Abstract
Opioid use disorder (OUD) and deaths from drug overdoses have reached unprecedented levels. Given the enormous impact of the opioid crisis on public health, a more thorough, in-depth understanding of the consequences of opioids on the brain is required to develop novel interventions and pharmacological therapeutics. In the brain, the effects of opioids are far reaching, from genes to cells, synapses, circuits, and ultimately behavior. Accumulating evidence implicates a primary role for the extracellular matrix (ECM) in opioid-induced plasticity of synapses and circuits, and the development of dependence and addiction to opioids. As a network of proteins and polysaccharides, including cell adhesion molecules, proteases, and perineuronal nets, the ECM is intimately involved in both the formation and structural support of synapses. In the human brain, recent findings support an association between altered ECM signaling and OUD, particularly within the cortical and striatal circuits involved in cognition, reward, and craving. Furthermore, the ECM signaling proteins, including matrix metalloproteinases and proteoglycans, are directly involved in opioid seeking, craving, and relapse behaviors in rodent opioid models. Both the impact of opioids on the ECM and the role of ECM signaling proteins in opioid use disorder, may, in part, depend on biological sex. Here, we highlight the current evidence supporting sex-specific roles for ECM signaling proteins in the brain and their associations with OUD. We emphasize knowledge gaps and future directions to further investigate the potential of the ECM as a therapeutic target for the treatment of OUD.
Keywords: opioids, extracellular matrix, addiction, neuroinflammation, opioid use disorder (OUD), sex differences
Introduction
In the United States, rates of opioid use disorder (OUD) and deaths from overdose have continued to climb over recent years, particularly in adolescents and young adults. Accompanying a rise in deaths from drug overdose has been a steady increase in the number of people diagnosed with OUD. Current estimates reflect more than 3 million people have OUD, with an estimated 200,000 new diagnoses annually. Despite the enormous public health impact of OUD, we lack a basic understanding of the neurobiological mechanisms that contribute to OUD and the associated health consequences. OUD is a chronic, relapsing brain disease that can be managed by long-term medical interventions and maintenance therapies such as methadone or buprenorphine. Yet, ∼90–95% of people with OUD relapse despite treatment, as cravings and other challenges such as protracted withdrawal, persist for weeks, months, and years (Smyth et al., 2010; Kadam et al., 2017; Montiel Ishino et al., 2020). Discovering new interventions and therapeutics for the treatment of OUD will require massive, parallel efforts, across multiple clinical and basic research domains. A critical effort will be necessary to further define the diverse array of consequences of chronic opioid use on the brain and body, along with an in-depth investigation into the cellular and molecular mechanisms in the brain involved in opioid reward, craving, and relapse.
Opioids lead to long-lasting changes in gene transcription, protein signaling, receptor activity, synaptic morphology and plasticity, as well as neural circuit function that contribute to the development of addiction (Hearing, 2019; Li et al., 2019; Madayag et al., 2019; Song et al., 2019; Valentinova et al., 2019; Koob, 2020; Jiang et al., 2021; Seney et al., 2021; Tavares et al., 2021; Trieu et al., 2022; Xue et al., 2022). A major class of signaling proteins involved in opioid-induced neural plasticity, include cell adhesion molecules (CAMs), matrix metalloproteinases (MMPs), and proteoglycans, and these proteins provide structural support to neurons, astrocytes, microglia in the formation of the extracellular matrix (ECM) and perineuronal nets (PNNs). ECM signaling proteins are involved in neurotransmission, synaptic plasticity, and vascular integrity in the brain. Over the recent decade, the ECM has become a focus as a major contributor to long-lasting neuroadaptations accompanying various processes, including learning, stress, and opioid use disorder.
Role for Extracellular Matrix Signaling Proteins in Opioid Use Disorder
In the brain, the ECM is critical in the regulation of synaptic function, blood-brain barrier integrity, and cell-to-cell communication. The scaffolding of the ECM comprises polysaccharides and glycoproteins that provide the necessary structure to support communication between neurons, astrocytes, and microglia, and helps facilitate both the formation of new synapses and tuning of synaptic functions (Dityatev et al., 2010; Ferrer-Ferrer and Dityatev, 2018). In particular, the ECM signaling proteins, MMPs, are implicated in opioid reward and addiction (Ishiguro et al., 2006). MMPs are multifunctional proteases involved in a variety of cellular pathways and processes including inflammation, cell migration, and angiogenesis (Visse and Nagase, 2003).
Opioids likely augment the activity of MMPs in the brain, substantially remodeling the ECM, potentially leading to opioid-induced changes in astrocyte-neuronal communication, synaptic plasticity, and trafficking of excitatory receptors (Figure 1; Michaluk et al., 2009, 2011; Huntley, 2012). For example, opioids lead to increased expression of both MMP-2 and MMP-9 in cell lines (Gach et al., 2011), and notably, in the rodent (Chioma et al., 2021), and human (Kovatsi et al., 2013; Seney et al., 2021) brain. Both MMP-2 and MMP-9-dependent signaling may be important for opioid-induced degradation in the integrity of the blood-brain barrier and an increase in neuroinflammation associated with OUD in the human brain (Huntley, 2012; Dal-Pizzol et al., 2013; Song et al., 2015; Rempe et al., 2016; Hannocks et al., 2019; Seney et al., 2021; Akol et al., 2022). Indeed, OUD is associated with alterations in ECM signaling and dopaminergic, GABAergic, and opioidergic neurotransmission, along with increased neuroinflammation in the human dorsolateral prefrontal cortex and nucleus accumbens (Seney et al., 2021), major neural substrates for cognition, impulsivity, and reward. Consistent with this, intravenous self-administration of heroin leads to elevated activity of MMP-2 and MMP-9 in the nucleus accumbens of both male and female rats (Chioma et al., 2021). Notably, MMP activity returns to below baseline levels following extinction of heroin self-administration behavior (Chioma et al., 2021). Opioid-induced increases in MMP activity are preferential to dendritic spines of dopamine receptor 1-expressing (D1+) medium spiny neurons (Chioma et al., 2021). In D1+ medium spiny neurons, MMP-9 activity seems to be acutely upregulated by heroin, returning to control levels after the removal of the drug and/or drug-cue (Chioma et al., 2021). As one of the major cell types in the nucleus accumbens that regulates drug reward-related behaviors, D1+ medium spiny neurons and associated MMP activity may serve as a key mechanism in the response to both opioid-induced and context-dependent neural plasticity (Smith et al., 2014). In mice, opioid administration also increases MMP-9 activity to modulate dopaminergic neurotransmission from the ventral tegmental area to nucleus accumbens (Nakamoto et al., 2012).
