladder cancer-a review. Urol. Oncol. 2022;40:133–148. doi: 10.1016/j.urolonc.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Ying X., Liu B., Yuan Z., Huang Y., Chen C., Jiang X., Zhang H., Qi D., Yang S., Lin S., et al. METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin. Transl. Med. 2021;11:e675. doi: 10.1002/ctm2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patra S.K. Roles of OCT4 in pathways of embryonic development and cancer progression. Mech. Ageing. Dev. 2020;189:111286. doi: 10.1016/j.mad.2020.111286. [DOI] [PubMed] [Google Scholar]
- 61.Pan G., Thomson J.A. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell. Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 62.Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A.A., et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell. Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 63.Chu J.M., Ye T.T., Ma C.J., Lan M.D., Liu T., Yuan B.F., Feng Y.Q. Existence of internal N7-methylguanosine modification in mRNA determined by differential enzyme treatment coupled with mass spectrometry analysis. ACS. Chem. Biol. 2018;13:3243–3250. doi: 10.1021/acschembio.7b00906. [DOI] [PubMed] [Google Scholar]
- 64.Meyer K.D. DART-seq: an antibody-free method for global m(6)A detection. Nat. Methods. 2019;16:1275–1280. doi: 10.1038/s41592-019-0570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchand V., Ayadi L., Ernst F.G.M., Hertler J., Bourguignon-Igel V., Galvanin A., Kotter A., Helm M., Lafontaine D.L.J., Motorin Y. AlkAniline-seq: profiling of m(7) G and m(3) C RNA modifications at single nucleotide resolution. Angew. Chem. Int. Ed. Engl. 2018;57:16785–16790. doi: 10.1002/anie.201810946. [DOI] [PubMed] [Google Scholar]
- 66.Marchand V., Ayadi L., Bourguignon-Igel V., Helm M., Motorin Y. AlkAniline-seq: a highly sensitive and specific method for simultaneous mapping of 7-methyl-guanosine (m(7)G) and 3-methyl-cytosine (m(3)C) in RNAs by high-throughput sequencing. Methods. Mol. Biol. 2021;2298:77–95. doi: 10.1007/978-1-0716-1374-0_5. [DOI] [PubMed] [Google Scholar]
- 67.Enroth C., Poulsen L.D., Iversen S., Kirpekar F., Albrechtsen A., Vinther J. Detection of internal N7-methylguanosine (m7G) RNA modifications by mutational profiling sequencing. Nucleic. Acids. Res. 2019;47:e126. doi: 10.1093/nar/gkz736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin S., Liu Q., Jiang Y.Z., Gregory R.I. Nucleotide resolution profiling of m(7)G tRNA modification by TRAC-Seq. Nat. Protoc. 2019;14:3220–3242. doi: 10.1038/s41596-019-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vinther J. No evidence for N7-methylation of guanosine (m(7)G) in human let-7e. Mol. Cell. 2020;79:199–200. doi: 10.1016/j.molcel.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 70.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsara O., Schneider R.J. m(7)G tRNA modification reveals new secrets in the translational regulation of cancer development. Mol. Cell. 2021;81:3243–3245. doi: 10.1016/j.molcel.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orellana E.A., Liu Q., Yankova E., Pirouz M., De Braekeleer E., Zhang W., Lim J., Aspris D., Sendinc E., Garyfallos D.A., et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell. 2021;81:3323–3338.e14. doi: 10.1016/j.molcel.2021.06.031. e3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Yang C., Zhao Y., Chi Q., Wang Z., Sun B. Overexpressed methyltransferase-like 1 (METTL1) increased chemosensitivity of colon cancer cells to cisplatin by regulating miR-149-3p/S100A4/p53 axis. Aging. 2019;11:12328–12344. doi: 10.18632/aging.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamoto M., Fujiwara M., Hori M., Okada K., Yazama F., Konishi H., Xiao Y., Qi G., Shimamoto F., Ota T., et al. tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS. Genet. 2014;10:e1004639. doi: 10.1371/journal.pgen.1004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai S., Huang Y., Liu T., Xu Z.H., Liu T., Chen L., Wang Z.W., Luo F. Development and validation of RNA binding protein-applied prediction model for gastric cancer. Aging (Albany NY) 2021;13:5539–5552. doi: 10.18632/aging.202483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou W., Li J., Lu X., Liu F., An T., Xiao X., Kuo Z.C., Wu W., He Y. Derivation and validation of a prognostic model for cancer dependency genes based on CRISPR-Cas9 in gastric adenocarcinoma. Front. Oncol. 2021;11:617289. doi: 10.3389/fonc.2021.617289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L., Yang Y., Wang Z., Xu C., Huang J., Li G. Prognostic role of METTL1 in glioma. Cancer. Cell. Int. 2021;21:633. doi: 10.1186/s12935-021-02346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng Y., Zhou Z., Lin S., Yu B. METTL1 limits differentiation and functioning of EPCs derived from human-induced pluripotent stem cells through a MAPK/ERK pathway. Biochem. Biophys. Res. Commun. 2020;527:791–798. doi: 10.1016/j.bbrc.2020.04.115. [DOI] [PubMed] [Google Scholar]
- 79.Greenberger S., Bischoff J. Pathogenesis of infantile haemangioma. Br. J. Dermatol. 2013;169:12–19. doi: 10.1111/bjd.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y., Kong L., Pei Z., Li F., Li C., Sun X., Shi B., Ge J. m7G methyltransferase METTL1 promotes post-ischemic angiogenesis via promoting VEGFA mRNA translation. Front. Cell. Dev. Biol. 2021;9:642080. doi: 10.3389/fcell.2021.642080. [DOI] [PMC free article] [PubMed] [Google Scholar]