Abstract
Diffuse midline gliomas are a rare relatively new classification of primary central nervous system tumors which include astrocytomas, oligodendrogliomas, and glioblastomas. The T2-FLAIR mismatch sign is regarded as a highly specific imaging feature of IDH-mutant, 1p/19q non-codeleted astrocytomas. The case presented herein demonstrates this sign, however, in a non-IDH mutated diffuse midline glioma with a H3K27M mutation, a World Health Organization Grade IV neoplasm. Although preoperative diagnosis can provide important treatment and prognostic information, it is often quite difficult particularly in primary central nervous system tumors.
Keywords: T2-FLAIR mismatch, Adult brainstem glioma, Diffuse midline glioma, H3K27M mutation, IDH mutation, Astrocytoma
Introduction
Diffuse midline gliomas (DMG) are a rare new classification of primary CNS tumors, according to the 2016 World Health Organization (WHO). Adult brainstem gliomas are a subtype of DMGs which previously were grouped with their pediatric counterparts despite differences in behavior. A specific subtype of adult brainstem gliomas is characterized by the K27M mutation which are designated as WHO grade IV, regardless of histological characteristics.
DMGs are often classified on the presence of an IDH mutation which include oligodendrogliomas and IDH-mutant astrocytomas whereas lack of an IDH mutation is classically seen in glioblastomas. Oligodendrogliomas contain a 1p/19q co-deletion, whereas IDH-mutant astrocytomas do not. The T2-FLAIR mismatch sign has been reported to have near-perfect specificity for the IDH-mutant, 1p/19q non-codeleted astrocytoma. The case reported herein demonstrates this T2-FLAIR mismatch sign, however in a non-IDH mutated DMG with a H3K27M mutation.
Case report
A 38-year-old man with no prior medical history presented to an outside institution with two weeks of mild right-sided hearing loss and a one-week history of progressive bilateral headache without sensitivity to light or sound. The physical examination was unremarkable without any focal neurological deficit.
The patient underwent a head CT without contrast (Fig. 1) at an outside hospital demonstrating a large heterogeneously attenuating mass measuring 6.1 × 3.3 × 4.7 cm centered within the left thalamus with superior extension into the body of the left lateral ventricle. There is mass effect on the third ventricle with resultant moderate obstructive hydrocephalus. No periventricular hypodensity to suggest transependymal flow was noted.
Fig. 1.

Axial CT of the head without contrast at the level of the lateral ventricles demonstrates a large heterogenous mass (red arrows) originating from the left thalamus and extending superiorly into the lateral ventricle. There is also rightward mass effect on the third ventricle with resultant moderate obstructive hydrocephalus. (Color figures are available online.)
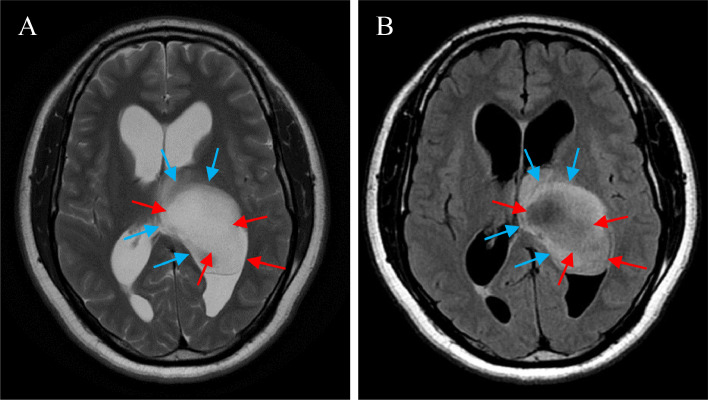
A next day MRI demonstrated a predominantly T1 hypointense mass with surrounding subtle hyperintense signal. T2-weighted imaging demonstrated the same mass with predominantly T2 hyperintense signal and surrounding subtle hypointense signal (Fig. 2A). On FLAIR sequencing, the T2 hyperintense regions demonstrated suppression (Fig. 2B). There is incomplete peripherally based restricted diffusion (images not shown). No blooming artifact was noted on the SWAN sequence (images not shown). Postcontrast sequences demonstrated minimal contrast enhancement (Figs. 3A-F). A stereotactic biopsy was then performed by the neurosurgical team.
