Abstract
Patient: Female, 61-year-old
Final Diagnosis: Acute disseminated encephalomyelitis (ADEM)
Symptoms: Altered mental status • lethargy
Medication: —
Clinical Procedure: —
Specialty: General and Internal Medicine • Neurology
Objective:
Unknown etiology
Background:
Acute disseminated encephalomyelitis (ADEM) is a disorder of the central nervous system which has been associated with preceding infection as well as vaccinations. We present a case of a 61-year-old woman with ADEM after receiving her initial vaccination for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This case highlights management of this acute condition.
Case Report:
A 61-year-old woman with history of hypertension and anxiety presented with progressive generalized weakness and difficulty with communication which began a few weeks ago, shortly after receiving the Pfizer vaccine for the novel coronavirus (COVID-19). On arrival, she was found to be encephalopathic and tachypneic, ultimately requiring emergent intubation. During her hospital course, an MRI of her brain was obtained which showed nonspecific acute versus subacute leukoencephalopathy involving the brainstem and deep white matter. Her cerebrospinal fluid showed elevated protein but was otherwise unremarkable. Further testing to rule out tick-borne illnesses, viral etiology, and multiple sclerosis were negative. Electroencephalography showed nonspecific diffuse cerebral dysfunction but no seizures or epileptiform discharges. She was treated with 5 doses of methylprednisolone 1 g and intravenous immunoglobulin (IVIG) 2 g/kg over 5 days. She had marked improvement in her neurologic status after treatment.
Conclusions:
In conclusion, ADEM should be acknowledged as a rare but potential complication related to COVID-19 vaccination. A proper history and physical exam in addition to a thorough work-up are necessary for prompt recognition of this condition. Initial treatment should consist of steroids followed by IVIG versus plasmapheresis for those not responsive to steroids.
Keywords: COVID-19; Encephalomyelitis, Acute Disseminated; Vaccination
Background
While acute disseminated encephalomyelitis can occur at any age, it is more common in pediatric patients ages 5–8 years and more rarely in adults ages 30–50 years, equally in men and women; the diagnosis is made usually after a viral illness or vaccination administration [1,2]. Many pathogens have been linked to ADEM, including viral and bacterial etiologies including Coronavirus, Coxsackievirus, Cytomegalovirus, Epstein-Barr, Herpes Simplex, and Measles [1]. Rarely, ADEM cases have been documented following organ transplant [1].
Recently, there have been cases of ADEM reported that are thought to be related to both the novel coronavirus and the coronavirus vaccines. A case of post-vaccination ADEM has been reported in a 56-year-old woman after receiving the Pfizer-BioMTech COVID-19 vaccine and in a 46-year-old woman after Sinovac vaccination [3,4]. There are also published cases of ADEM related to SARS-CoV-2 infection itself in both adults and children; neurological manifestations of the viral infection have shown ADEM as well as other encephalopathies, stroke, and the peripheral nervous system, including brachial plexopathy, nerve palsies, and myopathies [5–8].
The diagnosis of ADEM is based on clinical and radiologic features that may be difficult to distinguish from multiple sclerosis. Reported recurrent or multiphasic forms, besides just the monophasic course of illness, further makes diagnosis challenging [1]. Symptoms of encephalopathy with neurologic deficits usually begin within 2–4 weeks after the initiating event [1]. Both children and adults have changes in mental status, ataxia, and motor deficiencies. Additionally, adults demonstrate more sensory deficiencies [1]. There is great variability in the severity of ADEM and its presentation. It may present without specific symptoms such as irritability and somnolence, or rapid development of symptoms leading to coma and decerebrate rigidity [1].
Differential diagnosis includes multiple sclerosis, viral encephalopathies, progressive multifocal leukoencephalopathy, posterior reversible encephalopathy syndrome, cerebral autosomal dominant arteriopathy, toxic encephalopathies, adult-onset leukodystrophies, brain abscesses, tuberculomas, neurocysticercosis, histoplasmosis, toxoplasmosis, [1,2], human polyomavirus, tick-borne etiologies, immune-mediated vasculitis, and Alzheimer disease.