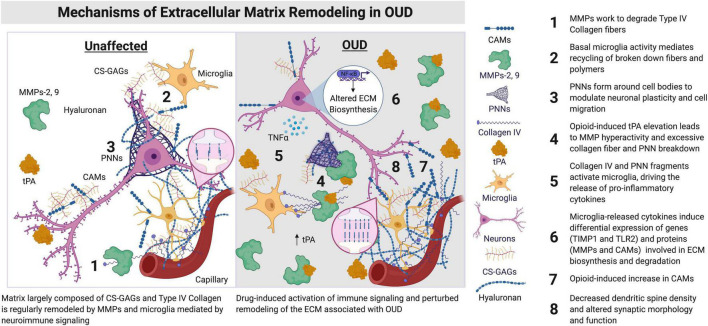
FIGURE 1.
Synaptic morphology and function are regulated by ECM signaling proteins and microglia. The components of the ECM lie proximal to brain capillaries and vessels, condensed as PNNs around cell bodies, including neurons, astrocytes, and microglia, along with synapses and dendrites of neurons. ECM components are also distributed amongst cells of the brain within the parenchyma. Hyaluronan is primarily located in the neural interstitial matrix of the parenchyma. Hyaluronan is involved in the regulation of inflammation and myelination in the brain, including remyelination after insult or injury. Opioids lead to an increase in neuroimmune activation by microglia and other immune cell types in the brain. An induction of immune activation in the brain can lead to increased expression and activity of tPAs, MMPs, CAMs, and Collagen IV (Webersinke et al., 1992; Roberts et al., 2018). Augmented activity of these ECM signaling proteins remodels the ECM, with consequences on dendritic spine morphology, including the reduction of spine number in key regions associated with OUD (e.g., prefrontal cortex and nucleus accumbens). CAMs, cell adhesion molecules; CS-GAGs, chondroitin sulfate glycosaminoglycans; ECM, extracellular matrix; MMPs, matrix metalloproteinases; NF-κB, nuclear factor kappa B; OUD, opioid use disorder; PNNs, perineuronal nets; TIMP, tissue inhibitor of metalloprotease; TIMP1, TIMP metallopeptidase inhibitor 1; TLR2, toll-like receptor 2; tPA, type plasminogen activator. Figure created using BioRender.
Changes in MMP-2 and MMP-9 have been found in the blood from people being treated for morphine dependency (Najafi et al., 2018). While MMP-2 activity is increased in the serum of morphine-dependent patients, MMP-9 activity is decreased (Najafi et al., 2018). Other studies report elevated MMP-9 in blood from patients with OUD during opioid withdrawal (Salarian et al., 2018). Interestingly, both studies suggest MMP-9 reflects a possible treatment response, as the expression and activity of the MMP-9 are reduced by methadone therapy (Salarian et al., 2018) and other treatments (Najafi et al., 2018). Changes in MMP expression in the blood of patients being treated for opioid dependency and addiction may reflect functional alterations in the central nervous system that are critical in the development of tolerance and physical dependence. For example, MMP-9 is increased in the brain and spinal cord of mice administered morphine across multiple days and contributes to the development of morphine tolerance for nociception (Nakamoto et al., 2012) and physical dependence (Liu et al., 2010). Pharmacological blockade of MMP activity or knockout of MMP-9 prevents the development of morphine tolerance for nociception (Nakamoto et al., 2012). Morphine-induced upregulation of MMP-2 and MMP-9 production has been indicated in ECM maintenance, particularly as it pertains to type IV collagen degradation and recycling (Gach et al., 2012). Specifically, opioid-induced alterations in MMP-2 activity are driven by the nitric oxide/nitric oxide synthase (NO/NOS) system, which in turn is regulated by receptor families independent of the μ-opioid receptor, thereby indicating a need for further research in opioid receptor crosstalk and subsequent downstream signaling cascades. Of note, NO/NOS-related mechanisms are involved in opioid-induced inhibition of MMP-9 activity in an opioid-receptor-dependent manner (Gach et al., 2012). Therefore, ECM protein levels in the context of opioid use are tightly regulated by mechanisms dependent and independent of opioid receptor activity and are intertwined with the NO/NOS system. Taken together, these findings suggest that increases in MMP-2 and MMP-9 expression following opioid administration may be critical for behavioral tolerance and dependence as well as drug- and context-induced neural plasticity. Future studies should examine MMP-2 and MMP-9 in preclinical addiction model behaviors to examine their validity as potential therapeutic targets.
A subset of MMPs, including MMP-2 and MMP-9, are activated by the serine protease tissue-type plasminogen activator (tPA), a key regulator of drug-induced synaptic plasticity and remodeling in major reward pathways of the brain (Calabresi et al., 2000; Sternlicht and Werb, 2001; Samson and Medcalf, 2006). Opioid administration leads to increases in tPA levels in the prefrontal cortex, hippocampus, and nucleus accumbens (Nagai et al., 2004). Importantly, the increases in tPA and MMPs during opioid administration are critical for the development of opioid tolerance (Yan et al., 2007; Nakamoto et al., 2012). tPA is also involved in locomotor sensitization to morphine (Bahi and Dreyer, 2008) and regulates the acquisition and maintenance of morphine self-administration behaviors (Yan et al., 2007), presumably via the modulation of dopamine neurotransmission in the striatum (Nagai et al., 2004; Yan et al., 2007). While increases in tPA and MMP are consistently found following opioid administration (Figure 1), the specific roles of tPA and MMP in opioid seeking, craving, and relapse behaviors, as related to OUD are unknown, requiring more studies into the potential crosstalk between tPA and MMP pathways in brain and behavioral plasticity associated with chronic opioid use.
