Abstract
Antibodies against enterocin A were obtained by immunization of rabbits with synthetic peptides PH4 and PH5 designed, respectively, on the N- and C-terminal amino acid sequences of enterocin A and conjugated to the carrier protein KLH. Anti-PH4-KLH antibodies not only recognized enterocin A but also pediocin PA-1, enterocin P, and sakacin A, three bacteriocins which share the N-terminal class IIa consensus motif (YGNGVXC) that is contained in the sequence of the peptide PH4. In contrast, anti-PH5-KLH antibodies only reacted with enterocin A because the amino acid sequences of the C-terminal parts of class IIa bacteriocins are highly variable. Enterocin A and/or pediocin PA-1 structural and immunity genes were introduced in Lactococcus lactis IL1403 to achieve (co)production of the bacteriocins. The level of production of the two bacteriocins was significantly lower than that obtained by the wild-type producers, a fact that suggests a low efficiency of transport and/or maturation of these bacteriocins by the chromosomally encoded bacteriocin translocation machinery of IL1403. Despite the low production levels, both bacteriocins could be specifically detected and quantified with the anti-PH5-KLH (anti-enterocin A) antibodies isolated in this study and the anti-PH2-KLH (anti-pediocin PA-1) antibodies previously generated (J. M. Martínez, M. I. Martínez, A. M. Suárez, C. Herranz, P. Casaus, L. M. Cintas, J. M. Rodríguez, and P. E. Hernández, Appl. Environ. Microbiol. 64:4536–4545, 1998). In this work, the availability of antibodies for the specific detection and quantification of enterocin A and pediocin PA-1 was crucial to demonstrate coproduction of both bacteriocins by L. lactis IL1403(pJM04), because indicator strains that are selectively inhibited by each bacteriocin are not available.
Bacteriocins produced by lactic acid bacteria (LAB) have received considerable research attention due to their potential application in the food industry as natural food preservatives (20, 26, 29, 42). In fact, the role of LAB and their bacteriocins as food biopreservatives may increase in the future as a result of consumer awareness of the potential risks derived not only from food-borne pathogens but also from the artificial chemical preservatives currently used to control them (28).
The application of bacteriocins in food biocontrol is mainly oriented towards two alternative directions: (i) the use of bacteriocin-producing LAB or (ii) the direct addition of bacteriocin preparations, either synthetic or purified from the culture supernatant of the producer strains. Such applications could be greatly facilitated with the development of efficient procedures for detection, quantification, and purification of bacteriocins (34). Up to now, bioassays that assess the inhibitory effect of bacteriocins on indicator microorganisms have been most commonly used for detection and quantification of bacteriocin activity. Although the importance of these biologically based methods in the bacteriocin field is undeniable, they also have some major drawbacks, such as lack of specificity (44) and low reproducibility (7).
The generation of antibodies against bacteriocins may allow the detection and quantification of bacteriocins in different substrates by the use of immunochemical assays (8, 33, 44, 45). Recently, we have reported the generation of polyclonal antibodies directed to chemically synthesized fragments deduced from the sequence of mature pediocin PA-1 (33, 34). The use of these peptide-directed antibodies combined with the choice of suitable immunoassay formats has provided specific and sensitive methods for the quantification of pediocin PA-1 and for the rapid isolation of strains producing it.
The application of bacteriocin-producing LAB in foods may have some limitations, such as narrow antimicrobial spectrum, low-level or unstable production, and inability to grow in foods in which the bacteriocin(s) would be particularly effective (1). In this context, interest in the heterologous production of LAB bacteriocins is growing rapidly (2, 6, 12, 27, 28, 50). Furthermore, the antimicrobial efficiency of a bacteriocin may be enhanced by combining it with other bacteriocins, seen for combinations of sakacin A and nisin A (41), pediocin PA-1 and nisin A (19), and pediocin PA-1 and lacticin 481, lacticin B, or lacticin F (35).
In this work, we describe the development of sensitive and specific rabbit polyclonal antibodies against two synthetic amino acid fragments of enterocin A, peptides PH4 (residues 1 to 14) and PH5 (residues 37 to 47). Additionally, we report the heterologous (co)production of enterocin A and pediocin PA-1, two bacteriocins that contain the N-terminal class IIa consensus amino acid motif (YGNGVXC) and a closely related inhibitory spectrum (4, 5, 11, 16, 18, 21, 36, 40).
MATERIALS AND METHODS
Microbiological techniques, strains, and plasmids.
The LAB strains and plasmids used in this work are listed in Table 1. Lactococcal strains were grown in M17 medium (47) supplemented with 0.5% (wt/vol) glucose (GM17 medium) at 30°C, and the rest of strains were grown in MRS broth (Oxoid Unipath, Ltd., Basingstoke, United Kingdom) at 32°C. Agar plates were made by the addition of 1.5% (wt/vol) agar to broth media. Chloramphenicol (Sigma-Aldrich, St. Louis, Mo.) was added to the cultures of Lactococcus lactis IL1403-derived recombinant strains as a selective agent (5 μg ml−1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source and/or referenceb |
|---|---|---|
| Strains | ||
| E. faecium | ||
| T136 | (Enterocins A and B) ADT indicator | OC (11) |
| P21 | (Enterocins A and B) | OC |
| P13 | (Enterocin P) ADT indicator | OC (11) |
| AA13 | (Enterocin P) | OC (22) |
| G16 | (Enterocin P) | OC (22) |
| L50 | (Enterocins L50A and L50B) | OC (15) |
| E. faecalis INIA 4 | (Enterocin AS-48) | INIA (30) |
| P. acidilactici | ||
| 347 | (Pediocin PA-1) | OC (40) |
| Ped− | P. acidilactici 347 isogenic strain (Non-pediocin PA-1 producer) | OC (33) |
| P. pentosaceus FBB61 | (Pediocin A) | TNO (37) |
| L. sakei | ||
| 706 | (Sakacin A) | NHL (23) |
| LTH673 | (Sakacin P) | NHL (48) |
| 148 | (Lactocin S) | OC (38) |
| L. lactis | ||
| BB24 | (Nisin A) | OC (39) |
| MG1614 | (Non-bacteriocin producer) | IFR (17) |
| IL1403 | Plasmid-free host strain | INRA (13) |
| Plasmids | ||
| pMC117 | Emr, pMG36e derivative carrying the pediocin operon under control of the lactococcal promoter P32 | 12 |
| pMG36c | Cmr, pMG36e derivative, gene expression vector | RuG-MG Laboratory collection |
| pHB04 | Cmr, pMG36c derivative carrying entA and orf2 under control of P32 | RuG-MG Laboratory collection |
| pJM03 | Cmr, pMG36c derivative carrying pedA and pedB under control of P32 | This work |
| pJM04 | Cmr, pHB04 derivative carrying pedA and pedB under control of P32 | This work |
The bacteriocin(s) produced is shown in parentheses. Em, erythromycin; Cm, chloramphenicol; r, resistance.
