Abstract
Background
In the Investigation of the Management of Pericarditis (IMPI) randomized control, 2x2 factorial trial, Mycobacterium indicus pranii (MIP) immunotherapy, adjunctive corticosteroids or MIP combined with corticosteroids was compared to standard tuberculosis (TB) therapy for tuberculous pericarditis (TBP). While MIP and/or the combination of MIP and corticosteroids had no impact on all-cause mortality or pericarditis related outcomes, corticosteroids reduced the incidence of constrictive pericarditis at 12 months. Data suggests that both adjunctive therapies modulate the immune and inflammatory responses to pulmonary TB. Whether they affect systemic antigen-specific T cell responses, key immune mediators of Mycobacterium tuberculosis control, in patients with TBP is unknown.
Methods
Participants with definite or probable TBP were randomly assigned to receive five injections of MIP or placebo at 2-week intervals and either 6 weeks of oral prednisolone or placebo. Frequencies of CD4 and CD8 T cells expressing IFN-γ, IL-2 or TNF in response to MIP or purified protein derivative stimulation were measured by intracellular cytokine staining and flow cytometry up to 24 weeks post treatment.
Results
Immunotherapy with MIP did not significantly modulate frequencies of Th1 CD4 and CD8 T cells compared to placebo. Adjunctive prednisolone also did not change mycobacteria-specific CD4 or CD8 T cell responses. By contrast, combinatorial therapy with MIP and prednisolone was associated with a modest increase in frequencies of multifunctional and single cytokine-expressing CD4 T cell responses at 6 and 24 weeks post treatment.
Conclusions
Consistent with the lack of a significant clinical effect in the IMPI trial, MIP immunotherapy did not significantly modulate mycobacteria-specific T cell responses. Despite the positive effect of prednisolone on hospitalizations and constrictive pericarditis in the IMPI trial, prednisolone did not significantly reduce pro-inflammatory T cell responses in this sub-study. The modest improvement of mycobacteria-specific T cell upon combinatorial therapy with MIP and prednisolone requires further investigation.
Keywords: Tuberculous pericarditis, Mycobacteriumindicus pranii immunotherapy, Adjunctive corticosteroid, T cell responses
Introduction
Tuberculous pericarditis (TBP), a severe manifestation of tuberculosis (TB) disease, is the most common pericardial disease in Africa [1]. The human immunodeficiency virus (HIV) pandemic further fuels the high burden of TBP, especially in Sub-Saharan Africa where the prevalence of HIV is high and TB is endemic.
Despite adequate antimicrobial treatment, the outcome of TBP remains poor. A lack of understanding of the underlying immune response associated with tuberculous pericarditis and the mechanisms that underpin existing and experimental treatment interventions have hampered the discovery and improvement of current therapies for TBP.
Elimination of Mycobacterium tuberculosis (Mtb) bacilli is a key requirement for TBP treatment and ultimate cure. The first line of chemotherapy is a standard 4-drug anti-TB regimen for a minimum duration of six months. However, despite adherence to treatment, the overall mortality rate in patients with TBP after a six-month course of chemotherapy was 26% in a sub-Saharan cohort [2]. This low treatment success rate may be due to poor penetration of drugs into the pericardial space [3]. Standard TB drugs may not be sufficient to adequately clear Mtb bacilli and motivate for adjunctive interventions to improve cure rates. Since the bacterial burden within the pericardium is positively correlated with mortality rates in TBP [4], and persistence of mycobacterial antigens contribute to prolonged pro-inflammatory immune responses in symptomatic TPB [5], [6], enhancing the immune systems’ own ability to kill and eliminate Mtb via therapeutic vaccination could substantially accelerate antigens clearance and shorten destructive inflammatory responses.
Mycobacterium indicus pranii (MIP, formerly known as Mycobacterium w) is a non-tuberculous mycobacterium which shares antigens with M. leprosis and Mtb. MIP has been effectively used to treat leprotic lesions [7]. Adjunctive MIP immunotherapy has also received attention as a treatment for TB. A significantly higher rate of sputum conversion was observed 4 weeks into treatment (67.1%) in pulmonary TB patients who received adjunctive MIP vaccination in addition to standard anti-TB therapy, compared to the placebo group who received anti-TB drugs only (57%, p = 0.0002) [8]. In a guinea pig model, reductions in lung bacterial load and pathology were associated with increased frequencies of CD4 and CD8 T cells after MIP boosting [9]. In a murine model, intranasal MIP vaccination induced an increase in IFN-γ+IL-2+TNF+ polyfunctional CD4 T cells in the lungs [10]. Further, adoptive transfer of lung resident T cells isolated from MIP vaccinated mice into naïve wildtype mice reduced the Mtb load in lungs of recipient mice [10]. These studies suggest that Th1-cytokine expressing T cells induced by MIP vaccination may improve immune control of Mtb bacilli.
Management of TBP also requires symptom relief and prevention of heart failure, which can be achieved using invasive procedures such as pericardiocentesis to drain fluid from the pericardium or pericardiectomy to remove fibrotic tissue and restore elasticity of the heart [11]. Corticosteroids are the only non-invasive form of treatment available to reduce symptoms caused by excessive inflammatory responses. A review of four randomized controlled trials including HIV-negative TBP patients suggests a modest reduction in all-cause mortality by approximately 20% (risk ratio (RR) 0.80, 95% CI 0.59–1.09) and a significant reduction of pericarditis related mortality (RR 0.39, 95% CI 0.19 to 0.80) in patients who received corticosteroids compared to placebo controls [12].
