Abstract
Late presentation of aortopulmonary window (APW) beyond infancy is uncommon and many of these cases are inoperable due to development of progressive pulmonary hypertension and Eisenmenger syndrome. Outcome data in this cohort is thus sparse and the aim of this study was to analyze the outcomes in patients with APW operable beyond 1 year of age. Between September 2016 and March 2020, in a single center, 12 consecutive patients older than 1 year, undergoing surgery for APW, were included in the study. The median age and weight at presentation were 7.5 years (interquartile range (IQR) 4–9.5) and 15 kg (IQR 11.7–19.5). Ten (83.3%) patients had type 1 APW (proximal type) and 2 (16.6%) had a type 2 APW (distal type). Eight (66.6%) patients had associated lesions. Transaortic patch closure of APW was done in all cases. Seven (58.3%) patients were extubated within 3.5 h of admission in intensive care. There were no early deaths or during follow-up. The median follow-up duration was 20.5 months (IQR 7.5–24), and all patients were in New York Heart Association (NYHA) class I at last follow-up. Follow-up echocardiography did not reveal any significant residual shunts necessitating any additional procedure and a consistent decrease in pulmonary artery pressures. Surgery in patients with APW beyond 1 year of age is possible in selected patients. The early and intermediate surgical outcomes in patients who remain operable are excellent.
Keywords: Congenital heart disease, Aorta, Pulmonary hypertension, Outcomes
Introduction
Aortopulmonary window (APW) is a rare anomaly where there is an abnormal communication between the main pulmonary artery (MPA) and the ascending aorta with two semilunar valves, representing 0.1–0.2% of all congenital cardiac structural anomalies [1]. Mori and colleagues classified APW into three types: proximal or type I, distal or type II, and total or type III [2].
Surgery and rarely interventional approaches are the only option to treat this congenital structural heart defect. With a large, unrestrictive left-to-right shunt, an early closure is always advocated to prevent early development of severe pulmonary arterial hypertension (PAH) [3, 4]. However, late presentation beyond infancy and even adulthood has been described and some of these patients do remain operable until late [5–7]. There are reports of development of Eisenmenger syndrome in untreated APW patients who survive into adulthood [8]. The aim of the study was to analyze the outcome of patients beyond 1 year of age who underwent surgical repair for APW and associated anomalies, if any, at our institution.
Case series
Between September 2016 and March 2020, 17 patients with APW were operated at our hospital. Twelve patients greater than 1 year of age, who underwent surgery for APW, were included in this study. The Institutional Ethics Committee granted an exemption from review due to the retrospective nature of the study (NSHEV/INV/Non-Reg/2021/003; dated 24 March 2021). Data collected included demographic details and intraoperative and postoperative outcomes. Follow-up data included clinical examination of the New York Heart Association (NYHA) class and echocardiographic findings including residual lesions, if any.
The aim of the study was to analyze the outcomes of patients with APW operated beyond 1 year of age. The other objectives were to report the co-existing pathologies in these patients as well as the changes in right ventricular systolic pressure (RVSP) over a follow-up period of 1 year.
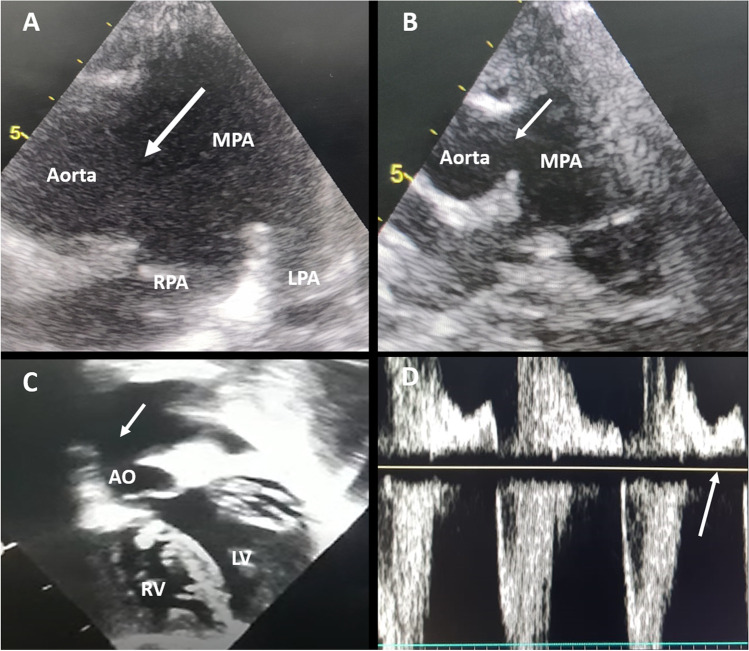
Operability of APW was determined on the basis of signs and symptoms of increased pulmonary blood flow, mainly recurrent lower respiratory tract infection, indicating presence of left-to-right shunt. Examination findings of room air saturation > 92%, absence of cyanosis, bounding peripheral pulses, hyperdynamic precordium, down and out apical impulse (clinical cardiomegaly), and mid-diastolic murmur over apex added to the history. Contrast-enhanced echocardiographic parameters of operability included left heart volume overload characterized by left ventricular internal dimension in diastole > + 2Z score, left atrial (LA) dilatation characterized by LA to aorta ratio > 1.4, brisk pulmonary venous return, flow acceleration across the mitral valve, direction of shunt (left-to-right shunt indicated operability), and significant flow reversal in the descending thoracic aorta (indicates diastolic stealing of blood into the pulmonary artery (PA) which favors operability) (Fig. 1a–d). All the patients had clear left-to-right shunt across the APW and there was no gradient across the APW which implied that all the patients had severe hyperkinetic PAH.
