Abstract
This study reports genome wide characterization and development of first set of microsatellite markers through in silico analysis of eight sequenced Xanthomonas axonopodis pv. punicae strains available in the public database. SSR survey resulted in identification of ~ 4638 perfect SSRs, with mean marker frequency 901 SSRs/Mb and densitiy of 11,006 bp/Mb aross the eight genomes. Frequency distribution graphs revealed hexa-nucleotide repeats were more prominent fowllowed by tri-, tetra-, di- and penta-nucleotides in the analysed genomes. We desinged 2927 SSR primers that are specific to the strain LMG 859 and ePCR confirmed on seven other Xap genomes. This resulted in identification of 542 informative SSRs that are producing single amplicons, from which 66 primers were successfully validated through wet lab experiments on eight Xap isolates of pomegranate. Furthermore, utility of these SSRs were demostrated by analysing molecular diversity among 22 Xap isolates using 20 Xap_SSR primers. SSRs revealed moderate genetic diversity among Xap isolates (61%) and grouped 11 isolates that are repersenting six different states into one cluster. This proved the earlier evidence of wider spread of ST3 type Xap acoss India using Multi locus Sequence Typing (MLST) technique. In summary, Xap_SSR will serve as powerful genomics tools that would helps in monitoring of population dynamics, taxonomy, epidomology and quarantine aspects in bacterial blight pathogen through development of microsatellite based Multilocus Variable number of Tandem repeat analysis (MLVA) in future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03209-z.
Keywords: Microsatellite, Xanthomonas axonopodis, Bacterial blight, Molecular diversity, Pomegranate, ePCR
Introduction
Pomegranate (Punica granatum L.) is an economically important perennial fruit crop, which is predominantly cultivated in tropical and subtropical regions of the world comprising India, Pakistan, Afghanistan, Iran, Israel, Spain, Turkey and South Africa (Kumar et al. 2019). Globally, India ranks first in pomegranate cultivation with an area 2.83 lakh ha and production of 31.83 lakh MT (http://agricoop.gov.in 2019–20). The 70% of pomegranate area in India is in Maharashtra state, followed by Karnataka, Gujarat, Telangana, Andhra Pradesh and Rajasthan. Although India is leading in pomegranate area and production, its average productivity remains low (12 t/ha). This could be due to losses encountered though various biotic and abiotic stresses during different growing seasons. Among the various biotic stresses that constrain pomegranate production, bacterial blight caused by Xanthomonas axonopodis pv. punicae is considered to be most devastating disease in India. A rapid increase in pomegranate area with monoculture of variety Bhagawa might be a reason for enhanced vulnerability to the disease (NRCP 2015). This disease alone can cause up to 30–100% yield losses under congenial conditions and has become a serious threat to pomegranate export due to its significant effect on the fruit quality (Sharma et al. 2012a, b). In India, this disease has witnessed severe economic losses since 2000 (Sharma et al. 2017). Currently, India is exporting its produce to the ASEAN, Gulf, European Union and Pacific Rim countries (Japan, South Korea and China). There is a great prospect of increasing export to other countries through producing export quality fruits by managing bacterial blight disease. However, with recent expansion of this disease to other pomegranate-growing countries, such as Pakistan, South Africa and Turkey, has gained international attention (Akhtar and Bhatti 1992; Petersen et al. 2010; Icoz et al. 2014).
Bacterial blight was first reported in India by Hingorani and Mehta (1952). The pathogen is known to attacks all aerial parts of the plant including leaves, twigs, and fruits leading to considerable reduction in fruit yield, quality and market value (Kumar et al. 2019). Visual symptoms of bacterial blight appears in the form of one to several small, regular to irregular greyish black water-soaked spots on leaves that later turn into dark brown to black spots with yellow halo or a water-soaked margin (Chand and Kishun, 1991). Pomegranate fruits remain vulnerable to blight at all the growth stages. Sharma et al. (2012a) reported that the bacterial blight infection on fruits is restricted to the rind, initially a water-soaked lesions will be noticed on the fruit pericarp, which later turn into brown to black spots that enlarge and coalesce to cover large areas. During dry conditions, along the lesions small L- or Y-shaped cracks appear on the fruits to split open entire fruit, thus rendering the fruit unmarketable in fresh form.
Presently the disease diagnosis relies heavily on visual inspection of plant parts and ooze tests. This pathogen is known to survive on the infected plant parts and debris in the soil for up to one year and it can easily disseminate through rain splashes, irrigation water, insect vectors and pruning tools (Sharma et al. 2015). Previous report confirmed X. axonopodis pv. punicae and Xanthomonas axonopodis pv. citri, the causal agent of citrus canker have > 99% genome identity (Sharma et al. 2012b). With respect to disease epidemiology as that of citrus canker pathogen, bacterial blight pathogen can naturally dispersed within individual trees or between neighboring trees in droplets, by splashing or wind‐driven dispersal, resulting in aggregated disease patterns. In addition to regular short‐range propagation, extreme weather events and human activities sporadically cause long‐distance dispersal, which can have a major epidemiological impact (Irey et al. 2006).
Therefore, now a days, genotyping using advanced genomics tools have gained popularity for assessing dispersal patterns of bacteria. For this, studying the population structures of bacterial at spatial scales is important. Deciphering the genetic structure of bacterial populations at smaller spatial scales is still challenging. Earlier, various marker techniques were employed for Xap population studies viz., Enterobacter Repetitive Intergenic Consensus (ERIC)–PCR, Random Amplified Polymorphic DNA (RAPD), Inter Simple Sequence Repeat (ISSR) (Mondal and Mani 2009; Raghuwanshi et al. 2013; Chavan et al. 2017), and more recently Multilocus Sequence Typing (MLST) based on housekeeping genes (Kumar et al. 2019). These all techniques have low resolution with respect to studying monomorphic pathogens. In contrast, Leduc et al. (2015) reported a new techniques called Multilocus Variable number of Tandem repeat analysis (MLVA) named MLVA-31, which is based on long tandem repeat sequences (i.e., minisatellites) and suited to global epidemiology analyses of the pathogen. MLVA-14 (14 loci) based on short tandem repeat sequences (i.e., microsatellites) which provides a very high discriminatory power, allowing the analysis of population structure of X. citri pv. citri at small to medium spatio-temporal scales. Recently, Pruvost et al. (2019) reported culture-independent molecular epidemiology study of X. citri pv. citri using microsatellite genotyping technique to detect population diversification within lesions or polyclonal infections. Hence, microsatellite genotyping has proved useful for outbreak investigation in X. citri pv. citri’s native area (Vernière et al. 2014). Therefore, to study bacterial population at micro-geographic scales it requires high‐resolution markers that can be genotyped with a high throughput. Wherein, simple sequence repeats (SSR) represents an ideal molecular marker system for analyzing prokaryotic genomes because of their high polymorphism, abundant, easy to use and generate reproducible fragments (Field and Wills, 1996). Recently, microsatellite and minisatellite based DNA fingerprinting was applied for fast and reliable molecular detection of many fungal pathogens in woody plants (Luchi et al. 2020). In this context, whole genome sequence of pathogens would serve as an important genomic resource for developing mini or microsatellite based markers (Sharma et al. 2012b). Jaiswal and Pandey (2014) reported in silico mining of SSRs from whole genome sequence of Xanthomonas sp viz., X. axonopodis pv. citri, X. oryzae pv. oryzae and X. campestris pv. campestris. However, till date no report available on genome-wide characterization of microsatellites from X. axonopodis pv. punicae. Therefore, here we performed SSR mining in the whole genome sequences of eight Xap strain available at NCBI database. We further examined distribution and abundance of SSRs in the genome. The primary objectives of this study include the development of genome-wide informative microsatellite markers through in silico comparative genome analysis of sequenced Xap genomes, and to demonstrate the utility of SSR markers over MLST for molecular diversity analysis in Xap isolates. The markers generated through this study will serve as a potential genomic resource for development of microsatellite based MLVA for future epidemiology and outbreak study of X. axonopodis pv. punicae in native pomegranate growing areas of India.