Whether or not MIP immunotherapy and corticosteroid reduce mortality and pericarditis related outcomes in patients with TBP was tested in the Investigation of the Management of Pericarditis (IMPI) trial (ClinicalTrials.gov identifier: NCT00810849). In the 1400-patient randomized control trial with a 2-by-2 factorial design, there was no reduction in mortality for either MIP, prednisolone or MIP and prednisolone combined. However, corticosteroid treated patients had a reduced hospitalization rate (20.7% vs 25.2%, p = 0.04) and incidence of constrictive pericarditis (4.4% vs 7.8%, p = 0.009) compared to the placebo group. Both interventions increased the incidence of cancer in patients in those with advanced HIV (CD4 < 50) [13].
We present the results of a planned IMPI trial sub-study, in which we characterized the influence of adjunctive MIP and corticosteroid therapy on mycobacteria-specific CD4 and CD8 T cell responses in a subgroup of TPB patients. We hypothesized that MIP would increase mycobacteria-specific CD4 and CD8 T cell responses while corticosteroid therapy would suppress these responses.
Methods
Study design and participants
The sub-study involved participants enrolled in the IMPI trial at the University of Cape Town between August 2012 and November 2013. All participants provided written informed consent. Ethics approval was granted from the Faculty of Health Sciences Human Research Ethics Committee approval (HREC 032/2009).
TBP was considered definite if there was pericardial biopsy or fluid culture evidence of TB. Probable TBP was assumed where a] pericardial fluid biochemical and cellular analysis was consistent with TB (i.e., a lymphocyte predominant exudate with raised adenosine deaminase (ADA), or b] TB was confirmed on a non-pericardial fluid sample (e.g. sputum) or the Tygerberg Index score was ≥ 6 [14]. Approximately 70% of the trial population was HIV infected and the majority were not yet on anti-retroviral therapy at the time of enrolment.
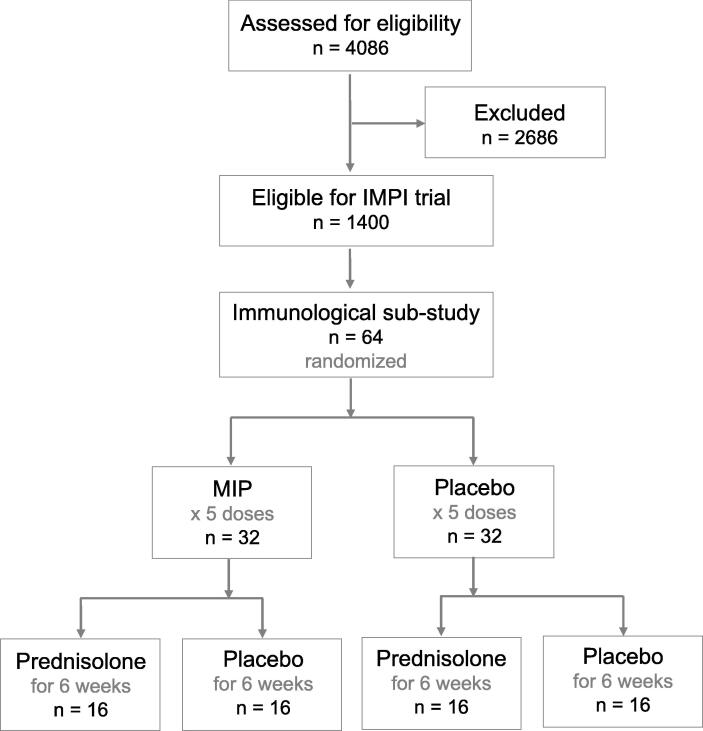
In the first step of the factorial randomization, participants were randomly assigned to receive five doses of 0.01 mL intradermal MIP immunotherapy or placebo at enrolment, 2, 4, 6 weeks and at 3 months. In the second step, the same participants were further randomly assigned to receive a tapering dose of daily oral prednisolone or placebo for 6 weeks starting at a dose of 120 mg/d in the first week of enrolment, followed by a reduction of 30 mg/d each subsequent week until the 5th week when participants received 15 mg/d and 5 mg/d in the 6th week. This allowed for an analysis and comparison of participant groups receiving placebo, steroids alone, MIP alone and or the two interventions together (Fig. 1). Blood was collected at baseline (within 7 days of TB treatment start), 2, 6 and 24 weeks after treatment initiation (Fig. 1).
Fig. 1.
Consort diagram of 2-by-2 factorial design of the immunogenicity sub-study of the IMPI trial. A total of 64 tuberculous pericarditis (TBP) patients were randomly assigned to receive either 5 injections of Mycobacterium indicus pranii (MIP) or saline. The MIP or placebo groups were further subdivided by randomization to receive either 6 weeks of oral prednisolone or placebo. Blood samples were collected at baseline, 2, 6 and 24 weeks after treatment initiation.
Most participants who were not on ART at the time of randomization were referred to dedicated ART clinics for commencement of therapy in line with local guidelines. The South African National Anti-Retroviral Guidelines 2004 document recommended commencement of ART within 12 weeks of starting TB treatment unless the attending clinician felt the need for earlier commencement on the basis of the patients clinical status and CD4 count.
