Abstract
An aerobic enrichment culture was developed by using vinyl chloride (VC) as the sole organic carbon and electron donor source. VC concentrations as high as 7.3 mM were biodegraded without apparent inhibition. VC use did not occur when nitrate was provided as the electron acceptor. A gram-negative, rod-shaped, motile isolate was obtained from the enrichment culture and identified based on biochemical characteristics and the sequence of its 16S rRNA gene as Pseudomonas aeruginosa, designated strain MF1. The observed yield of MF1 when it was grown on VC was 0.20 mg of total suspended solids (TSS)/mg of VC. Ethene, acetate, glyoxylate, and glycolate also served as growth substrates, while ethane, chloroacetate, glycolaldehyde, and phenol did not. Stoichiometric release of chloride and minimal accumulation of soluble metabolites following VC consumption indicated that the predominant fate for VC is mineralization and incorporation into cell material. MF1 resumed consumption of VC after at least 24 days when none was provided, unlike various mycobacteria that lost their VC-degrading ability after brief periods in the absence of VC. When deprived of oxygen for 2.5 days, MF1 did not regain the ability to grow on VC, and a portion of the VC was transformed into VC-epoxide. Acetylene inhibited VC consumption by MF1, suggesting the involvement of a monooxygenase in the initial step of VC metabolism. The maximum specific VC utilization rate for MF1 was 0.41 μmol of VC/mg of TSS/day, the maximum specific growth rate was 0.0048/day, and the Monod half-saturation coefficient was 0.26 μM. A higher yield and faster kinetics occurred when MF1 grew on ethene. When grown on ethene, MF1 was able to switch to VC as a substrate without a lag. It therefore appears feasible to grow MF1 on a nontoxic substrate and then apply it to environments that do not exhibit a capacity for aerobic biodegradation of VC.
Contamination of groundwater with vinyl chloride (VC) occurs primarily via anaerobic reductive dechlorination of tetrachloroethene, trichloroethene, and 1,1,1-trichloroethane (45). The maximum contaminant level for VC in drinking water is 2 μg/liter, which is lower than for any other volatile organic compound (34). This is consistent with the fact that VC is a known human carcinogen. Reductive dechlorination of VC to ethene (11, 16) and anaerobic oxidation of VC under iron-reducing and methanogenic conditions (4, 6) often occur at relatively low rates. The potential for persistence of VC has long been a concern with the exclusive reliance on anaerobic dechlorination as a method for groundwater remediation.
In contrast, it is generally accepted that VC is readily biodegradable under aerobic conditions. Cometabolism of VC has been demonstrated with numerous primary substrates, including ethene (17, 28), ethane (17), methane (8, 12), propane (30, 32), propylene (14), isoprene (15), 3-chloropropanol (8), and ammonia (37, 44). Under such conditions, cometabolism of VC occurs faster and with less apparent toxicity than cometabolism of more chlorinated alkenes. Aerobic biodegradation of VC during microcosm studies has also been widely reported, although it is not clear if the presence of organic matter other than VC served the role of a primary substrate (5, 9, 13). In spite of its apparent aerobic biodegradability, only a few organisms with the ability to use VC as a sole substrate capable of supporting growth have been isolated (23–25). These isolates have proven to be unstable in their sustained use of VC. The absence of VC for even short periods (i.e., less than 1 day) results in a complete loss of their ability to resume VC biodegradation (25). We hypothesized that other isolates which are capable of more robust use of VC as a sole organic substrate exist.
The isolate we obtained is capable of using VC as a growth substrate under aerobic conditions and resumes use of VC after periods of at least 24 days when none is present. In addition, we report on the kinetics of VC utilization (yield, maximum specific utilization rate, growth rate, and Monod half-saturation coefficient), growth of the isolate on other substrates (including ethene and acetate), the effect of oxygen deprivation on VC utilization, and the likely involvement of a monooxygenase in initiating VC biodegradation.
(Some preliminary results of this study were presented at the 99th Annual Meeting of the American Society for Microbiology, Chicago, Ill., 1999.)
MATERIALS AND METHODS
Chemicals and medium.
VC gas (99.5%, containing <0.5% phenol to inhibit polymerization) was obtained from Aldrich; ethene (99.9%) and ethane (99.9%) were obtained from Matheson. All other chemicals used were of reagent grade. VC-epoxide was synthesized by reacting VC with 3-chloroperoxybenzoic acid dissolved in chloroform-d (27). The epoxide was identified by gas chromatographic analysis of headspace samples (see below) and comparison of peaks in vials with and without VC.
The minimal salts medium (MSM) described by Hartmans et al. (25) was used for suspended cultures, but the amount of (NH4)2SO4 was reduced to 0.67 g/liter. No vitamins or other complex growth factors were added to the MSM.
Analytical methods.
Experiments involving VC, ethene, and ethane were performed with 70- and 160-ml serum bottles (Wheaton) sealed with slotted gray butyl rubber septa (diameter, 20 mm; Wheaton) and aluminum crimp caps. Autoclaved controls (121°C for 15 min) were used to evaluate abiotic losses, including adsorption and diffusion through the septa.
Consumption of VC, ethene, and ethane was monitored by gas chromatographic analysis of headspace samples (0.1-ml samples from 70-ml bottles; 0.5-ml samples from 160-ml bottles) (16). A Hewlett-Packard 5890 Series II gas chromatograph was used; it was equipped with a flame ionization detector and a 2.44-m by 3.175-mm column packed with 1% SP-1000 on 60/80 Carbopak B (Supelco). The gas chromatograph response to a headspace sample was calibrated to give the total mass of the compound (M) in that bottle (20). Assuming the headspace and aqueous phases were in equilibrium, the total mass present was converted to an aqueous-phase concentration with the following equation:
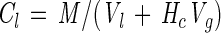 |
1 |
where Cl is the concentration in the aqueous phase (in micromolar units), M is the total mass present (in micromoles per bottle), Vl is the volume of the liquid in the bottle (in liters), Vg is the volume of the headspace in the bottle (in liters), and Hc is the Henry's constant (gas concentration [in moles per cubic meter]/aqueous concentration [in moles per cubic meter]) at 23°C (0.925 for VC and 7.24 for ethene [17]). Aqueous-phase detection limits were 19 nM for VC and 2.6 nM for ethene. The validity of assuming equilibrium between headspace and aqueous phases was verified during kinetic tests (see below).
