Abstract
Aims
Cardiac transplant recipients often suffer from type 2 diabetes mellitus (T2DM) but its influence on graft failure and post-transplant mortality remains unknown. The aim of this study was to investigate the long-term effects of pre-transplant T2DM in patients after heart transplantation (HTX).
Methods
This study included a total of 376 adult patients who received HTX at Heidelberg Heart Center between 01/01/2000 and 01/10/2016. HTX recipients were stratified by diagnosis of T2DM at the time of HTX. Patients with T2DM were further subdivided by hemoglobin A1c (HbA1c ≥ 7.0%). Analysis included donor and recipient data, immunosuppressive drugs, concomitant medications, post-transplant mortality, and causes of death. Five-year post-transplant mortality was further assessed by multivariate analysis (Cox regression) and Kaplan–Meier estimator.
Results
About one-third of all HTX recipients had T2DM (121 of 376 [32.2%]). Patients with T2DM showed an increased 5-year post-transplant mortality (41.3% versus 29.8%; P = 0.027) and had a higher percentage of death due to graft failure (14.9% versus 7.8%; P = 0.035). Multivariate analysis showed T2DM (HR: 1.563; 95% CI: 1.053–2.319; P = 0.027) as an independent risk factor for 5-year mortality after HTX. Kaplan–Meier analysis showed a significantly better 5-year post-transplant survival of patients with T2DM and a HbA1c < 7.0% than patients with T2DM and a HbA1c ≥ 7.0% (68.7% versus 46.3%; P = 0.008) emphasizing the clinical relevance of a well-controlled T2DM in HTX recipients.
Conclusion
Pre-transplant T2DM is associated with higher graft failure and increased 5-year mortality after HTX.
Keywords: diabetes mellitus, graft failure, HbA1c, heart transplantation, mortality, survival
Introduction
Type 2 diabetes mellitus (T2DM) is a common comorbidity in patients with advanced heart failure and is often associated with a variety of extracardiac diseases such as obesity, impaired wound healing with increased risk of infection, thromboembolic complications, and renal dysfunction (1–8). Given these risk factors, T2DM is considered a relative contraindication for listing for heart transplantation (HTX), depending on the patient’s diabetes status and severity of end-organ damage (4–8).
Encouraged by reasonable post-transplant outcomes of patients without evidence of end-organ damage from T2DM at the time of HTX (9–12), an increasing number of patients with T2DM were listed for HTX and subsequently transplanted. This development was supported by the growing number of patients with advanced heart failure and T2DM over the last decades (4–8). These early studies, however, included rather small numbers of carefully selected diabetic patients not necessarily reflecting clinical reality (9–12). It is therefore not surprising that recent literature is inconclusive as some studies found an elevated post-transplant mortality in patients with pre-transplant T2DM (13–16), whereas others could not observe such effect (17–21). Differences in study design, sample size, length of follow-up and analyzed post-transplant outcomes may have contributed to these inconsistencies (13–21). In addition, it should be noted that there was a distinct change in the composition of HTX study populations over time, since the reported rates of diabetic HTX recipients increased markedly from 13.7% to 18.3% in former studies (22, 23) up to 28.8–30.7% in recent studies (24, 25).
Another important aspect is the clinical management of patients with T2DM as a poorly controlled hemoglobin A1c (HbA1c) may be associated with increased post-transplant mortality. Furthermore, there might be an essential difference between T2DM patients with oral anti-diabetic medications and T2DM patients with insulin therapy. Yet, these questions have not been sufficiently answered in the literature. We therefore sought to investigate the effects of pre-transplant T2DM on survival and causes of death after HTX in a large contemporary population of HTX recipients.
Patients and Methods
Patients
We performed this study in accordance with the ethical standards of the Declaration of Helsinki in its current form. Approval was granted by the institutional review board (IRB) of Heidelberg University (ethical approval number: S-286/2015, Version 1.2, 28-07-2020). Written informed consent was obtained from patients for inclusion in the Heidelberg HTX Registry allowing the clinical and scientific use of data. According to the ethical approval, no additional written informed consent was required for this observational study as merely routine clinical data were analyzed (26–35).
We screened all adult patients (≥18 years) for pre-transplant T2DM who received HTX at Heidelberg Heart Center, Heidelberg, Germany, between 01/01/2000 and 01/10/2016. Patients with type 1 diabetes mellitus or other forms of diabetes than T2DM were excluded. We also did not include patients who received a second HTX. The remaining patients were stratified by diagnosis of T2DM at the time of HTX: patients with T2DM at the time of HTX (“T2DM group”) and patients without T2DM at the time of HTX (“no T2DM group”). Patients with T2DM at the time of HTX were further divided into patients with and without insulin therapy as well as into patients with a HbA1c < 7.0% at the time of HTX and patients with a HbA1c ≥ 7.0% at the time of HTX.
Follow-Up
Post-transplant follow-up was performed according to Heidelberg Heart Center’s routine clinical protocol. After hospital discharge, patients were monthly seen at the HTX outpatient-clinic during the first six post-transplant months, then bimonthly until the end of the first year after HTX, and thereafter three to four times annually (with additional visits when clinically required). Routine follow-up included medical history, physical examination, 12-lead electrocardiogram, echocardiography, endomyocardial biopsy, and blood tests including immunosuppressive drug monitoring (26–35).
Post-transplant Medications
Post-transplant medication including immunosuppressive drug therapy was administered according to Heidelberg Heart Center’s usual standard of care. Patients perioperatively received an anti-thymocyte globulin-based immunosuppression induction therapy. Cyclosporine A and azathioprine were administered as the initial immunosuppressive regimen prior to 2001. From 2001 onward, mycophenolate mofetil subsequently replaced azathioprine, and tacrolimus consecutively replaced cyclosporine A from 2006 onward. Steroids were tapered incrementally during the initial post-transplant months and were discontinued 6 months after HTX (unless clinically needed) (26–35).
Statistical Analysis
Data were analyzed with SAS (Version 9.4, SAS Institute, Cary, NC, United States) and expressed as count (n) with percentage (%) or as mean ± standard deviation (SD). For measures of association, difference of mean or hazard ratio (HR) with 95% confidence interval (CI) were used. Depending on the variable type (categorical variables or continuous variables) and the underlying question, we applied chi-squared test, Fisher’s exact test, Student’s t-test, Mann–Whitney U-test, analysis of variance (ANOVA), or Kruskal–Wallis test, as appropriate. The Kaplan–Meier estimator was used to graphically show 5-year post-transplant survival. A P-value of < 0.050 was considered statistically significant (26–35).
The primary outcome of this study was 5-year mortality after HTX which was compared between patients with and without T2DM at the time of HTX. We could obtain 5-year follow-up data from all patients requiring no censoring. Five-year post-transplant mortality of patients with T2DM was further assessed by stratification into patients with and without insulin therapy as well as into patients with a HbA1c < 7.0% at HTX and patients with a HbA1c ≥ 7.0% at HTX. Causes of death within 5 years after HTX were grouped into the following categories: graft failure, acute rejection, infection/sepsis, malignancy, and thromboembolic event/bleeding. We applied univariate analyses to search for intergroup differences including recipient data, previous open-heart surgery, principal diagnosis for HTX, donor data, transplant sex mismatch, perioperative data, immunosuppressive drug therapy, and concomitant medications. Analysis of 5-year mortality after HTX further included a multivariate analysis (Cox regression model) to investigate the impact of the eight variables which were statistically significant in the univariate analysis: recipient age (years), recipient body mass index (kg/m2), recipient arterial hypertension (in total), recipient dyslipidemia (in total), recipient T2DM (in total), recipient previous coronary artery bypass graft (CABG) surgery (in total), ischemic cardiomyopathy (CMP) as principal diagnosis for HTX (in total), and cardiac amyloidosis as principal diagnosis for HTX (in total). We did not include additional parameters in this multivariate analysis for 5-year mortality after HTX in order to avoid biased regression coefficients and to ensure a stable number of events (deceased patients) per analyzed variable. Given the long study period (01/01/2000–01/10/2016), we additionally performed a sensitivity analysis to test the robustness of our findings and to examine a possible era effect using a subgroup of patients with tacrolimus and mycophenolate mofetil as the immunosuppressive drug regimen was changed from 2006 onward (26–35).
Results
Baseline Characteristics of Patients With Type 2 Diabetes Mellitus at Heart Transplantation
This study included a total of 376 HTX recipients. About one-third of these patients (121 of 376 [32.2%]) had T2DM at the time of HTX. Patients with T2DM at the time of HTX were further divided into patients with a HbA1c < 7.0% at the time of HTX (67 of 121 [55.4%]) and patients with a HbA1c ≥ 7.0% at the time of HTX (54 of 121 [44.6%]).
