The key pathogenic features of pulmonary hypertension are endothelial apoptosis and vascular inflammation, which are caused by the down-regulation of Amphiregulin, accompanied by HIF-1a and BAD-mediated up-regulation of the target genes such as RRP1B, PHB2, and NCOA6.
Abstract
Pulmonary hypertension (PH) is a vascular disease characterized by elevated pulmonary arterial pressure, leading to right ventricular failure and death. Pathogenic features of PH include endothelial apoptosis and vascular inflammation, which drive vascular remodeling and increased pulmonary arterial pressure. Re-analysis of the whole transcriptome sequencing comparing human pulmonary arterial endothelial cells (PAECs) isolated from PH and control patients identified AREG, which encodes Amphiregulin, as a key endothelial survival factor. PAECs from PH patients and mice exhibited down-regulation of AREG and its receptor epidermal growth factor receptor (EGFR). Moreover, the deficiency of AREG and EGFR in ECs in vivo and in vitro heightened inflammatory leukocyte recruitment, cytokine production, and endothelial apoptosis, as well as diminished angiogenesis. Correspondingly, hypoxic mice lacking Egfr in ECs (cdh5cre/+ Egfrfl/fl) displayed elevated RVSP and pulmonary remodeling. Computational analysis identified NCOA6, PHB2, and RRP1B as putative genes regulating AREG in endothelial cells. The master transcription factor of hypoxia HIF-1⍺ binds to the promoter regions of these genes and up-regulates their expression in hypoxia. Silencing of these genes in cultured PAECs decreased inflammation and apoptosis, and increased angiogenesis in hypoxic conditions. Our pathway analysis and gene silencing experiments revealed that BCL2-associated agonist of cell death (BAD) is a downstream mediator of AREG. BAD silencing in ECs lacking AREG mitigated inflammation and apoptosis, and suppressed tube formation. In conclusion, loss of Amphiregulin and its receptor EGFR in PH is a crucial step in the pathogenesis of PH, promoting pulmonary endothelial cell death, influx of inflammatory myeloid cells, and vascular remodeling.
Introduction
Inflammation is a biological process mediated by immune cells such as neutrophils, monocytes and macrophages, and soluble molecular mediators like IL-6, IFN-β, and TNF-α (Iqbal et al, 2016; Vasamsetti et al, 2018). Inflammation is a defensive response against various stimuli such as pathogens and irritants. However, inflammation often propagates pathogenesis in various inflammatory diseases such as diabetes, atherosclerosis, and pulmonary hypertension (PH) (Tuder et al, 1994; Galkina & Ley, 2009; Price et al, 2012; Graham et al, 2013, 2018; Pugliese et al, 2015, 2017; Tsalamandris et al, 2019; Vasamsetti et al, 2020). PH is a progressive cardiopulmonary vascular disease, characterized by elevated mean pulmonary arterial pressure at rest and lung vascular remodeling. PH and its severe subtype pulmonary arterial hypertension (PAH) are thought to be driven by excessive expansion of smooth muscle cells, apoptosis of pulmonary artery endothelial cells (PAECs) (Tuder, 2017), and exaggerated inflammation (Rabinovitch et al, 2014; Amsellem et al, 2017; Florentin et al, 2018, 2021). However, the molecular interconnections linking these cellular phenotypes are not fully understood.
Endothelial dysfunction is crucial to the development of PH (Ranchoux et al, 2018). The pathogenic rise in pulmonary pressure is due to progressive loss of small pulmonary arterioles. The initial triggers caused by genetic or environmental factors can lead to endothelial injury and impaired vascular regeneration. It is thought that during the initial phase of PH, pulmonary vascular ECs are more likely to undergo apoptosis and possibly senescence (Culley et al, 2021) followed by the selection of ECs that exhibit resistance to apoptosis and a hyperproliferative response (Sakao et al, 2005; Michelakis, 2006). Furthermore, endothelial metabolic changes that may regulate EC survival play crucial roles in PH pathogenesis (Bertero et al, 2016). Yet, a knowledge gap exists, regarding the molecular pathways that regulate survival and regeneration of pulmonary ECs, leading to exaggerated inflammation, after vascular injury.
Our analysis of previously published unbiased whole genome transcriptome data (Rhodes et al, 2015) to discover cell survival genes revealed that PAECs isolated from the lungs of patients with PAH displayed a sixfold reduction in the expression of AREG compared with PAECs isolated from age-matched healthy patients. AREG encodes Amphiregulin, which mediates cell survival and proliferation (Piepkorn et al, 1994; Enomoto et al, 2009). Prior studies demonstrated the participation of Amphiregulin in a wide variety of physiological processes (Schneider & Wolf, 2009) such as mammary gland development and ductal morphogenesis during puberty. Estrogens induce Amphiregulin, and through the estrogen receptor alpha (ERα), induce proliferation of the mammary epithelium (Ciarloni et al, 2007; LaMarca & Rosen, 2007). Other studies reported the role of Amphiregulin in cancer cell proliferation (Berasain & Avila, 2014). The contributions of this growth factor in inflammatory disease pathogenesis such as psoriasis and rheumatoid arthritis have also been reported (Bhagavathula et al, 2005; Yamane et al, 2008). In addition, Amphiregulin has been shown to increase cardiac fibrosis and aggravate cardiac dysfunction in a mouse model of myocardial infarction (Liu et al, 2018), and promote fibroblast activation in pulmonary fibrosis (Ding et al, 2016; Liu et al, 2016b). The anti-apoptotic and protective roles of epidermal growth factor receptor (EGFR) in diseases, such as acute lung injury and liver injury (Meng et al, 2020; Wu et al, 2020b), have been shown. However, whether the loss of Amphiregulin promotes endothelial apoptosis and vascular inflammation in PH is not known.
In the present study, we aimed to define the function of Amphiregulin and its receptor the EGFR in (A) PAEC survival and proliferation as well as (B) inflammation in PH. To do so, we used computational genomic and multicolor flow cytometric analyses of samples obtained from PAH patients and multiple PAH animal models. We observed significant down-regulation of Amphiregulin and EGFR in PAECs in patients with PAH. In vitro, EGFR and AREG silencing in PAECs increased inflammation and apoptosis and suppressed their proliferation and tube formation ability. Conversely, Amphiregulin exposure dampened inflammation, protected these cells against apoptosis, and encouraged angiogenesis ability. These mechanisms correlated with the findings in vivo of mice lacking Egfr in endothelial cells. Pathway analysis identified NCOA6, PHB2, and RRP1B as AREG inhibitor genes in endothelial cells. These genes, which were up-regulated in hypoxia, were found to have binding motifs for HIF-1⍺ in their promoter regions. Silencing of these genes in PAECs diminished inflammatory cytokine production and apoptosis as well as increased tube formation ability. In addition, we found that BCL2-Associated Agonist of Cell Death (BAD), a downstream mediator of AREG, when silenced, reversed increased inflammation, endothelial apoptosis, and suppressed tube formation observed in absence of AREG. Altogether, these results define Amphiregulin and EGFR as PAEC survival factors and potential therapeutic targets to reduce inflammation in PH.
Results
AREG expression is decreased in PH
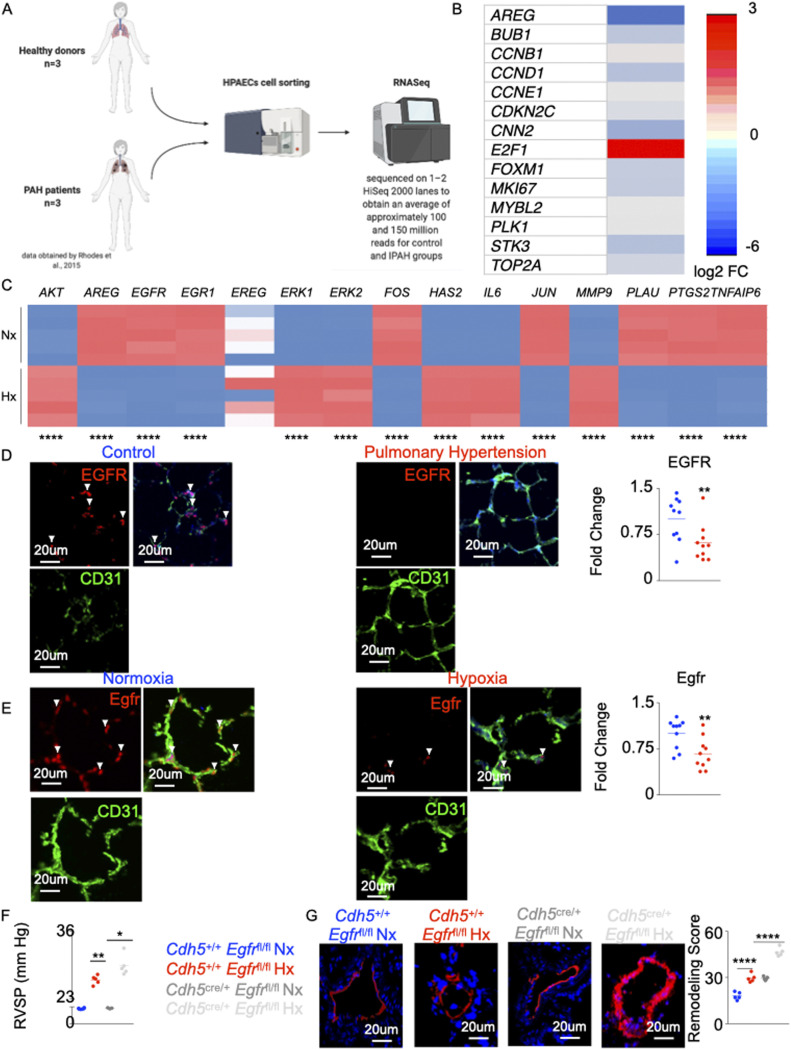
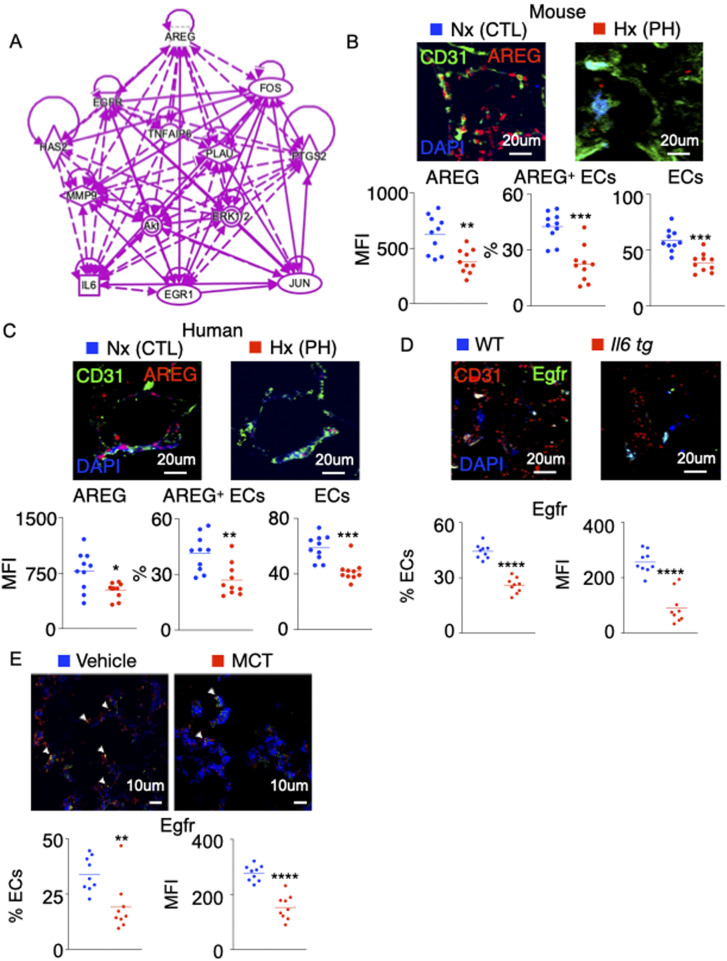
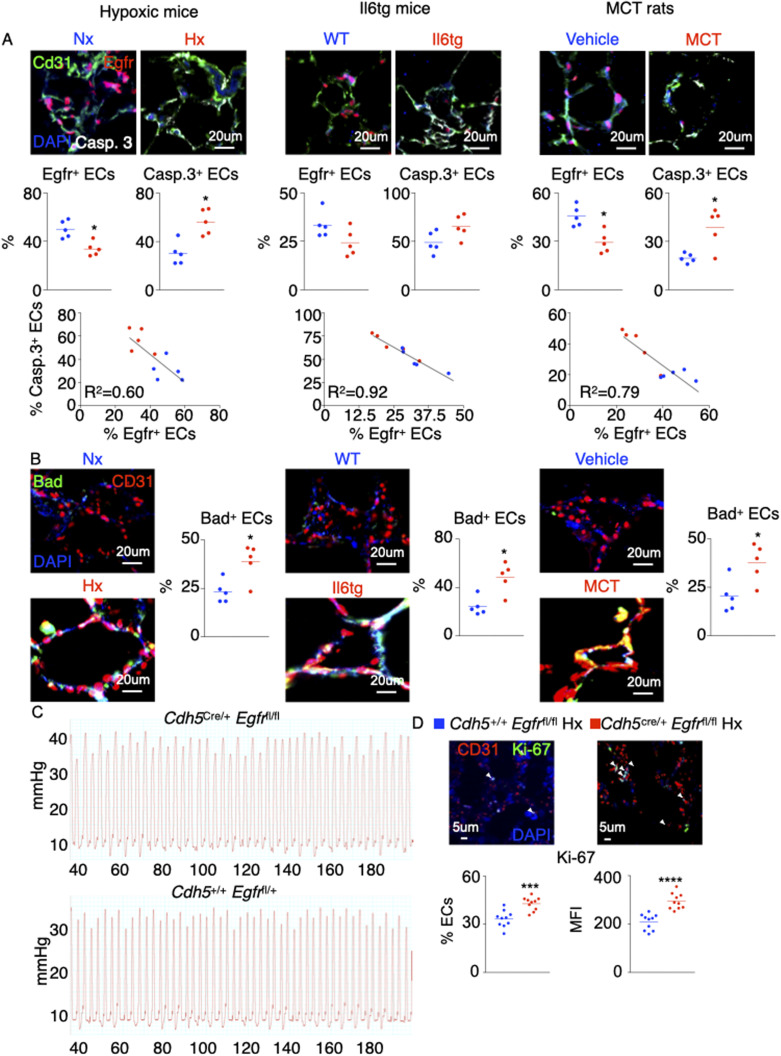
It is suggested that PH is characterized by initial PAEC apoptosis followed by hyperproliferation of apoptosis-resistant ECs (Tuder et al, 1994, 2001; Hirose et al, 2000; Nicolls et al, 2005). To understand the mechanisms of PAEC loss in PH, we analyzed RNA sequencing data (dbGaP genotype files: phs000998.v1.p1 NHLBI/iPSC_PulmonaryHypertension) (Chavakis & Dimmeler, 2002; Rhodes et al, 2015) (Fig 1A). The analysis of the RNA-Seq data comparing PAEC of seven control subjects and six patients with idiopathic pulmonary arterial hypertension revealed down-regulation of most genes encouraging EC survival (Fig 1B). Among these pro-survival genes, PAEC isolated from the lungs of patients with PAH had sixfold reduction in the expression of AREG compared with those isolated from age-matched healthy patients (Fig 1B). To understand if AREG signaling is altered in PH, we cultured human PAECs under hypoxic conditions, and analyzed PAECs in patients with PAH and mice with PH. We identified genes downstream to AREG using Ingenuity Pathway Analysis (IPA) (Fig S1A). Correspondingly, hypoxia significantly decreased AREG expression as well as these downstream genes in cultured PAECs (Fig 1C). In addition, using immunofluorescence imaging, we showed that AREG expression in pulmonary ECs of hypoxic mice and PAH patients is down-regulated compared with the controls (Fig S1B and C). Amphiregulin encoded by AREG binds to the EGFR. Using confocal imaging, we observed down-regulation of EGFR in PAECs of PAH patients (Fig 1D), C57BL/6 mice under hypoxic conditions (Fig 1E), Il6tg mice (Florentin et al, 2021) (Fig S1D) and monocrotaline-injected rats (Fig S1E) compared with age-matched control patients and mice under normoxic conditions, respectively. Finally, we demonstrated a concomitant decrease in Egfr expression and increase in caspase 3 levels in hypoxic and Il6tg mice, and in monocrotaline-injected rats (Fig S2A upper panel). Moreover, there were inverse correlations between Egfr and caspase 3 expression (Fig S2A lower panel). In addition, we found an increased Bad expression in pulmonary ECs across all rodent PH models, suggesting that Egfr regulates the expression of Bad, protecting ECs against apoptosis (Fig S2B).