Chloride ion concentrations were measured with an ion-selective electrode (Orion) attached to a pH-millivolt meter (Corning). A response curve was constructed using NaCl standards ranging from 0.29 to 30 mM, prepared in MSM. The reproducibility of the electrode in this concentration range was ±1.9%. Matrix effects were less than 10%, based on standard additions of NaCl to samples (1).
Standard methods (1) were used to determine total suspended solids (TSS) and volatile suspended solids (VSS). Soluble chemical oxygen demand (COD) was measured with a Hach kit (range, 0 to 150 mg/liter). Samples for soluble COD were prepared by filtration (filter pore size, 0.45 μm; Gellman). Acetate use was monitored on a high-performance liquid chromatograph (Waters) equipped with a Supelcogel H column (25 cm by 4.6 mm; Supelco), using 0.1% phosphoric acid as the mobile phase.
Culture maintenance, isolation, and identification.
Routine maintenance of all cultures included purging the headspace of bottles with pure oxygen between feedings and adjusting the pH to 7.1 with 8 M NaOH. An isolate capable of growth on VC was obtained from an enrichment culture by streaking it onto trypticase soy agar plates (BBL) incubated at room temperature (23°C). Creamy-white colonies that grew after 24 h were placed into sterile liquid medium and fed VC. After the liquid cultures consumed several additions of VC and exhibited a substantial increase in optical density, additional transfers into fresh medium were made with VC as the only carbon source.
The BBL CRYSTAL identification system was used to characterize the VC-grown isolate by subculturing it on Luria-Bertani enrichment plates (Difco), followed by an overnight incubation at 37°C under aerobic conditions. Single colonies (less than 12 h old) with diameters between 2 and 3 mm were added aseptically to BBL inoculum fluid and vortexed for 10 to 30 s at maximum speed. The inoculum fluid (2 ml) was added to the base plate and incubated at 40 to 60% humidity for 18 h. Reactions were read using the BBL CRYSTAL panel viewer, and results were compared to the BBL identification chart.
Chromosomal-DNA preparation, PCR, and sequencing applications.
For chromosomal-DNA preparations, a 1.5-ml sample of strain MF1 grown in the presence of VC as the sole carbon source was pelleted with a microcentrifuge (model 5415C; Eppendorf) at 14,000 rpm for 10 min, and DNA was extracted using a Wizard genomic DNA purification kit (catalog no. A1120; Promega). Preparations were analyzed on a 1% agarose gel containing 0.5 μg of ethidium bromide per ml. The 16S rRNA gene was amplified by PCR using the forward (5′-TGGAGAGTTTGATCCTGGCTCAGATTGAACGCT-3′) and reverse (5′-TACGGCTACCTTGTTACGACTTCACCCCAGTCA-3′) primers (20 pmol/μl) corresponding to Escherichia coli positions 005 and 1540, respectively. Target DNA (25 ng) was cycled once on a Tetrad thermocycler (model PTC-225; MJ Research) at 98°C for 3 min and then for 30 times at 98°C for 2 min, 50°C for 2 min, and 72°C for 2 min, followed by a 10-min extension at 72°C. Samples were analyzed on a 1.2% agarose gel containing 0.5 μg of ethidium bromide per μl. Reaction mixtures were purified to remove excess buffer, nucleotides, and enzyme by using a PCR purification kit (catalog no. 28104; Qiagen).
PCR products were sequenced using the previously described primers by combining 8 μl of Dye Terminator sequencing mix (Perkin-Elmer), 4 μl of template DNA (0.5 μg), and 1 μl of primer (3.0 pmol total). Samples were reacted on the thermocycler and analyzed on a sequencer (model 377XL DNA sequencer; Applied Biosystems). Cycling conditions were one cycle at 96°C for 60 s and then 25 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. Reactions were precipitated by adding 80 μl of 70% ethanol containing 0.5 mM MgCl2, followed by a 10-min incubation at room temperature without light exposure. Samples were centrifuged (as described above; 14,000 rpm, 10 min) and resuspended in 80 μl of 70% ethanol for 10 min at room temperature. Reaction mixtures were pelleted (as described above; 14,000 rpm, 5 min) and placed in a speed vacuum (model SC210A; Sevant) for 10 min on medium heat to remove residual ethanol.
Sequencing data were edited, assembled, and searched for open reading frames (Sequencer 4.0; Gene Codon Corp.). Additional primers for gap filling were designed using the program Oligo 4.0 (Molecular Biology Insights), and results were analyzed for protein and nucleic acid homology by comparison against sequences in the GenBank database.
Growth experiments.
Growth of the isolate on VC, ethene, and ethane was evaluated with 160-ml serum bottles that were modified by connecting a 1-cm-inside-diameter test tube at a right angle to the side of each bottle near the base, resulting in a final bottle volume of 173 ml. These modified serum bottles resemble culture flasks with a side arm (e.g., Bellco Biotechnology or Ace Glass), making it possible to monitor growth by determining optical density at 620 nm (Milton Roy Spec 20D spectrophotometer). Triplicate serum bottles containing 110 ml of sterile medium were used for all treatments, including controls.
Growth of the isolate on 20 mM sodium acetate, sodium chloroacetate, sodium glycolate, and sodium glyoxylate was tested with 250-ml Erlenmeyer flasks containing 100 ml of sterile medium. Two flasks containing live culture and one autoclaved control were established for each substrate. Due to the volatility of glycolaldehyde and phenol, growth on these substrates was tested in sealed 160-ml serum bottles containing 30 and 60 ml of medium, respectively, and a headspace of pure oxygen.
Kinetic experiments.