The disease is characterized by inflammation and demyelination of the central nervous system including the brain and spinal cord, particularly at the perivenous location of pathologic change [1,9]. Histologically, acute and early ADEM inflammatory lesions spread radially outward from blood vessels and macrophages and are concentrated around vessel, while in multiple sclerosis inflammatory lesions are discontinuous and macrophages are bordering plaques [10]. Another difference between ADEM and multiple sclerosis is that in ADEM, plaque boarders are poorly delineated while multiple sclerosis demonstrates sharply edged plaques seen on MRI [10]. Long-term effects on myelin are different as well; in ADEM, if a single attack of the disease occurs there is nonspecific gliosis with no myelin loss, but in multiple sclerosis lesions remain forever [10]. While ADEM is generally thought to be a demyelination with preservation of axons, axonal damage has occurred in some instances [1].
Established pediatric criteria concluded that ADEM is the first clinical event resulting in symptomatic encephalopathy with acute or subacute onset. Recurrent ADEM is a new demyelinating event occurring at least 3 months after initial ADEM event involving the same regions of CNS seen on MRI. Multiphasic ADEM occurs 3 months after the initial event but when there is recurrence involving new CNS areas visualized on MRI [1,11]. A molecular mimicry hypothesis has been proposed that suggests there is an autoimmune reaction from post-infectious myelin protein auto-antibodies [2,10,12,13]. Alternatively, in the setting of a foreign antibody, there is increased vascular congestion and increased central nervous system vasculature permeability, which leads to the recommendation of anti-inflammatory modalities being a mainstay of treatment [1,14].
Besides high-dose steroids, further treatment modalities include plasma exchange and i.v. immunoglobulin [1]. Fifteen percent of adult patients will have a recurrence of the disease, 25% develop multiple sclerosis within 5 years of initial ADEM presentation, and most individuals do not have further disease progression past 3 months [2,15].
Here, we report a rare condition of ADEM after SARS-CoV-2 vaccine with severe encephalopathy. This case is of interest in comparison to other reported cases as our patient’s symptom onset was delayed from the time of her vaccination. However, after ruling out multiple other etiologies as a cause for her encephalopathy, post-vaccination ADEM was suspected to be the ultimate cause of her altered mentation.
Case Report
A 61-year-old woman with past medical history significant for hypertension and anxiety presented to the emergency department reporting generalized weakness and altered mental status progressively worsening over the past 6–8 weeks. Two weeks prior to the emergency room visit, the patient’s husband had noted a change in the patient’s speech as she was having difficulty communicating and believed she was altered from her baseline. The patient was active and an avid runner but was unable to participate in her normal activities given her weakness. Of note, the patient had received the first dose of the Pfizer-BioNTech SARS-CoV-2 vaccination 8–10 weeks ago, around the time her symptoms started.
On presentation, the patient was noted to be hypertensive with blood pressure of 183/113 and tachypneic with a respiratory rate in the 30s. Arterial blood gas showed a pH of 7.65, pCO2 of <10, and pO2 of 124 while on nasal cannula. Given her encephalopathy and tachypnea, the decision was made to emergently intubate the patient. Her laboratory results on admission showed a white blood cell count of 10.1 K/uL and hemoglobin of 12.6 g/dL. Her comprehensive metabolic panel was significant for potassium of 3.2 mmol/L, bicarbonate of 11 mmol/L, chloride of 120 mmol/L. Additional tests, including procalcitonin, cortisol, glucose level, thyroid function tests, antinuclear antibody screen, and COVID RNA nasopharyngeal swab, were within normal limits. Her urinalysis was unremarkable, but her urine toxicology was positive for tetrahydrocannabinol. Blood cultures and methicillin-resistant Staphylococcus aureus screen were negative but respiratory culture did grow Klebsiella oxytoca. A lumbar puncture was performed; initial cerebrospinal fluid analysis was significant for elevated protein of 61 mg/dL, but the cell count, differential, glucose, and cultures were unremarkable. A meningitis/encephalitis panel which looked for organisms not limited to Haemophilus influenza, Listeria monocytogenes, Neisseria meningitis, Escherichia coli, Varicella-Zoster virus, Herpes simplex virus 1 and 2 came back negative (Table 1).
Table 1.
