Highlights
-
•
Based on a nationwide quantitative online survey among office-based physicians in Germany, we found a high divergence between preference for and use of treatment options for stroke prevention in atrial fibrillation.
-
•
In particular, vitamin K antagonists were used more often than preferred whereas non-vitamin K oral anticoagulants, on average, were used less often than preferred.
-
•
Oral anticoagulant attributes and patient characteristics related to efficacy and safety, as well as patients’ kidney function were most important when selecting a specific oral anticoagulant.
-
•
Federal and regional governance instruments likely influenced treatment decision-making process.
Keywords: Atrial fibrillation, Stroke prevention, Anticoagulant agents, Clinical practice, Physician preferences, Physician prescribing patterns
Abstract
In Germany, there is little real-world evidence on physicians’ choice of oral anticoagulants (OACs). Our study aimed at assessing preferences for and prescribing patterns of treatment options for stroke prevention in atrial fibrillation in clinical practice in Germany.
We conducted a nationwide quantitative online survey among office-based physicians in Germany. Physicians were asked about their preference for and use of treatment options as well as factors influencing their choice of a specific OAC.
A total of n = 953 physicians was surveyed in September and October 2020 (general physicians: 36.0%; internists: 37.3%; cardiologists: 23.7%; neurologists: 10.5%; multiple specialties possible). Preference and use were highest for non-vitamin K oral anticoagulants (NOACs); followed by vitamin K antagonists (VKAs). Most preferred OACs were apixaban (39.3%), rivaroxaban (28.5%) and edoxaban (14.7%). Most used OACs were apixaban (24.3%), rivaroxaban (21.2%) and phenprocoumon (21.4%). NOACs were preferred more often than used (85.6% > 68.6%). VKAs were preferred less often than used (9.6% < 23.5%). OAC attributes and patient characteristics related to efficacy and safety, as well as patients’ kidney function were most important when selecting a specific OAC. Federal and regional governance instruments likely influenced treatment decision-making.
We found a high divergence between preferences for and use of available treatment options in clinical practice. Further exploration of the importance of OAC attributes, patient characteristics as well as federal and regional governance instruments for physicians’ choice of a specific OAC may help to further optimize the healthcare of patients with atrial fibrillation in the long-term.
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults and affects about 1.8 million people in Germany (Kip et al. 2015). The disease is associated with increased morbidity and mortality and places a significant burden on those affected. Overall, AF increases the risk of stroke five-fold (Hindricks et al., 2021). Therefore, prevention of stroke and systemic embolism is a core tenet of the treatment of AF.
In patients with non-valvular AF, vitamin K antagonists (VKAs) or oral non-vitamin K anticoagulants (NOACs) are recommended pharmacotherapies for stroke prevention in AF. In their pivotal trials, the NOACs apixaban, dabigatran, edoxaban, and rivaroxaban were at least non-inferior to the VKA warfarin in preventing stroke or systemic embolic events (Connolly et al., 2009, Giugliano et al., 2013, Granger et al., 2011, Patel et al., 2011).
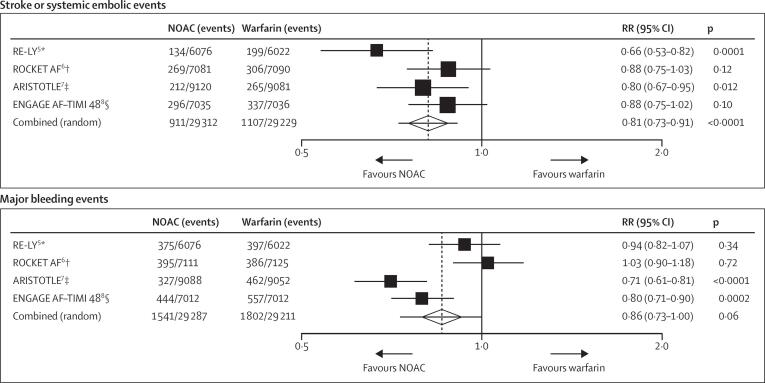
A meta-analysis of these trials reports a favorable risk–benefit profile of NOACs compared to warfarin, with significant reduction in stroke, intracranial bleeding, and mortality, a similar risk for major bleeding, but an increased risk for gastrointestinal bleeding (Ruff et al. 2014) (see Fig. 1). Real-world data support these findings (Escobar et al., 2019, Ntaios et al., 2017).
Fig. 1.
Stroke or systemic embolic events and major bleeding events reported by pivotal studies of NOACs Abbreviations: RE-LY – Randomized Evaluation of Long-Term Anticoagulation Therapy, ROCKET AF – Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation, ARISTOTLE – Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation, ENGAGE AF-TIMI 48 – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48, RR – Relative risk, NOAC – Non-vitamin K oral anticoagulants: p – p-value.