Materials and methods
Source of Xanthomonas axonopodis pv. punicae isolates
Twenty two Xap isolates were obtained from ICAR–National Research Center on Pomegranate, Solapur, India. These isolates were collected over periods (2013–2017) from six pomegranate growing states of India and were well maintained in 40% glycerol stock at − 80 °C for long term storage at Pathology Lab, NRCP, Solapur. The details of the isolates are given in Table 1, and each isolate was designated with tentative Xap code number. The same isolates were also used previously for genotyping experiments using MLST technique (Kumar et al. 2019).
Table 1.
Details of Xanthomonas axonopodis pv. punicae isolates used in this study
| Sl. No | Isolate | Place of collection | State | Year of collection |
|---|---|---|---|---|
| 1 | XAP-92 | Kullu | Himachal Pradesh | 2013 |
| 2 | XAP-93 | Sangali | Maharashtra | 2014 |
| 3 | XAP-94 | Mahebubnagar | Telangana | 2014 |
| 4 | XAP-96 | Kopargaon, Ahmednagar | Maharashtra | 2014 |
| 5 | XAP-98 | Yeola, Nashik | Maharashtra | 2015 |
| 6 | XAP-99 | Kauthali, Solapur | Maharashtra | 2015 |
| 7 | XAP-100 | Kasegaon, Pandharpur, Solapur | Maharashtra | 2015 |
| 8 | XAP-101 | Shirwal, Akkalkot, Solapur | Maharashtra | 2015 |
| 9 | XAP-102 | Borgaon, Akkalkot, Solapur | Maharashtra | 2015 |
| 10 | XAP-103 | Chale, Pandharpur, Solapur | Maharashtra | 2015 |
| 11 | XAP-104 | Gopalpur, Pandharpur, Solapur | Maharashtra | 2015 |
| 12 | XAP-105 | Wadegaon, Sangola, Solapur | Maharashtra | 2015 |
| 13 | XAP-106 | Kasegaon, Pandharpur, Solapur | Maharashtra | 2015 |
| 14 | XAP-107 | Mohol, Solapur | Maharashtra | 2015 |
| 15 | XAP-112 | Gulburga | Karnataka | 2016 |
| 16 | XAP-114 | Mangalwedha, Solapur | Maharashtra | 2016 |
| 17 | XAP-115 | Bhognipur, Kanpur | Uttar Pradesh | 2016 |
| 18 | XAP-116 | KVK, Danta, Badmer | Rajasthan | 2016 |
| 19 | XAP-118 | Hiriyur, Chitradurga | Karnataka | 2016 |
| 20 | XAP-120 | Mangewadi, Sangola, Solapur | Maharashtra | 2017 |
| 21 | XAP-124 | Kandhari, Ghansangvi, Jalna | Maharashtra | 2017 |
| 22 | XAP-125 | Kandhari, Ghansangvi, Jalna | Maharashtra | 2017 |
Pathogenicity tests
For the pathogenicity test, 6-month-old pomegranate plants cv. Bhagawa are used that were raised in polybags of size 12 × 16 cm, filled with equal proportion of sand: soil: farmyard manure and were inoculated with isolates as per the protocol described by Sharma et al. (2017). Before proceeding for challenge inoculation, all the plants are briefly covered with transparent polybags for 24 h. Later, leaves were sprayed with bacterial cell suspension of 1 × 106 CFU/ml and plants were covered with transparent polybags for 48 h to maintain humidity. Negative control plants were inoculated with sterile distilled water. All the plants were incubated in a polyhouse at 30 ± 2 °C and 65–70% relative humidity. The plants were visually observed at regular intervals for symptoms. The symptomatic plants were confirmed for pathogen identity using Xap specific XopQ primer (F: GCGAGGAACTTGGAATGCTC and R: AGGTCGAAGGCTTTTTGCG). The PCR program was set as: initial denaturation at 94 °C for 4 min, followed by 35 cycles of denaturation (94 °C for 15 s), annealing (58 °C for 30 s), extension (72 °C for 45 s) and the final extension at 72 °C for 10 min. The PCR product was resolved on 2% agarose gel and visualised under UV.
Designing of X. axonopodis pv. punicae specific SSRs from genome sequences
Complete genome sequence of X. axonopodis pv. punicae strain LMG859 (Sharma et al. 2012b) and other related strains viz., LMG7439, LMG7504, BD0022, BD0023, BD0025, Bagalkot (Radhika et al. 2021) and NCPPB3563 were retrieved from the NCBI database (http://www.ncbi.nlm.nih.gov). These genome sequences were surveyed sequentially for the presence of perfect and compound SSR repeats using Krait: ultra-fast SSR search module (Du et al. 2018). For SSR search, parameters such as 2–6 nucleotide motifs with minimum repeat unit of 12 for mono-, 6 for di-, 4 for tri-, 3 for tetra-, 3 for penta- and 2 for hexa-nucleotides were used. The two microsatellites interrupted by 100 bases were defined as compound microsatellites. Microsoft Excel was used to draw frequency distribution graphs based on Krait statistics results. Primer designing was performed for the reference genome sequence of LMG859 strain using the Primer module of Krait. Primers were designed to generate amplicons of 100–400 bp in length with the following parameters: primer length (bp) 18–20, with 19 as the optimum; GC content (%) 40–70; Tm (°C) 52–60, with 55 as the optimum. The other parameters used were as that of default program values.
In silico PCR validation of Xap_SSRs
To evaluate amplification efficiency and specificity of newly designed Xap_SSRs and to map the designed marker on genome sequence of LMG859 strain, the GMATA (Genome-wide Microsatellite Analyzing Tool Package) software (Wang and Wang, 2016) was used to performed an in silico amplification by calling the ePCR algorithm (Schuler, 1997). The settings for e-PCR were margin 3000, no gap in primer sequence, no mismatch in primer sequence, allowed size range of 100–1000, word size (-w) 12 and contiguous word (-f) 1. The output file (.emap) provided the detailed amplification patterns of the markers with calculated amplicon sizes and target positions on single chromosome, and identified single-locus and multi-locus markers. Finally, all the LMG859 specific Xap_SSR primers were ePCR confirmed on genome sequences of other seven Xap strains viz., LMG7439, LMG7504, BD0022, BD0023, BD0025, Bagalkot and NCPPB3563.
Wet-lab validation and genotyping of Xap_SSRs
Genomic DNA was extracted from pure cultures of 22 Xap isolates using column based HiPer® Bacterial genomic DNA extraction Kit (Himedia, India). The quality and concentration of DNA samples were determined on 0.8% agarose gel using uncut lambda DNA as a standard. Final dilution of 10 ng/l was made for subsequent PCR amplifications. For PCR experiment, a sub set of 66 Xap_SSR primers were selected that are physically located on chromosome of strain LMG859 using GenomeVx (Conant and Wolfe 2008) (Table S1). For initial screening DNA samples of eight Xap isolates, namely, XAP-92, XAP-93, XAP-94, XAP-96, XAP-98, XAP-99, XAP-100 and XAP-101 were selected for PCR using Prime-96™ Thermal Cycler (Himedia, India). Subsequently, twenty primers were selected based on their clear amplification profile to screen on 22 Xap isolates to evaluate molecular diversity (Table S2). Each PCR reaction was performed with 10 ng of genomic DNA, in 10 μl reaction volume containing 1.0 μl of 10X PCR buffer, 1 μl (1 mM dNTP mix), 0.5 μl each of forward and reverse primers (10 pmol), 0.2 μl of Taq DNA polymerase 5U/ μl (Himedia, India) and 1 μl (10 ng) of template DNA. PCR condition was set as initial denaturation at 94 °C for 5 min, followed by 36 cycles of 1 min at 94 °C, 1 min at 55 °C, and 2 min at 72 °C and a final extension of 7 min at 72 °C. PCR products were separated on 3% metaphor agarose gels containing 0.5 μg/ml EtBr and 1X (TBE) running buffer at 130 V for 4 h, visualized and gel documented using Vilbert dourmet system (France).