Comparison of recipient data showed a higher age (P < 0.001), a higher body mass index (P < 0.001), a higher percentage of arterial hypertension (P < 0.001), and a higher percentage of dyslipidemia (P < 0.001) in the T2DM group. We did not observe a statistically significant difference between both groups concerning recipient male sex, chronic obstructive pulmonary disease, or severe chronic kidney disease (all P ≥ 0.050). Further evaluation of end-organ damage and clinical status of patients at the time of HTX showed no statistically significant difference between patients with or without T2DM concerning total bilirubin, hemoglobin, hospitalization before HTX, days on waiting list, high urgency status on waiting list, inotropic support, intra-aortic balloon pump, or initial hospital stay after HTX (all P ≥ 0.050).
In terms of principal diagnoses for HTX, significantly more patients with ischemic CMP were found in the T2DM group (P < 0.001), whereas significantly more patients with cardiac amyloidosis (P < 0.001) were observed in the opposite group. In addition, patients with T2DM had a significantly higher percentage of CABG surgery before HTX (P = 0.003). Baseline characteristics stratified by T2DM at HTX are shown in Table 1.
TABLE 1.
Baseline characteristics – stratified by T2DM at HTX.
| Parameter | All (n = 376) | T2DM (n = 121) | No T2DM (n = 255) | Difference | 95% CI | P-value |
| Recipient data | ||||||
| Age (years), mean ± SD | 51.9 ± 10.4 | 56.0 ± 7.3 | 49.9 ± 11.0 | 6.1 | 4.2–8.0 | <0.001* |
| Male sex, n (%) | 287 (76.3%) | 99 (81.8%) | 188 (73.7%) | 8.1% | –0.6 – 16.8% | 0.085 |
| Body mass index (kg/m2), mean ± SD | 25.2 ± 4.3 | 26.9 ± 4.4 | 24.4 ± 3.9 | 2.5 | 1.6 – 3.4 | <0.001* |
| Arterial hypertension, n (%) | 207 (55.1%) | 91 (75.2%) | 116 (45.5%) | 29.7% | 19.9 – 39.5% | <0.001* |
| Dyslipidemia, n (%) | 242 (64.4%) | 102 (84.3%) | 140 (54.9%) | 29.4% | 20.5 – 38.3% | <0.001* |
| COPD, n (%) | 94 (25.0%) | 37 (30.6%) | 57 (22.4%) | 8.2% | –1.5 – 17.9% | 0.085 |
| Severe chronic kidney disease^, n (%) | 40 (10.6%) | 17 (14.0%) | 23 (9.0%) | 5.0% | –2.1 – 12.1% | 0.139 |
| Principal diagnosis for HTX | ||||||
| Ischemic CMP, n (%) | 126 (33.5%) | 60 (49.6%) | 66 (25.9%) | 23.7% | 13.3 – 34.1% | <0.001* |
| Non-ischemic CMP, n (%) | 187 (49.7%) | 53 (43.8%) | 134 (52.5%) | 8.7% | –2.1 – 19.5% | 0.113 |
| Valvular heart disease, n (%) | 16 (4.3%) | 5 (4.1%) | 11 (4.3%) | 0.2% | –4.1 – 4.5% | 0.935 |
| Cardiac amyloidosis, n (%) | 47 (12.5%) | 3 (2.5%) | 44 (17.3%) | 14.8% | 9.4 – 20.2% | <0.001* |
| Previous open-heart surgery | ||||||
| CABG surgery, n (%) | 47 (12.5%) | 24 (19.8%) | 23 (9.0%) | 10.8% | 2.9 – 18.7% | 0.003 * |
| Other surgery°, n (%) | 41 (10.9%) | 17 (14.0%) | 24 (9.4%) | 4.6% | –2.5 – 11.7% | 0.178 |
| VAD surgery, n (%) | 29 (7.7%) | 11 (9.1%) | 18 (7.1%) | 2.0% | –4.0 – 8.0% | 0.490 |
| Donor data | ||||||
| Age (years), mean ± SD | 44.0 ± 12.8 | 45.0 ± 12.5 | 43.6 ± 12.9 | 1.4 | –1.4 – 4.2 | 0.321 |
| Male sex, n (%) | 126 (33.5%) | 43 (35.5%) | 83 (32.5%) | 3.0% | –7.3 – 13.3% | 0.566 |
| Body mass index (kg/m2), mean ± SD | 25.0 ± 4.5 | 25.5 ± 3.9 | 24.8 ± 4.7 | 0.7 | –0.2 – 1.6 | 0.127 |
| Transplant sex mismatch | ||||||
| Mismatch, n (%) | 186 (49.5%) | 68 (56.2%) | 118 (46.3%) | 9.9% | –0.9 – 20.7% | 0.072 |
| Donor (m) to recipient (f), n (%) | 12 (3.2%) | 6 (5.0%) | 6 (2.4%) | 2.6% | –1.7 – 6.9% | 0.179 |
| Donor (f) to recipient (m), n (%) | 174 (46.3%) | 62 (51.2%) | 112 (43.9%) | 7.3% | –3.5 – 18.1% | 0.184 |
| Perioperative data | ||||||
| Ischemic time (min), mean ± SD | 248.1 ± 59.1 | 250.6 ± 60.7 | 247.0 ± 58.4 | 3.6 | –9.4 – 16.6 | 0.588 |
| Biatrial HTX, n (%) | 5 (1.3%) | 1 (0.8%) | 4 (1.6%) | 0.8% | –1.4 – 3.0% | 0.557 |
| Bicaval HTX, n (%) | 146 (38.8%) | 45 (37.2%) | 101 (39.6%) | 2.4% | –8.1 – 12.9% | 0.653 |
| Total orthotopic HTX, n (%) | 225 (59.9%) | 75 (62.0%) | 150 (58.8%) | 3.2% | –7.3 – 13.7% | 0.559 |
CABG, coronary artery bypass graft; CI, confidence interval; CMP, cardiomyopathy; COPD, chronic obstructive pulmonary disease; f, female; HTX, heart transplantation; m, male; n, number; SD, standard deviation; T2DM, type 2 diabetes mellitus; VAD, ventricular assist device; ^, estimated glomerular filtration rate < 30 ml/min/1.73 m2; °, congenital, valvular or ventricular surgery; *, statistically significant (P < 0.050).
Analysis of baseline characteristics stratified by HbA1c at the time of HTX indicated no statistically significant differences between T2DM patients with a HbA1c < 7.0% and T2DM patients with a HbA1c ≥ 7.0% regarding recipient data, principal diagnosis for HTX, previous open-heart surgery, donor data, and perioperative data, except for donor (m) to recipient (f) sex mismatch which was significantly higher in T2DM patients with a HbA1c < 7.0% (P = 0.024). Baseline characteristics stratified by HbA1c at HTX are presented in Table 2.
TABLE 2.
Baseline characteristics – stratified by HbA1c at HTX.