Figure 1. Deficiency in AREG/Egfr aggravates pulmonary hypertension.
RNA sequencing data (dbGaP genotype files: phs000998.v1.p1 NHLBI/iPSC_PulmonaryHypertension) (Rhodes et al, 2015) were analyzed. Lungs of pulmonary arterial hypertension patients and healthy controls were collected (n = 7 for controls and 6 for pulmonary arterial hypertension patients). Lungs of hypoxic and normoxic mice were harvested (n = 5 for each group). (A) The flowchart depicts the analysis of the RNASeq data obtained by Rhodes et al (2015). (B) Heat map depicting expression of the cell survival genes (expressed as log2 FC) obtained from the RNASeq analysis. (C) Heat map showing expression of the genes downstream to AREG using qRT-PCR in human pulmonary endothelial cell line. (D, E) Confocal imaging of human (D) and mouse (E) lungs showing expression of EGFR (green) in CD31 (white)-expressing endothelial cells. Smooth muscle actin (SMA) (red) was used to stain the medial layer. The arrows depict EGFR+ vascular endothelial cells. (F, G) In normoxic and hypoxic Cdh5cre/+ Egfrfl/fl and littermate control mice, right ventricular systolic pressure (F) and lung vasculature remodeling (G) were quantified. n = 5 per group. Data are shown as mean. *P < 0.05, **P < 0.01, ****P < 0.001.
Figure S1. Egfr expression is decreased in mouse and rat models of pulmonary hypertension.
(A) Schematic representing genes downstream to AREG determined using the Ingenuity Pathway Analysis Software. The solid and dotted arrows represent either direct or indirect activations, respectively. (B, C) AREG expression was evaluated in pulmonary ECs of hypoxic mice (B) and pulmonary hypertension patients (C) by confocal imaging. (D, E) Egfr-expressing ECs and Egfr MFI were assessed in lung ECs of Il6tg mice housed in hypoxic conditions (D) and monocrotaline-injected rats (E) by confocal microscopy. n = 10 replicates per condition. Data are shown as mean. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure S2. The levels of pulmonary endothelial Egfr, caspase 3, and Bad are altered across 3 rodent models of pulmonary hypertension.
(A, B) Wild-type and Il6tg mice were exposed to hypoxia for 3 wk. Rats were injected with either monocrotaline or vehicle diluent. (A, B) The levels of pulmonary endothelial Egfr, caspase 3, (A) and Bad (B) were assessed by confocal imaging. (C) Representative hemodynamics traces of hypoxic Cdh5Cre/+ Egfrfl/fl and Cdh5+/+ Egfrfl/fl mice. (D) Ki-67–expressing ECs and Ki-67 MFI were assessed in lung ECs of the control and Egfr KO mice. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, ***P < 0.005, ****P < 0.001.
Deficiency of EGFR in ECs aggravates PH
The role of Amphiregulin/EGFR in cardiovascular diseases has been reported (Makki et al, 2013; Peng et al, 2016; Liu et al, 2016a). However, the importance of these molecules in PH remains understudied. Because we observed that AREG and EGFR are down-regulated in pulmonary endothelial cells in patients and mice with PH, we hypothesized that EC-specific Egfr deficiency worsens PH pathogenesis. To this end, we generated mice that lack Egfr in EC (Cdh5cre/+ Egfrfl/fl), notably using Cdh5cre mice (Payne et al, 2018) that do not incur off-target ablation of expression in immune cells seen with other endothelial-specific Cre mouse lines (De Palma et al, 2005; Chen et al, 2016). These mice exhibited elevated right ventricular systolic pressure (RVSP) (Figs 1F and S2C) and exacerbated lung remodeling (Fig 1G) compared with control Cdh5+/+ Egfrfl/fl mice under hypoxic conditions. Although normoxic Cdh5cre/+ Egfrfl/fl had similar RVSP, they displayed worsened lung remodeling compared with Cdh5+/+ Egfrfl/fl mice housed under normoxic conditions.
The loss of AREG or its receptor EGFR increases EC apoptosis
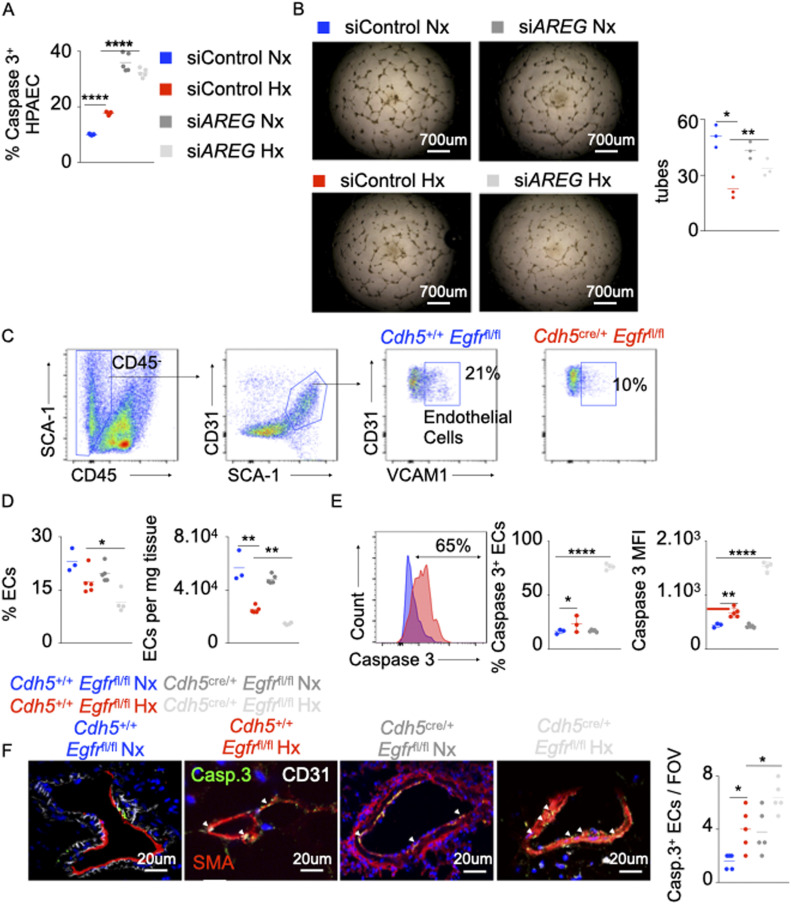
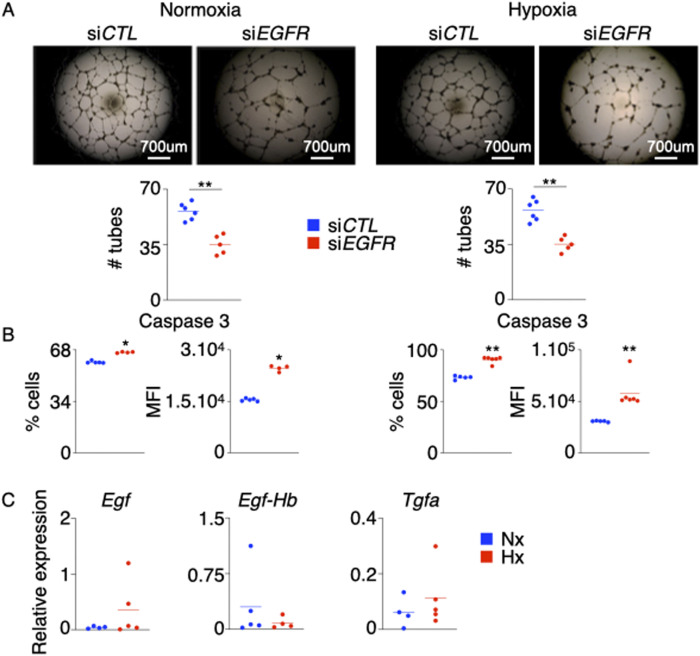
Various studies showed that Amphiregulin and Amphiregulin receptor EGFR play an important role in survival and proliferation of vascular smooth muscle cells (Kato et al, 2003) and endothelial cells (Bordoli et al, 2011; Lee et al, 2016), and increase atherogenesis (Dreux et al, 2006; Bordoli et al, 2011). However, the contributions of Amphiregulin and EGFR in the setting of PH are not known. To this end, the expression of AREG in PAECs was knocked down using siRNA. Hypoxia-induced endothelial apoptosis was further aggravated in the absence of AREG (Fig 2A). Similarly, AREG silencing decreased angiogenic tube formation ability (Fig 2B). In line with this observation, mice lacking Egfr in ECs (cdh5cre/+ Egfrfl/fl) displayed decreased number of PAECs under hypoxic conditions (Fig 2C and D). PAECs in these mice also displayed greater apoptotic activity compared with the littermate control mice (cdh5+/+ Egfrfl/fl) (Fig 2E and F). Concomitantly, we found that ECs from hypoxic Cdh5cre/+ Egfrfl/fl mice had higher expression of Ki-67 than the WT controls suggesting that Egfr-deficient ECs are hyperproliferative (Fig S2D). Next, we sought to determine the impact of EGFr on endothelial biology in vitro. We silenced Egfr in HPAECs in vitro using siRNA. We observed that HPAECs lacking Egfr, placed either in normoxia or hypoxia, are less angiogenic (Fig S3A) and more apoptotic (Fig S3B) than ECs transfected with scrambled siRNA. These data are consistent with what we observed when we knocked down AREG in vitro. In addition, we measured the gene expression of the other EGFr ligands such as Egf, Egf-Hb, and Tgfa in the lungs of normoxic and hypoxic mice (Fig S3C). We did not see any statistical difference in the expression of these ligands in normoxia v. hypoxia. In aggregate, these findings indicate that the Amphiregulin/EGFR signaling controls PAEC survival and proliferation.
Figure 2. The loss of AREG and Amphiregulin receptor epidermal growth factor receptor (EGFR) increases EC apoptosis.
(A, B) Pulmonary arterial endothelial cells were transfected with either scrambled siRNA (siCTL) or siRNA against AREG (siAREG) and placed in hypoxia or normoxia. (A) The cells were stained for caspase 3, and apoptosis was measured by flow cytometry. (B) Pulmonary arterial endothelial cells were plated on Matrigel, and tubular structures were photographed 4–6 h after plating. The number of tubes was quantified. (C, E) Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice were placed in normoxia or hypoxia for 21 d, and lung vascular endothelial cells were analyzed by flow cytometry. (C, D) Flow cytometric quantification of lung vasculature endothelial cells. (E, F) The percentage of apoptotic lung vascular endothelial cells was assessed using caspase 3 staining by flow cytometry (E) and confocal microscopy (F). n = 5 per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ****P < 0.001.
Figure S3. EGFR silencing in HPAECs decreases their angiogenic ability and increases cell death.
HPAECs were transfected with either siEGFR or siCTL and were exposed to normoxia (left panel) or hypoxia (right panel) for 24 h. (A, B) Angiogenesis was assessed by tube formation assay (A), and apoptosis was measured by flow cytometry (B). (C) Egf, Egf-Hb, and Tgfa were measured in the lungs of hypoxic mice. Data are shown as mean. **P < 0.01.