Molecular diversity analysis
The genotypic data scored for 22 Xap isolates using 20 Xap-SSR primers were used to estimate the important genetic diversity parameters, such as number of alleles (Na), effective number of alleles (Ne), and polymorphic information content (PIC) using GenAlEx v. 6.5 software (Peakall and Smouse 2012). Furthermore, the UPGMA (Unweighted pair group method with an arithmetic mean) based neighbor joining tree and principal coordination analysis (PCoA) was performed using DARwin ver. 6.0.13 software (Perrier and Jacquemoud-Collet 2006), based on Jaccard’s dissimilarity coefficients (Jaccard 1908).
Results
Pathogenicity test of Xap isolates
All isolates were pathogenic on pomegranate cv. Bhagawa and induced symptoms such as regular to irregular greyish water-soaked lesions or oily spots appeared on abaxial surface and that looked transparent yellow when observed against light. The lesions further enlarged and turned dark brown to black, eventually becoming visible on both leaf surfaces. These lesions coalesced to form larger lesions which resulted in brighten appearance (Figs. S1 and S2). The symptoms observed on inoculated plants were similar to those on naturally infected plants. The Koch postulates were proved through reisolation of yellow pigmented colonies of bacteria from inoculated plants and their identity was confirmed by XopQ PCR with an amplicon size of 190 bp (Fig. S3).
Identification of X. axonopodis pv. punicae specific SSRs
In the present study, whole genome sequences of eight strains of X. axonopodis pv. punicae, available at NCBI were searched for microsatellites. For SSR survey, the genome sizes varied between 5.08 Mb (BD0023) and 5.43 Mb (Bagalkot) among eight strains. A total of 4599 (BD0023) to 4824 (Bagalkot) perfect SSRs were detected with minimum and maximum frequencies of 887.3 SSRs/Mb (Bagalkot) to 904.4 (LMG7504) and densities of 10,826.6 bp/Mb (Bagalkot) to 11,054.9 bp/Mb (LMG7504), respectively among eight strains (Table 2). Apart from this, we noticed 348 (BD0023) to 364 (LMG859) compound SSRs distributed across the genomes.
Table 2.
In silico mining of SSRs in the genomes sequences of eight Xanthomonas axonopodis pv. punicae strains
| SSR mining in genomes of different Xap strains | LMG859 | LMG7439 | LMG7504 | BD0022 | BD0023 | BD0025 | Bagalkot | NCPPB3563 | Mean |
|---|---|---|---|---|---|---|---|---|---|
| Examined sequences size (bp): | 5,137,220 | 5,105,751 | 5,088,472 | 5,087,847 | 5,087,738 | 5,087,838 | 5,436,898 | 5,135,943 | 51,45,963 |
| Total number of perfect SSRs: | 4645 | 4617 | 4602 | 4600 | 4599 | 4601 | 4824 | 4619 | 4638 |
| Total length of perfect SSRs (bp): | 56,742 | 56,338 | 56,253 | 56,157 | 56,147 | 56,174 | 58,863 | 56,337 | 56,626 |
| Relative abundance of SSRs (loci/ Mb): | 904.2 | 904.3 | 904.4 | 904.1 | 903.9 | 904.3 | 887.3 | 899.4 | 901 |
| Relative density for SSRs (bp/Mb): | 11,045.3 | 11,034.2 | 11,054.9 | 11,037.5 | 11,035.7 | 11,040.8 | 10,826.6 | 10,969.2 | 11,006 |
| Total number of compound SSRs | 364 | 354 | 355 | 349 | 348 | 353 | 362 | 349 | 354 |
Examination of the number of SSRs and their relative abundance (loci/Mb) across eight Xap genomes revealed, Bagalkot strain had the highest number of SSRs (4824) compared to other strains (~ 4600) (Table 3, Table & Fig. S4). However, with respect to relative abundance of SSRs, Bagalkot (887.3) and NCPPB3563 (899.4) showed lowest values as compared to other strains (~ 904, 11,030) (Table 2). Similarly, the frequency distribution for different SSR repeating units revealed, abundance of hexa-nucleotides, followed by tri-, tetra-, di- and penta-nucleotides among Xap genomes (Table 3). It was interesting to note that Bagalkot strain had the higher number of hexa- (4451), tri- (210), tertra- (90) and penta-nucleotide (23) repeats as compared to other strains; however, it has maintained the relative abundance same as that of other strains.
Table 3.
Numbers and density of the SSRs identified in the genomes of eight Xanthomonas citri pv. punicae strains
| Different Xap strains | SSR numbers | Relative abundance (loci/Mb) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome size (MB) | MNR | DNR | TNR | TTR | PNR | HNR | Total | MNR | DNR | TNR | TTR | PNR | HNR | Total | |
| LMG859 | 5.14 | 4 | 51 | 194 | 85 | 20 | 4291 | 4645 | 0.8 | 9.9 | 37.8 | 16.5 | 3.9 | 835.3 | 904.2 |
| LMG7439 | 5.10 | 4 | 49 | 190 | 83 | 19 | 4272 | 4617 | 0.8 | 9.6 | 37.2 | 16.3 | 3.7 | 836.7 | 904.3 |
| LMG7504 | 5.09 | 2 | 48 | 194 | 83 | 20 | 4255 | 4602 | 0.4 | 9.4 | 38.1 | 16.3 | 3.9 | 836.2 | 904.4 |
| BD0022 | 5.09 | 1 | 48 | 197 | 83 | 19 | 4252 | 4600 | 0.2 | 9.4 | 38.7 | 16.3 | 3.7 | 835.7 | 904.1 |
| BD0023 | 5.09 | 3 | 49 | 196 | 83 | 19 | 4249 | 4599 | 0.6 | 9.6 | 38.5 | 16.3 | 3.7 | 835.1 | 903.9 |
| BD0025 | 5.09 | 4 | 48 | 193 | 83 | 20 | 4253 | 4601 | 0.8 | 9.4 | 37.9 | 16.3 | 3.9 | 835.9 | 904.3 |
| Bagalkot | 5.44 | – | 50 | 210 | 90 | 23 | 4451 | 4824 | – | 9.2 | 38.6 | 16.5 | 4.2 | 818.7 | 887.3 |
| NCPPB3563 | 5.14 | 3 | 49 | 199 | 82 | 19 | 4267 | 4619 | 0.6 | 9.5 | 38.7 | 16.0 | 3.7 | 830.8 | 899.4 |
MNR, DNR, TNR, TTR, PNR, and HNR indicate mono-, di-, tri-, tetra-, penta-, and hexa-nucleotide SSRs, respectively
With respect to distribution for most abundant motif categories in LMG859 strain, the motif categories ACCGCG/ACGCGG type had the highest occurrence (9.6%), followed by CCG/ACC (2.95%), CG/AG (1.1%), CCGG/AGCC (0.78%) and AGCGC/CCGCG (0.22%). Specifically, the major motifs of mono- to hexa-nucleotide repeats were C, CG, CCG, CCGG, AGCGC/CCGCG and ACCGCG, respectively, in which ACCGCG motifs were rich with relative abundance of 49.64 loci/Mb in the genome, followed by CCG (15.57 loci/Mb) and CG (9.73 loci/Mb). Overall the SSRs with CG-rich repeats were found more dominated in the Xap genome.
