Highlights
-
•
There is a distinct microbiota of the upper reproductive tract.
-
•
There are differences in the microbiota composition between the ovary, fallopian tube, and fimbriae.
-
•
Ovarian cancer appears to have a unique microbiota.
Keywords: Ovary microbiome, Fallopian tube microbiome, Upper reproductive tract microbiome, Ovarian cancer
Abstract
Objective
The microbiome of the female upper reproductive tract (URT) has not been characterized. We hypothesize that distinct bacterial species may be identified in different areas of the URT in women with or without ovarian cancers.
Methods
Postmenopausal women scheduled for salpingooophorectomy were prospectively identified. We excluded those who used antibiotics within three months of surgery or had a diagnosed gynecologic cancer. Bacteria were extracted from tissue samples of the proximal fallopian tube, fimbriae and ovaries of 10 women. Using molecular-phylogenetic methods based on the highly conserved 16S bacteria rRNA gene, we assessed the complexity of URT microbiota in tissue samples by high throughput sequencing of the V1-V3 region of the 16S gene. Sequences were processed through QIIME and an average of 69,625 reads per sample was obtained after quality filtering. Multivariate analyses were conducted using PRIMER VI software.
Results
The initial analysis of samples suggests that bacteria exist in the URT. Analysis of similarity matrix (ANOSIM) suggests that the microbiome differs in the areas examined (ANOSIM R = 0.26, p = 0.015). The microbiome differs significantly between the fallopian tube and ovary (ANOSIM R = 0.23, p = 0.02). The proximal fallopian tube microbiome also differs from the fimbriae (ANOSIM R = 0.66, p = 0.025). There were borderline differences in the microbial profiles of the specimens with and without epithelial ovarian cancer (p = 0.06).
Conclusions
We identified distinct microbiota of the ovaries and fallopian tubes with a profile unique to women with epithelial ovarian cancer. Further investigation is necessary to determine whether the microbiome is related to ovarian carcinogenesis.
1. Introduction
In the female reproductive phase of life, there are opportunities for the migration of substances between the lower and upper reproductive tract (URT): 1. Sperm are permitted passage from the vagina and ascend to the fallopian tube where conception may occur. 2. During menses, women are susceptible to the migration of organisms that cause pelvic inflammatory disease (PID), a constellation of salpingitis, oophoritis and endometritis. 3. Retrograde menstruation has been well documented and is accepted as one of the etiologies of endometriosis of the pelvis and abdomen. 4. Bacteria have been detected in the uterine cavity of healthy non-pregnant women (Swidsinski et al., 2013). 5. Bacterial infections of the amniotic cavity are responsible for 40–50% of cases of preterm delivery (Epstein et al., 2000). It is therefore unlikely that the URT—the fallopian tubes and ovaries—are sterile structures.
A vast array of microbes, characterized as communities, have been identified in numerous anatomic sites (Ding and Schloss, 2014). These microbial communities are unique to each site. The interactions within the microbiome, the compounds produced and their downstream effects on anatomic function and disease course are of scientific interest and possibly have clinical value. Microbes and the microbiota may contribute to carcinogenesis by altering the balance of proliferation and death of the host cell, perturbating immune system function, and influencing metabolism within the host (Garrett, 2015). The microbiome of the reproductive tract has been presumed to be limited to the lower genitalia, namely the external genitalia, vagina, cervix and uterus. The vaginal microbiome influences susceptibility to sexually transmitted diseases, influences the course of preterm labor and pregnancy outcomes (Fettweis et al., 2019, Lewis et al., 2017). High risk oncogenic human papilloma viruses are important in cervical carcinogenesis (Norenhag et al., 2020). Research has catalogued the placental microbiome and found that pregnancy does not occur in a sterile environment (Aagaard et al., 2014). The taxonomic profile of the placenta actually has more similarities with the non-pregnant oral microbiome than with the urogenital tract (Prince et al., 2016).
