Abstract
Spontaneous intracranial hypotension can be caused by spinal dural tears or CSF-venous fistulas. It is rare for patients to have more than one type of leak at any given time. Here, we illustrate 3 examples of dural tears that co-existed with CSF-venous fistulas, with both being seen on dynamic CT myelography. To our knowledge, coexistent CSF-venous fistulas and dural tears have not been previously illustrated on dynamic CT myelography, even though this is one of the most commonly used modalities to work-up patients with CSF leaks. We discuss the clinical importance of the rare co-occurrence of these leaks with regard to diagnosis and treatment, as well as implications for understanding and classifying CSF leaks.
Keywords: Dynamic CT myelography, Lateral decubitus, Fast CSF leak, Extradural fluid collection, CSF-venous fistula
Abbreviations: CVF, CSF-venous fistula; SIH, spontaneous intracranial hypotension; SLEC, spinal longitudinal extradural collection
Introduction
Spinal CSF leaks are the underlying cause of spontaneous intracranial hypotension (SIH). In recent years, knowledge of CSF leak types and optimal imaging techniques for their localization have evolved substantially. Farb et al. recently introduced a classification scheme for CSF leaks, which includes ventral dural tears (type I), proximal nerve root sleeve tears (type II), CSF-venous fistulas (CVF, type III), and distal nerve root sleeve tears (type IV) [1]. Type I and II leaks are associated with spinal longitudinal extradural collections (SLECs). In contrast, type III and IV leaks are SLEC-negative, because leaked CSF either enters a vein (type III) or disperses in the peripheral extraspinal space (type IV). A similar classification scheme was previously introduced by Schievink et al. [2].
Digital subtraction myelography (DSM) and/or dynamic CT myelography (CTM) are used to identify CSF leaks at many centers. The precise technique for either modality varies among institutions and is tailored to the type of leak the patient is suspected to have, which is largely dependent on the presence or absence of a SLEC [3,4]. It is usually assumed that patients with SIH have a single type of leak accounting for their symptoms. Schievink et al. recently showed that CVFs can rarely co-occur with dural tears [5]. This finding was seen in 5 of 398 SLEC-positive patients who underwent DSM in a single study, with the CVFs being at or near the site of dural tear in all 5 cases. Relatively few institutions routinely perform DSM, so it is important to know whether other imaging modalities can detect these rare combined dural tears and CVFs. Here, we describe 3 SLEC-positive patients with co-existing dural leaks and CSF-venous fistulas, with both lesions demonstrated on dynamic CTM.
Case examples
Patient 1
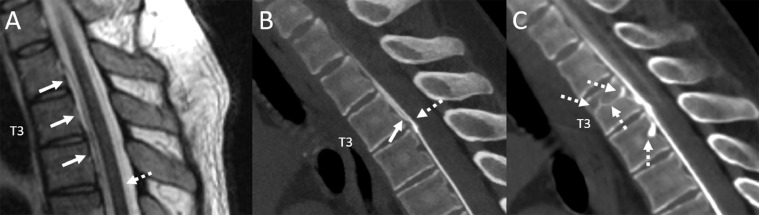
A 46-year-old man presented with years of orthostatic headaches. Spine MRI revealed a SLEC extending from T2 to T4 (Fig. 1). Prone dynamic CT myelogram showed fast CSF leak from a ventral dural tear at T3-T4. On serial CT acquisitions, there was also a CVF involving the basivertebral veins at T3 and T4 (Fig. 1). The patient underwent surgical treatment of both the dural tear and CVF with symptomatic improvement on 6-month follow-up.
Fig. 1.
Sagittal T2W thoracic spine MRI shows a thin ventral epidural fluid collection spanning T2 through T4 (A, arrows). Sagittal reconstructed image from a prone dynamic CT myelogram (B) demonstrates contrast leak into the ventral fluid collection at the T3-T4 interspace, compatible with fast leak from a ventral dural tear (B, dashed arrow), with superior flow of leaked epidural contrast (B, solid arrow). Sagittal image from a subsequent scan obtained one minute later shows a complex CSF-venous fistula involving the basivertebral veins at T3 and T4 (C, dashed arrows).
Patient 2
A 67-year-old woman presented with 5 years of orthostatic headaches and tinnitus. MRI brain showed brain sag and pachymeningeal enhancement (not shows). Spine MRI showed a ventral SLEC extending from C2 through T8 (Fig. 2). Prone dynamic CT myelogram showed both a fast leak related to a ventral dural tear at T8, as well as separate CVF at T6 (Fig. 2). The patient underwent surgical repair of a ventral dural tear at T8 and ligation of a CSF-venous fistula at T6 with symptomatic improvement at 3-month follow-up.
Fig. 2.
Sagittal T2W MRI demonstrates a thin ventral extradural fluid collection (A, arrows). Sagittal reconstructed image from a subsequent prone dynamic CT myelogram shows a ventral leak at the T8-T9 interspace with clearly distinct epidural contrast (B, solid arrow) and intrathecal contrast (B, dashed arrow). Axial image from the same exam demonstrates a T6 CSF-venous fistula with opacification of intraosseous veins (C, dashed arrows), as well as the right paraspinal vein (C, solid arrow).