Stimulation of whole blood
Blood was collected into heparinized tubes and stimulated with heat-killed MIP (5x107 CFU/mL; Cadila Pharmaceuticals, Ahmedabad, India), PPD (10 μg/mL, Statens Serum Institute, Copenhagen, Denmark) or PHA (5 μg/mL, Sigma-Aldrich, St. Louis, Mo) in the presence of anti-CD28 and anti-CD49d (at 1 μg/mL for each antibody, BD Bioscience) co-stimulatory antibodies at 37˚C, 5% CO2. The negative control was left unstimulated. After 7h Brefeldin A (10 μg/mL, Sigma-Aldrich) was added and the blood was incubated for another 5h, before red cells were lysed and white cells fixed using FACS lysing solution (BD). White cells were cryopreserved in 20% DMSO in FCS and RMPI1640 with L-Glutamine (Lonza Bioscience).
Immunofluorescent staining and flow cytometric analysis
Cryopreserved, stimulated cells were carefully thawed, washed with PBS (Lonza) and permeabilized in BD Perm/Wash (BD Biosciences) to enable labelling of intracellular cytokines. Cells were stained with fluorescent antibodies to surface and intracellular proteins (Suppl. Tab. 1) in a total volume of 20 μL in BV buffer (BD Biosciences) for 1hr at 4 °C. Stained cells were washed twice with BD Perm/Wash buffer and acquired on a BD LSR II flow cytometer.
Data analysis
Flow cytometry data were analyzed using FlowJo version 9.9.6. Background cytokine responses in unstimulated samples were subtracted from stimulated samples using Pestle V2 and SPICE V6.1 [15]. GraphPad Prism v7 (GraphPad, SanDiego, CA) was used for data presentation. Statistical analyses were performed using R version 3.6. Wilcoxon signed-rank was used for paired and Mann-Whitney U tests for un-paired analyses. P-values were corrected for multiple comparisons using Bonferroni Correction or Benjamini–Hochberg method (Suppl. Tables 2 & 3).
Results
Participants
A total of 64 TBP patients were enrolled into this immunological sub-study of the IMPI trial; 16 participants in each of the 4 randomized treatment groups. There were no significant differences between the 4 study groups in sex, age, distribution of definite or probable TBP, proportion of participants with HIV negative or positive status, or those receiving anti-retroviral therapy (ART) or CD4 T cell counts (Table 1). Of the 41 participants who were HIV infected, none were on ART at the time of enrolment. All had commenced therapy at their 3 month follow up visit in line with local guidelines.
Table 1.
Clinical and demographical characteristics of participants.
| Demographics |
Placebo (n = 16) |
Prednisolone (n = 16) |
MIP (n = 16) |