The loss of AREG and its receptor EGFR in ECs increases their inflammatory phenotype
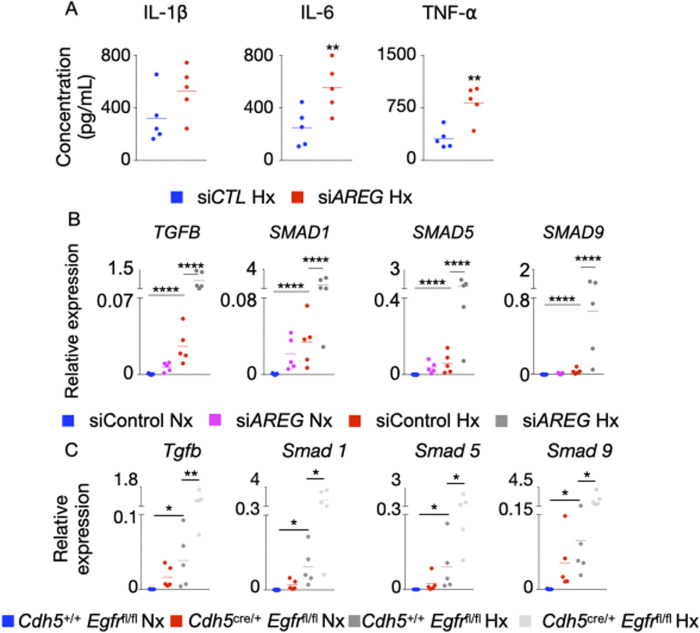
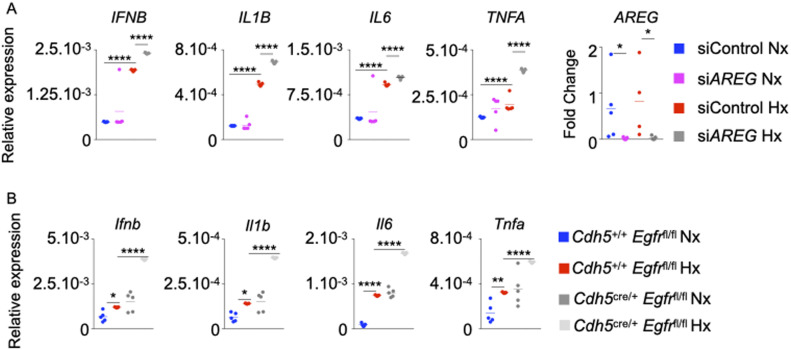
Various studies reported that resting and healthy endothelial cells prevent blood coagulation, control blood flow, and inhibit inflammation (Pober & Sessa, 2007). Endothelial cell dysfunction characterized by augmented apoptosis is associated with inflammation (Chavakis & Dimmeler, 2002). As discussed above, the disruption of Amphiregulin/EGFR signaling exacerbated PAEC apoptosis in hypoxic conditions. However, if loss of EGFR in PAECs results in exaggerated inflammation in PH is not known. AREG silencing in PAECs significantly elevated IL-1β, IL-6, and TNF-α concentrations (Fig S4A) and the expression of the genes encoding these cytokines under hypoxia (Fig 3A). Next, we assessed inflammation in the lungs of Cdh5cre/+ Egfrfl/fl mice that lack Egfr in PAECs in normoxia and hypoxia. These mice displayed elevated expression of inflammatory cytokines in the lungs compared with Cdh5+/+ Egfrfl/fl mice (Fig 3B). Given prior paradoxical links of Amphiregulin to fibrosis (Lu et al, 2006; de Vries & Noelle, 2010; Zaiss et al, 2013; Liu et al, 2016b, 2018, 2020), we sought to delineate the contributions of Amphiregulin in lung fibrosis in the context of PH. We first checked the expression of TGFB, SMAD1, SMAD5, and SMAD9, which are involved in fibrosis, in normoxic and hypoxic human PAECs transfected with siAREG. We found that the expression of these genes was increased in hypoxic PAECs treated with siAREG compared with control, highlighting the importance of AREG in vitro in modulating fibrosis (Fig S4B). We found similar increase of these genes in the lungs of Cdh5cre/+ Egfrfl/fl mice housed under hypoxic conditions compared with Cdh5+/+ Egfrfl/fl mice (Fig S4C). These data indicate that Amphiregulin reduces the expression of the genes responsible for fibrosis in pulmonary endothelial cells. As a whole, these data indicate that Amphiregulin and EGFR are important for maintaining an anti-inflammatory phenotype of PAECs.
Figure S4. AREG and Egfr deficiencies increase pro-inflammatory cytokine expression and modulate pro-fibrotic gene expression in pulmonary arterial endothelial cells.
(A, B) Human pulmonary arterial endothelial cells were transfected with siAREG for 72 h and placed in hypoxia or normoxia for 24 h. (A) The supernatants were collected at 72 h post treatment, and IL-1β, IL6, and TNF-α levels were measured by ELISA. (B) TGFB and SMAD1, 5, and 9 expression was assessed by RT-qPCR. (C) Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice were placed in either normoxic or hypoxic conditions for 21 d, and lungs were harvested. Tgfb and Smad1, 5, and 9 gene expression was quantified by RT-qPCR. Data are shown as mean. *P < 0.05, **P < 0.01, ****P < 0.001.
Figure 3. The loss of AREG and Amphiregulin receptor epidermal growth factor receptor (EGFR) in ECs increases their inflammatory phenotype.
(A) Pulmonary arterial endothelial cells were transfected with either scrambled siRNA (siCTL) or siRNA against AREG (siAREG) and placed in normoxic or hypoxic conditions. IFNB, IL1B, IL6, TNFA, and ARE expression was assessed by qRT-PCR. (B) Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice were placed in normoxia or hypoxia for 21 d, and lungs were harvested. Ifnb, Il1b, Il6, and Tnfa expression was evaluated in whole lungs by qRT-PCR. (A, B) n = 5 samples (A)/mice (B) per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ****P < 0.001.
The loss of AREG or its receptor EGFR in PAECs recruits inflammatory myeloid cells into the lungs in hypoxic mice
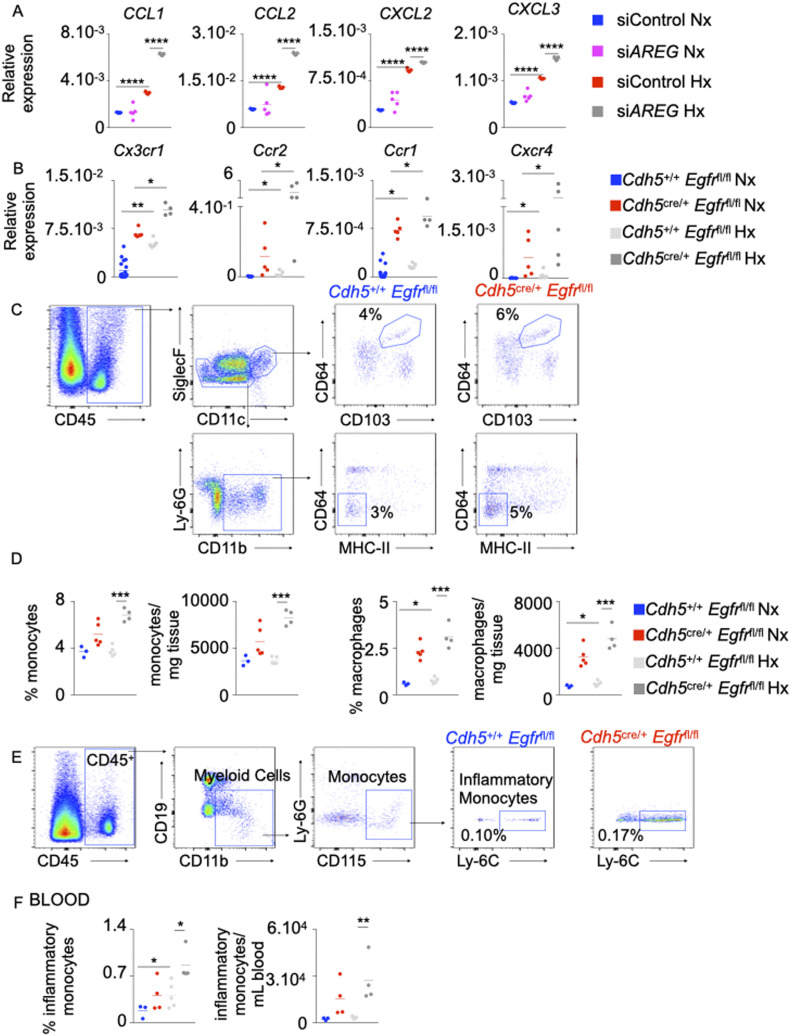
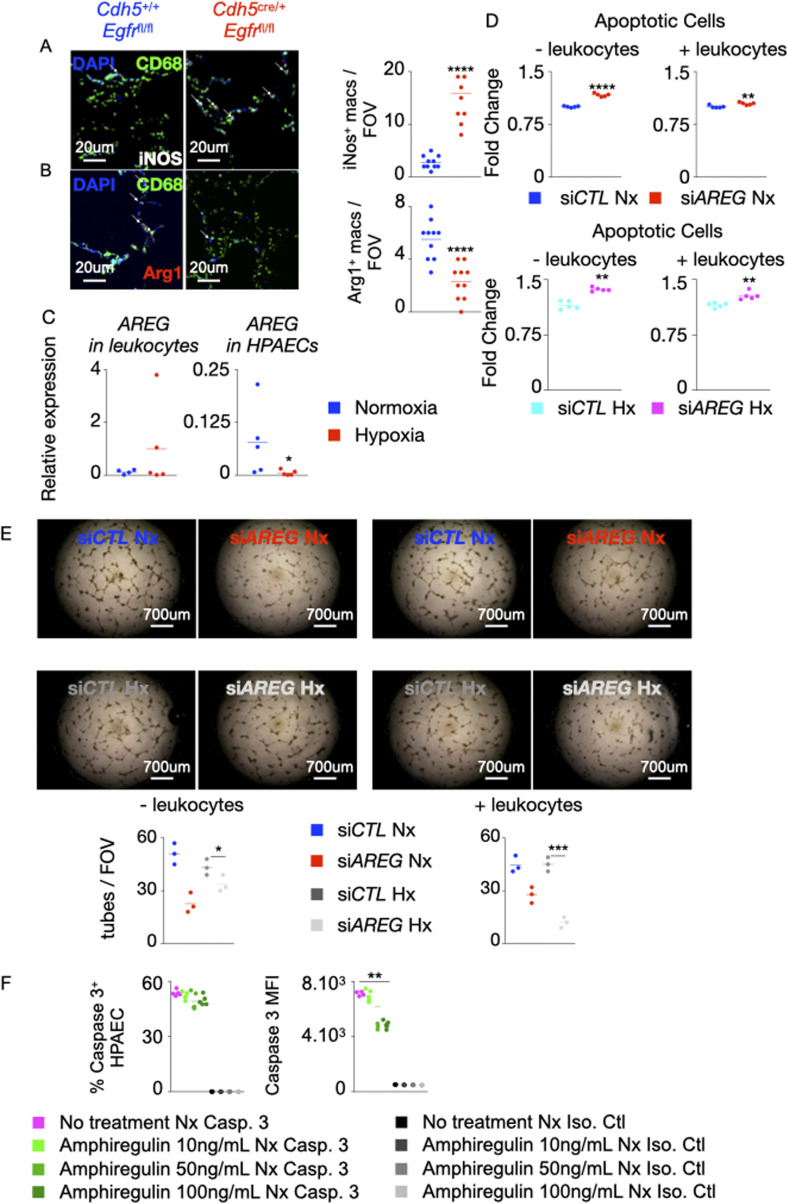
One of the hallmarks of PH is local lung inflammation mediated by the influx of inflammatory myeloid cells, such as monocytes, into the lungs (Amsellem et al, 2017; Florentin et al, 2018). The contribution of Amphiregulin/EGFR in cellular inflammation in PH has not been studied. We observed that siRNA-mediated knockdown of AREG in PAECs significantly increased the expression of CCL1, CCL2, CXCL2, and CXCL3 (Fig 4A). These chemokines promote myeloid cell influx at the sites of inflammation. Correspondingly, the lungs of Cdh5cre/+ Egfrfl/fl mice showed higher expression of chemokine receptors such as Cx3cr1, Ccr2, Ccr1, and Cxcr4 (Fig 4B). Consistent with high expression of the chemokines in PAECs in the absence of AREG and the chemokine receptors in PAECs of Cdh5cre/+ Egfrfl/fl mice, there was a higher abundance of monocytes and macrophages in the lungs of mice lacking Egfr in ECs compared with control (Fig 4C and D). In addition, to understand if Egfr deficiency controls macrophage phenotype, we stained lung sections from Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice with antibodies against iNOS and Arg-1, a pro-inflammatory and pro-resolution marker, respectively. We found that lung macrophages of Cdh5cre/+ Egfrfl/fl mice expressed lower amounts Arg1 and higher levels of iNOS, indicating that the macrophages in these mice are more inflammatory (Fig S5A and B). Of note, Egfr deficiency in EC also increased circulatory inflammatory monocyte frequency and number (Fig 4E and F). Then, we interrogated the possible role of exogenous Amphiregulin produced by recruited immune cells in the development of PH, which could neutralize or counter any potential deficiency in the endothelial cells. To this end, we first measured the expression of AREG in leukocytes and PAECs under normoxia and hypoxia by qRT-PCR. We observed that leukocytes expressed AREG at high levels (Fig S5C). To understand if Amphiregulin secreted by leukocytes could counteract the effects of AREG deficiency in endothelial cells, we co-cultured PAECs after AREG silencing with leukocytes isolated from human blood. We found that the presence of leukocytes did not have any impact on apoptosis of normoxic and hypoxic human PAECs (Fig S5D) or their angiogenic ability (Fig S5E). Altogether, these data suggest that the expression of AREG/EGFR in PAECs prevents the recruitment of inflammatory monocytes into the lungs in PH.
Figure 4. The loss of AREG and Egfr in pulmonary arterial endothelial cells recruits inflammatory myeloid cells into the lungs in hypoxic mice.