Patient 3
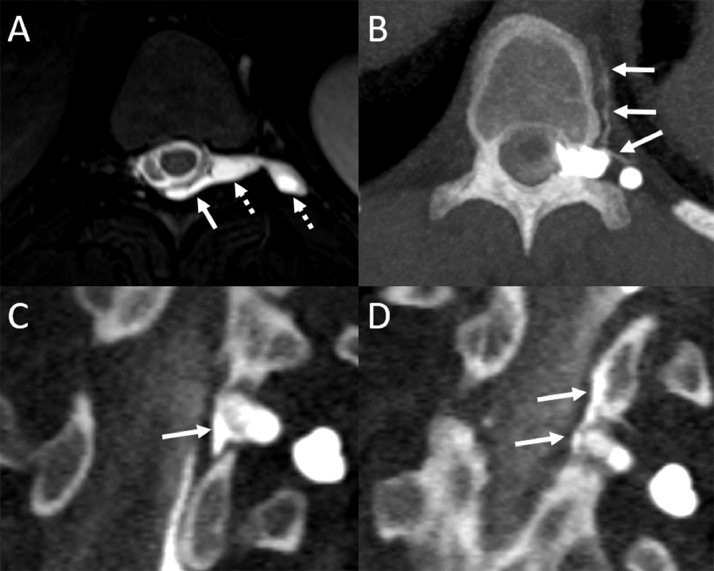
A 26-year-old woman presented with 20 years of orthostatic headaches with brain sag on MRI (not shown). Spine MRI showed a large left T10 meningeal diverticulum (Fig. 3) and an adjacent SLEC. Left lateral decubitus dynamic CT myelogram showed both a fast leak from the left T10 proximal nerve root sleeve, as well as a CVF arising from an adjacent left T10 meningeal diverticulum (Fig. 3). She underwent combined surgical repair of both the CVF and proximal nerve root sleeve tear with symptomatic improvement and resolution of MRI findings of brain sag on 3-month follow-up.
Fig. 3.
Axial T2W MR image shows a large left T10 meningeal diverticulum (A, dashed arrows) and adjacent dorsal epidural fluid collection (A, solid arrow). Multiple consecutive axial (A) and coronal reformatted (C-D) images from a left lateral decubitus dynamic CT myelogram show a left T10 CSF-venous fistula arising from the meningeal diverticulum (B, arrows). There is an additional adjacent dural leak from the proximal nerve root sleeve along the same left T10 diverticulum (C, solid arrows). Epidural contrast extends superiorly on a subsequently obtained image (D, solid arrows).
Discussion
We have illustrated 3 examples of dynamic CTM identifying co-existing fast leaks (dural tears) and CVFs in SLEC-positive patients. In these instances, the CVFs were entirely unexpected, because SLEC-positive patients are presumed to have an isolated fast leak from a dural tear based on conventional understanding and spinal leak nomenclature [6]. In 2 cases (patients 1 and 2), dynamic CTM was performed in the prone position since the patient had a ventral epidural fluid collection, indicative of a ventral dural tear. The associated CVF was also ventrally located, draining into the basivertebral and/or paraspinal veins near the dural leak. In the other case (patient 3), lateral decubitus dynamic CTM was performed, because the patient had a large lateral meningeal diverticulum. In this case, the meningeal diverticulum harbored both a proximal nerve root sleeve tear and a CVF.
It is important to note that these cases are not felt to be secondary to leaked contrast being “taken up” by paraspinal or epidural veins, but rather thought to be caused by true CVFs (abnormal intrathecal-venous connections). Although direct communications between the epidural space and adjacent veins have been described, the patients in this series all had CSF-venous fistulas discovered during surgical exploration [7].
The presented cases are clinically important for several reasons. First, our findings corroborate the phenomena of co-existent ventral and lateral dural tears with CVFs. Second, as recently suggested by Schievink et al., CVFs appear to occur at or near the site of dural tear in these cases [5]. Third, we show that these combined lesions can be detected on dynamic CTM, which is more widely available than DSM. Institutions performing dynamic CTM in SLEC-positive patients for fast leak localization should evaluate exams for the presence of a CVF in addition to dural tear. The presence of a CVF may suggest the need for surgical management to ensure that both sources of leak are treated (rather than epidural blood patch or transvenous embolization, which can be effective for dural tears or CVFs, respectively) [8].