| Parameter | T2DM (n = 121) | HbA1c < 7.0% (n = 67) | HbA1c ≥ 7.0% (n = 54) | Difference | 95% CI | P-value |
| Recipient data | ||||||
| Age (years), mean ± SD | 56.0 ± 7.3 | 57.0 ± 7.1 | 54.8 ± 7.5 | 2.2 | –0.4 – 4.8 | 0.093 |
| Male sex, n (%) | 99 (81.8%) | 51 (76.1%) | 48 (88.9%) | 12.8% | –0.4 – 26.0% | 0.070 |
| Body mass index (kg/m2), mean ± SD | 26.9 ± 4.4 | 26.2 ± 4.3 | 27.6 ± 4.5 | 1.4 | –0.2 – 3.0 | 0.086 |
| Arterial hypertension, n (%) | 91 (75.2%) | 51 (76.1%) | 40 (74.1%) | 2.0% | –13.5 – 17.5% | 0.796 |
| Dyslipidemia, n (%) | 102 (84.3%) | 57 (85.1%) | 45 (83.3%) | 1.8% | –11.3 – 14.9% | 0.794 |
| COPD, n (%) | 37 (30.6%) | 17 (25.4%) | 20 (37.0%) | 11.6% | –4.9 – 28.1% | 0.166 |
| Severe chronic kidney disease^, n (%) | 17 (14.0%) | 9 (13.4%) | 8 (14.8%) | 1.4% | –11.1 – 13.9% | 0.828 |
| Principal diagnosis for HTX | ||||||
| Ischemic CMP, n (%) | 60 (49.6%) | 35 (52.2%) | 25 (46.3%) | 5.9% | 11.9 – 23.7% | 0.516 |
| Non-ischemic CMP, n (%) | 53 (43.8%) | 27 (40.3%) | 26 (48.1%) | 7.8% | –9.9 – 25.5% | 0.387 |
| Valvular heart disease, n (%) | 5 (4.1%) | 3 (4.5%) | 2 (3.7%) | 0.8% | –6.3 – 7.9% | 0.832 |
| Cardiac amyloidosis, n (%) | 3 (2.5%) | 2 (3.0%) | 1 (1.9%) | 1.1% | –4.3 – 6.5% | 0.690 |
| Previous open-heart surgery | ||||||
| CABG surgery, n (%) | 24 (19.8%) | 15 (22.4%) | 9 (16.7%) | 5.7% | –8.4 – 19.8% | 0.433 |
| Other surgery°, n (%) | 17 (14.0%) | 9 (13.4%) | 8 (14.8%) | 1.4% | –11.1 – 13.9% | 0.828 |
| VAD surgery, n (%) | 11 (9.1%) | 6 (9.0%) | 5 (9.3%) | 0.3% | –10.0 – 10.6% | 0.954 |
| Donor data | ||||||
| Age (years), mean ± SD | 45.0 ± 12.5 | 46.1 ± 10.9 | 43.6 ± 14.3 | 2.5 | –2.2 – 7.2 | 0.295 |
| Male sex, n (%) | 43 (35.5%) | 25 (37.3%) | 18 (33.3%) | 4.0% | –13.1 – 21.1% | 0.649 |
| Body mass index (kg/m2), mean ± SD | 25.5 ± 3.9 | 25.6 ± 4.0 | 25.3 ± 3.7 | 0.3 | –1.1 – 1.7 | 0.589 |
| Transplant sex mismatch | ||||||
| Mismatch, n (%) | 68 (56.2%) | 38 (56.7%) | 30 (55.6%) | 1.1% | –16.7 – 18.9% | 0.898 |
| Donor (m) to recipient (f), n (%) | 6 (5.0%) | 6 (9.0%) | 0 (0.0%) | 9.0% | 2.1 – 15.9% | 0.024* |
| Donor (f) to recipient (m), n (%) | 62 (51.2%) | 32 (47.7%) | 30 (55.6%) | 7.9% | –10.0 – 25.8% | 0.394 |
| Perioperative data | ||||||
| Ischemic time (min), mean ± SD | 250.6 ± 60.7 | 245.9 ± 64.3 | 256.4 ± 56.1 | 10.5 | –11.2 – 32.2 | 0.338 |
| Biatrial HTX, n (%) | 1 (0.8%) | 0 (0.0%) | 1 (1.9%) | 1.9% | –1.7 – 5.5% | 0.263 |
| Bicaval HTX, n (%) | 45 (37.2%) | 21 (31.3%) | 24 (44.4%) | 13.1% | –4.2 – 30.4% | 0.138 |
| Total orthotopic HTX, n (%) | 75 (62.0%) | 46 (68.7%) | 29 (53.7%) | 15.0% | –2.3 – 32.3% | 0.092 |
CABG, coronary artery bypass graft; CI, confidence interval; CMP, cardiomyopathy; COPD, chronic obstructive pulmonary disease; f, female; HbA1c, hemoglobin A1c; HTX, heart transplantation; m, male; n, number; SD, standard deviation; T2DM, type 2 diabetes mellitus; VAD, ventricular assist device; ^, estimated glomerular filtration rate < 30 ml/min/1.73 m2; °, congenital, valvular or ventricular surgery; *, statistically significant (P < 0.050).
Medical Treatment of Patients With Type 2 Diabetes Mellitus at Heart Transplantation
Analysis of the immunosuppressive drug therapy showed no statistically significant differences between patients with or without T2DM at the time of HTX regarding the administration of cyclosporine A, tacrolimus, azathioprine, or mycophenolate mofetil (all P ≥ 0.050). We also found no statistically significant differences between both groups concerning the administration of acetylsalicylic acid, beta blockers, ivabradine, calcium channel blockers, angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers, or statins (all P ≥ 0.050). Medications after HTX stratified by T2DM at HTX are given in Table 3.
TABLE 3.
Medications after HTX – stratified by T2DM at HTX.
| Parameter | All (n = 376) | T2DM (n = 121) | No T2DM (n = 255) | Difference | 95% CI | P-value |
| Immunosuppressive drug therapy | ||||||
| Cyclosporine A, n (%) | 124 (33.0%) | 36 (29.8%) | 88 (34.5%) | 4.7% | –5.3 – 14.7% | 0.359 |
| Tacrolimus, n (%) | 252 (67.0%) | 85 (70.2%) | 167 (65.5%) | 4.7% | –5.3 – 14.7% | 0.359 |
| Azathioprine, n (%) | 46 (12.2%) | 16 (13.2%) | 30 (11.8%) | 1.4% | –5.8 – 8.6% | 0.687 |
| Mycophenolate mofetil, n (%) | 330 (87.8%) | 105 (86.8%) | 225 (88.2%) | 1.4% | –5.8 – 8.6% | 0.687 |
| Steroids, n (%) | 376 (100.0%) | 121 (100.0%) | 255 (100.0%) | 0.0% | n. a. | n. a. |
| Concomitant medications | ||||||
| ASA, n (%) | 47 (12.5%) | 14 (11.6%) | 33 (12.9%) | 1.3% | –5.8 – 8.4% | 0.707 |
| Beta blocker, n (%) | 85 (22.6%) | 25 (20.7%) | 60 (23.5%) | 2.8% | –6.1 – 11.7% | 0.534 |
| Ivabradine, n (%) | 44 (11.7%) | 14 (11.6%) | 30 (11.8%) | 0.2% | –6.7 – 7.1% | 0.956 |
| Calcium channel blocker, n (%) | 110 (29.3%) | 42 (34.7%) | 68 (26.7%) | 8.0% | –2.1 – 18.1% | 0.109 |
| ACE inhibitor/ARB, n (%) | 159 (42.3%) | 52 (43.0%) | 107 (42.0%) | 1.0% | –9.7 – 11.7% | 0.852 |
| Diuretic, n (%) | 376 (100.0%) | 121 (100.0%) | 255 (100.0%) | 0.0% | n. a. | n. a. |
| Statin, n (%) | 211 (56.1%) | 73 (60.3%) | 138 (54.1%) | 6.2% | –4.5 – 16.9% | 0.257 |
| Gastric protection †, n (%) | 376 (100.0%) | 121 (100.0%) | 255 (100.0%) | 0.0% | n. a. | n. a. |
ACE inhibitor, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; CI, confidence interval; HTX, heart transplantation; n, number; n. a., not applicable; T2DM, type 2 diabetes mellitus; †, gastric protection defined as proton pump inhibitor (PPI) or histamine receptor (H2) blocker.
Likewise, we did not find any statistically significant differences between T2DM patients with a HbA1c < 7.0% and T2DM patients with a HbA1c ≥ 7.0% concerning the immunosuppressive drug therapy or the concomitant medications (all P ≥ 0.050). Medications after HTX stratified by HbA1c at HTX are shown in Table 4.
TABLE 4.
Medications after HTX – stratified by HbA1c at HTX.
| Parameter | T2DM (n = 121) | HbA1c < 7.0% (n = 67) | HbA1c ≥ 7.0% (n = 54) | Difference | 95% CI | P-value |
| Immunosuppressive drug therapy | ||||||
| Cyclosporine A, n (%) | 36 (29.8%) | 17 (25.4%) | 19 (35.2%) | 9.8% | –6.6 – 26.2% | 0.241 |
| Tacrolimus, n (%) | 85 (70.2%) | 50 (74.6%) | 35 (64.8%) | 9.8% | –6.6 – 26.2% | 0.241 |
| Azathioprine, n (%) | 16 (13.2%) | 9 (13.4%) | 7 (13.0%) | 0.4% | –11.7 – 12.5% | 0.940 |
| Mycophenolate mofetil, n (%) | 105 (86.8%) | 58 (86.6%) | 47 (87.0%) | 0.4% | –11.7 – 12.5% | 0.940 |
| Steroids, n (%) | 121 (100.0%) | 67 (100.0%) | 54 (100.0%) | 0.0% | n. a. | n. a. |
| Concomitant medications | ||||||
| ASA, n (%) | 14 (11.6%) | 9 (13.4%) | 5 (9.3%) | 4.1% | –7.1 – 15.3% | 0.476 |
| Beta blocker, n (%) | 25 (20.7%) | 18 (26.9%) | 7 (13.0%) | 13.9% | –0.1 – 27.9% | 0.060 |
| Ivabradine, n (%) | 14 (11.6%) | 9 (13.4%) | 5 (9.3%) | 4.1% | –7.1 – 15.3% | 0.476 |
| Calcium channel blocker, n (%) | 42 (34.7%) | 21 (31.3%) | 21 (38.9%) | 7.6% | –9.5 – 24.7% | 0.386 |
| ACE inhibitor/ARB, n (%) | 52 (43.0%) | 27 (40.3%) | 25 (46.3%) | 6.0% | –11.7 – 23.7% | 0.508 |
| Diuretic, n (%) | 121 (100.0%) | 67 (100.0%) | 54 (100.0%) | 0.0% | n. a. | n. a. |
| Statin, n (%) | 73 (60.3%) | 44 (65.7%) | 29 (53.7%) | 12.0% | –5.5 – 29.5% | 0.181 |
| Gastric protection †, n (%) | 121 (100.0%) | 67 (100.0%) | 54 (100.0%) | 0.0% | n. a. | n. a. |
ACE inhibitor, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; CI, confidence interval; HbA1c, hemoglobin A1c; HTX, heart transplantation; n, number; n. a., not applicable; T2DM, type 2 diabetes mellitus; †, gastric protection defined as proton pump inhibitor (PPI) or histamine receptor (H2) blocker.