In a prior study, the endometrium of 58 predominantly Caucasian, premenopausal women was tested at the time of hysterectomy for microbiota using quantitative polymerase chain reaction (PCR) assays for 12 bacterial species: Lactobacillus iners, L crispatus, L jensenii, Gardnerella vaginalis, Atopobium vaginae, Megasphaera spp, Prevotella spp, Leptotrichia/Sneathia, BVAB1, BVAB2, BVAB3 and a broad-range 16S rRNA gene assay (Mitchell et al., 2015). Bacteria were detected in 95% of the endometrial cavity specimens; however, the quantity of colonization was lower in the upper endocervix and endometrium than the vagina. These observations support the presence of bacteria in the upper genital tract and encourage clarification of the presence and impact of the microbial environment of the fallopian tubes and ovaries (Mitchell et al., 2015). The ovarian and intratumoral microbiome in women with ovarian cancer has received recent attention and investigation (Nejman et al., 2020, Poore et al., 2020, Zhou et al., 2019, Banerjee et al., 2017, Wang et al., 2020). However, additional prospective studies across a wide range of populations are needed to further elucidate the site-specific differences of the microbiota in the URT and its role in ovarian malignancy.
Our objective was to assess and characterize the status of microbial communities in the URT, namely the ovary, fallopian tube and fimbriae in post-menopausal women. A secondary objective was to compare the microbiota of the URT in women with ovarian cancer compared to women with benign disease.
2. Methods
The School of Medicine Institutional Review Board at University of North Carolina, Chapel Hill approved this prospective study. Post-menopausal women with a uterus who were scheduled for at least a unilateral salpingo-oophorectomy were prospectively identified by review of the gynecologic surgery schedule. Women were excluded if: (1) they had received antibiotics within three months of the date of the operative procedure, (2) there was a known diagnosis of cervical or uterine cancer, (3) the operation was a risk reducing procedure for ovarian cancer or (4) the adnexal specimen was delivered through the vagina. A questionnaire that assessed reproductive history and gynecological health was administered pre-operatively.
Specimens were transferred from the abdomen to the pathology department under sterile conditions. 7 mm biopsies of the mucosal aspect of the proximal fallopian tube, fimbriae and ovarian serosa were collected and flash frozen. Subjects were classified as having either ovarian or fallopian tube cancer based on the final pathology report.
2.1. Microbiota analysis
DNA Extraction: Genomic DNA was extracted from tissue samples of the proximal fallopian tube, fimbriae and ovary using a modified protocol of the Qiagen DNeasy Blood and Tissue Kit. Briefly, tissue samples were incubated in lysozyme (20 mg/ml) and kit buffer ATL for 30 min at 37 °C, followed by the addition of proteinase K and incubation at 56 °C overnight. Samples were further processed by bead-beating in tubes containing 0.5 mm stainless steel beads (Bullet Blender, Next Advance, Averill Park, NY) for 4 min. The supernatant from each sample was combined with buffer AL and 100% ethanol, and the manufacturer’s protocol was followed for the remainder of the extraction.
Library Preparation and Sequencing: For amplicon library preparation, we amplified the V1-V3 region of the bacterial 16S rRNA using a universal reverse primer and a unique forward primer for each sample. Amplification was performed using fusion primers comprising Ion Torrent adapter 5′- CCATCTCATCCCTGCGTGTCTCCGACTCAG −3′ for the forward primer and 5′- CCTCTCTATGGGCAGTCGGTGAT −3′ for the reverse primer, and universal bacterial primer 8F 5′-AGAGTTTGATCCTGGCTCAG-3′ and 338R 5́-GCTGCCTCCCGTAGGAGT-3́. The forward primer also includes a 10 bp IonXpress™ barcode, unique to each sample. For PCR, two replicates were prepared for each sample, each containing 5x MyTaq Reaction Buffer (Bioline, Taunton, MA), 0.375 µM each of unique forward primer and universal reverse primer, MyTaq HS DNA Polymerase (Bioline, Taunton, MA) and 30 ng of DNA template. PCR was performed with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s and extension at 72 °C for 90 s, followed by a final 10 min extension at 72 °C.