In order to provide a consistent source of culture for the kinetic experiments, the isolate was grown in a VC-fed batch reactor, consisting of a 2.3-liter glass bottle (Belco Biotechnology) capped with a gray butyl rubber septum (diameter, 30 mm), held in place with a screw cap. The bottle contained 1.5 liter of culture. Two septa were also placed in the side of the bottle. The reactor was operated in a semicontinuous mode, as follows: 100 ml of VC was added and consumed in approximately 14 days, 150 ml of culture was removed and replaced with fresh medium, the pH was adjusted, the headspace was purged with oxygen, and more VC was added. The process resulted in an average cell retention time of 140 days. Aseptic conditions were maintained during all manipulations. Between feedings, the reactor was stored at room temperature on a gyratory shaker table. After several months of operation in this mode, the biomass concentration in the reactor stabilized at approximately 162 mg of TSS/liter (138 mg of VSS/liter).
Reactor effluent samples (25 ml) were distributed to serum bottles (70 ml) for the kinetic experiments. Bottles were agitated on a gyratory shaker table (150 rpm) between headspace samplings. VC depletion curves were then evaluated using the Monod equation:
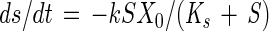 |
2 |
where S is the substrate concentration (in micromolar units) at time t (in days), calculated using equation 1 for Cl; X0 is the initial TSS concentration (in milligrams per liter); k is the maximum specific substrate utilization rate (in micromoles of substrate per milligram of TSS per day); and Ks is the Monod half-saturation coefficient (in micromolar units). The effect of mass transfer on the evaluation of kinetic parameters was determined by solving equation 2 simultaneously with the following equation (41, 42):
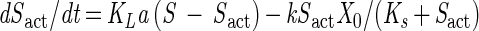 |
3 |
where Sact is the actual liquid-phase concentration of volatile substrate experienced by the culture and KLa is the mass transfer coefficient (34.5 h−1 [±5.24] for VC and 55.0 h−1 [±3.85] for ethene). KLa values were measured under conditions identical to those of the kinetic experiments, using a previously described laboratory procedure (41, 42).
During kinetic tests, residual VC in the syringe from monitoring higher concentrations carried over to subsequent measurements of lower concentrations. To prevent this problem, a separate clean syringe (free of residual VC) was used during measurement of low VC concentrations.
Kinetic parameters (k and Ks) were determined by a weighted, nonlinear least-squares method using the software Aquasim (38). The fit of equation 2 to kinetic data was initiated with the simplex optimization method and then fully optimized by the secant method, which reports the standard deviation of each parameter (36). Each data point was weighted with its standard deviation, which was calculated using an inference procedure (31). An iterative approach was used to arrive at a set of weights such that parameter estimates converged (31). For a given substrate, equation 2 was fitted simultaneously to multiple depletion curves to arrive at one set of parameters that described the entire data set. This procedure is in contrast to the more conventional approach of fitting each depletion curve individually and reporting arithmetically averaged parameters. Linear, absolute relative sensitivity functions for k and Ks were also calculated with Aquasim.
Maximum specific growth rates (μmax) were calculated as follows:
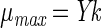 |
4 |
where Y is the yield coefficient (in milligrams of substrate per milligram of TSS). Experiments were also conducted to measure the endogenous decay rate for the VC-grown isolate, based on changes in oxygen uptake rates over time. However, the rate of change in uptake rates was too low to be detectable by this method, so endogenous decay was not included in equation 4.
The kinetics of ethene use were determined in similar tests, using biomass accumulated during the experiment in which ethene served as the sole substrate. The initial biomass concentration in the kinetic runs was 392 mg of TSS/liter (343 mg of VSS/liter). Approximately 25 μmol of ethene was added to duplicate serum bottles; the rate of depletion was monitored, and the data were fit to equation 2. The effect of mass transfer on evaluation of kinetic parameters for ethene was determined as described above for VC.
Nucleotide sequence accession number.
The complete sequence (1,526 bases) of the 16S rRNA of strain MF1 has been deposited in the GenBank database under accession no. AF193514.
RESULTS
Enrichment culture and isolation.
The culture used in this study originated from a mixed culture inoculated with activated sludge (Urbana, Illinois, Wastewater Treatment Plant) and enriched on ethane as the sole substrate, followed by several transfers into fresh medium (18). An aliquot of this enrichment culture was then fed VC as the sole substrate. Following a lag period of 80 days, it began consuming repeated additions of VC and has since been maintained through numerous transfers over a 4-year period. Duplicate subcultures of the enrichment were examined for their tolerance to VC. The amount fed was gradually increased to as much as 7.3 mM VC, with no apparent inhibition (data not shown).
The enrichment culture was not able to consume VC using nitrate as a terminal electron acceptor. Duplicate cultures supplied with KNO3 and no oxygen were initially provided with 390 μM VC. Following 180 days of incubation, the aqueous VC concentration decreased to only 340 μM, while live control cultures supplied with oxygen regularly consumed repeated additions of 400 μM VC.
Strain MF1 was isolated from the VC enrichment culture. It is gram negative, rod shaped, and motile. Evaluation of MF1 using the BBL CRYSTAL test resulted in positive reactions for mannose, galactose, p-nitrophenyl phosphate, proline nitroanilide, p-nitrophenyl phosphorylcholine, γ-l-glutamyl p-nitroanilide, urea, glycine, citrate, malonate, catalase, cytochrome c oxidase, pigmentation, and arginine (borderline). Negative reactions were observed for arabinose, sucrose, melibiose, rhamnose, sorbitol, mannitol, adonitol, inositol, p-nitrophenyl α-β-glucoside, p-nitrophenyl β-galactoside, p-nitrophenyl bis-phosphate, p-nitrophenyl xyloside, p-nitrophenyl α-arabinoside, p-nitrophenyl-β-glucuronide, p-nitrophenyl-N-acetyl glucosaminide, esculin, p-nitro-dl-phenylalanine, tetrazolium, lysine, and indole. These results match closest to those for Pseudomonas aeruginosa in the BBL CRYSTAL database (99.3% confidence).
Based on the sequence of strain MF1's 16S rRNA gene, the closest match using BLAST (GenBank) was to Pseudomonas aeruginosa. The highest degree of homology was to P. aeruginosa strain AL98, which is a potent degrader of cis-1,4-polyisoprene (29).
Growth on VC and other substrates.