ePCR validation of Xap specific SSR primers
Based on the results of SSR survey across Xap genomes, we selected the first reference genome of LMG859 strain for SSR primer designing. As a result we successfully designed 2927 Xap_SSR primers (Table S1). The majority of these primers were specific to hexa-nucleotide motifs (2661 primers, 90.91%), followed by tri- (159, 5.43%), di- (49, 1.67%), tetra- (45, 1.54%) and penta- (13, 0.44%) repeats, respectively. To assess the amplification specificity of developed Xap_SSRs, in silico ePCR was performed for 2927 Xap_SSR primers across eight Xap genomes. As a result 2906 (99.28%) primers got validated, of which 542 produced one allele, while remaining had more than one allele (920 with two, 1234 with three and 212 with ≥ 4 alleles). Concerning individual genome, total 2927 SSRs (100%) were validated in LMG859 genome, whereas 2916 SSRs amplified (99.62%) in BD0022, BD0023 and BD0025 genomes, 2903–2904 (99.21%) in LMG7439 and LMG7504, and 2884–2885 (98.57%) in Bagalkot and NCPPB3563 genomes, respectively. Of these, 522–558 primers showed single locus amplifications across the Xap genomes. The genome-wide details of four groups of SSRs producing, i.e., single, two, three and ≥ four alleles are presented in Table 4.
Table 4.
Experimental validation of Xap_SSRs through ePCR or eMapping across the eight Xap genomes
| Sl. no | Different Xap strains evaluated for ePCR | e PCR validation of XAP_SSRs primers across genomes | |||||
|---|---|---|---|---|---|---|---|
| Allele no | |||||||
| Number of LMG859 primers got validated | One | Two | Three | ≥ Four | Total (%) | ||
| 1 | LMG859 | 2927 | 545 (18.62%) | 931 (31.81%) | 1230 (42.02%) | 221 (7.55%) | 100 |
| 2 | LMG7439 | 2904 | 546 (18.80%) | 933 (32.13%) | 1214 (41.80%) | 211 (7.27%) | 100 |
| 3 | LMG7504 | 2903 | 550 (18.95%) | 924 (31.83%) | 1216 (41.89%) | 213 (7.34%) | 100 |
| 4 | BD0022 | 2916 | 551(18.90%) | 901 (30.90%) | 1251 (42.90%) | 213 (7.30%) | 100 |
| 5 | BD0023 | 2916 | 558 (19.14%) | 894 (30.66%) | 1251 (42.90%) | 213 (7.30%) | 100 |
| 6 | BD0025 | 2916 | 542 (18.59%) | 936 (32.10%) | 1224 (41.98%) | 214 (7.34%) | 100 |
| 7 | Bagalkot | 2884 | 522 (18.10%) | 920 (31.90%) | 1242 (43.07%) | 200 (6.93%) | 100 |
| 8 | NCPPB3563 | 2885 | 524 (18.16%) | 921 (31.92%) | 1227 (42.53%) | 213 (7.38%) | 100 |
| Average | 2906 | 542 | 920 | 1232 | 212 | ||
Wet lab validation of Xap_SSRs distributed across the genome
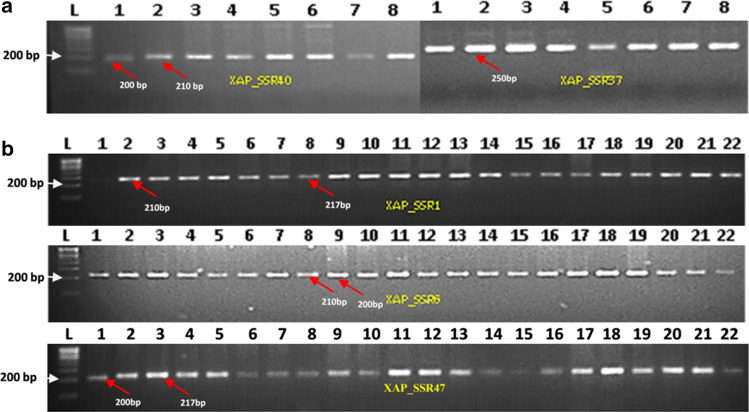
A sub set of 66 Xap primers were screened (Table S1) that are distributed throughout genome, as shown in physical positions of markers on LMG859 chromosome by Circos graph (Fig. 1). Out of 66 primers, 57 (86.36%) showed clear amplifications on eight Xap isolates and nine primers did not show amplification (Fig. 2a, Table S1). Furthermore, 20 primers were selected randomly based on their clear amplification profiles and screened on 22 Xap isolates for molecular diversity analysis.
Fig. 1.
Circos graph depicting physical locations of 66 Xap_SSRs on the chromosome of reference strain (LMG859)
Fig. 2.
Gel image showing a initial PCR validation of SSRs on eight Xap isolates, and b screening on 22 Xap isolates (where, L-100 bp DNA ladder for lanes 1–22 Xap isolates as listed in Table 1)
Molecular diversity analysis among Xap isolates
The SSR assay produced a total of 40 alleles across 22 isolates (Table 5). The ‘Ne’ ranged from 1.11 (Xap_SSR61) to 1.77 (Xap_SSR3) with an average of 1.43 alleles per locus. Similarly, the ‘I’ ranged from 0.21 (XAP_SSR61) to 0.63 (Xap_SSR3) with mean of 0.46. Based on the PIC values, 12 SSRs (Xap_SSRs 1, 3, 8, 9, 10, 22, 25, 32, 34, 40, 54 and 63) exhibited moderate level of polymorphism (0.5 < PIC > 0.25), whereas eight (Xap_SSRs 13, 18, 26, 37, 50, 57, 61 and 70) were less polymorphic (PIC < 0.25). Representative gel images of SSR profiles on 22 Xap isolates are shown in Fig. 2b.
Table 5.