Another class of ECM proteins called cell adhesion molecules (CAMs) may be involved in opioid reward-related behaviors and OUD. CAMs facilitate interactions between the ECM and various cell types in the brain. CAMs bind to other cell adhesion proteins and neighboring neurons to regulate neuronal growth, synaptic plasticity, and function. In the brain, some of the more common CAMs include neural CAM (NCAM), and the Cadherin family, including cadherin-2 (CDH-2) (Polanco et al., 2021). In the hippocampus, knockdown of neural CAMs (NCAMs) decreases the formation of conditioned place preference to morphine (Ishiguro et al., 2006). Following a lethal dose of heroin, levels of NCAMs are increased in the hippocampus of postmortem brains from people with heroin addiction, which positively correlate with blood levels of heroin at the time of death (Weber et al., 2006). Levels of CDH-2 in peripheral plasma have been shown to be a potential biomarker for methadone treatment outcome, correlating with treatment success (Liu et al., 2020), while hippocampal RNA expression of CDH-2 is increased following oxycodone self-administration (Zhang et al., 2015). However, this effect is specific to adult, but not adolescent mice, suggesting developmental stage may moderate the role of CAMs in opioid self-administration. Thus, opioids may lead to rapid increases in CAMs in a dose- and age-dependent manner in the brain, although whether CAMs directly contribute to neuroadaptations associated with OUD is still unknown, as these changes could be due to the acute effects of opioids. Future studies should examine the specific nature of CAM interactions concerning opioid use and relapse, with a specific focus on NCAMs and CDH-2 as potential biomarkers of opioid use.
An Interplay Between the Extracellular Matrix, Microglia, and Neuroinflammation in Opioid Use Disorder
The ECM, in conjunction with microglia and astrocytes, is integral in both pro- and anti-inflammatory responses in the brain. Several lines of evidence link pro-inflammatory cytokine signaling and microglial activity to susceptibility to opioid craving and reward processing (Bland et al., 2009; Hofford et al., 2019). Consistent with this, a recent study from our research group identified significant alterations in transcripts enriched for neuroinflammatory and ECM signaling in the dorsolateral prefrontal cortex and nucleus accumbens of people with OUD (Seney et al., 2021). For example, transcripts that are upregulated in both brain regions of people with OUD are enriched for tumor necrosis factor alpha (TNF-α) signaling via positive regulation of nuclear factor kappa B (NF-κB) (Seney et al., 2021). This finding further supports NF-κB-dependent activation of pro-inflammatory TNF-α signaling associated with OUD. While neuroinflammation may play a distinct role in OUD, of particular importance is the impact of neuroinflammatory cytokine signaling on ECM remodeling activity. In human and rodent brain, chondroitin sulfate glycosaminoglycans (CS-GAGs) accumulate around the synapse in response to inflammation (Li et al., 2013) and may be increased following chronic opioid use in human brain (Seney et al., 2021). Indeed, the CS-GAG pathway is enriched in both the dorsolateral prefrontal cortex and nucleus accumbens of people with OUD (Seney et al., 2021). This poses the possibility that opioids and/or withdrawal from opioids leads to the aggregation of CS-GAGs at the synapses of neurons in regions involved in cognition and reward processing in response to alterations in the homeostatic regulation of inflammatory activity (Figure 1).
Other factors involved in ECM signaling may also contribute to opioid reward-related behaviors and could be associated with OUD. For example, both TIMP metallopeptidase inhibitor 1 (TIMP1) and toll-like receptor 2 (TLR2) are involved in the remodeling of the ECM via inhibition of MMPs (Visse and Nagase, 2003; Ries, 2014) and were recently identified as hub genes (i.e., highly connected gene) within gene networks in the nucleus accumbens that were specifically associated with OUD (Seney et al., 2021). Possibly, chronic opioid use accompanied by periods of withdrawal induce the release of pro-inflammatory cytokines, in turn activating TLR2 and TIMP1, leading to remodeling of the ECM and altering synaptic plasticity and function. Activation of pro-inflammatory cascades by opioids are likely regulated by microglia, as cell type specific enrichment of markers demonstrate a potential primary role for microglia associated with OUD in the dorsolateral prefrontal cortex and nucleus accumbens (Seney et al., 2021). Notably, the same study found enrichment of integrin signaling pathways in OUD, suggesting integrins could be involved in the migration of microglia and/or the adherence of the ECM to microglia and neurons. Collectively, these findings provide strong support for the involvement of the ECM and microglia-dependent neuroinflammation (Shen et al., 2022) in OUD. Future studies combining new single nuclei sequencing technologies with histochemical approaches will be critical for further investigating the potential role of microglia and other cell types in inflammation and ECM remodeling related to OUD in the human brain.
Other studies provide additional support for an important, functional role of microglia in OUD. Pharmacological inhibition of microglia (e.g., via AV411 compound, minocycline, or ibudilast) in rodent models reduces opioid seeking and reward-related behaviors and attenuates the subjective measures of opioid withdrawal in humans (Hutchinson et al., 2008, 2009; Bland et al., 2009; Schwarz et al., 2011; Arezoomandan et al., 2016; Cooper et al., 2016; Pan et al., 2016). Reactivity of microglia to opioids may depend on “off-target” binding of opioid metabolites (e.g., morphine-3-glucuronide) to the toll-like receptor 4 (TLR4), initiating intracellular cascades involved in pro-inflammatory cytokine release and activation of the canonical NF-κB pathway that regulate opioid reward-related and analgesia behaviors (Zhang et al., 2017, 2020; Green et al., 2022). For example, opioid-induced hyperalgesia and the development of tolerance depends on the release of the cytokine interleukin-33 (IL-33) by astrocytes in the brain and spinal cord (Hu et al., 2021). The release of IL-33 into the extracellular space activates astrocytes and microglia through NF-κB-dependent signaling (Molofsky et al., 2015), and is recently identified as a modulator of microglial-dependent degradation of the ECM (Nguyen et al., 2020). Therefore, IL-33 is one molecular intermediary by which microglia induce ECM remodeling and promote synaptic plasticity in an experience-dependent manner. Other pro-inflammatory cytokines may also be involved in opioid-induced synaptic and behavioral plasticity, including TNF and interferon alpha (Wang et al., 2018; Hofford et al., 2019; Seney et al., 2021).
