Abstract
Sometimes, to move forward, it is necessary to look back. Collagen type I is one of the most commonly used biomaterials in tissue engineering and regenerative medicine. There are a variety of collagen scaffolds and biomedical products based on collagen have been made, and the development of new ones is still ongoing. Materials, where collagen is in the fibrillar form, have some advantages: they have superior mechanical properties, higher degradation time and, what is most important, mimic the structure of the native extracellular matrix. There are some standard protocols for the formation of collagen fibrils in vitro, but if we look more carefully at those methods, we can see some controversies. For example, why is the formation of collagen gel commonly carried out at 37 °C, when it was well investigated that the temperature higher than 35 °C results in a formation of not well-ordered fibrils? Biomimetic collagen materials can be obtained both using culture medium or neutralizing solution, but it requires a deep understanding of all of the crucial points. One of this point is collagen extraction method, since not every method retains the ability of collagen to reconstitute native banded fibrils. Collagen polymorphism is also often overlooked in spite of the appearance of different polymorphic forms during fibril formation is possible, especially when collagen blends are utilized. In this review, we will not only pay attention to these issues, but we will overview the most prominent works related to the formation of collagen fibrils in vitro starting from the first approaches and moving to the up-to-date recipes.
Keywords: Collagen type I, Collagen fibrils, Collagen polymorphism, Collagen gel, Compressed collagen
Abbreviations: ECM, extracellular matrix
Graphical abstract
1. Introduction
Fundamental aspects of the formation of collagen fibrils stirred the minds of a great number of scientists throughout the 20th century. Despite the fact that all the answers to the fundamental questions in this field have not been received yet, the great efforts today are directed to the development of collagen materials that allow solving applied problems in tissue engineering and regenerative medicine.
Collagen type I is a crucial component of the extracellular matrix (ECM). In most soft and hard connective tissues, collagen fibrils and their networks comprise the majority of the ECM providing not only physical supportsupport for cells but also defining cellular behaviors and tissue function [1,2]. Cell adhesion, proliferation, migration and gene expression are strongly affected by the structure and properties of ECM. Mechanical properties and nanoscale topography are critical parameters in the regulation of cell response. In collagen lattice, these parameters are defined by the structure of the fibrils, which have rather complex three-dimensional organization. But in a longitudinal direction, their main structural characteristic is the existence of D-periodical banding. This banding is an attribute of a very ordered structure, which settles both surface properties and nanomechanical characteristics.
D-periodical banding results in the regular organization of collagen-binding sites on the fibril surface. There are three main collagen receptor families: collagen-binding integrins, discoidin domain receptors (DDR) and collagen-binding immune receptors [[3], [4], [5], [6], [7]]. Collagen binding integrins are heterodimers which include four different α subunits (α1, α2, α10 and α11) bound to a common β1 subunit: α1β1, α2β1, α10β1, and α11β1. The major functional motif recognized by these integrins is GFOGER, the other motifs with a GxOGER sequence, where x = hydrophobic residue, include GROGER, GLOGER, GMOGER [3,8,9]. The discoidin domain receptors DDR1 and DDR2 are two homologous of tyrosine kinase receptors. Both of them bind the major fibrillar collagen types I-III through sequences GVMGFO [10]. Collagen-binding immune-related receptors such as leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) and platelet glycoprotein VI (GPIV) bind GPO-rich tracts, when osteoclast-associated receptors (OSCAR) binds a defined consensus recognition sequence GxOGPxGFx [11,12]. Thanks to D-periodical banding mentioned collagen-recognition motifs are present on collagen fibril surfaces in a regular manner. Even though there is not enough data establishing the relationship of such regularity with cell functioning, there are some works where the ability of D-periodicity to direct cells behaviour is shown. Poole et al. observed a strong correlation of cell elongation and motion directionality with the orientation of D-periodic collagen microfibrils, whereas neither directed motility nor cell body alignment, was observed on aligned collagen lacking D-periodicity [13].
Nanoscale mechanics of the fibrils is also related to D-periodicity. Experiments on the osteogenesis imperfecta mouse model demonstrated that indentation-type nanomechanical properties of tendon microfibrils correlate with the value of D-periodical banding [14,15].
Thus, to design biomimetic materials for tissue engineering and regenerative medicine aiming to supportsupport physiological cell functioning, remodeling of native ECM with its fine structure and nanotopography including D-periodicity is essential.
A unique property of collagen is the ability to self-assembly in vitro at optimal conditions into native banded fibrils. There are a certain number of protocols that allow creating optimal conditions to initiate self-assembly. They differ in chemical composition and physical parameters, the kinetics of the process is also different. However, there is one notable controversy that can be easily seen from a comparison of protocols suggested before and after “the age of tissue engineering” (here and further this term is used to refer to the period when tissue equivalents began to be actively developed). When formation of collagen fibril in vitro was used to investigate the nature of fibrillization, some protocols were developed to obtain native banded fibrils. In those protocols were a notification that the temperature over 35 °C results in the formation of poorly banded and not well-ordered fibrils [16,17]. Nowadays, the most popular protocols for the formation of fibrillar collagen materials even without cells carried out fibrillization at a temperature of 37 °C. To understand the reasons for this controversy and probably to address it, we will consider the most prominent protocols in more detail.
Here we will discuss some important points for the development of fibrillar collagen materials, including collagen extraction methods since not every method retains the ability of collagen to self-assembly. Another point is collagen polymorphism which we have to keep in mind when we use collagen blends with some other substances. But the main attention in this article we will pay to the analysis of collagen fibril formation methods trying to understand the reasons for utilization of certain compositions and physical parameters. This review aims to give the researchers more understanding and more freedom for the development of new effective biomimetic materials.
2. Collagen structure
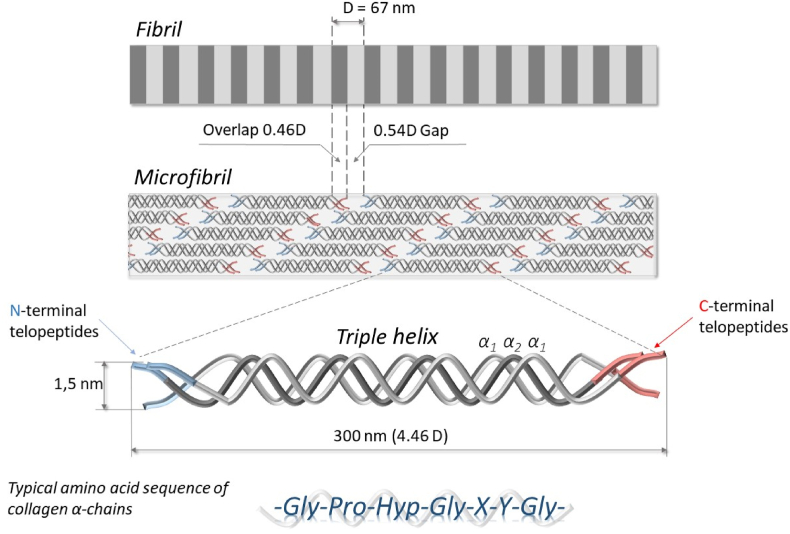
The main common characteristic of all collagens is a unique structural element – the collagen triple helix, which is also termed a triple-helical domain (COL) [4,7]. The triple-helical domain of collagen type I has a semi-rigid rod-like structure of about 300 nm length and 1.5 nm width. It is composed of three left-handed helical polypeptide α-chains comprising around 1014–-1020 amino acids. Two of them are identical - α1-chains and another distinct - α2-chain [7,18]. Each α-chain adopts a specific left-handed helical conformation called polyproline II (PPII), which is a secondary structure class comparable with the α-helix and β-structure [19]. Three collagen α-chains (α1, α1, α2) wrap each other with one-residue stagger forming a right-handed triple helix.
Repeating amino acid motif -(Gly-Xaa-Yaa)- is one of the most essential features of collagen α-chains, where Proline (Pro,P) is the most common residue in the Xaa position, and its derivative 4(R)-hydroxyproline (4Hyp,O) is the most common residue in the Yaa position. The presence of Glycine (Gly,G) in every third position is a crucial point for the formation of collagen triple helix. Triple helix places every third residue on each chain close to the common helical axis and is only stereochemically possible if the smallest amino acid (glycine) occupies this position. Both Pro and 4Hyp residues stabilize the collagen triple helix through stereochemical restrictions imposed by their imino acid rings [7].
Another specific feature of the collagen triple helix is the distribution of hydrogen bonds. Collagen α-chains do not bear direct intramolecular hydrogen bonds between the main-chain amide and the carbonyl groups like it occurs in coiled-coil chains of other polypeptides like keratin [20]. Instead, all the hydrogen bonds are formed only between the different polypeptide chains. There are different types of inter-strand hydrogen bonds. One of them is direct hydrogen bonds between carbonyl and amino groups of neighboring chains (Xaa)C = O···H – N(Gly), another is water-bridged hydrogen bonds (Gly)C =O···(H2O)···H––N(Xaa). First set of hydrogen bonds occurs once per every Gly-Xaa-Yaa triplet; the second set is only possible when Xaa is not an imino acid. N–-H groups play a key role in the formation of a network of interstrand hydrogen bonds that stabilize both helical and fibrillar structures. Another type of hydrogen bonds (Gly/Yaa)Cα – H···O = C(Xaa/Gly), which is weaker than direct bonding with N–-H groups, but which is rather abundant, also make a significant contribution to the stabilization of collagen structure [4,7,21,22].
Intrastrand stabilization of the triple helix is provided by n→π∗ interactions: the oxygen of a peptide bond donates electron density from its lone pairs into the antibonding orbital of the carbonyl in the subsequent peptide bond of the same chain [1,22,23].
The triple-helical domain except for the helical region has two non-helical ends called telopeptides. Telopeptides are composed of around 20 amino acid residues without repeating -(Gly-X-Y)-motif [24]. In native tissues, they actively participate in the fibril formation process providing structural integrity. Outside the cell, lysine and hydroxylysine residues in the N- and C-telopeptides can be converted to aldehydes by lysyl oxidases and then undergo a series of reactions to form covalent intra- and intermolecular cross-links [22,[25], [26], [27], [28], [29], [30]]. Reconstitution of native fibrils in vitro from atelocollagen, collagen lacking the telopeptides, is possible, however, initiation of the fibrillization is delayed and the fibrillar net has a looser structure [22,31]. In some cases, the formation of fibrils with changed morphology from atelocollagen is occurring.
Collagen fibril formation in vivo and in vitro are not equivalent processes. In vivo, this process is much more complex and up to now, it is a field of investigation. There are two main streams of hypotheses about how collagen molecules assemble into fibrils: (i) precipitation from a solution of ‘bulk’ collagen by liquid crystalline ordering of molecules [32] or (ii) ‘nucleation and propagation’ in which a finite number of collagen molecules form a nucleus that then grows in length and diameter to become the mature fibril [33]. They are not mutually exclusive and it is possible that elements of both assembly mechanisms exist in vivo [34,35].
In vivo, сells exert exquisite control over self-assembly of collagen fibrils to generate highly organized collagen supramolecular structures. Fibril formation, also, involves many other molecules such as different types of collagens (fibrillar collagens type V, XI and fibril-associated collagens type XII, XIV) and proteoglycans (e.g. decorin, lumican, osteoglycin, keratocan, fibromodulin, biglycan) [24,[34], [35], [36]]. During biosynthesis collagen acquires a number of specific posttranslational modifications that are critical for its structure and biological functions. These include hydroxylation of specific proline and lysine residues, glycosylation of specific hydroxylysine residues, and finally formation of covalent intra- and intermolecular cross-linking [29,[37], [38], [39], [40]].
