Abstract
The effects of different carbohydrates or mixtures of carbohydrates as substrates on bacterial growth and exopolysaccharide (EPS) production were studied for the yoghurt starter culture Streptococcus thermophilus LY03. This strain produces two heteropolysaccharides with the same monomeric composition (galactose and glucose in the ratio 4:1) but with different molecular masses. Lactose and glucose were fermented by S. thermophilus LY03 only when they were used as sole energy and carbohydrate sources. Fructose was also fermented when it was applied in combination with lactose or glucose. Both the amount of EPS produced and the carbohydrate source consumption rates were clearly influenced by the type of energy and carbohydrate source used, while the EPS monomeric composition remained constant (galactose-glucose, 4:1) under all circumstances. A combination of lactose and glucose resulted in the largest amounts of EPS. Measurements of the activities of enzymes involved in EPS biosynthesis, and of those involved in sugar nucleotide biosynthesis and the Embden-Meyerhof-Parnas pathway, demonstrated that the levels of activity of α-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase are highly correlated with the amount of EPS produced. Furthermore, a weaker relationship or no relationship between the amounts of EPS and the enzymes involved in either the rhamnose nucleotide synthetic branch of the EPS biosynthesis or the pathway leading to glycolysis was observed for S. thermophilus LY03.
Food hydrocolloids play an important role in the rheological properties of food products (29, 34). Examples are plant carbohydrates such as modified starch and guar gum, animal proteins like gelatin and casein, and microbial exopolysaccharides (EPS) such as xanthan and gellan. The addition of food additives is not always allowed, so natural yoghurts and fermented milks are dependent on the EPS-producing capacities of the starter strains used. EPS are microbial polysaccharides that are secreted extracellularly and are either associated with the cell surface in the form of capsules or released into the medium in the form of slimes (11). The thermophilic yoghurt strains Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus produce heteropolysaccharides that consist of a repeating unit of neutral sugars (glucose, galactose, and rhamnose) in different ratios (1, 3, 6, 11, 13, 15, 17–21, 24, 32; G. T. Lamothe, F. Stingele, J.-R. Neeser, and B. Mollet, Abstr. Am. Soc. Microbiol. Conf. Streptococcal Genet., abstr. no. 2A-16, p. 67, 1998). Some of these polysaccharides display pseudoplastic and thixotropic properties (4, 5, 6, 13). It has been postulated that their rheological properties are influenced not only by the amount of EPS but also by its structure. Recently, the structures of the repeating units of some EPS produced by L. delbrueckii subsp. bulgaricus (21) and S. thermophilus (3, 13, 15, 24, 32) have been elucidated. However, there is still a lack of knowledge of the physiological aspects of EPS production. Although EPS production is growth associated in thermophilic lactic acid bacterium strains (11, 12), the EPS biosynthesis pathway is very complex. Contradictory results have been reported in the literature concerning the influence of the carbohydrate source present in the medium on monomeric composition and hence the structure of the EPS produced. Grobben et al. showed that the proportions of glucose and fructose as carbohydrate sources influenced both the amount and monomeric composition of the EPS produced by L. delbrueckii subsp. bulgaricus NCFB 2772 (19). This was reflected in the activities of the enzymes involved, namely, UDP-glucose pyrophosphorylase, dTDP-glucose pyrophosphorylase, and the rhamnose nucleotide synthetic enzyme system. Escalante et al. found that UDP-glucose pyrophosphorylase was correlated with EPS production in a ropy strain of S. thermophilus but not, although the enzyme was present, in a nonropy strain and that the Leloir enzyme UDP-galactose 4-epimerase was not correlated with EPS biosynthesis in any strain (14). We need more knowledge of the nutritional factors influencing the activities of the enzymes involved in the biosynthetic pathway of EPS and the regulation of the energy and carbon flows between the Embden-Meyerhof-Parnas (EMP) pathway and the biosynthesis of EPS.
The yoghurt strain S. thermophilus LY03 produces a heteropolysaccharide consisting of galactose and glucose in the ratio 4:1. Its production is growth associated, and both physical and chemical conditions have been optimized to obtain maximum EPS production (8). In this paper, we report the differences in EPS production and monomeric composition between cultures of S. thermophilus LY03 grown on different carbohydrates or mixtures of carbohydrates. The effects of growth conditions on the activities of 10 enzymes involved in the EMP pathway and the biosynthesis of sugar nucleotides as the precursors for EPS biosynthesis were evaluated for the first time. A correlation between the activities of EPS precursor-forming enzymes and the amounts of EPS produced was unambiguously demonstrated.
MATERIALS AND METHODS
Bacterial strains.
S. thermophilus LY03, an industrial yoghurt starter culture, was used as the EPS-producing strain throughout this study (12). S. thermophilus NR, another industrial starter culture, was used as a non-EPS-producer. Both strains were kindly provided by V. Marshall (The University of Huddersfield, Huddersfield, United Kingdom). The strains were stored at −80°C in MRS broth (Oxoid, Basingstoke, England) containing 25% (vol/vol) glycerol. Before experimental use, the bacteria were propagated twice in MRS broth at 42°C for 12 h.
Growth on different carbohydrate sources.
A customized MRS broth was used as the basic EPS production medium; it contained 30 g of peptone (Oxoid) per liter, 12 g of yeast extract (Merck) per liter, 8 g of Lab Lemco (Oxoid) per liter, 2 g of K2HPO4 per liter, 5 g of sodium acetate per liter, 2 g of triammonium citrate per liter, 0.2 g of MgSO4 · 7H2O per liter, 0.038 g of MnSO4 · H2O per liter, and 1 ml of Tween 80 per liter (7). Different initial concentrations of specific energy and/or carbohydrate sources were tested: 0.22 and 0.29 M for lactose, 0.42 M for glucose, and 0.06, 0.11, 0.17, 0.22, 0.28, and 0.42 M for galactose. Furthermore, equivalent amounts of fructose (0.06, 0.11, 0.17, 0.22, 0.28, and 0.42 M), rhamnose (0.06, 0.12, 0.18, 0.24, 0.30, and 0.46 M), maltose (0.03, 0.06, 0.09, 0.12, 0.15, and 0.22 M), and sucrose (0.03, 0.06, 0.09, 0.12, 0.15, and 0.22 M) were applied. Also, additions of (i) 0.14 M glucose, galactose, or fructose and 0.15 M rhamnose to 0.15 M lactose; (ii) 0.14 M fructose to 0.22 M lactose, and (iii) 0.07 M lactose, 0.14 M fructose, and 0.15 M rhamnose to 0.28 M glucose were examined.
Fermentation conditions.
For all fermentations but one (0.22 M lactose plus 0.14 M fructose) the optimal initial carbon/complex-nitrogen ratio as determined earlier was applied (7). For the control strain, S. thermophilus NR, lactose (0.22 M) was used as the sole energy and carbohydrate source. The pH was controlled at 6.2 ± 0.1 by automatic addition of 10 N NaOH. The pH and the amount of base added were monitored online. Temperature was kept constant at 42 ± 0.1°C. To keep the fermentation broth homogenous, agitation was performed at 100 rpm with a stirrer composed of three standard impellers.
