Abstract
Background:
Renal ischemia reperfusion injury (IRI) predictably causes acute kidney injury (AKI) after shock, major cardiovascular procedures and in all kidneys procured for transplantation. The earliest events of IRI are triggered by molecules released from injured cells, DAMPs (Damage Associated Molecular Patterns), that bind pattern recognition receptors (PRRs) consitutively expressed on many cells within the kidney. Activation of PRR signaling leads to production of proinflammatory molecules, which incite a cascade of inflammatiory events leading to AKI. Renal tubular epithelial cells (RTECs) are particularly susceptible to ischemic injury and proximal RTEC injury is pathognomonic of renal IRI. To better understand how injured RTECs contribute to the cycle of deleterious inflammation in the setting of renal IRI, this study asked whether DAMPs released from injured RTECs induced PRR signals in healthy RTECs.
Methods:
Human renal tuble epithelial cells (RTECs) were necrosed ex vivo to release intracellular DAMPs and resulting necrotic supernatant used to stimulate healthy RTECs, T lymphocytes and monocytes.
Results:
DAMPs released from necrosed RTECs upregulated PRRs known to be associated with renal IRI and activated MAPK signaling pathways. Pro-inflammatory cytokines were upregulated in response to necrotic supernatant and this upregulation was abrogated by MEK1 inhibition. The RTEC-derived DAMPs were also potent inducers of T cell activation/proliferation and monocyte migration.
Conclusions:
This is the first study to our knowledge to show that endogenous DAMPs released from injured RTECs directly activate PRR signaling in healthy RTECs. These findings provide new insights directed to therapeutics for renal IRI.
1. Introduction
Renal ischemia-reperfusion injury (IRI) occurs frequently following cardiovascular procedures, in states of sepsis/shock, and universally in all kidneys procured for transplant. The outcome of renal IRI is often acute kidney injury (AKI), a disorder that significantly increases morbidity/mortality and leads to chronic kidney disease.1–3 Understanding the mechanisms that contribute to renal IRI and subsequent AKI holds promise for development of new therapeutic strategies to improve patient outcomes and to improve organ utilization for transplantation.
A major shift in our understanding of renal IRI came with the discovery that the innate immune system could be triggered by endogenous DAMPs (damage associated molecular patterns).4,5 DAMPs are endogenous molecules that are released from injured cells and incite a sterile inflammatory response. Once released, they ligate and activate pattern recognition receptors (PRRs) expressed on immune and parenchymal cells.6–9 Activation of PRR signaling pathways ultimately produces proinflammatory cytokines and cell death signaling within the affected cells.8–14 Activation of innate immune responses in the kidney by DAMPs released from injured cells leads to a cascade of renal injury that manifests as AKI.
Experimental models have elucidated several PRRs important to renal IRI, namely the membrane-bound toll like receptors (TLRs) TLR2, TLR4, and the cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) NOD2 and NLRP3.15–23 Many of the cell signaling pathways induced by DAMP-induced PRR ligation have been elucidated through experiments using genetic deletion of specific DAMPs and PRRs on immune cells.24–31 DAMPs have been identified in the intracellular debris released from dead and dying cells, and synthetic versions of DAMPs have been used experimentally to replicate renal inflammation and AKI. 8,13,28,32–39
Despite a large body of work, there is little empiric evidence that cellular contents released from dead/dying renal tubular cells can induce PRR signaling and activate inflammatory responses in the cells that surround them. There are very few studies that use renal parenchymal cells as the source of DAMPs,32,40 and there are no studies that we are aware of that use necrotic supernatant from renal tubular epithelial cells (RTECs) to stimulate PRR signaling in uninjured renal tubular epithelium. In this study we show that endogenous DAMPs from RTECs can directly stimulate healthy RTECs to induce PRR expression, proinflammatory cytokines/chemokines, cellular proliferation, and immune cell migration.