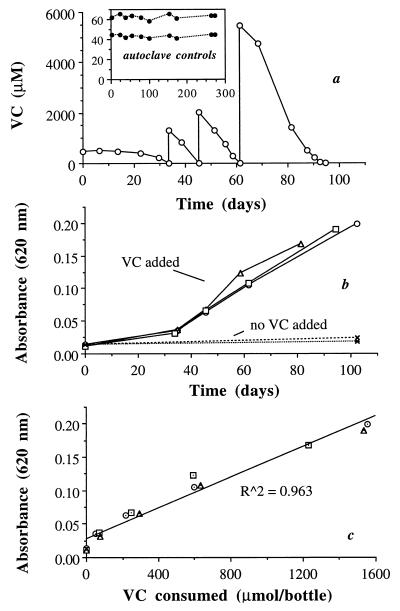
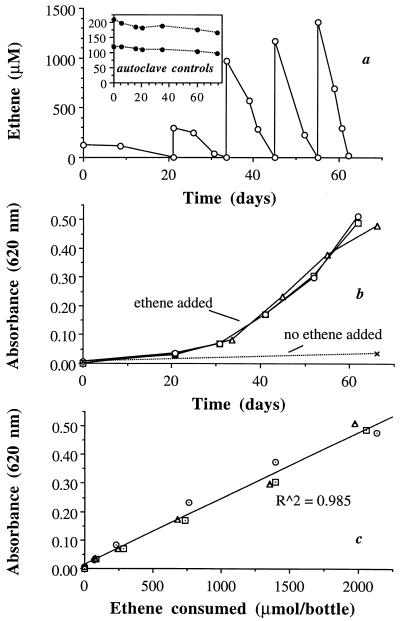
Strain MF1 used VC (Fig. 1) and ethene (Fig. 2) as sole substrates, with average observed yields (Yobs) reported in Table 1. The autoclave control results (Fig. 1a and 2a, insets) demonstrated that consumption of VC and ethene in the live bottles was a biotic process, and the gray butyl rubber septa were very effective in retaining VC and ethene. Over a 274-day period, there was no statistically significant loss of VC from the controls; loss of ethene averaged 20% over 75 days. Although gray butyl rubber septa are not appropriate for use with polychlorinated ethenes (data not shown), they are very effective at retaining VC and ethene.
FIG. 1.
Use of VC as a growth substrate by strain MF1, indicated by repeated consumption of VC (a) (results from only one of three bottles are shown; results from the other two were similar), an increase in absorbance in the bottles fed VC (b), and a direct correlation between the amount of VC consumed per bottle and an increase in absorbance (c). Results with autoclave controls are shown in the inset, demonstrating no loss of VC through the gray butyl rubber septa.
FIG. 2.
Use of ethene as a growth substrate by strain MF1, indicated by repeated consumption of ethene (a) (results from only one of three bottles are shown; results from the other two were similar), an increase in absorbance in the bottles fed ethene (b), and a direct correlation between the amount of ethene consumed per bottle and an increase in absorbance (c). Results with autoclave controls are shown in the inset, demonstrating no major loss of ethene through the gray butyl rubber septa.
TABLE 1.
Comparison of isolates capable of growth on VC and ethene as sole substrates
| Substrate | Parameter | Value for MF1a | Value for M. aurum L1b |
|---|---|---|---|
| VC | Yobs (mg of TSS/mg of VC) | 0.20 (±0.007) | 0.22 |
| k (μmol of VC/mg of TSS/day) | 0.41 (±0.003) | 270 | |
| μmax (per day) | 0.0048 | 0.96c | |
| Ks (μM) | 0.26 (±0.037) | 3.2 | |
| Ethene | Yobs (mg of TSS/mg of ethene) | 0.72 (±0.076) | 0.77 |
| k (μmol of ethene/mg of TSS/day) | 0.89 (±0.022) | NRd | |
| μmax (per day) | 0.018 | NR | |
| Ks (μM) | 3.9 (±0.38) | NR |
This study. Numbers in parentheses are 1 standard deviation.
From Hartmans and de Bont (24); standard deviations were not reported.
Ranges from 0.72 to 1.4 day−1.
NR, not reported.
In addition to growing on VC and ethene, strain MF1 grew on acetate and glyoxylate within 2 days of incubation. Growth on glycolate also occurred, but it did so after a longer incubation period (5 days). No increase in absorbance was observed in autoclaved controls or uninoculated medium. The isolate did not grow on chloroacetate, glycolaldehyde, and phenol after 2 weeks of incubation. Growth on phenol was of particular interest because it was present in the VC gas to prevent polymerization. Growth on ethane did not occur, even after more than 100 days of incubation, although a low rate of ethane consumption was noted (greater than losses from autoclaved controls).
After strain MF1 was grown on ethene for more than 60 days (Fig. 2), its ability to revert to VC as a growth substrate was tested. An initial addition of 450 μM VC was consumed in 3.1 days, without a lag period (data not shown). The amount of VC added was gradually increased to 2.6 mM. Each addition was consumed at a similar rate. This suggests a successful switch to VC as the sole substrate. However, absorbance was not monitored while VC was added, so additional work is needed to determine if strain MF1 actually does grow on VC following an initial growth period on ethene.
Extent of VC biodegradation.
The extent of VC biodegradation was determined based on the stoichiometry of chloride ion release and accumulation of soluble COD. Chloride measurements were taken at the beginning and end of the growth experiment (Fig. 1), with VC serving as the sole substrate. The net increase in chloride at the end of the growth experiment accounted for 97% of the VC consumed, indicating nearly complete dechlorination (Table 2).
TABLE 2.
Percent chloride recovered following growth of strain MF1 on VC
| Bottle | Mass of Cl− (mmol/bottle)
|
VC consumed (mmol/bottle) | Cl− recovered (%)a | |
|---|---|---|---|---|
| Initial | Final | |||
| 1 | 0.1028 | 1.6030 | 1.5528 | 96.6 |
| 2 | 0.0843 | 1.2994 | 1.2280 | 98.9 |
| 3 | 0.0969 | 1.5644 | 1.5363 | 95.5 |
| Avg ± SD | 97. ± 1.8 | |||
(Final Cl− mass − initial Cl− mass)/(VC consumed) · 100. The average percentage of Cl− consumed ± the standard deviation was 97.0 ± 1.8.
