Summary
Background
Glycans play essential functional roles in the nervous system and their pathobiological relevance has become increasingly recognized in numerous brain disorders, but not fully explored in traumatic brain injury (TBI). We investigated longitudinal glycome patterns in patients with moderate to severe TBI (Glasgow Coma Scale [GCS] score ≤12) to characterize glyco-biomarker signatures and their relation to clinical features and long-term outcome.
Methods
This prospective single-center observational study included 51 adult patients with TBI (GCS ≤12) admitted to the neurosurgical unit of the University Hospital of Pecs, Pecs, Hungary, between June 2018 and April 2019. We used a high-throughput liquid chromatography–tandem mass spectrometry platform to assess serum levels of N-glycans up to 3 days after injury. Outcome was assessed using the Glasgow Outcome Scale-Extended (GOS-E) at 12 months post-injury. Multivariate statistical techniques, including principal component analysis and orthogonal partial least squares discriminant analysis, were used to analyze glycomics data and define highly influential structures driving class distinction. Receiver operating characteristic analyses were used to determine prognostic accuracy.
Findings
We identified 94 N-glycans encompassing all typical structural types, including oligomannose, hybrid, and complex-type entities. Levels of high mannose, hybrid and sialylated structures were temporally altered (p<0·05). Four influential glycans were identified. Two brain-specific structures, HexNAc5Hex3DeoxyHex0NeuAc0 and HexNAc5Hex4DeoxyHex0NeuAc1, were substantially increased early after injury in patients with unfavorable outcome (GOS-E≤4) (area under the curve [AUC]=0·75 [95%CI 0·59-0·90] and AUC=0·71 [0·52-0·89], respectively). Serum levels of HexNAc7Hex7DeoxyHex1NeuAc2 and HexNAc8Hex6DeoxyHex0NeuAc0 were persistently increased in patients with favorable outcome, but undetectable in those with unfavorable outcome. Levels of HexNAc5Hex4DeoxyHex0NeuAc1 were acutely elevated in patients with mass lesions and in those requiring decompressive craniectomy.
Interpretation
In spite of the exploratory nature of the study and the relatively small number of patients, our results provide to the best of our knowledge initial evidence supporting the utility of glycomics approaches for biomarker discovery and patient phenotyping in TBI. Further larger multicenter studies will be required to validate our findings and to determine their pathobiological value and potential applications in practice.
Funding
This work was funded by the Italian Ministry of Health (grant number GR-2013-02354960), and also partially supported by a NIH grant (1R01GM112490-08).
Keywords: Traumatic brain injury, Glycosylation, Glycomics, Glycomarkers, Biomarkers, Serum, LC-MS, Prognosis
Research in context.
Evidence before this study
Diagnosis, characterization, and therapeutic decision-making in patients with moderate and severe traumatic brain injury (TBI) remain a challenge. Novel molecular alterations may provide insight into the evolution of secondary injury and repair that could significantly impact medical practice and patient outcome. We searched PubMed from inception to October 18, 2021 for research studies on glycomics in TBI using the search terms “glycomics” AND “traumatic brain injury.” Although 2 studies reported alterations in the concentrations of glycans after experimental brain injury in rats, we found no clinical studies on glycomics in patients after TBI and specifically, no assessments of the temporal profile of glycomic changes and their relationship with injury characteristics, TBI endophenotype, and/or outcome.
Added value of this study
In this pilot single-center study, we performed a characterization of changes in the concentrations of glycans in serial blood samples of a relatively small cohort of patients with moderate to severe TBI. We identified glycome expression patterns that may represent TBI-specific glycofingerprints and reflect molecular events related to brain injury mechanism, response and/or recovery. Future studies using independent larger cohorts of patients will be required to confirm the reproducibility of these findings and corroborate their utility in clinical practice.
Implications of all the available evidence
Our findings support the use of an mass spectroscopy-based glycomic profiling as a informative approach to uncover and characterize glycomic TBI signatures. Our work paves the way for future studies exploring pathways of secondary injury and/or repair associated with altered glycans after TBI along with providing potential clues toward mechanistic links with other diseases.
Alt-text: Unlabelled box
Introduction
Traumatic brain injury (TBI) is a critical global public health problem, representing a major cause of death and disability, especially among young adults.1 Clinical assessment and imaging form the diagnostic cornerstones and are currently used to guide management, treatment decisions and predict outcome for patients with TBI.2 Nonetheless, this approach is insufficient to unveil the complex heterogeneity of TBI and to inform the development and implementation of effective precision medicine-based therapies.
The use of blood-based biomarkers yields substantial potential for better patient characterization helping to identify underlying interindividual pathophysiological variability and define more accurate disease phenotypes to guide personalized clinical management and identify new therapies.3, 4, 5 During the past decades, promising brain injury protein markers have emerged that have been integrated into clinical guidelines and cleared by a regulatory agency.6,7 However, the fact that such proteins are primarily byproducts of the injury-induced damage rather than intrinsic participants in pathological mechanisms has led to a considerable growing interest in identifying additional novel ‘actionable’ biomarkers rooted in the disease pathogenesis. These ‘mechanistic’ biomarkers which generate a detectable injury-specific molecular signature may be more informative and more effectively used in clinical practice for both prognostication and for endophenotyping, and ideal for optimizing drug development and clinical trial design.4
Recent evidence from both pre-clinical and clinical studies has identified characteristic changes in protein glycosylation (i.e., the addition of complex sugars [glycans]) in many human diseases, providing insight into disease state, mechanism and progression (further details can be found in supplementary information).8, 9, 10 In particular, N-glycan branching modulating protein activity exerts pleiotropic effects in the brain, and alterations have been shown to affect development, neuroinflammatory responses, myelination, neuronal excitability and promote neurodegeneration.11, 12, 13, 14 Despite this converging evidence of the relevance of N-glycosylation in characterization and pathogenesis of brain disease and its potential for the discovery and elucidation of novel markers and therapeutic targets, to date the glycomics signatures of TBI are unknown.