2. Methods:
RTECs
Two sources of primary human RTECs were used in these studies. In each experiment the 2 sources of primary RTECs were compared and found to produce identical results. Human primary proximal RTECs were purchased from ATCC (PCS 400–010). These cells were thawed and passaged in DMEM:F12+Glutamine+HEPES (Gibco, Waltham, MA) with the addition of 1% v/v insulin, transferrin, selenium (Invitrogen, Carlsbad, CA), 0.05uM hydrocortisone (Sigma, St. Louis, MO), 20ng/mL EGF (Sigma, St. Louis, MO), 32ng/mL Tri-iodo-thryonine (Sigma, St. Louis, MO), 0.5% heat inactivated FBS (Genesee Scientific, El Cajon, CA). An additional source of primary RTECs was obtained from human kidneys not used for transplantation. The kidneys used for research were provided by our local organ bank, LifeSharing™ (San Diego, CA), were discarded and deidentified, thus not subject to IRB review (per NIH Human Subjects guidelines). All kidneys were tested for pathogens by nucleic acid testing (NAT) per organ bank protocol. Any kidneys found harboring pathogens and any public health service (PHS) high-risk kidneys were not used. Kidneys with >20% fibrosis based on procurement pathologist reading were also not used. Following procurement, the kidneys were transported to the laboratory in UW preservative solution (Bridge to Life, Columbia, SC) at 4°C and processed immediately. The RTECs were isolated using our published methods.22,23 Briefly, the cortex was dissected under aseptic conditions, minced, and the slurry suspended in 1mg/mL solution of sterile collagenase (Sigma, St. Louis, MO). Cells were then strained through a 100um filter (Fisher Scientific, Waltham, MA) and RTECs isolated by Percoll gradient centrifugation.
T cell and monocyte lines
The human T cell line Kit225 was originally obtained from S. Burakoff, Dana Farber Cancer Institute, Boston, MA and frozen aliquots grown in our laboratory. The cells were maintained in 10K media- 10% heat inactivated fetal bovine serum (Omega, Tarzana CA), 1% PenStrep (Gibco, Waltham, MA), 0.05mM 2-mercaptoethanol (Sigma, St. Louis, MO), 15mM HEPES (Gibco, Waltham, MA) in RPMI 1640 + L-glutamine (Gibco, Waltham, MA)- with the addition of 18units/mL recombinant human IL-2. The human monocyte cell line Thp1 was a gift of A. Gavin, Scripps Research, La Jolla, CA. The Thp1 cells were maintained and passaged in the 10K media.
Generation of necrotic supernatant:
To generate necrotic supernatant, RTECs were stimulated with 0.1mM hypoxanthine (Sigma, St. Louis MO) and 2.5mU/mL of xanthine oxidase (Sigma, St. Louis MO) in RPMI for 2 hours, washed 3 times with 30ml sterile PBS and then resuspended in DMEM:F12+L-glutamine+15mM HEPES (Gibco, Waltham, MA) at a concentration of 106cells/ 100uL media. The cell mixture was then transferred to a sterile Eppendorf tube and frozen for 24hrs at −80°C. The cells were then freeze-thawed 3 times and resuspended in 500ul fresh DMEM:F12 and cell debris removed by centrifugation at 17 000xg for 15 seconds. The supernatant was transferred to a fresh Eppendorf tube, tested for protein content and fresh DMEM:F12 added to bring the total volume of supernatant up to 1mL of supernatant normalized for protein content. A DMEM:F12 control was used by adding equal volumes of DMEM:F12 to an Eppendorf tube and freeze thawing 3 times.
Neutral Red Assay for RTEC proliferation
The RTECs, 5×104/well, were added to a 96 well plate (in quadruplicate for each treatment) in 200uL of the DMEM:F12 growth media and placed in the incubator (37°C/5%CO2) overnight. The following day, the media was removed and replaced with fresh DMEM:F12 without growth supplements and the RTECs rested for another 24 hours. After the 24hr rest, media was removed and replaced with 200uL of necrotic supernatant or DMEM control. The cells were placed back in the incubator for 24 hours, removed from the incubator, washed twice with 200uL of sterile PBS and neutral red assay performed as has been previously published.41
RTEC stimulation with necrotic supernatant
Primary human RTECs were isolated from human kidneys or purchased from ATCC (PCS 400–010). RTECs were seeded into 12 well biocoat plates (Corning, Tewksburg, MA) and grown to 70% confluency. 1 hour prior to stimulation, growth media was removed and replaced with fresh DMEM:F12 and cells placed in incubator at 37°C and 5% CO2. After 1 hour, media was removed and 500uL (for cell signling, RNA) or 650uL (for protein expression) of necrotic supernatant or DMEM:F12 control was added to each well and the cells placed back in the incubator for 30 minutes (cell signaling), 3 hours (RNA) or 12 hours (protein expression). After each time point, cells were washed with 2mL of sterile, room temperature PBS and then lysed using appropriate lysis buffer for each downstream application. Lysates were saved at −80 (cell signaling, protein expression) or −20 (RNA) until further analysis.