OC, our collection; IFR, Institute of Food Research, Norwich Laboratory (Norwich, United Kingdom); INIA, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (Madrid, Spain); INRA, Institut National de la Recherche Agronomique (Jouy-en-Josas, France); NHL, Laboratory of Microbial Gene Technology, Agricultural University (As, Norway); RuG-MG, Department of Molecular Genetics, University of Groningen, (Groningen, The Netherlands); TNO, Nutrition and Food Research (Zeist, The Netherlands).
The supernatants were obtained by centrifugation of overnight cultures at 12,000 × g for 10 min at 4°C, adjusted to pH 6.2 with 1 N NaOH, filtered through 0.2-μm-pore-size filters (Whatman International, Ltd., Maidstone, United Kingdom), and stored at −20°C until use. The antimicrobial activity of the supernatants or eightfold concentrated supernatants was evaluated by an agar diffusion test (ADT). The ADT was performed as previously described (33). Enterococcus faecium P13 (enterocin A sensitive, pediocin PA-1 sensitive) and E. faecium T136 (enterocin A resistant, pediocin PA-1 sensitive) were used as indicator microorganisms.
Molecular cloning.
Plasmid DNA was isolated from L. lactis IL1403 as described by Leenhouts et al. (31). All DNA-modifying enzymes were purchased from Roche Molecular Biochemicals (Mannheim, Germany) and were used as recommended by the supplier. All DNA manipulations were carried out according to procedures described by Maniatis et al. (32). Electroporation of L. lactis was performed according to the method of Holo and Nes (24) with a gene pulser (Bio-Rad Laboratories, Hercules, Calif.).
A PCR fragment (613 bp) containing the pedA and pedB genes was obtained from plasmid pMC117 with the primers PedA2 (5′-AACTGCAGAGCTCTCGGAGGAATTTTGAAATGAAAAAAATTGAAAAATTAACTG-3′) and PedA3 (5′-AACTGCAGCATGCTCTAGACTATTGGCTAGGCCACGTATTGG-3′). The PCR product was cloned as a PstI/SphI fragment into plasmid pMG36c or as a SacI/XbaI fragment into plasmid pHB04, resulting in the plasmids pJM03 and pJM04, respectively. After transformation of L. lactis IL1403 with the ligation mixtures, the bacteriocinogenicity of the recombinant cells grown in GM17 agar was tested by overlaying plates with MRS semisolid agar seeded with the indicator strains E. faecium P13 and T136. The plasmid from a representative colony from each cloning experiment was extracted and analyzed by restriction enzyme analysis, and the inserted PCR fragment was checked by nucleotide sequencing.
Immunological materials.
Two enterocin A fragments, peptides PH4 (residues 1 to 14, NH2-TTHSGKYYGNGYYC-COOH; 20 mg) and PH5 (residues 37 to 47, NH2-GFLGGAIPGKC-COOH; 20 mg) were synthesized by 9-fluorenylmethoxy carbonyl chemistry with an Applied Biosystems 431A automated solid-phase synthesizer (Perkin-Elmer, Foster City, Calif.) in the Protein Chemistry Facility at the Centro de Biología Molecular Severo Ochoa (Madrid, Spain) under the direction of J. Vázquez. Purity of the peptides (higher than 95%) was monitored by reverse-phase high-performance liquid chromatography (RP-HPLC), and peptide identity was confirmed by mass spectrometry with a MALDI-TOF mass spectrometer (Shimadzu Scientific Instruments, Inc., Columbia, Md.). Polyclonal antibodies of predetermined specificity against pediocin PA-1, anti-PH2-KLH antibodies (33), were used for detection and quantitation of pediocin PA-1. The amino acid sequence of peptide PH2 (residues 34 to 44 of pediocin PA-1) was NH2-ATGGHQGNHKC-COOH. Ovalbumin (OA) (grade III and fraction VII), Tween 20, glutaraldehyde, Freund's adjuvants, and ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid] substrate were obtained from Sigma. The Imject Activated Immunogen Conjugation kit containing maleimide-activated keyhole limpet hemocyanin (KLH), conjugation buffer, and gel filtration columns was obtained from Pierce Chemical Co. (Rockford, Ill.). Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase was obtained from Cappel Laboratories (West Chester, Pa.). Pure nisin A (30,000 U mg−1) was purchased from NBS Biologicals (Hartfield, United Kingdom). Female New Zealand White rabbits were purchased from a local supplier (Granja San Bernardo, Navarra, Spain).
Preparation of immunoconjugates and immunization.