(A) Human pulmonary arterial endothelial cells were transfected with either scrambled siRNA (siCTL) or siRNA against AREG (siAREG) and placed in normoxic or hypoxic conditions. CCL1, CCL2, CXCL2, and CXCL3 expression was measured by qRT-PCR. (B) Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice were placed in normoxic or hypoxic conditions for 21 d, and lungs were harvested. Cx3cr1, Ccr2, Ccr1, and Cxcr4 expression was assessed in whole lungs by qRT-PCR. (C, D, E, F) Number and frequency of alveolar macrophages (CD45+, CD11c+, CD103+, and CD64+) and monocytes (CD45+, Siglec F−, CD11b+, MHC-II−, and CD64−) were assessed in the lungs (C, D) and blood (E, F) of hypoxic Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice by flow cytometry. (A, B, C, D, E, F) n = 5 replicates (A) and 5 mice (B, C, D, E, F) per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Figure S5. Absence of Egfr in murine lung ECs increases the number of pro-inflammatory macrophages.
(A, B) Lung sections of mice conditionally lacking Egfr in ECs were stained for CD68 (green), iNOS (white, A) and Arg1 (red, B). (A, B) The numbers of iNOS+ (A) and Arg1+ (B) macrophages were quantified by confocal imaging. (C, D, E) Human pulmonary arterial endothelial cells (PAECs) were transfected with either scrambled siRNA (siCTL) and siRNA against AREG (siAREG) and placed in a transwell chamber. They were then cultured in hypoxic conditions with or without leukocytes for 24 h. (C) AREG expression was assessed in leukocytes and HPAECs by qPCR. (D) Apoptosis of normoxic and hypoxic HPAECs was quantified by flow cytometry and shown as fold change compared with the level of apoptosis in siCTL PAECs. (E) HPAECs were plated on Matrigel, and tube formation was measured. (F) Human PAECs were treated with increasing concentrations (10–100 ng/ml) of recombinant amphiregulin or vehicle and placed under normoxic conditions. PAECs apoptosis was assessed by measuring caspase 3+ cells and caspase 3 MFI by flow cytometry. Isotype control was used to determine caspase 3 positivity. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
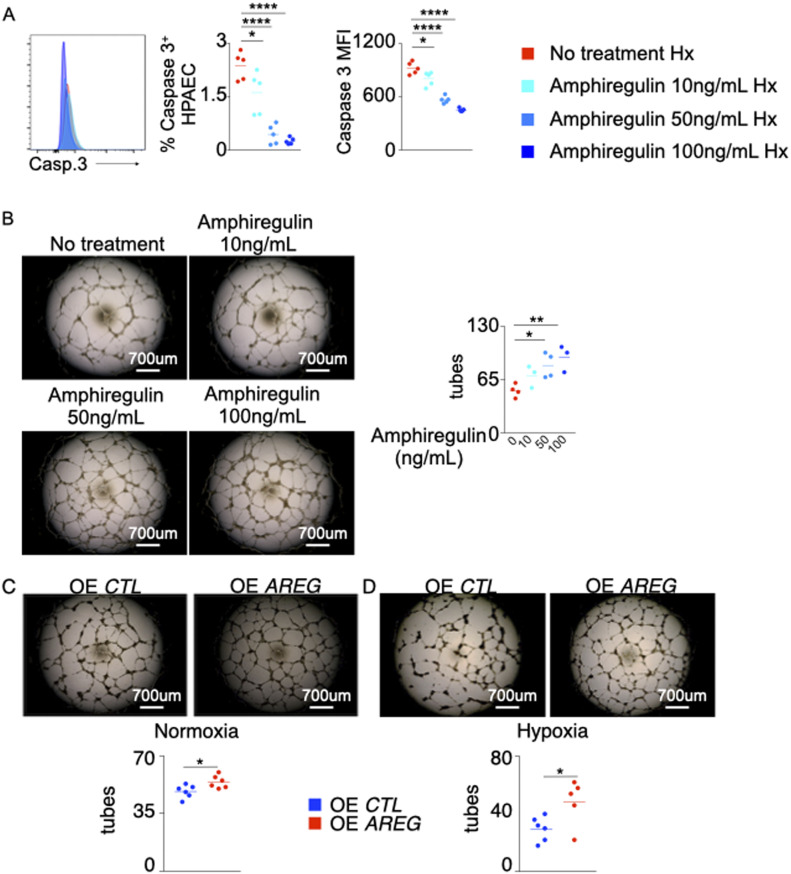
Amphiregulin treatment decreases PAEC apoptosis and increases tube formation
Many studies have reported that increased AREG expression stimulates cell migration and proliferation as well as reduces apoptosis in various diseases including cancers (Fontanini et al, 1998; Jiang et al, 2019). Here, we wanted to discern whether recombinant Amphiregulin treatment in hypoxic PAECs would rescue angiogenesis and prevent apoptosis. To this end, PAECs were treated with various concentrations of recombinant Amphiregulin under hypoxia. Hypoxic PAECs treated with recombinant Amphiregulin were less apoptotic than untreated hypoxic PAECs (Figs 5A and S5F). Correspondingly, recombinant Amphiregulin exposure increased the tube formation ability of PAECs under hypoxic conditions (Fig 5B). Conversely, we observed that both hypoxic and normoxic HPAECs overexpressing AREG showed greater tube formation ability compared with HPAECs transfected with an empty vector (Fig 5C and D).
Figure 5. Amphiregulin treatment decreases pulmonary arterial endothelial cell (PAEC) apoptosis and increases tube formation.
PAECs were treated with increasing concentrations (10–100 ng/ml) of recombinant Amphiregulin or vehicle and placed under hypoxic conditions. (A) PAEC apoptosis was assessed by measuring caspase 3+ cells and caspase 3 mean fluorescence intensity (MFI) by flow cytometry. (B) The cells were plated on Matrigel after the treatment, and tube formation was assessed. (C, D) PAECs were transfected with either an empty plasmid (OE CTL) or a plasmid encoding AREG (OE AREG) and placed in either normoxic (C) or hypoxic (D) conditions. The tube formation ability of the transfected PAECs was assessed. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ****P < 0.001.
Hypoxia Inducible Factor-1 alpha (HIF-1α) negatively regulates AREG expression in hypoxic PAECs
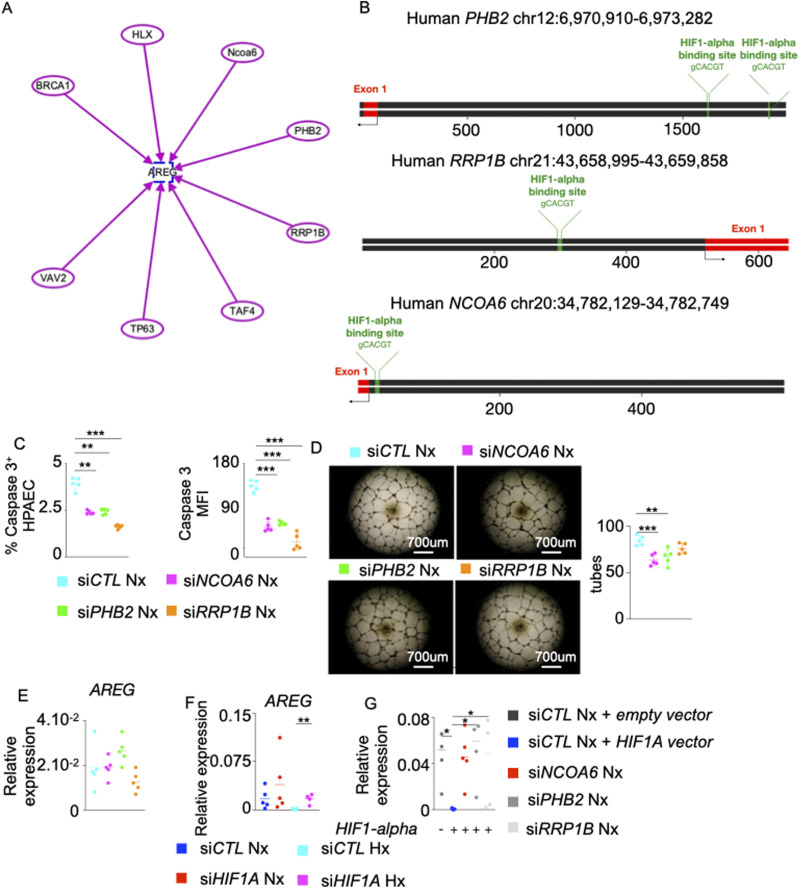
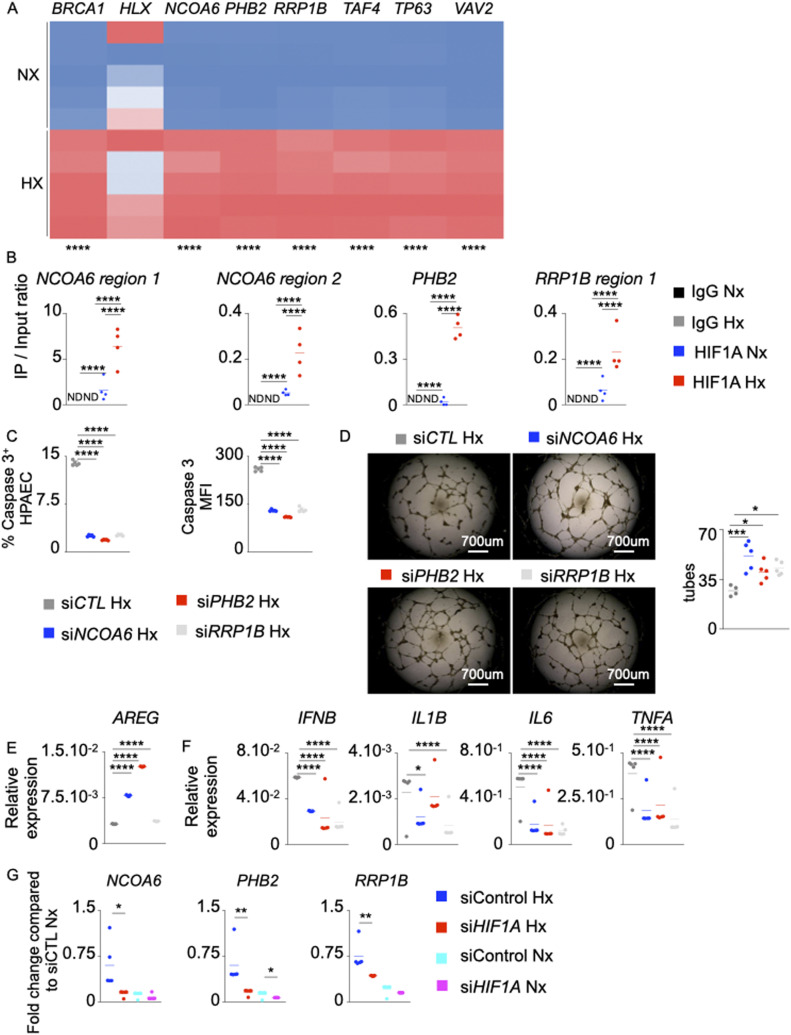
To understand the mechanisms of AREG down-regulation in endothelial cells under hypoxic conditions, we used IPA to identify possible inhibitors of AREG. We found seven possible candidates including BRCA1 (Lamber et al, 2010), HLX (Martin et al, 2013), NCOA6 (Qi et al, 2004), PHB2 (Park et al, 2011), RRP1B (Lee et al, 2014), TAF4 (Fadloun et al, 2007), TP63 (Wang et al, 2011), and VAV2 (Menacho-Márquez et al, 2013) (Fig S6A). All these genes except HLX were induced in PAECs under hypoxic conditions (Fig 6A). In silico analysis revealed that the promoter regions of PHB2, RRP1B, and NCOA6 carry binding motifs for HIF-1⍺ (Fig S6B). We experimentally demonstrated the binding of HIF-1⍺ to the promoter regions of PHB2, RRP1B, and NCOA6in PAECs (Fig 6B). Interestingly, we observed increased HIF-1⍺ binding to the promoter regions of these genes in hypoxic conditions. Silencing these genes in PAECs decreased hypoxia-mediated apoptosis (Figs 6C and S6C), increased tube formation ability (Fig 6D), augmented AREG expression (Fig 6E), and decreased PAEC inflammation (Fig 6F). Although there was a modest increase in AREG expression in siRRP1B-treated PAECs, hypoxia-induced cytokine production was blunted. Of note, knocking down these genes in normoxic human PAECSs also decreased their tube formation ability (Fig S6D). However, the treatment did not have a significant impact on AREG expression (Fig S6E), indicating HIF-1⍺–mediated augmentation of the activities of these genes in hypoxia. In addition, siRNA knockdown of HIF1A decreased the expression of NCOA6, PHB2, and RRP1B (Fig 6G); however, the treatment increased AREG expression (Fig S6F) in PAECs in hypoxic conditions, confirming the regulation of these genes by HIF-1⍺. To further understand if HIF1α-mediated AREG down-regulation is PHB2-, NCOA6-, and RRP1B-dependent, we silenced these genes in PAECs overexpressing HIF1A. Such knockdown abrogated HIF1α-induced AREG down-regulation (Fig S6G). Altogether, these data suggest that HIF-1⍺ suppresses AREG expression in hypoxic conditions by activating NCOA6, PHB2, and RRP1B.
Figure S6. AREG suppression by NCOA6, PHB2 and RRP1B is HIF-1⍺-mediated.
,(A) Schematic representing AREG and its upstream genes including BRCA1, HLX, NCOA6, PHB2, RRP1B, TAF4, TP63, and VAV2 was generated using the Ingenuity Pathway Analysis Software. Each arrow represents the activation of AREG by each gene. (B) Schematic depicting HIF-1⍺–binding sites in PHB2, RRP1B, and NCOA6 gene promoter regions. This schematic was designed using UCSC Genome Browser website (https://genome.ucsc.edu) and Snapgene software (https://www.snapgene.com). (C, D, E) Pulmonary arterial endothelial cells (PAECs) were transfected with scrambled siRNA (siCTL) or siRNA against NCOA6 (siNCOA6), PHB2 (siPHB2) or RRP1B (siRRP1B) and placed in normoxia for 24 h. (C) Apoptotic PAECs were quantified by measuring caspase 3+ cells and caspase 3 MFI by flow cytometry. (D) PAECs were plated on Matrigel, and tube formation was assessed. (E) AREG expression was assessed by qPCR. (F) Human PAECs were transfected with either scrambled siRNA (siCTL), or siRNA against HIF1A and placed in normoxic or hypoxic conditions for 24 h. AREG expression was quantified by qPCR. (G) Human PAECs were transfected with a lentivirus overexpressing HIF1-A for 48 h. The cells were transfected with siRNA against NCOA6, PHB2, or RRP1B and cultured in normoxic conditions for 48 h. AREG expression was assessed by qPCR. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ***P < 0.005.