In terms of diabetes medications, metformin was the most common oral anti-diabetic drug in patients with T2DM at the time of HTX (49 of 121 [40.5%]). In addition, almost half of patients with T2DM at the time of HTX received insulin therapy (58 of 121 [47.9%]). Analysis of diabetes medications stratified by HbA1c at the time of HTX showed a significantly higher percentage of regular insulin (P = 0.009) and insulin glargine (P = 0.028) in T2DM patients with a HbA1c ≥ 7.0%. An overview of the diabetes medications of T2DM patients stratified by HbA1c at HTX is displayed in Table 5.
TABLE 5.
Overview of diabetes medications.
| Parameter | T2DM (n = 121) | HbA1c < 7.0% (n = 67) | HbA1c ≥ 7.0% (n = 54) | Difference | 95% CI | P-value |
| Oral anti-diabetic medications | ||||||
| Alpha glucosidase inhibitors | ||||||
| Acarbose, n (%) | 3 (2.5%) | 2 (3.0%) | 1 (1.9%) | 1.1% | –4.3 – 6.5% | 0.690 |
| Biguanides | ||||||
| Metformin, n (%) | 49 (40.5%) | 27 (40.3%) | 22 (40.7%) | 0.4% | –17.2 – 18.0% | 0.961 |
| DPP-4 inhibitors | ||||||
| Saxagliptin, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Sitagliptin, n (%) | 11 (9.1%) | 9 (13.4%) | 2 (3.7%) | 9.7% | –0.1 – 19.5% | 0.064 |
| Vildagliptin, n (%) | 3 (2.5%) | 1 (1.5%) | 2 (3.7%) | 2.2% | –3.6 – 8.0% | 0.437 |
| GLP-1 receptor agonists | ||||||
| Dulaglutide, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Exenatide, n (%) | 1 (0.8%) | 0 (0.0%) | 1 (1.9%) | 1.9% | –1.7 – 5.5% | 0.263 |
| Liraglutide, n (%) | 2 (1.7%) | 1 (1.5%) | 1 (1.9%) | 0.4% | –4.2 – 5.0% | 0.878 |
| Meglitinides | ||||||
| Nateglinide, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Repaglinide, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| SGLT-2 inhibitors | ||||||
| Dapagliflozin, n (%) | 1 (0.8%) | 0 (0.0%) | 1 (1.9%) | 1.9% | –1.7 – 5.5% | 0.263 |
| Empagliflozin, n (%) | 1 (0.8%) | 1 (1.5%) | 0 (0.0%) | 1.5% | –1.4 – 4.4% | 0.367 |
| Sulfonylureas | ||||||
| Glibenclamide, n (%) | 2 (1.7%) | 1 (1.5%) | 1 (1.9%) | 0.4% | –4.2 – 5.0% | 0.878 |
| Glimepiride, n (%) | 12 (9.9%) | 8 (11.9%) | 4 (7.4%) | 4.5% | –6.0 – 15.0% | 0.407 |
| Gliquidone, n (%) | 5 (4.1%) | 3 (4.5%) | 2 (3.7%) | 0.8% | –6.3 – 7.9% | 0.832 |
| Thiazolidinediones | ||||||
| Pioglitazone, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Rosiglitazone, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Insulin therapy | ||||||
| Rapid-acting insulin | ||||||
| Insulin aspart, n (%) | 9 (7.4%) | 5 (7.5%) | 4 (7.4%) | 0.1% | –9.3 – 9.5% | 0.991 |
| Insulin glulisine, n (%) | 1 (0.8%) | 0 (0.0%) | 1 (1.9%) | 1.9% | –1.7 – 5.5% | 0.263 |
| Insulin lispro, n (%) | 3 (2.5%) | 1 (1.5%) | 2 (3.7%) | 2.2% | –3.6 – 8.0% | 0.437 |
| Short-acting insulin | ||||||
| Regular insulin, n (%) | 45 (37.2%) | 18 (26.9%) | 27 (50.0%) | 23.1% | 6.1 – 40.1% | 0.009* |
| Intermediate-acting insulin | ||||||
| NPH insulin, n (%) | 13 (10.7%) | 6 (9.0%) | 7 (13.0%) | 4.0% | –7.3 – 15.3% | 0.479 |
| Long-acting insulin | ||||||
| Insulin degludec, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n. a. | n. a. |
| Insulin detemir, n (%) | 4 (3.3%) | 1 (1.5%) | 3 (5.6%) | 4.1% | –2.7 – 10.9% | 0.214 |
| Insulin glargine, n (%) | 41 (33.9%) | 17 (25.4%) | 24 (44.4%) | 19.0% | 2.2 – 35.8% | 0.028* |
CI, confidence interval; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; n, number; n. a., not applicable; NPH, Neutral Protamine Hagedorn; SGLT-2, sodium-glucose transport protein 2; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
Survival of Patients With Type 2 Diabetes Mellitus at Heart Transplantation
Patients with T2DM at the time of HTX had a significantly higher 5-year all-cause mortality after HTX (41.3% versus 29.8%, difference: 11.5%, 95% CI: 1.1 – 21.9%, P = 0.027). Regarding the causes of death within 5 years after HTX, significantly more patients with T2DM died from graft failure (14.9% versus 7.8%, difference: 7.1%, 95% CI: 0.1 – 14.1%, P = 0.035). For further evaluation of the association between T2DM and graft failure, we performed a log rank test between patients with and without T2DM at HTX in regard to graft failure within 5 years after HTX analyzing the number of patients with graft failure and the time from HTX until graft failure. Patients with T2DM at the time of HTX had a significantly higher rate of graft failure within 5 years after HTX (P = 0.019). In contrast, we did not observe statistically significant differences between T2DM groups concerning acute rejection, infection/sepsis, malignancy, or thromboembolic event/bleeding (all P ≥ 0.050). Causes of death within 5 years after HTX stratified by T2DM at HTX are given in Table 6.
TABLE 6.
Causes of death within 5 years after HTX – stratified by T2DM at HTX.
| Parameter | All (n = 376) | T2DM (n = 121) | No T2DM (n = 255) | Difference | 95% CI | P-value |
| Graft failure, n (%) | 38 (10.1%) | 18 (14.9%) | 20 (7.8%) | 7.1% | 0.1 – 14.1% | 0.035* |
| Acute rejection, n (%) | 4 (1.1%) | 1 (0.8%) | 3 (1.2%) | 0.4% | –1.7 – 2.5% | 0.757 |
| Infection/Sepsis, n (%) | 66 (17.5%) | 20 (16.5%) | 46 (18.0%) | 1.5% | –6.6 – 9.6% | 0.719 |
| Malignancy, n (%) | 8 (2.1%) | 5 (4.1%) | 3 (1.2%) | 2.9% | –0.9 – 6.7% | 0.064 |
| Thromboembolic event/bleeding, n (%) | 10 (2.7%) | 6 (5.0%) | 4 (1.6%) | 3.4% | –0.8 – 7.6% | 0.056 |
| All causes, n (%) | 126 (33.5%) | 50 (41.3%) | 76 (29.8%) | 11.5% | 1.1 – 21.9% | 0.027* |
CI, confidence interval; HTX, heart transplantation; n, number; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
Analysis of causes of death within 5 years after HTX stratified by HbA1c at the time of HTX showed a significantly higher 5-year all-cause mortality after HTX in T2DM patients with a HbA1c ≥ 7.0% (53.7% versus 31.3%, difference: 22.4%, 95% CI: 5.1–39.7%, P = 0.013). Patients with T2DM and a HbA1c ≥ 7.0% at the time of HTX also had a higher percentage of death due to graft failure (18.5% versus 11.9%), infection/sepsis (24.1% versus 10.5%), and thromboembolic event/bleeding (9.3% versus 1.5%) within 5 years after HTX. Causes of death within 5 years after HTX stratified by HbA1c at HTX are provided in Table 7.