PH4 and PH5 were conjugated to maleimide-activated KLH (peptide-KLH; 1:2 [wt/wt]), employing the Imject Activated Immunogen Conjugation kit, for use as immunogens. The PH4 and PH5 fragments were also conjugated to OA (peptide-OAG; 12:1 [mol/mol]) by the glutaraldehyde method (3, 10) for use as solid-phase antigens. Rabbits were immunized with PH4-KLH and PH5-KLH according to a previously described scheme (33). Rabbits were bled via marginal ear veins on days 28 and 63, and a final bleed was performed on day 72 by cardiac puncture.
ELISAs.
An indirect enzyme-linked immunosorbent assay (ELISA) and noncompetitive indirect ELISA (NCI-ELISA) were performed as described by Martínez et al. (33). Briefly the indirect ELISA was performed for antiserum titration. Flat-bottom polystyrene microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight (at 4°C) with 100 μl of PH4-OAG (5 μg ml−1) or PH5-OAG (5 μg ml−1) in 0.1 M sodium carbonate-bicarbonate buffer (pH 9.6) coating buffer (CB). Plates were washed three times with 300 μl of PBST washing solution (0.05% Tween 20 in 0.01 M phosphate-buffered saline [PBS] [pH 7.4]). Wells were blocked for 30 min at 37°C with 300 μl of 1% (wt/vol) OA (grade III) in PBS (OA-PBS) and then washed six times. Next, 50 μl of serially diluted serum was added to each well and incubated for 1 h at 37°C. Unbound antibody was removed by washing the wells four times, after which 100 μl of goat anti-rabbit IgG peroxidase conjugate (diluted 1:500 in OA-PBS) was added to each well. Plates were incubated for 30 min at 37°C and washed eight times, and the amount of bound peroxidase present was determined with ABTS substrate. Absorbance was read at 405 nm. The titer of each serum was arbitrarily set as the reciprocal of the maximum dilution that yielded at least twice the absorbance of the same dilution of nonimmune control serum.
For NCI-ELISA, wells of microtiter plates were coated with 100 μl (each) of different concentrations of different controls (enterocin A, pediocin PA-1, nisin A, PH4-OAG, PH5-OAG, and OA) in CB or 100 μl (each) of the supernatants of the LAB strains to be tested diluted in CB 1:1 (vol/vol). The plates were maintained for 3 h at 40°C and then blocked and washed as described for the antiserum titration procedure. Next, 50 μl of antiserum, diluted 1:200 in PBS for anti-PH4-KLH serum and 1:300 in PBS for anti-PH5-KLH serum, was added, and the plates were incubated for 1 h at 37°C. After the washing step and addition of the goat anti-rabbit IgG peroxidase conjugate (diluted 1:500 in OA-PBS), the amount of bound peroxidase was determined with ABTS substrate as described above.
For quantification of enterocin A and pediocin PA-1 in the supernatants diluted in CB 1:1 (vol/vol), NCI-ELISA was used as described above. Different concentrations of both bacteriocins in MRS or GM17 with chloramphenicol (5 μg ml−1) were employed to construct standard curves. The standard concentrations (in MRS when employing supernatants from Enterococcus and Pediococcus strains or in GM17 with 5 μg of chloramphenicol ml−1 when employing supernatants from Lactococcus) were diluted in CB 1:1 (vol/vol) before coating. For quantification of enterocin A, anti-PH5-KLH serum was diluted 1:300 in PBS, while for quantification of pediocin PA-1, anti-PH2-KLH serum (33) was diluted 1:1,000 in PBS.
Purification of enterocin A and pediocin PA-1.
The bacteriocins produced by L. lactis IL1403(pHB04) (Ent A+) and Pediococcus acidilactici 347 (Ped PA-1+), were purified to homogeneity as previously described (14, 27, 33). Final concentrations of the purified bacteriocins were estimated by using the extinction coefficient of enterocin A (A280 of 2.1 corresponds to 1 mg ml−1) and pediocin PA-1 (A280 of 3.1 corresponds to 1 mg ml−1).
RESULTS
Purification of enterocin A and pediocin PA-1.
The results of the purification of enterocin A and pediocin PA-1 are summarized in Table 2. The final specific activities of enterocin A and pediocin PA-1 were approximately 40,000- and 385,000-fold higher than those in the culture supernatants of L. lactis IL1403(pHB04) and P. acidilactici 347, respectively. The recovery of bacteriocin activity was approximately 7% of the initial activity for enterocin A and 820% for pediocin PA-1. The final amounts of enterocin A and pediocin PA-1, each purified from 1 liter of culture, were 27 and 406 μg, respectively.
TABLE 2.
Purification of enterocin A and pediocin PA-1 from L. lactis IL1403(pHB04) and P. acidilactici 347 supernatants, respectively
| Supernatant and purification stage | Vol (ml) | Total A254a | Total activity (105 bacteriocin units) | Sp actb | Increase in sp actc (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| L. lactis IL1403(pHB04) | ||||||
| Culture supernatant | 1,000 | 30,500 | 15.8 | 52 | 1 | 100 |
| Fraction | ||||||
| Ammonium sulfate precipitation | 100 | 1,910 | 11.6 | 606 | 12 | 73 |
| Gel filtration chromatography | 200 | 980 | 7.4 | 752 | 14 | 47 |
| Cation-exchange chromatography | 50 | 6.9 | 1.2 | 16,726 | 322 | 7 |
| Hydrophobic-interaction chromatography | 10 | 2.75 | 6.3 | 2.3 × 105 | 4,390 | 40 |
| RP-HPLC | 0.75 | 0.06 | 1.2 | 2.1 × 106 | 39,956 | 7 |
| P. acidilactici 347 | ||||||
| Culture supernatant | 1,000 | 23,900 | 61.0 | 255 | 1 | 100 |
| Fraction | ||||||
| Ammonium sulfate precipitation | 100 | 1,260 | 49.2 | 3,904 | 15 | 81 |
| Gel filtration chromatography | 200 | 390 | 37.0 | 9,487 | 37 | 61 |
| Cation-exchange chromatography | 50 | 16.7 | 30.5 | 1.8 × 105 | 706 | 50 |
| Hydrophobic-interaction chromatography | 10 | 9.3 | 29.1 | 3.1 × 105 | 1,216 | 48 |
| RP-HPLC | 1.15 | 0.51 | 500.0 | 98.0 × 106 | 384,314 | 820 |
A254 is the absorbance at 254 nm multiplied by the volume in milliliters.