Marker statistics for twenty XAP_SSRs loci screened on 22 Xap isolates
| Sl. no | Marker | Na | Ne | MAF | I | PIC |
|---|---|---|---|---|---|---|
| 1 | Xap_SSR1 | 2 | 1.42 | 0.82 | 0.47 | 0.25 |
| 2 | Xap _SSR3 | 2 | 1.77 | 0.68 | 0.63 | 0.34 |
| 3 | Xap _SSR8 | 2 | 1.45 | 0.81 | 0.49 | 0.26 |
| 4 | Xap _SSR9 | 2 | 1.42 | 0.82 | 0.47 | 0.25 |
| 5 | Xap _SSR10 | 2 | 1.66 | 0.73 | 0.59 | 0.32 |
| 6 | Xap _SSR13 | 2 | 1.25 | 0.89 | 0.35 | 0.18 |
| 7 | Xap _SSR18 | 2 | 1.20 | 0.91 | 0.31 | 0.15 |
| 8 | Xap _SSR22 | 2 | 1.54 | 0.77 | 0.54 | 0.29 |
| 9 | Xap _SSR25 | 2 | 1.57 | 0.76 | 0.55 | 0.30 |
| 10 | Xap _SSR26 | 2 | 1.32 | 0.86 | 0.41 | 0.21 |
| 11 | Xap _SSR32 | 2 | 1.66 | 0.73 | 0.59 | 0.32 |
| 12 | Xap _SSR34 | 2 | 1.71 | 0.71 | 0.61 | 0.33 |
| 13 | Xap _SSR37 | 2 | 1.36 | 0.84 | 0.44 | 0.23 |
| 14 | Xap _SSR40 | 2 | 1.42 | 0.82 | 0.47 | 0.25 |
| 15 | Xap _SSR50 | 2 | 1.32 | 0.86 | 0.41 | 0.21 |
| 16 | Xap _SSR54 | 2 | 1.54 | 0.77 | 0.54 | 0.29 |
| 17 | Xap _SSR57 | 2 | 1.20 | 0.91 | 0.31 | 0.15 |
| 18 | Xap _SSR61 | 2 | 1.11 | 0.95 | 0.21 | 0.09 |
| 19 | Xap _SSR63 | 2 | 1.54 | 0.77 | 0.54 | 0.29 |
| 20 | Xap _SSR70 | 2 | 1.20 | 0.91 | 0.31 | 0.15 |
| Mean | 2 | 1.43 | 0.82 | 0.46 | 0.24 |
Na number of alleles, Ne number of effective alleles, MAF major allele frequency, I shannon’s information index, PIC polymorphism information content
Cluster analysis was performed based on Jaccards dissimilarity values that ranged from 0.14 (between XAP-125 and XAP-103) to 0.75 (XAP-107 and XAP-96) (Table S3). As shown in Fig. 3a, the phylogenetic tree grouped all isolates into four major clusters. Cluster 1 comprised 11 Xap isolates represented from five different states, i.e., Maharashtra, Karnataka, Telangana, Himachal Pradesh, Uttar Pradesh and Rajasthan. Cluster 2 contained 6 isolates, mainly from Solapur district and Jalna district of Maharashtra. Cluster 3 comprised two isolate from Solapur district of Maharashtra. Similarly, Cluster 4 constituted isolates from Ahmednagar and Nashik districts of Maharashtra. With respect to genetic dissimilarity, isolates of cluster 1 showed 26–66% diversity, cluster 2 (14–48%), cluster 3 (24%) and cluster 4 (35–54%) diversity. Furthermore, the result PCoA also divided 22 Xap isolates into four clusters (Fig. 3b). The axes 1 and 2 explained 19.39 and 14.25% of the total variance, respectively. Results of PCoA were in good agreement with the phylogenetic analysis. The isolates falling under cluster 1 are very sparsely distributed based on their genetic distances suggesting higher diversity (66%). Axis 1 explained higher variance and clearly separated cluster 1 form other three clusters 2, 3, and 4. However, Axis 2 could clearly separate cluster 2 from cluster 3. Among the clusters isolates belong to cluster 4 found most diverse as depicted in PCoA plot based on their genetic distances.
Fig. 3.
Dendrogram (a) and PCA plot (b) showing genetic relationships between 22 Xap isolates based on SSR marker data
Discussion
Bacterial blight is a major problem for pomegranate cultivation (Petersen et al. 2010; Mondal et al. 2012), and underlying reasons for frequent outbreaks of blight in India are poorly understood. Currently, limited literature is available with respect to epidemiology of pomegranate bacterial blight and no literature available on genetic differences that exist within the pathovar that these might be related to their differential virulence patterns (Mondal and Mani 2009). However, conflicting reports are available in X. axonopodis pv. punicae, pertaining to pathogenic and genetic variability (Raghuwanshi et al. 2013; Chavan et al. 2017). The identification of disease causing ability of distinct bacterial strain is very important with respect to understanding the evolution of pathogenicity, the emergence and spread. This can be accomplished through pathogenicity test of isolates collected from different geographical locations. In this study, all the 22 isolates tested were found pathogenic on pomegranate cv. Bhagawa showing typical symptoms as that of naturally infected plants. Also re-isolated typical colonies as that of bacterial blight pathogen from inoculated plant and PCR confirmed using diagnostic markers. Similarly, Kumar et al. (2019) also previously performed pathogenicity tests of X. axonopodis punicae isolates collected from different states of India and confirmed that all isolates were pathogenic on pomegranate cultivar, Bhagawa.
In-order to devise effective disease and crop management practices for bacterial blight in pomegranate studying the nature of pathogen, its population biology and genetic diversity is very important (Kumar et al. (2019). Therefore, it is imperative to undertake characterization of this pathogen at the molecular level (Eknath et al. 2015). Understanding pathogen genetic diversity and population structure on the landscape scale is crucial to assess the potential of pathogen populations to spread, increase aggressiveness, development of bactericide resistance, and overcome host resistance. Earlier, various research groups employed diverse DNA marker systems to understand genetic diversity among different Xap isolates, i.e., ERIC-PCR, RAPD, ISSR, BOX- PCR and MLST (Mondal and Mani 2009; Raghuwanshi et al. 2013; Kiran Kumar and Khan 2016; Chavan et al. 2017; Kumar et al. 2019). All these techniques were found inadequate in terms of genome coverage and hence remain inconclusive regarding the information they generated.
Recent advances made through NGS technologies has resulted in whole genome sequencing of many Xap strains in addition to first strain LMG 859 (Sharma et al. 2012b). This has opened unprecedented opportunities for large-scale development of microsatellite markers for basic and applied research. In the context, short repetitive regions called variable number of tandem repeats (VNTRs) could be valuable tool for molecular studies, which being potentially polymorphic could effectively differentiate bacterial strains (Baldi and La Porta 2017). Therefore, among the various molecular marker systems employed, the SSR are reported to be highly informative and reproducible (Belkum et al. 1998).
SSR survey in the whole genome sequences of eight Xap strains revealed 4599–4824 perfect SSRs with minor variations in SSR content and distributions. Gur-Arie et al. (2000) discovered a total of 2,35,495 SSRs resulting from a genome-wide scan of E. coli strain K12. However, Jaiswal and Pandey (2014) reported limited number of SSR motifs of 640, 377 and 541 SSRs in the whole genomes of X. axonopodis pv. citri, X. campestris pv. campestris, X. oryzae pv. oryzae, respectively, using SSRIT software. The differences in SSR counts observed could be result of use of different SSR mining software. This was very clearly elucidated earlier by comparing five software (MISA, TRA, TROLL, SSRIT and SSR primer) for mining EST–SSRs in cacao (Riju et al. 2009).
In this study, Krait software was used for mining and designing of SSRs from Xap genome. Du et al. (2018) developed this Krait software as an ultrafast tool for genome-wide survey of microsatellites and clearly demonstrates its efficiency in Homo sapiens (3.25 Gb), Arabidopsis thaliana (119.67 Mb) and Escherichia coli (4.64 Mb) genomes by comparing the run time with the other SSR-identification tools, such as MISA, MSDB, MSAT commander, GMATA and SciRoKo. The frequency distribution for different SSR repeat units in the eight Xap genomes revealed, abundance of hexa-nucleotides, followed by tri-, tetra-, di- and penta-nucleotides. Tardiani et al. (2014) identified 429 SSRs from complete genome sequence of strain GPE PC73 that causes leaf scald of sugarcane (Xanthomonas albilineans). In line with our observations, the authors reported abundance of hexa- nucleotides (33%) followed by tri-nucleotides (26%).