TABLE 7.
Causes of death within 5 years after HTX – stratified by HbA1c at HTX.
| Parameter | T2DM (n = 121) | HbA1c < 7.0% (n = 67) | HbA1c ≥ 7.0% (n = 54) | Difference | 95% CI | P-value |
| Graft failure, n (%) | 18 (14.9%) | 8 (11.9%) | 10 (18.5%) | 6.6% | –6.3 – 19.5% | 0.312 |
| Acute rejection, n (%) | 1 (0.8%) | 1 (1.5%) | 0 (0.0%) | 1.5% | –1.4 – 4.4% | 0.367 |
| Infection/Sepsis, n (%) | 20 (16.5%) | 7 (10.5%) | 13 (24.1%) | 13.6% | 0.1 – 27.1% | 0.045* |
| Malignancy, n (%) | 5 (4.1%) | 4 (6.0%) | 1 (1.9%) | 4.1% | –2.6 – 10.8% | 0.258 |
| Thromboembolic event/bleeding, n (%) | 6 (5.0%) | 1 (1.5%) | 5 (9.3%) | 7.8% | –0.4 – 16.0% | 0.050 |
| All causes, n (%) | 50 (41.3%) | 21 (31.3%) | 29 (53.7%) | 22.4% | 5.1 – 39.7% | 0.013* |
CI, confidence interval; HbA1c, hemoglobin A1c; HTX, heart transplantation; n, number; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
Multivariate analysis showed a more than 50% increased risk of death within 5 years after HTX in patients with T2DM at the time of HTX (HR: 1.563; 95% CI: 1.053–2.319; P = 0.027), while the other seven included variables (recipient age, recipient body mass index, recipient arterial hypertension, recipient dyslipidemia, previous CABG surgery, ischemic CMP as principal diagnosis for HTX, and cardiac amyloidosis as principal diagnosis for HTX) showed no statistically significant effect on 5-year post-transplant mortality. Multivariate analysis for 5-year mortality after HTX is given in Table 8.
TABLE 8.
Multivariate analysis for 5-year mortality after HTX.
| Parameter | Hazard Ratio | 95% CI | P-value |
| Recipient age (years) | 1.018 | 0.996 – 1.041 | 0.103 |
| Recipient body mass index (kg/m2) | 1.012 | 0.969 – 1.058 | 0.592 |
| Recipient arterial hypertension (in total) | 0.704 | 0.404 – 1.228 | 0.217 |
| Recipient dyslipidemia (in total) | 0.935 | 0.548 – 1.596 | 0.805 |
| Recipient T2DM (in total) | 1.563 | 1.053 – 2.319 | 0.027 * |
| Previous CABG surgery (in total) | 0.783 | 0.434 – 1.412 | 0.415 |
| Ischemic CMP (in total) | 1.718 | 0.993 – 2.972 | 0.053 |
| Cardiac amyloidosis (in total) | 1.697 | 0.985 – 2.923 | 0.057 |
CABG, coronary artery bypass graft; CI, confidence interval; CMP, cardiomyopathy; HTX, heart transplantation; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
Kaplan–Meier survival analysis displayed a significantly inferior 5-year post-transplant survival of patients with T2DM at the time of HTX (58.7%) in comparison to patients without T2DM at the time of HTX (70.2%, difference: 11.5%, 95% CI: 1.1 – 21.9%, P = 0.015). Patients with insulin therapy had in fact a lower 5-year post-transplant survival (53.4%) than patients without insulin therapy (63.5%) but this difference did not reach statistical significance (P = 0.243). Further stratification of T2DM patients at the time of HTX showed a significantly lower 5-year post-transplant survival of patients with a HbA1c ≥ 7.0% at HTX (46.3%) in comparison to patients with a HbA1c < 7.0% at the time of HTX (68.7%, difference: 22.4%, 95% CI: 5.1–39.7%, P = 0.008). Kaplan–Meier estimators are shown in Figures 1, 2.
FIGURE 1.
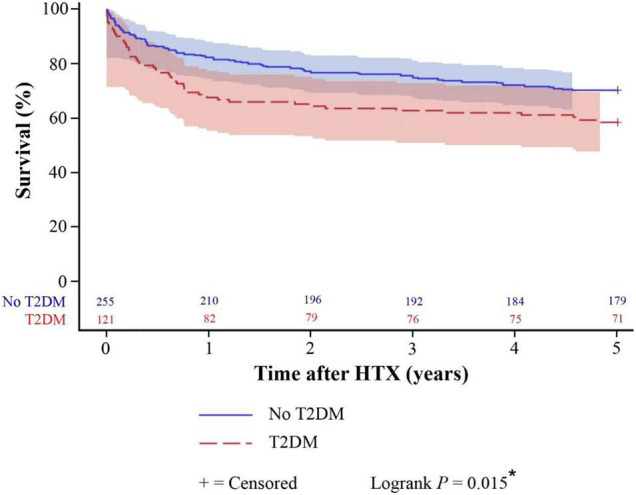
Five-year post-transplant survival of patients with and without T2DM at HTX (Kaplan–Meier estimator). Patients with T2DM at HTX had a significantly worse 5-year post-transplant survival in the Kaplan–Meier survival analysis (58.7%) compared to patients without T2DM at HTX (70.2%, P = 0.015). HTX, heart transplantation; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
FIGURE 2.
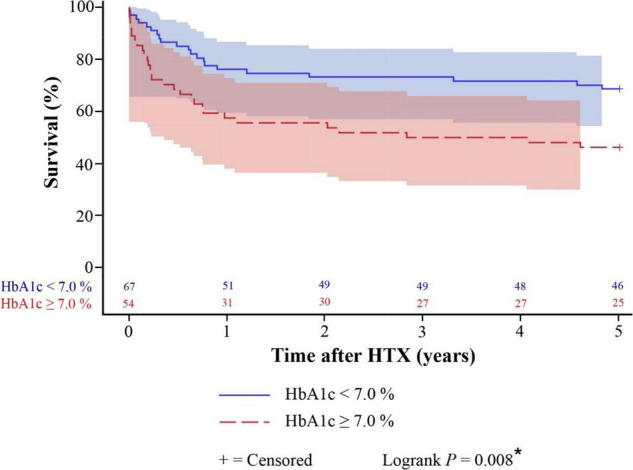
Five-year post-transplant survival of patients with T2DM stratified by HbA1c at HTX (Kaplan–Meier estimator). Stratification of patients with T2DM at HTX showed a significantly lower 5-year post-transplant survival of patients with a HbA1c ≥ 7.0% at HTX (46.3%) in comparison to patients with a HbA1c < 7.0% at HTX (68.7%, P = 0.008). HbA1c, hemoglobin A1c; HTX, heart transplantation; T2DM, type 2 diabetes mellitus; *, statistically significant (P < 0.050).
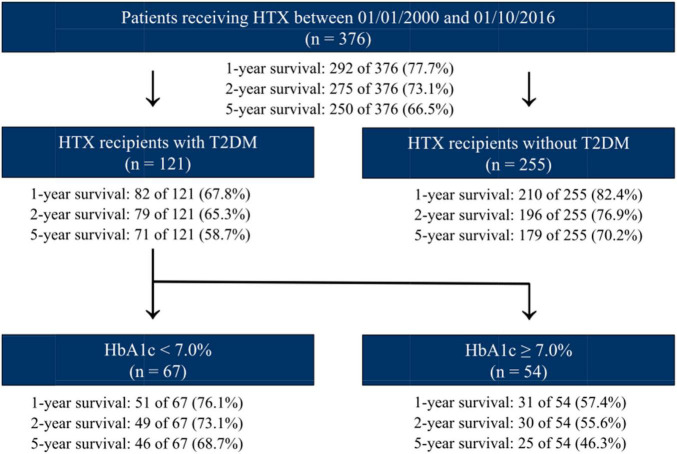
Additional survival analysis revealed that patients without T2DM at the time of HTX had the best 1-year (210 of 255 [82.4%]), 2-year (196 of 255 [76.9%]), and 5-year post-transplant survival (179 of 255 [70.2%]), followed by patients with T2DM at HTX and a HbA1c < 7.0% at HTX who showed a broadly similar 1-year (51 of 67 [76.1%]), 2-year (49 of 67 [73.1%]), and 5-year post-transplant survival (46 of 67 [68.7%]). Of note, patients with T2DM at HTX and a HbA1c ≥ 7.0% at HTX had the worst 1-year (31 of 54 [57.4%]), 2-year (30 of 54 [55.6%]), and 5-year post-transplant survival (25 of 54 [46.3%]). An overview of 5-year post-transplant survival stratified by T2DM at HTX and HbA1c at HTX is provided in Figure 3.