MEK inhibition
RTECs were grown to 70% confluency in 12 well biocoat plates (Becton Dickinson, Bedford MA). One hour prior to stimulation, the growth media was removed and replaced with fresh DMEM:F12 or 1000nM MEK1 inhibitor GDC0623 (Selleckchem, Cat #S7553) dissolved in DMEM:F12. After 1 hour, the media was replaced with DMEM:F12, necrotic supernatant, or necrotic supernatant+MEK1 inhibitor and the cells placed back in the incubator for 3 hours. After 3 hours, cells were washed twice with 2mL of sterile PBS and 300uL of RNA lysis buffer (Zymo Research, Irvine CA) was added directly to the plates. Lysates were stored at −80 until processing.
T cell proliferation/activation
Kit225 cells (1×105) were added to round-bottom wells (CoStar, Corning, NY) in either RPMI, necrotic supernatant or growth media (10K+IL2) for 48 hours. Proliferation was measured using an MTT assay from Promega (Madison, WI) according to the manufacturer’s instructions. MTT was added for 4 hours and then solubilized for at least 1 hour prior to reading at 570nm. Treatments were run in triplicate. Activation markers (CD44, CD69, and CD25) were assessed by FACS 24 hours after stimulation. Antibodies were obtained from BioLegend (San Diego, CA).
Ki67 expression
RTECs were grown in 12 well plates to 70% confluency. Growth media was removed and replaced with 2mL of fresh DMEM:F12 media and the cells placed back in the incubator (5% CO2, 37°C) for 1 hour. The media was then removed and 700uL of necrotic supernatant, DMEM control, or growth media was added to each well and the cells placed in the incubator for 24 hours. After 24 hours cells were washed twice with 2mL of sterile PBS and Ki67 expression was analyzed via FACS. Ki-67-PerCP antibody was purchased from BioLegend (San Diego, CA)
Monocyte/T cell migration assay
Necrotic supernatant or RPMI 1640 + L-glutamine (Gibco, Waltham, MA) (600uL) was added to the bottom chamber of a 5mm pore Transwell (Costar, Kennebunk ME) permeable support plate. Thp1 cells were added to the top chamber at a concentration of 5×105 cells/100uL RPMI media and the plates placed in the incubator for 4 hours, after which time the cells from the lower chamber were collected and then live cells counted.
qPCR
RNA was created using the Zymo Research Quick RNA mini prep (Zymo Research, Irivine CA), according to the manufacturer’s instructions. cDNA was created using the SuperScript III First Strand Synthesis System (Thermo Fisher, Waltham, MA). cDNA concentrations were standardized to 200ng/uL using DPEC-treated water (Thermo Fisher, Waltham, MA). All primers were purchased from Genecopoeia (Rockville, MD). Master mix was created using the following formula: 1uL primer+5uL PowerUp SYBR green master mix (Thermo Fisher, Waltham, MA). 6uL of this master mix was added to 4uL of cDNA in a 96 well PCR reaction plate (Thermo Fisher, Waltham, MA). RT-qPCR was then run on a Quant Studio 3 Real Time PCR system (Thermo Fisher, Waltham, MA). Reactions were run in triplicates. Data was analyzed on the Quant Studio software. GAPDH was used as a housekeeping gene.
Luminex for cell signaling molecules
Phospho-proteins were detected using the Bio-Rad MAPK 9plex cell signaling kit (Bio-Rad, Hercules CA), and Millipore NF-kb 6 plex assay (Millipore, Burlington, MA). β-actin (Bio-Rad, Hercules, CA) was used as a loading control, and units presented are unit of phosphorylated-protein fluorescence/β-actin fluorescence. Phosphotase treated lysates were provided in the 9plex kit and were used as negative controls. Protein was standardized to 200ug/mL and 50uL of sample was added in duplicate to a 96 well plate. The protocol was carried out according to the manufacturer’s instructions. Samples were analyzed on the Bio-Plex Magpix (Bio-Rad, Hercules, CA).
Luminex for chemokines/cytokines
Necrotic supernatant was prepared as described above, protein content standardized to 1mg/ml, and analyzed for chemokines/cytokines using the ProcartaPlex Inflammation 20-plex Human panel (Thermo Fisher, Waltham, MA) according to the manufacturer’s instructions.
Mass Spectrometry
Necrotic supernatant was prepared as above and 100mcg of total protein added to 400uL of 1:1 methanol:acetonitrile. Protein mixture was placed at 4° C overnight, then spun down at 17,500xg for 10min and supernatant removed. Protein was then analyzed using tandem mass spectrometry (MS/MS) on the ESI-TRAP instrument (n=3). The MASCOT search results based on protein family summary were assessed and the filters used for the search were a significance threshold of p< 0.05 and an Ion cut-off of 48. Pathway analyses were done using the DAVID Bioinformatic Resources 6.8 Software (https://david.ncifcrf.gov/.)