Taking LMG859 strain as reference, we observed from mono- to hexa-nucleotides the frequency of motif types, i.e., C, CG, CCG, CCGG, AGCGC/CCGCG and ACCGCG were found higher. Similarly, Jaiswal and Pandey (2014) reported higher frequency for (GC)n and (CG)n motifs in three Xanthomas species, i.e., X. axonopodis pv. citri, X. oryzae pv. oryzae, X. campestris pv. Campestris. For trinucleotide repeats they found (CGC)n followed by (GCC)n, (GCG)n, (CAC)n, (CCG)n, (CCA)n, (TGC)n, (GCC)n (CAA)n, (TGG)n, (GCA)n were more abundant in X. campestris pv. campestris and others are present in less frequent repeats spread in all three genomes. For hexa-nucleotide (ATGGCC) was found higher and other hexa nucleotide repeats were present in equal frequencies in X. axonopodis pv. citri.
Through ePCR 2906 (99.28%) primers were successfully got validated across Xap genomes, of which 542 produced single alleles. Previous evidence suggests that SSRs developed in silico have been successfully validated through experimental evaluations in many plant genomes (Cui et al. 2017; Wang et al. 2018). Therefore, the Xap specific SSR primers designed here represent an important genomic resource for resolving the conflicting patterns available on the pathogenic and genetic variability of X. axonopodis pv. punicae. However, limited numbers of informative SSRs were reported in other pathogenic bacterial genomes, i.e., Xanthomonas albilineans and Xylella fastidiosa (Della Coletta-Filho et al. 2001; Tardiani et al. 2014). SSR markers have also proven very useful to characterize other species of animal pathogenic bacteria, such as Mycobacterium tuberculosis (Le Fleche et al. 2002), Salmonella enterica (Liu et al. 2003), Nisseria meningitidis (Yazdankhah et al. 2005).
For wet lab validation, 66 Xap_SSRs were selected that are specifically targeting di- and tri-nucleotides and distributed evenly across LMG859 genome. Since, it has been proposed that SSRs are the hot spots for recombination (Templeton et al. 2000) and especially di- and tri- nucleotide repeats are preferential sites for recombination due to their high affinity for recombination enzymes (Biet et al. 1999). As a molecular markers, di- and tri-nucleotides are more important than the other SSRs and are one of the most sought-after markers because of their higher mutation rates.
Genetic diversity analysis of 22 Xap isolates elucidated a total 40 alleles with an average of 2 alleles per locus. The mean values of ‘I’ and PIC as 0.46 and 0.24, respectively, indicated moderate allelic diversity. The possible reason for lesser number of alleles and PIC values for SSR markers is poor resolving power of metaphor gels. Therefore, the SSRs developed here could show a higher level of polymorphism when assayed on polyacrylamide gel and capillary systems. The Jaccard’s dissimilarity values implied towards a wider genetic diversity (14–75%) among 22 Xap isolates. Similarly, Gadhe et al. (2016) studied the variability among five Xap isolates from major pomegranate growing regions of Maharashtra, India based on RAPD assay and they found high level of genomic variability among the isolates even within the same geographical region. More recently, MLST analysis of 24 isolates from major Indian states revealed the occurrence of genetically homogenous clone of X. axonopodis pv. punicae with narrow genetic differences among them (Kumar et al. 2019). Since, multi-locus sequence typing lacks resolution for the so-called ‘monomorphic’ bacteria, which display very low to null levels of diversity when sequencing a few housekeeping genes (Maiden 2006; Achtman and Wagner 2008).
SSR based cluster analysis of 22 Xap isolates revealed four clusters, Cluster 1 comprised 11 Xap isolates from five different states, i.e., Maharashtra (XAP-93, XAP-99, XAP-100, XAP-104 and XAP-106), Karnataka (XAP-112 and XAP-118), Telangana (XAP-94), Himachal Pradesh (XAP-92), Uttar Pradesh (XAP-115) and Rajasthan (XAP-116). Based on Jaccards dissimilarity values, the 11 Xap isolates belonging to CL-I revealed 26 to 66% genetic diversity. Cluster 2 had 6 isolates mainly from Sangola (XAP-105, XAP-120), Pandharpur (XAP-103), Mohol (XAP-107), Mangalwedha (XAP-114) regions of Solapur district and Ghansangvi (XAP-125) region of Jalna district showing 14–48% genetic diversity. Cluster 3 contained isolates (XAP-101 and XAP-102) from Akkalkot regions of Solapur showing 24% diversity, while Cluster 4 harbored isolates from Ghansangvi (XAP-124) region of Jalna, Kopargaon (XAP-96) region of Ahmednagar and Yeola (XAP-98) region of Nashik districts belonging to Maharashtra state showing 35–54% diversity. Kiran Kumar and Khan (2016) performed RAPD based genetic diversity analysis for eighteen Xap isolates collected from Karnataka and other parts of India. They found four sub clusters within first two major cluster suggesting two lineages and four races/strains.
Among 22 isolates analyzed, XAP-125 (Jalna) and XAP-103 (Solapur) registered a higher genetic similarity (86%), whereas the isolates XAP-107 (Solapur) with XAP-96 (Ahmednagar) were found to be highly divergent with lowest genetic similarity of 25%. It is interesting to note that in cluster 4, XAP-96 (Ahmednagar) and XAP-98 (Nasik) grouped together with higher similarity (65%), and also XAP-101 and XAP-102 grouped together separately as cluster 3 with higher similarity (76%), confirming presence of divergent strains in Maharashtra. Our results concur with an earlier ISSR based molecular characterization of four isolates collected from Western Maharashtra (Raghuwanshi et al. 2013). The PCoA also separated total 22 Xap isolates into four clusters, and the axes 1 and 2 explained 19.39 and 14.25% of the genotypic variance, respectively. These results corroborated with the phylogenetic analysis.
Recently, Kumar et al. (2019) performed molecular characterization of 24 representative Xap isolates collected from major Indian states using MLST based on nine housekeeping genes. The study also grouped all the isolates into four clusters (ST1, ST2, ST3 and ST4), with ST3 being the most widespread in Indian states. The reason for these striking allelic similarities among the Xap isolates from diverse geographical locations strongly suggested that the bacterial blight dissemination most likely occurred with planting material of elite and popular cultivar ‘Bhagawa’ transported from one place to another. Similarly, higher similarity was reported among X. axonopodis pv. punicae isolates by earlier researchers based on other marker techniques, such as RAPD, BOX–PCR and MLST. Here, we report higher genetic diversity among Xap isolates using genome-wide SSR markers. A finding from the present study needs to be reconfirmed on larger Xap collections. Here, we found moderate allelic diversity for SSR markers based on PIC values, due to use of agarose gel based SSR detection systems. This limitation can be overcome by capillary-based genotyping of large numbers of Xap isolates. The allele count observed for the SSRs in this study was very low (2 alleles/locus) as compared to that found in Xanthomonas pathogens of other crops. For instance, Tardiani et al. (2014) reported 12 highly polymorphic SSRs in Xanthomonas albilineans genome, revealing average number 4.5 polymorphic alleles per locus that ranged from 2 to 12 alleles. Similarly, Lin et al. (2005) identified and deployed a set of 34 SSR loci targeting SSR motif length (> 3 to 15) through genome-wide search in Xylella fastidiosa and evaluated genetic diversity among 43 isolates collected from almond, oleander, citrus and grape. They found SSRs are more powerful in distinguishing genetically similar isolates.