In addition to cytokines, specific substrates of the ECM are involved in microglial activation and neuronal functions related to opioid actions. The formation and integrity of perisynaptic ECM scaffolds and PNNs are regulated by microglia (Crapser et al., 2020a,b, 2021; Strackeljan et al., 2021). PNNs are located proximal to both neurons and glial cells, and in some cortical regions of the brain form dense nets that surround GABAergic interneurons (Kosaka and Heizmann, 1989; Brückner et al., 1993; Sorg et al., 2016; Jorgensen, 2021). In rats, PNNs are significantly reduced in the medial prefrontal cortex and nucleus accumbens following extinction from heroin operant self-administration behavior (Van den Oever et al., 2010). Specifically, the ECM proteins, tenascin-R (TNR) and brevican (BCAN) are downregulated during heroin abstinence, yet are upregulated in the medial prefrontal cortex and nucleus accumbens in response to cue-induced reinstatement (Van den Oever et al., 2010). TNR and BCAN are preferentially expressed in PNNs that surround GABAergic interneurons in the medial prefrontal cortex and nucleus accumbens. Following reinstatement heroin-seeking behavior, these GABAergic interneurons displayed elevated spiking activity and enhanced inhibition of pyramidal neurons in the medial prefrontal cortex (Van den Oever et al., 2010). Therefore, TNR and BCAN may be key proteins of the ECM signaling pathways involved in the function of PNNs that modulate GABAergic cell activity during opioid reward-related behaviors, long-term abstinence from opioids, and potentially involved in opioid craving and relapse (Van den Oever et al., 2010; Xue et al., 2014; Favuzzi et al., 2017; Roura-Martínez et al., 2020). Taken together, there is a complex interplay between microglia, ECM, and neuroinflammation, and further studies examining these interactions related to OUD could be valuable for identifying new approaches for developing effective therapeutics.
Future Investigations Into Biological Sex as a Potential Mediator of Extracellular Matrix Remodeling and Synaptic Plasticity in Opioid Use Disorder
Susceptibility to OUD and the severity of the related symptoms are the result of a complex interplay of biological and psychosocial factors. Earlier studies describe sex-specific differences in the frequency of use of opioids and the prevalence of clinical diagnoses of OUD. For example, higher rates of opioid use and OUD have been reported in men compared to women (Lee and Ho, 2013), although women may have accelerated progression from initial use to dependence (Kosten et al., 1993; Brady and Randall, 1999). Additionally, risk and frequency of opioid overdoses and propensity to use heroin has been described in men compared to women, while women may be more likely to misuse prescription opioids (Parlier-Ahmad et al., 2021). Comorbid psychiatric disorders, such as major depression, are also more prevalent in women compared to men diagnosed with OUD (Parlier-Ahmad et al., 2021).
Preclinical rodent models of opioid-related behaviors support sex-specific effects in opioid seeking, craving, and relapse, along with opioid withdrawal. Indeed, female rats tend to acquire morphine or heroin self-administration behavior quicker and display higher motivation to self-administer opioids compared to males (Lynch and Carroll, 1999; Cicero et al., 2003; Roth et al., 2004). Oxycodone self-administration is also significantly greater in female than male rats for both oral (Sharp et al., 2021) and intravenous self-administration (Kimbrough et al., 2020). Further, female rats exhibit higher sensitivity to the rewarding effects of morphine at far lower doses (Karami and Zarrindast, 2008). During withdrawal from opioids, female and male rats exhibit similarly elevated somatic symptoms (i.e., foot licks, grooming, and writhing) for nearly 48 h following opioid cessation (Gipson et al., 2021). Only increased body temperature was specific to female rats during withdrawal relative to males (Gipson et al., 2021). However, other studies report exaggerated somatic opioid withdrawal symptoms in both the severity and duration in male rodents (Cicero et al., 2002; Diaz et al., 2005). Activity of mu and/or kappa opioid receptors may also be involved in sex-specific effects of opioids (Barrett et al., 2002; Negus et al., 2002). Given this evidence highlighting sex as a critical factor in OUD and opioid actions, more studies are required to investigate the sex-specific cellular and molecular mechanisms involved in opioid reward, treatment response to opioids, and the development of dependence and tolerance to opioids. Few studies have directly examined the role of sex in ECM signaling in response to opioids and associated with OUD.
Sexual dimorphism in ECM signaling pathways that regulate synaptic plasticity and neuroinflammation has been found in fish, birds, mice, and humans. In zebrafish, gene expression patterns for genes associated with the production of ECM signaling proteins are overrepresented in males (Wong et al., 2014), while sex differences in the number and formation of PNNs are observed in zebra finches (Cornez et al., 2015). Sex specific transcriptomic differences are found in mouse sensory neurons, specifically in genes related to neurotransmission, inflammation, and ECM reorganization, suggesting potential sex differences in susceptibility to neuroinflammation and ECM (Mecklenburg et al., 2020; Batzdorf et al., 2022) and OUD (Cahill and Taylor, 2017; Jang et al., 2020). In humans, differences in ECM signaling markers and remodeling are also found in blood serum depending on age and sex, irrespective of disease-related factors (Kehlet et al., 2018). While these few studies on sex-specific effects in ECM are sparse, they are particularly relevant considering the sexual heterogeneity in OUD.
Conclusion
In this review, we highlight the current understanding of the interactions between ECM signaling, neuroinflammation, and synaptic plasticity, as it contributes to opioid seeking, craving, and relapse behaviors. Overall, there is a need for additional research investigating the potential role for biological sex at the intersection of ECM signaling and remodeling, synaptic plasticity, neuroinflammation, and opioids. Targeting specific ECM signaling proteins (e.g., MMPs and CAMs) during opioid administration and/or withdrawal could be a viable therapeutic approach. Preclinical models of opioid self-administration, opioid tolerance, and withdrawal, as well as pain and analgesia, provide tractable approaches that can provide depth into the potential roles of the ECM in opioid-related neurobiology and behavior. The inclusion of sex as a biological variable in these studies should aid in the discovery of novel therapeutic targets for the treatment of opioid dependence and OUD, while also supporting more inclusive options for interventions and therapeutics.