Statistical analyses:
The number of replicates for each experiment are stated in the figure legends. Student’s 2 tailed T test were used to compare all data with p<0.05 considered significant and >0.05 indicating no significant differences between 2 groups. For experiments with MEK1 inhibition, p values were calculated comparing necrotic supernatant to necrotic supernatant with MEK1 inhibition. For the pathway analysis of mass spectrometry data, all p values were corrected using the Benjamini-Hochberg method for false discovery rate and only corrected values of p <0.05 were considered significant.
3. Results
3.1. Endogenous DAMPs increase PRR expression in RTECs
The PRRs TLR2, TLR4, NLRP3 and NOD2 are constitutively expressed in RTECs and play an important role as triggers of cellular injury following renal IRI.18,19,21–23 Fig 1 shows that necrotic supernatant from injured primary human RTECs increased expression in healthy primary human RTECs of the TLRs and NLRs that have been implicated as triggers of AKI. As shown in Fig 1, we found that exposure of healthy RTECs to necrotic supernatant derived from injured RTECs caused significant upregulation of TLR2, TLR4, and NOD2. There was no effect however on NOD1 nor on NLRP3 expression.
Figure 1. DAMPs released from necrosed RTECs induce TLR2, TLR4, and NOD2 mRNA in RTECs.
Fresh primary human RTECs were treated with either control media (media) or supernatant from necrosed RTECs (Nec sup) in 12 well plates by replacing the culture media with the control media or necrotic supernatant (normalized for protein content) for 3 hours. mRNA expression was analyzed via RT-qPCR. Graphs are representative of 3 separate experiments. Error bars are SDs of triplicates. *** p<0.001; ** p<0.01
3.2. Endogenous DAMPs activate MAPK signaling pathways in RTECs
Cell stress signaling is induced by activation of PRRs, and is integrated through MAPK signaling pathways.42 Three well-characterized MAPK signaling pathways are expressed in mammalian cells: the ERK/MEK, the c-JUN N-terminal kinase 1, 2, and 3 (JNK1/2/3) and the p38 MAPK pathways.43 We asked whether exposure to necrotic supernatant induced MAPK signaling in the primary human RTECs and whether there was specificity in the signaling pathways induced by the exposure to necrotic supernatant.
As shown in Fig 2, we found significant activation of MEK1 (Fig 2A), but not JNK (Fig 2B) after exposure to the necrotic supernatant. To confirm the absence of JNK activation we also analyzed (seen in Fig 2B) its downstream target p53 and saw no significant activation.44 We did note (Fig 2A) that HSP27 was phosphorylated after stimulation with the necrotic supernatant, suggesting that the p38 pathway might also be induced, however p38 was not significantly activated (Fig 2B). Exposure to the necrotic supernatant also caused significant activation of the MAPK target NF-kB (Fig 2A).
Figure 2. DAMPs released from necrosed RTECs activate MAPK signaling in RTECs.
A) Primary human RTECs were stimulated with media vs. necrotic supernatant for 30 minutes, washed twice and then lysed in 12 well plates. pMAPKs were then analyzed using Luminex. Y axis is luminescence standardized to the luminescence of beta actin, which served as a loading control. Graphs are representative of 3 separate experiments. Error bars represent SD of duplicates. *p<0.05, **p<0.01. B. RTECs were stimulated in same way as Fig 2A., analyzed for phospho- p38, JNK, and p53. Phosphotase treated lysates were used as a negative control. Data is representative of 3 separate experiments. Error bars are SDs of duplicates.
3.3. Endogenous DAMPs cause upregulation of proinflammatory cytokines in RTECs
TLR and NLR activation by DAMPs released by ischemic tissue induce downstream signaling pathways that produce proinflammatory cytokines and chemokines associated with the influx of innate and adaptive effector cells seen during reperfusion. While inflammatory cytokines are produced by many cells in the kidney, they are also known to be produced directly by RTECs.45–47 Since we noted that the necrotic supernatant induced NF-kB signaling, we next asked whether exposure to necrotic supernatant also induced proinflammatory cytokine/chemokine production in the primary human RTECs. As shown in Fig 3, we found that necrotic supernatant causes significant production of IL1β, IL6, IL8 and MCP1 mRNA in the RTECs (Fig 3A). To confirm these findings at the protein level, RTECs were stimulated with necrotic supernatant and ELISA performed to quantitate protein upregulation. IL1β, IL6, IL8 and MCP1 protein levels were all significantly higher than control media after stimulation with necrotic supernatant, mirroring the findings seen at the mRNA level (Fig 3B).