In this pilot study, using a high-sensitive liquid chromatography–tandem mass spectroscopy (LC-MS/MS) approach15, 16, 17, 18 we performed a profile and comparative characterization of the N-glycome in serial serum samples from patients with moderate to severe TBI. We hypothesized that the pathophysiological cascades triggered by TBI would result in characteristic changes in the blood N-glycans that can potentially be used to predict outcome, as assessed by the Glasgow Outcome Scale-Extended (GOS-E) score at 12 months post-injury. Furthermore, we investigated the relationship of blood N-glycans with injury and patient characteristics.
Methods
Study patients
This research is part of the Novel Biomarkers for Improved Characterization, Disease Tracking and Outcome Prediction in TBI, a prospective study designed to use a granular, innovative, multimarker strategy to advance characterization of patients with moderate to severe TBI.19
Between June 2018 and April 2019, we prospectively included 51 consecutive patients admitted to the neurosurgical unit of the University Hospital of Pecs. Eligible patients were adults (≥18 years) with a diagnosis of moderate-to-severe TBI (Glasgow Coma Scale [GCS] score of ≤12 on admission) due to a blunt mechanism (i.e., closed trauma). Exclusion criteria were pregnancy, GCS score equal to 3 associated with bilateral fixed and dilated pupils, normal head computed tomography (CT) and/or neurological comorbidities that could affect brain injury biomarker concentrations, such as neurodegenerative disorders, history of stroke or cerebrovascular events.
All patients underwent head CT examinations upon presentation and were managed according to international guidelines.20,21 The CT scans were acquired with the use of the Siemens SOMATOM Perspective 128 scanner. None of the enrolled patients was claustrophobic. Initial head CT scans were classified according to the Marshall Classification and the NINDS CDE Neuroimaging Working Group consensus recommendations (NIHCT). Study procedures included a detailed collection of clinical data, with variables coded in accordance with The National Institute of Neurological Disorders and Stroke (NINDS) Common Data Elements (CDE) scheme (https://commondataelements.ninds.nih.gov/). The clinical outcome was assessed (blinded for glycomics levels) using the GOS-E score at 12 months after injury by telephone or in-person structured interview. Patients were assigned upper good recovery (8), lower good recovery (7), upper moderate disability (6), lower moderate disability (5), upper severe disability (3), lower severe disability (3), vegetative state (2), or death (1).22 For the purposes of the analysis, GOS-E scores were dichotomized into favorable outcomes (GOS-E >4) and unfavorable outcomes (GOS-E≤4) outcomes.
The study protocol was approved by the Local Ethics Committee (IRB#: IRB00003108 - U Pecs, Med Ctr IRB #1) (approval protocol number: 7179 – PTE 2018) and written informed consent to participate in the study was obtained from patients or their next of kin.
Sample collection
Approximately 5 mL of blood was drawn from each subject on admission (median time between injury and first blood sampling, 13.5 h, interquartile range [IQR] 7.7-17.7) and daily (between 7 and 9 AM) over the study duration. Blood samples were collected by venipuncture in gel separator tubes and centrifuged (4,000 rpm for 10 min) at room temperature (RT) within 60 min, according to a standardized protocol. Serum was processed, aliquoted, and stored at−80°C until shipment on dry ice to the laboratory at the Texas Tech University (Lubbock, TX, USA). To avoid the influence of common preanalytical factors, storage conditions including time and temperature were monitored, and specimens were not subjected to previous freeze-thaw cycles. Serum samples were used to more accurately monitor changes in low abundant proteins that would have been masked by the higher level of fibrinogen.23 All scientists involved in the analysis were blinded to the patient characteristics.
Analytical methods
The digestion and purification of N-glycans followed our previously published protocol.24 Briefly, 10 µL human blood serum of each patient was mixed with 10 times diluted sodium phosphate buffer and denatured in a 90°C water bath for 20 min. Next, 1.0 µL PNGase F was added to the mixture and digested overnight in a 37°C water bath. Released N-glycans were then suspended in 90% icy ethanol, and after centrifugation, the supernatant containing N-glycans were collected, dried and reduced with the ammonium-borane complex at 60°C reaction temperature for 1 h. Reduced N-glycans were washed with methanol to remove excess reducing reagent.
The purified reduced N-glycans were solid-phase permethylated as previously described.9,24,25 Sodium hydroxide beads were packed in spin columns with DMSO solution and washed with DMSO. The dried N-glycans were resuspended in 30 µL DMSO, 20 µL iodomethane and a trace amount of water (ca. 1.2 µL). The sample solution was then loaded to spin columns and incubated for 25 min at room temperature. Additional 20 µL iodomethane was added into the spin column. After incubation for 15 min, spin columns were centrifuged at 1.8 k rpm. The collected solutions were dried overnight in a vacuum drier. Reduced and permethylated N-glycans extracted from human blood sera were reconstituted in 20% ACN containing 0.1% formic acid before injection in LC−MS.
All samples were analyzed with C18-LC–MS conditions for glycan profiles and quantitative analysis using Ultimate 3000 nano-LC system (Dionex, Sunnyvale, CA, USA) coupled with LTQ Orbitrap Velos mass spectrometer (Thermo Scientific, San Jose, CA, USA). Glycan profiles were obtained on reversed-phase Acclaim PepMap capillary column (150 mm × 75mm i.d.) packed with 100 Å C18-bounded phase (Dionex) at 55°C under optimized LC conditions. The mobile phase for separation was composed of 98% HPLC water, 2% ACN, 0.1% formic acid; on the other hand, mobile phase B consisted of 100% ACN and 0.1% formic acid. The flow rate was 0.35 µL/min with a gradient elution of 20% mobile phase B over 10 min, then increased to 42% B (10–11 min), 42–55% B (11–48 min), 55–90% B (48–49 min), 90% B (49–54 min), 90–20% B (54– 55 min), 20% B (55–60 min). Full MS spectra were obtained in the mass range of 700–2000 m/z using positive ionization mode. The resolution of the instrument was set to 100,000 with a mass accuracy of 5 ppm.