Specific activity is the number of bacteriocin units divided by total A254.
Increase in specific activity is specific activity of a fraction divided by the specific activity of the culture supernatant.
Sensitivity of the rabbit anti-peptide antibodies for enterocin A.
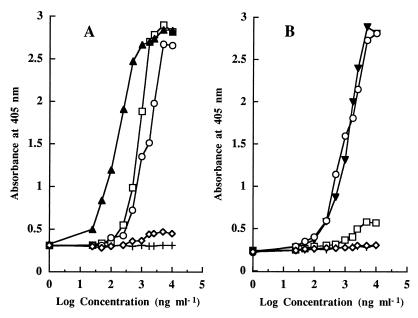
Two regions of the enterocin A linear sequence were chosen for the production of synthetic peptides. Synthetic peptides PH4 (amino acid residues 1 to 14) and PH5 (amino acid residues 37 to 47) were conjugated to KLH and used to immunize rabbits. On day 72 of the immunization process after six doses of the immunogen were administered, the animals had apparent titers in serum ranging from 12,800 to 102,400. The highest serum immunogen titers for fragments PH4 and PH5, respectively, were used throughout this work. The sensitivity of the anti-PH4-KLH and anti-PH5-KLH antibodies for enterocin A was initially determined by NCI-ELISA (Fig. 1). The anti-PH4-KLH antibodies showed a high recognition of PH4-OAG and enterocin A, with absorbance values higher than 2 with 4 μg of antigen ml−1. More importantly, the antibodies recognized the pediocin PA-1 present in the wells of the microtiter plates (absorbance value of 2.7 with 4 μg of pediocin ml−1. These results suggest that a large number of antibodies recognized the consensus sequence of the class IIa bacteriocins. In the case of the anti-PH5-KLH antibodies, serum titers showed a high recognition of PH5-OAG and enterocin A, with absorbance values higher than 2 with 4 μg of antigen ml−1 but with weak or no recognition of pediocin PA-1 (absorbance values from 0.5 to 0.6 with 4 to 10 μg of pediocin ml−1). Both antibodies could not detect the presence of equivalent concentrations of OA or pure nisin A in the wells of the microtiter plates, because absorbance values smaller than 0.5 were obtained with 10 μg of antigen ml−1.
FIG. 1.
NCI-ELISA detection with anti-PH4 (A) or anti-PH5 (B) antibodies of enterocin A (○), pediocin PA-1 (□), nisin A (◊), PH4-OAG (▴), PH5-OAG (▾), and pure OA (+) in CB.
Immunoreactivity of the rabbit anti-peptide antibodies to different bacteriocins.
The specificities of the serum polyclonal antibodies in neutralized and filter-sterilized supernatants of 16-h cultures of representative LAB strains were evaluated by NCI-ELISA (Table 3). The anti-PH4-KLH antibodies showed a high cross-reactivity (>75%) with the supernatants of the E. faecium enterocin A producers T136 and P21. The cross-reactivity was medium to low (5 to 25%) with the supernatants of the strains producing pediocin PA-1, enterocin P, and sakacin A, three class IIa bacteriocins with the N-terminal consensus amino acid motif YGNGVXC. A negligible to no reaction was observed with the supernatants of Lactobacillus sakei LTH673, a producer of sakacin P, another bacteriocin of the pediocin family, P. acidilactici Ped− (non-bacteriocin producer), Pediococcus pentosaceus FBB61 (pediocin A), E. faecium L50 (enterocin L50A and L50B), Enterococcus faecalis I4 (enterocin AS-48), L. sakei 148 (lactocin S), L. lactis BB24 (nisin A) and L. lactis MG1614 (non-bacteriocin producer). The anti-PH5-KLH antibodies showed a high cross-reactivity (>75%) with the supernatants of the enterocin A producers E. faecium T136 and P21, but did not react with the supernatants of the other LAB strains tested.
TABLE 3.
Reactivities of anti-PH4 and anti-PH5 serum polyclonal antibodies against culture supernatants of LAB as determined by NCI-ELISA
| Microorganism (bacteriocin) | Cross-reactivity (%)a
|
|
|---|---|---|
| Anti-PH4 | Anti-PH5 | |
| E. faecium T136 (enterocins A and B) | 100 | 100 |
| E. faecium P21 (enterocins A and B) | 78.8 | 76.4 |
| P. acidilactici 347 (pediocin PA-1) | 22.9 | NR |
| P. acidilactici Ped− (non-pediocin PA-1 producer) | NR | 1.1 |
| E. faecium P13 (enterocin P) | 8.2 | NR |
| E. faecium AA13 (enterocin P) | 9.5 | NR |
| E. faecium G16 (enterocin P) | 13.9 | 1.5 |
| L. sakei 706 (sakacin A) | 6.9 | NR |
| L. sakei LTH673 (sakacin P) | NR | NR |
| E. faecium L50 (enterocins L50A and L50B) | NR | NR |
| P. pentosaceus FBB61 (pediocin A) | NR | NR |
| E. faecalis INIA 4 (enterocin AS-48) | NR | NR |
| L. sakei 148 (lactocin S) | NR | NR |
| L. lactis BB24 (nisin A) | NR | NR |
| L. lactis MG1614 | NR | NR |
Cross-reactivity is calculated as follows: [(absorbance reading produced by a culture supernatant above the absorbance reading produced by MRS/absorbance reading produced by the supernatant of E. faecium T136 above the absorbance reading produced by MRS) × 100]. NR, no reaction.