PCR product visualization and clean-up was performed on 2% E-Gel Size Select (Life Technologies). To confirm proper band size, each cleaned PCR product was quantified using the Agilent 2100 Bioanalyzer and Quant-iT PicoGreen dsDNA Kit (Invitrogen), following the manufacturer’s protocol. Samples were pooled in equimolar ratios to make a library for sequencing on the Ion Torrent NGS system. The pooled library was quantified using the Bioanalyzer and Picogreen Assay. For quality control, appropriate negative and positive controls were included in the DNA extraction, PCR and sequencing steps.
Bioinformatics and Data Analysis: The 16S rRNA sequences were filtered to remove low quality reads and processed through QIIME (Caporaso et al., 2010). An average of 69,625 reads per sample was obtained after quality filtering. Sequences were assigned to operational taxonomic units (OTUs) using the Greengenes database (DeSantis et al., 2006). Multivariate analyses and bacterial diversity metrics were conducted in Qiime and PRIMER VII software (PRIMER-E, Plymouth Marine Laboratory). Bray Curtis similarity matrixes were used for nMDS and cluster analysis. Discriminating taxa between the groups were identified with Metastats, and p values were corrected for multiple hypothesis testing (White et al., 2009, Benjamini and Hochberg, 1995).
3. Results
We present data on 25 samples collected from 2 Black and 8 White postmenopausal women. Supplemental table S1 displays the sample distribution among patients. The median age at the time of the surgical procedure was 54 years old (range 44–78 years). A median of three biopsies were received per subject and were collected at the discretion of the pathologist. Two of the subjects had a diagnosis of high grade serous ovarian cancer, and biopsies of normal fallopian tube and ovary from the affected side were obtained.
3.1. Microbiota composition in upper reproductive tract
Analyses of 25 samples from the proximal fallopian tube, fimbriae and ovary found that bacteria exist in the URT of postmenopausal women. We observed significant differences between the microbiota profiles of the ovary, fallopian tube and fimbriae (Anosim R = 0.26, p = 0.015). The microbiota composition differed significantly between the fallopian tube and ovary (Anosim R = 0.23, p = 0.02) while the fallopian tube bacterial composition also differed from the fimbriated end (Anosim R = 0.66, p = 0.025). See Supplemental Figures S1A and S1B.
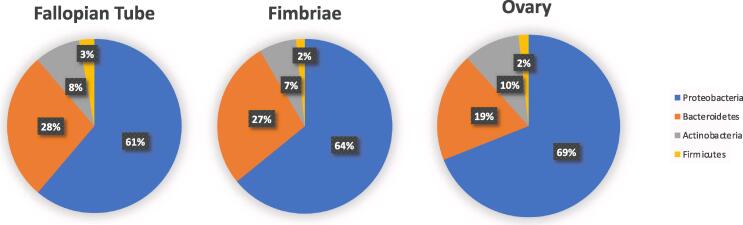
At the phylum level, the bacterial distribution in the URT consisted of Actinobacteria, Proteobacteria, Bacteoidetes, and Firmicutes, with Proteobacteria being the most abundant (Fig. 1). The abundance of Proteobacteria (p = 0.005) and Actinobacteria (p = 0.065) were higher in the fimbriae than the fallopian tube. Similarly, Bacteroidetes (p = 0.018) and Proteobacteria (p = 0.032) were more abundant in the fimbriae compared to the ovary. Compared to the ovary, the fallopian tube had increased Actinobacteria (p = 0.026) and reduced relative abundance of Bacteroidetes (p = 0.07).
Fig. 1.
Composition of microbiota in the upper reproductive tract. Relative abundance of phylum level taxa.