Glycan compositions were identified by searching the experimental m/z value of monoisotopic peaks of N-glycan ions against the default database built in the MultiGlycan software. Mass accuracy of 5 ppm was employed, isotopic envelope tolerance was set to 6 ppm. For the quantitative glycan profiling, acquired.raw files from LC-MS were processed via Skyline (MacCoss Lab Software) with a transition list generated through MultiGlycan software. All possible charge states and adducts of a glycan composition were considered and added as the output quantitation results and were evaluated manually. The glycan quantitative results were normalized and reported as relative abundances, which were calculated by using the peak area of each glycan structure versus the total structures identified in each sample.
Statistical analysis
As this research was exploratory, all subjects enrolled in the study were included to maximize the sample size.26,27 Baseline characteristics were summarized using standard descriptive statistics. Continuous variables were described as mean (standard deviation [SD]) or median (IQR), as appropriate, and categorical data were summarized as absolute frequencies and percentages. The association between categorical variables was evaluated using the Fisher's exact test. Distributions of glycans levels were compared between patients with different clinical characteristics (e.g., patients with mass lesions vs. those with diffuse injury, patients requiring decompressive craniectomy vs. those conservatively treated, and patients with GOS-E >4 vs. those with GOS-E≤4) with Mann-Whitney U tests. Correlations between quantitative variables were quantified using Spearman's rank correlation coefficients, and the Friedman test was used to test significant glycan changes across time. To determine significant trends in glycan data, we used Jonckheere-Terpstrsa test for non-parametric trend analysis. Multivariate analysis (MVA) methods were used to evaluate the relationship with outcome and identify relevant variables responsible for class discrimination.28,29 For these analyses, a series of data pre-processing steps were performed. The glycan data were log-transformed and pareto scaled and the noise level in the data was reduced by averaging glycomics data on each day (see the Supplementary material). Initial exploration of the data was performed using principal component analysis (PCA). PCA was used to overview, to lower the dimensionality of the data, to identify natural clustering and to detect potential outliers. Subsequently, we conducted a supervised multivariate analysis using orthogonal partial least squared discriminant analysis (OPLS-DA). OPLS-DA was used to maximize detection of glycans (X-variables) associated with differentiation between the predefined groups (outcome). The OPLS-DA model was validated using a three-fold “leave-one-out” (LOO) methodology. In a subsequent modeling step, the loadings associated with each model were limited with the R2VXAdj metric (i.e., explained fraction of the variation of X variables for the predictive component) to only the top 10 relevant variables. Correcting for multiple testing was not required at this stage, as the number of variables was kept constant. Using the above method, only 10 variables, namely the ones with the highest R2VXAdj value (which is adjusted for degrees of freedom and therefore accounts for a high number of variables), were taken into consideration. Thus, the common glycans (n=4) derived from the three new OPLS-DA models were identified as highly influential variables driving class distinction (Supplementary material). As criterion to assess the quality of the computed models, we adopted R2X > 0.4 for PCA, and R2Y (goodness of fit parameter) and Q2 (predictive ability parameter) > 0.5 for OPLS-DA.30 Receiver operating characteristic (ROC) curves were used to evaluate the ability of the subset of identified glycans, separately, to predict the probability of having an unfavorable outcome (GOS-E 1 to 4) 12 months after TBI. Two sided tests were used and p-values < 0.05 were considered statistically significant. Traditional statistical analysis was performed using R (http://www.r-project.org, version 3.5.1) in RStudio (http://www.rstudio.com, version 1.1.456), and SIMCA® 17 Software (Umetrics® Suite, SIMCA® 17 by Sartorius Stedim Data Analytics AB, Umeå, Sweden) was used for multivariate data analysis.
Role of the funding source
The funder had no role in the study design, collection, analysis or interpretation of data, nor in the writing of the report or in publication decisions. All authors had full access to the study data and the corresponding author had final responsibility to submit the manuscript for publication.
Results
Patient characteristics
The demographic and clinical characteristics of the 51 patients included in this study are summarized in Table 1. In our TBI cohort, ∼76% of the subjects were men and the average patient age was 56·51±16·40 years. Most patients (∼76%) sustained TBI due to a fall, and 17% sustained injuries through automobile or bicycle accident. Twenty (40%) patients had extra-axial lesions, 12 (24%) concurrently sustained extracranial injuries, and 6 (12%) had cerebral edema. In all, 61% of the population was discharged to home or an acute rehabilitation facility, and 31% discharged to a long-term acute care facility. The overall mortality rate was ∼20% at 12 months after injury.
Table 1.
Demographic and clinical characteristics of TBI patients.