Figure 6. Hypoxia inducible factor-1⍺ (HIF-1⍺) negatively regulates AREG expression in pulmonary arterial endothelial cells (PAECs) under hypoxic conditions.
(A) Heat map showing BRCA1, HLX, NCOA6, PHB2, RRP1B, TAF4, TP63, and VAV2 expression assessed by qRT-PCR in normoxic and hypoxic PAECs. (B) The binding of HIF-1⍺ to the NCOA6, PHB2, and RRP1B promoter regions in PAECs was assessed by ChIP qPCR. (C, D, E, F, G) PAECs were transfected with scrambled siRNA (siCTL) or siRNA against NCOA6 (siNCOA6), PHB2 (siPHB2), or RRP1B (siRRP1B) and placed in hypoxia for 24 h. (C) Apoptotic PAECs were quantified by measuring caspase 3+ cells and caspase 3 MFI by flow cytometry. (D) PAECs were plated on Matrigel, and tube formation was assessed. (E, F) AREG (E), IFNB, IL1B, IL6, and TNFA (F) expression was assessed by qRT-PCR. (G) PAECs were transfected with either scrambled siRNA (siCTL) or siRNA against HIF1A (siHIF1A) and placed in hypoxia or normoxia for 24 h. NCOA6, PHB2, and RRP1B expression was quantified by qRT-PCR. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
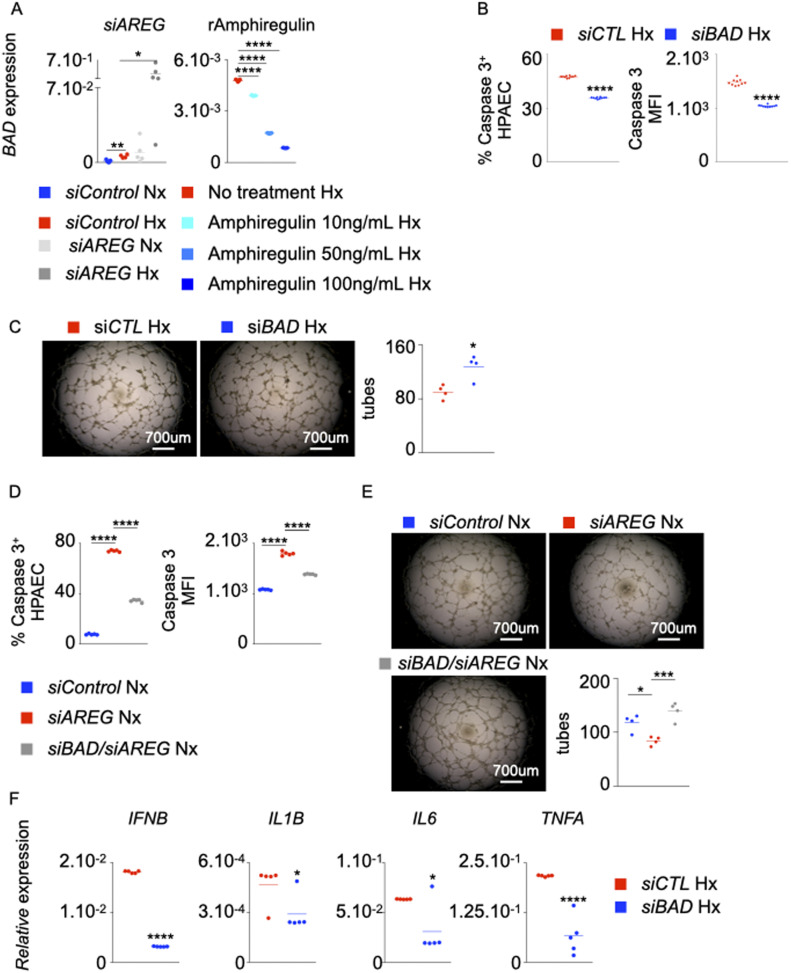
AREG deficiency depends on BAD for exaggerated inflammation, enhanced apoptosis, and depressed angiogenic potential of PAECs
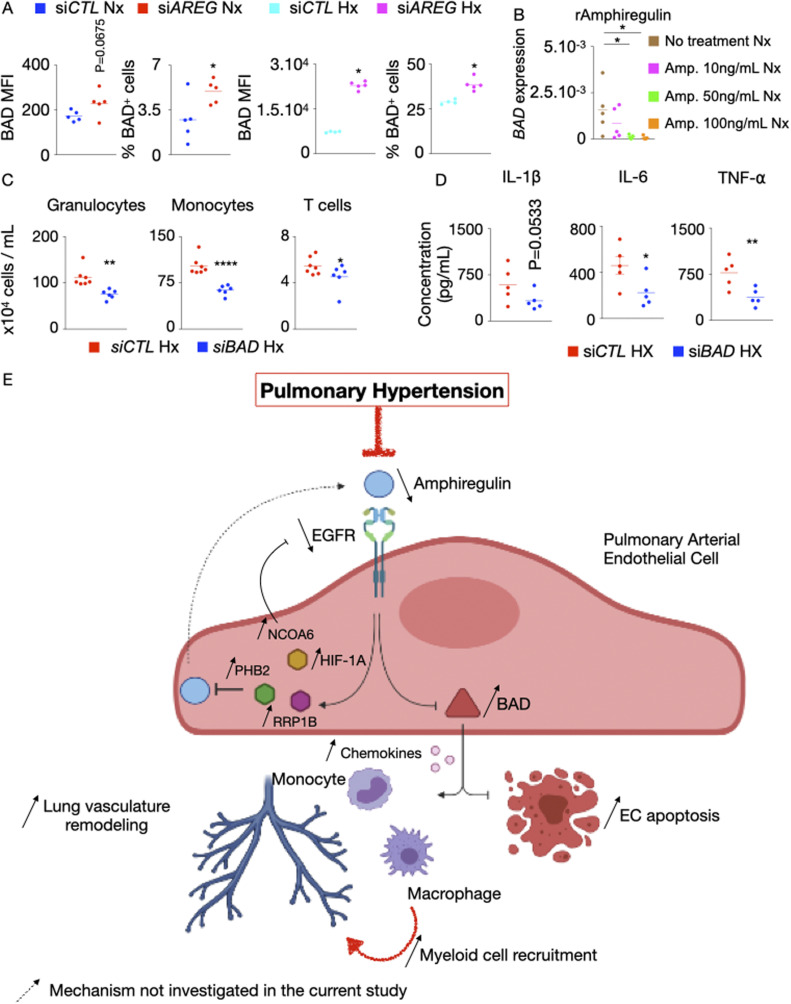
We sought to investigate EGFR/Amphiregulin downstream signaling in PH patients. Our in silico analysis revealed BAD as a gene downstream to Amphiregulin/EGFR signaling, controlling cellular apoptosis. siAREG treatment augmented the expression of BAD mRNA (Fig 7A, left panel) and protein (Fig S7A) in PAECs cultured under normoxic and hypoxic conditions. Conversely, the exposure of PAECs to recombinant Amphiregulin diminished BAD levels in these cells in hypoxic (Fig 7A, right panel) and normoxic conditions (Fig S7B). In addition, siRNA knockdown of BAD in hypoxic PAECs reduced hypoxia-induced apoptosis (Fig 7B), improved their tube formation ability in hypoxia (Fig 7C) and decreased the number of recruited inflammatory immune cells (Fig S7C). Moreover, siBAD significantly prevented siAREG-mediated apoptosis (Fig 7D) and reversed the decrease of normoxic EC tube formation by AREG knockdown (Fig 7E). In addition, BAD knockdown in PAECs decreased their inflammatory phenotype in hypoxia, as evidenced by diminished mRNA (Fig 7F) and protein (Fig S7D) levels of inflammatory cytokines such as IFN-β, IL-1β, IL-6, and TNF-α. In aggregate, these data demonstrate that AREG deficiency depends critically on BAD expression for enhanced PAEC apoptosis, increased inflammation, and suppressed tube formation ability.
Figure 7. BAD is essential for exaggerated inflammation, enhanced apoptosis, and suppressed tube formation ability of ECs in the absence of AREG.
(A) BAD expression in pulmonary arterial endothelial cells (PAECs) was quantified after AREG silencing (left panel) and recombinant Amphiregulin treatment (right panel). (B, C, F) PAECs were transfected with either scrambled siRNA (siCTL) or siRNA against BAD (siBAD) and placed in hypoxia for 24 h. (B, C) PAECs apoptosis was assessed by measuring caspase 3+ and caspase 3 MFI cells by flow cytometry (B), and tube formation ability was determined by a Matrigel assay (C). (D, E) PAECs were transfected with either scrambled siRNA (siCTL), siRNA against AREG (siAREG) or siRNA against both AREG and BAD (siBAD/AREG) and placed in normoxic conditions for 24 h. (D, E) Apoptosis (D) and tube formation (E) were examined. (F) IFNB, IL1B, IL6, and TNFA expression was assessed by qRT-PCR. n = 5 replicates per condition. Data are shown as mean. *P < 0.05, ***P < 0.005, ****P < 0.001.
Figure S7. BAD increases inflammation and leukocyte recruitment in hypoxic HPAECs.
(A) BAD expression and the frequency of BAD+ cells were determined by flow cytometry after AREG silencing in normoxic and hypoxic pulmonary arterial endothelial cells (PAECs). (B) PAECs were treated with increasing concentrations (10–100 ng/ml) of recombinant amphiregulin or vehicle and placed under normoxic conditions. BAD expression was measured by RT-qPCR. (C, D) HPAECs were co-cultured in a transwell with leukocytes and then treated with either control or BAD siRNA. (C) Granulocytes, monocytes, and T cells were enumerated by flow cytometry. (D) Cytokine concentrations were assessed by ELISA. (E) Mechanisms of increased PAEC apoptosis and exaggerated inflammation in the absence of AREG and epidermal growth factor receptor (EGFR) in pulmonary hypertension (PH). Our data support a model whereby decreased amphiregulin and EGFR expression in PAECs promote PH. Specifically, in the steady state, amphiregulin binds to the EGFR, which decreases the expression of BCL2-associated agonist of Cell Death (BAD), resulting in PAEC survival and suppressed inflammation. In PH, HIF-1⍺ binds to the promoters of NCOA6, PHB2, and RRP1B and increases their expression. These genes down-regulate AREG, resulting in augmented BCL2 expression. This pro-apoptotic gene, in turn, incites apoptosis and chemokine production. Elevated levels of the chemokines recruit inflammatory myeloid cells in lung vasculature. Mechanisms that were not investigated in the present study are labeled with a dotted arrow. The cartoon was designed with the online Biorender software (https://biorender.com). n = 5 replicates per condition. Data are shown as mean. *P < 0.05, **P < 0.01.
Discussion
Genetic and environmental stimuli trigger PAEC apoptosis (White et al, 2014; Diebold et al, 2015; Vaillancourt et al, 2015), which is one of the inciting factors driving pulmonary vasculature remodeling and PAH. However, the mechanisms of endothelial apoptosis in PAH are understudied. Our analysis of RNA sequencing data comparing PAEC from healthy and PAH patients suggested that Amphiregulin is a potential regulator of endothelial survival. Although Amphiregulin is known to act as a pro-survival molecule, the roles of endothelial AREG and its receptor EGFR have not been studied. In this study, we sought to elucidate the role of the EGFR ligand Amphiregulin in controlling endothelial pathobiology in PH. In this study, we characterized endothelial apoptosis and inflammation in PH and identified Amphiregulin and EGFR as key molecules regulating endothelial survival and suppressing inflammation in hypoxic conditions and PH. In addition, our study highlighted the orchestration of pulmonary remodeling and hemodynamic manifestations in PH by endothelial EGFR. Mechanistically, we showed that AREG expression and inflammatory monocyte recruitment is dependent on HIF1⍺-mediated NCOA6, PHB2, and RRP1B expression. Last, we showed that the regulation of apoptosis and inflammatory chemokine production by BAD in absence of AREG resulting in exacerbation of PH pathogenesis (Fig S7E). In aggregate, these results define a protective role of endothelial Amphiregulin and EGFR in PH, suggesting the notion of Amphiregulin as a therapy of PH for further investigation.
In addition, we acknowledge that PH can be generated through a multitude of mechanisms including chronic hypoxia, thromboembolic disorders, left heart failure, and primary remodeling of the pulmonary vasculature in PPH. We used a hypoxic mouse model for the present study. This mouse model recapitulates the WSPH Group 3 PH. However, this model also is known to recapitulate some aspects of WSPH Group 1 PH (PAH), as it has been widely used to understand inflammatory mechanisms that are important in both groups 1 and 3 PH (Stenmark et al, 2009; Gomez-Arroyo et al, 2012; Ikeda et al, 2019). In addition, we have used two different animal models of PAH: (A) Il6tg mice expressing Il6 in pneumocytes that develop severe angioproliferative PAH in hypoxic conditions and (B) Monocrotaline (MCT) exposure in rats exhibiting endothelial dysfunction followed by robust angioproliferative remodeling in small pulmonary arterioles.