In vitro, fibril formation process is less complex. The major structural element making self-assembly possible is the alternation of charged and hydrophobic sidechains on the helix surface, which is determined by amino acid sequences [41]. In vitro, the pathway of fibril formation is not unique and depends on fibrilization conditions [17,36]. Self-assembly into a highly ordered fibril is one of the most essential features of collagen macromolecules, which enable us to obtain native structures in vitro.
3. Collagen extraction methods
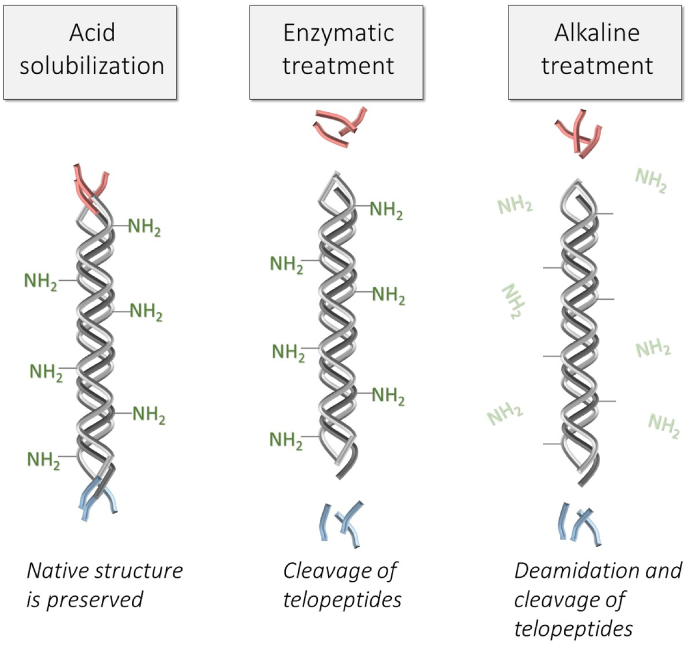
An important detail that we should pay attention to when designing collagen materials is the method of collagen extraction. There are three main methods: acidic, enzymatic and alkaline extraction, which got their name depending on the method of solubilization or the method of pretreatment. All of them result in different structures of obtained collagens (Fig. 1). Collagen can be solubilized both by the action of acid or enzymes, but more often enzymes are used for tissue pretreatment with following acid solubilization. Acid can break collagen into monomers only if tissue has low-level covalent crosslinks, like a rat or mouse tail tendon [42]. The tissue, like skin, which is an attractive source for industrial production of collagen, is extensively cross-linked and the older animals have a higher number of stable crosslinks [42,43]. Acid solubilization of young animal hides yields 4–-6% of collagen (relative to wet weight hide) [44], the yield from the tissue of mature animals is much lower. Thus, to obtain collagen from extensively cross-linked tissues enzymatic or alkaline pretreatment is needed.
Fig. 1.
Schematic representation of the main differences in the molecular structure of collagen extracted by different methods.
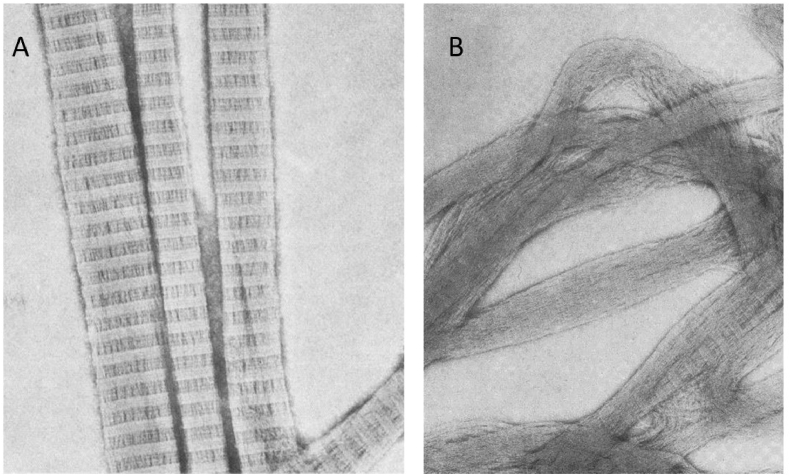
Only acid solubilization allows to obtain collagen with the intact molecular structure: with preserved telopeptides and NH2- side groups of asparagine and glutamine amino acids. Acid collagen solution easily undergoes self-assembly at optimal conditions with the appearance of native banded fibrils. The formation of gel from this type of collagen is possible even at a very low collagen concentration of 1–-2 mg/ml.
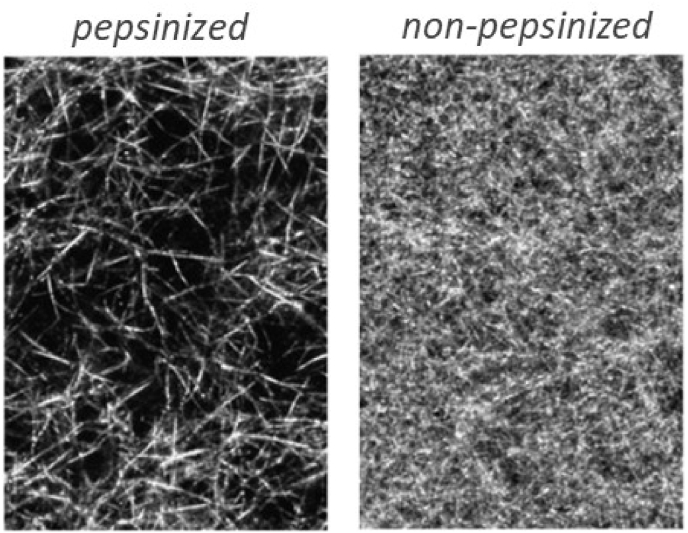
For enzymatic solubilization or pretreatment proteolytic enzymes, such as pepsin, trypsin, chymotrypsin or papain can be used (non-denaturing conditions should be provided), but pepsin is preferred because it is easy to separate from the solubilized collagen [45]. Depend on enzymes the specific sites of telopeptides are more or less cleaved [46,47]. Other parts of the molecule remain intact, thus, as a result of enzymatic treatment, atelocollagen is obtained. Telopeptides play an important role in the fibril formation process [28], so atelocollagen undergoes fibrillization in some different way: the initiation of the process is delayed in comparison with the intact collagen, the fibrillar lattice has a lower density of fibrils and larger diameters of pores (Fig. 2) [31]. Thus, gels from enzyme-treated collagen have weaker mechanical properties in comparison with gels obtained from acid-soluble collagen [46]. However, enzyme-treated collagen may have some advantages, because from atelocollagen it is possible to obtain gels with a high collagen content (e.g. 15–-20 mg/ml) by simply mixing of collagen with neutralizing solution [45].
Fig. 2.
Structure of collagen gels obtained from pepsinized (telopeptide-free) and non-pepsinized (telopeptide-containing) mouse tail collagen. Concentration of collagen in both gels is 1.7 mg/ml. Reproduced from Ref. [31] with a permission of publisher.
It is possible to reconstitute native banded fibrils from enzyme-treated collagen [48], however, some authors reported that obtained fibrils are less well organized [49]. Some polymorphic forms, such as obliquely banded fibrils, fibrous long spacing (FLS) and segment long spacing (SLS) also can be obtained from enzyme-treated collagen [[50], [51], [52]].
Alkaline treatment is widely used in the industrial production of collagen because this method gives a high product yield. However, this type of treatment leads to the deamidation of asparagine and glutamine amino acids and cleavage of telopeptides [53,54] so alkali-treated collagen losses the ability to form native banded fibrils in vitro. Just some kind of fibrous structures can be observed at acidic pH for this type of collagen [54]. Nevertheless, alkali-treated collagen maintains its property as biological adherent molecules: cells adhere to substrata from those collagens via collagen-binding integrins [54].
A comparison of different types of collagen is shown in Table 1 and Fig. 1. Only acid-soluble and enzyme-treated collagens can form native banded fibrils. Alkali-treated collagen losses the ability to self-assembly, so the production of biomimetic collagen materials from this type of collagen is restricted. Gelation of enzyme-treated collagen occurs at much higher concentrations in comparison with acid-soluble collagen solution, however, in some cases it can be considered as an advantage.
Table 1.
Fibril-forming properties of different types of collagen.
| Acid-soluble | Enzyme-treated | Alkali-treated | |
|---|---|---|---|
| Molecular structure | intact | telopeptides cleaved | deamidized asparagine and glutamine amino acids and telopeptides cleaved |
| Gelation | Possible at low concentration of collagen | Possible at high concentration of collagen | at acidic pH with the appearance of non-banded filaments |
| Ability to form native banded fibrils | Yes | Yes, but less well organized | No |
| Adhesion of cells to substrata from this type of collagen | Good | Good | Good |
| Advantages | Ability to form native banded well-ordered collagen fibrils at optimal conditions Ability to form stable collagen gels at very low concentration of collagen |
Ability to form collagen gels with a high concentration of collagen Obliquely banded fibrils, FLS and SLS can be obtained |
Suitable for the production of non-fibrous collagen materials (films, sponge, etc.) |
4. Collagen polymorphism
The phenomenon of aggregation of collagen fibrils with different structures is called collagen polymorphism [55]. More often we deal with the native type of collagen fibrils, and, in this article, we will focus on them either. However, some other polymorphic forms exist both in vitro and in vivo. During fabrication of fibrillar collagen scaffolds, especially when we use different collagen blends or some new specific conditions, we must account for collagen polymorphism. And even if we observe gelling or an increase in the turbidity of the collagen solution, the appearance of the native type of the fibrils has to be proven. Cells are rather sensitive to the topography of the scaffolds, thus the fine structure of the fibrils should be taken into account for correct interpretation of cell behavior.
4.1. Native collagen fibrils
Collagen triple helices in native fibrils are related to each other by a stagger, which results in the observation of the period on electron microscopy images (Fig. 3), (Fig. 4a). This period is referred to as D, which is about 67 nm and includes 234 ± 1 amino acid residues. However, in different tissues, D- periodicity may differ, but more often it is ranging from 60 to 70 nm [14]. The collagen triple helix has a length of about 300 nm referred to as 4.46D. During fibril formation, collagen molecules on the adjacent levels are staggered by D. At the same time, the nearest macromolecules lying on the same level (in a straight line) are displaced axially relative to one another by 5D. Since the length of each molecule is 4.46D, the axial displacement, which is called “gap” or “hole”, is equal 5D – 4.46D = 0.54D. Staggering of the molecules by D on the adjacent levels gives rise to “overlap” region of 0.46D [22,26,56].
Fig. 3.
Simplified representation of collagen fibril hierarchical structure.
Fig. 4.