The fermentor inoculum was always prepared in two steps. First, 10 ml of customized MRS broth was inoculated with 100 μl of a freshly prepared S. thermophilus LY03 culture. After 12 h of incubation at 42°C, it was used to inoculate 100 ml of production medium. After 12 h of growth at 42°C, this second preculture was used to inoculate the fermentor (10 liters). All fermentations were done in 15-liter laboratory fermentors (Biostat C; B. Braun Biotech International, Melsungen, Germany), with a working volume of 10 liters. They were computer controlled (MicroMFCS for Windows NT software), and were sterilizable in situ. Sterilization was performed at 121°C for 20 min. The energy and carbohydrate source was sterilized separately (20 min at 121°C) and aseptically pumped into the fermentor. Samples were aseptically withdrawn from the fermentor to determine cell number (CFU), biomass (cell dry mass [CDM]), EPS production (polymer dry mass [PDM]), lactic acid concentration, galactose concentration, and residual substrate (carbohydrate) concentration (lactose, glucose, fructose) as described elsewhere (12). Isolation of both high-molecular-mass EPS (HMM-EPS) and low-molecular-mass EPS (LMM-EPS) and monomer analysis were done as previously described (7). Averaged standard deviations of 20.0 and 5.0% were observed for EPS isolation and monomer analysis, respectively.
Samples of 50 ml were taken to prepare cell extracts for measuring enzyme activity (see below) at three or four time points: once or twice during the exponential growth phase, at the end of the exponential growth phase when EPS production reached its maximum, and during the stationary phase or beyond the EPS maximum production level.
The maximum specific growth rate (μmax, per hour) was calculated as the maximum slope from the linearized values of the biomass (grams of CDM per liter) as a function of fermentation time (hours). The maximum carbohydrate consumption rates (rmax, per hour) were calculated from the residual concentration of the carbohydrate in the medium. To confirm the reliability of the experiments, one fermentation, which was chosen at random, was repeated twice and yielded comparable results for all parameters (see also Table 1).
TABLE 1.
Influence of the carbohydrate source on cell growth and EPS production during S. thermophilus LY03 fermentationsa
| Carbohydrate source(s) | μmax (h−1) (r2) | rmax (h−1) (r2) | Biomassmax (g of CDM · liter−1) (h) | (HMM-EPS)max
|
(LMM-EPS)max
|
(Total EPS)max
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg of PDM · liter−1 (h) | Gal-Glu | mg of PDM · liter−1 (h) | Gal-Glu | mg of PDM · liter−1 (h) | Gal-Glu HMM | Gal-Glu LMM | ||||
| 0.22 M lactose | 1.1 (0.997) | 0.3 (0.999) | 4.5 (10.00) | 821 (8.50) | 4.14:1.00 | 766 (10.00) | 4.19:1.00 | 1,142 (10.00) | 3.91:1.00 | 4.02:1.00 |
| 0.15 M lactose + 0.14 M fructose | 0.9 (0.982) | 0.5 (0.986) | 4.3 (10.75) | 754 (10.00) | 4.09:1.00 | 348 (26.50) | 4.07:1.00 | 787 (10.00) | 3.82:1.00 | 3.94:1.00 |
| 1.0 (0.942) | ||||||||||
| 0.15 M lactose + 0.14 M galactose | 1.0 (0.994) | 0.3 (0.998) | 4.6 (7.50) | 537 (6.50) | 3.83:1.00 | 335 (25.00) | 3.78:1.00 | 615 (6.50) | 3.87:1.00 | 4.02:1.00 |
| 0.22 M lactose + 0.14 M fructose | 1.0 (0.996) | 0.1 (0.997) | 4.2 (15.25) | 674 (12.25) | 3.78:1.00 | 360 (15.25) | 4.19:1.00 | 884 (12.25) | 4.18:1.00 | 4.17:1.00 |
| 0.2 (0.944) | ||||||||||
| 0.15 M lactose + 0.14 M glucose | 1.1 (0.997) | 0.1 (0.997) | 4.3 (9.75) | 1,234 (10.75) | 3.89:1.00 | 480 (9.00) | 4.97:1.00 | 1,436 (10.75) | 3.89:1.00 | 3.97:1.00 |
| 1.0 (0.998) | ||||||||||
| 0.42 M glucose | 1.3 (0.991) | 0.3 (0.999) | 4.7 (12.00) | 340 (9.50) | 4.12:1.00 | 454 (22.00) | 4.03:1.00 | 504 (10.75) | 4.06:1.00 | 4.12:1.00 |
| 0.28 M glucose + 0.14 M fructose | 1.0 (0.999) | 0.3 (0.996) | 5.8 (16.25) | 671 (12.50) | 3.78:1.00 | 550 (26.00) | 3.69:1.00 | 776 (15.00) | 4.02:1.00 | 4.08:1.00 |
| 0.2 (0.999) | ||||||||||
| 0.28 M glucose + 0.14 M galactose | 0.8 (0.980) | 0.5 (0.971) | 5.4 (12.00) | 669 (12.00) | 3.74:1.00 | 462 (26.00) | 4.02:1.00 | 797 (10.75) | 4.00:1.00 | 4.00:1.00 |
| 0.7 (0.995)b | 0.5 (0.970)b | 5.1 (12.50)b | 603 (12.50)b | 3.90:1.00b | 433 (25.00)b | 4.12:1.00b | 625 (12.50)b | 4.13:1.00b | 4.07:1.00b | |
| 0.28 M glucose + 0.07 M lactose | 1.0 (0.997) | 0.3 (0.992) | 4.0 (8.00) | 731 (7.00) | 3.68:1.00 | 433 (25.00) | 3.88:1.00 | 934 (7.00) | 4.03:1.00 | 3.98:1.00 |
| 0.3 (0.960) | ||||||||||
Fermentations were performed with MRS broth at 42°C and at a controlled pH of 6.2. The biokinetic parameters μmax and rmax were estimated through modelling of the experimental data (the correlation coefficient r2 is given in parentheses beside these data).
Where two fermentations were carried out, two values are reported. The maximum amount of the biomass and the amounts of HMM-EPS, LMM-EPS, and total EPS produced are given. The harvesting times corresponding to the maximum values are given in parentheses. The composition of the EPS material is represented as the ratio of galactose to glucose.
Preparation of cell extracts.
Samples, freshly taken from the fermentation broth (50 ml), were centrifuged at 20,000 × g for 20 min at 4°C, and the supernatant fluid was decanted. The cell pellet was washed with potassium phosphate buffer (0.05 mol liter−1, pH 7.0) and then resuspended in 6 ml of phosphate buffer and held on ice. The chilled cells were lysed using a Vibra-Cell sonicator (Sonic & Materials Inc., Danbury, Conn.) with a microtip setting (sonic power, 375 W; output control, 5) for 6 min and a 50% duty cycle consisting of 30 s of sonication pulses and 30 s of rest (to cool the suspension). Cell debris was removed by centrifugation (20,000 × g for 20 min at 4°C), and the supernatant fluid (the cell extract) was stored at −80°C and used as a source of enzymes.