At the genus level, the distribution of the microbiota also varied significantly by tissue site. Key genera that differed between the ovary, fallopian tube and fimbriae are shown in a heatmap, Fig. 2. Actinoplanes, Arthrobacter, Bradyrhizobium, Gemmatimonas, Limnobacter, Roseobacter, Saccharopolyspora, and Mycobacterium sp. were differentially abundant between the ovary and the fallopian tube after correction for multiple hypothesis testing. For the fallopian tube and fimbriae, Acidovorax, Bradyrhizobium, Mesorhizobium, Mycobacterium and Ralstonia were differentially abundant after multiple testing correction.
Fig. 2.
Distribution of differentially abundant genus level microbiota in upper reproductive tract tissue. FT = fallopian tube. OV = ovary. FIM = fimbriae.
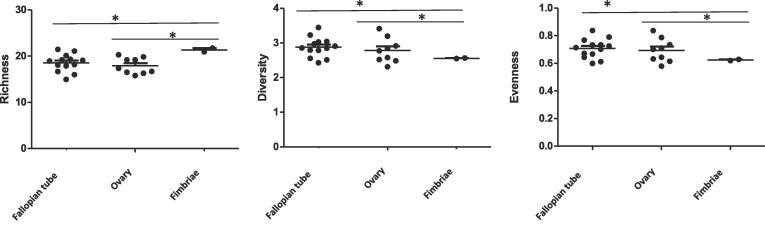
Diversity measures such as richness (abundance), evenness (distribution of the different kinds of bacteria) and diversity (combination of richness and evenness) provide additional insight into the microbial structure. We assessed microbiota diversity in the ovary, fallopian tube and fimbriae. We found significant differences in microbiota richness (p = 0.005), evenness (p = 0.009) and diversity (p = 0.001) between the fallopian tube and fimbriae. Similarly, microbiota richness was higher in the fimbriae than the ovary (p = 0.002) while evenness (p = 0.039) and diversity (p = 0.09) were lower in the ovary than the fimbriae, Fig. 3.
Fig. 3.
Comparison of microbiota richness, evenness and diversity in upper reproductive tract. *p < 0.05.
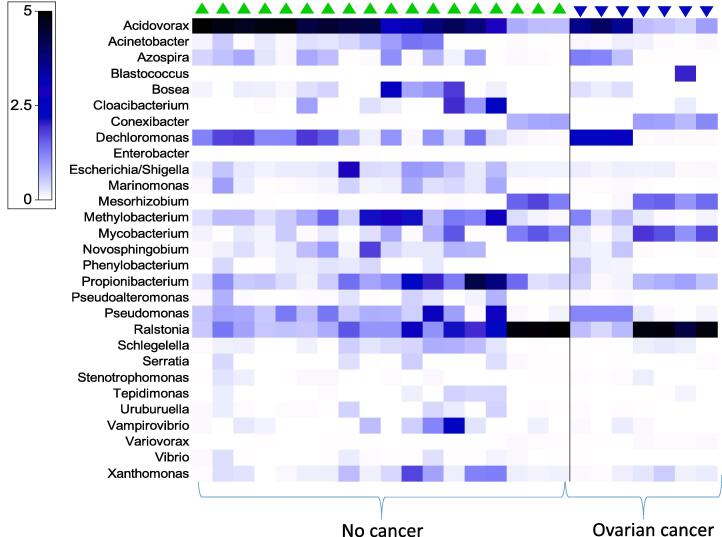
Next, we assessed whether the microbiota abundance in the URT differed by ovarian cancer status as shown in Fig. 4. Cluster analysis revealed differences nearing statistical significance (Anosim R = 0.15, p = 0.06) between the microbial profile of subjects with ovarian cancer and those without. Analysis of tissue sites by ovarian cancer status showed some significant differences in the microbiota profiles (Anosim R = 0.35, p = 0.004). The relative abundance of thirty two genus level taxa differed significantly relative to ovarian cancer status. Acidovorax, Acinetobacter, Aeromonas, Cloacibacterium, Conexibacter, Mariomonas, Methylobacterium, Propionibacterium, Pseudoalteromonas, Vibrio and Xanthomonas sp. had significantly lower relative abundance in URT tissues of ovarian cancer than control tissues without ovarian cancer. Bosea, Mesorhizobium, Mycobacterium, Ralstonia and Variovorax were significantly more abundant in URT tissues of ovarian cancer than controls after multiple testing correction.