FIGURE 3.
Overview of 5-year post-transplant survival stratified by T2DM at HTX and HbA1c at HTX. Patients without T2DM at HTX showed the best 1-, 2-, and 5-year post-transplant survival, followed by patients with T2DM at HTX and a HbA1c < 7.0% at HTX, whereas patients with T2DM at HTX and a HbA1c ≥ 7.0% at HTX had the worst 1-, 2-, and 5-year post-transplant survival. HbA1c, hemoglobin A1c; HTX, heart transplantation; n, number; T2DM, type 2 diabetes mellitus.
Sensitivity Analysis
In order to investigate a possible era effect and to examine the robustness of our findings, we performed a sensitivity analysis with a subgroup of patients who were administered tacrolimus and mycophenolate mofetil as immunosuppressive drug therapy [252 of 376 patients (67.0%)]. This analysis provided similar results supporting the robustness of our findings and reducing the likelihood of a potential era effect.
Discussion
Frequency and Clinical Relevance of Type 2 Diabetes Mellitus at Heart Transplantation
Given the unknown prognostic effect of T2DM at the time of HTX on post-transplant outcomes, we performed this large study with 376 HTX recipients to investigate the frequency and clinical relevance of pre-transplant T2DM. A total of 121 HTX recipients (32.2%) had pre-transplant T2DM. This is in line with recent studies describing a similar percentage of pre-transplant diabetic patients (24, 25). Chamarthi et al. (24) reported a pre-transplant diabetic rate of 28.8% (46 of 160) at Brigham and Women’s Hospital, Harvard Medical School, between January 2000 and July 2012. Similarly, Feng et al. (25) published a pre-transplant diabetic rate of 30.7% (75 of 244) at Stanford University Medical Center between January 2008 and July 2018. In contrast, older studies covering earlier eras of HTX reported a considerably lower rate of pre-transplant diabetic patients ranging between 13.7 and 18.3% (22, 23). These data evidently highlight the rising percentage of diabetic HTX recipients (22–25).
From a clinician’s perspective, this change is of high relevance as patients with T2DM face an increased risk of morbidity and mortality (1–8, 13–16, 36). Diabetic patients have a higher risk for post-transplant infections requiring hospitalization and often suffer from further deterioration of renal function, especially in combination with calcineurin inhibitors which are known nephrotoxic drugs (20, 21, 25, 37–39). In order to evaluate the degree of end-organ damage as well as the clinical status of patients at the time of HTX, we compared patients with and without T2DM at the time of HTX. We found no statistically significant differences between both groups in regard to severe chronic kidney disease, total bilirubin, hemoglobin, hospitalization before HTX, days on waiting list, high urgency status on waiting list, inotropic support, intra-aortic balloon pump, or initial hospital stay after HTX highlighting the importance of careful evaluation of T2DM patients before listing for HTX. Alternatively, patients with T2DM and severe end-organ damage may be considered for left ventricular assist device (LVAD) implantation (21). However, especially in younger patients, this may not be a valid long-term solution given the known LVAD complications (40, 41). Hence, patients with T2DM should be carefully evaluated before listing for HTX, particularly in regard to severe end-organ damage, and should receive optimal individualized diabetes management before and after HTX (1–8).
Clinical Management of Patients With Type 2 Diabetes Mellitus at Heart Transplantation
In order to reduce microvascular and macrovascular complications in patients with T2DM, the 2019 European Society of Cardiology (ESC) guidelines on diabetes recommend a targeted HbA1c < 7.0% (1). We therefore compared in our study the diabetes medications of T2DM patients with a HbA1c < 7.0% and T2DM patients with a HbA1c ≥ 7.0%. We found no significant difference between both groups in regard to oral anti-diabetic medications of which metformin was the most common oral anti-diabetic drug in patients with T2DM at the time of HTX. In terms of insulin therapy which was administered to almost half of patients with T2DM at the time of HTX, T2DM patients with a HbA1c ≥ 7.0% had a significantly higher percentage of regular insulin (P = 0.009) and insulin glargine (P = 0.028).
With the introduction of new anti-diabetic medications such as dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose transport protein 2 (SGLT-2) inhibitors, diabetes management has improved and a targeted HbA1c < 7.0% has become more achievable (1, 3, 25, 42–48). In addition to their excellent glucose-lowering profile, these novel agents exhibit multiple beneficial effects via reduction of body weight, blood pressure, major cardiovascular events and even mortality (1, 3, 25, 42–48). However, data regarding the safety and efficacy of these new drugs in HTX recipients with T2DM are limited to studies with small sample sizes (42, 45).
Cehic et al. (42) examined 22 HTX recipients with T2DM who were treated with empagliflozin. They observed no genitourinary infections and treatment with empagliflozin was associated with reductions in body mass index and HbA1c (42). Similar findings were reported by Sammour et al. (45) who evaluated the safety and efficacy of GLP-1 receptor agonists and SGLT-2 inhibitors in HTX recipients with T2DM. Among 21 patients, they found a significant reduction of body weight, HbA1c, and low-density lipoprotein-cholesterol with no adverse events leading to discontinuation of either therapy (45).
In our study, only a minority of patients with pre-transplant T2DM received DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT-2 inhibitors, as the majority of patients with oral anti-diabetic medications were still on metformin. This is in line with a recent study by Feng et al. (25) reporting likewise only a few HTX recipients on GLP-1 receptor agonists or SGLT-2 inhibitors. Further studies with large contemporary populations of HTX recipients with T2DM are therefore needed to determine the safety and efficacy of these medications but one should keep in mind that the use of anti-diabetic medications is just one part of diabetes management. A multimodal approach including nutrition counseling, increased physical activity, weight loss, smoking cessation in addition to anti-diabetic medications with new pharmacologic strategies is required to reduce the burden of morbidity and mortality in HTX recipients with T2DM.
Post-transplant Mortality of Patients With Type 2 Diabetes Mellitus at Heart Transplantation
Data regarding the impact of pre-transplant T2DM on mortality after HTX are inconclusive (9–21). Several studies reported an increased post-transplant mortality in patients with T2DM at the time of HTX (13–16), whereas others studies did not find a relevant difference (17–21).
In our study with a large contemporary population of HTX recipients, patients with pre-transplant T2DM had a significantly increased 5-year all-cause mortality after HTX (41.3% versus 29.8%, P = 0.027), along with a higher rate of death due to graft failure (14.9% versus 7.8%, P = 0.035). Multivariate analysis showed a more than 50% increased risk for 5-year mortality after HTX in these patients (HR: 1.563; 95% CI: 1.053–2.319; P = 0.027).
Discrepancies between former studies regarding post-transplant mortality in patients with T2DM at the time of HTX may result from differences in diabetes status (13–21). A large study of the United Network of Organ Sharing (UNOS) database with 20,412 HTX recipients including 3,687 diabetic patients reported a significantly better post-transplant survival in non-diabetic HTX recipients than in diabetic HTX recipients in general (P < 0.001) but there was no statistically significant difference in post-transplant survival between non-diabetic HTX recipients and diabetic HTX recipients with uncomplicated diabetes status (P = 0.080) (21).
Stratification of patients with T2DM by HbA1c at HTX in our study showed a significantly higher 5-year all-cause mortality after HTX in T2DM patients with a HbA1c ≥ 7.0% (53.7% versus 31.3%, P = 0.013). Patients with T2DM and a HbA1c ≥ 7.0% also had a higher percentage of death due to graft failure (18.5% versus 11.9%), infection/sepsis (24.1% versus 10.5%), and thromboembolic event/bleeding (9.3% versus 1.5%) within 5 years after HTX highlighting the vulnerability of these patients. As insulin therapy is often needed in patients with advanced T2DM, we also compared patients with and without insulin therapy. HTX recipients with insulin therapy had in fact a lower 5-year post-transplant survival (53.4%) than patients without insulin therapy (63.5%) but this difference did not reach statistical significance (P = 0.243). This is in line with a report by Czerny et al. (13) who also found no significant influence of insulin therapy on survival after HTX.
Furthermore, a key message of our study is the finding that patients with T2DM and a HbA1c < 7.0% had a similar 5-year survival after HTX in comparison to patients without T2DM indicating that comparable long-term post-transplant survival rates of HTX recipients with T2DM are achievable if these patients receive optimal diabetes management and are followed-up closely after HTX.