The Amphiregulin/EGFR axis has been shown to play a pivotal role in the pathogenesis of various inflammatory diseases (Rayego-Mateos et al, 2018; Stolarczyk & Scholte, 2018). Mechanistically, Amphiregulin binds to the EGFR and activates downstream signaling pathways, such as AKT, MEK1/2, ERK1/2, and NF-κB pathways (Heldin et al, 1998; Heldin & Westermark, 1999; Rosenkranz & Kazlauskas, 1999). Our data show that normoxic mice lacking Egfr, specifically in endothelial cells, display greater systemic inflammation because of increased numbers of recruited pro-inflammatory leukocytes such as monocytes and macrophages, and heightened endothelial cell death compared with wild-type normoxic mice. Interestingly, such increased endothelial inflammation and apoptosis do not result in worse RVSP in absence of hypoxia, indicating that EGFR deficiency alone without noxious environmental trigger is not sufficient to drive PH pathogenesis. In the context of hypoxia, the proliferation of cultured human pulmonary microvascular endothelial cells has been shown to depend on EGFR activation (Toby et al, 2010; Pool et al, 2019). In line with this finding, our in vitro studies indicate that Amphiregulin produced by ECs acts in an autocrine fashion and promotes cellular survival and proliferation. We observed that the presence of leukocytes does not reverse the decreased ability of endothelial tube formation in absence of AREG. Although amphiregulin secreted by leukocytes can affect endothelial tube formation capability, there are other factors secreted by leukocytes that alters angiogenic ability of ECs. Furthermore, when cultured in contact with HPAECs lacking AREG, inflammatory cells will heighten inflammation in ECs, which may impair angiogenesis (Hanumegowda et al, 2012; Quarck et al, 2015). The protective autocrine function of Amphiregulin is relevant to a key observation made by Rhodes et al (2015), who reported decreased expression in IQSEC1, a guanidine nucleotide exchange protein recruited by EGFR, in PAH patients (Rhodes et al, 2015). Yet, in contrast to prior in vitro findings reporting up-regulation of EGF/EGFR in hypoxic endothelial cells (Toby et al, 2010; Pool et al, 2019), we found that the expression of EGFR was attenuated in pulmonary endothelial cells in human and murine subjects with PH. Importantly, whereas the in vitro response to hypoxia may be more one-sided and may reflect more uncomplicated phenotypes, the evolution of endothelial cells in vivo in PH is known to be complex, including initial apoptosis followed by development of hyperproliferative cells (Michelakis, 2006). Nonetheless, a similarly complex endothelial evolution of EGFR expression and activity may underlie this discrepancy, and future time-course studies of EGFR in vivo should be considered.
EGFR has multiple ligands. Besides Amphiregulin, EGFR interacts with EGF, TGF-α, and heparin-binding EGF (Wieduwilt & Moasser, 2008). Binding of these ligands to EGFR results in different physiological responses (Makki et al, 2013; Singh et al, 2016). Our study does not decipher the contributions of these EGFR ligands in PH. Specifically, Amphiregulin is involved in the pathogenesis of various cardiovascular diseases. Amphiregulin has been shown to increase cardiac fibrosis and aggravate cardiac dysfunction in a mouse model of myocardial infarction (Liu et al, 2018). We have not assessed the role of Amphiregulin produced by PAECs in vivo, and PAEC-specific AREG-deficient mice will be required to answer this question. Moreover, future studies are warranted to decipher the contributions of Amphiregulin produced by other pulmonary cells such as smooth muscle cells (Heialy et al, 2013; Deacon & Knox, 2015), which are crucial to the development of PH.
It has also been reported that TGF-α–dependent EGFR signaling promotes PAH (Le Cras et al, 2003) and that the inhibition of the EGFR attenuated PH in a rat model (Merklinger et al, 2005). Our findings that endothelial-specific deficiency of EGFR in fact promotes PH point to a cell type-specific role for this pleiotropic receptor. Correspondingly, in the development of hepatocellular carcinoma, EGFR has been shown to elicit opposing physiological functions depending on cell type (Timchenko, 2015). Future work in animal models carrying genetic depletions of EGFR across different cellular compartments in the lung may reveal further distinct actions of EGFR, converging upon the final manifestation of PH. In addition, it has been proposed that at the initial stage of PH, pulmonary ECs undergo apoptosis followed by the development of hyperproliferative cells (Michelakis, 2006). During the initial stage of PH, low endothelial expression of amphiregulin/EGFR contributes to elevated EC apoptosis, whereas in the latter stage, amphiregulin promotes EC proliferation and fibrosis. Moreover, EGFR exerts opposing effects depending on cell type as stated above (Timchenko, 2015). Thus, our findings support the notion that targeting AREG expressed by endothelial cells in the early stage of PH in patients could be beneficial. This could be achieved by using siRNA against AREG formulated in 7C1 nanoparticles, which specifically target endothelial cells (Dahlman et al, 2014; Sager et al, 2016).
Unexpectedly, in endothelial cells, we found that EGFR is involved in suppressing inflammation. Genetic and siRNA-mediated endothelial deletion of EGFR and AREG resulted in increased production of inflammatory cytokines and chemokines responsible for myeloid cell recruitment. In agreement with this, the lungs of cdh5cre/+ Egfrfl/fl mice harbored augmented number of monocytes compared with age-matched control. Conversely, in an animal model of atherosclerosis, Egfr expressed in atheromas facilitates monocyte recruitment and their subsequent differentiation into macrophages (Lamb et al, 2004). The discrepancy in Egfr-mediated inflammatory potential can again be explained by tissue and cell specificity. Studies from our and other groups have shown that monocytes infiltrated into the lungs in PH increase inflammation (Rabinovitch et al, 2014; Pugliese et al, 2015; Amsellem et al, 2017; Kumar et al, 2017; Florentin et al, 2018) and worsen PH burden. Similarly, aggravation of PH in the absence of EGFR in PAECs could result from not only accelerated apoptosis but also heightened inflammation. However, mice lacking monocytes and Egfr will be necessary to precisely dissect these mechanisms further. In addition, we used Cdh5cre/+ Egfrfl/fl mice, which have a conditional deficiency of Egfr in endothelial cells. We acknowledge that we did not use inducible Cre mice, such as Cdh5 ER-Cre, which would allow us to avoid any developmental adaptation. Further analysis using the latter mouse model is required to decipher the possibility of developmental adaptation that a conditional deletion of Egfr in endothelial cells might generate.
While investigating the mechanisms of AREG down-regulation in hypoxic conditions, we observed that the expression of various upstream transcription co-regulators, including NCOA6, RRP1B, and PHB2 was augmented in hypoxic PAECs. These molecules are found to be up-regulated in the hypoxic microenvironments in tumors (Cheng et al, 2014; Xu et al, 2019; Wu et al, 2020a). Interestingly, the functions of these transcription factors in inflammation and PH are largely unknown. We observed that HIF-1⍺ increases the expression of these transcription factors by directly binding to their promoter regions. Furthermore, silencing of NCOA6, RRP1B, and PHB2 abrogated AREG down-regulation, endothelial apoptosis, and inflammation in hypoxic condition. Additional studies will be necessary to ascertain the exact roles of these transcription factors in PH pathogenesis.
Finally, with the help of IPA and in vitro assays, we found that BCL2-Associated Agonist of Cell Death (BAD), a molecule downstream to EGFR/Amphiregulin, plays a pivotal role in enhancing apoptosis and inflammation and suppressing the angiogenic potential of ECs in the absence of AREG/EGFR. Correspondingly, BAD expression has been shown to be up-regulated in various subtypes of PH (Liu et al, 2016c; Deng et al, 2017; Opitz & Kirschner, 2019). Specifically, increased Bad expression has been associated with heightened endothelial apoptosis and lung remodeling in rat models of chronic thromboembolic pulmonary hypertension (CTEPH) (Deng et al, 2017) and World Symposium on Pulmonary Hypertension (WSPH) Group 3 PH (Liu et al, 2016c). Thus, our report connects these two seemingly disparate pathways of pathogenic molecules, describing an AREG-mediated BAD down-regulation and prevention of endothelial apoptosis in PH. Further studies using endothelial-specific deletion of Bad will be required to evaluate BAD as a potential druggable target to dampen inflammation, promote endothelial survival, and reduce PH pathogenesis.
Materials and Methods
Animals
All animal experiments were conducted following NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult females C57BL/6 wild-type and lung-specific Il-6–overexpressing transgenic mice (Il6tg) (10–12 wk old) were obtained from the Jackson Laboratory and maintained under a standard light cycle (12 h light/dark). Il6tg mice express pulmonary-specific Il-6, leading to secondary systemic increases of Il-6 (Steiner et al, 2009). Egfrfl/fl mice were gifts from Dr. Matthias Nahrendorf. cdh5cre/+ male and Egfrfl/fl female mice were bred to generate Cdh5+/+ Egfrfl/fl and Cdh5cre/+ Egfrfl/fl mice. To induce PH, mice were placed in hypoxic chambers under 10% O2 for 3 wk. Adult, 8–10-wk-old male Sprague–Dawley rats were obtained from Charles River laboratory and maintained under a standard light cycle (12 h light/dark). To induce PH, rats were injected with monocrotaline (60 mg/kg).
Organ harvesting and flow cytometry
Mice were euthanized and perfused thoroughly with 30 ml of ice-cold PBS through the left ventricle. One lobe of the lungs was harvested, minced and digested in an enzymatic mixture of collagenase I, collagenase XI, DNase I, and hyaluronidase (Sigma-Aldrich) for leukocyte analysis, and collagenase IV and DNase I for endothelial cell separation, under agitation at 37°C for an hour. Cells were passed through 40-μm cell strainers, washed in 10 ml of FACS buffer that is made of PBS containing 0.5% bovine serum albumin and centrifuged at 4°C. Peripheral blood was collected through cardiac puncture, and RBC lysis buffer was used to lyse erythrocytes for 3 min at room temperature. After lysis, FACS buffer was added and the cells were centrifuged. Supernatant was discarded and pellet was dissolved in FACS buffer. Total viable cell numbers per mg of tissue for lungs and per mL of blood were counted using a hemocytometer after dissolving the single cell suspension in Trypan Blue (Cellgro, Mediatech Inc.). The staining of the single cell suspension was performed with the following antibodies diluted in FACS buffer. These antibodies were purchased from eBioscience, BioLegend, or BD Biosciences. The following panel of antibodies was used to analyze myeloid cell population: anti-CD45.2 (104), CD11b (M1/70), CD115 (AFS98), Ly6G (1A8), and Ly6C (AL-21). The following panel of antibodies was used to analyze endothelial cell population: anti-CD45.2 (104), Sca-1 (M1/70), CD31 (MEC13.3), and Vcam1 (429). Monocytes were identified as CD11b+, Ly6G−, and CD115+. Endothelial cells were defined as CD45.2−, Sca-1+, CD31+, and Vcam1+. Apoptosis experiments were carried out using PE Active Caspase 3 Apoptosis kit from BD Biosciences. A Fortessa Flow Cytometer (BD) was used to acquire data. Data were analyzed with FlowJo software (Tree Star).
Immunofluorescence
After 30 ml of cold PBS perfusion through the left ventricle, one lobe of the lungs was excised and fixed in 4% (PFA, methanol-free, Electron Microscopy Science) for 1 h and then incubated in 30% sucrose PBS at 4°C. Then, the tissue was embedded in Tissue-Tek OCT compound (Sakura Finetek) and frozen on dry ice. For immunofluorescence staining, 0.1% Triton X-100 PBS was used to permeabilize 20-μm-thick sections for 1 h at room temperature. To prevent off-site binding of antibodies, the sections were blocked in 2% BSA PBS for 1 h. Next, sections were incubated with anti-SMA-Cy3 (Millipore) for 1 h to detect smooth muscle cells in lung vasculature. The sections were subsequently washed with PBS containing 0.5% BSA, and counterstained and fixed with vector shield DAPI to visualize nuclei, and images were taken using Confocal laser scanning immunofluorescence microscopy (CLSM). Image analysis was performed using ImageJ software (Fiji). The degree of pulmonary arteriolar muscularization was assessed in lung sections stained for α-SMA by the calculation of the proportion of fully and partially muscularized peripheral (<100 mm in diameter) pulmonary arterioles among total peripheral pulmonary arterioles, as previously described (Ingersoll et al, 2010). Medial thickness was also measured in α-SMA–stained vessels (<100 mm in diameter) using ImageJ software (Fiji) and expressed as arbitrary units. All measurements were performed blinded to condition.
qRT-PCR
One half of a lobe of the lungs was flash frozen in liquid nitrogen. RNeasy RNA isolation kit (QIAGEN) was used to extract total mRNA. Total mRNA concentration was measured using a NanoDrop device. Then, cDNA was generated from 100 ng of mRNA with the high capacity RNA to cDNA synthesis kit (Applied Biosystems). SYBRgreen primers (IDT) were used to quantify the expression of genes using Quantitative RT-PCR. Data were expressed as Δ Ct values of genes normalized to the house-keeping gene β-actin. Final results were then showed as relative expression of genes compared with β-actin. The relative expression of genes was calculated as follows: Relative expressiongene = POWER(2 − (Ctgene − Ctbeta actin)).
Catheterization and hemodynamics
The right jugular vein was dissected. Salivary and lymphatic tissues were separated out to visualize and isolate a section of the vessel. Using 4-0 silk suture, the cranial end of the vessel was tied, and a loose tie on the caudal end of the vessel was made. An incision large enough to pass and insert the catheter between the two ligatures was made using a 22-G needle. The catheter was advanced towards the heart until desired and stable signal appears. RVSP was recorded. Heart was flushed with 10 ml of PBS, and the right ventricle (RV) was separated from the left ventricle (LV). Both ventricles were weighed, and RV/LV ratios were calculated.
RNAseq data and ingenuity pathway analysis
Dr. Marlene Rabinovitch kindly provided the expression values of genes differentially expressed in PAEC of patients with PAH and age-matched control patients. CLC Genomics and IPA (QIAGEN Inc.) (Krämer et al, 2013) were used to analyze the data. We assessed the fold change in the expression of the genes involved in endothelial cell survival. Genes with at least twofold differences were selected for the pathway analysis. Using IPA, we focused on gene sets whose expression was down-regulated in PH patients.