Fig. 1.
a–d Preoperative echocardiographic assessment of AP window. a High parasternal view showing large distal type AP window (white arrow) encroaching RPA origin. b Parasternal long axis view showing large intermediate type AP window (white arrow). c Subcostal coronal view showing large AP window (white arrow) with significantly dilated LV. d Pulse wave Doppler study at the level of the descending thoracic aorta showing holo-diastolic flow reversal (white arrow). Abbreviations: AP window, aortopulmonary window; RPA, right pulmonary artery; LV, left ventricle
Contrast-enhanced computed tomography (CECT) was done when there was concomitant aortic arch anomaly. None of the patients needed cardiac catheterization.
Of the 12 patients, there were 7 males (58%) with a median age of 7.5 years (interquartile range (IQR) 4–9.5) and median body weight was 15 kg (IQR 11.7–19.5). All 12 patients presented with history of recurrent respiratory infections and features of congestive cardiac failure. Chest X-ray (CXR) showed cardiomegaly and plethoric lung fields in all patients. CECT was done in 2 out of 12 patients.
Ten (83.3%) patients had type 1 APW (proximal type) and 2 (16.6%) had type 2 APW (distal type). The interrupted aortic arch (IAA) was one type A and one type B. The associated anomalies seen in our study included ventricular septal defect (VSD) in 2 (16.6%) patients, IAA in 2 (16.6%), subaortic membrane in 2 (16.6%), supramitral membrane in 1 (8.3%), and subaortic membrane with significant aortic regurgitation, which was repaired, in 1 (8.3%).
All patients, except the 2 with IAA, underwent elective procedures through median sternotomy under moderately hypothermic (28–32 °C) cardiopulmonary bypass (CPB) with aorto-bicaval cannulation and antegrade del Nido cardioplegia. The 2 patients with IAA underwent surgery with bifurcated aortic line with direct cannulation of innominate artery and a second arterial inflow to ductus for lower body perfusion along with bicaval venous cannulas. The arch repair was done under antegrade cerebral perfusion in these 2 patients with distal body under circulatory arrest at a temperature of 20 °C. Whole-body circulation was started after arch repair by adjusting the innominate artery cannula and APW was repaired during rewarming.
The median size of APW was 20 mm (IQR 18.75–20) and was nonrestrictive in all patients. All APW repair was done with a transaortic approach with Dacron Patch (Bard Sauvage knitted polyester fabric), using 6/0 polypropylene continuous sutures. The median CPB time was 81.5 min (IQR 71.5–85.2) and the median cross clamp (XCl) time was 50 min (IQR 44.2–61.7). Antegrade cerebral perfusion was required in 2 patients and the median antegrade cerebral perfusion time was 67.5 min (IQR 60.7–74.2).
The PA pressure was recorded intraoperatively by putting a needle directly into the MPA before and after the procedure. The mean PA pressure reduced from 65 ± 9.9 mmHg pre-procedure to 23.9 ± 2.5 mmHg in the immediate post-procedure period, and the difference was statistically significant (< 0.00001).
The elective inotropic support consisted of dobutamine (5 μg/kg/min) and epinephrine (0.05 μg/kg/min), if the postoperative direct mean PA pressure was ≤ 25 mmHg. Milrinone (0.5 μg/kg/min) and/or sodium nitroprusside (0.5 μg/kg/min) was added if required, when the mean PA pressure was more than 25 mmHg and titrated with hemodynamics. In the intensive care unit (ICU), appropriate care was taken to prevent PAH crisis by avoiding unnecessary suction of the endotracheal tube, keeping the patient sedated, and gradual weaning from ventilation without letting the patient struggle. Any central venous pressure (CVP) rise or rise of airway pressures was taken as indirect evidence of rise of PA pressures and corrective actions such as increase of pulmonary vasodilators, paralyzing and sedating the patient, and increasing the fraction of inspired oxygen (FiO2) were done.