In addition to these diagnostic and treatment implications, these cases provide interesting insights into the pathogenesis of CVFs. CVFs have been increasingly recognized since their initial description in 2014, but the mechanism by which they develop remains unclear [9]. SIH is associated with connective tissue disorders as well as congenital vascular disease such as Klippel-Trenaunay syndrome [10]. Some have speculated that CVFs form at the site of prior dural tears due to abnormal venous incorporation occurring during the healing process [1]. This theory would be consistent with the identification of CVFs adjacent to dural tears in these patients. It may be that imaging patients before a dural defect has completely healed can reveal a dural leak and CVF, whereas imaging after a dural defect has healed shows only the newly developed CVF. Such a mechanism could also account for the apparently de novo emergence of some CVFs [5]. Of course, this remains speculative and requires further study. Since there are so few reports of co-occurring dural tears and CVFs, there are likely additional mechanisms by which CVFs develop. For example, persistent spinal arachnoid granulations along the nerve root sleeves may also play a role in CVF pathogenesis [11].
We are uncertain whether these cases are best classified as subtypes of type I and type II leaks or if these should be classified as a new/transitional leak type. Regardless, it is clear that CVFs can be seen in SLEC-positive patients both on DSM and dynamic CTM. Radiologists performing either procedure should be aware of this rare but clinically important phenomenon, as definitive management likely requires surgery.
Author contribution
All authors contributed to drafting and editing the manuscript and have approved the submitted version.
Ethical standards
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Compliance with ethical standards was followed.
Data regarding the study are available upon request.
Patient consent
Informed consent was obtained from all individual participants included in the study. Consent was provided for publication.
Footnotes
Competing Interests: All authors declare no conflict of interest.
Funding: No funding was received for this study.
References
- 1.Farb R.I., Nicholson P.J., Peng P.W., Massicotte E.M., Lay C., Krings T., et al. Spontaneous Intracranial Hypotension: A Systematic Imaging Approach for CSF Leak Localization and Management Based on MRI and Digital Subtraction Myelography. AJNR Am J Neuroradiol. 2019;40(4):745–753. doi: 10.3174/ajnr.A6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schievink W.I., Maya M.M., Jean-Pierre S., Nuno M., Prasad R.S., Moser F.G. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87(7):673–679. doi: 10.1212/WNL.0000000000002986. [DOI] [PubMed] [Google Scholar]
- 3.Kim D.K., Carr C.M., Benson J.C., Diehn F.E., Lehman V.T., Liebo G.B., et al. Diagnostic Yield of Lateral Decubitus Digital Subtraction Myelogram Stratified by Brain MRI Findings. Neurology. 2021;96(9) doi: 10.1212/WNL.0000000000011522. e1312-e8. [DOI] [PubMed] [Google Scholar]
- 4.Mamlouk M.D., Ochi R.P., Jun P., Shen P.Y. Decubitus CT Myelography for CSF-Venous Fistulas: A Procedural Approach. AJNR Am J Neuroradiol. 2021;42(1):32–36. doi: 10.3174/ajnr.A6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schievink W.I., Maya M.M., Moser F., Prasad R., Wadhwa V., Cruz R., et al. Multiple Spinal CSF Leaks in Spontaneous Intracranial Hypotension: Do They Exist? Neurol Clin Pract. 2021;11(5) doi: 10.1212/CPJ.0000000000001084. e691-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoorn J.T., Luetmer P.H., Carr C.M., Lane J.I., Lehman V.T., Morris J.M., et al. Predicting High-Flow Spinal CSF Leaks in Spontaneous Intracranial Hypotension Using a Spinal MRI-Based Algorithm: Have Repeat CT Myelograms Been Reduced? AJNR Am J Neuroradiol. 2016;37(1):185–188. doi: 10.3174/ajnr.A4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffington C.W., Nichols L., Moran P.L., Blix E.U. Direct connections between the spinal epidural space and the venous circulation in humans. Reg Anesth Pain Med. 2011;36(2):134–139. doi: 10.1097/AAP.0b013e31820d41ab. [DOI] [PubMed] [Google Scholar]
- 8.Brinjikji W., Savastano L.E., Atkinson J.L.D., Garza I., Farb R., Cutsforth-Gregory J.K. A Novel Endovascular Therapy for CSF Hypotension Secondary to CSF-Venous Fistulas. AJNR Am J Neuroradiol. 2021;42(5):882–887. doi: 10.3174/ajnr.A7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schievink W.I., Moser F.G., Maya M.M. CSF-venous fistula in spontaneous intracranial hypotension. Neurology. 2014;83(5):472–473. doi: 10.1212/WNL.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 10.Madhavan A.A., Kim D.K., Carr C.M., Luetmer P.H., Covington T.N., Cutsforth-Gregory J.K., et al. Association Between Klippel-Trenaunay Syndrome and Spontaneous Intracranial Hypotension: A Report of 4 Patients. World Neurosurg. 2020;138:398–403. doi: 10.1016/j.wneu.2020.03.148. [DOI] [PubMed] [Google Scholar]
- 11.Kido D.K., Gomez D.G., Pavese A.M., Jr., Potts D.G. Human spinal arachnoid villi and granulations. Neuroradiology. 1976;11(5):221–228. doi: 10.1007/BF00328377. [DOI] [PubMed] [Google Scholar]