ChIP sequencing
To assess the interaction between HIF1-α and NCOA6, PHB2, and RRP1B, we first located the promoter regions of NCOA6, PHB2, and RRP1B with the help of UCSC genome browser Website (http://genome.ucsc.edu, University of California Santa Cruz). Then, we looked for potential HIF-1α–binding sites onto these promoter regions using Transfac software (GeneXplain). The primers for each HIF1-α binding site were designed using the NCBI Primer Blasts. PAECs were plated onto 10 cm dishes and for 24 h in hypoxia or normoxia. Cells were then harvested and resuspended in PBS at a concentration of 1 × 106 cells/ml. Cells were then fixed and DNA was extracted and immunoprecipitated by a ChIP grade HIF1-α antibody (Rb polyclonal; Novusbio) as previously described (Dahl & Collas, 2008). Finally, real-time PCR was run to quantify the amount of HIF1A bound to NCOA6, PHB2, and RRP1B promoter regions in each condition.
PAEC culture and AREG inhibition
Human PAECs were obtained from Promocell and grew in Endothelial Basal Medium-2 (Promocell). The cells were further expanded to reach passage 3. At passage 3, cells were transfected with siRNA against AREG (IDT) for 72 h. After 48 h of siRNA treatment, cells were put in hypoxic chamber for 24 h. Cells were then analyzed for gene expression, apoptosis and in vitro tube formation assay.
Amphiregulin treatment
PAECs were cultured for 96 h in the presence or absence of 10, 50, or 100 ng/ml of recombinant Amphiregulin (Sigma-Aldrich) that has been previously reconstituted in filtered PBS containing 0.1% BSA. Cells were then used for apoptosis and in vitro angiogenesis assays.
In vitro angiogenesis assays
Matrigel with reduced growth factors was pipetted into pre-chilled 96-well plate (50 μl Matrigel per well) and polymerized for 30 min at 37°C. PAECs following different treatments (2 × 104 cells per well) were resuspended in 100 μl of basic media, and seeded in Matrigel-coated 96-well plate. After 4–6 h of incubation, tubular structures were photographed using Olympus inverted fluorescent microscope with a 20× magnification. The number of branch points was quantified in technical triplicate.
Statistical analysis
Data were compiled in PRISM software (GraphPad), and statistics were generated and represented as mean ± SEM. Normality of data distribution was determined by Shapiro–Wilk testing. For normally distributed data, statistical significance between two categories of analyzed samples was calculated using two-tailed t test. For non-normally distributed data, statistical significance was calculated using Mann–Whitney U test. For multiple category comparisons, one-way ANOVA was used with post hoc Bonferroni testing. Differences with P-values less than 0.05 were considered statistically significant.
Study approval
All experimental procedures involving the use of human lung tissue included the relevant receipt of written informed consent and were approved by the Committee for Oversight of Research and Clinical Training Involving Decedents No. 101, at the University of Pittsburgh, as well as the Institutional Review Board of the University of Pittsburgh No. REN17020169/IRB020810. All experimental procedures involving the use of human peripheral blood included the relevant receipt of written informed consent and were approved by the Institutional Review Board of the University of Pittsburgh No. REN16070123/PRO11070366 as well as the Institutional Review Board of the University of Pittsburgh No. REN17030011/IRB0306040. Ethical approval for this study and informed consent conformed to the standards of the Declaration of Helsinki.
Data Availability
For data sharing and information, please contact Dr. Partha Dutta, the corresponding author at duttapa@pitt.edu.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Health grants R00HL121076-03 (to P Dutta), R01HL143967 (to P Dutta), R01HL142629 (to P Dutta), and R01AG069399 (to P Dutta), AHA Transformational Project Award (19TPA34910142 to P Dutta), AHA Innovative Project Award (19IPLOI34760566 to P Dutta), and ALA Innovation Project Award (IA-629694 to P Dutta) as well as the VMI Postdoctoral Training Program in Translational Research and Entrepreneurship in Pulmonary and Vascular Biology T32 funded by the National, Heart, Lung and Blood Institute (NHLBI) (to J Florentin). The work was also supported by NIH grants R01 HL124021 and HL 122596, as well as the American Heart Association Established Investigator Award 18EIA33900027 (SY Chan). We thank the NIH supported microscopy resources in the Center for Biologic Imaging (NIH grant 1S10OD019973-01). We thank Dr. Marlene Rabinovitch, Stanford University, who kindly provided RNA sequencing data. Additionally, we acknowledge Dr. Matthias Nahrendorf, Massachusetts General Hospital for Systems Biology, Harvard University, for kindly providing us with the Egfrfl/fl mice. We thank the Center for Organ Recovery & Education (CORE) as well as organ donors and their families for the generous donation of tissues used in this study. We used Biorender application (https://biorender.com) to generate Figs 1A and S7E.
Author Contributions
J Florentin: conceptualization, data curation, formal analysis, investigation, and writing—original draft, review, and editing.
J Zhao: data curation.
Y-Y Tai: data curation.
W Sun: data curation.
LL Ohayon: data curation.
SP O’Neil: data curation.
A Arunkumar: data curation.
X Zhang: data curation.
J Zhu: methodology.
Y Al Aaraj: resources.
A Watson: resources.
J Sembrat: resources.
M Rojas: resources.
SY Chan: resources, funding acquisition, and writing—review and editing.
P Dutta: conceptualization, resources, supervision, funding acquisition, project administration, and writing—review and editing.
Conflict of Interest Statement
SY Chan has served as a consultant for United Therapeutics and Acceleron Pharma. SY Chan is a director, officer, and shareholder in Synhale Therapeutics. SY Chan has held research grants from Actelion, Bayer, and Pfizer. SY Chan has filed patent applications regarding the targeting of metabolism in pulmonary hypertension. The authors declare no other conflicts of interest.
References
- Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, Latiri M, Dubois-Rande JL, Lipskaia L, Combadiere C, et al. (2017) Roles for the cx3cl1/cx3cr1 and ccl2/ccr2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 56: 597–608. 10.1165/rcmb.2016-0201OC [DOI] [PubMed] [Google Scholar]
- Berasain C, Avila MA (2014) Amphiregulin. Semin Cell Dev Biol 28: 31–41. 10.1016/j.semcdb.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, et al. (2016) Vascular stiffness mechanoactivates yap/taz-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335. 10.1172/jci86387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavathula N, Nerusu KC, Fisher GJ, Liu G, Thakur AB, Gemmell L, Kumar S, Xu ZH, Hinton P, Tsurushita N, et al. (2005) Amphiregulin and epidermal hyperplasia: Amphiregulin is required to maintain the psoriatic phenotype of human skin grafts on severe combined immunodeficient mice. Am J Pathol 166: 1009–1016. 10.1016/s0002-9440(10)62322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoli MR, Stiehl DP, Borsig L, Kristiansen G, Hausladen S, Schraml P, Wenger RH, Camenisch G (2011) Prolyl-4-hydroxylase phd2- and hypoxia-inducible factor 2-dependent regulation of amphiregulin contributes to breast tumorigenesis. Oncogene 30: 548–560. 10.1038/onc.2010.433 [DOI] [PubMed] [Google Scholar]
- Chavakis E, Dimmeler S (2002) Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol 22: 887–893. 10.1161/01.atv.0000017728.55907.a9 [DOI] [PubMed] [Google Scholar]
- Chen L, Li J, Wang F, Dai C, Wu F, Liu X, Li T, Glauben R, Zhang Y, Nie G, et al. (2016) Tie2 expression on macrophages is required for blood vessel reconstruction and tumor relapse after chemotherapy. Cancer Res 76: 6828–6838. 10.1158/0008-5472.Can-16-1114 [DOI] [PubMed] [Google Scholar]
- Cheng J, Gao F, Chen X, Wu J, Xing C, Lv Z, Xu W, Xie Q, Wu L, Ye S, et al. (2014) Prohibitin-2 promotes hepatocellular carcinoma malignancy progression in hypoxia based on a label-free quantitative proteomics strategy. Mol Carcinog 53: 820–832. 10.1002/mc.22040 [DOI] [PubMed] [Google Scholar]
- Ciarloni L, Mallepell S, Brisken C (2007) Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci U S A 104: 5455–5460. 10.1073/pnas.0611647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley MK, Zhao J, Tai YY, Tang Y, Perk D, Negi V, Yu Q, Woodcock CSC, Handen A, Speyer G, et al. (2021) Frataxin deficiency promotes endothelial senescence in pulmonary hypertension. J Clin Invest 131: e136459. 10.1172/jci136459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JA, Collas P (2008) A rapid micro chromatin immunoprecipitation assay (chip). Nat Protoc 3: 1032–1045. 10.1038/nprot.2008.68 [DOI] [PubMed] [Google Scholar]
- Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, et al. (2014) In vivo endothelial sirna delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol 9: 648–655. 10.1038/nnano.2014.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8: 211–226. 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- de Vries VC, Noelle RJ (2010) Mast cell mediators in tolerance. Curr Opin Immunol 22: 643–648. 10.1016/j.coi.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon K, Knox AJ (2015) Human airway smooth muscle cells secrete amphiregulin via bradykinin/cox-2/pge2, inducing cox-2, cxcl8, and vegf expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 309: L237–L249. 10.1152/ajplung.00390.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Zhong Z, Wu D, Chen Y, Lian N, Ding H, Zhang Q, Lin Q, Wu S (2017) Role of foxo1 and apoptosis in pulmonary vascular remolding in a rat model of chronic thromboembolic pulmonary hypertension. Scientific Rep 7: 2270. 10.1038/s41598-017-02007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold I, Hennigs J, Miyagawa K, Li C, Nickel N, Kaschwich M, Cao A, Wang L, Reddy S, Chen P-I, et al. (2015) Bmpr2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608. 10.1016/j.cmet.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Liu T, Wu Z, Hu B, Nakashima T, Ullenbruch M, Gonzalez De Los Santos F, Phan SH (2016) Bone marrow cd11c+ cell-derived amphiregulin promotes pulmonary fibrosis. J Immunol 197: 303–312. 10.4049/jimmunol.1502479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA (2006) The epidermal growth factor receptors and their family of ligands: Their putative role in atherogenesis. Atherosclerosis 186: 38–53. 10.1016/j.atherosclerosis.2005.06.038 [DOI] [PubMed] [Google Scholar]
- Enomoto Y, Orihara K, Takamasu T, Matsuda A, Gon Y, Saito H, Ra C, Okayama Y (2009) Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol 124: 913-920.e1-7. 10.1016/j.jaci.2009.08.044 [DOI] [PubMed] [Google Scholar]
- Fadloun A, Kobi D, Pointud JC, Indra AK, Teletin M, Bole-Feysot C, Testoni B, Mantovani R, Metzger D, Mengus G, et al. (2007) The tfiid subunit taf4 regulates keratinocyte proliferation and has cell-autonomous and non-cell-autonomous tumour suppressor activity in mouse epidermis. Development 134: 2947–2958. 10.1242/dev.005041 [DOI] [PubMed] [Google Scholar]
- Florentin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, Zhang Y, Watson A, Sembrat J, Rojas M, et al. (2018) Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol 200: 3612–3625. 10.4049/jimmunol.1701287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin J, Zhao J, Tai YY, Vasamsetti SB, O’Neil SP, Kumar R, Arunkumar A, Watson A, Sembrat J, Bullock GC, et al. (2021) Interleukin-6 mediates neutrophil mobilization from bone marrow in pulmonary hypertension. Cell Mol Immunol 18: 374–384. 10.1038/s41423-020-00608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini G, De Laurentiis M, Vignati S, Chinè S, Lucchi M, Silvestri V, Mussi A, De Placido S, Tortora G, Bianco AR, et al. (1998) Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage i-iiia non-small-cell lung cancer: Amphiregulin and microvessel count are independent prognostic indicators of survival. Clin Cancer Res 4: 241–249. 10.1109/TWC.2007.05152 [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27: 165–197. 10.1146/annurev.immunol.021908.132620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, et al. (2012) A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991. 10.1152/ajplung.00362.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, et al. (2013) Transforming growth factor-beta signaling promotes pulmonary hypertension caused by schistosoma mansoni. Circulation 128: 1354–1364. 10.1161/circulationaha.113.003072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BB, Kumar R, Mickael C, Kassa B, Koyanagi D, Sanders L, Zhang L, Perez M, Hernandez-Saavedra D, Valencia C, et al. (2018) Vascular adaptation of the right ventricle in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 59: 479–489. 10.1165/rcmb.2018-0095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanumegowda C, Farkas L, Kolb M (2012) Angiogenesis in pulmonary fibrosis: Too much or not enough? Chest 142: 200–207. 10.1378/chest.11-1962 [DOI] [PubMed] [Google Scholar]
- Heialy SA, Risse P-A, Zeroual MA, Roman HN, Tsuchiya K, Siddiqui S, Laporte SA, Martin JG (2013) T cell–induced airway smooth muscle cell proliferation via the epidermal growth factor receptor. Am J Respir Cell Mol Biol 49: 563–570. 10.1165/rcmb.2012-0356OC [DOI] [PubMed] [Google Scholar]
- Heldin CH, Ostman A, Ronnstrand L (1998) Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378: F79–F113. 10.1016/s0304-419x(98)00015-8 [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316. 10.1152/physrev.1999.79.4.1283 [DOI] [PubMed] [Google Scholar]
- Hirose S, Hosoda Y, Furuya S, Otsuki T, Ikeda E (2000) Expression of vascular endothelial growth factor and its receptors correlates closely with formation of the plexiform lesion in human pulmonary hypertension. Pathol Int 50: 472–479. 10.1046/j.1440-1827.2000.01068.x [DOI] [PubMed] [Google Scholar]
- Ikeda KT, Hale PT, Pauciulo MW, Dasgupta N, Pastura PA, Le Cras TD, Pandey MK, Nichols WC (2019) Hypoxia-induced pulmonary hypertension in different mouse strains: Relation to transcriptome. Am J Respir Cell Mol Biol 60: 106–116. 10.1165/rcmb.2017-0435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al. (2010) Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115: e10–e19. 10.1182/blood-2009-07-235028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal AJ, Fisher EA, Greaves DR (2016) Inflammation-a critical appreciation of the role of myeloid cells. Microbiol Spectr 4: 325–342. 10.1128/microbiolspec.MCHD-0027-201610.1128/microbiolspec.MCHD-0027-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Zhao W, Tang Q, Wang B, Li X, Feng Z (2019) Over expression of amphiregulin promoted malignant progression in gastric cancer. Pathol Res Pract 215: 152576. 10.1016/j.prp.2019.152576 [DOI] [PubMed] [Google Scholar]