| TBI(n=51) | 12-month Outcome |
p-valuea | ||
|---|---|---|---|---|
| Unfavorable (GOS-E 1-4) (n=11) | Favorable (GOS-E 5-8) (n=40) | |||
| Age, years mean (SD) | 56·51 (16·40) | 59·63 (11·48) | 55·65 (17·53) | 0·48 |
| (range) | (20-89) | |||
| Sex, n (%) | ||||
| Female | 12 (23·53) | 1 (9·09) | 11 (27·50) | 0·42 |
| Male | 39 (76·47) | 10 (90·91) | 29 (72·50) | |
| Race, n (%) | ||||
| Caucasian | 51 (100) | 11 (100) | 40 (100) | |
| GCS | ||||
| Median (IQR) | 12 (3-12) | 3 (3-8) | 12 (12-12) | 0·0001 |
| Trauma history, n (%) | ||||
| Motor Vehicle Accident | 3 (5·88) | 0 (0) | 3 (7·50) | |
| Motor Bicycle Accident | 8 (15·68) | 3 (27·27) | 5 (12·50) | 0·77 |
| Fall | 37 (72·55) | 8 (72·73) | 29 (72·50) | |
| Gun Shoot | 1 (1·96) | 0 (0) | 1 (2·50) | |
| Assault | 1 (1·96) | 0 (0) | 1 (2·50) | |
| Other | 1 (1·96) | 0 (0) | 1 (2·50) | |
|
Time to first sample withdrawal, h, Median (IQR) |
13.5 (7.7-17.7) | 14.1 (9.3-22.7) | 14.1 (8.2-18.7) | 0.66 |
| Extracranial injures, n (%) | 12 (23·53) | 5 (45·45) | 7 (17·50) | 0·05 |
| Image Findings, n (%) | ||||
| Contusion | 11 (22) | 1 (9·09) | 10 (25) | |
| DAI | 2 (4) | 0 (0) | 2 (5) | 0·6 |
| Extra-axial lesions | 20 (39) | 4 (36·36) | 16 (40) | |
| Mixed Lesions | 18 (35) | 6 (54·55) | 12 (30) | |
| Marshall Score, n (%) | ||||
| Diffuse Injury | ||||
| 1 | 2 (3·92) | 0 (0) | 2 (5) | |
| 2 | 34 (66·67) | 4 (36·36) | 30 (75) | 0·02 |
| Mass lesions | ||||
| 5 | 14 (27·45) | 7 (63·64) | 7 (17·50) | |
| 6 | 1 (1·96) | 0 (0) | 1 (2·50) | |
| Edema, n (%) | ||||
| Yes | 6 (11·76) | 2 (18·18) | 4 (10) | |
| No | 45 (88·24) | 9 (81·82) | 36 (90) | 0·6 |
| Decompressive Craniectomy, n (%) | ||||
| Yes | 10 (19·61) | 6 (54·55) | 4 (10) | |
| No | 41 (80·39) | 5 (45·45) | 36 (90) | 0·004 |
| GOS-E 12 months, n (%) | ||||
| Poor outcome | ||||
| 1 | 10 (19·61) | 10 (90·90) | - | |
| 3 | 1 (1·96) | 1 (9·1) | - | |
| Good outcome | ||||
| 6 | 2 (3·92) | - | 2 (5) | |
| 7 | 2 (3·92) | - | 2 (5) | |
| 8 | 36 (70·59) | - | 36 (90) | |
p values of the t-Test or Mann-Whitney U test, as appropriate, for continuous variables, Fisher's exact test for categorical variables, for differences between the 2 groups [unfavorable versus favorable outcome]).
DAI, Diffuse axonal Injury; GCS, Glasgow Coma Scale; GOS-E, Glasgow Outcome Scale-Extended; IQR, interquartile range.
Serum N-glycan analysis/ glycomics profiles of TBI samples
Our glycomics profiling of pooled serum samples identified 94 N-glycans which include all typical structural types, including oligomannose, hybrid, and complex-type entities (refer to Supplementary Table for the full list of N-glycans and their relative abundances over time). Figure 1 presents a representative N-glycan spectrum for the TBI patients. The dominant type was a biantennary fully sialylated complex N-glycan (HexNAc4Hex5DeoxyHex0NeuAc2) (Supplementary Table 1).
Figure 1.
Representative serum N-glycan profile of TBI patients indicating the major glycans. Results from pooled serum samples (n = 158) are shown. Representative glycan structures were assigned to each peak.
Further investigations were performed on the glycomics temporal profile demonstrating changes in the levels of oligomannose (high mannose) and sialylated structures (p<0·05) (Suppl. Figure 1A and B), and a trend toward temporal changes of both fucosylated and sialylated glycans (p=0·06). The analysis of glycan features also revealed that levels of hybrid glycans increased overtime (12% Day 0, 17% Day 3, p=0.03) while a decrease in complex forms was noted (86% Day 0, 80% Day3, p=0·06) (Suppl. Figure 1C). In addition, hybrid triantennary, and complex mono/biantennary and triantennary glycan concentrations were increased at the later timepoints (p=0·008, p=0·02 and p=0·04, respectively) (Suppl. Figure 1D).
Associations between glycomics profiles and clinical outcomes: multivariate statistical analysis
Of the 51 patients recruited, 22% had an unfavorable outcome at 12 months after injury, and of these ∼91% died. Forty patients (78%) had a favorable outcome, and of these 90% had a full recovery. Patients were similar across the groups with respect to their demographic characteristics (Table 1).
To find potential glycosignatures, the temporal profiles of glycans in patients with favorable and unfavorable outcome were compared using PCA (2 PC, R2 = 0·68, Q2 = 0·32) which revealed a degree of natural separation (Figure 2A). The relatively low Q2 value indicates that TBI patients are glycomically variable. Supervised OPLS-DA modelling maximized the variations between favorable and unfavorable outcome groups in glycomics analysis showing a clear separation as visualized in Figure 2B (R2Y = 0·97, Q2 = 0·83, p=0·04). In addition, the scores plot indicated that patients with favorable outcomes (GOS-E 5-8) tended to cluster together, while subjects with unfavorable outcomes (GOS-E 1-4) show a wider spread over the sampling period. The loadings plot indicated that different species of glycans characterized the two groups, monoantennary, and triantennary complex N-glycans in GOS-E 1-4, and tetraantennary and multiantennary complex N-glycans in GOS-E 5-8 (Figure 2C). Four highly influential glycans were identified. More specifically, GOS-E 1-4 was associated with two triantennary complex glycans (HexNAc5Hex3DeoxyHex0NeuAc0 - X5.3.0.0 and HexNAc5Hex4DeoxyHex0NeuAc1 - X5.4.0.1), while GOS-E 5-8 was associated with two tetraantennary complex glycans (HexNAc7Hex7DeoxyHex1NeuAc2 - X7.7.1.2 and HexNAc8Hex6DeoxyHex0NeuAc0 - X8.6.0.0).
Figure 2.