Regarding the impact of diabetes on ventricular ejection fraction and cardiac allograft vasculopathy, results have been controversially discussed (4, 11, 13, 17, 49). Higgins et al. (17) reported that diabetic HTX recipients had an increased rate of transplant coronary artery disease (42% versus 13%; P = 0.02) as well as a lower left ventricular ejection fraction at 3 years after HTX (54% versus 61%; P = 0.03). In contrast, Munoz et al. (11) found no statistically significant difference in transplant coronary artery disease by the fourth year of follow-up (31% in diabetic HTX recipients versus 33% in non-diabetic HTX recipients). Similar results were reported by Czerny et al. (13) who also reported no statistically significant difference in transplant coronary artery disease (15% in diabetic HTX recipients versus 14% in non-diabetic HTX recipients) at 5 years after HTX.
In our study, survival of T2DM patients declined markedly within the first year after HTX, some patients with T2DM even died from graft failure within the first 3 months after HTX. As the development of cardiac allograft vasculopathy usually takes several months to years after HTX this may indicate that adverse graft survival in HTX recipients is rather related to generally impaired global organ function (13). However, given the importance of this aspect and the lack of contemporary knowledge, there is an urgent need for future studies focusing on the development of cardiac allograft vasculopathy by analyzing catheterization data of HTX recipients with T2DM.
Study Limitations
The findings of this study were derived from a large single-center registry (Heidelberg HTX Registry) including the highly elaborated data of 376 patients who received HTX at Heidelberg Heart Center. Given the known limitations of such a study design, our results should be interpreted carefully and within the context of the existing literature. However, we would like to point out that our study was comparable to multicenter studies in sample size and our patients received standardized treatment and follow-up, decreasing the likelihood of potential selection bias and confounders (26–35).
In order to detect long-term effects of T2DM in HTX recipients, we selected adult HTX recipients who received HTX at Heidelberg Heart Center between 01/01/2000 and 01/10/2016, enabling a minimum post-transplant follow-up of 5 years. This study included data of HTX recipients over a period of more than 20 years. A possible era effect as a result of changes in medical and surgical care may have therefore affected our results. As tacrolimus replaced cyclosporine A as the main immunosuppressive agent from 2006 onward, we investigated a possible era effect by comparing the immunosuppressive drug therapy of HTX recipients with or without T2DM. We could neither detect a statistically significant difference between HTX recipients with or without T2DM regarding the use of cyclosporine A or tacrolimus, nor concerning the use of azathioprine or mycophenolate mofetil supporting the robustness of our findings (26–35).
Our results should be interpreted as hypothesis-generating, especially in the context of post-transplant survival. We can therefore neither proof nor disproof a causal relationship between T2DM at the time of HTX and increased 5-year post-transplant mortality but merely indicate an association. In addition, the effects of the recently introduced SGLT-2 inhibitors on long-term post-transplant mortality in HTX recipients remain unknown and require further investigation, preferably in form of large multicenter trials.
Conclusion
The number of HTX recipients with pre-transplant T2DM has continuously been growing over the last decades. Many of these patients suffer from impaired wound healing, infections, renal dysfunction, thromboembolic complications, cardiac rejections, and cardiac allograft vasculopathy. Management of HTX recipients with T2DM is therefore very challenging but data about this topic are still very limited. In order to investigate the effects of pre-transplant T2DM on survival and causes of death after HTX, we performed a large study with a contemporary population of 376 HTX recipients including 121 patients with T2DM (32.2%). We observed a significantly higher 5-year all-cause mortality after HTX in patients with pre-transplant T2DM (41.3% versus 29.8%, P = 0.027) along with a higher percentage of death due to graft failure (14.9% versus 7.8%, P = 0.035). Multivariate analysis indicated pre-transplant T2DM as a significant risk factor for 5-year mortality after HTX (HR: 1.563; 95% CI: 1.053–2.319; P = 0.027). Stratification of HTX recipients with pre-transplant T2DM showed no statistically significant difference in 5-year survival between patients with and without insulin therapy (P = 0.243) but patients with pre-transplant T2DM and a HbA1c < 7.0% had a significantly better 5-year survival than patients with a HbA1c ≥ 7.0% (P = 0.008). Of note, patients with T2DM and a HbA1c < 7.0% had a similar 5-year survival after HTX compared to patients without T2DM. Therefore, patients with T2DM can successfully undergo HTX if they receive optimal diabetes management before and after HTX.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Heidelberg University, Germany (ethical approval number: S-286/2015, Version 1.2, 28-07-2020). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RR, CG, TB, and LK: conceptualization, methodology, and investigation. RR, CG, MH, FD, TB, and LK: formal analysis, validation, and data curation. RR, CG, and LK: writing – original draft preparation, review and editing. RR, CG, FD, and LK: visualization. RR and FD: funding acquisition. RR, PE, WS, GW, SK, JS, NF, and LK: supervision and resources. All authors: contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Viola Deneke and Berthold Klein for their assistance and advice.
Funding
This work was supported by the Physician-Scientist-Program of the Faculty of Medicine, University of Heidelberg (RR and FD) and the German Heart Foundation/German Foundation of Heart Research (RR). For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program “Open Access Publikationskosten” as well as by Heidelberg University.
References
- 1.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Professional Practice Committee [ADAPPC], Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S17–38. 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. (2017) 389:2239–51. 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 4.Cimato TR, Jessup M. Recipient selection in cardiac transplantation: contraindications and risk factors for mortality. J Heart Lung Transplant. (2002) 21:1161–73. 10.1016/s1053-2498(02)00428-x [DOI] [PubMed] [Google Scholar]
- 5.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. (2010) 29:914–56. 10.1016/j.healun.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 6.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. (2010) 122:173–83. 10.1161/CIRCULATIONAHA.109.858076 [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. Listing criteria for heart transplantation: international Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant. (2006) 25:1024–42. 10.1016/j.healun.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. (2016) 35:1–23. 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 9.Faglia E, Favales F, Mazzola E, Pizzi G, De Maria R, Mangiavacchi M, et al. Heart transplantation in mildly diabetic patients. Diabetes. (1990) 39:740–2. 10.2337/diab.39.6.740 [DOI] [PubMed] [Google Scholar]
- 10.Ladowski JS, Kormos RL, Uretsky BF, Griffith BP, Armitage JM, Hardesty RL. Heart transplantation in diabetic recipients. Transplantation. (1990) 49:303–5. 10.1097/00007890-199002000-00015 [DOI] [PubMed] [Google Scholar]
- 11.Munoz E, Lonquist JL, Radovancevic B, Baldwin RT, Ford S, Duncan JM, et al. Long-term results in diabetic patients undergoing heart transplantation. J Heart Lung Transplant. (1992) 11:943–9. [PubMed] [Google Scholar]
- 12.Rhenman MJ, Rhenman B, Icenogle T, Christensen R, Copeland J. Diabetes and heart transplantation. J Heart Transplant. (1988) 7:356–8. [PubMed] [Google Scholar]
- 13.Czerny M, Sahin V, Fasching P, Zuckermann A, Zimpfer D, Kilo J, et al. The impact of diabetes mellitus at the time of heart transplantation on long-term survival. Diabetologia. (2002) 45:1498–508. 10.1007/s00125-002-0960-0 [DOI] [PubMed] [Google Scholar]
- 14.Kilic A, Weiss ES, George TJ, Arnaoutakis GJ, Yuh DD, Shah AS, et al. What predicts long-term survival after heart transplantation? An analysis of 9,400 ten-year survivors. Ann Thorac Surg. (2012) 93:699–704. 10.1016/j.athoracsur.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 15.Kirklin JK, Naftel DC, Bourge RC, McGiffin DC, Hill JA, Rodeheffer RJ, et al. Evolving trends in risk profiles and causes of death after heart transplantation: a ten-year multi-institutional study. J Thorac Cardiovasc Surg. (2003) 125:881–90. 10.1067/mtc.2003.168 [DOI] [PubMed] [Google Scholar]