For the optimization of the cell lysis, preliminary experiments were done, based on measurements of the intracellular lactate dehydrogenase activity. They indicated that sonication (6 min, 50% duty cycle), compared with the results of a glass-bead treatment carried out according to the method of Hansen et al. (22), gave the most reproducible and optimum results for release of proteins. These results were obtained within the limited time required to avoid great losses of enzymatic activities due to extensive heating during sonication. The reaction mixture for the measurement of the lactate dehydrogenase activity contained (for 1 ml) 10 mM dithiothreitol, 0.5 mM NADH, 50 mM sodium pyruvate, and 50 mM 3-morpholinopropane sulfonic acid, dissolved in 950 μl of ultrapure water, plus 50 μl of cell extract. Reaction mixtures were incubated at 30°C, and the oxidation of NADH was monitored spectrophotometrically at 340 nm.
Enzyme assays.
Enzyme assays were done at 37°C in a volume of 1.0 ml (unless otherwise indicated), and the formation or disappearance of NAD(P)H was monitored by measuring the absorbance at 340 nm (ɛ340 = 6,220 M−1 · cm−1). The protein concentration of cell extracts was determined using a commercial kit (DC protein assay; Bio-Rad Laboratories, Hercules, Calif.) that is based on the method of Lowry et al. (25). In all of the assays, the reaction velocity was linearly proportional to the amount of cell extract. All enzyme activity measurements were done in triplicate and expressed as mean values and standard deviations. Specific activities are expressed as nanomoles of substrate converted into product during 1 min for 1 mg of total cell protein.
The α-phosphoglucomutase reaction mixture contained 179 μmol of glycylglycine (pH 7.4), 0.67 μmol of β-NAD phosphate, 0.02 μmol of glucose 1,6-diphosphate, 30 μmol of MgCl2, 43 μmol of l-cysteine, 1 U of glucose 6-phosphate dehydrogenase, and cell extract. The reaction was initiated by adding 5.0 μmol of α-glucose 1-phosphate (28).
The reaction mixture for β-phosphoglucomutase was exactly the same as that for α-phosphoglucomutase, except that β-glucose 1-phosphate was used as the substrate.
The UDP-glucose pyrophosphorylase forward reaction mixture contained 50 μmol of Tris · HCl (pH 7.5), 8 μmol of MgCl2, 1.58 mg of cysteine hydrochloride (pH 7.5), 0.5 μmol of NAD, 1.25 μmol of UTP, 25 μg of UDP-glucose dehydrogenase, and cell extract. The reaction was initiated by adding 1 μmol of α-glucose 1-phosphate (19).
The UDP-galactose 4-epimerase reaction mixture contained 40 μmol of glycylglycine-NaOH (pH 8.5), 5 μmol of MgCl2, 0.6 μmol of NAD, 25 μg of UDP-glucose dehydrogenase, and cell extract. The reaction was initiated with 0.2 μmol of UDP-galactose (14, 19).
The dTDP-glucose pyrophosphorylase forward reaction mixture contained 50 μmol of Tris · HCl (pH 7.5), 8 μmol of MgCl2, 1.58 mg of cysteine hydrochloride (pH 7.5), 1.25 μmol of dTTP, and cell extract. The reaction was initiated by adding 1 μmol of α-glucose 1-phosphate and stopped by adding 50 μl of 1 M HCl. The samples were assayed by high-pressure liquid chromatography as described for monomer analysis (7, 19).
The activity of dTDP-glucose 4,6-dehydratase was assayed in a reaction mixture (0.5 ml) containing 25 μmol of sodium phosphate (pH 7.0), 0.05 μmol of NAD, and cell extract. The reaction was initiated with 0.25 μmol of dTDP-glucose. At different time intervals, samples (50 μl) were withdrawn, added to 750 μl of 0.1 N NaOH, and reincubated for 15 min (at 37°C). Formation of dTDP-6-deoxy-d-xylo-4-hexulose (ɛ320 = 4,600 M−1 · cm−1) was determined spectrophotometrically at 320 nm (2, 36).
The dTDP-rhamnose synthetic enzyme (26), which is responsible for the conversion of dTDP-6-deoxy-d-xylo-4-hexulose to dTDP-l-rhamnose, was assayed by monitoring NADPH oxidation in a mixture of 0.5 μmol of NADPH, 50 μmol of Tris · HCl (pH 8.0), and cell extract. The reaction was initiated by the addition of 0.3 μmol of dTDP-glucose and stopped by adding 300 μl of 0.5 M NaOH. The mixture was then reincubated (at 37°C) for 10 min (19).
The phosphoglucose isomerase reverse reaction mixture contained 50 μmol of potassium phosphate (pH 6.8), 5 μmol of MgCl2, 0.4 μmol of NADP, 0.01 ml of glucose 6-phosphate dehydrogenase (180 U · ml−1), and cell extract. The reaction was initiated by adding 2.5 μmol of fructose 6-phosphate (19, 30).
The reaction mixture for the 6-phosphofructokinase assay contained 50 μmol of Tris · HCl (pH 7.5), 5 μmol of MgCl2, 50 μmol of KCl, 0.15 μmol of NADH, 1.25 μmol of ATP, 50 μg of aldolase, 20 μg of triosephosphate isomerase-glycerol phosphate dehydrogenase, and cell extract. The reaction was initiated by adding 1 μmol of fructose 6-phosphate (19, 35).
The fructose 1,6-bisphosphatase reaction mixture contained 50 μmol of glycylglycine (pH 8.5), 5 μmol of MgCl2, 0.4 μmol of NADP, 25 μg of phosphoglucose isomerase, 25 μg of glucose 6-phosphate dehydrogenase, and cell extract. The reaction was initiated by adding 2 μmol of fructose 1,6-bisphosphate (19, 35).
Statistical significance of correlations.
Statistical significance of correlations between enzyme activities and amounts of EPS is based on a correlation test. It consists of a simple test of hypothesis that decides whether the correlation is significantly different from 0 or not. The test is performed by seeing how the absolute correlation value (r) compares to a critical value according to a chosen level of probability (P value). If the correlation is significant, then it can be concluded that the two factors are indeed associated, but if the correlation is found to be insignificant, it is concluded that the two factors might not be related at all. In the latter case, the correlation might be due to sampling randomness.
RESULTS
Effect of the energy and carbohydrate source on bacterial growth.
Different initial concentrations of specific energy sources and/or carbohydrate sources, in particular, lactose, glucose, galactose, fructose, rhamnose, maltose, and sucrose, were first tested as potential substrates for S. thermophilus LY03. S. thermophilus LY03 displayed a homofermentative metabolism and grew on both lactose and glucose. Galactose, fructose, rhamnose, maltose, and sucrose as sole energy sources were not fermented by S. thermophilus LY03 and neither was the galactose moiety of lactose. Fructose was fermented by the bacterium when it was used in combination with lactose or glucose. When rhamnose was applied as an additional carbohydrate source, it was not fermented and could not be found in the EPS produced. Therefore, fermentations with additional rhamnose were not further studied.