Quantification of the homologous and heterologous (co)production of enterocin A and pediocin PA-1.
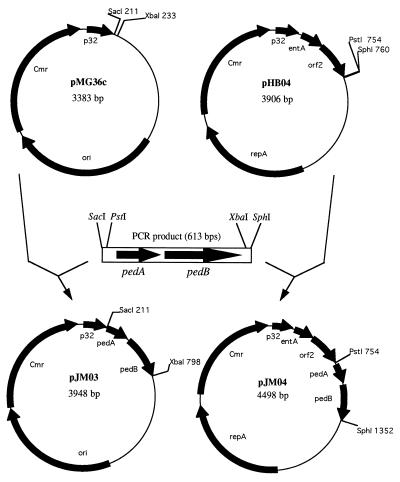
A PCR fragment carrying the pedA and pedB genes was cloned in plasmids pMG36c and pHB04. After transformation of L. lactis IL1403 with the ligation mixtures, eight colonies produced bacteriocin, as evidenced by halos on the indicators. The plasmid from a representative colony from each cloning experiment was examined by restriction enzyme analysis, and the correctness of the inserted PCR fragment was confirmed by nucleotide sequencing. Maps of the two resulting plasmids, pJM03 and pJM04, are given in Fig. 2.
FIG. 2.
Construction of plasmids pJM03 and pJM04. Sizes of plasmids are given in base pairs. Only relevant restriction enzyme sites are given. p32, strong lactococcal promoter (51); ori, origin of replication; Cmr, chloramphenicol resistance marker; repA, gene encoding plasmid pWV01 replication protein; entA, enterocin A structural gene; orf2, enterocin A immunity gene; pedA, pediocin PA-1 structural gene; pedB, pediocin PA-1 immunity gene.
The concentration of enterocin A and pediocin PA-1 in the supernatants of 16-h cultures of E. faecium T136 and P21, P. acidilactici 347, and L. lactis IL1403 carrying either pHB04, pJM03, or pJM04 was evaluated by NCI-ELISA employing the PH2-KLH- and the PH5-KLH-generated antibodies (Table 4). The detection limits of enterocin A and pediocin PA-1 in the supernatants were below 50 ng ml−1. The concentrations of enterocin A and pediocin PA-1 in the supernatants of L. lactis IL1403(pHB04 (Ent A+) and L. lactis IL1403(pJM03) (Ped PA-1+), were determined to be around 7% of those found in the supernatants of E. faecium T136 (2,483 ng of Ent A ml−1) and P. acidilactici 347 (1,724 ng of Ped PA-1 ml−1), respectively. The concentration of bacteriocins in the supernatant of L. lactis IL1403(pJM04) (Ent A+ and Ped PA-1+) was 93 ng of enterocin A ml−1 and 87 ng of pediocin PA-1 ml−1, which is approximately 4 and 5%, respectively, of the concentrations found in the wild-type bacteriocin producers E. faecium T136 and P. acidilactici 347.
TABLE 4.
Pediocin PA-1 and enterocin A concentrations in culture supernatants as determined by NCI-ELISA with anti-PH2 and anti-PH5 antibodies
| Microorganism (bacteriocin) | Concn in supernatant with:
|
|||
|---|---|---|---|---|
| Anti-PH2
|
Anti-PH5
|
|||
| Pediocin PA-1 (ng/ml) | Cross-reactivitya (%) | Enterocin A (ng/ml) | Cross-reactivityb (%) | |
| E. faecium T136 (enterocin A and enterocin B) | NRc | 2,483 | 100 | |
| E. faecium P21 (enterocin A and enterocin B) | NR | 1,896 | 76.4 | |
| P. acidilactici 347 (pediocin PA-1) | 1,724 | 100 | NR | |
| L. lactis IL1403(pHB04) (enterocin A) | NR | 187 | 7.5 | |
| L. lactis IL1403(pJM03) (pediocin PA-1) | 115 | 6.7 | NR | |
| L. lactis IL1403(pJM04) (pediocin PA-1 and enterocin A) | 87 | 5.1 | 93 | 3.8 |
| L. lactis IL1403 | NR | NR | ||
Cross-reactivity is calculated as follows: [(concentration of pediocin PA-1 in a culture supernatant/concentration of pediocin PA-1 in the supernatant of P. acidilactici 347) × 100].
Cross-reactivity is calculated as follows: [(concentration of enterocin A in a culture supernatant/concentration of enterocin A in the supernatant of E. faecium T136) × 100].
NR, no reaction.
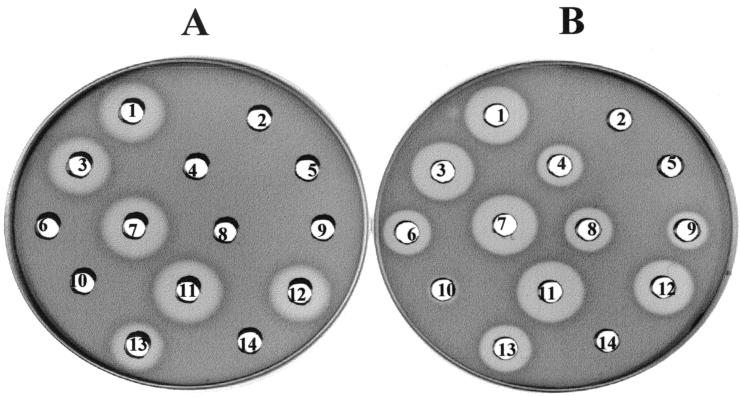
The enterocin A and/or pediocin PA-1 production by the recombinants strains was confirmed by ADT (Fig. 3). The concentrations of enterocin A and pediocin PA-1 found in the eightfold-concentrated supernatants of the recombinant strains were in accordance with those determined by NCI-ELISA. However, the production of enterocin A by L. lactis IL1403(pJM04) could only be demonstrated immunologically with the anti-PH5-KLH antibodies, since no enterocin A-sensitive, pediocin PA-1-resistant strain was available.