- Kato M, Inazu T, Kawai Y, Masamura K, Yoshida M, Tanaka N, Miyamoto K, Miyamori I (2003) Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, a7r5. Biochem Biophys Res Commun 301: 1109–1115. 10.1016/s0006-291x(03)00093-7 [DOI] [PubMed] [Google Scholar]
- Krämer A, Green J, Pollard J Jr., Tugendreich S (2013) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30: 523–530. 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, et al. (2017) Tgf-β activation by bone marrow-derived thrombospondin-1 causes schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun 8: 15494. 10.1038/ncomms15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca HL, Rosen JM (2007) Estrogen regulation of mammary gland development and breast cancer: Amphiregulin takes center stage. Breast Cancer Res 9: 304. 10.1186/bcr1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DJ, Modjtahedi H, Plant NJ, Ferns GA (2004) Egf mediates monocyte chemotaxis and macrophage proliferation and egf receptor is expressed in atherosclerotic plaques. Atherosclerosis 176: 21–26. 10.1016/j.atherosclerosis.2004.04.012 [DOI] [PubMed] [Google Scholar]
- Lamber EP, Horwitz AA, Parvin JD (2010) Brca1 represses amphiregulin gene expression. Cancer Res 70: 996–1005. 10.1158/0008-5472.Can-09-2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cras TD, Hardie WD, Fagan K, Whitsett JA, Korfhagen TR (2003) Disrupted pulmonary vascular development and pulmonary hypertension in transgenic mice overexpressing transforming growth factor-alpha. Am J Physiol Lung Cell Mol Physiol 285: L1046–L1054. 10.1152/ajplung.00045.2003 [DOI] [PubMed] [Google Scholar]
- Lee M, Dworkin AM, Gildea D, Trivedi NS, Moorhead GB, Crawford NPS (2014) Rrp1b is a metastasis modifier that regulates the expression of alternative mrna isoforms through interactions with srsf1. Oncogene 33: 1818–1827. 10.1038/onc.2013.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Shin KJ, Park S-A, Park KS, Park S, Heo K, Seo Y-K, Noh D-Y, Ryu SH, Suh P-G (2016) G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget 7: 70898–70911. 10.18632/oncotarget.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Jiang XM, Zhang J, Li B, Li J, Xie DJ, Hu ZY (2016. a) Pulmonary artery denervation improves pulmonary arterial hypertension induced right ventricular dysfunction by modulating the local renin-angiotensin-aldosterone system. BMC Cardiovasc Disord 16: 192. 10.1186/s12872-016-0366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, De Los Santos FG, Ding L, Wu Z, Phan SH (2016. b) Amphiregulin promotes fibroblast activation in pulmonary fibrosis. FASEB J 30: 50.56. [Google Scholar]
- Liu Y, Cao Y, Sun S, Zhu J, Gao S, Pang J, Zhu D, Sun Z (2016. c) Transforming growth factor-beta1 upregulation triggers pulmonary artery smooth muscle cell proliferation and apoptosis imbalance in rats with hypoxic pulmonary hypertension via the pten/akt pathways. Int J Biochem Cell Biol 77: 141–154. 10.1016/j.biocel.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Liu L, Jin X, Hu CF, Zhang YP, Zhou Z, Li R, Shen CX (2018) Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating egfr-dependent pathway. Basic Res Cardiol 113: 12. 10.1007/s00395-018-0669-y [DOI] [PubMed] [Google Scholar]
- Liu L, Song S, Zhang YP, Wang D, Zhou Z, Chen Y, Jin X, Hu CF, Shen CX (2020) Amphiregulin promotes cardiac fibrosis post myocardial infarction by inducing the endothelial-mesenchymal transition via the egfr pathway in endothelial cells. Exp Cell Res 390: 111950. 10.1016/j.yexcr.2020.111950 [DOI] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. (2006) Mast cells are essential intermediaries in regulatory t-cell tolerance. Nature 442: 997–1002. 10.1038/nature05010 [DOI] [PubMed] [Google Scholar]
- Makki N, Thiel KW, Miller FJ Jr. (2013) The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci 14: 20597–20613. 10.3390/ijms141020597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Popov N, Aguilo F, O’Loghlen A, Raguz S, Snijders AP, Dharmalingam G, Li S, Thymiakou E, Carroll T, et al. (2013) Interplay between homeobox proteins and polycomb repressive complexes in p16ink4a regulation. EMBO J 32: 982–995. 10.1038/emboj.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menacho-Márquez M, García-Escudero R, Ojeda V, Abad A, Delgado P, Costa C, Ruiz S, Alarcón B, Paramio JM, Bustelo XR (2013) The rho exchange factors vav2 and vav3 favor skin tumor initiation and promotion by engaging extracellular signaling loops. PLoS Biol 11: e1001615. 10.1371/journal.pbio.1001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C, Wang S, Wang X, Lv J, Zeng W, Chang R, Li Q, Wang X (2020) Amphiregulin inhibits tnf-α-induced alveolar epithelial cell death through egfr signaling pathway. Biomed Pharmacother 125: 109995. 10.1016/j.biopha.2020.109995 [DOI] [PubMed] [Google Scholar]
- Merklinger SL, Jones PL, Martinez EC, Rabinovitch M (2005) Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation 112: 423–431. 10.1161/circulationaha.105.540542 [DOI] [PubMed] [Google Scholar]
- Michelakis ED (2006) Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: Heterogeneous bmp signaling may have therapeutic implications. Circ Res 98: 172–175. 10.1161/01.RES.0000204572.65400.a5 [DOI] [PubMed] [Google Scholar]
- Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF (2005) Autoimmunity and pulmonary hypertension: A perspective. Eur Respir J 26: 1110–1118. 10.1183/09031936.05.00045705 [DOI] [PubMed] [Google Scholar]
- Opitz I, Kirschner MB (2019) Molecular research in chronic thromboembolic pulmonary hypertension. Int J Mol Sci 20: 784. 10.3390/ijms20030784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Zhao Y, Yoon S, Xu J, Liao L, Lydon J, DeMayo F, O’Malley BW, Katzenellenbogen BS (2011) Repressor of estrogen receptor activity (rea) is essential for mammary gland morphogenesis and functional activities: Studies in conditional knockout mice. Endocrinology 152: 4336–4349. 10.1210/en.2011-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S, De Val S, Neal A (2018) Endothelial-specific cre mouse models. Arterioscler Thromb Vasc Biol 38: 2550–2561. 10.1161/ATVBAHA.118.309669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Tian X, Qian Y, Skibba M, Zou C, Liu Z, Wang J, Xu Z, Li X, Liang G (2016) Novel egfr inhibitors attenuate cardiac hypertrophy induced by angiotensin ii. J Cell Mol Med 20: 482–494. 10.1111/jcmm.12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M, Lo C, Plowman G (1994) Amphiregulin-dependent proliferation of cultured human keratinocytes: Autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J Cell Physiol 159: 114–120. 10.1002/jcp.1041590115 [DOI] [PubMed] [Google Scholar]
- Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815. 10.1038/nri2171 [DOI] [PubMed] [Google Scholar]
- Pool CM, Jin Y, Trittmann JK, Chen B, Liu Y, Nelin LD (2019) Hypoxic pulmonary endothelial cells release epidermal growth factor which results in vascular smooth muscle cell arginase 2 expression and proliferation. FASEB J 33: 845.812. 10.1096/fasebj.2019.33.1_supplement.845.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S, Humbert M (2012) Inflammation in pulmonary arterial hypertension. Chest 141: 210–221. 10.1378/chest.11-0793 [DOI] [PubMed] [Google Scholar]
- Pugliese SC, Kumar S, Janssen WJ, Graham BB, Frid MG, Riddle SR, El Kasmi KC, Stenmark KR (2017) A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension. J Immunol 198: 4802–4812. 10.4049/jimmunol.1601692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR (2015) The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252. 10.1152/ajplung.00238.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Kashireddy P, Zhu YT, Rao SM, Zhu YJ (2004) Null mutation of peroxisome proliferator-activated receptor-interacting protein in mammary glands causes defective mammopoiesis. J Biol Chem 279: 33696–33701. 10.1074/jbc.M401266200 [DOI] [PubMed] [Google Scholar]
- Quarck R, Wynants M, Verbeken E, Meyns B, Delcroix M (2015) Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur Respir J 46: 431–443. 10.1183/09031936.00009914 [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Guignabert C, Humbert M, Nicolls MR (2014) Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175. 10.1161/circresaha.113.301141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, Yuan JX, Perez VdJ (2018) Endothelial dysfunction in pulmonary arterial hypertension: An evolving landscape (2017 grover conference series). Pulm Circ 8: 2045893217752912. 10.1177/2045893217752912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayego-Mateos S, Rodrigues-Diez R, Morgado-Pascual JL, Valentijn F, Valdivielso JM, Goldschmeding R, Ruiz-Ortega M (2018) Role of epidermal growth factor receptor (egfr) and its ligands in kidney inflammation and damage. Mediators Inflamm 2018: 8739473. 10.1155/2018/8739473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Im H, Cao A, Hennigs JK, Wang L, Sa S, Chen PI, Nickel NP, Miyagawa K, Hopper RK, et al. (2015) Rna sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 356–366. 10.1164/rccm.201408-1528OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz S, Kazlauskas A (1999) Evidence for distinct signaling properties and biological responses induced by the pdgf receptor alpha and beta subtypes. Growth Factors 16: 201–216. 10.3109/08977199909002130 [DOI] [PubMed] [Google Scholar]
- Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, et al. (2016) Rnai targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med 8: 342ra80. 10.1126/scitranslmed.aaf1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF (2005) Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180. 10.1096/fj.04-3261fje [DOI] [PubMed] [Google Scholar]
- Schneider MR, Wolf E (2009) The epidermal growth factor receptor ligands at a glance. J Cell Physiol 218: 460–466. 10.1002/jcp.21635 [DOI] [PubMed] [Google Scholar]
- Singh B, Carpenter G, Coffey RJ (2016) Egf receptor ligands: Recent advances. F1000Res 5: F1000. 10.12688/f1000research.9025.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB (2009) Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244. 10.1161/circresaha.108.182014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF (2009) Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032. 10.1152/ajplung.00217.2009 [DOI] [PubMed] [Google Scholar]
- Stolarczyk M, Scholte BJ (2018) The egfr-adam17 axis in chronic obstructive pulmonary disease and cystic fibrosis lung pathology. Mediators Inflamm 2018: 1067134. 10.1155/2018/1067134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko NA (2015) Cell-type specific functions of epidermal growth factor receptor are involved in development of hepatocellular carcinoma. Hepatology 62: 314–316. 10.1002/hep.27863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD (2010) Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606. 10.1152/ajplung.00122.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-A, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D (2019) The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol 14: 50–59. 10.15420/ecr.2018.33.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF (2001) The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med 22: 405–418. 10.1016/s0272-5231(05)70280-x [DOI] [PubMed] [Google Scholar]
- Tuder RM, Groves B, Badesch DB, Voelkel NF (1994) Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285. [PMC free article] [PubMed] [Google Scholar]
- Tuder RM (2017) Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649. 10.1007/s00441-016-2539-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt M, Ruffenach G, Meloche J, Bonnet S (2015) Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol 31: 407–415. 10.1016/j.cjca.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Vasamsetti SB, Coppin E, Zhang X, Florentin J, Koul S, Götberg M, Clugston AS, Thoma F, Sembrat J, Bullock GC, et al. (2020) Apoptosis of hematopoietic progenitor-derived adipose tissue-resident macrophages contributes to insulin resistance after myocardial infarction. Sci Transl Med 12: eaaw0638. 10.1126/scitranslmed.aaw0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasamsetti SB, Florentin J, Coppin E, Stiekema LCA, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ESG, et al. (2018) Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity 49: 93–106.e7. 10.1016/j.immuni.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, et al. (2011) Residual embryonic cells as precursors of a barrett’s-like metaplasia. Cell 145: 1023–1035. 10.1016/j.cell.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, et al. (2014) Endothelial apoptosis in pulmonary hypertension is controlled by a microrna/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194. 10.1161/HYPERTENSIONAHA.113.03037 [DOI] [PubMed] [Google Scholar]
- Wieduwilt MJ, Moasser MM (2008) The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 65: 1566–1584. 10.1007/s00018-008-7440-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhao K-q, Wang W, Cui L-n, Hu L-l, Jiang X-x, Shuai J, Sun Y-p (2020. a) Nuclear receptor coactivator 6 promotes htr-8/svneo cell invasion and migration by activating nf-κb-mediated mmp9 transcription. Cell Prolif 53: e12876. 10.1111/cpr.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Chen J, Hu X, Zhu Y, Xie S, Wu C, Pei Z, Xiong S, Peng Y (2020. b) Amphiregulin alleviated concanavalin a-induced acute liver injury via il-22. Immunopharmacol Immunotoxicol 42: 473–483. 10.1080/08923973.2020.1810271 [DOI] [PubMed] [Google Scholar]
- Xu L, Luo H, Wang R, Wu WW, Phue J-N, Shen R-F, Juhl H, Wu L, Alterovitz W-l, Simonyan V, et al. (2019) Novel reference genes in colorectal cancer identify a distinct subset of high stage tumors and their associated histologically normal colonic tissues. BMC Med Genet 20: 138. 10.1186/s12881-019-0867-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S, Ishida S, Hanamoto Y, Kumagai K-I, Masuda R, Tanaka K, Shiobara N, Yamane N, Mori T, Juji T, et al. (2008) Proinflammatory role of amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. J Inflamm (Lond) 5: 5. 10.1186/1476-9255-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Gröne A, Sibilia M, van Bergen en Henegouwen PMP, Roovers RC, Coffer PJ, Sijts AJ (2013) Amphiregulin enhances regulatory t cell-suppressive function via the epidermal growth factor receptor. Immunity 38: 275–284. 10.1016/j.immuni.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For data sharing and information, please contact Dr. Partha Dutta, the corresponding author at duttapa@pitt.edu.