CSF meningitis/encephalitis PCR panel.
| Organism | Result |
|---|---|
| E. coli K 1 | Not detected |
| Haemophilus influenza | Not detected |
| Listeria monocytogenes | Not detected |
| Neisseria meningitis | Not detected |
| Varicella-Zoster Virus | Not detected |
| Enterovirus | Not detected |
| Herpes simplex virus 1 | Not detected |
| Herpes simplex virus 2 | Not detected |
| Human herpesvirus 6 | Not detected |
| Human parechovirus | Not detected |
| Streptococcus agalactiae | Not detected |
| Streptococcus pneumoniae | Not detected |
| Cryptococcus neoformans/gattii | Not detected |
| Cytomegalovirus | Not detected |
Imaging studies, including computed tomography of the abdomen and pelvis, as well as computed tomography angiography of the chest, did not show any acute pathology. MRI without contrast of the brain and cervical spine was significant for diffuse, near symmetric acute leukoencephalopathy process involving the deep white matter extending downward through the brainstem into the cerebellar white matter tracts (Figures 1–3). Electroencephalogram showed nonspecific diffuse cerebral dysfunction which is nonspecific without evidence of seizures or epileptiform discharges. At this time, further work-up was pursued to determine the cause of the patient’s encephalopathy and MRI findings. A Tick-Borne Disease antibody panel was negative for Ehrlichia chaffeensis, Anaplasma phagocytophilum, Babesia microti, and Lyme disease; serum studies were also negative for human polyomavirus 2, myeloperoxidase antibodies, serine protease 3 antibodies, contactin-associated protein-2 IgG antibody, leucine-rich glioma inactivated 1 protein IgG antibody, and myelin oligodendrocyte glycoprotein antibody. Additional CSF analysis was unremarkable for human polyomavirus 2, and 14-3-3 Protein Tau. A multiple sclerosis panel was obtained which was negative for oligoclonal bands and showed 4.0 mg/dL of immunoglobulin G in the CSF.
Figure 1.
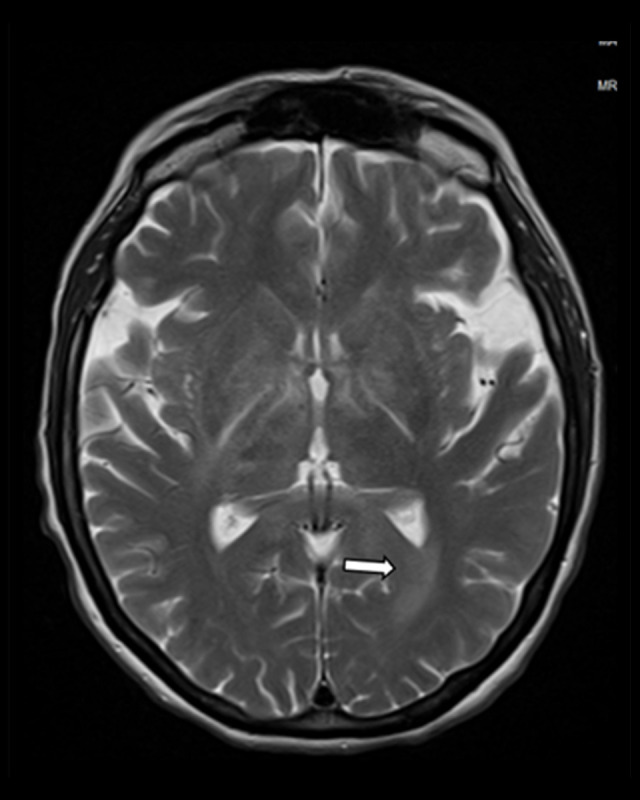
MRI brain without contrast showing diffuse near symmetric acute leukoencephalopathy process involving the deep white matter.
Figure 2.

MRI brain with contrast showing diffuse near symmetric acute leukoencephalopathy process involving the deep white matter.
Figure 3.

MRI brain without contrast showing diffuse near symmetric acute leukoencephalopathy process involving the deep white matter at the level of the cerebellum.
Given the patient’s MRI findings, the diagnosis was made of acute disseminated encephalomyelitis. As her work-up was overall unremarkable and her symptoms began shortly after her COVID-19 vaccination, her ADEM was thought to be related to the vaccine. She was treated with 5 doses of methylprednisolone 1 g in addition to intravenous immunoglobulin (IVIG) 2 g/kg over 5 days. There was significant improvement in the patient’s mentation with treatment and she was subsequently extubated. She was alert, oriented, and able to follow commands but remained generally weak. She required tracheostomy and percutaneous endoscopic gastrostomy tube placement. Repeat MRI brain performed 1 month later did not show any further progression of her disease process (Figures 4, 5).
Figure 4.

MRI brain 1 month after patient’s initial presentation, with stable findings.
Figure 5.