MIP+ Prednisolone (n = 16) |
P-value |
|---|---|---|---|---|---|
| Sex, n (%) | 0.243 | ||||
| Male | 9 (56) | 14 (88) | 10 (62) | 10 (62) | |
| Female | 7 (44) | 2 (12) | 6 (38) | 6 (38) | |
| Age (years) | 0.494 | ||||
| Range Median (IQR) | 24 – 60 3115.25 (27.75–43) |
24 – 54 33.510.5 (30–40.5) |
23 – 51 329.25 (28.5–37.75) |
24 – 58 3910.5 (34–44.5) |
|
| Clinical characteristics | |||||
| Tuberculous pericarditis, n (%) | 0.531 | ||||
| Definite | 7 (44) | 5 (31) | 8 (50) | 9 (56) | |
| Probable | 9 (56) | 11 (69) | 8 (50) | 7 (44) | |
| HIV | 0.507 | ||||
| Negative | 7 (44) | 5 (31) | 5 (31) | 3 (19) | |
| Positive | 9 (56) | 11 (69) | 11 (69) | 13 (81) | |
| Anti-retroviral therapy (ART) | 9 (56) | 11 (69) | 9 (57) | 12 (75) | 0.774 |
| CD4 count, median (cells/mL) (IQR) |
377 545.25 (123.75–669) |
247.5251.25 (104–355.25) |
211.5357 (111.25–468.25) |
218292.25 (137.25–429.5) |
0.196 |
Q = Quartile, IQR = interquartile range; HIV = human immunodeficiency virus, p-values were calculated using Chi-square test or ANOVA.
Cytokine+ CD4 and CD8 T cell responses prior to treatment
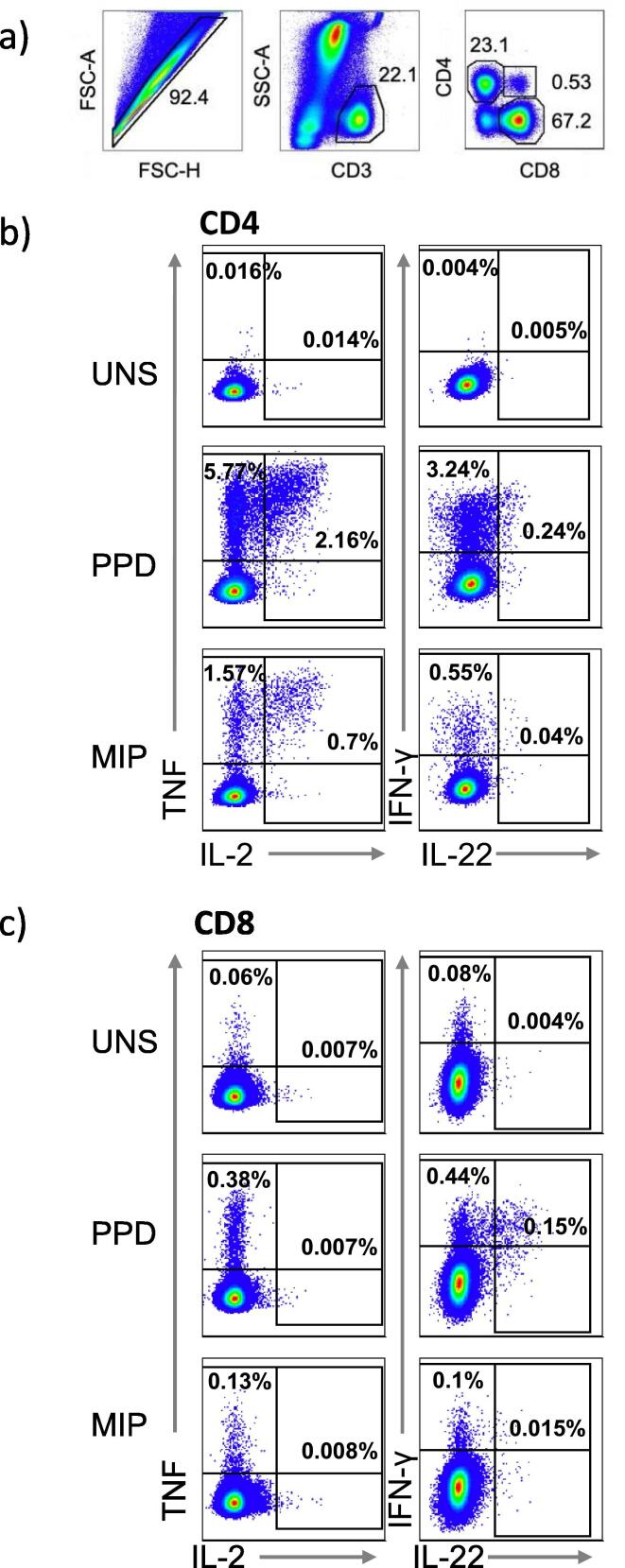
To characterize mycobacteria-specific T cell responses in the 4 groups, we measured co-expression of IFN-γ, IL-2, IL-22 and TNF by CD4 and CD8 T cells using flow cytometry after blood stimulation with PPD or heat-killed MIP (Fig. 2). Frequencies of PPD- or MIP-specific cytokine-expressing CD4 T cells above those of the unstimulated control were readily detectable and responses to PPD were generally higher than those to MIP (Suppl. Fig. 2 and Fig. 4; PHA control Suppl. Fig. 3). Responses by CD8 T cells were mostly restricted to single IFN-γ + cells and much lower than cytokine-expressing CD4 T cell frequencies (Suppl. Fig. 4, Fig. 5). Overall, IL-22 expression was low or not detected, and were therefore not included in the primary analyses (Suppl. Fig. 6). As a result, we focused our primary analyses on PPD-specific (herein referred to as mycobacteria-specific) CD4 T cell responses.
Fig. 2.
Flow cytometry gating strategy to identify cytokine expressing CD4 and CD8 T cells. Doublets were excluded by forward scatter (FSC) area versus height gating. T cells were identified as CD3 positive and side scatter (SSC)-low cells and further subdivided into CD4 or CD8 positive cells (A). Interferon-gamma (IFN-γ), Interleukin-2 (IL-2), Interleukin-22 (IL-22) and tumor necrosis factor (TNF) expression in unstimulated (UNS) samples or after PPD and MIP stimulation in CD4 (B) and CD8 (C) T cells.
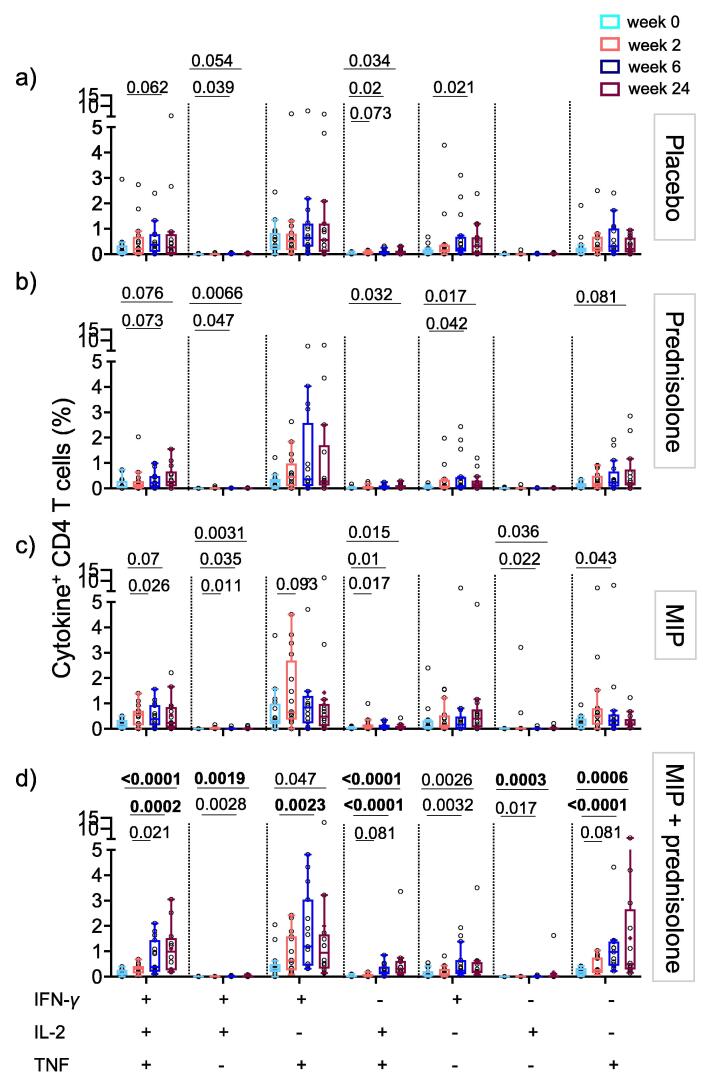
Fig. 4.