(A) Score plot of the principal component analysis (PCA) model. PCA scores plot (R2 X = 0·68, Q2 = 0·32) colored according to outcome, poor (red circles), good (green circles). The score plot displays the relationships/group separation of the subjects. Dots represent average of the samples up to day 3 taken from TBI patients. t [1], principal component 1; t [2], principal component 2. (B) Score plot of the orthogonal partial least squared discriminant analysis (OPLS-DA) model. OPLS-DA scores plot, with one orthogonal and one aligned component (R2Y = 0·97, Q2 = 0·83, p=0·04), comparing glycomics patterns from patients with poor outcome (red circles) to those with good outcome (green circles). The two axes (scores t [1] and t [2]) represent the two latent variables of the model. (C) Loading plot of the OPLS-DA model. Loading plot of the OPLS-DA model visualizing the relationship between variables (i.e., glycans) and showing how the X-variables relate to each other as well as to group belonging (Y-variable symbolized by group star). X-variables located near a group star are positively associated with that group.
Prognostic accuracy of the glycomarkers and correlation with patient characteristics
HexNAc5Hex3DeoxyHex0NeuAc0 and HexNAc5Hex4DeoxyHex0NeuAc1 levels at 24 h after injury were significantly higher in patients with unfavorable outcome (GOS-E≤4) vs. patients with favorable outcome (GOS-E>4) at 12 months post-injury (p= 0·01 and p=0·038, respectively, Mann-Whitney test, see Table 2), while concentrations at later timepoints did not differ between these subpopulations. In addition, initial assessment of HexNAc5Hex3DeoxyHex0NeuAc0 was effective at tracking injury magnitude, showing higher levels in patients who died within 3 days (early mortality) compared to those with favorable outcome or who had unfavorable outcome/died later (p=0.012, Jonckheere-Terpstra trend test) (Suppl. Figure2). In contrast, there were no significant differences in the levels of HexNAc7Hex7DeoxyHex1NeuAc2 and HexNAc8Hex6DeoxyHex0NeuAc0 after injury across outcome groups (p > 0·05). However, overall, their serum levels were persistently increased in patients with favorable outcome but undetectable in patients with unfavorable outcome (Table 2).
Table 2.
Group differences for the 4 identified highly influential glycans. Univariate statistical analyses were performed using Mann-Whitney tests.
| N-Glycan Compositions | Composition ID | Good E (n=40) | Poor E (n=11) | p-value | Good Day 1 (n=36) | Poor Day 1 (n=12) | p-value | Good Day 2 (n=36) | Poor Day 2 (n=12) | p-value | Good Day 3 (n=36) | Poor Day 3 (n=12) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
HexNAc5Hex3DeoxyHex0NeuAc0 | 5-3-0-0 | 0·92% | 1·35% | 0·01 | 0·81% | 0·91% | NS | 0·77% | 1% | NS | 0·8% | 0·98% | NS |
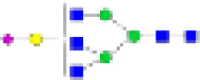 |
HexNAc5Hex4DeoxyHex0NeuAc1 | 5-4-0-1 | 0·45% | 0·62% | 0·038 | 0·42% | 0·38% | NS | 0·4% | 0·37% | NS | 0·37% | 0·35% | NS |
 |
HexNAc7Hex7DeoxyHex1NeuAc2 | 7-7-1-2 | 0·03% | 0·00% | NS | 0·03% | 0·00% | NS | 0·03% | 0·00% | NS | 0·02% | 0·00% | NS |
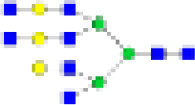 |
HexNAc8Hex6DeoxyHex0NeuAc0 | 8-6-0-0 | 0·07% | 0·00% | NS | 0·09% | 0·01% | NS | 0·1% | 0·00% | NS | 0·02% | 0·00% | NS |
ROC curves demonstrated that HexNAc5Hex3DeoxyHex0NeuAc0 and HexNAc5Hex4DeoxyHex0NeuAc1 levels at 24 h after TBI were able to discriminate unfavorable from favorable outcome with an AUC of 0·75 (95% CI 0·59 to 0·90) and 0·71 (95% CI 0·52 to 0·89), respectively (Figure 3).
Figure 3.
Prognostic accuracy of the glycomarkers. ROC curves for glycan 5-3-0-0 (A) and 5-4-0-1 (B) concentrations day of injury for poor outcome (GOS-E 1-4) at 12 months after moderate to severe TBI.
Age was positively correlated with HexNAc5Hex3DeoxyHex0NeuAc0 (r= 0.46; p<0.001) and HexNAc5Hex4DeoxyHex0NeuAc1 (r= 0·30; p<0·05), and negatively with HexNAc7Hex7DeoxyHex1NeuAc2 (r= - 0·31; p<0·05) andHexNAc8Hex6DeoxyHex0NeuAc0 (r= -0·32; p<0·05). Acute serum HexNAc5Hex4DeoxyHex0NeuAc1 concentrations were significantly higher in patients with mass lesions vs. those with diffuse injury (0·6% vs 0·4%; p<0·05) and in patients requiring decompressive craniectomy vs. those conservatively treated (0·6% vs 0·4%; p<0·05). No other associations were found between the glycomarkers and patient characteristics.
Discussion
The advent of cutting-edge highly sensitive technologies has greatly advanced our understanding of the complexity and heterogeneity of glycan structure and properties.15, 16, 17, 18,31 This has allowed pathobiological mechanisms and underpinning to be deciphered at the glycome level.15 In this study, we used a high-throughput PGC-LC-MS platform to perform large-scale N-glycan profiling of patients with moderate to severe TBI and identified and quantified 94 N-glycans in blood after injury. Specifically, we observed temporal changes in the glycome expression patterns that may represent TBI glycofingerprints.
Several mechanisms could underlie these observations. Since glycosylation is cell type–and site-specific, and highly influenced by the physiological status of cells,32 the changes seen in serum glycans can be attributed, in part. to the biosynthetic and metabolic crisis that occurs after TBI. In response to acute brain injury, multiple mechanisms result in mitochondrial dysfunction and increased oxidative stress with a shift from aerobic to anaerobic metabolism in neurons. By starving the hexosamine pathway of glucose and glutamine, glycolysis and glutaminolysis reduce UDP-GlcNAc biosynthesis - a critical precursor in the N-glycan branching - and block Golgi branching activity, ultimately, leading to altered glycosylation.33, 34, 35 Importantly, there is now substantial evidence that reduced N-glycan branching affects cell development and growth, and promotes pro-inflammatory differentiation, that in turn affect mitochondrial integrity and function.34 Breaking this vicious cycle might represent a promising therapeutic target.