None of the patients developed pulmonary hypertensive crisis in the postoperative period and none needed inhaled nitric oxide. Inotropes were gradually tapered off over a duration of 24–48 h.
All patients were weaned off ventilatory support after ensuring that there was no significant bleeding (less than 2 ml/kg/h for the first 2–4 h after receiving in ICU), acceptable hemodynamics, and appropriate neurological status.
Seven (58.3%) patients were extubated within 3.5 h of admission in intensive care. The median duration of mechanical ventilation was 3.1 h (IQR 2.15–17.35).
The median ICU stay was 2 days (IQR 2–2.2) while the median postoperative hospital stay was 5 days (IQR 5–6). There were no early deaths or in the follow-up period.
All patients underwent detailed echocardiography prior to discharge. The postoperative PA pressures were estimated by using RVSP calculated from the tricuspid regurgitation (TR) gradient. Two patients had evidence of small hemodynamically insignificant residual shunt (left to right) at APW level and remained so in the follow-up period. Two patients had mild right ventricular (RV) dysfunction which improved in the follow-up period. All patients were discharged on furosemide and enalapril. Of the 12 patients, 7 (58.3%) had moderate PAH on pre-discharge echocardiogram and received additional oral sildenafil.
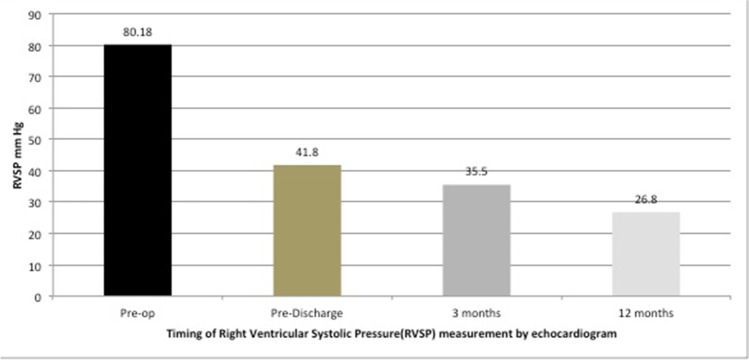
The median follow-up duration was 20.5 months (IQR 7.5–24). All patients were in NYHA class I at the last follow-up. Follow-up echocardiography did not reveal any significant residual shunts necessitating any additional procedure. There was significant reduction in the RVSP values before discharge and at the 3-month and 1-year follow-up (f-ratio 48.8; p < 0.0001) (Fig. 2). At 3 months, 5/12 (41.6%) patients were on enalapril only and 7/12 (58.3%) were on both enalapril and sildenafil. In 8 (66.6%) patients (4/5 in the enalapril group and 4/7 in the enalapril and sildenafil group), the PA pressure had normalized on echocardiographic assessment at 3 months, and it was possible to stop these drugs.
Fig. 2.
Change of right ventricular systolic pressure (RVSP) with time
Discussion
The main finding of our study was that surgery can be carried out in a selective cohort of patients presenting late with APW with an excellent in-hospital and intermediate-term outcome. In our series, 10 (83.3%) patients had type 1 APW (proximal type) and 2 (16.6%) had type 2 APW (distal type) according to the Mori classification [3].
Assessment of operability in late-presenting left-to-right shunts is always of paramount importance. In the developing world, this scenario is not uncommon and there are some reports of late-presenting APW beyond infancy and even adulthood [5–8].
Most of the developed world literature regarding APW repair mostly includes infants and rarely young adults [3, 4]. Operability in late-presenting left-to-right shunts is determined by using clinical examination including baseline saturation, CXR findings, echocardiographic evidence, and, if needed, cardiac catheterization and objective documentation of pulmonary vascular resistance (PVR). In borderline cases, cardiac catheterization with or without pulmonary vasoreactivity testing establishes operability of a patient. A baseline pulmonary vascular resistance index (PVRI) of < 6 WU/m2 and a resistance ratio of < 0.3 have been used as indicators of favorable outcome following biventricular repair, with no need for vasoreactivity testing [9]. Echocardiographic parameters of operability included left heart volume overload characterized by left ventricular internal dimension in diastole > + 2Z score, LA dilatation characterized by LA to aorta ratio > 1.4, brisk pulmonary venous return, flow acceleration across the mitral valve, direction of shunt (left-to-right shunt indicated operability), and significant flow reversal in the descending thoracic aorta (indicates diastolic stealing of blood into the PA which favors operability) [10]. In our series, all the patients were operable from the clinical, radiological, and echocardiographic criteria.
As an institutional protocol, we do not perform oximetry studies and PVRI calculations in clearly operable shunt lesions. Occasionally, we carry out PVRI measurements postoperatively in the presence of moderate or severe residual PAH.