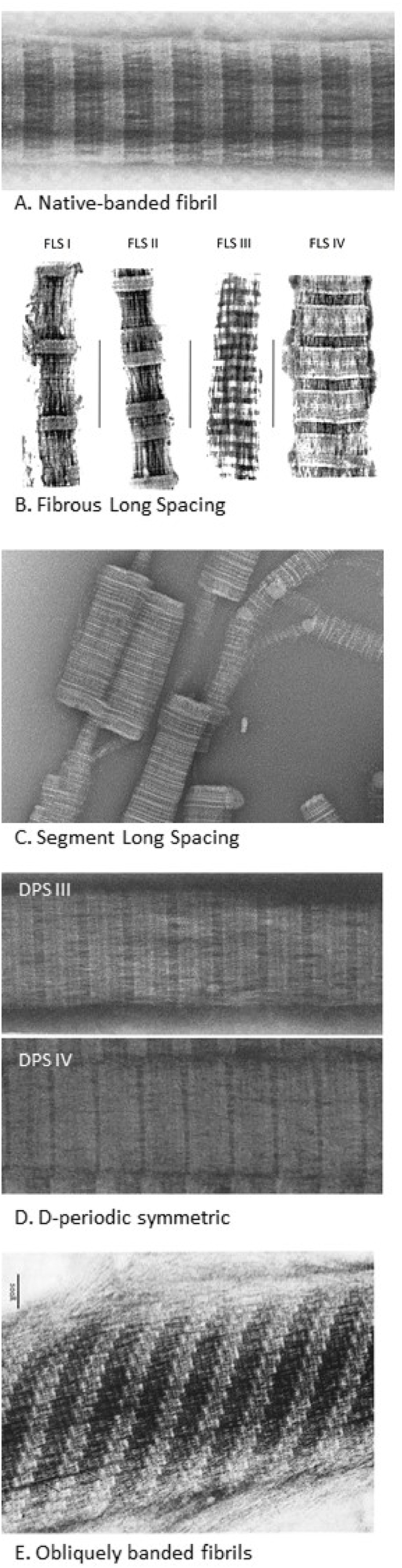
Collagen polymorphism. TEM images of different polymorphic forms of reconstituted collagen fibrils: A) Native type (reprinted from Ref. [16]), B) FLS (reproduced from Ref. [51] with a permission of publisher). C) SLS (reprinted from Ref. [65] with a permission of publisher), D) DPS III and IV (reprinted from Ref. [16]), E) Obliquely banded fibrils (reprinted from Ref. [52] with a permission of publisher).
The stagger of the collagen molecules is related to the sequence of the side groups on the surface of the α-chains. As it was shown by Hulmes and Miller [41], interactions between two neighboring chains are maximal when amino acids of opposite charge and large hydrophobic amino acids of two α-chains staggered by 0D, 1D, 2D, 3D, 4D. This structural feature gives rise to the formation of not only native banded fibrils but different well-ordered polymorphic forms.
Model explaining the longitudinal organization of collagen molecules inside fibril was established by Hodge & Petruska in 1963 [56]. Their two-dimensional model considered the structure of collagen microfibrils as a stack of five triple-helical molecules are offset by D = 67 nm between neighboring strands (Fig. 3). It took much more time to establish a three-dimensional model of the collagen fibril. One of the first models of lateral organization was proposed by Hulmes and Miller in 1979, where they suggested quasi-hexagonal molecular packing in collagen fibrils [57]. This model has required clarification of some important details, which finally was made by Orgel and coworkers in 2001. The molecular packing in this model results in the arrangement of collagen monomers to form supertwisted, right-handed microfibrils that interdigitate with neighboring microfibrils. Also, it was noted that segments containing telopeptides provide a corrugated arrangement of cross-linked molecules that strengthen and stabilize the native fibrils [22,26,27].
To reconstitute collagen native fibrils, it is necessary to provide optimal conditions. Now we will overview different polymorphic forms of collagen fibrils and then return to the very careful description of the methods enabling to obtain native fibrils in vitro.
4.2. Fibrous lLong sSpacing (FLS)
This type of fibril is characterized by large periodicity in the banding pattern observed on the electron images (Fig. 4b), which for FLS fibrils obtained in vitro varies from 100 to 260 nm; in vivo FLS with periodicity 100–-150 nm and 250 nm was found [[58], [59], [60]]. First FLS was observed in citrate extracts of animals’ skin and rat tail tendons, which were dialysis against water [61]. The next experiments showed that dialysis against the water of highly purified collagen does not lead to the formation of FLS, so the proposal of glycoproteins impact was made [62]. Then FLS was obtained by precipitation of collagen in the presence of α1-acid glycoprotein [33,58,59,63], chondroitin sulphate [60] and heparin [50] followed by dialysis of the resulting mixture. It is worth noting, the experiments where FLS was obtained in the presence of chondroitin sulphate and heparin were performed with atelocollagen (collagen with enzymatically removed telopeptides). It is proposed that telopeptides hinder the interaction of glycosaminoglycans with some specific areas in collagen fibrils.
For the formation of FLS in vitro α1-acid glycoprotein is more commonly utilized. In early experiments with α1-acid glycoprotein, it was suggested that glycoproteins are incorporated into FLS fibrils and its content is almost equal to collagen content [63], but up to now the exact role of the a1-acid glycoprotein in fibril formation and morphology of FLS is still under discussion [59].
Four distinct forms of FLS fibers have been observed: FLS I, FLS II, FLS III, FLS IV from transmission electron microscope analysis. All forms have symmetrical banding patterns and thus involve antiparallel relationships between molecules as well as axial stagger between them [64].
4.3. Segment lLong sSpacing (SLS)
SLS is collagen polymorphic forms represented mostly by short crystallites (Fig. 4c). Depending on the morphology of the banding pattern there are symmetric and asymmetric SLS crystallites with a length equal to collagen triple helix (280–-300 nm) exist. The formation of SLS crystallites can be induced in vitro under mildly acidic conditions (pH ∼3.0) by the addition of adenosine triphosphoric acid (ATP) [65] or from mildly alkaline salt extracts of collagen by dialysis against citrate buffer [33]. However, it is not only the way, Harries et al. reported that the polysulphonated diazo dyes Direct red (Sirius red) and Evans blue can also induce the formation of SLS-like aggregates of collagen, but under markedly different ionic conditions to those employed in the presence of ATP [65].
4.4. D-periodic symmetric (DPS)
DPS fibrils are characterized by the same size of the D-periodicity as in native collagen fibrils (67 nm), but with symmetric banding pattern (Fig. 4d). The banding pattern of DPS can be considered as a superposition of two native type patterns arranged anti-parallel with a specific stagger [64]. The same as for FLS there are four distinct DPS forms observed from transmission electron microscope analysis, differing in the fine structure of the pattern: DPS-I, DPS-II, DPS-III, DPS-IV.
DPS appears to be produced either by precipitation at low pH or after enzyme treatment [55]. In one of the first experiments, DPS forms were observed after precipitation of acid-soluble collagen at pH 4.5–-5.5 in the presence of citrate-phosphate buffer or acetate buffer with sufficient NaCl to bring ionic strength 0.23 [66]. Later, DPS-III and DPS-IV were registered while precipitation at pH = =6.5 and pH = =8.5 in the presence of phosphate and NaCl at a similar value of ionic strength [16].
4.5. Obliquely banded fibrils
In one of the first experiments, fibrils with oblique banding patterns were reconstituted from collagen obtained from chick xiphoid cartilage by neutral extraction [67]. These fibrils, which are also called tactoids, had wide bands inclined at an angle of 61⁰±7⁰ to the longitudinal axis of the fibril (Fig. 4e) [51]. The main repeating period D was similar to that in the native banded fibrils equal 67-nm [52]. However, the structure of these tactoids was represented by sub-fibrils staggered to each other with a displacement of 9–-10 nm (D/7). The width of sub-fibrils could vary in a considerable range from 4 to 40 nm, but the stagger displacement stayed constant [55]. The oblique pattern was either right-, left-handed or it occurs frequently in a V-shaped.
Further, similar fibrils were obtained from enzymatically treated rat tail collagen [68], so it was proposed that the enzyme might affect the interaction of tropocollagen molecules via telopeptides resulting in a formation of oblique banding patterns.
Another type of collagen fibrils obtained from cartilage collagen is tactoids with a sub-fibril displacement of 11–-12 nm (D/6). Doyle et al. considered these tactoids as a special case of an oblique striated polymorphic form [55] when Bruns et al. suggested that these forms could arise from DPS-III sub-fibrils displaced by D/3 to each other [52].
4.6. Fibrils with periodicity 22 nm (D/3)
Collagen fibrils with periodicity 22 nm (D/3) have been observed in vivo in embryonic tissue and tissue culture [55,69,70]. In vitro, such kind of fibrils was obtained by precipitation of collagen at a certain concentration of NaCl [70]. In this experiment, the concentration of NaCl in the range between 0.02 and 0.2 M led to the formation of fibrils with an axial period of about 64 nm, when at the concentration of 0.35 M most of the fibrils had a period of about 22 nm and at 0.5 M non-striated fibrils were formed. However, some controversial data about NaCl concentration we can see from data which was obtained by Harris et al. [71]. When collagen was mixed with a solution of NaCl in a phosphate buffer (pH = =7), depending on NaCl concentration initial aggregates (0.01–-0.05 M NaCl), disordered native banded fibrils (0.75 M NaCl) and spindle-shaped fibrils with periodicity 67 nm (0.1–-0.5 M NaCl) were formed [71]. Probably the presence of phosphate buffer, in this case, contributed to the formation of native banded fibrils or the collagen in the experiments of Gross [70] was obtained with the use of enzymes.
Doyle et al. [55] proposed that D/3 is a kind of DPS structures since the distribution of the bulky terminal peptides has an approximate D/3 (22 nm) periodicity, so the formation of D/3 fibrils occurs due to hydrophobic interactions which are important for specifying DPS.
5. Formation of native collagen fibrils in vitro before the “age of tissue engineering”
In 1942, Schmitt et al. [72] on the base of electron microscopic images showed that, reconstituted collagen fibrils have the same banded structure characteristic as native ones. This research initiated intensive studies on the fine structure of collagen and collagen fibrillogenesis, using as a tool the reconstitution of collagen fibrils under various experimental conditions [73]. During that period different procedures were used to form native collagen fibrils, the most common were as follows [64]:
-
1)
dialysis of collagen solution against 0.225 M sodium citrate (pH 3.7), and then against tap water [74];
-
2)
dialysis of collagen solution in 0.5 M acetic acid at 4 °C against 0.02 M sodium phosphate (pH 7.4) with several changes (this procedure is commonly used in the purification of collagen during extraction [75,76];
-
3)
dialysis of collagen solution in 0.5 M acetic acid at 4 °C against 0.15 M NaCI for 24hr, at which time the outer dialysate was neutralized with NaOH [51];
-
4)
exposure of the acetic acid-collagen solution to ammonia vapor [73];
-
5)
neutralization of acid collagen solution (commonly with NaOH) with the following heating.
Dialysis methods are used rarely now for the formation of collagen materials, however, there are some approaches to make heterostructural scaffolds by dialysis of the solution having layers with different collagen concentrations [77].
Exposure of acetic acid-collagen solution to ammonia vapor is an effective method to obtain collagen fibrils. However, it is more often used in special cases when it is needed to modify the material surface with the thin layer of collagen fibrils [78], or when the solutions with a very high concentration of collagen is utilized [79]. Therefore, ammonia vapors are widely used for the formation of fibrillar collagen structures with a liquid crystalline order. The liquid crystalline order occurs spontaneously in highly concentrated acidic collagen solutions, which persists after sol/gel transition triggered by pH increase. Incubation of acidic collagen solution in ammonia vapors induces a raise in pH without diluting the sample. The formation of collagen materials with a liquid crystalline order is not discussed in detail in this article, but they deserve special attention because they mimic collagen connective tissue organisations [[80], [81], [82], [83]].
One of the most convenient methods to obtain collagen materials with native collagen fibrils in vitro is neutralization with the following heating. The reconstitution of native type collagen fibrils by heating was first described by Gross, Highberger and Schmitt [84] and Jackson and Fessler [85]. Soon it began obvious that there are a number of parameters that may influence the fibrillar structure while precipitation of collagen by heating: collagen concentration, amount and kind of salts, pH and temperature [70].