However, a higher serum concentration of oligomannosides early after injury could also represent a biomarker of brain injury. Unlike most tissues, where they are trimmed (glycan processing) during the N-glycan maturation, in the brain oligomannosides are carried to the cell surface on recognition molecules, such as neural cell-adhesion molecule, L1 and adhesion molecule on glia (AMOG).14 In this respect, importantly, oligomannosides are concentrated in synapses and at the level of the blood–brain barrier, where they appear to play a role in its formation and maintenance.14,36,37 Thus, increased concentrations of oligomannosides have the potential to be autonomously associated with multiple injury sites in the brain critically affecting function and recovery. Future studies are necessary to confirm this hypothesis, increase our ability to correctly interpret the glycomics data, and better define any potential clinical utility.
Another potential interpretation of our findings is that the serum glycan patterns may reflect distinct pathobiological pathways linked to different types of brain damage. In line with this, we found decreased HexNAc5Hex4DeoxyHex0NeuAc1 concentrations in patients with diffuse injury (i.e., diffuse axonal injury38) vs. those with mass lesions—two very distinct and important TBI endophenotypes. This hypothesis is also consistent with recent data in patients with multiple sclerosis indicating that N-glycan branching regulates oligodendrogenesis, promotes myelination and myelin repair, and that low serum levels of GlcNAc, a rate-limiting metabolite for N-glycan branching, are associated with demyelination and axon damage.39 The mechanisms that may drive these processes remain to be elucidated. Nonetheless, together with previous findings, our data raise the possibility that alterations in the glycosylation biosynthetic pathways can profoundly impact white matter damage and repair/ regeneration, and thereby, clinical outcome.
To gain insight into the relationship between N-glycan profiles and outcome and to identify novel prognostic biomarkers, we used a multivariate data analysis approach capable of handling and leveraging the complex intercorrelations of the investigated glycome profiles and which identified four promising candidates (Table 2). Among these glycans, HexNAc5Hex3DeoxyHex0NeuAc0 and HexNAc5Hex4DeoxyHex0NeuAc1 are brain-specific18,40 and were substantially increased early after injury in patients with unfavorable outcome. It is conceivable that these increased concentrations correspond to injury severity. Interestingly, HexNAc5Hex4DeoxyHex0NeuAc1 appears to be sialylated, which may suggest different mechanisms linking this marker to unfavorable outcome. There is growing evidence that glycosylation plays a major role in modulating the immune response.41 In particular, the inflammatory phenotypes of several immune cells, including monocytes, B cells, T cells, and also microglia are regulated by sialic acid–containing glycans, for example through the binding with siglecs (sialic acid-binding immunoglobulin-like lectins).42,43 Therefore, HexNAc5Hex4DeoxyHex0NeuAc1 could affect and regulate neuroinflammation. This will be an important avenue for future investigation, particularly given the link between chronic neuroinflammation and neurodegenerative diseases.44 On the other hand, sialylated N-glycan structures exerting relevant effects on voltage gated Na+ and K+ channels, modulating cell excitability, and thus controlling neural transmission and excitability of neural circuits.45 Given evidence showing that spreading depolarizations (i.e., pathological waves of neuronal depolarization occurring after brain injury) are associated with unfavorable outcome after TBI, a possibility is that HexNAc5Hex4DeoxyHex0NeuAc1 could have a direct effect on outcome through this mechanism.46,47 Glycosylated forms of the Sur1-TRPM4 ion channel on the surface of neurons have also been reported and the importance of this channel in the development of cerebral edema after TBI is emerging.48,49 These are areas of great interest, and future work should assess whether HexNAc5Hex4DeoxyHex0NeuAc1 or other glycans might represent a mechanistic therapeutic target and biomarker to inform and guide treatment.
While our work provides the first framework of the “clinical validity” of glycomarkers in TBI- how well the identified glycans relates to the clinical outcome of interest (GOS-E)-, whether they can inform and improve medical decision-making beyond existing standards (“clinical utility”) remains to be determined. The pathway toward future clinical implementation includes the establishment of thresholds for normality, validating the performance, and assessing the added (independent) and complementary prognostic value when compared and combined with traditional blood-based TBI biomarkers. This may also reveal opportunities for multimarker strategies useful in refining patient endophenotyping, risk stratification, overcoming and/or augmenting traditional classification approaches and transforming clinical practice in the field of TBI.
Our study has several limitations. Given its exploratory nature and a rather small sample size, our findings should be interpreted with caution. Although we applied a rigorous approach to identify and validate the glycomarkers, further confirmation in larger cohorts will increase statistical power (reducing the probability of type II error) and permit meaningful multivariate analyses. To this end, it would be of great interest to investigate the potential influence of therapeutic interventions, as well as to assess the independent prognostic value of glycomarkers over and above established outcome predictors (e.g., age, injury severity [GCS], and radiological information). Another limitation is that the patients analyzed were homogeneous across race, relatively old, with injuries caused by falls and mainly representing the extremes of the injury spectrum. Such characteristics are in line with the changing epidemiological landscape of TBI observed in large multicenter studies (e.g., CENTER-TBI50) and are likely to reflect socioeconomic health determinants of high-income countries.51 Nonetheless, these factors may affect the generalizability of our results. Future studies including more heterogenous/real-world like cohorts also from middle-income and low-income countries are necessary. While we suggested associations between TBI characteristics and outcome after injury, we did not quantify glycomarker levels in either normal or ICU control subjects without TBI, and/or compare injury to control. The longitudinal design of the study allows each patient to act as their own control while providing initial information about distinct glycan dynamics associated with different patient phenotypes. However, to guide glycosignature interpretation and use in the clinic, large-scale studies are needed to generate valid normative reference intervals as well as ascertain potential age-sex-race-specific effects on glycan blood levels. A more accurate definition of the dynamic range and temporal ordering of glycomarkers after TBI will also be essential to characterize the optimal prognostic window and specific correlations with pathological cascades as well as potential relationships between time-dependent glycan changes and injury progression/sub-acute clinical consequences (e.g., secondary injuries). Further work is warranted. Furthermore, although putative brain specific glycomarkers were identified, additional supportive evidence in this regard could be obtained in studies of cerebrospinal fluid in patients after injury. We also did not directly verify putative relationships with pathophysiological mechanisms. This would likely require pre-clinical and clinical studies in which glycans measurements are correlated with metabolic, neurophysiological and neuropathological assessments as well as developmental processes and spatial diversity of glycosylation in the brain.18 Indeed, in this regard, our work suggests the need for reverse translation of our clinical findings in both in vitro TBI models such as neuronal stretch,52 and across a spectrum of in vivo pre-clinical TBI models.53, 54, 55 Spatial analysis using advanced imaging methods to define brain region specificity of glycome expression patterns would also be informative. Finally, evaluation of glycoprotein changes was beyond the scope of the current analysis. However, since changes in glycosylation influence protein function as well as protein solubility, antigenicity, and half-life, this remains to be addressed.