FIG. 3.
ADT with E. faecium T136 (Ent Ar Ped PA-1s) (A) or E. faecium P13 (Ent As Ped PA-1s) (B) as indicator microorganisms to test the bacteriocin activity of supernatants (Enterococcus and Pediococcus), eightfold-concentrated supernatants (L. lactis IL1403), or pure bacteriocin in GM17 broth with 5 μg of chloramphenicol ml−1. Spots: 1, L. lactis IL1403(pJM03); 2, L. lactis IL1403; 3, L. lactis IL1403(pJM04); 4, L. lactis IL1403(pHB04); 5, P. acidilactici Ped−; 6, E. faecium T136; 7, P. acidilactici 347; 8, 2.5 μg of enterocin A ml−1; 9, 0.5 μg of enterocin A ml−1; 10, 0.1 μg of enterocin A ml−1; 11, 2.5 μg of pediocin PA-1 ml−1; 12, 0.5 of μg pediocin PA-1 ml−1; 13, 0.1 μg of pediocin PA-1 ml−1; 14, GM17 broth with 5 μg of chloramphenicol ml−1.
DISCUSSION
With the polyclonal anti-PH4-KLH and anti-PH5-KLH antibodies, it was possible to detect not only the peptides PH4 and PH5 but also enterocin A, either purified to homogeneity or in the supernatant of the wild-type E. faecium producer strains T136 and P21. Both antibodies displayed maximum cross-reactivity (100%) with the supernatant of E. faecium T136 and a reaction of around 75 to 80% with the supernatant of E. faecium P21. This difference could be attributed to higher production of enterocin A by E. faecium T136. This is not rare, because considerable differences in bacteriocin production have, for instance, also been found among different wild-type pediocin PA-1 producers (34). Anti-PH5-KLH antibodies could not detect the other class IIa bacteriocins tested, namely pediocin PA-1, enterocin P, sakacin A, and sakacin P. This result is concordant with the fact that these antibodies were raised against amino acid residues 37 to 47 in the C-terminal part of the enterocin A molecule, a region with high sequence diversity among class IIa bacteriocins. In contrast, the anti-PH4-KLH antibodies displayed medium to low cross-reactivity (5 to 25%) with supernatants containing pediocin PA-1, enterocin P, and sakacin A. This fact suggests that a number of the antibodies were able to recognize the consensus sequence of the class IIa bacteriocins, which is present in the peptide PH4 (covering residues 1 to 14 of enterocin A). However, they were not able to detect sakacin P in the supernatant of L. sakei LTH673. This fact may be explained by differences in the structural conformation preferentially adopted by this part of the sakacin P molecule, causing poor access of the antibodies to the bacteriocin and/or by low bacteriocin production by this particular strain. Further analysis with purified bacteriocins and elucidation of the relation between the amount of each bacteriocin present in the supernatants and its immunodetection could facilitate a better understanding of how anti-peptide antibodies recognize the sequence against which they have been raised.
L. lactis IL1403, a plasmid-free strain that does not produce bacteriocin but harbors chromosomal genes analogous to those encoding the lactococcin A secretion apparatus, IcnC and IcnD (43, 52), was selected as the host for the heterologous (co)production of enterocin A and pediocin PA-1. Introduction of pHB04 or pJM03 into IL1403 led to heterologous production and secretion of enterocin A or pediocin PA-1 in the respective transformants, while transformation with pJM04 resulted in coproduction of both bacteriocins.
Detection and quantification of enterocin A and pediocin PA-1 production by the L. lactis recombinant strains were done using the ADT and NCI-ELISA. E. faecium T136 (Ent Ar Ped PA-1s) and E. faecium P13 (Ent As Ped PA-1s) were employed as indicator strains in the ADT, while anti-PH2-KLH antibodies (33) and anti-PH5-KLH antibodies were used for the recognition of pediocin PA-1 and enterocin A by NCI-ELISA, respectively. The production of enterocin A and pediocin PA-1 by the wild-type and the recombinant single bacteriocin producers could be carried out using either the ADT or the NCI-ELISA. Both assays showed a similar sensitivity, detecting concentrations of bacteriocin as low as 90 ng ml−1 in the supernatant of the recombinant strains. However, the immunological method proved to be critical for detection of coproduction of enterocin A and pediocin PA-1 by L. lactis IL1403(pJM04), because no enterocin A-sensitive, pediocin PA-1-resistant strain is available.
The low level of bacteriocin production by the recombinant strains, around 7% for single bacteriocin production and 4 to 5% for coproduction, compared with the levels achieved by the wild types, is in accordance with the yields previously obtained for lactococcin A (25, 49) and pediocin PA-1 (27) when expressed in L. lactis IL1403. This reduction in bacteriocin activity may be due to the low copy number of the chromosomal IcnC and IcnD analogs in IL1403 and/or to the fact that their gene products are not identical to the equivalent lactococcin A translocation apparatus (25, 27, 43, 52), which may result in a less-efficient secretion process. Considerable increases in the production of bacteriocins containing the lactococcin A leader have been described after the introduction of plasmid copies of the IcnC and IcnD genes in IL1403 (28, 50, 52). Moreover, in IL1403, lactococcin A-leader-directed secretion of pediocin PA-1 by IcnC and IcnD is more efficient than the equivalent process directed by the pediocin PA-1 translocation machinery (12, 28). If the same were true for enterocin A, an alternative to increase enterocin A and pediocin PA-1 (co)production in IL1403 would be the introduction of the IcnC and IcnD genes together with chimeric genes encoding fusions between the lactococcin A leader and the mature part of enterocin A or pediocin PA-1.