Figure 3. DAMPs released from necrosed RTECs induce proinflammatory cytokines in RTECs.
A) Primary human RTECs were stimulated with media vs. necrotic supernatant for 3 hours and mRNA expression analyzed by RT-qPCR. B) RTECs were stimulated with media vs necrotic supernatant for 12 hours and cytokine expression analyzed via ELISA. Graphs are representative of 3 separate experiments. Error bars are SD of triplicates ***p<0.001
3.4. Inhibition of MEK1 blocks upregulation of inflammatory cytokines
To further investigate the role of the MEK/ERK signaling in the response to necrotic supernatant, we stimulated the primary human RTECs with the necrotic supernatant in the presence or absence of the MEK1 inhibitor GDC0623. We noted, as shown in Fig 4, that MEK1 inhibition caused a significant reduction in the upregulation of proinflammatory cytokines IL1b, IL6, IL8 and MCP1, suggesting that MEK/ERK signaling pathways were indeed selectively activated by the necrotic supernatant.
Figure 4. Inhibition of MEK1 prevents necrotic supernatant-induced expression of pro-inflammatory cytokines in RTECs.
Primary human RTECs were pretreated with either DMEM:F12 or MEK1 inhibitor (1000nM) for 1 hour and then stimulated for 3hrs with either media or necrotic supernatant in the presence or absence of the MEK1 inhibitor. mRNA was then assessed via RT-qPCR. Error bars are SD of triplicates. Graph is representative of 3 separate experiments. **p<0.01, ***p<0.001
3.5. RTECs proliferate in response to endogenous DAMPs
DAMPs have been found to cause both cell proliferation and cell death.48–50 Understanding the conditions that lead to these outcomes is an area that holds great potential for therapeutic targets. Here we tested the impact of exposure to the necrotic supernatant on viability of healthy primary human RTECs. As shown in Fig 5A, the exposure to necrotic supernatant caused significant proliferation of the primary human RTECs. As shown in Fig 5B, necrotic supernatant caused significant increase in Ki67 expression, confirming the proliferative response in the necrotic supernant group compared to DMEM:F12 control.
Figure 5. DAMPs released from necrosed RTECs cause proliferation of RTECs.
A) Primary human RTECs were stimulated with media or necrotic supernatant for 24 hours and then a neutral red assay performed to assess for cell proliferation. Error bars are SD of quadruplicates. B) RTECs were stimulated with media or necrotic supernatant for 24 hours and then Ki67 expression was analyzed via FACS. Graphs are representative of 3 separate experiments. *p<0.05, **p<0.01
3.6. T cells are activated and proliferate in response to endogenous DAMPs
To determine whether T cells were activated by exposure to necrotic supernatant, Kit225 cells were exposed to necrotic supernatant and proliferation assessed by MTT assay. As shown in Fig 6A, exposure to necrotic supernatant significantly increased T cell proliferation. These cells are known to proliferate in IL2-containing growth media, which was used as a positive control. We also asked whether exposure to the necrotic supernatant induced markers of T cell activation. The cell surface activation markers CD69, CD44, and CD25, are well-known to be upregulated in response to T cell activation.51,52 As shown in Fig 6B, cell surface expression of CD44 and CD69 significantly increased after exposure to necrotic supernatant.
Figure 6. DAMPs released from necrosed RTECs cause proliferation and activation of T cells.