Given their relative efficacy, safety and convenience, the European Society of Cardiology recommends NOACs as first-line treatment for stroke prevention in AF (Hindricks et al., 2021). In contrast, the Drug Commission of the German Medical Association recommends NOACs primarily in specific cases, e.g., poor anticoagulation control, an acute arrhythmia or ablation, or an increased risk of intracerebral hemorrhage or drug and food interactions (AkdÄ 2019).
Each oral anticoagulant (OAC) is associated with a different bundle of attributes. For instance, apixaban and dabigatran were superior to warfarin in reducing stroke or systemic embolic events and apixaban and edoxaban were associated with less major bleeding events. Patients treated with phenprocoumon require regular testing of the international normalized ratio (INR) and concomitant adjustment of dosage. Although home testing is generally possible, INR testing is usually performed at a physician’s office (Cromheecke et al. 2000). In addition, there are different dosing options (e.g., once vs. twice per day) and dietary restrictions. Furthermore, patient characteristics such as age, comorbidity, and polypharmacy must be considered when treating AF (Wang and Bajorek, 2016).
The use of treatment options for stroke prevention in AF is also subject to the regulatory framework. In Germany, legal regulations are driven by federal and regional governance instruments. On a federal level, a new procedure for Health Technology Assessment and Price Negotiation was introduced in 2011 with the Act on the Reform of the Market for Medicinal Products (AMNOG). The “AMNOG benefit assessment” compares the actual patient-relevant benefit of a new drug with that of an established drug or treatment strategy (IQWIG 2021). On a regional level, the Association of Statutory Health Insurance Physicians (ASHIP) is responsible for ensuring that outpatient medical care is available in appropriate quality and regional accessibility and that outpatient healthcare providers handle the limited financial resources of the statutory health insurance system cost-efficiently. Despite legal regulations, physicians generally enjoy therapeutic freedom to use the therapy they deem appropriate for the individual patient. However, physicians’ general preference for treatment options for stroke prevention in AF and their actual use may differ in clinical practice (Andrade et al. 2016).
In Germany, there is little real-world evidence on physicians’ choice of OACs. A better understanding of physicians’ decision-making may help to further optimize the healthcare of patients with AF in the long-term. Given this background, our study aimed at assessing physicians’ familiarity with, preference for and use of treatment options for stroke prevention in AF as well as factors potentially influencing their choice of a specific OAC under real-world conditions in Germany.
2. Methods
2.1. Study design
Our study was designed as a quantitative online survey by the research and consulting company IGES Institut GmbH (IGES) and conducted in cooperation with the market research company Schlesinger Group Germany GmbH (Schlesinger).
As a field organization, Schlesinger recruits sophisticated target groups for qualitative and quantitative surveys. Healthcare professionals (HCPs) such as physicians, pharmacists, and nurses can join closed HCP panels, which are deeply profiled by Schlesinger. The HCP panel for physicians contains, among others, detailed information on the primary specialty, the years in practice and the practice setting. In total, the HCP panel for physicians consists of approximately 25,000 physicians located nationwide in Germany.
Physicians eligible to prescribe treatment for stroke prevention in AF were invited by Schlesinger to participate in our survey. Prerequisite for inclusion was a relevant specialist group, namely, general medicine, internal medicine, cardiology, or neurology, as well as a predominantly office-based activity. Participation in our closed survey was possible for all registered and approved HCP panel members. The specific selection of physicians was therefore made exclusively through Schlesinger and could not be influenced by third parties.
Recruitment was aimed at a target number of 1,000 physicians; a composition of about 300 general physicians, 550 internists and cardiologists, and 100 neurologists was anticipated beforehand. Recruitment began in September 2020 and should be completed within two months. All physicians were compensated for their participation by Schlesinger.