In this study, heterologous production of enterocin A and/or pediocin PA-1 in L. lactis IL1403 was achieved. Although both bacteriocins were (co)produced at low levels, the heterologous system developed was very useful in demonstrating the specificity and sensitivity of anti-peptide antibodies of predetermined specificity against enterocin A (anti-PH5-KLH) and pediocin PA-1 (anti-PH2-KLH), despite the low concentration of the bacteriocins and their high sequence similarity. The use of peptide-directed antibodies to detect and quantify homologous or heterologous bacteriocin coproduction avoids dependence on the availability of indicator strains selectively inhibited by each of the bacteriocins. The antibodies also offer potential alternative methods for the (industrial-scale) purification of bacteriocins to homogeneity by the use of immunoaffinity chromatography strategies (46). Finally, these antibodies could be employed for immunolocalization of bacteriocins in bacterial strains and in those foods in which bacteriocins have been naturally produced or added (9).
ACKNOWLEDGMENTS
This work was partially supported by grant ALI97-0559 from the Comisión Interministerial de Ciencia y Tecnología (CICYT), Madrid, Spain. J.M.M. holds a fellowship from the Comunidad Autónoma de Madrid.
We are grateful to J. Vázquez (Centro de Biología Molecular Severo Ochoa, Madrid, Spain) for the chemical synthesis of the peptides and to Harma Karsens for the construction of pHB04. We thank Juan M. Rodríguez for helpful discussions.
REFERENCES
- 1.Abee T, Krockel L, Hill C. Bacteriocins: mode of action and potentials in food preservation and control of food poisoning. Int J Food Microbiol. 1995;28:169–185. doi: 10.1016/0168-1605(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 2.Allison G E, Worobo R W, Stiles M E, Klaenhammer T R. Heterologous expression of the lactacin F peptides by Carnobacterium piscicola LV17. Appl Environ Microbiol. 1995;61:1371–1377. doi: 10.1128/aem.61.4.1371-1377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avrameas S, Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969;6:53–56. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- 4.Aymerich T, Holo H, Havarstein L S, Garriga M, Nes I F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhunia A K, Johnson M C, Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988;65:261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 6.Biet F, Berjeaud J M, Worobo R W, Cenatiempo Y, Fremaux C. Heterologous expression of the bacteriocin mesentericin Y105 using the dedicated transport system and the general secretion pathway. Microbiology. 1998;144:2845–2854. doi: 10.1099/00221287-144-10-2845. [DOI] [PubMed] [Google Scholar]
- 7.Blom H, Katla T, Hagen B F, Axelsson L. A model assay to demonstrate how intrinsic factors affect diffusion of bacteriocins. Int J Food Microbiol. 1997;38:103–109. doi: 10.1016/s0168-1605(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 8.Bouksaim M, Fliss I, Meghrous J, Simard R, Lacroix C. Immunodot detection of nisin Z in milk and whey using enhanced chemiluminescence. J Appl Microbiol. 1998;81:176–184. doi: 10.1046/j.1365-2672.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouksaim M, Lacroix C, Bazin R, Simard R E. Production and utilization of polyclonal antibodies against nisin in an ELISA and for immuno-location of nisin in producing and sensitive bacterial strains. J Appl Microbiol. 1999;87:500–510. doi: 10.1046/j.1365-2672.1999.00842.x. [DOI] [PubMed] [Google Scholar]
- 10.Briand J P, Muller S, van Regenmortel M H V. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985;78:59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- 11.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernández P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 12.Chikindas M L, Venema K, Ledeboer A M, Venema G, Kok J. Expression of lactococcin A and pediocin PA-1 in heterologous hosts. Lett Appl Microbiol. 1995;21:183–189. doi: 10.1111/j.1472-765x.1995.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 13.Chopin A, Chopin M C, Moillo-Batt A, Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984;11:260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- 14.Cintas L M, Casaus P, Havarstein L S, Hernández P E, Nes I F. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol. 1997;63:4321–4330. doi: 10.1128/aem.63.11.4321-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cintas L M, Casaus P, Holo H, Hernández P E, Nes I F, Havarstein L S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol. 1998;180:1988–1994. doi: 10.1128/jb.180.8.1988-1994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ennahar S, Aoude-Werner D, Sorokine O, van Dorsselaer A, Bringel F, Hubert J-C, Hasselmann C. Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl Environ Microbiol. 1996;62:4381–4387. doi: 10.1128/aem.62.12.4381-4387.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez C F, Kunka B S. Plasmid-associated bacteriocin production and sucrose fermentation in Pediococcus acidilactici. Appl Environ Microbiol. 1987;53:2534–2538. doi: 10.1128/aem.53.10.2534-2538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanlin M B, Kalchayanand N, Ray P, Ray B. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J Food Prot. 1993;56:252–255. doi: 10.4315/0362-028X-56.3.252. [DOI] [PubMed] [Google Scholar]
- 20.Helander I M, von Wright A, Mattila-Sandhlom T M. Potential of lactic acid bacteria and novel antimicrobials against Gram-negative bacteria. Trends Food Sci Technol. 1997;8:146–150. [Google Scholar]
- 21.Henderson J T, Chopko A L, Wassenaar P D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC1.0. Arch Biochem Biophys. 1992;295:5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- 22.Herranz C, Mukhopadhyay S, Casaus P, Martínez J M, Rodríguez J M, Nes I F, Cintas L M, Hernández P E. Biochemical and genetic evidence of enterocin P production by two Enterococcus faecium-like strains isolated from fermented sausages. Curr Microbiol. 1999;39:282–290. doi: 10.1007/s002849900460. [DOI] [PubMed] [Google Scholar]