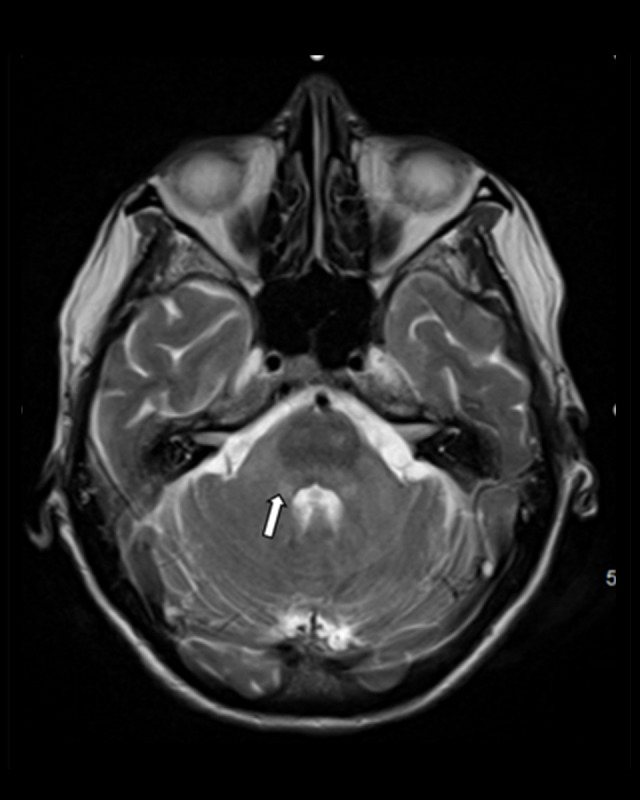
MRI brain 1 month after patient’s initial presentation, with stable findings at the level of the cerebellum.
Discussion
Acute disseminated encephalomyelitis remains a rare illness which predominantly affects children but occasionally affects the adult population. Most of the cases are associated with preceding vaccinations or immunizations. The incidence for post-vaccination ADEM remains low at approximately 0.1 to 0.2 per 100 000 vaccinated individuals [16]. The mRNA vaccine for SARS-CoV-2 is relatively new but has been shown to have an association with adverse events, but these events remain rare. With respect to neurologic complications, there were 17 cases of strokes, 32 cases of GBS, 190 cases of facial palsy, 9 cases of transverse myelitis, and 6 cases of acute disseminated encephalomyelitis reported in the VAERS database as of March 2021 [17].
There are only a few published case reports discussing ADEM potentially caused by COVID-19 vaccination, and more cases reported after SARS-CoV-2 infection in both adults and children [5–8]. A recent case report discussed the case of a 56-year-old woman presenting with left-sided weakness 2 weeks after receiving the Pfizer COVID-19 vaccination [4]. Brain MRI showed an area of hyperintensity on fluid attenuated inversion recovery (FLAIR) sequences involving the left cerebellar peduncle, with a modest mass effect on the fourth ventricle. The patient underwent extensive work-up including CSF, microbiologic, and serologic studies, which were all unremarkable. A cytokine panel and screening panel for demyelinating disorders were negative. The patient was treated with steroids with gradual improvement in her symptoms. Another case of an ADEM-like presentation was reported involving a 46-year-old woman with a past medical history of Hashimoto’s thyroiditis [3]. She presented to the hospital after experiencing a seizure and had received the Sinovac COVID-19 vaccination 1 month prior. Her MRI showed scattered hyperintense lesions in the left thalamus, bilateral corona radiata, left diencephalon, and right parietal cortex on T2 and FLAIR sequences. Laboratory tests including thyroid panel, nasal swab for COVID 19, complement levels, anti-double-stranded DNA, extractable nuclear antigen (ENA) panel, anti-aquaporin-4 and anti-myelin oligodendrocyte (MOG) antibodies were all negative. CSF studies were negative as well. Her labs did come back showing an ANA of 1/100 and positive Anti-SOX1 antibody. She was treated with a 1 g/day steroid regimen for 7 days.