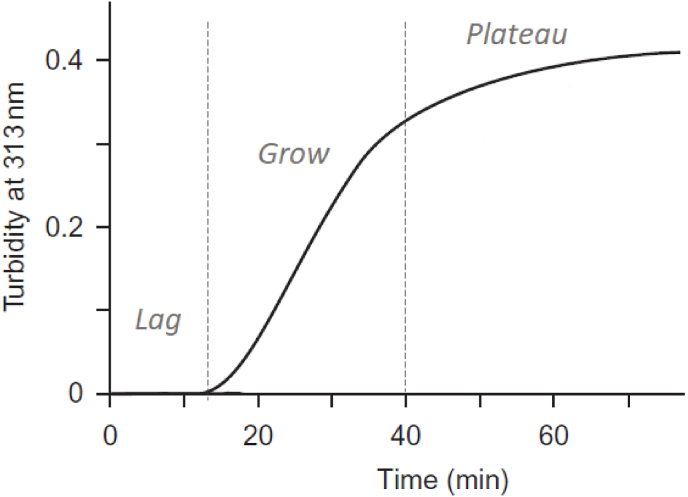
Wood and Keech conducted extensive research on the effect of experimental conditions, such as temperature, pH, ionic strength and concentration of collagen on the rate of fibril formation, its width and morphology [86]. They reported about the formation of native banded fibrils at a variety of conditions, however, the main attention was paid to the rate of fibril formation and fibrils width. The main tool which was used to estimate the rate of fibril formation was the turbidimetric assay. An increase in turbidity follows a sigmoid curve where three distinct regions can be identified (Fig. 5): a lag phase where no detectable change in turbidity; a growth phase where turbidity increases rapidly; and a plateau phase where a non-significant increase in turbidity or its constant value is observed. Gross&Kirk [87] in precipitation of neutral-salt solution and Wood&Keech [86] in precipitation of acid-soluble collagen showed that depend on fibrillization parameters the character of the turbidity curve significantly changes: the length of the lag phase, incline and length of the growth phase is sensitive to pH, temperature, collagen concentration, ionic strength and the presence of some other chemical agents. Wood and Keech used more purified collagen free of mucopolysaccharides and for all experimental conditions, they reported a formation of native fibrils with different widths depending on the experimental conditions. Also, they noted that the temperature accelerates fibril formation both during the lag and growth phase, but the width of the fibrils may change only if the temperature changes in the lag period. So, they proposed that most of the fibrils are formed in the lag period in two steps: the first step consists mainly of the formation of nuclei whereas the second consists of the growth of these nuclei into fibrils [86].
Fig. 5.
A typical turbidity curve showing an increase in optical density during collagen fibrils formation (collagen concentration 0.18 mg/ml, temperature 34 °°C [17,36]).
Another notable work that was focused on the estimation of exact parameters that allow reproducibly form native fibrils from intact soluble collagen was made by Williams, Gelman and Piez [16]. In this experiment, acid-soluble rat tail tendon collagen at different concentrations was mixed with a solvent where the concentration of sodium phosphate, NaCl and buffer was varied. At the same time influence of different pH and fibrillization temperature was investigated by careful analysis of transmission electron microscopic images and turbidimetric parameters. In the detailed images, it was shown that non-optimal conditions may lead to the formation of not only various polymorphic forms but also the formation of native banded but loosely packed and poorly aligned fibrils. Finally, they suggested the following optimal conditions. When collagen solution is mixed with the solvent the final concentration of salts should be: 30 mM sodium phosphate, 30 mM TES (N-[tris(hydroxymethyl)methyl-2-amino]ethanesulfonic acid), and 135 mM NaCl to give an ionic strength of 0.225. The optimal pH of the mixture is in the range of 7.0– to 7.5. The temperature in the range 20–-30 °C provides the formation of native well-banded collagen fibrils. Fibrillization at higher temperatures led to the appearance of non-banded and poorly aligned fibrils. They also noted that phosphate is required to obtain well-ordered fibrils. But phosphate only does not allow to control pH adequately, thus the use of another buffer is necessary.
From turbidimetric analyses, Williams et al. noted that the fibrillization process is strongly temperature-dependent and its rate is proportional to collagen concentration. All the experiments they carried out in a quite diluted solution of rat tail collagen with concentrations in the range from 0.023 to 0.94 mg/ml. From their results, fibril formation from a solution with a concentration higher 0.5 mg/ml led to the formation of native banded fibrils were accompanied by increasing amounts of mixed filamentous aggregates. In many cases to solve problems of regenerative medicine collagen scaffolds have to be produced from collagen solutions with significantly higher concentrations. Based on the abovementioned optimal conditions, but with the concentration of collagen in final solution 2–-2.5 mg/ml and the use of HEPES buffer instead TES, Torbet et al. developed scaffolds with magnetically aligned collagen lamellae, which was successfully applied for corneal stroma reconstitution [88,89].
In the study of Williams et al. [16], collagen fibril formation was initiated by mixing cold collagen solution with the cold solvent with the following heating of the blend. Their further experiments [28] were set at the same conditions. From a careful analysis of the turbidity change and electron microscopic images of newly fibrils they proposed a multistep collagen fibrillization process. It was suggested that fibril formation consists of three stages: 1) initiation – a temperature-dependent process occurring when neutralized collagen converted to intermediates; 2) linear growth – a temperature-independent process occurring when the initial intermediate spontaneously growth in longitudinal directions resulting in the appearance of non-banded filaments with a diameter 2–-4 nm; 3) lateral growth – a temperature-dependent process which is related with the rapid growth of the turbidity during which lateral filaments associations is occurring. The first two stages are occurring in the lag phase on the sigmoid turbidity curve, the third stage is in the linear growth phase.
Another evidence of a multistep theory was obtained from laser light scattering by Silver et al. [90,91]. The experiments were carried out on acid-soluble rat tail tendon collagen which was neutralized at 4 °C by adding to it an equal volume of potassium phosphate (K2HPO4/KH2P04) buffer, pH 7.6, ionic strength 0.4. Final collagen concentration was below 0.4 mg/ml. Two steps of collagen fibril formation were proposed: step I is characterized by the appearance of 4D-staggered linear dimers and trimers which are from theoretical estimation 570 nm and 845 nm long respectively; step II is characterized by the appearance of aggregates greater than 930 nm in length.
Later, important research was made by Holmes et al. [17] where they showed that assembly of collagen molecules into fibrils does not follow a unique pathway. Previous researches [25,92] made in this group proved different growth behavior which is characterized by the early appearance of a large number of native banded short fibrils, so-called ‘early fibrils’, which simultaneously increase in length and diameter during growth. Since these authors used another method to establish the conditions for fibril formation, it began obvious that not only temperature, pH, amount and kind of salts, but the order of the initial adjustment is also important for growth behavior.
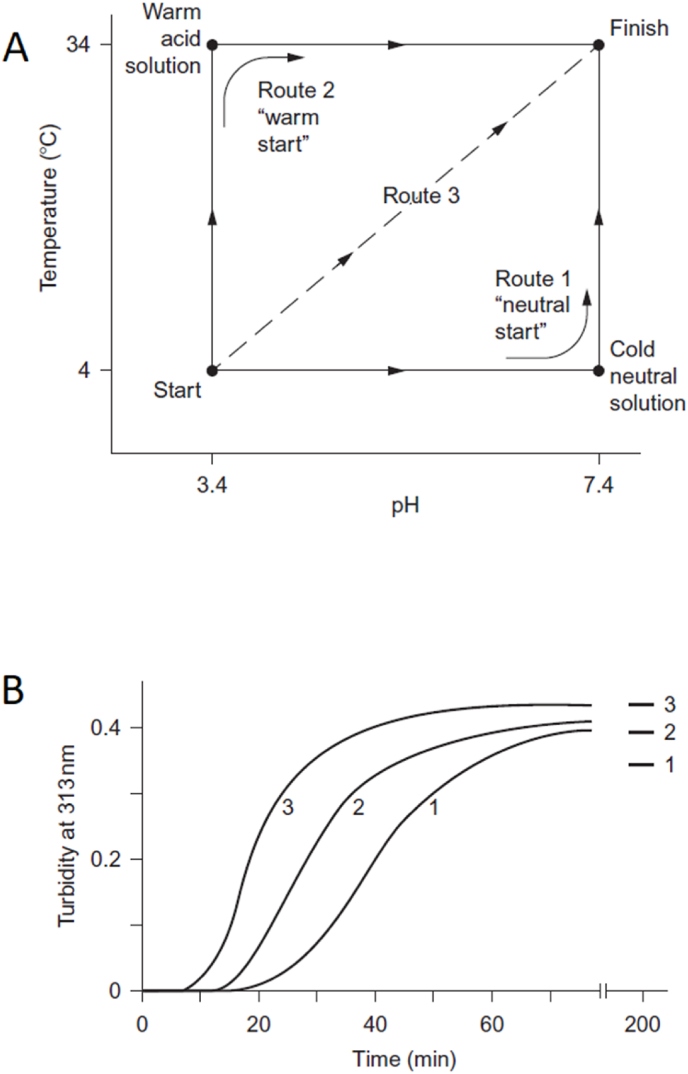
To establish the impact of the initial adjustment the same collagen solution was precipitated in different ways [17]. For the experiments following conditions for fibril formation were used: ionic strength I = =0.2 Na2HPO4/KH2PO4 (62 mM Na2HPO4, 14.6 mM KH2PO4), pH 7.4, T = =34 °C and 0.18 mg/ml collagen concentration in final solution.
Three different transfer routes were used to bring acid collagen solution to the above conditions (Fig. 6). Route 1 – Neutral start – neutralization-before-warming. Cold acid collagen solution was first mixed with cold buffer solution and then heated. This route was used by Williams and Gelman [16,28] and Silver et al. [90,91] in their researches. Route 2 – Warm start – warming-before-neutralization. Both acid collagen solution and the buffer were prewarmed to 34 °C and then mixed (prewarming at 35 °C and higher led to the formation of less well-ordered final fibrils). This route was used by Chapman and coworkers [25,92]. Route 3 – Simultaneous neutralization and warming. The cold acid collagen solution was mixed with prewarmed up to 37 °C buffer, the final temperature after mixing was 34 °C.
Fig. 6.
Alternative assembly routes observed in the reconstitution of type I collagen fibrils [17,36]. (A) Diagram to show three initiation routes to transfer the collagen from a cold acid solution to a warm neutral solution. Routes 1 and 2 involve a warming step and a neutralization step. Route 3 is a combined single step achieved by adding a warm buffer solution to the initial collagen solution. (B) Turbidimetric curves to show a comparison in the kinetics of fibril formation between the three routes. Both figures reprinted from Ref. [36] with a permission of publisher.
Depending on the initiation method different turbidity curves were observed. “"Warm start”" led to more rapid fibril formation than in the case of “neutral start”, but slower than in the case of “simultaneous neutralization and warming”.
“Neutral start” initiation resulted in a formation of thin non-banded filaments with diameter 2–-10 nm which occurred in increasing amount (but with no increase in diameter) during the early part of the turbidity growth phase. In the second part of the growth phase amount of these filaments decreased since they grew in width and transformed into the native banded fibrils. These results were in agreement with Williams et. al. and Gelman et. al. studies [16,28]. “Warm start” was characterized by a much earlier appearance of banded fibrils. From the beginning of the lag phase, native banded early fibrils with the shortest length of about 1 μm (∼∼15 D-periods) appeared. From previous studies, it was observed that the diameter of the early fibrils is slightly greater than 10 nm and the maximum cross-section comprises 40–-50 molecules [92]. Further fibril formation was occurring by the growth of early fibrils in both lateral and axial directions. “Simultaneous neutralization and warming” resulted in similar to “warm start” fibril formation behavior but with a higher rate of this process. In all these three routes, the final product was native banded fibrils. However, Holmes et al. supposed that the warm start and simultaneous neutralization and warming routes involve more ordered association and a more balanced combination of linear and lateral growth.
6. Fibrillar collagen scaffolds in the “age of tissue engineering”
One of the first experiments where collagen gel was used as a carrier for cells was made by Ehrmann and Gey [73]. They investigated the behavior of 29 cell and tissue cultures on collagen gel which was obtained by dialysis of collagen solution with its following equilibration with culture medium. It was shown that collagen reconstituted as a clear gel promotes a cellular outgrowth usually free from retraction.