A) Kit225 cells were stimulated with media vs. necrotic supernatant vs. media with 18units/mL IL2 for 48 hours and proliferation assayed by MTT assay. Data represents 1 of 3 separate experiments. B) Kit225 cells were stimulated with media vs. necrotic supernatant for 24 hours and cell surface expression of activation markers was evaluated by FACS. Error bars are SD of triplicates. P values presented are media vs. Nec Sup. **p<0.01, ***p<0.001
3.7. Endogenous DAMPs induce monocyte and T cell migration
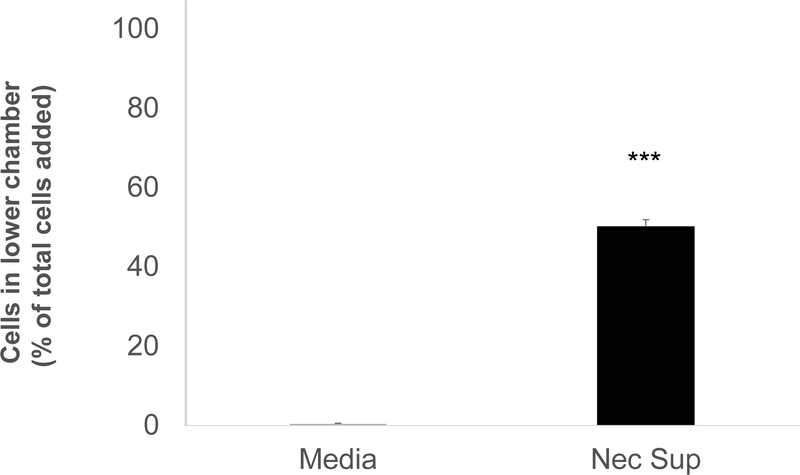
Monocytes/macrophages are thought to play an important role in both injury and recovery phases of renal IRI.6 Circulating monocytes have been shown to extravasate from the circulation and differentiate at the site of injury under the direction of chemotaxic and integrin signaling induced by the ischemic tissue.6 In these experiments we asked whether necrotic supernatant contained chemotactic signals that signaled monocyte migration. Using the human monocyte cell line Thp1, we added cells to the top of micropore chambers and then exposed them to necrotic supernatant. Cell migration was assessed by counting the cells in the lower chamber after 4 hours of stimulation. We saw no migration of Thp1 cells into control media, whereas approximately 50% of the cells migrated to the lower chamber in response to the chemotactic signals present in the necrotic supernatant (Fig 7).
Figure 7. DAMPs released from necrosed RTECs induce monocyte migration.
Thp1 cells were seeded in the upper chamber of a 5uM pore size exclusion chamber. The lower chamber was filled with necrotic supernatant or media. After 4 hours of incubation, cells in the lower chamber were counted. The data is representative of 3 separate experiments. Error bars are SD of duplicates. ***p<0.001
3.8. Analysis of necrotic supernatant
We screened the RTEC derived necrotic supernatants from 3 human donor kidneys using mass spectrometry. Many proteins were identified, and pathway analyses of all the proteins revealed that many of the proteins were common to pathogen-associated molecular patterns (PAMPs), phagosome degradation of biological material, and metabolic functions such as glycolysis, synthesis of amino acids and carbon metabolism (Table 1). We also identified 6 different heat shock proteins (HSPs) present in the necrotic supernatants (Table 2), as HSPs have previously been reported to act as DAMPs.20,53 The necrotic supernatant was also screened for relevant pro-inflammatory molecules. Using a 20-analyte Luminex panel, we found that there were substantial levels of ICAM1, p-selectin, GM-CSF, e-selectin, IL-6, IL-8, MCP1, IL-1β, MIP1β and IFNγ (Table 3). TNFα, IL-13, IL-12, IL-1α, IL-17A, IP10 and MIP1α were present at very low concentrations (<85pg/mL). IFNα, IL-4, and IL-10 and were not present (data not shown).
Table 1.
Pathway analysis of proteins in necrotic supernatant.
| Biological Pathway | P value | Benjamini |
|---|---|---|
| PAMPs | 9.60E-06 | 5.00E-04 |
| Phagosome | 6.50E-04 | 1.20E-02 |
| Glycolysis/Gluconeogenesis | 7.70E-04 | 1.20E-02 |
| Biosynthesis of amino acids | 9.40E-04 | 1.20E-02 |
| Regulation of actin cytoskeleton | 2.30E-03 | 2.40E-02 |
| Carbon metabolism | 3.50E-03 | 3.00E-02 |
Table 2.
Heat shock proteins are present in necrotic supernatant
| Protein ID | Protein Name |
|---|---|
| gi|5729877 | heat shock cognate 71 kDa protein isoform 1 |
| gi|662841 | heat shock protein 27 |
| gi|306891 | 90kDa heat shock protein |
| gi|153792590 | heat shock protein HSP 90-alpha isoform 1 |
| gi|167466173 | heat shock 70 kDa protein 1A/1B |
| gi|15010550 | heat shock protein gp96 precursor |
Table 3.
Pro-inflammatory proteins in necrotic supernatant
| Analyte | pg/mg protein | SEM |
|---|---|---|
| ICAM 1 | 17,565 | 1,051 |
| p-selectin | 9,767 | 1,140 |
| GM-CSF | 2,955 | 282 |
| e-selectin | 2,738 | 254 |
| IL-6 | 2,293 | 460 |
| IL-8 | 2,011 | 860 |
| MCP1 | 719 | 42 |
| IL-1β | 229 | 112 |
| MIP1β | 159 | 23 |
| IFNγ | 118 | 11 |
4. Discussion
The results of this study demonstrate that primary human RTECs upregulate TLRs and NLRs, MAPK signaling pathways and proinflammatory cytokine/chemokine production when exposed to supernatant from necrotic RTECs. This is the first study to our knowledge to show that human RTECs exposed to DAMPs derived from human RTECs are primed for proinflammatory signaling.