- 16.Radovancevic B, Konuralp C, Vrtovec B, Radovancevic R, Thomas CD, Zaqqa M, et al. Factors predicting 10-year survival after heart transplantation. J Heart Lung Transplant. (2005) 24:156–9. 10.1016/j.healun.2003.11.399 [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Pflugfelder PW, Kostuk WJ. Increased morbidity in diabetic cardiac transplant recipients. Can J Cardiol. (2009) 25:e125–9. 10.1016/s0828-282x(09)70071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang CC, Beniaminovitz A, Edwards N, Mancini DM. Morbidity and mortality in diabetic patients following cardiac transplantation. J Heart Lung Transplant. (2003) 22:244–9. 10.1016/s1053-2498(02)00567-3 [DOI] [PubMed] [Google Scholar]
- 19.Mancini D, Beniaminovitz A, Edwards N, Chen J, Maybaum S. Survival of diabetic patients following cardiac transplant. J Heart Lung Transplant. (2001) 20:168. 10.1016/s1053-2498(00)00311-9 [DOI] [PubMed] [Google Scholar]
- 20.Marelli D, Laks H, Patel B, Kermani R, Marmureanu A, Patel J, et al. Heart transplantation in patients with diabetes mellitus in the current era. J Heart Lung Transplant. (2003) 22:1091–7. 10.1016/s1053-2498(02)01219-6 [DOI] [PubMed] [Google Scholar]
- 21.Russo MJ, Chen JM, Hong KN, Stewart AS, Ascheim DD, Argenziano M, et al. Survival after heart transplantation is not diminished among recipients with uncomplicated diabetes mellitus: an analysis of the United Network of Organ Sharing database. Circulation. (2006) 114:2280–7. 10.1161/CIRCULATIONAHA.106.615708 [DOI] [PubMed] [Google Scholar]
- 22.Moro JA, Martínez-Dolz L, Almenar L, Martínez-Ortiz L, Chamorro C, García C, et al. Impacto de la diabetes mellitus en el paciente con trasplante cardiaco [Impact of diabetes mellitus on heart transplant recipients]. Rev Esp Cardiol. (2006) 59:1033–7. 10.1157/13093980 Spanish, [DOI] [PubMed] [Google Scholar]
- 23.Morgan JA, John R, Weinberg AD, Colletti NJ, Mancini DM, Edwards NM. Heart transplantation in diabetic recipients: a decade review of 161 patients at Columbia Presbyterian. J Thorac Cardiovasc Surg. (2004) 127:1486–92. 10.1016/j.jtcvs.2003.11.063 [DOI] [PubMed] [Google Scholar]
- 24.Chamarthi B, Zafrir B, Plutzky J, Givertz MM. Impact of pre-diabetes on heart transplant outcomes in patients with advanced heart failure. J Heart Lung Transplant. (2014) 33:215–7. 10.1016/j.healun.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng KY, Henricksen EJ, Wayda B, Moayedi Y, Lee R, Han J, et al. Impact of diabetes mellitus on clinical outcomes after heart transplantation. Clin Transplant. (2021) 35:e14460. 10.1111/ctr.14460 [DOI] [PubMed] [Google Scholar]
- 26.Darche FF, Helmschrott M, Rahm AK, Thomas D, Schweizer PA, Bruckner T, et al. Atrial fibrillation before heart transplantation is a risk factor for post-transplant atrial fibrillation and mortality. ESC Heart Fail. (2021) 8:4265–77. 10.1002/ehf2.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heil KM, Helmschrott M, Darche FF, Bruckner T, Ehlermann P, Kreusser MM, et al. Risk factors, treatment and prognosis of patients with lung cancer after heart transplantation. Life. (2021) 11:1344. 10.3390/life11121344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahm AK, Helmschrott M, Darche FF, Thomas D, Bruckner T, Ehlermann P, et al. Newly acquired complete right bundle branch block early after heart transplantation is associated with lower survival. ESC Heart Fail. (2021) 8:3737–47. 10.1002/ehf2.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, et al. Combined amiodarone and digitalis therapy before heart transplantation is associated with increased post-transplant mortality. ESC Heart Fail. (2020) 7:2082–92. 10.1002/ehf2.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, et al. Risk factors and survival of patients with permanent pacemaker implantation after heart transplantation. J Thorac Dis. (2019) 11:5440–52. 10.21037/jtd.2019.11.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivinius R, Helmschrott M, Ruhparwar A, Darche FF, Thomas D, Bruckner T, et al. Comparison of posttransplant outcomes in patients with no, acute, or chronic amiodarone use before heart transplantation. Drug Des Devel Ther. (2017) 11:1827–37. 10.2147/DDDT.S136948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, et al. Elevated pre-transplant pulmonary vascular resistance is associated with early post-transplant atrial fibrillation and mortality. ESC Heart Fail. (2020) 7:176–87. 10.1002/ehf2.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, et al. COPD in patients after heart transplantation is associated with a prolonged hospital stay, early posttransplant atrial fibrillation, and impaired posttransplant survival. Clin Epidemiol. (2018) 10:1359–69. 10.2147/CLEP.S171929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Erbel C, Gleissner CA, et al. Long-term use of amiodarone before heart transplantation significantly reduces early post-transplant atrial fibrillation and is not associated with increased mortality after heart transplantation. Drug Des Devel Ther. (2016) 10:677–86. 10.2147/DDDT.S96126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Klein B, Erbel C, et al. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des Devel Ther. (2014) 9:93–102. 10.2147/DDDT.S75464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. (2016) 37:37–61. 10.1210/er.2015-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almuti K, Haythe J, Tsao L, Naka Y, Mancini D. Does renal function deteriorate more rapidly in diabetic cardiac transplant recipients? Transplantation. (2007) 83:550–3. 10.1097/01.tp.0000253428.60083.df [DOI] [PubMed] [Google Scholar]
- 38.Klingenberg R, Gleissner C, Koch A, Schnabel PA, Sack FU, Zimmermann R, et al. Impact of pre-operative diabetes mellitus upon early and late survival after heart transplantation: a possible era effect. J Heart Lung Transplant. (2005) 24:1239–46. 10.1016/j.healun.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 39.Lachance K, White M, de Denus S. Risk factors for chronic renal insufficiency following cardiac transplantation. Ann Transplant. (2015) 20:576–87. 10.12659/AOT.893788 [DOI] [PubMed] [Google Scholar]
- 40.Kilic A, Acker MA, Atluri P. Dealing with surgical left ventricular assist device complications. J Thorac Dis. (2015) 7:2158–64. 10.3978/j.issn.2072-1439.2015.10.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long B, Robertson J, Koyfman A, Brady W. Left ventricular assist devices and their complications: a review for emergency clinicians. Am J Emerg Med. (2019) 37:1562–70. 10.1016/j.ajem.2019.04.050 [DOI] [PubMed] [Google Scholar]
- 42.Cehic MG, Muir CA, Greenfield JR, Hayward C, Jabbour A, Keogh A, et al. Efficacy and safety of empagliflozin in the management of diabetes mellitus in heart transplant recipients. Transplant Direct. (2019) 5:e450. 10.1097/TXD.0000000000000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ertuglu LA, Porrini E, Hornum M, Demiray A, Afsar B, Ortiz A, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors for diabetes after solid organ transplantation. Transpl Int. (2021) 34:1341–59. 10.1111/tri.13883 [DOI] [PubMed] [Google Scholar]
- 44.Grubić Rotkvić P, Cigrovski Berković M, Rotkvić L, Bulj N. Prevention of cardiac allograft vasculopathy - A new possible indication for SGLT-2 inhibitors? Med Hypotheses. (2020) 137:109594. 10.1016/j.mehy.2020.109594 [DOI] [PubMed] [Google Scholar]
- 45.Sammour Y, Nassif M, Magwire M, Thomas M, Fendler T, Khumri T, et al. Effects of GLP-1 receptor agonists and SGLT-2 inhibitors in heart transplant patients with type 2 diabetes: initial report from a cardiometabolic center of excellence. J Heart Lung Transplant. (2021) 40:426–9. 10.1016/j.healun.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 46.Schwarzenbach M, Bernhard FE, Czerlau C, Sidler D. Chances and risks of sodium-glucose cotransporter 2 inhibitors in solid organ transplantation: a review of literatures. World J Transplant. (2021) 11:254–62. 10.5500/wjt.v11.i7.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marfella R, Amarelli C, Cacciatore F, Balestrieri ML, Mansueto G, D’Onofrio N, et al. Lipid accumulation in hearts transplanted from nondiabetic donors to diabetic recipients. J Am Coll Cardiol. (2020) 75:1249–62. 10.1016/j.jacc.2020.01.018 [DOI] [PubMed] [Google Scholar]
- 48.Marfella R, D’Onofrio N, Trotta MC, Sardu C, Scisciola L, Amarelli C, et al. Sodium/glucose cotransporter 2 (SGLT2) inhibitors improve cardiac function by reducing JunD expression in human diabetic hearts. Metabolism. (2022) 127:154936. 10.1016/j.metabol.2021.154936 [DOI] [PubMed] [Google Scholar]
- 49.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. (2004) 23:S187–93. 10.1016/j.healun.2004.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.