The 2.3-liter reactor was used to evaluate how much of the VC fed during each cycle was converted to soluble organic products. This was done by comparing the COD of the VC consumed to the amount of soluble COD in the reactor effluent (the balance was presumably oxidized to CO2 or converted to cells). At the end of one feeding cycle, gas chromatographic measurements indicated that all of the VC added was consumed (in the headspace and liquid phase, which was in equilibrium with the headspace), and no other volatile compounds were detected. At this point, the soluble COD in the reactor effluent was 49.3 (±0.94) mg/liter. After another addition of VC (100 ml, equivalent to 357 mg of COD) was consumed, the soluble COD in the reactor effluent was 48.5 (±4.5) mg/liter, indicating no significant increase in concentration. These measurements were made when the reactor was operating in a steady-state mode (i.e., no significant changes in TSS or the rate of VC consumption). Therefore, based on an effluent soluble-COD concentration of 49 mg/liter and removal of 0.15 liter during each feeding cycle, the amount of soluble COD removed from the reactor each cycle was 7.4 mg (49 mg/liter × 0.15 liter). This represents only 2.3% of the COD fed as VC (100 ml = 357 mg of COD), indicating that 97.7% was mineralized or incorporated into cells. Thus, the chloride stoichiometry and soluble-COD results indicate nearly complete biodegradation of VC when it was used as a sole substrate.
Starvation experiments.
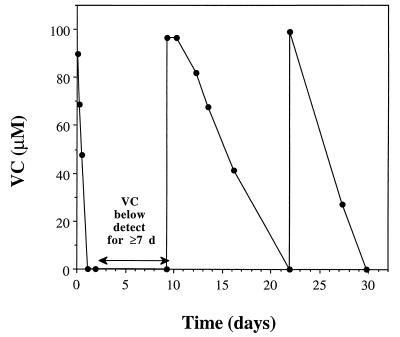
The ability of MF1 to resume biodegradation of VC after various periods of VC starvation was examined. Three sets of duplicate cultures were fed VC (25-ml culture in 70-ml serum bottle). Once the VC was consumed below the detectable level, none was added for 1, 3, or 7 days, although oxygen was always present in the bottles. When VC was added again, all of the bottles resumed use of VC, although the longer the starvation period, the lower the rate of biodegradation following resumption of VC feeding. Results for the 7-day starvation period are shown in Fig. 3.
FIG. 3.
Recovery of VC utilization by strain MF1 after a 7-day period when VC was below the detectable limit. The results shown are for a single serum bottle; similar results were obtained for a duplicate bottle.
Although VC levels were below detection when the cultures described above were starved, it is conceivable that trace amounts of VC or a metabolite remained in the medium and kept the culture induced for VC biodegradation. To investigate this possibility, the starvation experiment was repeated with another set of duplicate bottles. This time, however, once VC levels were below detection, the cultures were centrifuged (12,100 × g for 10 min), decanted, washed with MSM to remove any traces of VC or soluble metabolites, and then resuspended in MSM. After a 24-day starvation period, VC was added (115 μM). Following a lag of approximately 15 days, biodegradation of the VC resumed and all of it was completely consumed within 95 days, confirming the ability of MF1 to withstand extended periods of VC starvation.
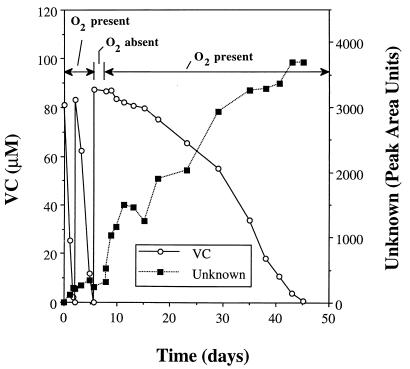
The effect of oxygen deprivation was also examined. Three sets of duplicate cultures rapidly consumed two initial doses of VC when it was added along with oxygen (Fig. 4). The bottles were then purged with N2 to remove residual oxygen, more VC was added, and the bottles were stored in an anaerobic glove box for various lengths of time (2.5, 5, or 9 days). In the absence of oxygen, no VC was consumed and no other volatile products were formed. At the end of their respective deprivation periods, oxygen (5 ml) was injected into the headspace of each culture. VC use resumed in the cultures deprived of oxygen for 2.5 days but at a much lower rate (Fig. 4). A second addition of VC (95 μM) was made on day 45, but only 23 μM was consumed by day 90 (data not shown). VC use did not recover in cultures deprived of oxygen for 5 and 9 days.
FIG. 4.
Effect of oxygen deprivation for 2.5 days on VC utilization by strain MF1. The results shown are for a single serum bottle; similar results were obtained for a duplicate bottle.
When oxygen was added after 2.5 days of depravation, VC consumption was accompanied by significant accumulation of an unknown volatile compound (Fig. 4). The unknown was presumptively identified as VC-epoxide, based on its coelution (retention time, 3.10 min) with chemically synthesized VC-epoxide. Its concentration was not high enough to permit identification by mass spectrometry.
A similar oxygen deprivation experiment was conducted with the enrichment culture. Withholding oxygen for more than 2 days significantly lowered the rate of VC use when oxygen was added back. However, in one set of bottles that were deprived of oxygen for 1 day, repeated and rapid consumption of VC did resume. In these duplicate bottles, the same unknown also accumulated shortly after oxygen was added back and VC degradation resumed. As VC use was reestablished, the amount of VC-epoxide declined to a level below detection within several days (data not shown).
Kinetic experiments.