Fermentations were then done with lactose and glucose as the sole substrate or in combination with other carbohydrates (Table 1). From these experiments, it was clear that S. thermophilus LY03 grew well on glucose and lactose and consumed glucose and lactose efficiently. This is reflected in the μmax values of 1.3 and 1.1 h−1, respectively, the maximum substrate consumption rates (rmax) of 0.3 and 0.3 h−1, respectively, and the maximum attainable biomass concentrations of 4.7 and 4.5 g of CDM · liter−1, respectively. Both the μmax values and the maximum attainable biomass concentrations were comparable in all other cases, i.e., approximately 0.93 h−1 and 4.6 g of CDM · liter−1, respectively, except that the maximum biomass values were higher than 5.0 g of CDM · liter−1 for the experiments with a combined energy source of glucose and fructose or glucose and galactose. When grown on lactose as the sole energy source, S. thermophilus LY03 used only the glucose moiety and galactose accumulated in the medium. In contrast, galactose was converted to lactic acid upon prolonged fermentation with lactose as the sole energy source as well as during fermentations with the following carbohydrate combinations: 0.15 M lactose plus 0.14 M galactose, 0.28 M glucose plus 0.14 M galactose, 0.22 M lactose plus 0.14 M fructose, and 0.28 M glucose plus 0.07 M lactose. In all cases, approximately 0.02 M galactose was converted into lactic acid from the end of the exponential growth phase and beyond.
When cultures were grown on both lactose and fructose, glucose and fructose, and lactose and glucose, both carbohydrate sources were consumed simultaneously during growth. They were converted into lactic acid homofermentatively. However, the rmax varied when different carbohydrate combinations were used. Indeed, explicit increases in rmax were seen from averages of 0.4 to 1.0 h−1 for both glucose and fructose, when we used the combinations of 0.15 M lactose plus 0.14 M glucose and 0.15 M lactose plus 0.14 M fructose, respectively. Lactose was only slowly consumed (rmax = 0.1 h−1) in the experiment with an increased initial energy source concentration of 0.22 M lactose plus 0.14 M fructose and in the fermentation with the carbohydrate combination of 0.15 M lactose plus 0.14 M glucose, indicating possible substrate inhibition or catabolite repression by easily fermentable substrates.
Effect of the energy and carbon source on EPS production.
For each of the fermentations mentioned above, the amount of EPS produced (both the HMM-EPS and LMM-EPS fractions), as well as the monomeric composition of the polysaccharide material, was determined (Table 1).
For all experiments, the monomeric compositions of both LMM-EPS and HMM-EPS were determined for each sample taken. No variation in EPS composition was observed; galactose and glucose were observed in all cases in a 4:1 ratio. Both the total maximum amount of EPS and the maximum amount of EPS from each fraction independently were clearly influenced by the nature of the carbohydrate source. Greater amounts of each EPS fraction and of the total EPS were observed with 0.22 M lactose as the sole carbohydrate source, and the greatest amounts of HMM-EPS and total EPS were found with the combination of 0.15 M lactose plus 0.14 M glucose, compared to amounts in the other fermentations. When glucose was used as the sole carbohydrate source, both HMM-EPS and the total amount of EPS were significantly lower than for all other fermentation conditions tested.
Activities of enzymes involved in the EMP pathway, sugar nucleotide biosynthesis, and EPS biosynthesis.
To determine whether the differences in EPS production between cultures of S. thermophilus LY03 grown on different carbohydrates as substrate sources could be related to precursor availability, the activities of 10 enzymes involved in the EMP pathway and in the biosynthesis and interconversion of sugar nucleotides were determined. For comparison, the same enzyme activities were also measured for S. thermophilus NR, a strain that does not produce EPS. Samples were taken at three or four time points (see Materials and Methods) during the different fermentations described above.
Enzyme activities and EPS production during fermentation of S. thermophilus LY03 on lactose, on a combination of lactose and another carbohydrate source, on glucose, or on a combination of glucose and another carbohydrate source and of the non-EPS-producer S. thermophilus NR are shown in Tables 2 through 6.
TABLE 2.
Amounts of HMM, LMM, and total EPS measured in fermented medium of the EPS-producing strain S. thermophilus LY03a
| Carbon source | Amt produced (mg of PDM liter−1) at indicated time point in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.22 M lactose
|
0.15 M lactose + 0.14 M fructose
|
0.15 M lactose + 0.14 M galactose
|
0.15 M lactose + 0.14 M glucose
|
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| HMM EPS | 0 | 821 | 495 | 296 | 754 | 404 | 214 | 537 | 496 | 877 | 1,234 | 1,133 |
| LMM EPS | 603 | 207 | 0 | 84 | 33 | 0 | 121 | 78 | 94 | 350 | 202 | 230 |
| Total EPS | 603 | 1,028 | 495 | 380 | 787 | 404 | 335 | 615 | 590 | 1,227 | 1,436 | 1,363 |
S. thermophilus LY03 was grown in batch cultures in MRS broth on lactose and combinations of lactose and glucose, lactose and galactose, and lactose and fructose as carbohydrate sources at 42°C and at a constant pH of 6.2. Samples were taken at three time points of the fermentation course, representing the exponential growth phase (point 1), the end of the exponential growth phase (point 2), and the stationary phase (point 3). Each value is the average of at least three measurements.
TABLE 6.
Activities of enzymes involved in the EMP pathway and the pathway leading to the biosynthesis of sugar nucleotides and EPS in cell extracts of S. thermophilus NRa
| Enzyme | Avg activity (nmol · mg of cell protein−1 · min−1) ± SD |
|---|---|
| α-Phosphoglucomutase | 154 ± 72 |
| β-Phosphoglucomutase | 0 ± 0 |
| UDP-glucose pyrophosphorylase | 18 ± 16 |
| UDP-galactose 4-epimerase | 52 ± 28 |
| dTDP-glucose pyrophosphorylase | 0 ± 1 |
| Dehydratase | 7 ± 2 |
| Epimerase reductase | 0 ± 0 |
| Phosphoglucose isomerase | 1,687 ± 56 |
| 6-Phosphofructokinase | 2,064 ± 68 |
| Fructose 1,6-bisphosphatase | 0 ± 0 |
S. thermophilus NR, a nonropy strain, was grown in batch cultures in MRS broth with 0.22 M lactose at 42°C and at a controlled pH 6.2. Samples were taken at the end of the exponential growth phase.