- 23.Holck A, Axelsson L, Birkeland S E, Aukrust T, Blom H. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Gen Microbiol. 1992;138:2715–2720. doi: 10.1099/00221287-138-12-2715. [DOI] [PubMed] [Google Scholar]
- 24.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holo H, Nilssen Ø, Nes I F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991;173:3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoover D H. Minimally processed fruits and vegetables: reducing microbial load by nonthermal physical treatments. Food Technol. 1997;51:66–71. [Google Scholar]
- 27.Horn N, Martínez M I, Martínez J M, Hernández P E, Gasson M J, Rodríguez J M, Dodd H. Production of pediocin PA-1 by Lactococcus lactis using the lactococcin A secretory apparatus. Appl Environ Microbiol. 1998;64:818–823. doi: 10.1128/aem.64.3.818-823.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn N, Martínez M I, Martínez J M, Hernández P E, Gasson M J, Rodríguez J M, Dodd H. Enhanced production of pediocin PA-1 and coproduction of nisin and pediocin PA-1 by Lactococcus lactis. Appl Environ Microbiol. 1999;65:4443–4450. doi: 10.1128/aem.65.10.4443-4450.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Appl Environ Microbiol. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joosten H M L J, Núñez M, Devreese B, van Beeumen J, Marugg J D. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Appl Environ Microbiol. 1998;64:4220–4223. doi: 10.1128/aem.62.11.4220-4223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leenhouts K J, Kok J, Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;55:394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 33.Martínez J M, Martínez M I, Suárez A M, Herranz C, Casaus P, Cintas L M, Rodríguez J M, Hernández P E. Generation of polyclonal antibodies of predetermined specificity against pediocin PA-1. Appl Environ Microbiol. 1998;64:4536–4545. doi: 10.1128/aem.64.11.4536-4545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez J M, Martínez M I, Herranz C, Suárez A, Fernández M F, Cintas L M, Rodríguez J M, Hernández P E. Antibodies to a synthetic 1-9-N-terminal amino acid fragment of mature pediocin PA-1: sensitivity and specificity for pediocin PA-1 and cross-reactivity against class IIa bacteriocins. Microbiology. 1999;145:2777–2787. doi: 10.1099/00221287-145-10-2777. [DOI] [PubMed] [Google Scholar]
- 35.Mulet-Powell N, Lacoste-Armynot A M, Viñas M, Simeon de Buochberg M. Interactions between pairs of bacteriocins from lactic bacteria. J Food Prot. 1998;61:1210–1212. doi: 10.4315/0362-028x-61.9.1210. [DOI] [PubMed] [Google Scholar]
- 36.Nieto Lozano J C, Nissen Meyer J, Sletten K, Pelaez C, Nes I F. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J Gen Microbiol. 1992;138:1985–1990. doi: 10.1099/00221287-138-9-1985. [DOI] [PubMed] [Google Scholar]
- 37.Piva A, Headon D H. Pediocin A, a bacteriocin produced by Pediococcus pentosaceus FBB61. Microbiology. 1994;140:697–702. doi: 10.1099/00221287-140-4-697. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez J M, Cintas L M, Casaus P, Suárez A, Hernández P E. PCR detection of the lactocin S structural gene in bacteriocin-producing lactobacilli from meat. Appl Environ Microbiol. 1995;61:2802–2805. doi: 10.1128/aem.61.7.2802-2805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez J M, Cintas L M, Casaus P, Horn N, Dodd H M, Hernández P E, Gasson M J. Isolation of nisin-producing Lactococcus lactis strains from dry fermented sausages. J Appl Bacteriol. 1995;78:109–115. doi: 10.1111/j.1365-2672.1995.tb02830.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez J M, Cintas L M, Martínez M I, Casaus P, Suárez A M, Hernández P E. Detection of pediocin PA-1 producing pediococci by rapid molecular biology procedures. Food Microbiol. 1997;14:363–371. [Google Scholar]
- 41.Schillinger U, Geisen R, Holzapfel W H. Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol. 1996;7:158–164. [Google Scholar]
- 42.Stiles M E. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:331–345. doi: 10.1007/BF00395940. [DOI] [PubMed] [Google Scholar]
- 43.Stoddard G W, Petzel J P, van Belkum M J, Kok J, McKay L L. Molecular analyses of the lactococcin A gene cluster from Lactococcus lactis subsp. lactis biovar diacetylactis WM4. Appl Environ Microbiol. 1992;58:1952–1961. doi: 10.1128/aem.58.6.1952-1961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suárez A M, Rodríguez J M, Hernández P E, Azcona-Olivera J I. Generation of polyclonal antibodies against nisin: immunization strategies and immunoassay development. Appl Environ Microbiol. 1996;62:2117–2121. doi: 10.1128/aem.62.6.2117-2121.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suárez A M, Rodríguez J M, Morales P, Hernández P E, Azcona-Olivera J I. Development of monoclonal antibodies to the lantibiotic nisin A. J Agric Food Chem. 1996;44:2936–2940. [Google Scholar]
- 46.Suárez A M, Azcona J I, Rodríguez J M, Sanz B, Hernández P E. One-step purification of nisin A by immunoaffinity chromatography. Appl Environ Microbiol. 1997;63:4990–4992. doi: 10.1128/aem.63.12.4990-4992.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tichaczek P S, Vogel R F, Hammes W P. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH 673. Microbiology. 1994;140:361–367. doi: 10.1099/13500872-140-2-361. [DOI] [PubMed] [Google Scholar]
- 49.van Belkum M J, Hayema B J, Jeeninga R E, Kok J, Venema G. Organization and nucleotide sequence of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991;57:492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Belkum M J, Worobo R W, Stiles M E. Double-glycine-type leader peptides directed secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Mol Microbiol. 1997;23:1293–1301. doi: 10.1046/j.1365-2958.1997.3111677.x. [DOI] [PubMed] [Google Scholar]
- 51.van der Vossen J M B M, van der Lelie D, Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987;53:2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venema K, Dost M H R, Beun P A H, Haandrikman A J, Venema G, Kok J. The genes for secretion and maturation of lactococcins are located on the chromosome of Lactococcus lactis IL1403. Appl Environ Microbiol. 1996;62:1689–1692. doi: 10.1128/aem.62.5.1689-1692.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]