Antigen-specific CD4 T cell responses increase during follow-up. Frequencies of cytokine co-expressing CD4 T cells in peripheral blood of tuberculous pericarditis patients after PPD stimulation in placebo (a) adjunctive prednisolone (b) MIP immunotherapy (c) or combinatorial prednisolone and MIP therapy (d). Frequencies observed in unstimulated blood were subtracted from those in PPD stimulated blood. Horizontal lines of graphs depict the median, boxes the IQR and whiskers the range (Tukey). Wilcoxon comparison of responses at 2 weeks (pink), 6 weeks (royal blue) and 24 weeks (purple) post treatment time points to baseline (light blue). We used the Bonferroni method to account for multiple testing and a p-value below 0.00238 (21 comparisons) was considered significant and highlighted in bold. Only p-values < 0.1 are shown.
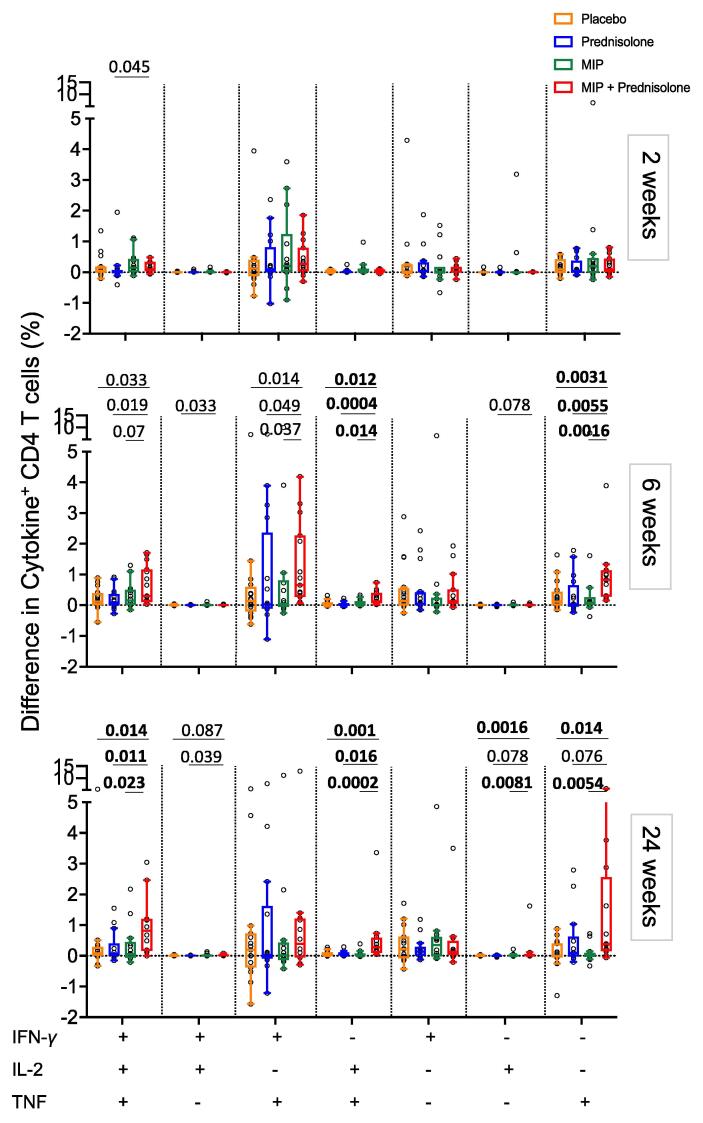
Fig. 5.
Comparison of changes in antigen-specific CD4 T cell responses relative to baseline between the treatment groups after PPD stimulation. Relative differences in frequencies of cytokine co-expressing CD4 T cells in peripheral blood after PPD stimulation between baseline (0 weeks) and 2 weeks (top), 6 weeks (middle) or 24 weeks (bottom), in the placebo (orange), prednisolone (blue), MIP (green) and MIP + prednisolone (red) treatment groups. Horizontal lines depict the median, boxes the IQR and whiskers the range (Tukey). Mann-Whitney was used to compare placebo (orange), prednisolone (blue) and MIP (green) cytokine values to MIP + prednisolone (red) treatment. We adjusted p-values using the Benjamini-Hochberg method (FDR adjustment within each cytokine combination across all treatment group). Only p-values < 0.1 are indicated (all p and q values in Suppl Tab 3) and p-values with associated q-values below 0.05 were considered significant and are highlighted in bold.
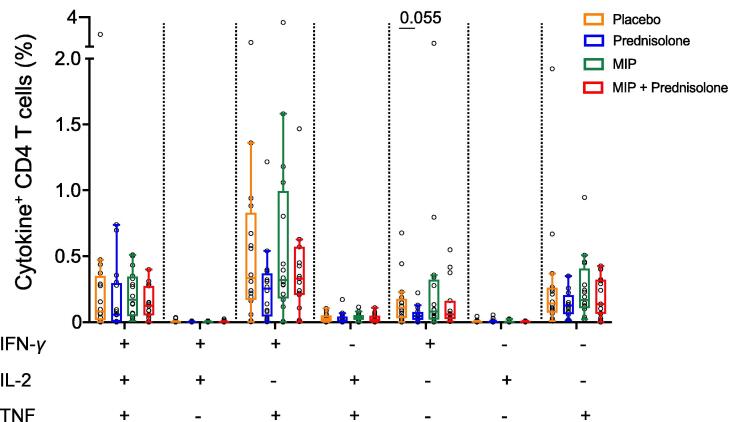
Variable TBP disease states including definite, probable, co-infection with or the absence of HIV infection can influence the magnitude and function of T cells that respond to stimulation. Frequencies of IFN-γ, IL-2 and TNF co-expressing CD4 T cell subsets, measured before treatment initiation, were highly heterogenous, but not significantly different, within each treatment group (Fig. 3 and Suppl. Table 2). We also observed unusually high proportions of double-positive CD4 + CD8 + CD3 + T cells in many TBP patients, with higher frequencies in HIV- than HIV + participants (Fig. 2 and Suppl. Fig. 1).