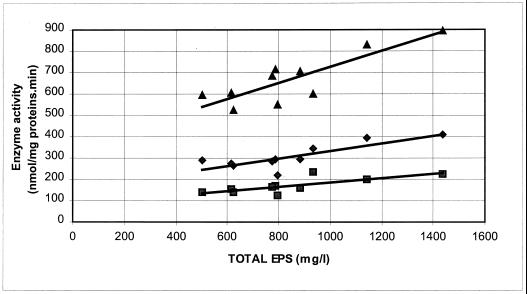
The activity of α-phosphoglucomutase was linked to EPS biosynthesis and the amount of EPS produced. Indeed, a maximum amount was reached for all fermentations at the end of the exponential growth phase. For all carbohydrate sources used, the specific activity paralleled the maximum total amount of EPS. A correlation (r) of 0.85 was found, which was statistically significant at a P of <0.01 (Fig. 1). For the non-EPS-producing strain, a very low specific activity of α-phosphoglucomutase was detected. Neither of the strains displayed β-phosphoglucomutase activity.
FIG. 1.
Relationship between the activities of the enzymes α-phosphoglucomutase (▴; y = 0.3716x + 354.76; r = 0.85), UDP-galactose 4-epimerase (⧫; y = 0.1764x + 157.53; r = 0.81), and UDP-glucose pyrophosphorylase (■; y = 0.1005x + 86.01; r = 0.80) and the total amount of EPS produced (total sample size was 10).
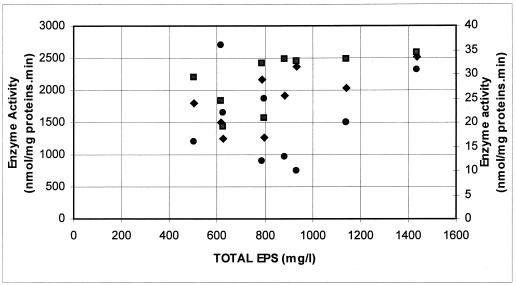
The same trend of activities during fermentation was observed for both UDP-glucose pyrophosphorylase (r = 0.80; P < 0.01) and UDP-galactose 4-epimerase (r = 0.81; P < 0.01) (Fig. 1). All other relationships between enzyme activities and the amounts of EPS gave a correlation coefficient, r, of less than 0.70 (P > 0.05) (Fig. 2). This result indicates a decreased level of or no significance for the involvement of the measured enzyme activities in EPS production compared to those of α-phosphoglucomutase, UDP-glucose pyrophosphorylase, and UDP-galactose 4-epimerase.
FIG. 2.
Relationship between the activities of the enzymes 6-phosphofructokinase (primary axis) (■; y = 0.8912x + 1426; r = 0.61), phosphoglucose isomerase (primary axis) (⧫; y = 1.0579x + 998; r = 0.69), and dehydratase (secondary axis) (●; y = 0.005x + 15.46; r = 0.13) and the total amount of EPS produced (total sample size was 10).
For the rhamnose nucleotide synthetic branch of EPS biosynthesis, a very low enzyme activity was measured for dTDP-glucose 4,6-dehydratase. The dTDP-glucose pyrophosphorylase and the dTDP-rhamnose synthetic enzyme system displayed almost no activity in cell extracts of both strains.
There was no substantial specific activity of fructose 1,6-bisphosphatase for any of the carbohydrate combinations tested for either strain. Furthermore, a clear decrease in the specific activities of both phosphoglucose isomerase and 6-phosphofructokinase was observed when galactose was present as an additional carbohydrate source during fermentation. Also, for the non-EPS-producing strain, the specific activities of these enzymes were comparable with the values observed for the S. thermophilus LY03 strain.
DISCUSSION
The yoghurt strain S. thermophilus LY03 produces a heteropolysaccharide consisting of galactose and glucose in the ratio 4:1. In this study, fermentation experiments were carried out, using different carbohydrates or combinations of carbohydrates as substrates for bacterial growth and EPS production.
Lactose and glucose were taken up by S. thermophilus LY03 and metabolized homofermentatively. Galactose was not used as energy source. Indeed, S. thermophilus metabolizes lactose in a homofermentative way and takes up lactose using a lactose-galactose antiport system that excretes the galactose moiety into the medium (10). S. thermophilus does not ferment galactose (23). However, for some fermentation experiments, very small amounts of galactose were also converted into lactic acid towards the end of the exponential growth phase. This may be explained by the fact that non-galactose-fermenting strains contain intact genes for the Leloir pathway. However, these genes are usually not transcribed (9).
The variations observed for the maximum carbohydrate consumption rates can be explained by substrate inhibition and/or catabolite repression of the more quickly metabolized energy sources. Indeed, de Vos and Vaughan have stated that the lac genes (lactose metabolism) may be under (glucose) catabolite repression control (10). For the one experiment carried out with a higher initial carbohydrate concentration, the low maximum carbohydrate consumption rates may also be explained by the fact that the carbohydrate/nitrogen ratio was not optimal for bacterial growth in this particular case (7).
The monomeric composition of the EPS produced by S. thermophilus LY03 was not affected by any of the carbohydrate sources used in the fermentation experiments. This is an important conclusion, given the contradictory reports in the literature concerning the influence of the carbohydrate source on EPS composition. The main reasons are most likely analytical artifacts encountered during composition analysis and carbohydrate quantification (11). Our results are in agreement with those of Escalante et al., who did not observe any variation in monomeric composition when using either lactose or glucose as an energy source for S. thermophilus (14).
Unlike the monomeric composition of the EPS, the amount of EPS produced was clearly influenced by the carbohydrate source. When glucose was used as the sole carbohydrate source, the amount of EPS produced by S. thermophilus LY03 was significantly lower while the maximum cell density was unchanged. Some authors also report the stimulation of EPS production without a concomitant increase of the biomass concentration by varying the carbohydrate source or increasing its concentration. For instance, L. delbrueckii subsp. bulgaricus NCFB 2772 produced considerably larger amounts of EPS when it was grown on glucose or lactose than when it was grown on fructose (19).
The amount of EPS produced seems to be correlated with the activities of three enzymes: α-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase. Some authors have reported the specific activities of enzymes involved in the biosynthesis of EPS from lactic acid bacteria (14, 16, 19, 27, 31). A relationship between EPS biosynthesis and the activity of the enzyme β-phosphoglucomutase was reported for L. lactis (31). Our results clearly showed that none of the S. thermophilus strains displayed β-phosphoglucomutase activity. The correlation that we observed between the amount of EPS and the activity of UDP-glucose pyrophosphorylase was also reported by Escalante et al. (14). They observed that UDP-glucose pyrophosphorylase was correlated with EPS production in a ropy S. thermophilus strain but not in a nonropy strain. Grobben et al. found that cultures of L. delbrueckii subsp. bulgaricus NCFB 2772 grown on glucose displayed a higher UDP-glucose pyrophosphorylase activity than cultures grown on fructose (19). They also observed that cells grown on a mixture of glucose and fructose showed enzyme activities similar to those of cells grown on glucose alone, while glucose was preferentially consumed as the energy source. Because cultures of L. delbrueckii subsp. bulgaricus NCFB 2772 grown on fructose produced EPS with a lower level of galactose and even though the activity of UDP-galactose 4-epimerase was only slightly lower in these cells, the authors concluded that this enzyme does not play an important role in the composition of the EPS produced. However, a relationship between the activity of the enzyme UDP-galactose 4-epimerase and EPS production was found for L. lactis subsp. cremoris (16). In contrast, the UDP-glucose pyrophosphorylase activity was inversely correlated with EPS production in the latter strain (16). UDP-galactose 4-epimerase activity was not correlated with EPS biosynthesis in any of the S. thermophilus strains tested by Escalante et al. (14).