Author Contributions
MHR, BRW, MKK, CDB, and RWL conducted a review of the literature and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the NHLBI T32HL007224 to MHR, and NIDA R01DA051390 and NHLBI R01HL150423 to RWL.
References
- Akol I., Kalogeraki E., Pielecka-Fortuna J., Fricke M., Löwel S. (2022). MMP2 and MMP9 activity is crucial for adult visual cortex plasticity in healthy and stroke-affected mice. J. Neurosci. 42 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezoomandan R., Khodagholi F., Haghparast A. (2016). Administration of the glial condition medium in the nucleus accumbens prolong maintenance and intensify reinstatement of morphine-seeking behavior. Neurochem. Res. 41 855–868. 10.1007/s11064-015-1762-3 [DOI] [PubMed] [Google Scholar]
- Bahi A., Dreyer J.-L. (2008). Overexpression of plasminogen activators in the nucleus accumbens enhances cocaine-, amphetamine- and morphine-induced reward and behavioral sensitization. Genes Brain Behav. 7 244–256. 10.1111/j.1601-183X.2007.00346.x [DOI] [PubMed] [Google Scholar]
- Barrett A. C., Smith E. S., Picker M. J. (2002). Sex-related differences in mechanical nociception and antinociception produced by A- and n-opioid receptor agonists in rats. Eur. J. Pharmacol. 452 163–173. 10.1016/s0014-2999(02)02274-4 [DOI] [PubMed] [Google Scholar]
- Batzdorf C. S., Morr A. S., Bertalan G., Sack I., Silva R. V., Infante-Duarte C. (2022). Sexual dimorphism in extracellular matrix composition and viscoelasticity of the healthy and inflamed mouse brain. Biology 11:230. 10.3390/biology11020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland S. T., Hutchinson M. R., Maier S. F., Watkins L. R., Johnson K. W. (2009). The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav. Immun. 23 492–497. 10.1016/j.bbi.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K. T., Randall C. L. (1999). Gender differences in substance use disorders. Psychiatr. Clin. North Am. 22 241–252. 10.1016/S0193-953X(05)70074-5 [DOI] [PubMed] [Google Scholar]
- Brückner G., Brauer K., Härtig W., Wolff J. R., Rickmann M. J., Derouiche A., et al. (1993). Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8 183–200. 10.1002/glia.440080306 [DOI] [PubMed] [Google Scholar]
- Cahill C. M., Taylor A. M. (2017). Neuroinflammation—a co-occurring phenomenon linking chronic pain and opioid dependence. Curr. Opin. Behav. Sci. 13 171–177. 10.1016/j.cobeha.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Napolitano M., Centonze D., Marfia G. A., Gubellini P., Teule M. A., et al. (2000). Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory: t-PA and synaptic plasticity. Eur. J. Neurosci. 12 1002–1012. 10.1046/j.1460-9568.2000.00991.x [DOI] [PubMed] [Google Scholar]
- Chioma V. C., Kruyer A., Bobadilla A. C., Angelis A., Ellison Z., Hodebourg R., et al. (2021). Heroin seeking and extinction from seeking activate matrix metalloproteinases at synapses on distinct subpopulations of accumbens cells. Biol. Psychiatry 89 947–958. 10.1016/J.BIOPSYCH.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero T. J., Aylward S. C., Meyer E. R. (2003). Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol. Biochem. Behav. 74 541–549. 10.1016/S0091-3057(02)01039-0 [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Nock B., Meyer E. R. (2002). Gender-linked differences in the expression of physical dependence in the rat. Pharmacol. Biochem. Behav. 72 691–697. 10.1016/S0091-3057(02)00740-2 [DOI] [PubMed] [Google Scholar]
- Cooper Z. D., Johnson K. W., Pavlicova M., Glass A., Vosburg S. K., Sullivan M. A., et al. (2016). The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers: ibudilast decreases withdrawal. Addict. Biol. 21 895–903. 10.1111/adb.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornez G., ter Haar S. M., Cornil C. A., Balthazart J. (2015). Anatomically discrete sex differences in neuroplasticity in zebra finches as reflected by perineuronal nets. PLoS One 10:e0123199. 10.1371/journal.pone.0123199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J. D., Arreola M. A., Tsourmas K. I., Green K. N. (2021). Microglia as hackers of the matrix: sculpting synapses and the extracellular space. Cell. Mol. Immunol. 18 2472–2488. 10.1038/s41423-021-00751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J. D., Ochaba J., Soni N., Reidling J. C., Thompson L. M., Green K. N. (2020a). Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington’s disease. Brain 143 266–288. 10.1093/brain/awz363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J. D., Spangenberg E. E., Barahona R. A., Arreola M. A., Hohsfield L. A., Green K. N. (2020b). Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine 58:102919. 10.1016/j.ebiom.2020.102919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Pizzol F., Rojas H. A., dos Santos E. M., Vuolo F., Constantino L., Feier G., et al. (2013). Matrix metalloproteinase-2 and Metalloproteinase-9 activities are associated with blood–brain barrier dysfunction in an animal model of severe sepsis. Mol. Neurobiol. 48 62–70. 10.1007/s12035-013-8433-7 [DOI] [PubMed] [Google Scholar]
- Diaz S. L., Kemmling A. K., Rubio M. C., Balerio G. N. (2005). Morphine withdrawal syndrome: involvement of the dopaminergic system in prepubertal male and female mice. Pharmacol. Biochem. Behav. 82 601–607. 10.1016/J.PBB.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Dityatev A., Schachner M., Sonderegger P. (2010). The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 11 735–746. 10.1038/nrn2898 [DOI] [PubMed] [Google Scholar]
- Favuzzi E., Marques-Smith A., Deogracias R., Winterflood C. M., Sánchez-Aguilera A., Mantoan L., et al. (2017). Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein Brevican. Neuron 95 639–655.e10. 10.1016/j.neuron.2017.06.028 [DOI] [PubMed] [Google Scholar]
- Ferrer-Ferrer M., Dityatev A. (2018). Shaping synapses by the neural extracellular matrix. Front. Neuroanat. 12:40. 10.3389/FNANA.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach K., Szemraj J., Wyrȩbska A., Janecka A. (2011). The influence of opioids on matrix metalloproteinase-2 and -9 secretion and mRNA levels in MCF-7 breast cancer cell line. Mol. Biol. Rep. 38 1231–1236. 10.1007/s11033-010-0222-z [DOI] [PubMed] [Google Scholar]