Fig. 2.
(A-B) Axial T2WI of the brain at the level of the lateral ventricles (A) demonstrates a predominantly T2 hyperintense mass (red arrows) extending from the region of the left thalamus into the left ventricle. There is subtle associated T2 hypointense signal peripherally (blue arrows). Axial FLAIR sequence at the same level (B) demonstrates suppression of the previously noted mass (red arrows). The previously described peripheral T2 hypointense signal reverts to a hyperintense signal on FLAIR sequencing. (Color figures are available online.)
Fig. 3.
A-F: Axial (A), coronal (C), and sagittal (E) T1-weighted precontrast images demonstrate a predominantly T1 hypointense mass (red arrows) with subtle peripheral T1 hyperintense signal (blue arrows) originating from the left thalamus and extending superiorly into the left lateral ventricle. Axial (B), coronal (D), and sagittal (F) T1-weighted postcontrast images demonstrate minimal contrast enhancement. (Color figures are available online.)
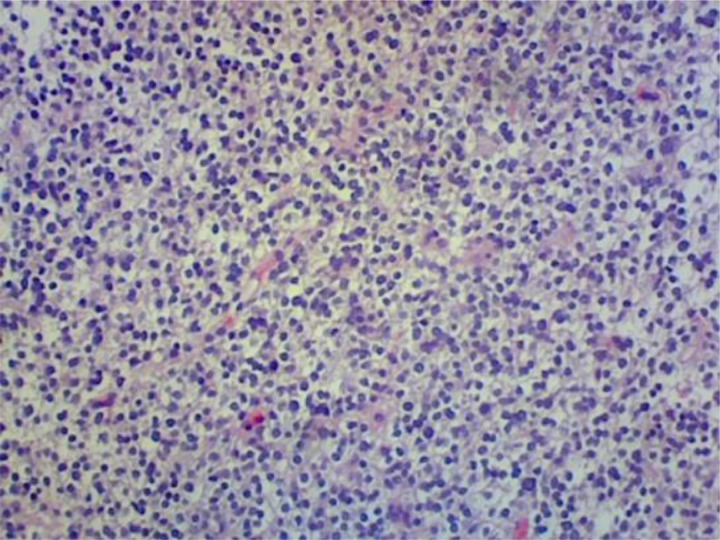
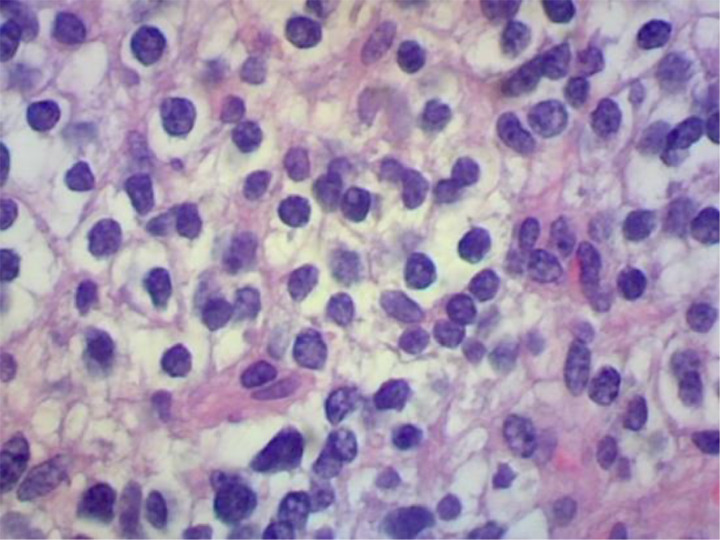
The initial pathology demonstrated a cellular neoplasm forming sheets of cells with oval nuclei (Fig. 4), dense chromatin pattern, mild variation of nuclear size (Fig. 5), and a low mitotic index. There are also delicate blood vessels that intervene within the neoplasm. Subsequent staining analysis demonstrated negative EMA but positive GFAP, OLIG2, and synaptophysin with a small percentage of ATRX staining. Staining for KI-67 revealed approximately 15% positivity (Fig. 6). Thus, most consistent with a WHO grade II oligodendroglioma. Additional outside laboratory testing demonstrated no IDH mutation and a 19q deletion with polysomy of chromosome 19, without codeletion of 1p.