Sreevatsan et al. (1997) in Mycobacterium tuberculosis noticed 1/10,000 polymorphic nucleotides and reported the lack of variation is the major limitation for MLST, thus making it difficult to achieve sufficient discriminatory power to distinguish between isolates (Sweet et al. 2012). In contrast, MLVA has been shown to allow revealing intraspecific genetic diversity in several human monomorphic pathogens. Leduc et al. (2015) reported MLVA-14 based on short tandem repeat sequences (i.e., microsatellites) provides a very high discriminatory power, allowing the analysis of the population structure of X. citri pv. citri at small to medium spatio-temporal scales. Microsatellite genotyping has proved useful for outbreak investigation in X. citri pv. citri’s native area (Vernière et al. 2014). Recently, Pruvost et al. (2019) reported culture‐independent microsatellite genotyping for molecular epidemiology study of X. citri pv. citri to detect population diversification within lesions or polyclonal infections. If intra pathovar variants that differ in terms or host range or pathological reaction type coexist, lesions may also be privileged sites for DNA exchange between genetically distant strains through horizontal gene transfer of a large conjugative plasmid. This leads to acquisition of adaptive traits in response to the selective pressure by copper sprays widely used for controlling plant bacterial diseases (Richard et al. 2017). To confirm this, recently Krishna et al. (2020) reported reduced efficacy of antibiotics and copper compounds against field populations of Xanthomonas axonopodis pv. punicae causing bacterial blight of pomegranate is due to the development of bactericide resistance through acquired genes. Therefore, pathogenic variability using microsatellite markers could be important genomic tools to study and understand the population dynamics, taxonomy, epidemiological and quarantine aspects of this pathogen, which could help to devise eco-friendly and cost-effective control measures to reduce the economic losses caused by this pathogen.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are highly grateful to the Indian Council of Agricultural Research (ICAR), New Delhi for extended financial support through ICAR–National Research Centre on Pomegranate, Solapur. Authors are also thankful to Ms. Anitha Alarimar, project assistant working in Plant Pathology Department, ICAR–NRCP, Solapur for extending her help in carrying out this work.
Funding
Funds to conduct current research work were provided by Indian Council of Agricultural Research, New Delhi, India as institutional grants to ICAR–National Research Centre on Pomegranate, Solapur, India.
Data availability
The data which supports our findings were listed in the supplementary files. All the relevant information obtained here will be freely available to any scientist for their research purpose.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Prakash G. Patil, Email: patilbt@gmail.com
Jyotsana Sharma, Email: jyotisharma128@yahoo.com.
Manjunatha Nanjundappa, Email: manju.ars@rediffmail.com.
N. V. Singh, Email: nripendras72@gmail.com
Abhishek Bohra, Email: abhi.omics@gmail.com.
Raghavendra Gunnaiah, Email: raghavendra.gunnaiah@gmail.com.
Shivani M. Jamma, Email: shivanijamma201@gmail.com
Jeer Vinayaka, Email: jvinayaka05@gmail.com.
Vipul R. Sangnure, Email: sangnurevipul@gmail.com
R. A. Marathe, Email: ramarathe28@gmail.com
References
- Achtman M, Wagner M. Microbial diversity and the genetic nature of microbial species. Nat Rev Microbiol. 2008;6:431–440. doi: 10.1038/nrmicro1872. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Bhatti MR. Occurrence of bacterial leaf spot of pomegranate in Pakistan. Pak J Agric Res. 1992;13:95–97. [Google Scholar]
- Baldi P, La Porta N. Xylella fastidiosa: host range and advance in molecular identification techniques. Front Plant Sci. 2017;8:944. doi: 10.3389/fpls.2017.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkum AV, Scherer S, Alphen LV, et al. Microbiology short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev. 1998;62:275–293. doi: 10.1128/MMBR.62.2.275-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biet E, Sun J, Dutreix M. Conserved sequence preference in DNA binding among recombination proteins: an effect of ssDNA secondary structure. Nucleic Acids Res. 1999;27:596–600. doi: 10.1093/nar/27.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand R, Kishun R. Studies on bacterial blight (Xanthomonas campestris pv. punicae) on Pomegranate. Indian Phytopathol. 1991;44:370–372. [Google Scholar]
- Chavan NP, Pandey R, Nawani N, et al. Genetic variability within Xanthomonas axonopodis pv. punicae, causative agent of oily spot disease of pomegranate. J Plant Pathol Microbiol. 2017;8:394. doi: 10.4172/2157-7471.1000394. [DOI] [Google Scholar]
- Conant GC, Wolfe KH. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 2008;24:861–862. doi: 10.1093/bioinformatics/btm598. [DOI] [PubMed] [Google Scholar]
- Cui J, Cheng J, Nong D, et al. Genome-wide analysis of simple sequence repeats in bitter gourd (Momordica charantia) Front Plant Sci. 2017;8:1103. doi: 10.3389/fpls.2017.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Coletta-Filho H, Takita MA, de Souza AA, et al. Differentiation of strains of Xylella fastidiosa by a variable number of tandem repeat analysis. Appl Env Microbiol. 2001;6:4091–4095. doi: 10.1128/AEM.67.9.4091-4095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Zhang C, Liu Q, et al. Krait: an ultrafast tool for genome-wide survey of microsatellites and primer design. Bioinformatics. 2018;34:681–683. doi: 10.1093/bioinformatics/btx665. [DOI] [PubMed] [Google Scholar]
- Eknath WJ, Dange AS, Pagar TA, et al. Assessment of the genetic diversity among oily spot (Xanthomonas axonopodis pv. punicae) pathogen of pomegranate by randomly amplified polymorphic DNA analysis. J Appl Nat Sci. 2015;7:1006–1011. doi: 10.31018/jans.v7i2.722. [DOI] [Google Scholar]
- Field D, Wills C. Long, polymorphic microsatellites in simple organisms. Proceed Biological Sci. 1996;263:209–215. doi: 10.1098/rspb.1996.0033. [DOI] [PubMed] [Google Scholar]
- Gadhe SK, Antre SH, Ghorpade BB, et al. Studies on molecular variability among Xanthomonas axonopodis pv. punicae isolates collected from different locations. Int J Pure Appl Biosci. 2016;4:160–66. doi: 10.1878/2320-7051.2311. [DOI] [Google Scholar]
- Gur-Arie R, Cohen CJ, Shelef L, et al. Simple sequence repeats in Escherichia coli: abundance, distribution, composition, and polymorphism. Genome Res. 2000;10:62–71. [PMC free article] [PubMed] [Google Scholar]
- Hingorani M, Mehta P. Bacterial leaf spot of pomegranate. Indian Phytopathololgy. 1952;5:55–56. [Google Scholar]
- Icoz SM, Polat I, Sulu G, et al. First report of bacterial blight of pomegranate caused by Xanthomonas axonopodis. pv punicae in Turkey. Plant Dis. 2014;98:1427. doi: 10.1094/PDIS-06-14-0656-PDN. [DOI] [PubMed] [Google Scholar]
- Irey M, Gottwald TR, Graham JH, et al. Post-hurricane analysis of citrus canker spread and progress towards the development of a predictive model to estimate disease spread due to catastrophic weather events. Plant Health Prog. 2006 doi: 10.1094/PHP-2006-0822-1001-RS. [DOI] [Google Scholar]
- Jaccard P. Nouvelles recherches sur la distribution fiorale. Bull Soc Vaud Sci Nat. 1908;44:223–270. [Google Scholar]
- Jaiswal M, Pandey A. In silico mining of simple sequence repeats in whole genome of Xanthomonas sp. J Computer Sci Syst Biol. 2014;7:203–08. doi: 10.4172/jcsb.1000157. [DOI] [Google Scholar]