2.2. Survey instrument and implementation
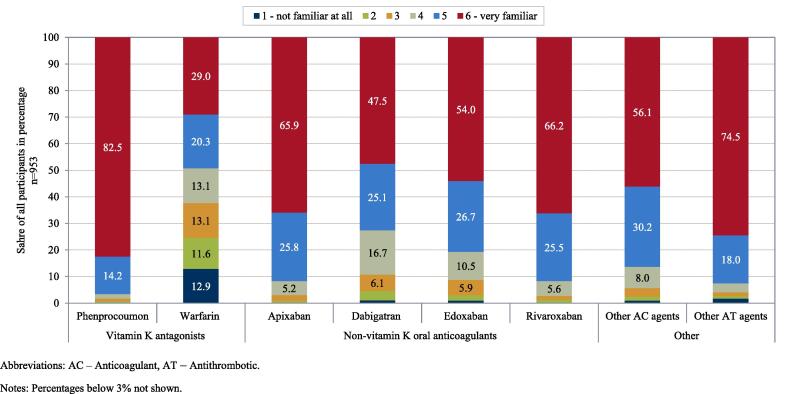
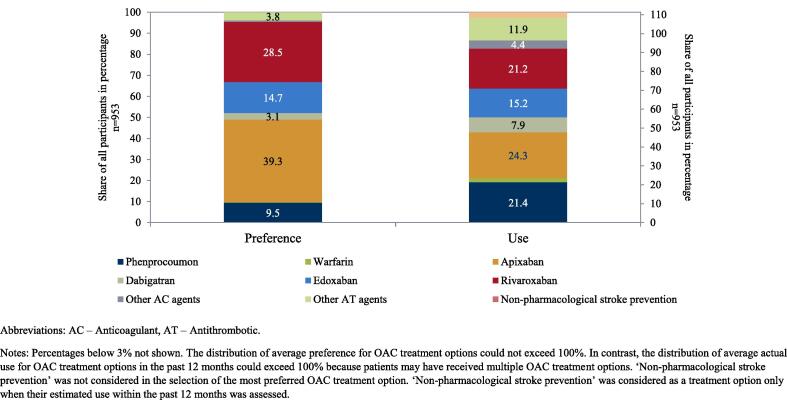
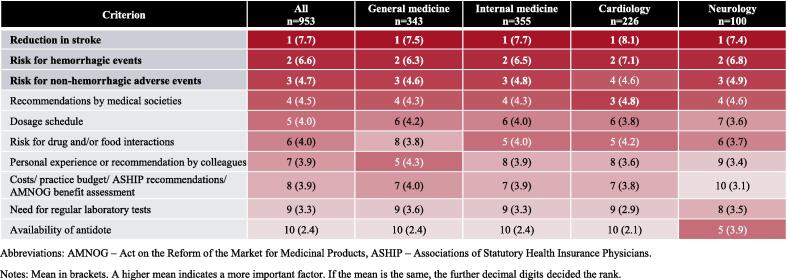
“Physicians’ familiarity with treatment options” was rated on a 6-point scale indicating their level of familiarity (see Fig. 2). “Physicians’ preference for treatment options” was assessed based on the most preferred option (see Fig. 3). “Physicians’ use of treatment options” was assessed by the estimated proportion of prescriptions in the 12 months prior to the time of the survey (see Fig. 3). Furthermore, “importance of OAC attributes” and “importance of patient characteristics” were assessed based on ten factors relevant in treatment of AF, respectively (see Fig. 4 and Fig. 5). Physicians were asked to rank these factors from 1 to 10 with respect to the importance for their choice of a specific OAC. Lastly, the “role of the regulatory framework” was assessed by means of five federal and regional governance instruments relevant in the German context on a 6-point scale to determine how strongly physicians perceive their role (see Fig. 6). Treatment options were limited to those approved in the German healthcare context, namely, the VKAs phenprocoumon (Marcumar®, Falithrom®, generics) and warfarin (Coumadin®), the NOACs apixaban (Eliquis®), dabigatran (Pradaxa®), edoxaban (Lixiana®), and rivaroxaban (Xarelto®), other anticoagulant agents (e.g., heparins), and other antithrombotic agents (e.g., acetylsalicylic acid). “Non-pharmacological stroke prevention” was considered as a treatment option only when their estimated use in the past 12 months was assessed. Physicians’ age, sex, and aspects related to their routine care situation (e.g., their experience in treating and anticoagulating patients with AF) were assessed as characteristics of participants (see supplement).
Fig. 2.
Physicians’ familiarity with OAC treatment options for stroke prevention in AF Abbreviations: AC – Anticoagulant, AT – Antithrombotic. Notes: Percentages below 3% not shown.
Fig. 3.
Physicians’ preference for treatment options for stroke prevention in AF and their estimated use within the past 12 months Abbreviations: AC – anticoagulant, AT – antithrombotic. Notes: Percentages below 3% not shown. The distribution of average preference for OAC treatment options could not exceed 100%. In contrast, the distribution of average actual use for OAC treatment options in the past 12 months could exceed 100% because patients may have received multiple OAC treatment options. ‘Non-pharmacological stroke prevention’ was not considered in the selection of the most preferred OAC treatment option. ‘Non-pharmacological stroke prevention’ was considered as a treatment option only when their estimated use within the past 12 months was assessed.
Fig. 4.