Mycobacteria-specific T cell cytokine responses are not modulated by MIP immunotherapy
This study evaluated the treatment effects of i) anti-inflammatory prednisolone, ii) MIP immunotherapy and iii) combinatorial prednisolone and MIP on mycobacteria-specific Th1 responses. Besides trial-specific treatment, all participants received anti-tuberculosis therapy and, depending on HIV status, received anti-retroviral therapy (Table 1). The effects of MIP and prednisolone on PPD-specific CD4 T cell responses were determined at 2, 6 and 24 weeks after treatment initiation and compared to baseline responses, before treatment start.
PPD-specific IFN-γ+IL-2+TNF+, IFN-γ+TNF+ and single positive IFN-γ+ or TNF+ CD4 T cell subsets predominated in all groups of participants (Fig. 4). The frequencies of PPD-specific Th1 CD4 T cells did not change in the placebo group, where participants only received standard anti-TB treatment and ART, if applicable (Fig. 4a).
Based on the anti-inflammatory properties of glucocorticoids, we hypothesized that prednisolone would dampen mycobacteria-specific T cell responses. However, frequencies of cytokine co-expressing CD4 T cells did not decrease at 6 or 24 weeks after treatment initiation relative to baseline (Fig. 4b).
To determine if MIP vaccination boosts mycobacteria-specific T cell responses, we compared the longitudinal responses in individuals who received MIP with those in placebo recipients. Following the first MIP vaccination, frequencies of polyfunctional or single positive PPD-specific CD4 T cells were not significantly higher than baseline responses (Fig. 4c). Repeated boosting with MIP also did not result in increases in PPD-specific CD4 T cell responses measured at 6 and 24 weeks post treatment in comparison to baseline.
Combination of prednisolone and MIP therapy increases mycobacteria-specific T cell responses
It is unclear whether simultaneous treatment with immunostimulatory MIP and immunosuppressive prednisolone would act antagonistically or synergistically in TBP. From week 6 until at least 24 weeks post-treatment, frequencies of cytokine-expressing polyfunctional or single cytokine-expressing CD4 T cells were significantly higher compared to baseline (Fig. 4d).
To factor in the highly heterogenous responses at baseline (Fig. 3), we further analyzed the differences in frequencies of cytokine-expressing T cells at the post-treatment time points relative to baseline [baseline cytokine values from each participant were subtracted from 2 week, 6 week or 24 week post treatment time points.] (Fig. 5 and Suppl. Fig. 7). Neither prednisolone nor MIP therapy alone resulted in marked and significantly modulated PPD-specific Th1 CD4 T cell responses throughout the 24 week period, relative to the placebo group (Suppl. Fig. 7, p-values in Suppl Tab. 3). The most consistent and marked changes were observed after combinatorial treatment, which resulted in increased mycobacteria-specific CD4 T cell responses relative to placebo, or relative to these interventions alone (Fig. 5 and Suppl. Fig. 7). The greatest increase in frequencies was apparent at 24 weeks after treatment for IFN-γ+IL-2+TNF+ CD4 T cells (in comparison to placebo p = 0.014; prednisolone p = 0.011; MIP p = 0.023), IL-2+TNF+ CD4 T cells (in comparison to placebo p = 0.001; prednisolone p = 0.016; MIP p = 0.0002) and single TNF+ CD4 T cells (in comparison to placebo p = 0.014; MIP p = 0.0054) (Fig. 5).
Fig. 3.
Heterogenous cytokine-expressing CD4 T cell responses between the four groups at study baseline, prior to treatment initiation. Frequencies of cytokine co-expressing CD4 T cells in peripheral blood of tuberculous pericarditis patients, detected after PPD stimulation at baseline in placebo (orange), prednisolone (blue), MIP (green) and MIP + prednisolone (red) treatment groups. Frequencies observed in unstimulated blood were subtracted from those in PPD stimulated blood. Horizontal lines within the boxes depict medians, boxes the IQR and whiskers the range (Tukey). Mann-Whitney test was used to compare responses between all four treatment groups. We adjusted p-values using the Benjamini-Hochberg method (false discovery rate (FDR) adjustment within each cytokine combination across all treatment group). Only p-values < 0.1 are shown (for all p-values refer to Suppl Tab. 2).
Discussion
In the large IMPI randomized controlled trial, while neither prednisolone, MIP, nor the combination of the two reduced all-cause mortality, prednisolone had a significant positive impact on hospitalizations and constrictive pericarditis. The hypothesis underlying the IMPI trial was that both interventions, through their modulation of the immune responses to Mtb bacilli, would reduce both mortality and TB pericarditis related morbidity. To further understand whether either intervention had an impact on T cell responses in trial participants, we investigated the systemic mycobacteria-specific T cell responses to patients with TBP. Our findings suggest that neither MIP immunotherapy nor prednisolone significantly modulated mycobacteria-specific T cell responses.