In conclusion, glycans play pivotal roles in the central nervous system and our results suggest that deciphering glycomics patterns after TBI could add another important dimension to our understanding of complex pathobiological processes of TBI, while yielding complementary information.
Contributors
SM, EC, KA, FHK, AB and YM conceived and designed the study. SM, VS, EC, KA and AB performed the data curation. SM and VS performed the formal analysis. SM and YM acquired the funding for the current study. SM, VS, EC, KA and AB contributed to the data collection. All authors contributed to the investigation. MG, SG, BGC, RM and YM performed the glycomic analysis. SM, VS, FHK, and YM designed the methodology. All authors contributed to the project administration. SM and YM supervised the study. SM drafted the manuscript. All authors made important intellectual contributions to the interpretation of data and helped revise the manuscript. All authors had full access to the data in the study and all authors accept responsibility to submit for publication.
Funding
This work was funded by the Italian Ministry of Health (grant number GR-2013-02354960), and also partially supported by a NIH grant (1R01GM112490-08).
Data sharing statement
De-identified data can be made available from the corresponding author SM upon reasonable request. Contact information for SM is included on the title page.
Declaration of interests
The authors declare no conflict of interest.
Acknowledgments
We are grateful to all patients their families for their invaluable contributions to this study. We would like to thank the ICU nurses and staff for assistance in collecting samples and data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101494.
Contributor Information
Stefania Mondello, Email: stm_mondello@hotmail.com.
Yehia Mechref, Email: Yehia.Mechref@ttu.edu.
Appendix. Supplementary materials
References
- 1.Injury GBDTB, Spinal Cord Injury C Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 3.Maas AI, Murray GD, Roozenbeek B, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurol. 2013;12(12):1200–1210. doi: 10.1016/S1474-4422(13)70234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29(11):1259–1272. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondello S, Gabrielli A, Catani S, et al. Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Inj. 2012;26(13-14):1629–1635. doi: 10.3109/02699052.2012.700083. [DOI] [PubMed] [Google Scholar]
- 6.Unden J, Ingebrigtsen T, Romner B, Scandinavian Neurotrauma C. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11:50. doi: 10.1186/1741-7015-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondello S, Sorinola A, Czeiter E, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. J Neurotrauma. 2021;38(8):1086–1106. doi: 10.1089/neu.2017.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everest-Dass AV, Moh ESX, Ashwood C, Shathili AMM, Packer NH. Human disease glycomics: technology advances enabling protein glycosylation analysis - part 2. Expert Rev Proteomics. 2018;15(4):341–352. doi: 10.1080/14789450.2018.1448710. [DOI] [PubMed] [Google Scholar]
- 9.Dong X, Mondello S, Kobeissy F, Talih F, Ferri R, Mechref Y. LC-MS/MS glycomics of idiopathic rapid eye movement sleep behavior disorder. Electrophoresis. 2018;39(24):3096–3103. doi: 10.1002/elps.201800316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zulueta A, Mingione A, Signorelli P, Caretti A, Ghidoni R, Trinchera M. Simple and complex sugars in parkinson's disease: a bittersweet taste. Mol Neurobiol. 2020;57(7):2934–2943. doi: 10.1007/s12035-020-01931-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee SU, Grigorian A, Pawling J, et al. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem. 2007;282(46):33725–33734. doi: 10.1074/jbc.M704839200. [DOI] [PubMed] [Google Scholar]
- 12.Mkhikian H, Grigorian A, Li CF, et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun. 2011;2:334. doi: 10.1038/ncomms1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt AU, Sy M, Bellmann-Strobl J, et al. Association of a marker of N-acetylglucosamine with progressive multiple sclerosis and neurodegeneration. JAMA Neurol. 2021;78(7):842–852. doi: 10.1001/jamaneurol.2021.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5(3):195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- 15.Peng W, Gutierrez Reyes CD, Gautam S, et al. MS-based glycomics and glycoproteomics methods enabling isomeric characterization. Mass Spectrom Rev. 2021 doi: 10.1002/mas.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara R, Chernykh A, Alagesan K, et al. Community evaluation of glycoproteomics informatics solutions reveals high-performance search strategies for serum glycopeptide analysis. Nat Methods. 2021;18(11):1304–1316. doi: 10.1038/s41592-021-01309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong X, Huang Y, Cho BG, et al. Advances in mass spectrometry-based glycomics. Electrophoresis. 2018;39(24):3063–3081. doi: 10.1002/elps.201800273. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Ha S, Kim M, et al. Spatial and temporal diversity of glycome expression in mammalian brain. Proc Natl Acad Sci USA. 2020;117(46):28743–28753. doi: 10.1073/pnas.2014207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondello S, Guedes VA, Lai C, et al. Circulating brain injury exosomal proteins following moderate-to-severe traumatic brain injury: temporal profile, outcome prediction and therapy implications. Cells. 2020;9(4) doi: 10.3390/cells9040977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 21.Nationa_Institute_for_Health_and_Care_Excellence. Head Injury:Triage, assessment, investigation and early management of head in-jury in children, young people and adults. 2014.