In 1972, Elsdale and Bard [93] represented a simple technique for the preparation of collagen substrata similar to soft-tissue matrices. They suggested precipitating the collagen solution with a fluid having the same composition as the tissue culture medium employed. The idea was to obtain native collagen fibrils by rising the ionic strength and pH to physiological levels by the mixture of Eagle's medium and 10% fetal calf serum. First, they obtained collagen solution in ten times diluted Eagle's medium with pH = =4. Then, collagen solution was placed on ice and an appropriate amount of serum and 10X Eagle's medium, the predetermined amount of 0.142 M NaOH to bring pH = =7.6 was added and mixed. The concentration of collagen in the final solution was 0.1% by weight. Then the mixture was dispersed into containers and left undisturbed for several minutes for setting. It is worth noting, that the setting of the gel occurred at room temperature. Finally, they obtained a collagen gel which they called hydrated collagen lattices (HCL). Investigation of HCL by transmission electron microscopy revealed that the structure of HCL is composed of native banded collagen fibrils. At the beginning of the precipitation, very fine fibrils appeared which then aggregated into bundles of up to 500 nm diameter with 64 nm periodicity.
The authors also suggested a simple technique to obtain an aligned fibrillar structure: the mix of collagen and medium was spread over the surface of a dish and left to set on the inclined surface, the excess of the mixture was drained and collected at the lowest point. Thus, they obtained a collagen lattice with bundles roughly aligned along the axis of draining.
Eugene Bell with coworkers was the first who described a formation of a tissue-like structure as a result of the contraction of collagen lattice by human fibroblasts [94]. Collagen lattice with incorporated fibroblasts was prepared as follows: 1.0 ml of concentrated McCoy's 5a medium, 1.0 ml of fetal calf serum and 0.25 ml of 0.1 M NaOH were mixed, then 1.5 ml of acid collagen solution with concentration of collagen 0.57 mg/ml and 1.0 ml of fibroblasts suspended in McCoy's medium were simultaneously added. The gel has started setting immediately after the collagen solution was added. Then it was placed in an incubator at 37 °C in a 95% air/5% CO2 atmosphere and after 10 min gel was completely set. When the gel with the cells has been just prepared it was almost transparent, but it has been getting opaque with time. That was the contraction phenomenon. Under the action of cells, collagen lattice contracted and the fluid was squeezed out, hence the reduction of the diameter of the lattice and increase of its density occurred. The tissue-like fabric which was finally obtained was similar to skin or dermis in color and texture. Authors predicted the useful application of this invention for wound healing and burns.
This study marked the beginning of a new stream in biomedical sciences and Eugene Bell is deservedly considered as a pioneer of tissue engineering. Within several next years, Bell with coworkers suggested a variety of products for regeneration of different tissues: skin-equivalent [[95], [96], [97]], a blood vessel model [98,99], bone-equivalent [100]. In the following, a huge number of research were made to develop different biomedical products for regenerative medicine which were based on collagen gel with cells.
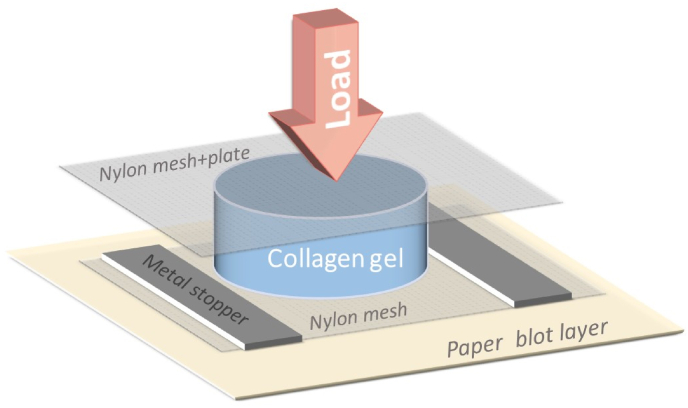
It took 27 years to make a significant next step in the engineering of tissue-like structures by using collagen self-assembly property. In 2005, Brown et al. [101] suggested ultrarapid engineering of biomimetic materials by plastic compression of collagen gel. Since collagen gel is hydrated collagen lattice, where fluid content is more than 99% and the main amount of which is held by capillary force, so, the fluid can be easily removed by application of the compressive load. The amount of the residual fluid depends on the level of the compression, thus the material with the desired density can be easily obtained. The authors suggested a very simple technique for the formation of dense collagen material. Collagen gel was placed onto the mesh and then loaded, so the fluid could freely flow out (Fig. 7). For example, the mean compression of collagen gel under 50 gg load during 5 min was about 88%, the thickness of the gel reduced from 3.6 mm to 23–-48 μm. The obtained material had a tissue-like structure similar to that which was obtained by Eugen Bell but without cell participation.
Fig. 7.
Simplified scheme representing the system for collagen gel compression.
To prepare collagen gel for following compression Brown et al. utilized a protocol with the use of a culture medium. The gel composed of rat tail tendon collagen in acetic acid with concentration of 2.16 mg/ml, 10x Eagle minimum essential medium (EMEM), Earle'’s balanced salt solution and NaOH (recipe of gel with incorporated cells contained Dulbecco'’s modified Eagle'’s medium (DMEM) with fetal calf serum). Then the gel was cast into a mould and set/stabilized at 37 °C in a CO2 incubator. The authors showed that cells can be introduced into the gel before compression and the viability of the cells after compression is not less than 82%.
6.1. The singularity of collagen fibrils deformation while compression
Analysis of the structure of compressed collagen revealed the appearance of smaller-scale structures within the compressed collagen sheet (Fig. 8) [101]. The sheet consists of many layers of compacted lamellae of collagen fibrils arranged parallel to the fluid-leaving surface with the highest density near this surface. In the following studies [102], it was shown that the fluid-leaving surface behaves as an ultrafiltration membrane, allowing fluid out but retaining collagen fibrils. It was hypothesized that the accumulation of collagen at the fluid-leaving surface produces anisotropic structuring resulting in an increase of hydraulic resistance of the surface. So, the compression can be divided into two phases: the primary load-dependent phase which is the determinant of flux at the beginning of compression, and the second phase where increasing hydraulic resistance of the surface become the key determinant of flux as the process proceeds.
Fig. 8.
Detailed SEM appearance of the edge of a single compressed collagen sheet showing the multiple layers of lamellae of collagen fibril networks (arrowed), typically 1–-5 μm. Reprinted from Ref. [101] with a permission of publisher.
In other experiments [103], structural anisotropy along the cross-section of collagen gel during compression was also shown by real-time birefringence mapping. It was revealed that the layers next to the fluid leaving surface had gotten more and more dense during compression. The authors denoted the region near the “"fluid leaving surface”" as a collapsed region because the structural changes in this region were irreversible. The deformation in the bulk of the gel was reversible. Based on their experiments supportingsupporting also with the previous observation from SEM [104], they proposed the following structural mechanism during compression of the gel:
-
1)
the fibrils in the collagen gel are well interconnected and during compression, they do not slip at these interconnections.
-
2)
the accommodation of macroscopic strain occurs due to the bending of fibrils at the interconnections. Between interconnections, fibrils do not bend.
-
3)
the bending of fibrils at the interconnections is reversible until collapse.
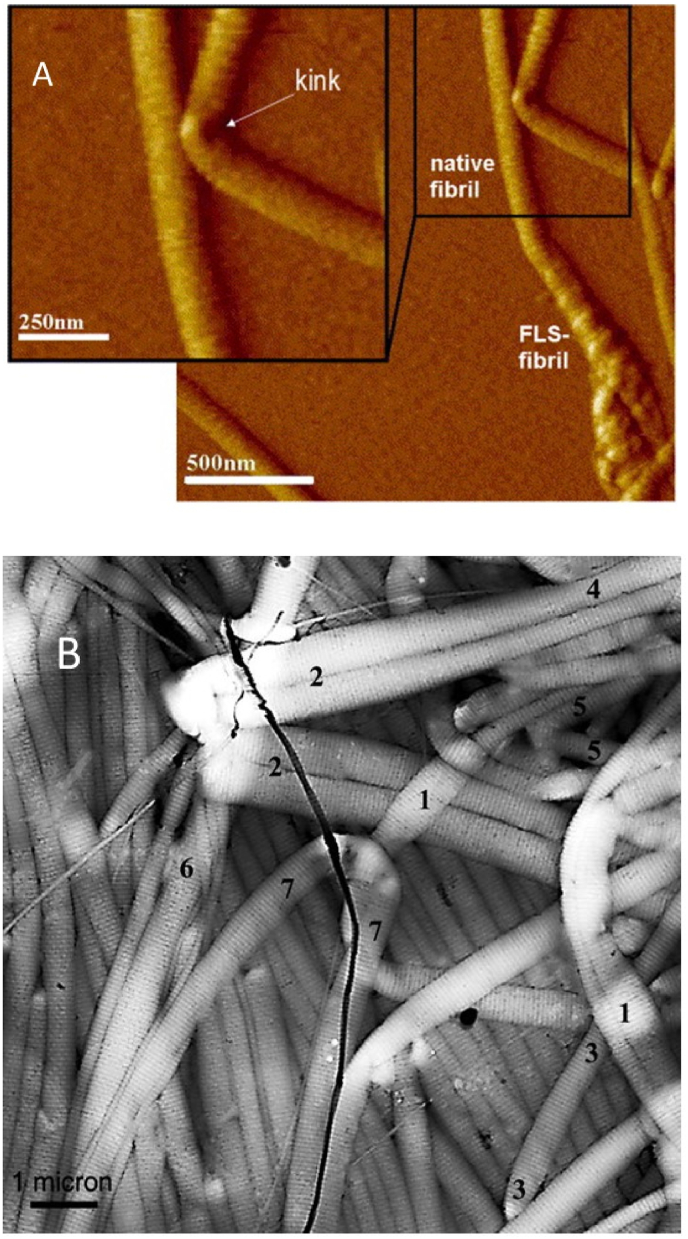
Another interesting detail in the deformation of collagen fibrils is that fibrils bend with the appearance of a kink (Fig. 9) that makes their mechanical behaviour similar to behaviour of a tube [105,106]. Gutsmann et al. suggested that collagen fibrils have a structure with different mechanical properties of the shell and the core, due to the density of the crosslinking: collagen molecules are more highly crosslinked near the fibril surface and more disordered or soft in the central region [105].
Fig. 9.
A) High-resolution AFM image of reconstituted collagen fibril showing the appearance of a kink in the bend. Reprinted from Ref. [106] with a permission of publisher. B) AFM image of an epoxy mold of compressed collagen fibrils. This replica illustrates the tubelike mechanical behavior of collagen fibrils. Reprinted from Ref. [105] with a permission of publisher.
The ability of collagen gel to be compressed without damage of fibrillar structure, the existence of “fluid leaving surface” behaving as an ultrafiltration membrane enable to design biomedical materials for wound healing which both have superior mechanical properties and keep wet conditions in the wound bed. Another advantage of compressed collagen is the simplicity of the production and ease of scalability. Based on compressed collagen, a variety of tissue-engineering constructs are already suggested: for regeneration of skin [[107], [108], [109]], bone [[110], [111], [112]], cornea [[113], [114], [115]], bladder [116,117], blood vessels [118].
7. The key points in the formation of collagen fibrils in vitro
Let's compare the protocols from those experiments where the appearance of native fibrils was investigated most carefully and the protocols which are now commonly used for the formation of fibrillar collagen scaffolds. They are the protocols described by Williams et al. [16], Holmes et al. [17], Elsdale and Bard [93], Eugen Bell [94] and Brown et al. [101] (Table 2). They can be divided into two groups: one of them utilize neutralizing buffers for collagen fibril formation and the others utilize the culture medium.