MIP immunotherapy had been reported to enhance Th1 cytokine expression in prior studies [10], [16]. In our study population, we found no significant boosting effect of MIP immunotherapy to enhance the ability of T cells to respond to mycobacterial antigens. The question remains why MIP immunotherapy did not further enhance mycobacteria-specific T cell responses. The bactericidal activity of anti-TB drugs may be associated with increased release of mycobacterial antigens, stimulating the immune response [17]. We propose that high levels of in vivo antigen exposure mediate sufficient stimulation of mycobacteria-specific T cells in TBP patients receiving chemotherapy, such that the effect of boosting with MIP, or even that in vitro stimulation with antigen, is negated. An alternative hypothesis is that proliferative capacity of T cells has progressively become attenuated upon repeated exposure to mycobacterial antigens [18], [19]. Excessive attempts to further boost the immune response through vaccination could render T cells progressively unresponsive, or even drive pathological T cell responses, as reported for repeat BCG vaccination in mice [20].
Glucocorticoids are widely used in the management of inflammatory diseases for their suppressive activity of pro-inflammatory responses by almost all immune cells [21]. Excessive, pathogenic T cell responses against mycobacterial antigens may contribute to tissue damage, promote fibrosis, and thus enhance severity of TBP. High-dose prednisolone was hypothesized to inhibit mycobacteria-specific T cell responses [22], thereby reducing immunopathology. However, we observed no significant reduction of T cell responsiveness to mycobacterial antigens in the prednisolone compared to the placebo control group. Although in the IMPI trial mortality was not reduced with prednisolone, the incidence of constrictive pericarditis was reduced by almost 50% regardless of HIV status, suggesting a significant steroid-mediated reduction of inflammation induced pericardial tissue injury. It is therefore possible that our 64 participant sub-study was underpowered to detect a significant decrease of T cell responsiveness after prednisolone treatment.
As another possibility, benefits attributed to prednisolone in the IMPI trial might be due to reduction of inflammatory responses in immune cells other than T cells. In a small study, reduced concentrations of pro-inflammatory cytokines produced by innate cells, including IL-1ß, IL-8 and lL-6, were seen in pericardial fluid of TBP patients after prednisolone treatment [23].
Intriguingly, combinatorial treatment of MIP and prednisolone did improve the functional responsiveness of mycobacteria-reactive CD4 T cells. CD4 T cells expressed higher levels of IFN-γ, IL-2 and TNF at completion of prednisolone treatment (6 weeks). It is possible that prednisolone therapy dampened excessive pro-inflammatory innate responses which continuously stimulated T cells, thus reconstituting T cell capacity to respond to boosting of MIP. As such, our results suggest that MIP boosting alone may not be successful during the first few weeks of anti-TB drug treatment when mycobacterial antigen load is high. However, such an effect was not reported in animal studies where MIP boosting was initiated at the same time as chemotherapy [9]. In TBP patients, prolonged antigen exposure over several weeks might have rendered T cells hyporesponsive. Further investigation is needed to elucidate the mechanisms behind the improved T cell responsiveness after combinatorial MIP and prednisolone treatment.
In addition, an important limitation of this study is that the mycobacterial T cell responses were only assessed in peripheral blood. There is evidence to suggest that both the pro-inflammatory and pro-fibrotic responses to TB pericarditis at gene and protein level are highly compartmentalized, with the most robust responses confined to the pericardium regardless of HIV status [24]. Furthermore, a comparison of cytokines and chemokines analyzed by multiplex assays in pericardial fluid, plasma and saliva in a small subset of IMPI trial participants, suggested important differences by compartment with the strongest response at the disease site [23]. This may also have contributed to the absence of any difference in T cell response in our analysis given that we did not assess T cell responses in pericardial fluid.
The relatively small sample size and diverse clinical disease states preclude definitive conclusions to be drawn about the efficacy results of monotherapy with MIP and prednisolone. We found that the ability of CD4 T cells to respond to stimulation with mycobacterial antigen highly differed between participants within one group. The highly heterogenous responses could be due to several factors: Pericarditis can only be confirmed via invasive procedures and probable TBP patients might have had pericarditis for reasons other than TB, which could have resulted in dilution of data [25]. Different stages of TB and/or HIV infection could affect the ability of T cells to respond to antigenic stimulation. Further potential factors influencing heterogenous immune response could result from various anti-retroviral drugs for a subgroup of participants.
In conclusion, this immunological sub-study supports the clinical result of the IMPI trial. MIP immunotherapy was not sufficient in boosting mycobacteria-specific T cell responses in TBP patients. High dose oral prednisolone treatment did not reduce pro-inflammatory cytokine responses in T cells. A combinatorial treatment of MIP and prednisolone significantly increased the ability of CD4 T cells to respond to Mtb antigens. The results of this study suggest that a combinatorial therapy of MIP and prednisolone may improve CD4 T cell function.
Funding
The immunological sub-study of the IMPI trial was funded by Mayosi Research Group and the Discovery Academic Foundation, the South African Medical Research Council, and the Lily and Ernst Hausmann Research Trust. PS received a fellowship from the Wellcome Centre for Infectious Diseases Research (CIDRI) in Africa.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful for the participation of the study participants of the IMPI trial and the IMPI team.
Footnotes
In memory of Bongani Mayosi MBChB DPhil.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2022.100177.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Mayosi BM, Volmink JA, Commerford PJ. Pericardial disease: An evidence-based approach to diagnosis and treatment. In: Yusuf S, Cairns J, Camm A, BJ F, editors. Evidence-based Cardiol. (2nd Ed., London, UK: BMJ Books; 2003, p. 735–48. https://doi.org/10.1002/9780470986882.ch50.