It is well known that ischemia causes RTEC injury/death with subsequent release of cellular debris into the microenvironment.8,9 The cellular debris released from injured RTECs contains various molecules, including DAMPs, that play an important role in the pathogenesis of renal IR injury.8,10,12,13,50,54,55 Consistent with this model, our data show that necrotic supernatant from human RTECs causes healthy RTECs to upregulate proinflammatory cytokines that recruit inflammatory cells, which contribute to the cascade of injurious events characteristic of renal IRI. 18,19,21–23 Preliminary studies using healthy cell supernatant also showed that there were no differences between healthy cell supernatant and media, further emphasizing the importance of the intracellular DAMPs as the source of pro-inflammatory signaling. Interestingly the data also demonstrate that the supernatant from necrotic RTECs can directly induce T cell activation and proliferation, as well as T cell and monocyte migration, mimicking the events associated with inflammatory cell infiltration following renal IRI.6,56
Since PRRs that induce cell death signaling are upregulated in health RTECs by the necrotic supernatant, we expected to see induction of apoptosis or necrosis in the healthy RTECs. Our findings, however, indicate that RTECs proliferate in response to necrotic supernatant. This observation is not without precedent, and indeed DAMPs are thought to be involved in both the initial injury phase as well as healing and regeneration.49,50,56–58 We also observed that the MEK1/ERK pathway was activated by the necrotic supernatant and that inhibition of MEK1 blocked upregulation of inflammatory mediators. Other MAPK pathways, such as JNK were not activated by necrotic supernatant, suggesting that DAMPs generated from RTECs might induce specific downstream signaling pathways. To fully address this question requires additional studies that identify putative ligands released from the necrotic RTECs under germ-free conditions. We did however find in the mass spectrometry analyses that 6 HSPs were common to all of the necrotic supernatant replicates, suggesting these may be promising targets of future studies.
In conclusion, we find that necrotic supernatant derived from human RTECs contains molecules that prime for TLR and NLR signaling, for production of proinflammatory mediators and for RTEC proliferation. These findings provide new insight into the mechanisms that contribute to the complex immune responses to renal IRI and perhaps offer potential avenues for therapeutic intervention.
Funding:
This research was funded via the following sources:
DM - NIH, NIDDK- R01 DK113162
DM - NIH, NIAID-R21 AI154471
Abbreviations:
- AKI
Acute Kidney Injury
- DAMPs
Damage Associated Molecular Patterns
- IRI
Ischemia Reperfusion Injury
- MAPK
Mitogen-activated protein kinase
- NLR
Nucleotide-binding oligomerization domain (NOD)-like receptors
- PRR
Pattern Recognition Receptor
- RTEC
Renal Tubule Epithelial Cell
- TLR
Toll-like Receptor
Footnotes
Disclosure:
The authors declare no conflicts of interest
References
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. [DOI] [PubMed] [Google Scholar]
- 2.Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matzinger P Tolerance, danger, and the extended family. Ann Rev Immunol. 1994;12(1):991–1045. [DOI] [PubMed] [Google Scholar]
- 5.Pradeu T, Cooper E. The danger theory: 20 years later. Front Immunol. 2012;3(287). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11(2):88–101. [DOI] [PubMed] [Google Scholar]
- 7.Dai H, Thomson AW, Rogers NM. Dendritic cells as sensors, mediators, and regulators of ischemic injury. Front Immunol. 2019;10:2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury J Clin Invest. 2011;121(11):4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22(3):416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulay SR, Kumar SV, Lech M, et al. How kidney cell death induces renal necroinflammation. Sem Nephrol. 2016;36(3):162–173. [DOI] [PubMed] [Google Scholar]
- 11.Anders HJ. Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol. 2010;21(8):1270–1274. [DOI] [PubMed] [Google Scholar]
- 12.Anders HJ. Of Inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol. 2016;27(9):2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. 2014;25(7):1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Plenter R, Nydam T, Jani A. Injury pathways that lead to AKI in a mouse kidney transplant model. Transplantation. 2020;104(9):1832–1841. [DOI] [PubMed] [Google Scholar]
- 15.Altemeier WA, Liles WC, Villagra-Garcia A, et al. Ischemia-reperfusion lung injury is attenuated in MyD88-deficient mice. PloS One. 2013;8(10):e77123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faubel S, Ljubanovic D, Reznikov L, et al. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kid Int. 2004;66(6):2202–2213. [DOI] [PubMed] [Google Scholar]