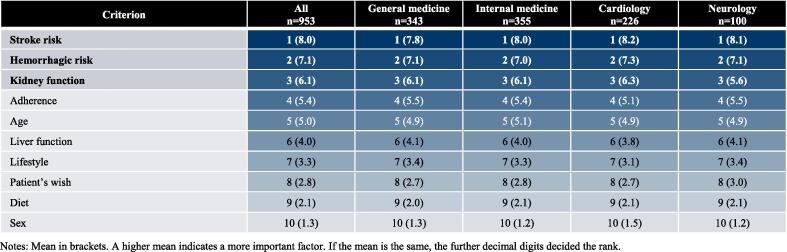
Importance of OAC attributes in physicians’ choice of treatment options for stroke prevention in AF. Abbreviations: AMNOG – Act on the Reform of the Market for Medicinal Products, ASHIP – Associations of Statutory Health Insurance Physicians. Notes: Mean in brackets. A higher mean indicates a more important factor. If the mean is the same, the further decimal digits decided the rank.
Fig. 5.
Importance of patient characteristics in physicians’ choice of treatment options for stroke prevention in AF. Notes: Mean in brackets. A higher mean indicates a more important factor. If the mean is the same, the further decimal digits decided the rank.
Fig. 6.
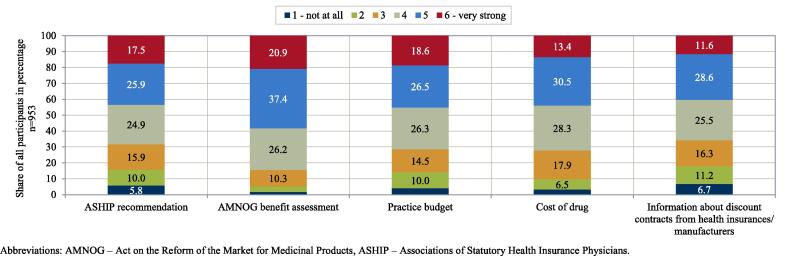
Role of regulatory framework in physicians’ choice of treatment options for stroke prevention in AF. Abbreviations: AMNOG – Act on the Reform of the Market for Medicinal Products, ASHIP – Associations of Statutory Health Insurance Physicians.
The survey was programmed as a web application and hosted by Schlesinger. Plausibility rules were used, where appropriate, to ensure a high quality of the participants’ responses. Furthermore, treatment options, OAC attributes, patient characteristics, and federal and regional governance instruments were displayed in random order to prevent the order of items from influencing the participants’ responses. In addition, a ‘cut-off’ was set for the speed of completing the online survey. Based on an estimated survey length of 15 to 20 min, surveys completed in less than 10% of 17 min were automatically sorted out. The suitability of the survey instrument was tested by two independent office-based physicians. In addition, the usability and technical functionality of the online survey was tested by two independent medical professionals before its fielding.
All data protection and data security requirements resulting from applicable national and international guidelines were taken into account during data collection and processing. Raw data were collected by Schlesinger and stored on dedicated servers located in the EU (Ireland). Fully completed online surveys were provided to IGES in anonymous form only for the purpose of statistical analysis; data were stored on own servers located in the EU (Germany).
2.3. Statistical analyses
All statistical analyses were purely descriptive in nature and performed using Stata 14. Continuous variables were summarized by mean and standard deviation. Depending on their scale level, continuous variables and categorical variables were expressed as frequencies and percentages in tabular form or by means of appropriate charts. Physicians’ preference for treatment options and their estimated use in the past 12 months were contrasted to show potential divergence. The distribution of average preference for treatment options could not exceed 100% as one option could be chosen by physicians. In contrast, the distribution of average estimated use in the past 12 months could exceed 100%, if physicians prescribed multiple treatment options to a single patient, e.g., a NOAC plus acetylsalicylic acid. Ranks of the importance of OAC attributes and patient characteristics were ordered by their mean, with a higher mean indicating a more important factor.
3. Results
The online survey took place between 03.09.2020 and 12.10.2020. The participation rate (ratio of unique visitors who agreed to participate divided by unique first online survey page visitors) and the completion rate (ratio of users who finished the online survey divided by users who agreed to participate) were 81.0% and 90.3%, respectively. A total of n = 953 primarily office-based physicians with fully completed questionnaires was surveyed.
General physicians and internists were the largest specialty groups (36.0%, 37.3%); followed by cardiologists (23.7%) and neurologists (10.5%). The single shares add up to more than 100% because some physicians held several specialties. Mean age of the participants was 54.4 years (± 8.2 years); 76.1% of them were men. Single practices and joint practices were the most common practice types (41.1%, 38.6%), with most practices located in North Rhine-Westphalia (22.0%), Bavaria (17.6%), Baden-Wuerttemberg (10.8), or Hesse (10.0%) (see supplement).