- 22.Wilson L, Boase K, Nelson LD, et al. A manual for the glasgow outcome scale-extended interview. J Neurotrauma. 2021;38(17):2435–2446. doi: 10.1089/neu.2020.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamczyk B, Struwe WB, Ercan A, Nigrovic PA, Rudd PM. Characterization of fibrinogen glycosylation and its importance for serum/plasma N-glycome analysis. J Proteome Res. 2013;12(1):444–454. doi: 10.1021/pr300813h. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33(12):1768–1777. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong X, Mondello S, Kobeissy F, Ferri R, Mechref Y. Serum glycomics profiling of patients with primary restless legs syndrome using LC-MS/MS. J Proteome Res. 2020;19(8):2933–2941. doi: 10.1021/acs.jproteome.9b00549. [DOI] [PubMed] [Google Scholar]
- 26.Fortea J, Carmona-Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018;17(10):860–869. doi: 10.1016/S1474-4422(18)30285-0. [DOI] [PubMed] [Google Scholar]
- 27.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 28.Backryd E, Ghafouri B, Carlsson AK, Olausson P, Gerdle B. Multivariate proteomic analysis of the cerebrospinal fluid of patients with peripheral neuropathic pain and healthy controls - a hypothesis-generating pilot study. J Pain Res. 2015;8:321–333. doi: 10.2147/JPR.S82970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olausson P, Gerdle B, Ghafouri N, Sjostrom D, Blixt E, Ghafouri B. Protein alterations in women with chronic widespread pain–An explorative proteomic study of the trapezius muscle. Sci Rep. 2015;5:11894. doi: 10.1038/srep11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triba MN, Le Moyec L, Amathieu R, et al. PLS/OPLS models in metabolomics: the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol Biosyst. 2015;11(1):13–19. doi: 10.1039/c4mb00414k. [DOI] [PubMed] [Google Scholar]
- 31.Kobeissy F, Kobaisi A, Peng W, et al. Glycomic and glycoproteomic techniques in neurodegenerative disorders and neurotrauma: towards personalized markers. Cells. 2022;11(3) doi: 10.3390/cells11030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudd PM, Merry AH, Wormald MR, Dwek RA. Glycosylation and prion protein. Curr Opin Struct Biol. 2002;12(5):578–586. doi: 10.1016/s0959-440x(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Filho AF, Sena WLB, Lima LRA, et al. Glycobiology modifications in intratumoral hypoxia: the breathless side of glycans interaction. Cellular Physiol Biochem. 2017;41(5):1801–1829. doi: 10.1159/000471912. [DOI] [PubMed] [Google Scholar]
- 34.Araujo L, Khim P, Mkhikian H, Mortales CL, Demetriou M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 2017;6 doi: 10.7554/eLife.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mkhikian H, Mortales CL, Zhou RW, et al. Golgi self-correction generates bioequivalent glycans to preserve cellular homeostasis. Elife. 2016;5 doi: 10.7554/eLife.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matus A, De Petris S, Raff MC. Mobility of concanavalin A receptors in myelin and synaptic membranes. Nat New Biol. 1973;244(139):278–280. doi: 10.1038/newbio244278a0. [DOI] [PubMed] [Google Scholar]
- 37.Heller M, von der Ohe M, Kleene R, Mohajeri MH, Schachner M. The immunoglobulin-superfamily molecule basigin is a binding protein for oligomannosidic carbohydrates: an anti-idiotypic approach. J Neurochem. 2003;84(3):557–565. doi: 10.1046/j.1471-4159.2003.01537.x. [DOI] [PubMed] [Google Scholar]
- 38.Moenninghoff C, Kraff O, Maderwald S, et al. Diffuse axonal injury at ultra-high field MRI. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sy M, Brandt AU, Lee SU, et al. N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J Biol Chem. 2020;295(51):17413–17424. doi: 10.1074/jbc.RA120.015595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronewitter SR, An HJ, de Leoz ML, Lebrilla CB, Miyamoto S, Leiserowitz GS. The development of retrosynthetic glycan libraries to profile and classify the human serum N-linked glycome. Proteomics. 2009;9(11):2986–2994. doi: 10.1002/pmic.200800760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9(6):593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 42.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 43.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278(33):31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 44.Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott H, Panin VM. The role of protein N-glycosylation in neural transmission. Glycobiology. 2014;24(5):407–417. doi: 10.1093/glycob/cwu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartings JA, Bullock MR, Okonkwo DO, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12):1058–1064. doi: 10.1016/S1474-4422(11)70243-5. [DOI] [PubMed] [Google Scholar]
- 47.Hartings JA, Watanabe T, Bullock MR, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134(Pt 5):1529–1540. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- 48.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013;288(5):3655–3667. doi: 10.1074/jbc.M112.428219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019;145(Pt B):230–246. doi: 10.1016/j.neuropharm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steyerberg EW, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- 51.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 52.Jackson TC, Kotermanski SE, Jackson EK, Kochanek PM. BrainPhys(R) increases neurofilament levels in CNS cultures, and facilitates investigation of axonal damage after a mechanical stretch-injury in vitro. Exp Neurol. 2018;300:232–246. doi: 10.1016/j.expneurol.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondello S, Shear DA, Bramlett HM, et al. Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: operation brain trauma therapy. J Neurotrauma. 2016;33(6):595–605. doi: 10.1089/neu.2015.4132. [DOI] [PubMed] [Google Scholar]
- 54.Kochanek PM, Bramlett HM, Dixon CE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33(6):513–522. doi: 10.1089/neu.2015.4113. [DOI] [PubMed] [Google Scholar]
- 55.Kochanek PM, Bramlett HM, Shear DA, et al. Synthesis of findings, current investigations, and future directions: operation brain trauma therapy. J Neurotrauma. 2016;33(6):606–614. doi: 10.1089/neu.2015.4133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