Fig. 4.
Low power photomicrograph demonstrates sheets of cells with oval nuclei.
Fig. 5.
High power photomicrograph demonstrates a dense chromatin pattern with varying nuclear size.
Fig. 6.
Photomicrograph demonstrates approximately 15% Ki-67 staining.
Additional staining demonstrated CD99 negative, NeuN negative, and S100 positive. The case was then sent out for external review with an ultimate diagnosis of diffuse midline glioma, H3K27M mutant (WHO grade IV).
Discussion
DMG are a new entity according to the 2016 WHO Classification of CNS tumors. The previous 2007 WHO Classification categorized all tumors with an astrocytic phenotype separately from those with an oligodendroglial phenotype, despite the clinical characteristics [1]. Now, the 2016 WHO classification categorizes all diffuse infiltrating gliomas (whether astrocytic or oligodendroglial) based on behavior as well-as shared genetic driver mutations in the IDH1 and IDH2 genes. In this new classification, DMGs include WHO grade II/III astrocytic tumors, grade II/III oligodendrogliomas, grade IV glioblastomas, and the diffuse gliomas of childhood [2].
The T2-FLAIR mismatch sign is defined by the presence of near complete or homogenous hyperintense signal on T2 weighted imaging and relatively hypointense signal on FLAIR sequences, with the exception of a hyperintense rim [3]. This imaging pattern has been found to be a highly specific feature of IDH-mutant, 1p/19q non-codeleted astrocytomas. Given that the initial imaging featured this T2-FLAIR mismatch sign, it was suspected that the pathology would be concordant with an IDH-mutant, 1p/19q non-codeleted astrocytoma. However, the special testing subsequently confirmed an H3K27M altered tumor. Since the first publication of the T2-FLAIR mismatch sign, additional studies have been published substantiating this phenomenon and the corresponding IDH mutant pathology [4], [5], [6]. However, there have been reports of false positive cases with varying pathologies including the H3K27M-mutant midline glioma, similar to our case [7,8]. Further studies have been performed reviewing the validity of this sign noting the possible differences of various imaging acquisition parameters and strictness of the inclusion criteria [9].
Adult brainstem gliomas are a subtype of diffuse midline gliomas arising from midline structures of the brainstem such as midbrain, pons, medulla, cerebellar peduncles, and cerebellopontine angle. Previously, pediatric diffuse gliomas were grouped with their adult counterparts despite differences in behavior yet similar histological patterns. A specific subgroup of these tumors is characterized by K27M mutations in the histone H3 gene H3F3A that demonstrate a diffuse growth pattern and a midline location [2]. This subgroup is termed DMG, H3K27M mutants which correspond to WHO grade IV, regardless of histological characteristics. H3K27M-mutant gliomas represent approximately 3% of adult infiltrating gliomas [10]. It is now known that H3K27M-mutant brainstem gliomas occur in all age groups, although are less well understood in adults [11].
When present in the adult population, the mean age of diagnosis is often the 4-5th decade. These tumors are currently not completely characterized clinically, but often demonstrate an overall aggressive clinical course. Of note, studies have shown that brainstem gliomas are generally less aggressive in adults than in children. However, it is still unknown whether H3K27M-mutant adult tumors exhibit similar behavior to their pediatric counterparts [11].
The pathologic spectrum of H3K27M-mutant gliomas includes both low-grade and high-grade tumors at various anatomic locations. Specifically, tumors arising from the thalamus have been shown to follow a less aggressive phenotype and are associated with a better prognosis for H3-mutant thalamic gliomas compared to H3-mutant brainstem gliomas at other anatomic sites [11,12].
Specific imaging characteristics of the adult H3K27M-mutant are emerging. Interestingly, studies have shown heterogeneity of both anatomic sites and imaging features in these tumors. Most tumors demonstrate an expansile T2-FLAIR hyperintense mass with variable contrast enhancement. Of note, tumors arising from the thalamus can show partial or no enhancement [11]. Rarely, leptomeningeal and subependymal spread can be seen [12]. Studies have shown no correlation between the presence of contrast enhancement and tumor grade [11]. Furthermore, a survival advantage for the nonenhancing tumors compared to those that enhanced has not been demonstrated [11].