Table 2.
Comparison of protocols for collagen fibril formation in vitro.
| Neutralizing salt solutions |
Culture medium |
||||
|---|---|---|---|---|---|
| Composition/ Reference | Williams et al. [16] | Holmes et al. [17] | Elsdale and Bard [93] | Bell et al. [94] | Brown et al. [101] |
| Concentrations of salts in the final solution | •30 mM Na2HPO4 •30 mM TES/HEPES∗ •135 mM NaCl •NaOH •collagen solution in acetic acid |
•62 mM Na2HPO4 •14.6 mM KH2PO4 •NaOH •collagen solution in acetic acid |
•10x Eagle's medium with 10% fetal calf serum •NaOH •collagen solution in 0.1X Eagle's medium |
•1.0 ml concentrated McCoy's 5a medium •1.0 ml serum •0.25 ml 0.1 M NaOH •1.5 ml collagen solution in acetic acid •1.0 ml fibroblasts suspended in McCoy's |
•0.3 ml 10x Eagle minimum essential medium (EMEM) •0.3 ml Earle'’s balanced salt solution •5 M NaOH •2.4 ml Collagen solution in acetic acid |
| Ionic strength | I = =0.225 | I = =0.2 | –- | –- | –- |
| pH | 7.0–-7.4 | 7.4 | 7.6 | Neutral (exact value not specified) | Neutral (exact value not specified) |
| Temperature | 26-30 °°C | 34 °°C | Room temperature | 37 °°C | 37 °°C |
| Gelation rate | Relatively slow gelation | Relatively slow gelation | Rapid gelation | Rapid gelation | Rapid gelation |
7.1. Protocols utilizing neutralizing salt solutions
Protocols of Williams et al. and Holmes et al. are based on compositions containing salts and NaOH for initiation of collagen fibril formation. The ionic strength and pH value are similar in both methods. The main difference is in the composition of the salts in neutralizing solution. In “Holmes protocol” the solution includes only phosphate salts whereas in “Williams protocol” it contains NaCl and TES. In the article of Williams et al., they regulated the ionic strength by adding NaCl and showed its importance for the formation of well-ordered fibrils. The same suggestion was made by some other authors [71]. However, Holmes et al. adjusted the same value of ionic strength by only phosphate salts and showed the formation of well-ordered native fibrils.
About the presence of special buffer for stabilization of pH value. Williams et al. reported that during fibril formation phosphate produced the best native banded fibrils, but pH control was inadequate. High concentrations (more than 40 mM in their experiments) of phosphate inhibited the rate of fibril formation, so they used additional TES buffer, which gave good pH control and did not have any obvious effect on the structure of collagen fibrils. From our experience, when we tried to use phosphate buffered saline (PBS) to form collagen gel, we noticed that after mixing acid collagen solution with PBS and proper amount of NaOH the required pH value 7.4 was stable just until fibrillization starts. During the fibrillization process pH uncontrollably was raising up. The addition of TES or HEPES buffer to the neutralizing solution prevented this undesirable effect, and the pH value near 7.4 was stable until collagen gel was completely formed. In some experiments [88,89], where the solution from “Williams protocol” was used for the formation of tissue engineering constructs HEPES buffer was utilized instead of TES. Both TES and HEPES buffers in concentrations up to 25 mM do not have toxic action on cells [119]. HEPES is commonly used in cell culture medium now. So, for the formation of fibrillar collagen scaffolds for tissue engineering both TES and HEPES buffers can be safely utilized at mentioned concentrations. Moreover, if it is necessary, any buffer can be removed from fibrillar collagen material by simple washing or immersion of the material in water.
Another important point that requires special attention is the temperature of the fibrillization. In both protocols, it is emphasized that fibrillization temperature should not be over 35 °C, because higher temperature leads to the formation of poorly banded and not well-ordered fibrils (Fig. 10). At the same time, very low temperature results in the formation of a high amount of filamentous aggregates [16]. Williams et al. recommended temperature diapason from 20 °C to 30 °C, Holmes et al. noted that the temperature should not be more than 35 °C.
Fig. 10.
TEM images of reconstituted collagen fibrils obtained in the experiments of Willians et. al. [16]: A) reconstituted native type collagen fibrils obtained at optimal conditions; B) poorly banded and loosely packed fibrils obtained at high temperature (37 °°C).
7.2. Protocols utilizing culture medium
All protocols utilizing the culture medium for collagen fibril formation are based on the idea that physiological conditions that occurred in the presence of culture medium initiate self-assembly process. So, collagen should be mixed with the culture medium in appropriate ratio to bring physiological ionic strength in the final solution. If concentrated 10x culture medium is utilized, the volume ratio of collagen to culture medium should be 9:1 (collagen:medium). There can be some small displacement of this proportion, but the idea should be the same. The value of pH should be adjusted around 7.0–-7.4 by adding NaOH. In some protocols for better stabilization of pH value special buffers, like HEPES, can be additionally added.
What about the temperature in these protocols? When Elsdale and Bard first introduced their protocols, they obtained collagen gels with well-ordered fibrils at room temperature. Eugen Bell for the formation of the tissue-like structure obtained collagen gel with incorporated cells in the conditions more preferred for cells than for the formation of well-ordered fibrils. In the protocol of Bell fibrillization occurred at 37 °C. The same thermal conditions were used by Brown for the preparation of collagen gels for the following compression and it is used now by other researchers to obtain compressed collagen. We noticed above that the temperature over 35 °C results in a formation of not well-ordered fibrillar structure. To eliminate this problem for acellular compressed collagen materials, fibrillization temperature can be easily reduced. For cellular materials, additional investigations should be made.
There is one more point which we have to pay attention to. The kinetics of the fibrillization process in the presence of a culture medium is different from the kinetics when neutralizing solution is utilized. After mixing collagen with culture medium gelation occurs rapidly, that is why it is recommended to perform all operations on ice. Depending on tasks rapid gelation can be both an advantage and disadvantage. If the fast gelling is causing an excessively negative effect, the protocol based on a neutralizing solution is preferred.
8. Conclusions
The development of biomimetic materials for tissue engineering and regenerative medicine gives opportunities to force physiological regeneration. From this point of view, compressed collagen and other materials where collagen is represented in a form of native fibrils look very attractive.
Compressed collagen gels can be obtained both using culture medium or neutralizing solution allowing native-like collagen fibrils and tissue-like structures. The use of neutralizing solution is more cost-effective, do not require a coolant and present delayed gelation. Delayed gelation can be an advantage for injectable forms of gels. Methods utilizing culture medium are more suitable for the materials with introduced cells, where the presence of cells nutrition elements is more important than the orderliness of the structure. To improve structural characteristic of such gels, a reduction of the fibrillization temperature in many cases may be acceptable.
Each task from medicine requires a special approach. In the case of collagen materials, the choice of fibril formation protocol may depend on the purpose, way of application, storage conditions, ease of handling, cost, etc. A better understanding of all the reasons to apply certain chemical compositions and physical parameters in the production of biomimetic materials can give more freedom to find the best solution for a unique task.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supportedsupported by the Ministry of Science and Higher Education of the Russian Federation (FMFU-2021-0008, State contracts no. 075-15-2021-1063).)
References
- 1.Chattopadhyay S., Raines R.T. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–833. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aszódi A., Legate K.R., Nakchbandi I., Fässler R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 3.Farndale R.W., Lisman T., Bihan D., Hamaia S., Smerling C.S., Pugh N., Konitsiotis A., Leitinger B., De Groot P.G., Jarvis G.E., Raynal N. Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochem. Soc. Trans. 2008;36:241–250. doi: 10.1042/BST0360241. [DOI] [PubMed] [Google Scholar]
- 4.Bella J. Collagen structure: new tricks from a very old dog. Biochem. J. 2016;473:1001–1025. doi: 10.1042/BJ20151169. [DOI] [PubMed] [Google Scholar]
- 5.Chow W.Y., Forman C.J., Bihan D., Puszkarska A.M., Rajan R., Reid D.G., Slatter D.A., Colwell L.J., Wales D.J., Farndale R.W., Duer M.J. Proline provides site-specific flexibility for in vivo collagen. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-31937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., Barnes M.J. The collagen-binding a-domains of integrins α1/β1 and α2/β1 recognize the same specific amino acid sequence, GFOGER, in native (triple- helical) collagens. J. Biol. Chem. 2000;275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Bella J., Hulmes D.J.S. In: Fibrous Proteins Struct. Mech. Subcell. Biochem. Parry D.A.D., Squire J.M., editors. Springer International Publishing; 2017. Fibrillar collagens; pp. 457–490. [DOI] [Google Scholar]
- 8.Bourgot I., Primac I., Louis T., Noël A., Maquoi E. Reciprocal interplay between fibrillar collagens and collagen-binding integrins: implications in cancer progression and metastasis. Front. Oncol. 2020;10:1–28. doi: 10.3389/fonc.2020.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaia Samir, Farndale Richard W. In: Adv. Exp. Med. Biol. Gullberg D., editor. Springer; 2014. Integrin recognition motifs in the human collagens; pp. 127–142. https://doi.org/doi: 10.1007/978-94-017-9153-3_9. [DOI] [PubMed] [Google Scholar]
- 10.Xu H., Raynal N., Stathopoulos S., Myllyharju J., Farndale R.W., Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L., Hinerman J.M., Blaszczyk M., Miller J.L.C., Conrady D.G., Barrow A.D., Chirgadze D.Y., Bihan D., Farndale R.W., Herr A.B. Structural basis for collagen recognition by the immune receptor OSCAR. Blood. 2016;127:529–537. doi: 10.1182/blood-2015-08-667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrow A.D., Raynal N., Andersen T.L., Slatter D.A., Bihan D., Pugh N., Cella M., Kim T., Rho J., Negishi-Koga T., Delaisse J.M., Takayanagi H., Lorenzo J., Colonna M., Farndale R.W., Choi Y., Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 2011;121:3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole K., Khairy K., Friedrichs J., Franz C., Cisneros D.A., Howard J., Mueller D. Molecular-scale topographic cues induce the orientation and directional movement of fibroblasts on two-dimensional collagen surfaces. J. Mol. Biol. 2005;349:380–386. doi: 10.1016/j.jmb.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 14.Fang M., Holl M.M.B. Variation in type I collagen fibril nanomorphology: the significance and origin. BoneKEy Rep. 2013;2:1–7. doi: 10.1038/bonekey.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp A.D., Harding C.C., Cabral W.A., Marini J.C., Wallace J.M. Effects of tissue hydration on nanoscale structural morphology and mechanics of individual Type I collagen fibrils in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2012;180:428–438. doi: 10.1016/j.jsb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams R., Gelman A., Poppke C., Piez K.A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J. Biol. Chem. 1978;253:6578–6585. [PubMed] [Google Scholar]