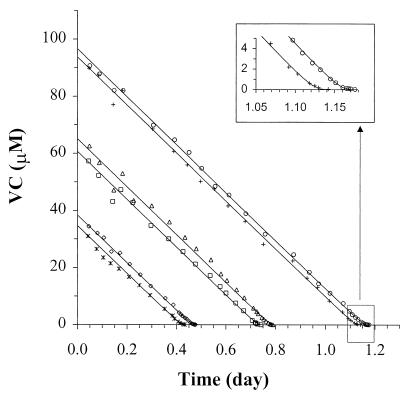
The kinetics of VC utilization of strain MF1 were evaluated using batch depletion data (Fig. 5). Initial VC concentrations were set high enough to demonstrate a maximum rate of utilization (i.e., zero order) but low enough to avoid significant growth. For the highest initial concentration, the increase in biomass was estimated to be only 2% (based on an initial biomass concentration of 205 mg of TSS/liter and the Yobs reported in Table 1). The frequency of sampling was increased with time as the rate of depletion slowed, in order to adequately characterize the nonlinear portion of the curve. Simultaneously fitting all of the depletion data to equation 2 resulted in the k and Ks values shown in Table 1, along with the calculated value for μmax using equation 4. Similar experiments were conducted with ethene as the growth substrate, with the resulting kinetic parameters shown in Table 1. Numerically computed sensitivity functions for k and Ks were not multiples of one another (data not shown), indicating that both parameters were uniquely identifiable from the experimental data (39).
FIG. 5.
VC batch depletion results used to determine k and Ks for strain MF1. Symbols represent data from six separate cultures fed various initial concentrations of VC; lines represent a simultaneous fit of equation 2 to the entire data set. The inset shows depletion at low VC concentrations for two of the bottles.
The aqueous-phase data used to determine k and Ks were calculated (using equation 1) based on the total masses of VC and ethene in the serum bottles, as determined by analysis of gas-phase samples. This approach assumes that the gas and aqueous phases are continuously in equilibrium, which would not be valid if the rate of mass transfer of the volatile substrate between phases was much lower than the rate of biodegradation. To evaluate this possibility, the solution to equation 3 (which includes biodegradation and mass transfer) was compared to the fit of equation 2 (which assumes equilibrium). In all cases, the curves for each model are indistinguishable after approximately 0.005 days, well before the first data points were measured for either VC or ethene (data not shown). This result demonstrated that the equilibrium assumption used to calculate aqueous-phase concentrations was appropriate and that mass transfer did not affect the evaluation of k or Ks.
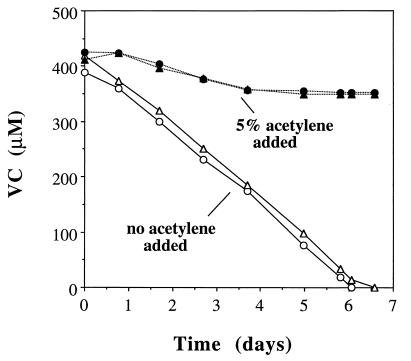
Effect of acetylene.
The presence of monooxygenase activity in MF1 was examined using acetylene as an inhibitor. Duplicate cultures without acetylene added consumed a single addition of VC within 6 days, while another set of duplicates fed the same amount of VC plus 5% acetylene consumed less than 10% of the VC after 13 days (Fig. 6). In order to evaluate potential effects of acetylene on metabolic processes other than monooxygenase activity, two sets of duplicate cultures were fed acetate (20 mM) as the sole substrate, one of which also received 5% acetylene. After 1.5 days of incubation, the absorbance (620 nm) in both sets of bottles was 0.45 (±0.016), indicating no apparent inhibitory effect of acetylene during growth on acetate.
FIG. 6.
Effect of acetylene on biodegradation of VC by strain MF1.
DISCUSSION
Strain MF1 appears to be the first Pseudomonas sp. reported that can use VC as a growth substrate. Castro et al. (8) demonstrated biodegradation of VC by a Pseudomonas sp., but the culture first had to be grown on 3-chloroproponol as the primary substrate. Cometabolism of VC began with a direct hydroxylation of the C-Cl bond to produce acetaldehyde. This finding is in contrast to the likely involvement of a monooxygenase in the initial step of VC metabolism by MF1.
M. aurum L1 is the best characterized of previously described isolates that use VC as a growth substrate (24). A comparison of MF1 and L1 reveals several differences and similarities (Table 1). Both organisms are able to grow on VC and ethene, with comparable yields on each substrate. However, the μmax for L1 grown on VC is several orders of magnitude higher, while the Ks for MF1 is approximately an order of magnitude lower. From this perspective, L1 may be viewed as a “μmax strategist” (40), having a competitive advantage at higher VC concentrations. MF1 may be viewed as a “Ks strategist” (40), because its lower Ks allows it to utilize lower concentrations of VC. Similar comparisons have been made between other organisms that use 1,2-dichloroethane (43) and dichloromethane (19) as growth substrates.
It should be noted that the kinetic parameters reported in Table 1 were measured under extant conditions (21), i.e., the ratio of substrate to biomass (on an electron equivalent basis) was comparatively low. The physiological state of cells used in extant tests has an impact on the rate of substrate depletion during batch tests. It is likely that some of the differences in k and Ks shown in Table 1 are attributable to differences in how MF1 and L1 were grown prior to measurement of the parameters. Even this consideration, however, is unlikely to alter the conclusion that MF1 grows much more slowly than L1 and has a lower Ks with VC.
L1 and MF1 also differ significantly in their ability to resume VC biodegradation following an absence of the substrate. L1 completely lost its ability to metabolize VC when addition to a reactor was discontinued for as little as 9 h (25). When addition of VC resumed, VC-epoxide accumulated, presumably to a toxic level that prevented further growth (24). In contrast, MF1 resumed VC utilization after 7 days without VC and VC-epoxide did not accumulate when VC was added after the starvation period. Depriving MF1 of oxygen for several days had a much more severe impact on resumption of VC utilization and did result in an accumulation of VC-epoxide. The ability of an organism to withstand periods of electron donor or acceptor starvation is an important factor for environmental applications, including biofiltration and in situ bioremediation.
The pathway for VC metabolism by MF1 is not yet known. VC-epoxide formation, a lack of VC metabolism in the absence of oxygen, and inhibition of VC use by acetylene strongly suggest that a monooxygenase is involved in the first metabolic step. Acetylene inhibition of monooxygenases has been demonstrated with a number of other substrates (3, 35). It is less clear how VC-epoxide is then transformed, although tests with several possible products provides some insight. Rearrangement to chloroacetaldehyde and oxidation to chloroacetate (22, 33) seems unlikely, since MF1 does not grow on chloroacetate. Hydrolysis and dechlorination of VC-epoxide to glycolaldehyde has been observed during cometabolic transformation of VC by Methylosinus trichosporium Ob3b (7) but is unlikely with MF1, since MF1 does not grow on glycolaldehyde. Another possibility is direct conversion of VC-epoxide to acetyl coenzyme A. A similar transformation involving epoxyethane dehydrogenase has been demonstrated during growth of Mycobacterium sp. E20 on ethene (10). More studies are needed to clarify the metabolic pathway utilized by MF1 when it grows on VC.