- Gach K., Wyrȩbska A., Szemraj J., Janecka A. (2012). The influence of opioid peptides on matrix metalloproteinase-9 and urokinase plasminogen activator expression in three cancer cell lines. Mol. Biol. 46 796–801. 10.1134/S0026893312060052 [DOI] [PubMed] [Google Scholar]
- Gipson C. D., Dunn K. E., Bull A., Ulangkaya H., Hossain A. (2021). Establishing preclinical withdrawal syndrome symptomatology following heroin self-administration in male and female rats. Exp. Clin. Psychopharmacol. 29 636–649. 10.1037/pha0000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. M., Sundman M. H., Chou Y. (2022). Opioid-induced microglia reactivity modulates opioid reward, analgesia, and behavior. Neurosci. Biobehav. Rev. 135:104544. 10.1016/j.neubiorev.2022.104544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannocks M.-J., Zhang X., Gerwien H., Chashchina A., Burmeister M., Korpos E., et al. (2019). The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 75–76 102–113. 10.1016/j.matbio.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Hearing M. (2019). Prefrontal-accumbens opioid plasticity: implications for relapse and dependence. Pharmacol. Res. 139 158–165. 10.1016/j.phrs.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford R. S., Russo S. J., Kiraly D. D. (2019). Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur. J. Neurosci. 50 2562–2573. 10.1111/ejn.14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.-M., Yang W., Zhang M.-T., Du L.-X., Tian J.-H., Zhu J.-Y., et al. (2021). Glial IL-33 signaling through an ST2-to-CXCL12 pathway in the spinal cord contributes to morphine-induced hyperalgesia and tolerance. Sci. Signal. 14:eabe3773. 10.1126/scisignal.abe3773 [DOI] [PubMed] [Google Scholar]
- Huntley G. W. (2012). Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 13 743–757. 10.1038/nrn3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. R., Lewis S. S., Coats B. D., Skyba D. A., Crysdale N. Y., Berkelhammer D. L., et al. (2009). Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav. Immun. 23 240–250. 10.1016/j.bbi.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. R., Northcutt A. L., Chao L. W., Kearney J. J., Zhang Y., Berkelhammer D. L., et al. (2008). Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav. Immun. 22 1248–1256. 10.1016/j.bbi.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H., Liu Q.-R., Gong J.-P., Hall F. S., Ujike H., Morales M., et al. (2006). NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology 31 572–584. 10.1038/sj.npp.1300855 [DOI] [PubMed] [Google Scholar]
- Jang D. G., Sim H. J., Song E. K., Kwon T., Park T. J. (2020). Extracellular matrixes and neuroinflammation. BMB Rep. 53 491–499. 10.5483/BMBRep.2020.53.10.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Yang X., He G., Wang F., Wang Z., Xu W., et al. (2021). CRHCeA→VTA inputs inhibit the positive ensembles to induce negative effect of opiate withdrawal. Mol. Psychiatry 26 6170–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen E. (2021). Role of Perineuronal Nets and Parvalbumin in Cortical Plasticity and Substance Abuse. ProQuest Diss. Theses. Available online at: https://ezproxy.bu.edu/login?qurl=https%3A%2F%2Fwww.proquest.com%2Fdissertations-theses%2Frole-perineuronal-nets-parvalbumin-cortical%2Fdocview%2F2572580378%2Fse-2%3Faccountid%3D9676 (accessed December 9, 2021). [Google Scholar]
- Kadam M., Sinha A., Nimkar S., Matcheswalla Y., De Sousa A. (2017). A comparative study of factors associated with relapse in alcohol dependence and opioid dependence. Indian J. Psychol. Med. 39 627–633. 10.4103/IJPSYM.IJPSYM_356_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami M., Zarrindast M. R. (2008). Morphine sex-dependently induced place conditioning in adult Wistar rats. Eur. J. Pharmacol. 582 78–87. 10.1016/J.EJPHAR.2007.12.010 [DOI] [PubMed] [Google Scholar]
- Kehlet S. N., Willumsen N., Armbrecht G., Dietzel R., Brix S., Henriksen K., et al. (2018). Age-related collagen turnover of the interstitial matrix and basement membrane: implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS One 13:e0194458. 10.1371/journal.pone.0194458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A., Kononoff J., Simpson S., Kallupi M., Sedighim S., Palomino K., et al. (2020). Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology 237 1545–1555. 10.1007/S00213-020-05479-Y/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F. (2020). Neurobiology of opioid addiction: opponent process, Hyperkatifeia, and negative reinforcement. Biol. Psychiatry 87 44–53. 10.1016/j.biopsych.2019.05.023 [DOI] [PubMed] [Google Scholar]
- Kosaka T., Heizmann C. W. (1989). Selective staining of a population of parvalbumin-containing GABAergic neurons in the rat cerebral cortex by lectins with specific affinity for terminal N-acetylgalactosamine. Brain Res. 483 158–163. 10.1016/0006-8993(89)90048-6 [DOI] [PubMed] [Google Scholar]
- Kosten T. A., Gawin F. H., Kosten T. R., Rounsaville B. J. (1993). Gender differences in cocaine use and treatment response. J. Subst. Abuse Treat. 10 63–66. 10.1016/0740-5472(93)90100-G [DOI] [PubMed] [Google Scholar]
- Kovatsi L., Batzios S., Nikolaou K., Fragou D., Njau S., Tsatsakis A., et al. (2013). Alterations in serum MMP and TIMP concentrations following chronic heroin abuse. Toxicol. Mech. Methods 23 377–381. 10.3109/15376516.2012.758681 [DOI] [PubMed] [Google Scholar]