- 17.Holmes D.F., Capaldi M.J., Chapman J.A. Reconstitution of collagen fibrils in vitro; the assembly process depends on the initiating procedure. Int. J. Biol. Macromol. 1986;8:161–166. doi: 10.1016/0141-8130(86)90020-6. [DOI] [Google Scholar]
- 18.Miller E.J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol. Cell. Biochem. 1976;13:165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- 19.Adzhubei A.A., Sternberg M.J.E., Makarov A.A. Polyproline-II helix in proteins: structure and function. J. Mol. Biol. 2013;425:2100–2132. doi: 10.1016/j.jmb.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Tsobkallo K., Aksakal B., Darvish D. Analysis of the contribution of the microfibrils and matrix to the deformation processes in wool fibers katherina. J. Appl. Polym. Sci. 2012;125:168–179. doi: 10.1002/app.36535. [DOI] [Google Scholar]
- 21.Bella J., Berman H.M. Crystallographic evidence for C(α)-H...O = C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996;264:734–742. doi: 10.1006/jmbi.1996.0673. [DOI] [PubMed] [Google Scholar]
- 22.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeRider M.L., Wilkens S.J., Waddell M.J., Bretscher L.E., Weinhold F., Raines R.T., Markley J.L. Collagen stability: insights from NMR spectroscopic and hybrid density functional computational investigations of the effect of electronegative substituents on prolyl ring conformations. J. Am. Chem. Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- 24.Hulmes D.J.S. In: Collagen Struct. Mech. Fratzl P., editor. Springer Science+Business Media; 2008. Collagen diversity, synthesis and assembly; pp. 15–47. [Google Scholar]
- 25.Capaldi M.J., Chapman J.A. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro. Biopolymers. 1982;21:2291–2313. doi: 10.1002/bip.360211115. [DOI] [PubMed] [Google Scholar]
- 26.Orgel J.P.R.O., Miller A., Irving T.C., Fischetti R.F., Hammersley A.P., Wess T.J. The in situ supermolecular structure of type I collagen. Structure. 2001;9:1061–1069. doi: 10.1016/0141-8130(87)90067-5. [DOI] [PubMed] [Google Scholar]
- 27.Orgel J.P.R.O., Irving T.C., Miller A., Wess T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelman R.A., Williams B.R., Piez K.A. Collagen fibril formation. Evidence for a multistep process. J. Biol. Chem. 1979;254:180–186. [PubMed] [Google Scholar]
- 29.Yamauchi M., Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–133. doi: 10.1042/BSE0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gjaltema R.A.F., Bank R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2017;52:74–95. doi: 10.1080/10409238.2016.1269716. [DOI] [PubMed] [Google Scholar]
- 31.Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., Deryugina E., Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin R., Farjanel J., Eichenberger D., Colige A., Kessler E., Hulmes D.J.S., Giraud-Guille M.M. Liquid crystalline ordering of procollagen as a determinant of three-dimensional extracellular matrix architecture. J. Mol. Biol. 2000;301:11–17. doi: 10.1006/jmbi.2000.3855. [DOI] [PubMed] [Google Scholar]
- 33.Gross J., Highberger J.H., Schmitt F.O. Collagen structures considered as states of aggregation of a kinetic unit. The tropocollagen particle. Proc. Natl. Acad. Sci. U.S.A. 1954;40:679–688. doi: 10.1073/pnas.40.8.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadler K.E., Holmes D.F., Trotter J.A., Chapman J.A. Collagen fibril formation. Biochem. J. 1996;11:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadler K.E. Fell Muir Lecture: collagen fibril formation in vitro and in vivo. Int. J. Exp. Pathol. 2017;98:4–16. doi: 10.1111/iep.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes D.F., Lu Y., Starborg T., Kadler K.E. first ed. Elsevier; 2018. Collagen Fibril Assembly and Function. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi M., Sricholpech M., Terajima M., Tomer K.B., Perdivara I. Glycosylation of type I collagen. Methods Mol. Biol. 2019;1934:127–144. doi: 10.1007/978-1-4939-9055-9_9. [DOI] [PubMed] [Google Scholar]
- 38.Takaluoma K., Hyry M., Lantto J., Sormunen R., Bank R.A., Kivirikko K.I., Myllyharju J., Soininen R. Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knock-out mice. J. Biol. Chem. 2007;282:6588–6596. doi: 10.1074/jbc.M608830200. [DOI] [PubMed] [Google Scholar]
- 39.Sricholpech M., Perdivara I., Yokoyama M., Nagaoka H., Terajima M., Tomer K.B., Yamauchi M. Lysyl hydroxylase 3-mediated glucosylation in type I collagen: molecular loci and biological significance. J. Biol. Chem. 2012;287:22998–23009. doi: 10.1074/jbc.M112.343954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torre-Blanco A., Adachi E., Romanic A.M., Prockop D.J. Copolymerization of normal type I collagen with three mutated type I collagens containing substitutions of cysteine at different glycine positions in the α1(I) chain. J. Biol. Chem. 1992;267:4968–4973. doi: 10.1016/s0021-9258(18)42925-0. [DOI] [PubMed] [Google Scholar]
- 41.Hulmes D.J.S., Miller A., Parry D.A.D., Piez K.A., Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J. Mol. Biol. 1973;79:137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- 42.Butzow J.J., Eichhorn G.L. Physical chemical studies on the age changes in rat tail tendon collagen. BBA - Protein Struct. 1968;154:208–219. doi: 10.1016/0005-2795(68)90273-0. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi M., Woodley D.T., Mechanic G.L. Aging and cross-linking of skin collagen. Biochem. Biophys. Res. Commun. 1988;152:898–903. doi: 10.1016/S0006-291X(88)80124-4. [DOI] [PubMed] [Google Scholar]
- 44.Kukhareva L.V., Paramonov B.A., Shamolina I.I., Semenova E.G. Method for preparing collagen for treatment of pathology of body tissues. Patent RU. 2002 [Google Scholar]
- 45.Daniels J.R., Park M., Knapp T.R., Clara S. Process for augmenting connective mammalian tissue with in situ polymerizable native collagen solution. Patent US. 1976 [Google Scholar]
- 46.Sato K., Ebihara T., Adachi E., Kawashima S., Hattori S., Irie S. Possible involvement of aminotelopeptide in self-assembly and thermal stability of collagen I as revealed by its removal with proteases. J. Biol. Chem. 2000;275:25870–25875. doi: 10.1074/jbc.M003700200. [DOI] [PubMed] [Google Scholar]
- 47.Qian J., Okada Y., Ogura T., Tanaka K., Hattori S., Ito S., Satoh J., Takita T., Yasukawa K. Kinetic analysis of the digestion of bovine type I collagen telopeptides with porcine pepsin. J. Food Sci. 2016;81:C27–C34. doi: 10.1111/1750-3841.13179. [DOI] [PubMed] [Google Scholar]
- 48.Lian J.B., Morris S., Faris B., Albright J. The effects of acetic acid and pepsin on the crosslinkages and ultrastructure of corneal collagen. Biochim. El Biophys. Acta. 1973;328:193–204. doi: 10.1016/0005-2795(73)90345-0. https://doi.org/https://doi.org/10.1016/0005-2795(73)90345-0. [DOI] [PubMed] [Google Scholar]
- 49.Comper W.D., Veis A. The mechanism of nucleation for in vitro collagen fibril formation. Biopolymers. 1977;16:2133–2142. doi: 10.1002/bip.1977.360161005. [DOI] [PubMed] [Google Scholar]
- 50.Stamov D., Salchert K., Springer A., Werner C., Pompe T. Structural polymorphism of collagen type I-heparin cofibrils. Soft Matter. 2009;5:3461–3468. doi: 10.1039/b908267k. [DOI] [Google Scholar]
- 51.Bruns R.R., Trelstad R.L., Gross J. Cartilage collagen: a staggered substructure in reconstituted fibrils. Science. 1973;181:269–271. doi: 10.1126/science.181.4096.269. 80- [DOI] [PubMed] [Google Scholar]
- 52.Bruns R.R. Supramolecular structure of polymorphic collagen fibrils. J. Cell Biol. 1976;68:521–538. doi: 10.1083/jcb.68.3.521. https://doi.org/https://doi.org/10.1083/jcb.68.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujii T. The effect of amines added to an alkali-pretreatment on the solubilisation of collagen and on the properties of gelatin. Hoppe. Seylers. Z. Physiol. Chem. 1969;350:1257–1265. doi: 10.1515/bchm2.1969.350.2.1257. [DOI] [PubMed] [Google Scholar]
- 54.Hattori S., Adachi E., Ebihara T., Shirai T., Someki I., Irie S. Alkali-treated the ligand collagen activity retained the adhesion triple via conformation integrin. J. Biochem. 1999;125:676–684. doi: 10.1093/oxfordjournals.jbchem.a022336. [DOI] [PubMed] [Google Scholar]
- 55.Doyle B.B., Hukins D.W.L., Hulmes D.J.S., Miller A., Woodhead-Galloway J. Collagen polymorphism: its origins in the amino acid sequence. J. Mol. Biol. 1975;91:79–99. doi: 10.1016/0022-2836(75)90373-3. [DOI] [PubMed] [Google Scholar]
- 56.Petruska J.A., Hodge A.J. A subunit model for the tropocollagen macromolecule. Proc. Natl. Acad. Sci. United States. 1964;51:871–876. doi: 10.1073/pnas.51.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulmes D.J.S., Miller A. Quasi-hexagonal molecular packing in collagen fibrils [34] Nature. 1979;282:878–880. doi: 10.1038/282878a0. [DOI] [PubMed] [Google Scholar]
- 58.Paige M.F., Rainey J.K., Goh M.C. Fibrous long spacing collagen ultrastructure elucidated-by atomic force microscopy. Biophys. J. 1998;74:3211–3216. doi: 10.1016/S0006-3495(98)78027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paige M.F., Goh M.C. Ultrastructure and assembly of segmental long spacing collagen studied by atomic force microscopy. Micron. 2001;32:355–361. doi: 10.1016/S0968-4328(00)00038-X. [DOI] [PubMed] [Google Scholar]
- 60.Chapman J.A., Armitage P.M. An analysis of fibrous long spacing forms of collagen. Connect. Tissue Res. 1972;1:31–37. doi: 10.3109/03008207209152053. [DOI] [Google Scholar]
- 61.Highberger J.H., Gros J., Schmitt F.O. Electron microscope observations of certain fibrous structures obtained from connective tissue extracts. J. Am. Chem. Soc. 1950;72:3321–3322. https://doi.org/doi.org/10.1021/ja01163a553. [Google Scholar]
- 62.Highberger J.H., Gross J., Schmitt F.O. The interaction of mucoprotein with soluble collagen; an electron microscope study. Proc. Natl. Acad. Sci. U.S.A. 1951;37:286–291. doi: 10.1073/pnas.37.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franzblau C., Schmid K., Faris B., Beldekas J., Garvin P., Kagan H.M., Baum B.J. The interaction of collagen with α1-acid glycoprotein. BBA - Protein Struct. 1976;427:302–314. doi: 10.1016/0005-2795(76)90306-8. [DOI] [PubMed] [Google Scholar]
- 64.Brodsky B., Eikenberry E.F. Methods Enzymol. 1982. Characterization of fibrous forms of collagen; pp. 127–174. [DOI] [PubMed] [Google Scholar]
- 65.Harris J.R., Lewis R.J. The collagen type I segment long spacing (SLS) and fibrillar forms: formation by ATP and sulphonated diazo dyes. Micron. 2016;86:36–47. doi: 10.1016/j.micron.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Bard J.B.L., Chapman J.A. Polymorphism in collagen fibrils precipitated at low pH. Nature. 1968;219:1279–1280. doi: 10.1038/2191279a0. [DOI] [PubMed] [Google Scholar]