The results shown in Table 1 for yields on VC and ethene are based on mass of cells per mass of substrate. Since VC is more oxidized than ethene, a more equitable comparison is based on electron equivalents. Expressed in this way, Yobs for MF1 is 0.22 eq of biomass per eq of VC, versus 0.30 eq of biomass per eq of ethene. Even on this basis, the yield on VC remains lower than on ethene, although the difference is smaller. The reason for this is not yet known, but the results suggest that there is a substantial energetic cost associated with VC metabolism, perhaps due to repair of proteins that become alkylated by VC-epoxide (2, 22, 26). Furthermore, the lower yield on VC than on ethene suggests that neither MF1 nor L1 have a mechanism to conserve the substantial free energy available from breaking the carbon-chlorine bond.
Whether or not the enrichment process that led to isolation of MF1 was purposeful or fortuitous requires further investigation. The process began with ethane as the sole substrate in order to examine the potential for ethane-promoted cometabolism of VC (18). One of the treatments during these experiments involved addition of VC alone. These bottles later started consuming repeated additions of VC in the absence of any added ethane and became the source of enrichment culture from which MF1 was isolated on VC as the sole substrate. How MF1 was able to survive during the extended enrichment process on ethane is not yet clear, especially in light of the fact that MF1 does not grow on ethane. We previously reported the same phenomenon with a different ethane enrichment culture capable of using VC as a sole substrate, although in that study we did not pursue an isolate from the enrichment (17). The source of inoculum for both ethane enrichment cultures was the same activated sludge reactor, although the samples were taken 2 years apart. We are currently exploring the potential for obtaining additional isolates capable of growth on VC as a sole substrate by beginning with an ethene enrichment culture, as well as by using VC from the start.
The ability of MF1 to grow on ethene and then switch to VC has implications for possible use of this organism in bioaugmentation of contaminated groundwater or inoculation of biofilters. Given its toxicity, relatively high cost in neat form, and low Yobs, VC is a poor substrate for growing an initial source of biomass. Ethene is less costly and far less toxic, and the apparent absence of a lag period when switching to VC makes it a preferential substrate.
ACKNOWLEDGMENTS
James M. Gossett generously provided guidance on determining the impact of mass transfer on the estimation of kinetic coefficients for volatile compounds. Michel Boufadel and Herman Senter provided helpful discussion on the nonlinear parameter estimations. The assistance of James Cashwell in measuring acetate is gratefully acknowledged.
This research was supported in part by a grant from the U.S. Environmental Protection Agency.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 16th ed. Washington, D.C.: American Public Health Association; 1989. [Google Scholar]
- 2.Barbin A, Brésil H, Croisy A, Jacquignon P, Malaveille C, Montesan R, Bartsch H. Liver-microsome-mediated formation of alkylating agents from vinyl bromide and vinyl chloride. Biochem Biophys Res Commun. 1975;67:596–603. doi: 10.1016/0006-291x(75)90854-2. [DOI] [PubMed] [Google Scholar]
- 3.Bedard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley P M, Chapelle F H. Kinetics of DCE and VC mineralization under methanogenic and Fe(III)-reducing conditions. Environ Sci Technol. 1997;31:2692–2696. [Google Scholar]
- 5.Bradley P M, Chapelle F H. Effect of contaminant concentration on aerobic microbial mineralization of DCE and VC in stream-bed sediments. Environ Sci Technol. 1998;32:553–557. [Google Scholar]
- 6.Bradley P M, Chapelle F H. Methane as a product of chloroethene biodegradation under methanogenic conditions. Environ Sci Technol. 1999;33:653–656. [Google Scholar]
- 7.Castro C E, Riebeth D M, Belser N O. Biodehalogenation: the metabolism of vinyl chloride by Methylosinus trichosporium OB-3b. A sequential oxidative and reductive pathway through chloroethylene oxide. Environ Toxicol Chem. 1992;11:749–755. [Google Scholar]
- 8.Castro C E, Wade R S, Riebeth D M, Bartnicki E W, Belser N O. Biodehalogenation: rapid metabolism of vinyl chloride by a soil Pseudomonas sp. direct hydrolysis of a vinyl C-Cl bond. Environ Toxicol Chem. 1992;11:757–764. [Google Scholar]
- 9.Davis J W, Carpenter C L. Aerobic biodegradation of vinyl chloride in groundwater samples. Appl Environ Microbiol. 1990;56:3878–3880. doi: 10.1128/aem.56.12.3878-3880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bont J A M, Harder W. Metabolism of ethylene by Mycobacterium E20. FEMS Microbiol Lett. 1978;3:89–93. [Google Scholar]
- 11.Distefano T D. The effect of tetrachloroethene on biological dechlorination of vinyl chloride: potential implication for natural bioattenuation. Water Res. 1999;33:1688–1694. [Google Scholar]
- 12.Dolan M E, McCarty P L. Small-column microcosm for assessing methane-stimulated vinyl chloride transformation in aquifer samples. Environ Sci Technol. 1995;29:1892–1897. doi: 10.1021/es00008a005. [DOI] [PubMed] [Google Scholar]
- 13.Edwards E A, Cox E E. Field and laboratory studies of sequential anaerobic-aerobic chlorinated solvent biodegradation. In: Alleman B C, Leeson A, editors. In situ and on-site bioremediation. New Orleans, La: Battelle Press; 1997. pp. 261–265. [Google Scholar]