Prognosis remains poor with a reported median overall survive ranging from 7 to 19.6 months [13]. Targeted therapies against H3K2M tumors are continually emerging, including chemotherapy agents such as panobinostat and JMJD3 inhibitors [11].
Conclusion
Identification of the T2-FLAIR mismatch sign on preoperative glioma imaging has been shown to be clinically useful in predicting IDH status with high specificity in determining IDH-mutant tumors. We present an interesting case in which the imaging demonstrates an example of the T2-FLAIR mismatch sign; although, the pathological diagnosis was confirmed to be an H3K27M-mutant tumor. We hope to bring awareness of the possibility of other pathological entities that can have a similar imaging appearance.
Acknowledgments
Acknowlegments
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.Acknowlegment
Patient consent
Informed written consent was obtained from the patient for publication of this Case Report and all imaging studies. Consent form on record.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 World Health Organization classification of tumors of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Patel SH, Poisson LM, Brat DJ, Zhou Y, Cooper L, Snuderl M, et al. T2–FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Research. 2017;23(20):6078–6085. doi: 10.1158/1078-0432.ccr-17-0560. [DOI] [PubMed] [Google Scholar]
- 4.Batchala PP, Muttikkal TJE, Donahue JH, Patrie JT, Schiff D, Fadul CE, et al. Neuroimaging-based classification algorithm for predicting 1P/19Q-codeletion status in IDH-mutant lower grade gliomas. Am J Neuroradiol. 2019 doi: 10.3174/ajnr.a5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broen MP, Smits M, Wijnenga MM, Dubbink HJ, Anten MHME, Schijns OEMG, et al. The T2-FLAIR mismatch sign as an imaging marker for non-enhancing IDH-mutant, 1p/19q-intact lower-grade glioma: a validation study. Neuro-Oncology. 2018;20(10):1393–1399. doi: 10.1093/neuonc/noy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasocki A, Gaillard F, Gorelik A, Gonzales M. MRI features can predict 1P/19Q status in intracranial gliomas. Am JNeuroradiol. 2018;39(4):687–692. doi: 10.3174/ajnr.a5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DR, Kaufmann TJ, Patel SH, Chi AS, Snuderl M, Jain R. There is an exception to every rule—T2-flair mismatch sign in gliomas. Neuroradiology. 2018;61(2):225–227. doi: 10.1007/s00234-018-2148-4. [DOI] [PubMed] [Google Scholar]
- 8.Juratli TA, Tummala SS, Riedl A, Daubner D, Hennig S, Penson T, et al. Radiographic assessment of contrast enhancement and T2/Flair mismatch sign in lower grade gliomas: Correlation with molecular groups. J Neuro-Oncol. 2018;141(2):327–335. doi: 10.1007/s11060-018-03034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain R, Johnson DR, Patel SH, Castillo M, Smits M, van den Bent MJ, et al. Real world” use of a highly reliable imaging sign: “T2-flair mismatch” for identification of IDH mutant astrocytomas. Neuro-Oncology. 2020;22(7):936–943. doi: 10.1093/neuonc/noaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dono A, Takayasu T, Ballester LY, Esquenazi Y. Adult diffuse midline gliomas: clinical, radiological, and genetic characteristics. J Clin Neurosci. 2020;82(Pt A):1–8. doi: 10.1016/j.jocn.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM, et al. Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol. 2018;77(4):302–311. doi: 10.1093/jnen/nly006. [DOI] [PubMed] [Google Scholar]
- 12.Qiu T, Chanchotisatien A, Qin Z, Wu J, Du Z, Zhang X, et al. Imaging characteristics of adult H3 K27M-mutant gliomas. Journal of Neurosurgery. 2020;133(6):1662–1670. doi: 10.3171/2019.9.jns191920. [DOI] [PubMed] [Google Scholar]
- 13.Yekula A, Gupta M, Coley N, U HS. Adult H3K27M-mutant diffuse midline glioma with gliomatosis cerebri growth pattern: case report and review of the literature. Int J Surg Case Rep. 2020;68:124–128. doi: 10.1016/j.ijscr.2020.02.046. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]