3.1. Familiarity with treatment options
Of all treatment options, familiarity was highest with phenprocoumon (‘6 – very familiar’: 82.5%; ‘5’: 14.2%) and lowest with warfarin (‘6 – very familiar’: 29.0%; ‘5’: 20.3%). Among NOAC agents, familiarity was highest with rivaroxaban (‘6 – very familiar’: 66.2%; ‘5’: 25.5%) and apixaban (‘6 – very familiar’: 65.9%; ‘5’: 25.8%). Familiarity with other anticoagulant agents was high among treatment options (‘6 – very familiar’: 74.5%; ‘5’: 18.0%).
3.2. Preference for and use of treatment options
NOACs were the most preferred treatment option by physicians. In total, preference for NOACs amounted to 85.6% (apixaban: 39.3%; rivaroxaban: 28.5%; edoxaban: 14.7%; dabigatran: 3.1%). Preference for apixaban was particularly high among neurologists (46.0%) (see supplement). VKAs were preferred by 9.6% of the respondents (phenprocoumon: 9.5%; warfarin: 0.1%). Preference for other treatment options played a rather minor role.
In line with physicians’ preference, NOACs were the most used treatment option. In total, use for NOACs amounted to 68.6% (apixaban: 24.3%; rivaroxaban: 21.2%; edoxaban: 15.2%; dabigatran: 7.9%). Use of VKAs amounted to 23.5% (phenprocoumon: 21.4%; warfarin: 2.1%). Use of apixaban was highest among cardiologists (26.8%) (see supplement). Again, use of other treatment options played a minor role.
VKAs were used more often than preferred. The average use of phenprocoumon exceeded the preference by 11.9 pp (= 21.4% minus 9.5%). NOACs were, on average, used less often than preferred. Use of apixaban was 15.0 pp below preference for apixaban (= 24.3% minus 39.3%). The highest difference was found among neurologists (-24.3 pp = 21.7% minus 46.0%) (see supplement). Use of rivaroxaban was 7.3 pp below preference for rivaroxaban (= 21.2% minus 28.5%). The highest difference was found among internists (-10.5 pp = 20.2% minus 30.7%) and general physicians (-7.7 pp = 22.3% minus 30.0%) (see supplement). In contrast, the use of dabigatran and edoxaban exceeded the preference for these NOACs by 4.8 pp (= 7.9% minus 3.1%) and 0.5 (= 15.2% minus 14.7%) pp, respectively.
3.3. Importance of OAC attributes
OAC attributes, which were rated as most important when selecting a specific OAC, were attributes related to efficacy (“reduction of stroke events”) and safety (“risk for hemorrhagic events” and “risk for non-hemorrhagic adverse events”) (average of ≥ 4.7 on a 10-point scale). “Need for regular laboratory tests” and “availability of antidote” were rated as least important (≤ 3.3 / 10).
When participants’ responses were stratified by specialty, the “availability of antidote” was rated more important by neurologists (3.9 / 10) compared to physicians from other specialties (2.1 – 2.4 / 10). “Personal experience or recommendation by colleagues” was rated more important among general physicians (4.3 / 10) compared to neurologists (3.4 / 10), cardiologists (3.6 / 10), and internists (3.9 / 10). “Risk for drug and/or food interactions” was rated more important by cardiologists (4.2 / 10) when compared to other specialties (4.0 – 3.7 / 10). “Costs/ practice budget/ KV recommendations/ AMNOG benefit assessment” were rated more important among general physician, internists and cardiologists compared to neurologists (3.8 – 4.0 / 10 vs. 3.1 / 10). The remainder of the responses were similar between specialties.
3.4. Importance of patient characteristics
Characteristics of patients with AF, which were rated the most important when selecting a specific OAC, were “risk of stroke”, “hemorrhagic risk” and “kidney function” (average of ≥ 6.1 on a 10-point scale). “Sex” and “diet” were rated as least important (≤ 2.1 / 10). The order of importance of patient characteristics did not change when participants’ responses were stratified by specialty.
3.5. Role of regulatory framework
The majority of physicians reported that their choice of a specific OAC was strongly influenced by federal and regional governance instruments (judged by the response categories ‘6 – very strong’ and ‘5’). “AMNOG benefit assessment” was reported to play the strongest role in physicians’ choice of a specific OAC (‘6 – very strong’: 20.9%; ‘5’: 37.4%). In contrast, “information about discount contracts by health insurers or manufacturers” were reported to play the lowest role (‘6 – very strong’: 11.6%; ‘5’: 28.6%). Overall, the role of federal and regional governance instruments was comparable between response categories.
4. Discussion
We conducted a nationwide quantitative online survey on preferences for and prescribing patterns of treatment options for stroke prevention in AF among predominantly office-based general physicians, internist, cardiologists, and neurologists in Germany.