As discussed earlier, the exact pathogenesis behind ADEM remains unknown. There have been several proposed mechanisms, including molecular mimicry, post-infectious etiology, autoimmune, as well as potential genetic predisposition to developing ADEM [16,18]. We presume that our patient had ADEM related to the COVID-19 vaccination as we ruled out other causes of her encephalopathy including sepsis, autoimmune causes, meningitis, viral etiologies, and her work-up was also negative for multiple sclerosis. Her CSF analysis showed elevated protein but there was no evidence of pleocytosis; although pleocytosis can be seen in ADEM, CSF can also be normal in some cases [19]. The onset of her symptoms was also shortly after receiving her vaccination, although the progression was slow. ADEM typically develops 1–2 weeks after vaccination or infection; however, there have been cases of ADEM developing after several months. Diagnostic criteria have mentioned ADEM can develop over 3 months with any acute or fluctuating symptoms as part of the same event [11]. There was a case involving a 61-year-old man who developed bilateral optic neuropathies 3 weeks after receiving an inactivated influenza vaccine [16]. Approximately 3 months later, this patient returned for evaluation as he was lethargic and confused; an MRI of his brain showed a symmetric signal abnormality involving the central gray matter [16]. His CSF did show 24×106 white blood cells/μL but the remainder of his work-up was negative including testing for HSV, TB, cryptococcal antigen, antinuclear antibodies, antineutrophil cytoplasmic antibodies, HIV, EBV, and CMV [16]. He was diagnosed with ADEM and treated with steroids with improvement in his mentation [16]. The mainstay of treatment focuses on immunosuppression. Patients are initially treated with steroids; plasmapheresis and intravenous immunoglobulin are reserved for those who do not respond to steroid therapy [16]. Patients with ADEM usually have a good prognosis, with most having full recovery or minor residual deficits.
Although ADEM should be recognized as a possible complication associated with vaccine administration, the overall benefit of vaccines from a public health standpoint should be considered. Currently there are a few cases of reported ADEM thought to be related to COVID-19 vaccination; however, it is difficult to make a direct association between the 2 as ADEM can occur spontaneously at times. Similarly, there were concerns of patients developing Guillain-Barre syndrome after COVID-19 vaccination; however, studies have not demonstrated an association between the vaccine and GBS [20]. Overall, vaccine administration should not be halted, as the benefits to the public outweigh the risks.
Conclusions
In conclusion, acute disseminated encephalomyelitis should be recognized as a rare but potential complication of the SARS-CoV-2 vaccination. However, post-vaccination encephalomyelitis accounts for less than 5% of present cases [16]. Overall, those eligible should receive the vaccination as the benefits outweigh the risks.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Tenembaum S, Chitnis T, Ness J, Hahn JS, International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 2.Almaghrabi N, Saab A. Adult-onset acute disseminated encephalomyelitis: A case report. Radiol Case Rep. 2021;16:2469–73. doi: 10.1016/j.radcr.2021.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozgen KG, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089–91. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogrig A, Janes F, Gigli GL, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siracusa L, Cascio A, Giordano S, et al. Neurological complications in pediatric patients with SARS-CoV-2 infection: A systematic review of the literature. Ital J Pediatr. 2021;47:123. doi: 10.1186/s13052-021-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novi G, Rossi T, Pedemonte E. Acute disseminated encephalomyelitis after SARS-COV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e949. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons T, Banks S, Bae C, et al. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267:2799–802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143:3104–20. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: An update. Arch Neurol. 2005;62:1673–80. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 10.Wender M. Acute disseminated encephalomyelitis (ADEM) J Neuroimmunol. 2011;231:92–99. doi: 10.1016/j.jneuroim.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, Banwell B, Tenembaum S, International Pediatric MS Study Group Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;2007;68:S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 12.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tselis A, Lisak R. Acute disseminated encephalomyelitis. In: Antel J, Birnbaum G, Hartung HP, Vincent A, editors. Clinical neuroimmunology. Oxford: 2005. pp. 147–71. [Google Scholar]
- 14.Steiner I, Kennedy PG. Acute disseminated encephalomyelitis: current knowledge and open questions. J Neurovirol. 2015;21(5):473–79. doi: 10.1007/s13365-015-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahmlow MR, Kantarci O. Fulminant demyelinating diseases. Neurohospitalist. 2013;3(2):81–91. doi: 10.1177/1941874412466873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh W, Cordato DJ, Kehdi E, et al. Post-vaccination encephalomyelitis: Literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315–22. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss AL, Samudralwar RD, Das RR, Nath A. ANA Investigates: Neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–57. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016;87:S38–45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ. Acute disseminated encephalomyelitis in children: Differential diagnosis from multiple sclerosis on the basis of clinical course. Korean J Pediatr. 2011;54(6):234–40. doi: 10.3345/kjp.2011.54.6.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koike H, Chiba A, Katsuno M. Emerging infection, vaccination, and Guillain-Barré syndrome: A review. Neurol Ther. 2021;10(2):523–37. doi: 10.1007/s40120-021-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