- 17.Ferhat M, Robin A, Giraud S, et al. Endogenous IL-33 contributes to kidney ischemia-reperfusion injury as an alarmin. J Am Soc Nephrol. 2018;29(4):1272–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Lee DW, Ravichandran K, et al. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2013;346(3):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leemans JC, Stokman G, Claessen N, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115(10):2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mkaddem SB, Werts C, Goujon JM, et al. Heat shock protein gp96 interacts with protein phosphatase 5 and controls toll-like receptor 2 (TLR2)-mediated activation of extracellular signal-regulated kinase (ERK) 1/2 in post-hypoxic kidney cells. J Biol Chem. 2009;284(18):12541–12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulskens WP, Teske GJ, Butter LM, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PloS One. 2008;3(10):e3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigeoka AA, Kambo A, Mathison JC, et al. Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol. 2010;184(5):2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shigeoka AA, Mueller JL, Kambo A, et al. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010;185(10):6277–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer SS, He Q, Janczy JR, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106(48):20388–20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Carpio DF, Zheng Y, et al. An essential role of the NF-κB/toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166(12):7128–7135. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. [DOI] [PubMed] [Google Scholar]
- 29.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5(8):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allam R, Scherbaum CR, Darisipudi MN, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23(8):1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azroyan A, Cortez-Retamozo V, Bouley R, et al. Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor. PloS One. 2015;10(3):e0121419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai C, Kotani H, Miyao M, et al. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol. 2016;186(4):829–843. [DOI] [PubMed] [Google Scholar]
- 35.Rabadi M, Kim M, Li H, et al. ATP induces PAD4 in renal proximal tubule cells via P2X7 receptor activation to exacerbate ischemic AKI. Am J Physiol Renal Physiol. 2018;314(2):F293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabadi MM, Ghaly T, Goligorksy MS, et al. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303(6):F873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Ma J, Wang P, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21(11):1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki K, Muto J, Taylor KR, et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284(19):12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. [DOI] [PubMed] [Google Scholar]
- 40.Liu N, Wang H, Han G, et al. Alleviation of apoptosis of bone marrow-derived mesenchymal stem cells in the acute injured kidney by heme oxygenase-1 gene modification. Int J Biochem Cell Biology. 2015;69:85–94. [DOI] [PubMed] [Google Scholar]
- 41.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–1131. [DOI] [PubMed] [Google Scholar]
- 42.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–758. [DOI] [PubMed] [Google Scholar]
- 44.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70(4):1061–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109(4):e102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeWolf SE, Shigeoka AA, Scheinok A, et al. Expression of TLR2, NOD1, and NOD2 and the NLRP3 inflammasome in renal tubular epithelial cells of male versus female mice. Nephron. 2017;137(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasimsetty SG, DeWolf SE, Shigeoka AA, et al. Regulation of TLR2 and NLRP3 in primary murine renal tubular epithelial cells. Nephron Clin Prac. 2014;127(1–4):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linkermann A, Stockwell BR, Krautwald S, et al. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14(11):759–767. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa S, Omura T, Yonezawa A, et al. Extracellular nucleotides from dying cells act as molecular signals to promote wound repair in renal tubular injury. Am J Physiol Renal Physiol. 2014;307(12):F1404–F1411. [DOI] [PubMed] [Google Scholar]
- 50.Sarhan M, Land WG, Tonnus W, et al. Origin and consequences of necroinflammation. Physiol Rev. 2018;98(2):727–780. [DOI] [PubMed] [Google Scholar]
- 51.Reddy M, Eirikis E, Davis C, et al. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293(1):127–142. [DOI] [PubMed] [Google Scholar]
- 52.Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7(5):213–221. [DOI] [PubMed] [Google Scholar]
- 53.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anders H-J, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22(6):1007–1018. [DOI] [PubMed] [Google Scholar]
- 55.Jordan SC, Ammerman N, Choi J, et al. Interleukin-6: An important mediator of allograft injury. Transplantation. 2020;104(12):2497–2506. [DOI] [PubMed] [Google Scholar]
- 56.Shankar AS, Hoorn EJ, Gribnau J, et al. Current state of renal regenerative therapies. Transplantation. 2019;103(2):250–261. [DOI] [PubMed] [Google Scholar]
- 57.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tingle SJ, Sewpaul A, Bates L, et al. Dual microRNA blockade increases expression of antioxidant protective proteins: implications for ischemia-reperfusion injury. Transplantation. 2020;104(9):1853–1861. [DOI] [PubMed] [Google Scholar]