Consequently, glucose or the glucose moiety from lactose hydrolysis seems to be the source of carbohydrate for heteropolysaccharide biosynthesis in lactic acid bacteria. Indeed, a high UDP-glucose pyrophosphorylase activity was always found in our experiments. In addition, the Leloir pathway enzyme UDP-galactose 4-epimerase, necessary to convert UDP-galactose to UDP-glucose, displayed a very high activity in the galactose-negative S. thermophilus LY03 strain examined, in particular when the EPS-producing and non-EPS-producing strains of S. thermophilus were compared. This indicates that the Leloir enzyme UDP-galactose 4-epimerase is involved in the biosynthesis of precursors for EPS production in EPS-producing galactose-negative S. thermophilus strains. This finding might also explain why accumulation of UDP-glucose in cells of S. thermophilus leads to the production of EPS and why UDP-galactose 4-epimerase activity increased upon accumulation of UDP-galactose going hand in hand with a decreased galactokinase activity (33).
The fact that rhamnose is not present in the EPS produced by S. thermophilus LY03 corresponds with the very low activities that we observed for the enzymes involved in the biosynthesis of dTDP-rhamnose. This finding is in agreement with the work of Grobben et al., who detected almost no activity for this enzyme system in cultures grown on fructose, which resulted in rhamnose-free EPS, and who detected high activities in cultures grown on glucose, which led to rhamnose-containing EPS (19).
The enzyme fructose 1,6-bisphosphatase did not display a substantial specific activity for any of the carbohydrate combinations or for the control strain, indicating that the flux of carbon to EPS production does not follow the route from fructose 1,6-bisphosphate through fructose 6-P to glucose 6-P and that all fructose 6-P is converted into lactic acid for energy generation. Finally, a clear decrease of the specific activities of both phosphoglucose isomerase and 6-phosphofructokinase was observed for S. thermophilus LY03, when galactose was present as an additional carbohydrate source during fermentation. These results suggest inhibition or repression of these enzymes by galactose and hence limitation of the carbon flux towards glycolysis, without a significant change in the amount of EPS.
Because the α-phosphoglucomutase enzyme is responsible for the linkage between sugar catabolism and sugar anabolism, it will be important to increase the carbon flux via phosphoglucomutase to a sufficiently high level to improve EPS production. This might be done via genetic engineering or process engineering. The same might be accomplished by overexpression of UDP-galactose 4-epimerase and/or UDP-glucose pyrophosphorylase.
TABLE 3.
Activities of enzymes involved in the EMP pathway and the pathway leading to the biosynthesis of sugar nucleotides and EPS in cell extracts of the EPS-producing strain S. thermophilus LY03a
| Enzyme | Avg activity (nmol · mg of cell protein−1 · min−1) ± SD at indicated time point with:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.22 M lactose
|
0.15 M lactose + 0.14 M fructose
|
0.15 M lactose + 0.14 M galactose
|
0.15 M lactose + 0.14 M glucose
|
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| α-Phosphoglucomutase | 581 ± 44 | 829 ± 7 | 715 ± 18 | 567 ± 72 | 717 ± 11 | 673 ± 30 | 429 ± 9 | 603 ± 7 | 523 ± 20 | 590 ± 42 | 897 ± 58 | 831 ± 6 |
| β-Phosphoglucomutase | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| UDP-glucose pyrophosphorylase | 137 ± 11 | 199 ± 2 | 175 ± 5 | 147 ± 21 | 171 ± 3 | 161 ± 8 | 108 ± 2 | 155 ± 8 | 142 ± 11 | 148 ± 11 | 224 ± 14 | 208 ± 2 |
| UDP-galactose 4-epimerase | 281 ± 60 | 396 ± 38 | 351 ± 44 | 252 ± 39 | 297 ± 6 | 277 ± 14 | 183 ± 4 | 275 ± 15 | 258 ± 16 | 277 ± 18 | 410 ± 25 | 382 ± 3 |
| dTDP-glucose pyrophosphorylase | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2 ± 1 | 1 ± 0 | 1 ± 1 | 3 ± 1 | 1 ± 0 |
| Dehydratase | 15 ± 1 | 20 ± 1 | 14 ± 2 | 8 ± 1 | 12 ± 1 | 8 ± 2 | 30 ± 2 | 36 ± 3 | 25 ± 1 | 18 ± 3 | 31 ± 1 | 17 ± 1 |
| Epimerase reductase | 0 ± 0 | 2 ± 1 | 1 ± 1 | 0 ± 0 | 2 ± 1 | 0 ± 0 | 0 ± 0 | 2 ± 0 | 0 ± 1 | 0 ± 0 | 2 ± 0 | 0 ± 0 |
| Phosphoglucose isomerase | 1,635 ± 253 | 2,034 ± 327 | 1,984 ± 76 | 2,167 ± 225 | 2,167 ± 294 | 2,061 ± 321 | 1,371 ± 197 | 1,496 ± 53 | 1,467 ± 50 | 2,084 ± 26 | 2,523 ± 103 | 2,112 ± 256 |
| 6-Phosphofructokinase | 1,832 ± 100 | 2,478 ± 148 | 2,444 ± 270 | 2,239 ± 230 | 2,425 ± 90 | 2,306 ± 154 | 1,778 ± 161 | 1,830 ± 65 | 1,795 ± 61 | 2,132 ± 27 | 2,582 ± 106 | 2,161 ± 262 |
| Fructose 1,6-bisphosphatase | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 3 ± 3 | 20 ± 3 | 1 ± 1 |
S. thermophilus LY03 was grown in batch cultures in MRS broth on lactose and combinations of lactose and glucose, lactose and galactose, and lactose and fructose as carbohydrate sources at 42°C and a constant pH of 6.2. Samples were taken at three time points of the fermentation course, representing the exponential growth phase (point 1), the end of the exponential growth phase (point 2), and the stationary phase (point 3). Each value is the average of at least three measurements.
TABLE 4.