However, none of the patients from this series had significant residual PAH in immediate postoperative period or during follow-up.
The first report of surgical closure of APW was described by Gross in 1952 without CPB. Surgical approaches to APW include transaortic, transpulmonary, and through the APW (sandwich method) [6]. All 12 patients in our series underwent repair through the transaortic approach. The arch repair in 2 patients was done under antegrade cerebral perfusion with distal body under circulatory arrest at a temperature of 20 °C, with an end-to-side anastomosis of the descending aorta to the ascending aorta.
Late-presenting APW with severe pulmonary hypertension preoperatively can have high PA pressures in postoperative period including periods of pulmonary hypertensive crisis. All patients in our series did not have any PA hypertension issues in the immediate postoperative period. Estimation of PA pressure was done by echocardiography using TR gradient. We used RVSP as a measure for estimating PAH postoperatively: RVSP < 35 mmHg, no PAH; ≥ 35–45, mild PAH; ≥ 45–60 mmHg, moderate PAH; and > 60 mmHg, severe PAH [7]. Of the 12 patients, 7 (58.3%) had moderate PAH on discharge and were started on additional sildenafil therapy. Of the 12 patients, 4 (33.3%) needed pulmonary vasodilators beyond 3 months of follow-up.
Current results of surgical repair done early in infancy are excellent [4]. However, many studies have shown that selected patients with APW who remain operable beyond 1 year of age can undergo surgery with satisfactory results [5, 6] (Table 1). Our experience in this study was similar with no mortality in the early and intermediate follow-up period. All patients in our series are in NYHA class I in the follow-up period (median 20.5 months, IQR 7.5–24).
Table 1.
Comparison with published literature
The major limitation of our study is the small sample size. Moreover, our study was single-center and retrospective in nature. Despite these limitations, the study provides an important insight into management of late-presenting APW. A larger, multi-center prospective study is warranted to gather more evidence.
Conclusion
APW does present beyond 1 year of age, especially in the developing countries. Selected patients who remain operable can be safely operated with excellent early- and intermediate-term results. However, a long-term follow-up is needed to assess the regression of raised PA pressures.
Funding
None.
Declarations
Ethics committee approval
As the study was retrospective and record-based, it was exempted from Ethics Committee review (reference number: NSHEV/INV/Non-Reg/2021/003. Date: 24/03/2021).
Human and animal rights consent
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The need for informed consent was waived off by the Ethics Committee.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kutsche LM, Van Mierop L. Anatomy and pathogenesis of aorticopulmonary septal defect. Am J Cardiol. 1987;59:443–447. doi: 10.1016/0002-9149(87)90953-2. [DOI] [PubMed] [Google Scholar]
- 2.Mori K, Ando M, Takao A, Ishikawa S, Imai Y. Distal type of aortopulmonary window. Report of 4 cases.Br Heart J. 1978;40:681–689. [DOI] [PMC free article] [PubMed]
- 3.McElhinney DB, Reddy VM, Tworetzky W, Silverman NH, Hanley FL. Early and late results after repair of aortopulmonary septal defect and associated anomalies in infants < 6 months of age. Am J Cardiol. 1998;81:195–201. [DOI] [PubMed]
- 4.Backer CL, Mavroudis C. Surgical management of aortopulmonary window: a 40-year experience. Eur J Cardiothorac Surg. 2002;21:773–779. doi: 10.1016/S1010-7940(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 5.Talwar S, Siddharth B, Gupta SK, et al. Aortopulmonary window: results of repair beyond infancy. Interact Cardiovasc Thorac Surg. 2017;25:740–744. [DOI] [PubMed]
- 6.Gowda D, Gajjar T, Rao JN, et al. Surgical management of aortopulmonary window: 24 years of experience and lessons learned. Interact Cardiovasc Thorac Surg. 2017;25:302–309. doi: 10.1093/icvts/ivx099. [DOI] [PubMed] [Google Scholar]
- 7.Myers PO, Lador F, Hachulla A-L, et al. Unrestrictive Aortopulmonary window: extreme presentation as non-Eisenmenger in a 30-year-old patient. Circulation. 2016;133:1907-1910. [DOI] [PubMed]
- 8.El Dick J, El-Rassi I, Tayeh C, Bitar F, Arabi M. Aortopulmonary window in adults: A rare entity leading to Eisenmenger syndrome. Echocardiography. 2019;36:1173-1178. [DOI] [PubMed]
- 9.Myers PO, Tissot C, Beghetti M. Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circ J .2014;78: 4–11. [DOI] [PubMed]
- 10.Awasthy N, Radhakrishnan S. Stepwise evaluation of left to right shunts by echocardiography. Indian Heart J. 2013;65:201–218. doi: 10.1016/j.ihj.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]