Fig. 4.
Distribution of differentially abundant genus level upper reproductive tract microbiota by ovarian cancer status.
4. Discussion
This is a proof of principle study that demonstrates the presence of a microbiome in the fallopian tubes and ovarian surface epithelium. We identified Firmicutes, Proteobacteria, Actinobacteria and Bacteroidetes phyla in the URT. These phyla are comparable to that reported from other sites of the human body; however, the relative abundances are different with Proteobacteria being the most abundant while Firmicutes was the least common phylum in the URT. Highly abundant members of the Proteobacteria phyla such as Ralstonia, Mycobacterium, and Variovorax sp. were associated with ovarian cancer.
Although, the URT was previously thought to be sterile (Teisala, 1987), our findings along with the limited literature on the topic suggests otherwise. Pelzer et al. reported that commensal bacteria may exist in URT without evidence of infection (Pelzer et al., 2013). They observed that the ovarian follicular fluid from women undergoing in vitro fertilization contained a variety of bacteria such as Actinomyces, Staphylococcus and Bifidobacterium sp. The endometrial cavity in non-pregnant women undergoing hysterectomy also harbors low bacteria that is not associated with inflammation (Mitchell et al., 2015). Similarly, Verstraelen et al. found that the Bacteroides sp was a dominant resident of the non-pregnant endometrium (Verstraelen et al., 2016). Together, these observations support the notion that the URT is home to commensal bacteria.
We noted that the microbiota distribution differed by URT site. There were differences in microbiota composition between the ovary, fallopian tube and fimbriae. We also observed that the bacteria diversity was different between the URT sites and identified several taxa that contribute to these differences in microbiota composition. Interestingly, the microbiota abundances for several taxa were significantly reduced in URT tissues from patients with ovarian cancer compared to URT tissues from control patients without ovarian cancer. This would suggest that bacterial dysbiosis is associated with ovarian cancer, consistent with prior studies (Zhou et al., 2019, Banerjee et al., 2017).
Postmenopausal women were selected for this study to eliminate the variable hormonal influence of the menstrual cycle. To minimize distortions of the native adnexal microbiome, women who had taken antibiotics within three months of surgery were excluded as were those subjects with surgical specimens delivered through the vagina. To account for potential bias in processing of tissue samples for microbiota analysis, we included appropriate positive and negative controls in the DNA extraction, PCR amplification and sequencing steps. Positive controls included the pooled sample of known bacteria. Negative controls include water and reagent controls. These controls yielded expected results.
All specimens were transferred in a sterile container to the pathology department. Strategies to assess possible microbial contamination of the specimens via transfer from ‘sterile’ gloves, containers or instruments will need to be considered in future studies. Contamination in the pathology area is a possibility; however, if gross contamination occurred, the abundance and diversity of organisms present would have been more than detected. Finally, the small sample size limits meaningful sub-analyses.
Ravel at al. characterized the vaginal microbiome of 400 sexually active women (Ravel et al., 2011). There was almost equal representation from White, Hispanic, Asian and Black ethnic groups. The bacterial communities clustered into five groups: four were dominated by Lactobacillus iners, L. crispatus, L. gasseri, or L. jensenii. The authors noted a statistically significant difference in the proportions of each community group between the four ethnic groups (Ravel et al., 2011). It will be interesting to investigate if ethnicity is also a driver of the microbiome of the URT and whether a malignancy of the URT transforms the characteristics of the microflora of the lower genital tract, particularly the species composition of the vaginal communities.