Amounts of HMM, LMM, and total EPS measured in fermented medium of the EPS-producing strain S. thermophilus LY03a
| Carbon source | Amt (mg of PDM liter−1) at the indicated time point in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.42 M glucose
|
0.28 M glucose + 0.14 M fructose
|
0.28 M glucose + 0.14 M galactose
|
0.28 M glucose + 0.07 M lactose
|
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| HMM EPS | 0 | 340 | 180 | 0 | 671 | 256 | 433 | 669 | 524 | 556 | 731 | 320 |
| 0 | 598 | 19 | 603 | 500 | ||||||||
| LMM EPS | 79 | 61 | 271 | 75 | 0 | 335 | 133 | 12 | 12 | 124 | 203 | 413 |
| 0 | 0 | 106 | 22 | 13 | ||||||||
| Total EPS | 79 | 401 | 451 | 75 | 671 | 591 | 566 | 681 | 536 | 680 | 934 | 733 |
| 0 | 598 | 125 | 625 | 513 | ||||||||
S. thermophilus LY03 was grown in batch cultures in MRS broth on glucose and combinations of glucose and lactose, glucose and galactose, and glucose and fructose as carbohydrate sources at 42°C and a constant pH of 6.2. Samples were taken at three time points of the fermentation course, representing the exponential growth phase (point 1), the end of the exponential growth phase (point 2), and the stationary phase (point 3). When two samples were taken in the exponential growth phase (point 1), two values are reported. Each value is always the average of at least three measurements.
TABLE 5.
Activities of enzymes involved in the EMP pathway and the pathway leading to the biosynthesis of sugar nucleotides and EPS in cell extracts of the EPS-producing strain S. thermophilus LY03a
| Enzyme | Avg activity (nmol · mg of cell protein−1 · min−1) ± SD at indicated time point in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.42 M glucose
|
0.28 M glucose + 0.14 M fructose
|
0.28 M glucose + 0.14 M galactose
|
0.28 M glucose + 0.07 M lactose
|
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| α-Phosphoglucomutase | 348 ± 55 | 597 ± 21 | 592 ± 10 | 507 ± 29 | 685 ± 9 | 700 ± 5 | 394 ± 7 | 549 ± 16 | 521 ± 15 | 568 ± 28 | 600 ± 3 | 530 ± 17 |
| 488 ± 7 | 542 ± 20 | 372 ± 12 | 525 ± 19 | 496 ± 9 | ||||||||
| β-Phosphoglucomutase | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||||||
| UDP-glucose pyrophosphorylase | 79 ± 14 | 122 ± 11 | 141 ± 5 | 119 ± 7 | 166 ± 4 | 170 ± 4 | 99 ± 2 | 125 ± 0 | 130 ± 4 | 216 ± 2 | 234 ± 16 | 217 ± 6 |
| 122 ± 11 | 128 ± 5 | 93 ± 3 | 141 ± 20 | 124 ± 2 | ||||||||
| UDP-galactose 4-epimerase | 172 ± 16 | 289 ± 32 | 238 ± 4 | 199 ± 13 | 287 ± 8 | 297 ± 12 | 166 ± 4 | 218 ± 0 | 227 ± 7 | 328 ± 3 | 344 ± 24 | 383 ± 20 |
| 253 ± 59 | 269 ± 13 | 183 ± 5 | 265 ± 35 | 236 ± 4 | ||||||||
| dTDP-glucose pyrophosphorylase | 1 ± 1 | 2 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 1 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||||||
| Dehydratase | 11 ± 2 | 16 ± 2 | 11 ± 2 | 8 ± 2 | 12 ± 1 | 8 ± 2 | 17 ± 1 | 25 ± 1 | 19 ± 1 | 6 ± 2 | 10 ± 2 | 3 ± 4 |
| 14 ± 1 | 9 ± 1 | 18 ± 1 | 22 ± 1 | 15 ± 3 | ||||||||
| Epimerase reductase | 0 ± 0 | 2 ± 1 | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 0 ± 0 | 1 ± 0 | 0 ± 0 |
| 0 ± 0 | 1 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | ||||||||
| Phosphoglucose isomerase | 1,583 ± 296 | 1,807 ± 312 | 1,817 ± 245 | 1,839 ± 295 | 2,136 ± 365 | 1,910 ± 104 | 1,098 ± 81 | 1,275 ± 103 | 1,183 ± 95 | 1,934 ± 97 | 2,359 ± 91 | 2,041 ± 72 |
| 1,962 ± 284 | 2,080 ± 309 | 1,277 ± 254 | 1,258 ± 237 | 1,222 ± 51 | ||||||||
| 6-Phosphofructokinase | 1,928 ± 89 | 2,200 ± 85 | 2,224 ± 198 | 2,055 ± 48 | 2,381 ± 95 | 2,336 ± 127 | 1,344 ± 99 | 1,560 ± 127 | 1,501 ± 164 | 1,982 ± 27 | 2,457 ± 224 | 2,267 ± 121 |
| 2,392 ± 52 | 2,326 ± 90 | 1,307 ± 259 | 1,437 ± 120 | 1,495 ± 62 | ||||||||
| Fructose 1,6-bisphosphatase | 1 ± 1 | 5 ± 5 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 9 ± 2 | 11 ± 1 | 6 ± 3 |
| 8 ± 3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||||||
S. thermophilus LY03 was grown in batch cultures in MRS broth on glucose and combinations of glucose and lactose, glucose and galactose, and glucose and fructose as carbohydrate sources at 42°C and a constant pH of 6.2. Samples were taken at three time points of the fermentation course, representing the exponential growth phase (point 1), the end of the exponential growth phase (point 2), and the stationary phase (point 3). When two samples were taken in the exponential growth phase (point 1), two values are reported. Each value is always the average of at least three measurements.
ACKNOWLEDGMENTS
This work benefited from financial support from the Institut Yoplait International. We further acknowledge financing from the European Commission (grants FAIR CT98-4267 and INCO-Copernicus IC15-CT98-0905), the Flemish Institute for the Encouragement of Scientific and Technological Research in the Industry (IWT), the Fund for Scientific Research (FWO—Flanders), and the Research Council of the Vrije Universiteit Brussel (VUB).