- 67.Trelstad R.L., Igarashi S., Gross J., Kang A.H. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970;9:4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh S.K., Mitra H.P. Oblique banding pattern in collagen fibrils reconstituted in vitro after trypsin treatment. Biochim. Biophys. Acta. 1975;405:340–346. doi: 10.1016/0005-2795(75)90099-9. [DOI] [PubMed] [Google Scholar]
- 69.Fitton Jackson S., Smith R.H. Studies on the biosynthesis of collagen. I. The growth of fowl osteoblasts and the formation of collagen in tissue culture. J. Biophys. Biochem. Cytol. 1957;3:897–912. doi: 10.1083/jcb.3.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross J. The behavior of collagen units as a model in morphogenesis. J. Biophys. Biochem. Cytol. 1956;2:261–279. doi: 10.1083/jcb.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris J.R., Soliakov A., Lewis R.J. In vitro fibrillogenesis of collagen type I in varying ionic and pH conditions. Micron. 2013;49:60–68. doi: 10.1016/j.micron.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Schmitt F., Hall C.E., Jukus M.A. Electron microscope investigations of the structure of collagen. J. Cell. Comp. Physiol. 1942;20:11–33. https://doi.org/https://doi.org/10.1002/jcp.1030200103. [Google Scholar]
- 73.Ehrmann R.L., Gey G.O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J. Natl. Cancer Inst. 1956;16:1375–1403. doi: 10.1093/jnci/16.6.1375. [DOI] [PubMed] [Google Scholar]
- 74.Tkocz C., Kühn K. the formation of triple-helical collagen molecules from α1 or α2 polypeptide chains. Eur. J. Biochem. 1969;7:454–462. doi: 10.1111/j.1432-1033.1969.tb19631.x. [DOI] [PubMed] [Google Scholar]
- 75.Chandrakasan G., Torchia D.A., Piez K.A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J. Biol. Chem. 1976;251:6062–6067. doi: 10.1016/s0021-9258(17)33059-4. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi T., Nagai Y. Effect of pH on the stability of collagen molecule solution. J. Biochem. 1973;73:999–1006. doi: 10.1093/oxfordjournals.jbchem.a130184. [DOI] [PubMed] [Google Scholar]
- 77.Nam K., Sakai Y., Hashimoto Y., Kimura T., Kishida A. Fabrication of a heterostructural fibrillated collagen matrix for the regeneration of soft tissue function. Soft Matter. 2012;8:472–480. doi: 10.1039/c1sm06543b. [DOI] [Google Scholar]
- 78.Shved Y.A., Kukhareva L.B., Zorin I.M., Blinova M.I., Bilibin A.Y., Pinaev G.P. Interaction of cultured skin cells with the polylactide matrix coated with different collagen structural isoforms. Cell Tissue Biol. 2007;1:89–95. doi: 10.1134/S1990519X07010117. [DOI] [Google Scholar]
- 79.Ramtani S., Takahashi-IÑiguez Y., Helary C., Geiger D., Guille M.M.G. Mechanical behavior under unconfined compression loadings of dense fibrillar collagen matrices mimetic of living tissues. J. Mech. Med. Biol. 2010;10:35–55. doi: 10.1142/S0219519410003290. [DOI] [Google Scholar]
- 80.Giraud-Guille M.M. Liquid crystallinity in condensed type I collagen solutions. A clue to the packing of collagen in extracellular matrices. J. Mol. Biol. 1992;224:861–873. doi: 10.1016/0022-2836(92)90567-4. [DOI] [PubMed] [Google Scholar]
- 81.Giraud Guille M.M., Mosser G., Helary C., Eglin D. Bone matrix like assemblies of collagen: from liquid crystals to gels and biomimetic materials. Micron. 2005;36:602–608. doi: 10.1016/j.micron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Besseau L., Giraud-Guille M.M. Stabilization of fluid cholesteric phases of collagen to ordered gelated matrices. J. Mol. Biol. 1995;251:197–202. doi: 10.1006/jmbi.1995.0426. [DOI] [PubMed] [Google Scholar]
- 83.Price J.C., Roach P., El Haj A.J. Liquid crystalline ordered collagen substrates for applications in tissue engineering. ACS Biomater. Sci. Eng. 2016;2:625–633. doi: 10.1021/acsbiomaterials.6b00030. [DOI] [PubMed] [Google Scholar]
- 84.Gross J., Highberger J.H., Schmitt F.O. Extraction of collagen from connective tissue by neutral salt solutions. Proc. Natl. Acad. Sci. 1955;41:1–7. doi: 10.1073/pnas.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson D.S., Fessler J.H. Isolation and properties of a collagen soluble in salt solution at neutral pH. Nature. 1955;176:69–70. doi: 10.1038/176069a0. [DOI] [PubMed] [Google Scholar]
- 86.Wood G.C., Keech M.K. The formation of fibrils from collagen solutions. 2. A mechanism of collagen-fibril formation. Biochem. J. 1960;75:588–598. doi: 10.1042/bj0750598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gross J., Kirk D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J. Biol. Chem. 1958;233:355–360. doi: 10.1016/s0021-9258(18)64764-7. [DOI] [PubMed] [Google Scholar]
- 88.Torbet J., Malbouyres M., Builles N., Justin V., Roulet M., Damour O., Oldberg Å., Ruggiero F., Hulmes D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials. 2007;28:4268–4276. doi: 10.1016/j.biomaterials.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 89.Builles N., Janin-Manificat H., Malbouyres M., Justin V., Rovère M.R., Pellegrini G., Torbet J., Hulmes D.J.S., Burillon C., Damour O., Ruggiero F. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials. 2010;31:8313–8322. doi: 10.1016/j.biomaterials.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 90.Silver F.H., Langley K.H., Trelstad R.L. Type I collagen fibrillogenesis: initiation via reversible linear and lateral growth steps. Biopolymers. 1979;18:2523–2535. doi: 10.1002/bip.1979.360181011. [DOI] [PubMed] [Google Scholar]
- 91.Silver F.H. Type I collagen fibrillogenesis in vitro. Additional evidence for the assembly mechanism. J. Biol. Chem. 1981;256:4973–4977. doi: 10.1016/s0021-9258(19)69353-1. [DOI] [PubMed] [Google Scholar]
- 92.Holmes D.F., Chapman J.A. Axial mass distributions of collagen fibrils grown in vitro: results for the end regions of early fibrils. Biochem. Biophys. Res. Commun. 1979;87:993–999. doi: 10.1016/S0006-291X(79)80005-4. [DOI] [PubMed] [Google Scholar]
- 93.Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. U.S.A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bell E., Ehrlich H.P., Buttle D.J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–1054. doi: 10.1126/science.7008197. 80- [DOI] [PubMed] [Google Scholar]
- 96.Bell E., Sher S., Hull B., Merrill C., Rosen S., Chamson A., Asselineau D., Dubertret L., Coulomb B., Lapiere C., Nusgens B., Neveux Y. The reconstitution of living skin. J. Invest. Dermatol. 1983;81:2–10. doi: 10.1111/1523-1747.ep12539993. [DOI] [PubMed] [Google Scholar]
- 97.Bell E., Dedham M. Tissue-equivalent and method for preparation thereof. Patent US. 1984 [Google Scholar]
- 98.Weinberg C.B., Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. 80- [DOI] [PubMed] [Google Scholar]
- 99.Bell E., Dedham M. Fabrication of living blood vessels and glandular tissues. Patent US. 1985 [Google Scholar]
- 100.Bell E., Dedham M. Bone-equivalent and method for preparation thereof. Patent US. 1984 [Google Scholar]
- 101.Brown R.A., Wiseman M., Chuo C.B., Cheema U., Nazhat S.N. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005;15:1762–1770. doi: 10.1002/adfm.200500042. [DOI] [Google Scholar]
- 102.Hadjipanayi E., Ananta M., Binkowski M., Streeter I., Lu Z., Cui Z.F., Brown R.A., Mudera V. Mechanisms of structure generation during plastic compression of nanofibrillar collagen hydrogel scaffolds: towards engineering of collagen. J. Tissue Eng. Regen. Med. 2011;5:505–519. doi: 10.1002/term.343. [DOI] [PubMed] [Google Scholar]
- 103.Chandran P.L., Barocas V.H. Microstructural mechanics of collagen gels in confined compression: poroelasticity, viscoelasticity, and collapse. J. Biomech. Eng. 2004;126:152–166. doi: 10.1115/1.1688774. [DOI] [PubMed] [Google Scholar]
- 104.Allen T.D., Schor S.L., Schor A.M. Ultrastructural review of collagen gels, a model system for cell-matrix, cell-basement membrane and cell-cell interactions. Scanning Electron. Microsc. 1984:375–390. [PubMed] [Google Scholar]
- 105.Gutsmann T., Fantner G.E., Venturoni M., Ekani-Nkodo A., Thompson J.B., Kindt J.H., Morse D.E., Fygenson D.K., Hansma P.K. Evidence that collagen fibrils in tendons are inhomogeneously structured in a tubelike manner. Biophys. J. 2003;84:2593–2598. doi: 10.1016/S0006-3495(03)75064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strasser S., Zink A., Janko M., Heckl W.M., Thalhammer S. Structural investigations on native collagen type I fibrils using AFM. Biochem. Biophys. Res. Commun. 2007;354:27–32. doi: 10.1016/j.bbrc.2006.12.114. [DOI] [PubMed] [Google Scholar]
- 107.Hu K., Shi H., Zhu J., Deng D., Zhou G., Zhang W., Cao Y., Liu W. Compressed collagen gel as the scaffold for skin engineering, Biomed. Microdevices. 2010;12:627–635. doi: 10.1007/s10544-010-9415-4. [DOI] [PubMed] [Google Scholar]
- 108.Braziulis E., Diezi M., Biedermann T., Pontiggia L., Schmucki M., Hartmann-Fritsch F., Luginbühl J., Schiestl C., Meuli M., Reichmann E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. C Methods. 2012;18:464–474. doi: 10.1089/ten.tec.2011.0561. [DOI] [PubMed] [Google Scholar]
- 109.Helary C., Bataille I., Abed A., Illoul C., Anglo A., Louedec L., Letourneur D., Meddahi-Pellé A., Giraud-Guille M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials. 2010;31:481–490. doi: 10.1016/j.biomaterials.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 110.Bitar M., Salih V., Brown R.A., Nazhat S.N. Effect of multiple unconfined compression on cellular dense collagen scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007;18:237–244. doi: 10.1007/s10856-006-0685-1. [DOI] [PubMed] [Google Scholar]
- 111.Marelli B., Ghezzi C.E., Mohn D., Stark W.J., Barralet J.E., Boccaccini A.R., Nazhat S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 112.Marelli B., Ghezzi C.E., Zhang Y.L., Rouiller I., Barralet J.E., Nazhat S.N. Fibril formation pH controls intrafibrillar collagen biomineralization invitro and invivo. Biomaterials. 2015;37:252–259. doi: 10.1016/j.biomaterials.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Kim A., Lakshman N., Karamichos D., Matthew Petroll W. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Investig. Ophthalmol. Vis. Sci. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levis H.J., Brown R.A., Daniels J.T. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials. 2010;31:7726–7737. doi: 10.1016/j.biomaterials.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 115.Mi S., Chen B., Wright B., Connon C.J. Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J. Biomed. Mater. Res., Part A. 2010;95 A:447–453. doi: 10.1002/jbm.a.32861. [DOI] [PubMed] [Google Scholar]
- 116.Engelhardt E.-M., Stegberg E., Brown R.A., Hubbell J.A., Wurm F.M., Adam M., Frey P. Compressed collagen gel: a novel scaffold for human bladder cells. J. Tissue Eng. Regen. Med. 2010;4:123–130. doi: 10.1002/term.222. [DOI] [PubMed] [Google Scholar]
- 117.Micol L.A., Ananta M., Engelhardt E.M., Mudera V.C., Brown R.A., Hubbell J.A., Frey P. High-density collagen gel tubes as a matrix for primary human bladder smooth muscle cells. Biomaterials. 2011;32:1543–1548. doi: 10.1016/j.biomaterials.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 118.Ghezzi C.E., Marelli B., Muja N., Nazhat S.N. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 2012;8:1813–1825. doi: 10.1016/j.actbio.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 119.Itagaki A., Kimura G. TES and HEPES buffers in mammalian cell cultures and viral studies: problem of carbon dioxide requirement. Exp. Cell Res. 1974;83:351–361. doi: 10.1016/0014-4827(74)90349-8. [DOI] [PubMed] [Google Scholar]