The results of our survey yielded following key findings. 1) The most preferred treatment option for stroke prevention in AF were NOACs; followed by VKAs and other antithrombotic treatment options. The most preferred OAC was apixaban; followed by rivaroxaban and edoxaban. 2) Overall, there was a high divergence between physicians’ preference for treatment options and their actual use in the past 12 months. In particular, VKAs were used more often than preferred whereas NOACs, on average, were used less often than preferred. The most used agent was apixaban; followed by rivaroxaban and phenprocoumon. 3) OAC attributes related to efficacy (“reduction in stroke”) and safety (“risk for hemorrhagic events” and “risk for non-hemorrhagic adverse events”) were reported to be most important when selecting a specific OAC. 4) The most important patient characteristics were “risk of stroke”, “hemorrhagic risk” and “kidney function”. 5) Federal and regional governance instruments exerted a strong influence on physicians’ choice for a specific OAC, with the “AMNOG benefit assessment” playing the strongest role.
In our survey, reflective of a 12-month period before September/October 2020, the estimated ratio of prescriptions for NOACs and VKAs was 2.9 (= 68.6% / 23.5%). This result is in good accordance with official numbers on prescriptions for these treatment options in Germany. For instance, the number of defined daily doses prescribed in 2020 was about 600 million for NOACs and about 209 million for VKAs; the former was thus about three times higher than the latter (Häussler and Höer, 2021).
The observed divergence between preference for and use of treatment options for stroke prevention in AF is consistent with results reported in similar studies. Andrade et al. (2016) conducted a discrete choice experiment in to assess values and, preference and experience of patients who receive OAC therapy, and physicians who prescribe it. The Canadian study found that physicians preferred NOACs (namely apixaban, dabigatran, or rivaroxaban) over VKAs (namely warfarin). Contrast to their preference, physicians were most likely to prescribe warfarin. At the individual NOAC level, rivaroxaban and apixaban were most preferred by physicians (44% and 23%, respectively); followed by dabigatran (12%) and warfarin (12%). In contrast to Andrade et al. (2016), physicians in our study strongly preferred apixaban over rivaroxaban (39.3% vs. 28.5%); however, both agents were also most preferred in our study. When the decision of physicians in the Canadian study was solely based on the OAC attribute profile, they were more likely to prefer apixaban over rivaroxaban; that is, when physicians were blinded to specific NOAC agents.
The importance of OAC attributes related to efficacy in safety is also consistent with results reported in similar studies. (Rymer et al., 2021, Andrade et al., 2016) conducted a survey on differences in the preferences of clinicians and patients with AF regarding the use and dosing of NOACs. The US-American study found that OAC attributes related to efficacy (“reduced risk of stroke”, and “proven to work better than other medications”) and safety (“severe bleeding”) were among the most relevant factors in clinicians’ anticoagulation decision making. In Andrade et al. (2016), the preferences of physicians regarding OAC therapy were largely focused on OAC attributes related to safety and, to a lesser extent, also to efficacy. However, the authors argued that the impact of efficacy on actual treatment decision-making was only marginal as all agents show excellent efficacy; instead, treatment decision-making was more reliant on other OAC attributes such as bleeding, reversal, and food- drugs interactions.
Furthermore, patient characteristics may play a major role in physicians’ choice of a specific OAC reflecting pharmacological differences according to the respective summary of product characteristics and reported safety and efficacy profiles. Anguita-Sánchez et al. (2016) conducted a survey on physicians’ perceptions on factors that influence the choice of classic VKA or NOAC therapy in patients with AF in Spain. According to the authors, the presence of a high thrombotic or hemorrhagic risk of patients with AF are leading factors for selecting NOAC therapy. In our survey, stroke risk and hemorrhagic risk were found to be the most important patient characteristics in physicians’ choice of a specific OAC; followed by patients’ kidney function.
Moreover, the availability of antidotes to reverse the effects of NOACs may have influenced physicians’ choice of a specific OAC. In Germany, idarucizumab is available as an antidote to dabigatran. In addition, andexanet alfa is approved to reverse antithrombotic effects of apixaban and rivaroxaban. In our study, the “availability of antidote” was rated least important by most specialties. However, it was rated more important by neurologists. Also, preference for and use of dabigatran was slightly higher in this specialty. Dabigatran is the only NOAC that demonstrated a reduction in ischemic strokes in AF patients compared to warfarin in pivotal trials. Compared with the other specialties, neurologists are more specialized in (primary and secondary) stroke prevention in AF and the treatment is more focused on efficacy. They also use antidotes more frequently in clinical practice to reverse the antithrombotic effects of NOACs. Given their likely knowledge of the efficacy of available NOACs and their frequent use of antidotes in clinical practice, it seems plausible that dabigatran was preferred and used more frequently by neurologists.
Although OAC attributes related to the regulatory framework were not ranked as high as OAC attributes related to efficacy and safety, the majority of the physicians still stated that federal and regional governance instruments exerted a strong influence on their choice of a specific OAC. As these instruments provide a regulatory framework in terms of quality, accessibility and economic efficiency, they likely influenced the treatment decision-making process of physicians and, thus, the prescription of the relevant agents in our study.