- Lee C. W. S., Ho I. K. (2013). Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol. Pain 9:45. 10.1186/1744-8069-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Penzo M. A., Taniguchi H., Kopec C. D., Huang Z. J., Li B. (2013). Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16 332–339. 10.1038/nn.3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li C.-Y., Xi W., Jin S., Wu Z.-H., Jiang P., et al. (2019). Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron 101 748–761.e5. 10.1016/j.neuron.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Liu W.-T., Han Y., Liu Y.-P., Song A. A., Barnes B., Song X.-J. (2010). Spinal matrix metalloproteinase-9 contributes to physical dependence on morphine in mice. J. Neurosci. 30 7613–7623. 10.1523/JNEUROSCI.1358-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-L., Kuo H.-W., Fang C.-P., Tsung J.-H., Chen A. C. H. (2020). Adhesion molecules as potential novel biomarkers for opioid dependence. Curr. Pharm. Des. 26 253–259. 10.2174/1381612826666200116144147 [DOI] [PubMed] [Google Scholar]
- Lynch W. J., Carroll M. E. (1999). Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144 77–82. 10.1007/s002130050979 [DOI] [PubMed] [Google Scholar]
- Madayag A. C., Gomez D., Anderson E. M., Ingebretson A. E., Thomas M. J., Hearing M. C. (2019). Cell-type and region-specific nucleus accumbens AMPAR plasticity associated with morphine reward, reinstatement, and spontaneous withdrawal. Brain Struct. Funct. 224 2311–2324. 10.1007/s00429-019-01903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg J., Zou Y., Wangzhou A., Garcia D., Lai Z., Tumanov A. V., et al. (2020). Transcriptomic sex differences in sensory neuronal populations of mice. Sci. Rep. 10:15278. 10.1038/s41598-020-72285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P., Mikasova L., Groc L., Frischknecht R., Choquet D., Kaczmarek L. (2009). Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin 1 signaling. J. Neurosci. 29 6007–6012. 10.1523/JNEUROSCI.5346-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P., Wawrzyniak M., Alot P., Szczot M., Wyrembek P., Mercik K., et al. (2011). Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 124 3369–3380. 10.1242/jcs.090852 [DOI] [PubMed] [Google Scholar]
- Molofsky A. B., Savage A. K., Locksley R. M. (2015). Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42 1005–1019. 10.1016/j.immuni.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel Ishino F. A., McNab P. R., Gilreath T., Salmeron B., Williams F. (2020). A comprehensive multivariate model of biopsychosocial factors associated with opioid misuse and use disorder in a 2017–2018 United States national survey. BMC Public Health 20:1740. 10.1186/s12889-020-09856-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Yamada K., Yoshimura M., Ishikawa K., Miyamoto Y., Hashimoto K., et al. (2004). The tissue plasminogen activator-plasmin system participates in the rewarding effect of morphine by regulating dopamine release. Proc. Natl. Acad. Sci. U.S.A. 101 3650–3655. 10.1073/pnas.0306587101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi K., Komi D. E. A., Khazaie H., Moini A., Vaisi-Raygani A., Ahmadi H. R., et al. (2018). Investigation of serum levels and activity of matrix metalloproteinases 2 and 9 (MMP2, 9) in opioid and methamphetamine-dependent patients. Acta Med. Iran. 56 559–562. [Google Scholar]
- Nakamoto K., Kawasaki S., Kobori T., Fujita-Hamabe W., Mizoguchi H., Yamada K., et al. (2012). Involvement of matrix metalloproteinase-9 in the development of morphine tolerance. Eur. J. Pharmacol. 683 86–92. 10.1016/j.ejphar.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Negus S. S., Zuzga D. S., Mello N. K. (2002). Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50,488 by quadazocine. J. Pain 3 218–226. 10.1054/jpai.2002.124734 [DOI] [PubMed] [Google Scholar]
- Nguyen P. T., Dorman L. C., Pan S., Vainchtein I. D., Han R. T., Nakao-Inoue H., et al. (2020). Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182 388–403.e15. 10.1016/J.CELL.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Sun X., Jiang L., Hu L., Kong H., Han Y., et al. (2016). Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflammation 13:294. 10.1186/s12974-016-0754-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlier-Ahmad A. B., Martin C. E., Radic M., Svikis D. S. (2021). An exploratory study of sex and gender differences in demographic, psychosocial, clinical, and substance use treatment characteristics of patients in outpatient opioid use disorder treatment with buprenorphine. Transl. Issues Psychol. Sci. 7 141–153. 10.1037/tps0000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco J., Reyes-Vigil F., Weisberg S. D., Dhimitruka I., Brusés J. L. (2021). Differential spatiotemporal expression of type I and type II Cadherins associated with the segmentation of the central nervous system and formation of brain nuclei in the developing mouse. Front. Mol. Neurosci. 14:633719. 10.3389/fnmol.2021.633719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempe R. G., Hartz A. M., Bauer B. (2016). Matrix metalloproteinases in the brain and blood–brain barrier: versatile breakers and makers. J. Cereb. Blood Flow Metab. 36 1481–1507. 10.1177/0271678X16655551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries C. (2014). Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 71 659–672. 10.1007/s00018-013-1457-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Maniskas M. E., Bix G. J. (2018). Bilateral carotid artery stenosis causes unexpected early changes in brain extracellular matrix and blood-brain barrier integrity in mice. PLoS One 13:e0195765. 10.1371/journal.pone.0195765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Cosgrove K., Carroll M. (2004). Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev. 28 533–546. 10.1016/j.neubiorev.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Roura-Martínez D., Díaz-Bejarano P., Ucha M., Paiva R. R., Ambrosio E., Higuera-Matas A. (2020). Comparative analysis of the modulation of perineuronal nets in the prefrontal cortex of rats during protracted withdrawal from cocaine, heroin and sucrose self-administration. Neuropharmacology 180:108290. 10.1016/j.neuropharm.2020.108290 [DOI] [PubMed] [Google Scholar]
- Salarian A., Kadkhodaee M., Zahmatkesh M., Seifi B., Bakhshi E., Akhondzadeh S., et al. (2018). Opioid use disorder induces oxidative stress and inflammation: the attenuating effect of methadone maintenance treatment. Iran. J. Psychiatry 13 46–54. [PMC free article] [PubMed] [Google Scholar]