REFERENCES
- 1.Ariga H, Urashima T, Michihata E, Ito M, Morizono N, Kimura T, Takahashi S. Extracellular polysaccharide from encapsulated Streptococcus salivarius subsp. thermophilus OR 901 isolated from commercial yogurt. J Food Sci. 1992;57:625–628. [Google Scholar]
- 2.Breedveld M, Bonting K, Dijkhuizen L. Mutational analysis of exopolysaccharide biosynthesis by Lactobacillus sakei 0-1. FEMS Microbiol Lett. 1998;169:241–249. doi: 10.1111/j.1574-6968.1998.tb13324.x. [DOI] [PubMed] [Google Scholar]
- 3.Bubb W A, Urashima T, Fujiwara R, Shinnai T, Ariga H. Structural characterisation of the exocellular polysaccharide produced by Streptococcus thermophilus OR 901. Carbohydr Res. 1997;301:41–50. doi: 10.1016/s0008-6215(97)00083-9. [DOI] [PubMed] [Google Scholar]
- 4.Cerning J. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait. 1995;75:463–472. [Google Scholar]
- 5.Cerning J, Renard C M G C, Thibault J F, Bouillanne C, Landon M, Desmazeaud M, Topisirovic L. Carbon source requirements for exopolysaccharide production by Lactobacillus casei CG11 and partial structure analysis of the polymer. Appl Environ Microbiol. 1994;60:3914–3919. doi: 10.1128/aem.60.11.3914-3919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degeest B, Van De Ven S, De Vuyst L. Proceedings of the 11th Forum for Applied Biotechnology. 1997. Optimization of the production and isolation of exopolysaccharides from Streptococcus thermophilus and strong evidence for their growth-associated biosynthesis; pp. 1199–1206. Part I. [Google Scholar]
- 7.Degeest B, De Vuyst L. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of bacterial growth and exopolysaccharide production. Appl Environ Microbiol. 1999;65:2863–2870. doi: 10.1128/aem.65.7.2863-2870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degeest B, Van de Ven S, De Vuyst L. Process characteristics of exopolysaccharide production by Streptococcus thermophilus. Macromol Symp. 1999;140:43–52. [Google Scholar]
- 9.de Vos W M. Metabolic engineering of sugar catabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:223–242. doi: 10.1007/BF00395934. [DOI] [PubMed] [Google Scholar]
- 10.de Vos W M, Vaughan E E. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol Rev. 1994;15:217–237. doi: 10.1111/j.1574-6976.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 11.De Vuyst L, Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev. 1999;23:157–177. doi: 10.1111/j.1574-6976.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 12.De Vuyst L, Vanderveken F, Van de Ven S, Degeest B. Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J Appl Microbiol. 1998;84:1059–1068. doi: 10.1046/j.1365-2672.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 13.Doco T, Wieruszeski J M, Fournet B. Structure of an exocellular polysaccharide produced by Streptococcus thermophilus. Carbohydr Res. 1990;198:313–321. doi: 10.1016/0008-6215(90)84301-a. [DOI] [PubMed] [Google Scholar]
- 14.Escalante A, Wacher-Rodarte C, Garcia-Garibay M, Farrés A. Enzymes involved in carbohydrate metabolism and their role on exopolysaccharide production in Streptococcus thermophilus. J Appl Microbiol. 1998;84:108–114. doi: 10.1046/j.1365-2672.1997.00330.x. [DOI] [PubMed] [Google Scholar]
- 15.Faber E J, Zoon P, Kamerling J P, Vliegenthart J F G. The exopolysaccharides produced by Streptococcus thermophilus Rs and Sts have the same repeating unit but differ in viscosity of their milk cultures. Carbohydr Res. 1998;310:269–276. doi: 10.1016/s0008-6215(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 16.Forsén R, Häivä V M. Induction of stable slime-forming and mucoid states by p-fluorophenylalanine in lactic streptococci. FEMS Microbiol Lett. 1981;12:409–413. [Google Scholar]
- 17.Griffin A M, Morris V J, Gasson M J. The cpsABCDE genes involved in polysaccharide production in Streptococcus salivarius ssp. thermophilus strain NCBF 2393. Gene. 1996;183:23–27. doi: 10.1016/s0378-1119(96)00405-2. [DOI] [PubMed] [Google Scholar]
- 18.Grobben G J, Sikkema J, Smith M R, de Bont J A M. Production of extracellular polysaccharides by Lactobacillus delbrueckii ssp. bulgaricus NCFB 2772 grown in a chemically defined medium. J Appl Bacteriol. 1995;79:103–107. [Google Scholar]
- 19.Grobben G J, Smith M R, Sikkema J, de Bont J A M. Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl Microbiol Biotechnol. 1996;46:279–284. [Google Scholar]
- 20.Grobben G J, van Casteren W H M, Schols H A, Oosterveld A, Sala G, Smith M R, Sikkema J, de Bont J A M. Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl Microbiol Biotechnol. 1997;48:516–521. [Google Scholar]
- 21.Gruter M, Leeflang B R, Kuiper J, Kamerling J P, Vliegenthart J F G. Structural characterisation of the exopolysaccharide produced by Lactobacillus delbrueckii subspecies bulgaricus rr grown in skimmed milk. Carbohydr Res. 1993;239:209–226. doi: 10.1016/0008-6215(93)84216-s. [DOI] [PubMed] [Google Scholar]
- 22.Hansen R G, Albrecht G J, Bass S T, Seifert L L. UDP-glucose pyrophosphorylase (crystalline) from liver. Methods Enzymol. 1966;3:248–253. [Google Scholar]
- 23.Hutkins R W, Morris H A. Carbohydrate metabolism by Streptococcus thermophilus: a review. J Food Prot. 1987;50:876–884. doi: 10.4315/0362-028X-50.10.876. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine J, Chirat F, Wieruszeski J-M, Strecker G, Favre N, Neeser J-N. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Appl Environ Microbiol. 1997;63:3512–3518. doi: 10.1128/aem.63.9.3512-3518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Martins L O, Correia I S. Temperature profiles of gellan gum synthesis and activities of biosynthetic enzymes. Biotechnol Appl Biochem. 1993;20:385–395. [Google Scholar]
- 27.Poolman B, Royer T J, Mainzer S E, Schmidt B F. Carbohydrate utilization in Streptococcus thermophilus: characterisation of the genes for aldose 1-epimerase (mutarotase) and UDP glucose 4-epimerase. J Bacteriol. 1990;12:4037–4047. doi: 10.1128/jb.172.7.4037-4047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian N, Stanley G A, Hahn-Hägerdal B, Rådström P. Purification and characterization of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose- and glucose-utilizing cells. J Bacteriol. 1994;176:5304–5311. doi: 10.1128/jb.176.17.5304-5311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roller S, Dea I C M. Biotechnology in the production and modification of biopolymers for foods. Crit Rev Biotechnol. 1992;12:261–277. [Google Scholar]
- 30.Schreyer R, Böck A. Phosphoglucose isomerase from Escherichia coli K10: purification, properties and formation under aerobic and anaerobic condition. Arch Microbiol. 1980;127:289–298. doi: 10.1007/BF00427206. [DOI] [PubMed] [Google Scholar]
- 31.Sjöberg A, Hahn-Hägerdal B. β-Glucose-1-phosphate, a possible mediator for polysaccharide formation in maltose-assimilating Lactococcus lactis. Appl Environ Microbiol. 1989;55:1549–1554. doi: 10.1128/aem.55.6.1549-1554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stingele F, Neeser J-R, Mollet B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol. 1996;178:1680–1690. doi: 10.1128/jb.178.6.1680-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas T D, Crow V L. Lactose and sucrose utilization by Streptococcus thermophilus. FEMS Microbiol Lett. 1983;17:13–17. [Google Scholar]
- 34.Tombs M, Harding S E. An introduction to polysaccharide biotechnology. London, United Kingdom: Taylor & Francis Ltd.; 1998. [Google Scholar]
- 35.Van Dijken J P, Quale J R. Fructose metabolism in four Pseudomonas species. Arch Microbiol. 1977;114:281–286. doi: 10.1007/BF00446874. [DOI] [PubMed] [Google Scholar]
- 36.Zarkowsky H, Glaser L. The mechanism of 6-deoxyhexose synthesis. J Biol Chem. 1969;244:4750–4756. [PubMed] [Google Scholar]