The following limitations have to be kept in mind when interpreting the results of our study. First, aspects such as physicians’ experience in treating and anticoagulating patients with AF were not considered during recruitment. However, the proportion of physicians who did not treat patients with AF or had no or little experience treating or anticoagulating patients with AF was less than five percent each in our study (see supplement) case. Second, the majority of respondents prescribed OAC treatment options as a follow-up prescription (74.0%). For these physicians, the actual use of OAC treatment options during the 12 months prior to the survey may not reflect the “physician’s choice of an OAC treatment option but a continuation of an initially initiated therapy”. However, final therapy decision is mostly made by physicians alone (67.0%) or jointly by physicians and patients (27.1%). Thus, physicians’ role in OAC treatment decision-making was generally high at 94.1%. Third, our study did not aim at linking the importance of OAC attributes, patient characteristics as well as federal and regional governance instruments to specific OAC treatment options. Therefore, we could not examine whether individual factors more important in relation to VKAs, NOACs, or other OAC treatment options.
5. Conclusion
To our knowledge, our study is the first large-scale survey on preferences for and prescribing patterns of treatment for stroke prevention in AF among physicians under real-world conditions in Germany. Therefore, the results of our study provide relevant insights into physicians’ decision-making process. We found a high divergence between preferences for and use of available OAC treatment options among relevant office-based specialist groups. The favorable efficacy and safety profile of NOACs presumably affected physicians’ high preference for this treatment option, as OAC attributes and patient characteristics related to efficacy and safety played the most important role in the physicians’ choice of a specific OAC. A better understanding of factors influencing physicians’ choice of specific OACs may help to further optimize the healthcare of patients with AF in the long-term. Future research should thus further explore the importance of OAC attributes, patient characteristics as well as federal and regional governance instruments on physicians’ choice of specific OAC under real-world conditions in Germany.
6. Data sharing statement
The data set, including physician-level information, cannot be shared due to privacy reasons. Summary data can be provided upon request. The questionnaire of our survey can be found the supplement.
Funding
This study was funded by the Bristol-Myers Squibb GmbH & Co. KGaA and the Pfizer Pharma GmbH. The funding did not have any impact on the study design, data collection and analysis, or preparation of the manuscript.
Author contribution
AM, VW, JS, HW, and HG were involved in the design of the survey. AM was carrying out the statistical analysis. AM, VW, JS, HW, and HG were involved in the data interpretation. AM wrote the manuscript with contributions from VW, JS, HW, and HG. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Anja Mocek, Valeria Weber and Holger Gothe are employees of the vendor IGES Institut GmbH, which is a paid consultant to Bristol-Myers Squibb GmbH & Co. KGaA and Pfizer Pharma GmbH for designing the survey, carrying out the statistical analyses, interpreting the results and writing the manuscript. The vendor Schlesinger Group Germany GmbH was subcontracted and reimbursed by the vendor IGES Institut GmbH for recruiting participants and conducting the online survey. Johanna Schmölders is an employee of Bristol-Myers Squibb GmbH & Co. KGaA. Henning Witt is an employee of Pfizer Pharma GmbH. Fees for data provision as well as publication fees are paid by IGES Institut GmbH as incurred costs and reimbursed by Bristol-Myers Squibb GmbH & Co. KGaA and Pfizer Pharma GmbH. The funding for designing the survey, carrying out the statistical analyses, interpreting the results and writing the manuscript as well as reimbursement of fees was shared between Bristol-Myers Squibb GmbH & Co. KGaA and Pfizer Pharma GmbH. All authors had complete autonomy for the process of designing the survey, carrying out the statistical analyses, interpreting the results and writing the manuscript. This also includes the full right to publish the results without limitation.
Acknowledgements
We thank the Schlesinger Group Germany GmbH for the recruitment of participants and conducting the online survey. Furthermore, we thank all the physicians who participated in our study. Lastly, we thank Anne-Katharina Carlitz (IGES Institut GmbH) and Marie Engelhard (IGES Institut GmbH) for their analytical support, and Leilah Dismond (IGES Institut GmbH) for her publication support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101861.
Contributor Information
Anja Mocek, Email: anja.mocek@iges.com.
Valeria Weber, Email: valeria.weber@iges.com.
Johanna Schmölders, Email: johanna.schmoelders@bms.com.
Henning Witt, Email: henning.witt@pfizer.com.
Holger Gothe, Email: holger.gothe@iges.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- AkdÄ, 2019. Leitfaden “Orale Antikoagulation bei nicht valvulärem Vorhofflimmern” (3., überarbeitete Auflage 2019).