- 14.Ensign S, Hyman M, Arp D. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene grown Xanthobacter strain. Appl Environ Microbiol. 1992;58:3038–3046. doi: 10.1128/aem.58.9.3038-3046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewers J, Freier-Schroder D, Knackmuss H J. Selection of trichloroethylene (TCE) degrading bacteria that resist inactivation by TCE. Arch Microbiol. 1990;154:410–413. doi: 10.1007/BF00276540. [DOI] [PubMed] [Google Scholar]
- 16.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman D L, Herz S D. Use of ethylene and ethane as primary substrates for aerobic cometabolism of vinyl chloride. Water Environ Res. 1996;68:320–328. [Google Scholar]
- 18.Freedman D L, Verce M F. Ethene- and ethane-promoted biodegradation of vinyl chloride. In: Alleman B C, Leeson A, editors. In situ and on-site bioremediation. Vol. 3. Columbus, Ohio: Battelle Press; 1997. pp. 255–260. [Google Scholar]
- 19.Gisi D, Willi L, Traber H, Leisinger T, Vuilleumier S. Effects of bacterial host and dichloromethane dehalogenase on the competitiveness of methylotrophic bacteria growing with dichloromethane. Appl Environ Microbiol. 1998;64:1194–1202. doi: 10.1128/aem.64.4.1194-1202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gossett J M. Measurement of Henry's Law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 21.Grady C P L J, Smets B F, Barbeau D S. Variability in kinetic parameter estimates: possible causes and a proposed terminology. Water Res. 1996;30:742–748. [Google Scholar]
- 22.Guengerich F P, Crawford W M, Watanabe P G. Activation of vinyl chloride to covalently bound metabolites: roles of 2-chloroethylene oxide and 2-chloroacetaldehyde. Biochemistry. 1979;18:5177–5182. doi: 10.1021/bi00590a023. [DOI] [PubMed] [Google Scholar]
- 23.Hartmans S, de Bont J, Tramper J, Luyben K. Bacterial degradation of vinyl chloride. Biotechnol Lett. 1985;7:383–388. [Google Scholar]
- 24.Hartmans S, de Bont J A M. Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Appl Environ Microbiol. 1992;58:1220–1226. doi: 10.1128/aem.58.4.1220-1226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmans S, Kaptein A, Tramper J, de Bont J A M. Characterization of a Mycobacterium sp. and Xanthobacter sp. for the removal of vinyl chloride and 1,2-dichloroethane from waste gases. Appl Microbiol Biotechnol. 1992;37:796–801. [Google Scholar]
- 26.Henschler D. Halogenated alkenes and alkynes. In: Anders W, editor. Bioactivation of foreign compounds. New York, N.Y: Academic Press; 1985. pp. 317–347. [Google Scholar]
- 27.Kline S A, Solomon J J, van Duuren B L. Synthesis and reactions of chloralkene epoxides. J Org Chem. 1978;43:3596–3600. [Google Scholar]
- 28.Koziollek P, Bryniok D, Knackmuss H J. Ethene as an auxiliary substrate for the cooxidation of cis-1,2-dichloroethene and vinyl chloride. Arch Microbiol. 1999;172:240–246. doi: 10.1007/s002030050766. [DOI] [PubMed] [Google Scholar]
- 29.Linos A, Reichelt R, Keller U, Steinbuchel A. A gram-negative bacterium, identified as Pseudomonas aeruginosa AL98, is a potent degrader of natural and synthetic cis-1,4-polisoprene. FEMS Microbiol Lett. 2000;182:156–161. doi: 10.1111/j.1574-6968.2000.tb08890.x. [DOI] [PubMed] [Google Scholar]
- 30.Malachowsky K J, Phelps T J, Teboli A B, Minnikin D E, White D C. Aerobic mineralization of trichloroethylene, vinyl chloride, and aromatic compounds by Rhodococcus species. Appl Environ Microbiol. 1994;60:542–548. doi: 10.1128/aem.60.2.542-548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neter J, Kutner M H, Nachtsheim C J, Wasserman W. Applied linear statistical models. Chicago, Ill: Irwin; 1996. [Google Scholar]
- 32.Phelps T J, Malachowsky K, Schram R M, White D C. Aerobic mineralization of vinyl chloride by a bacterium of the order Actinomycetales. Appl Environ Microbiol. 1991;57:1252–1254. doi: 10.1128/aem.57.4.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plugge H, Safe S. Vinyl chloride metabolism—a review. Chemosphere. 1977;6:309–325. [Google Scholar]
- 34.Pontius F W. An update of the federal regs. J Am Water Works Assoc. 1996;88:36–45. [Google Scholar]
- 35.Prior S D, Dalton H. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1985;29:105–109. [Google Scholar]
- 36.Ralston M L, Jennrich R I. Dud, a derivative-free algorithm for nonlinear least squares. Technometrics. 1978;20:7–14. [Google Scholar]
- 37.Rasche M E, Hyman M R, Arp D J. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol. 1991;57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichert P. Concepts underlying a computer program for the identification and simulation of aquatic systems. Schriftenr. 1994. EAWAG (Swiss Fed. Inst. Environ. Sci. Technol.) Report CH-8600. [Google Scholar]
- 39.Robinson J A. Determining microbial kinetic parameters using nonlinear regression analysis: advantages and limitations in microbial ecology. Adv Microb Ecol. 1985;8:61–114. [Google Scholar]
- 40.Schlegel H G, Jannasch H W. Prokaryotes and their habitats. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. New York, N.Y: Springer-Verlag; 1992. pp. 75–125. [Google Scholar]
- 41.Smatlak C R. M.S. thesis. Ithaca, N.Y: Cornell University; 1995. [Google Scholar]
- 42.Smatlak C R, Gossett J M, Zinder S H. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ Sci Technol. 1996;30:2850–2858. [Google Scholar]
- 43.van den Wijngaard A J, Wind R D, Janssen D B. Kinetics of bacterial growth on chlorinated aliphatic compounds. Appl Environ Microbiol. 1993;59:2041–2048. doi: 10.1128/aem.59.7.2041-2048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannelli T, Logan M, Arciero D M, Hooper A B. Degradation of halogenated aliphatic compounds by the ammonia-oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990;56:1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel T M, Criddle C S, McCarty P L. Transformations of halogenated aliphatic compounds. Environ Sci Technol. 1987;21:722–736. doi: 10.1021/es00162a001. [DOI] [PubMed] [Google Scholar]