- 2.Mayosi B.M., Wiysonge C.S., Ntsekhe M., Gumedze F., Volmink J.A., Maartens G., et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. South African Med J. 2008;98:36–40. doi: 10.7196/SAMJ.287. [DOI] [PubMed] [Google Scholar]
- 3.Shenje J., Ifeoma Adimora-Nweke F., Ross I.L., Ntsekhe M., Wiesner L., Deffur A., et al. Poor penetration of antibiotics into pericardium in pericardial tuberculosis. EBioMedicine. 2015;2(11):1640–1649. doi: 10.1016/j.ebiom.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasipanodya J.G., Mubanga M., Ntsekhe M., Pandie S., Magazi B.T., Gumedze F., et al. Tuberculous pericarditis is multibacillary and bacterial burden drives high mortality. EBioMedicine. 2015;2(11):1634–1639. doi: 10.1016/j.ebiom.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherian G. Diagnosis of tuberculous aetiology in pericardial effusions. Postgrad Med J. 2004;80(943):262–266. doi: 10.1136/pgmj.2003.013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler N.O. Tuberculous pericarditis. JAMA J Am Med Assoc. 1991;266:99–103. doi: 10.1001/jama.1991.03470010103039. [DOI] [PubMed] [Google Scholar]
- 7.Talwar G, Singh P, Atrey N, C Gupta J. Making of a highly useful multipurpose vaccine. J Transl Sci 2016;2:69–73. https://doi.org/10.15761/jts.1000117.
- 8.Sharma S.K., Katoch K., Sarin R., Balambal R., Kumar Jain N., Patel N., et al. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomized trial. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-03514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A., Ahmad F.J., Ahmad F., Gupta U.D., Natarajan M., Katoch V., et al. Efficacy of Mycobacterium indicus pranii immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung. PLoS ONE. 2012;7(7):e39215. doi: 10.1371/journal.pone.0039215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A., Saqib M., Singh B., Pal L., Nishikanta A., Bhaskar S. Mycobacterium indicus pranii induced memory T-cells in lung airways are sentinels for improved protection against M.tb infection. Front Immunol. 2019;10:2359.;10 doi: 10.3389/fimmu.2019.02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isiguzo G., Du Bruyn E., Howlett P., Ntsekhe M. Diagnosis and management of tuberculous pericarditis: What Is New? Curr Cardiol Rep. 2020;22(1) doi: 10.1007/s11886-020-1254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiysonge C.S., Ntsekhe M., Thabane L., Volmink J., Majombozi D., Gumedze F., et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev. 2017;2017(9) doi: 10.1002/14651858.CD000526.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayosi B.M., Ntsekhe M., Bosch J., Pandie S., Jung H., Gumedze F., et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371(12):1121–1130. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayosi B.M., Burgess L.J., Doubell A.F. Tuberculous pericarditis. Circulation. 2005;112(23):3608–3616. doi: 10.1161/CIRCULATIONAHA.105.543066. [DOI] [PubMed] [Google Scholar]
- 15.Roederer M., Nozzi J.L., Nason M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytom Part A. 2011;79A(2):167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chahar M., Rawat K.D., Reddy P.V.J., Gupta U.D., Natrajan M., Chauhan D.S., et al. Potential of adjunctive Mycobacterium w (MIP) immunotherapy in reducing the duration of standard chemotherapy against tuberculosis. Indian J Tuberc. 2018;65(4):335–344. doi: 10.1016/j.ijtb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Mzinza D.T., Sloan D.J., Jambo K.C., Shani D., Kamdolozi M., Wilkinson K.A., et al. Kinetics of Mycobacterium tuberculosis-specific IFN-γ responses and sputum bacillary clearance in HIV-infected adults during treatment of pulmonary tuberculosis. Tuberculosis. 2015;95(4):463–469. doi: 10.1016/j.tube.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moguche A.O., Musvosvi M., Penn-Nicholson A., Plumlee C.R., Mearns H., Geldenhuys H., et al. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe. 2017;21(6):695–706.e5. doi: 10.1016/j.chom.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day C.L., Abrahams D.A., Lerumo L., Janse van Rensburg E., Stone L., O’rie T., et al. Functional capacity of Mycobacterium tuberculosis -specific T Cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187(5):2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz A., Fraga A.G., Fountain J.J., Rangel-Moreno J., Torrado E., Saraiva M., et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207(8):1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strehl C., Ehlers L., Gaber T., Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchimont D., Galon J., Gadina M., Visconti R., Zhou Y.-J., Aringer M., et al. Inhibition of Th1 Immune Response by Glucocorticoids: Dexamethasone Selectively Inhibits IL-12-Induced Stat4 Phosphorylation in T Lymphocytes. J Immunol. 2000;164(4):1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- 23.Shenje J., Lai R.P., Ross I.L., Mayosi B.M., Wilkinson R.J., Ntsekhe M., et al. Effect of prednisolone on inflammatory markers in pericardial tuberculosis: A pilot study. IJC Hear Vasc. 2018;18:104–108. doi: 10.1016/j.ijcha.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews K., Deffur A., Ntsekhe M., Syed F., Russell J.B.W., Tibazarwa K., et al. A compartmentalized profibrotic immune response characterizes pericardial tuberculosis, irrespective of HIV-1 infection. Am J Respir Crit Care Med. 2015;192(12):1518–1521. doi: 10.1164/rccm.201504-0683LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaisson R.E., Post W.S. Immunotherapy for tuberculous pericarditis. N Engl J Med. 2014;371(12):1155–1157. doi: 10.1056/NEJMe1409356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.