- Andrade J.G., Krahn A.D., Skanes A.C., Purdham D., Ciaccia A., Connors S. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can. J. Cardiol. 2016;32(6):747–753. doi: 10.1016/j.cjca.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Anguita-Sánchez, Manuel; Marco-Vera, Pascual; Alonso-Moreno, Francisco J.; Arribas-Ynsaurriaga, Fernando; Gállego-Culleré, Jaime; Honorato-Pérez, Jesús; Suárez-Fernández, Carmen, 2016. Percepción de los médicos sobre los factores que influyen en la elección de un dicumarínico o de un nuevo anticoagulante oral en pacientes con fibrilación auricular no valvular. In: Atencion primaria 48 (8), S. 527–534. doi: 10.1016/j.aprim.2015.11.004. [DOI] [PMC free article] [PubMed]
- Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B.S., Darius H., Diener H.-C., Joyner C.D., Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- Cromheecke M.E., Levi M., Colly L.P., de Mol B.JM., Prins M.H., Hutten B.A., Mak R., Keyzers K.CJ., Büller H.R. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. The Lancet. 2000;356(9224):97–102. doi: 10.1016/S0140-6736(00)02470-3. [DOI] [PubMed] [Google Scholar]
- Escobar C., Martí-Almor J., Pérez Cabeza A., Martínez-Zapata M.J. Direct oral anticoagulants versus vitamin k antagonists in real-life patients with atrial fibrillation. a systematic review and meta-analysis. Revista Española de Cardiología (English Edition) 2019;72(4):305–316. doi: 10.1016/j.rec.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Giugliano R.P., Ruff C.T., Braunwald E., Murphy S.A., Wiviott S.D., Halperin J.L., Waldo A.L., Ezekowitz M.D., Weitz J.I., Špinar J., Ruzyllo W., Ruda M., Koretsune Y., Betcher J., Shi M., Grip L.T., Patel S.P., Patel I., Hanyok J.J., Mercuri M., Antman E.M. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- Granger C.B., Alexander J.H., McMurray J.J.V., Lopes R.D., Hylek E.M., Hanna M., Al-Khalidi H.R., Ansell J., Atar D., Avezum A., Bahit M.C., Diaz R., Easton J.D., Ezekowitz J.A., Flaker G., Garcia D., Geraldes M., Gersh B.J., Golitsyn S., Goto S., Hermosillo A.G., Hohnloser S.H., Horowitz J., Mohan P., Jansky P., Lewis B.S., Lopez-Sendon J.L., Pais P., Parkhomenko A., Verheugt F.W.A., Zhu J., Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- Häussler, Bertram; Höer, Ariane, 2021. Arzneimittel-Atlas 2020. Der Arzneimittelverbrauch in der GKV: Medizinisch Wissenschaftliche Verlagsgesellschaft.
- Hindricks, Gerhard; Potpara, Tatjana; Dagres, Nikolaos; Arbelo, Elena; Bax, Jeroen J.; Blomström-Lundqvist, Carina et al., 2020. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. In: European heart journal 42 (5), S. 373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed]
- IQWIG, 2021. 1. Drug approval and early benefit assessment in Germany. Online verfügbar unter https://www.iqwig.de/en/presse/in-the-focus/new-drugs-approval-benefit-assessment-coverage/1-drug-approval-and-early-benefit-assessment-in-germany/, zuletzt aktualisiert am 20.07.2021, zuletzt geprüft am 27.07.2021.
- Kip, Miriam Julia; Schönfelder, Tonio; Bleß, Hans-Holger, 2015. Weißbuch Schlaganfallprävention und Vorhofflimmern. Stuttgart: Thieme.
- Ntaios G., Papavasileiou V., Makaritsis K., Vemmos K., Michel P., Lip G.Y.H. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2017;48(9):2494–2503. doi: 10.1161/STROKEAHA.117.017549. [DOI] [PubMed] [Google Scholar]
- Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W., Breithardt G., Halperin J.L., Hankey G.J., Piccini J.P., Becker R.C., Nessel C.C., Paolini J.F., Berkowitz S.D., Fox K.A.A., Califf R.M. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- Ruff C.T., Giugliano R.P., Braunwald E., Hoffman E.B., Deenadayalu N., Ezekowitz M.D., Camm A.J., Weitz J.I., Lewis B.S., Parkhomenko A., Yamashita T., Antman E.M. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. The Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- Rymer J.A., Webb L., McCall D., Hills M.T., Wang T.Y. Differences in preferences between clinicians and patients for the use and dosing of direct oral anticoagulants for atrial fibrillation. J. Am. Heart Assoc. 2021;10(11):e020697. doi: 10.1161/JAHA.120.020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int. J. Clin. Pharm. 2016;38(4):985–995. doi: 10.1007/s11096-016-0329-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.