The microbiome has a complicated role in the balancing act between human health and disease. The pathway by which organisms influence or shape a developing tumor environment or alter cellular metabolism is an evolving area of active research. Microbe driven cancers have been described, for example, Helicobacter pylori in gastric cancer and human papillomavirus in cervical, anal, head and neck cancers (Garrett, 2015). Cancers may develop over decades, and different microbes and microbiotas may participate at distinct stages of the neoplastic process. This study is an important first step in understanding the characteristics of the microbiota of the fallopian tubes, ovaries and other close structures. Based on these findings, further investigation of the unique microbiota profile of women with ovarian and fallopian tube cancers is warranted to explain these differences in the microbiota of the female genital tract and how it contributes to susceptibility of cancer and outcomes.
This work is generously supported by funding from:
-
1.
NIH – P30DK034987 Center for Gastrointestinal Biology and Disease (TK)
-
2.
NIH – RO1 CA136887 (TK)
-
3.
NIH – Program in Translational Medicine T32-CA244125 to UNC/WB
-
4.
Lineberger Comprehensive Cancer Center.
Author Contribution:
All authors had input in the writing and reviewing of the manuscript. All authors also contributed to the profiling and/or analysis of the microbiome data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
UNC CGIBD Microbiome Core for 16S sequencing. The authors acknowledge technical assistance from Dr. Larry Forney’s Lab at the University of Idaho.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.101017.
Contributor Information
Wendy R. Brewster, Email: wrbrewst@med.unc.edu.
Wesley C. Burkett, Email: wesley.burkett@unchealth.unc.edu.
Emily M. Ko, Email: Emily.ko@pennmedicine.upenn.edu.
Victoria Bae-Jump, Email: victoria_baejump@med.unc.edu.
Amber Nicole McCoy, Email: amber_mccoy@med.unc.edu.
Temitope O. Keku, Email: temitope_keku@med.unc.edu.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014;6(237) doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Tian T., Wei Z., Shih N., Feldman M.D., Alwine J.C., Coukos G., Robertson E.S. The ovarian cancer oncobiome. The ovarian cancer oncobiome. Oncotarget. 2017;8(22):36225–36245. doi: 10.18632/oncotarget.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57(1):289–300. [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T., Schloss P.D. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F.H., Goldenberg R.L., Hauth J.C., Andrews W.W. Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Fettweis J.M., Serrano M.G., Brooks J.P., Edwards D.J., Girerd P.H., Parikh H.I., Huang B., Arodz T.J., Edupuganti L., Glascock A.L., Xu J., Jimenez N.R., Vivadelli S.C., Fong S.S., Sheth N.U., Jean S., Lee V., Bokhari Y.A., Lara A.M., Mistry S.D., Duckworth R.A., Bradley S.P., Koparde V.N., Orenda X.V., Milton S.H., Rozycki S.K., Matveyev A.V., Wright M.L., Huzurbazar S.V., Jackson E.M., Smirnova E., Korlach J., Tsai Y.-C., Dickinson M.R., Brooks J.L., Drake J.I., Chaffin D.O., Sexton A.L., Gravett M.G., Rubens C.E., Wijesooriya N.R., Hendricks-Muñoz K.D., Jefferson K.K., Strauss J.F., Buck G.A. The vaginal microbiome and preterm birth. Nat. Med. 2019;25(6):1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett W.S. Cancer and the microbiota. Science. 2015;348(6230):80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F.M.T., Bernstein K.T., Aral S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017;129:643–654. doi: 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, C.M., Haick, A., Nkwopara, E., Garcia, R., Rendi, M., Agnew, K., et al., 2015. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstetr. Gynecol. 212:611.e1-.e9. [DOI] [PMC free article] [PubMed]
- Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L.T., Rotter-Maskowitz A., Weiser R., Mallel G., Gigi E., Meltser A., Douglas G.M., Kamer I., Gopalakrishnan V., Dadosh T., Levin-Zaidman S., Avnet S., Atlan T., Cooper Z.A., Arora R., Cogdill A.P., Khan M.A.W., Ologun G., Bussi Y., Weinberger A., Lotan-Pompan M., Golani O., Perry G., Rokah M., Bahar-Shany K., Rozeman E.A., Blank C.U., Ronai A., Shaoul R., Amit A., Dorfman T., Kremer R., Cohen Z.R., Harnof S., Siegal T., Yehuda-Shnaidman E., Gal-Yam E.N., Shapira H., Baldini N., Langille M.G.I., Ben-Nun A., Kaufman B., Nissan A., Golan T., Dadiani M., Levanon K., Bar J., Yust-Katz S., Barshack I., Peeper D.S., Raz D.J., Segal E., Wargo J.A., Sandbank J., Shental N., Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenhag J., Du J., Olovsson M., Verstraelen H., Engstrand L., Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127(2):171–180. doi: 10.1111/1471-0528.15854. [DOI] [PubMed] [Google Scholar]
- Pelzer, E.S., Allan, J.A., Waterhouse, M.A., Ross, T., Beagley, K.W., Knox, C.L., 2013. Microorganisms within Human Follicular Fluid: Effects on IVF. PLOS ONE. 8:e59062. [DOI] [PMC free article] [PubMed]
- Poore G.D., Kopylova E., Zhu Q., Carpenter C., Fraraccio S., Wandro S., Kosciolek T., Janssen S., Metcalf J., Song S.J., Kanbar J., Miller-Montgomery S., Heaton R., Mckay R., Patel S.P., Swafford A.D., Knight R. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567–574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prince A.L., Ma J., Kannan P.S., Alvarez M., Gisslen T., Harris R.A., Sweeney E.L., Knox C.L., Lambers D.S., Jobe A.H., Chougnet C.A., Kallapur S.G., Aagaard K.M. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 2016;214(5):627.e1–627.e16. doi: 10.1016/j.ajog.2016.01.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S.K., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., Brotman R.M., Davis C.C., Ault K., Peralta L., Forney L.J. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108(supplement_1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski, A., Verstraelen, H., Loening-Baucke, V., Swidsinski, S., Mendling, W., Halwani, Z., 2013. Presence of a Polymicrobial Endometrial Biofilm in Patients with Bacterial Vaginosis. PLOS ONE. 8:e53997. [DOI] [PMC free article] [PubMed]
- Teisala K. Endometrial microbial flora of hysterectomy specimens. Eur. J. Obstetr. Gynecol. Reprod. Biol. 1987;26(2):151–155. doi: 10.1016/0028-2243(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Verstraelen, H., Vilchez-Vargas, R., Desimpel, F., Jauregui, R., Vankeirsbilck, N., Weyers, S., et al., 2016. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 4:e1602. [DOI] [PMC free article] [PubMed]
- Wang Q.i., Zhao L., Han L.u., Fu G., Tuo X., Ma S., Li Q., Wang Y., Liang D., Tang M., Sun C., Wang Q., Song Q., Li Q. The differential distribution of bacteria between cancerous and noncancerous ovarian tissues in situ. J. Ovarian Res. 2020;13(1) doi: 10.1186/s13048-019-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J.R., Nagarajan, N., Pop, M., 2009. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. PLOS Comput. Biol. 5:e1000352. [DOI] [PMC free article] [PubMed]
- Zhou B.o., Sun C., Huang J., Xia M., Guo E., Li N.a., Lu H., Shan W., Wu Y., Li Y., Xu X., Weng D., Meng L.i., Hu J., Gao Q., Ma D., Chen G. The biodiversity Composition of Microbiome in Ovarian Carcinoma Patients. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-018-38031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.