- Kiran Kumar KC, Khan ANA. Molecular characterization of isolates of Xanthomonas axonopodis pv. punicae causing bacterial blight of pomegranate on the basis of RAPD-PCR. Greeen Farming. 2016;7:203–207. [Google Scholar]
- Krishna P, Prasanna Kumar MK, Channappa M, et al. Antibiotic resilience in Xanthomonas axonopodis pv. punicae causing bacterial blight of pomegranate. Curr Sci. 2020;119:10. doi: 10.18520/cs/v119/i9/1564-1569. [DOI] [Google Scholar]
- Kumar A, Sharma J, Munjal V, et al. Polyphasic phenotypic and genetic analysis reveals clonal nature of Xanthomonas axonopodis pv. punicae causing pomegranate bacterial blight. Plant Pathol. 2019 doi: 10.1111/ppa.13128. [DOI] [Google Scholar]
- Le Fleche P, Fabre M, Denoeud F, et al. High resolution, on-line identification of strains from the Mycobacterium tuberculosis complex based on tandem repeat typing. BMC Microbiol. 2002;27:2–37. doi: 10.1186/1471-2180-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc A, Traoré YN, Boyer K, et al. Bridgehead invasion of a monomorphic plant pathogenic bacterium: Xanthomonas citri pv. citri, an emerging citrus pathogen in Mali and Burkina Faso. Environ Microbiol. 2015;17:4429–4442. doi: 10.1111/1462-2920.12876. [DOI] [PubMed] [Google Scholar]
- Lin H, Civerolo EL, Hu R, et al. Multilocus simple sequence repeat markers for differentiating strains and evaluating genetic diversity of Xylella fastidiosa. Appl Env Microbiol. 2005;71:4888–92. doi: 10.1128/AEM.71.8.4888-4892.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lee MA, Ooi EE, et al. Molecular typing of Salmonella enterica serovar typhi isolates from various countries in Asia by a multiplex PCR assay on variable-number tandem repeats. J Clin Microbiol. 2003;41:94388–94394. doi: 10.1128/JCM.41.9.4388-4394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchi N, Ioos R, Santini A. Fast and reliable molecular methods to detect fungal pathogens in woody plants. Appl Microb Biotech. 2020;104:2453–2468. doi: 10.1007/s00253-020-10395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden MC. Multilocus sequence typing of bacteria. Annu RevMicrobiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- Mondal KK, Mani C. ERIC-PCR genomic fingerprints and their relationship with pathogenic variability of Xanthomonas campestris pv. punicae, the incitant of bacterial blight of pomegranate. Curr Microbiol. 2009;59:616–20. doi: 10.1007/s00284-009-9482-z. [DOI] [PubMed] [Google Scholar]
- Mondal KK, Rajendran TP, Phaneendra C, et al. The reliable and rapid polymerase chain reaction (PCR) diagnosis for Xanthomonas axonopodis pv. punicae in pomegranate. Afr J Microbiol Res Acad J. 2012;6:5950–956. doi: 10.5897/AJMR12.543. [DOI] [Google Scholar]
- NRCP (2015) National research centre on pomegranate vision 2050 document, Kegaon, Solapur, Maharashtra, India
- Peakall R, Smouse PE. GenAlEx 6.5 genetic analysis in excel population genetic software for teaching and research–an update. Bioinformatics. 2012;28:2537–539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier X, Jacquemoud-Collet JP (2006). DARwin software. Paris: Centre de Cooperation Internationale en Recherche Agronomique Pour le De’veloppement (CIRAD)
- Petersen Y, Mansvelt E, Venter E, et al. Detection of Xanthomonas axonopodis pv. punicae causing bacterial blight on pomegranate in South Africa. Australas Plant Pathol. 2010;39:544–546. doi: 10.1071/AP10034. [DOI] [Google Scholar]
- Pruvost O, Boyer K, Ravigné V, et al. Deciphering how plant pathogenic bacteria disperse and meet: Molecular epidemiology of Xanthomonas citri pv. citri at microgeographic scales in a tropical area of asiatic citrus canker endemicity. Evol Appl. 2019;12:1523–1538. doi: 10.1111/eva.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhika DH, Gunnaiah R, Lamani A, et al. Long read genome sequence resources of Xanthomonas citri pv punicae strain Bagalkot, causing pomegranate bacterial blight. Mol Plant Microbe Interact. 2021 doi: 10.1094/MPMI-01-21-0001-A. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi KS, Hujare BA, Chimote VP, et al. Characterization of Xanthomonas axonopodis pv. punicae isolates from western Maharashtra and their sensitivity to chemical treatments. Bioscan. 2013;8:845–850. [Google Scholar]
- Richard D, Tribot N, Boyer C, et al. First report of copper-resistant Xanthomonas citri pv. citri pathotype a causing asiatic citrus canker in Réunion, France. Plant Dis. 2017;101:503. doi: 10.1094/PDIS-09-16-1387-PDN. [DOI] [Google Scholar]
- Riju A, Rajesh MK, Sherin PTPF, et al. Mining of expressed sequence tag libraries of cacao for microsatellite markers using five computational tools. J Genet. 2009;88:217–225. doi: 10.1007/s12041-009-0030-1. [DOI] [PubMed] [Google Scholar]
- Schuler GD. Sequence mapping by electronic PCR. Genome Res. 1997;7:541–550. doi: 10.1101/gr.7.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Sharma KK, Jadhav VT. Diseases of pomegranate. In: Misra AK, Chowdappa P, Sharma P, Khetrapal RK, editors. Diseases of fruit crops, 181–224. New Delhi, India: Indian Phytopatholpgy Society; 2012. [Google Scholar]
- Sharma V, Midha S, Ranjan M, et al. Genome sequence of Xanthomonas axonopodis pv. punicae strain LMG 859. J Bacteriol. 2012 doi: 10.1128/JB.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Sharma J, Jadhav V (2015) In: recent advances in diagonosis and management of plant disease. 119–26
- Sharma J, Sharma KK, Kumar A, et al. Pomegranate bacterial blight: symptomatology and rapid inoculation technique for Xanthomonas axonopodis pv. punicae. J Plant Pathol. 2017;99:109–119. [Google Scholar]
- Sreevatsan S, Pan X, Stockbauer KE, et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proceed National Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet MJ, Scriven LA, Singleton I. Microsatellites for microbiologists. Adv Appl Microbiol. 2012;81:169–207. doi: 10.1016/B978-0-12-394382-8.00005-8. [DOI] [PubMed] [Google Scholar]
- Tardiani AC, Perecin D, Peixoto-Junior RF, et al. Molecular and pathogenic diversity among Brazilian isolates of Xanthomonas albilineans assessed with SSR marker loci. Plant Dis. 2014;98:540–546. doi: 10.1094/PDIS-07-13-0762-RE. [DOI] [PubMed] [Google Scholar]
- Templeton AR, Clark AG, Weiss KM, et al. Recombinational and mutational hotspots within the human lipoprotein lipase gene. Am J Hum Genet. 2000;66:69–83. doi: 10.1086/302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernière C, Bui Thi Ngoc L, Jarne P, et al. Highly polymorphic markers reveal the establishment of an invasive lineage of the citrus bacteria pathogen Xanthomonas citri pv. citri in its area of origin. Environ Microbiol. 2014;16:2226–2237. doi: 10.1111/1462-2920.12369. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang L. GMATA: an integrated software package for genome-scale SSR mining, marker development and viewing. Front Plant Sci. 2016;7:1350. doi: 10.3389/fpls.2016.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang S, Chen Y, et al. Comparative genome-wide characterization leading to simple sequence repeat marker development for nicotiana. BMC Genom. 2018;19:500. doi: 10.1186/s12864-018-4878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdankhah SP, Lindstedt BA, Caugant DA. Use of variable- number tandem repeats to examine genetic diversity of Neisseria meningitides. J Clin Microbiol. 2005;43:1699–1705. doi: 10.1128/JCM.43.4.1699-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data which supports our findings were listed in the supplementary files. All the relevant information obtained here will be freely available to any scientist for their research purpose.