- Samson A. L., Medcalf R. L. (2006). Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron 50 673–678. 10.1016/j.neuron.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Schwarz J. M., Hutchinson M. R., Bilbo S. D. (2011). Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J. Neurosci. 31 17835–17847. 10.1523/JNEUROSCI.3297-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M. L., Kim S. M., Glausier J. R., Hildebrand M. A., Xue X., Zong W., et al. (2021). Transcriptional alterations in dorsolateral prefrontal cortex and nucleus accumbens implicate neuroinflammation and synaptic remodeling in opioid use disorder. Biol. Psychiatry 90 550–562. 10.1016/J.BIOPSYCH.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp B. M., Fan X., Redei E. E., Mulligan M. K., Chen H. (2021). Sex and heredity are determinants of drug intake in a novel model of rat oral oxycodone self-administration. Genes Brain Behav. 20:e12770. 10.1111/GBB.12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Li Y., Zhu Q., Wang J., Huang Y., Liang J., et al. (2022). The immunomodulatory effect of microglia on ECM neuroinflammation via the PD-1/PD-L1 pathway. CNS Neurosci. Ther. 28 46–63. 10.1111/cns.13760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. C. W., Kupchik Y. M., Scofield M. D., Gipson C. D., Wiggins A., Thomas C. A., et al. (2014). Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat. Neurosci. 1712 1655–1657. 10.1038/nn.3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth B. P., Barry J., Keenan E., Ducray K. (2010). Lapse and relapse following inpatient treatment of opiate dependence. Ir. Med. J. 103 176–179. [PubMed] [Google Scholar]
- Song J., Shao D., Guo X., Zhao Y., Cui D., Ma Q., et al. (2019). Crucial role of feedback signals from prelimbic cortex to basolateral amygdala in the retrieval of morphine withdrawal memory. Sci. Adv. 5:eaat3210. 10.1126/sciadv.aat3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Wu C., Korpos E., Zhang X., Agrawal S. M., Wang Y., et al. (2015). Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 10 1040–1054. 10.1016/j.celrep.2015.01.037 [DOI] [PubMed] [Google Scholar]
- Sorg B. A., Berretta S., Blacktop J. M., Fawcett J. W., Kitagawa H., Kwok J. C. F., et al. (2016). Casting a wide net: role of perineuronal nets in neural plasticity. J. Neurosci. 36 11459–11468. 10.1523/JNEUROSCI.2351-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M. D., Werb Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17 463–516. 10.1146/annurev.cellbio.17.1.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strackeljan L., Baczynska E., Cangalaya C., Baidoe-Ansah D., Wlodarczyk J., Kaushik R., et al. (2021). Microglia depletion-induced remodeling of extracellular matrix and excitatory synapses in the hippocampus of adult mice. Cells 10:1862. 10.3390/cells10081862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares I., Costa-Pereira J. T., Martins I. (2021). Monoaminergic and opioidergic modulation of brainstem circuits: New insights into the clinical challenges of pain treatment? Front. Pain Res. 2:696515. 10.3389/fpain.2021.696515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu B. H., Remmers B. C., Toddes C., Brandner D. D., Lefevre E. M., Kocharian A., et al. (2022). Angiotensin-converting enzyme gates brain circuit-specific plasticity via an endogenous opioid. Science 375 1177–1182. 10.1126/science.abl5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinova K., Tchenio A., Trusel M., Clerke J. A., Lalive A. L., Tzanoulinou S., et al. (2019). Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nat. Neurosci. 22 1053–1056. 10.1038/s41593-019-0421-4 [DOI] [PubMed] [Google Scholar]
- Van den Oever M. C., Lubbers B. R., Goriounova N. A., Li K. W., Van der Schors R. C., Loos M., et al. (2010). Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology 35 2120–2133. 10.1038/npp.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R., Nagase H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92 827–839. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- Wang T.-Y., Lee S.-Y., Chang Y.-H., Chen S.-L., Chen P. S., Chu C.-H., et al. (2018). Correlation of cytokines, BDNF levels, and memory function in patients with opioid use disorder undergoing methadone maintenance treatment. Drug Alcohol Depend. 191 6–13. 10.1016/j.drugalcdep.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Modemann S., Schipper P., Trauer H., Franke H., Illes P., et al. (2006). Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience 138 1215–1223. 10.1016/j.neuroscience.2005.11.059 [DOI] [PubMed] [Google Scholar]
- Webersinke G., Bauer H., Amberger A., Zach O., Bauer H. C. (1992). Comparison of gene expression of extracellular matrix molecules in brain microvascular endothelial cells and astrocytes. Biochem. Biophys. Res. Commun. 189 877–884. 10.1016/0006-291x(92)92285-6 [DOI] [PubMed] [Google Scholar]
- Wong R. Y., McLeod M. M., Godwin J. (2014). Limited sex-biased neural gene expression patterns across strains in Zebrafish (Danio rerio). BMC Genomics 15:905. 10.1186/1471-2164-15-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X. B., Zong W. B., Glausier J. R., Kim S.-M., Shelton M. A., Phan B. N., et al. (2022). Molecular rhythm alterations in prefrontal cortex and nucleus accumbens associated with opioid use disorder. Transl. Psychiatry 12:123. 10.1038/s41398-022-01894-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.-X., Xue L.-F., Liu J.-F., He J., Deng J.-H., Sun S.-C., et al. (2014). Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J. Neurosci. 34 6647–6658. 10.1523/JNEUROSCI.5390-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Yamada K., Mizoguchi H., Noda Y., Nagai T., Nitta A., et al. (2007). Reinforcing effects of morphine are reduced in tissue plasminogen activator-knockout mice. Neuroscience 146 50–59. [DOI] [PubMed] [Google Scholar]
- Zhang H., Largent-Milnes T. M., Vanderah T. W. (2020). Glial neuroimmune signaling in opioid reward. Brain Res. Bull. 155 102–111. 10.1016/j.brainresbull.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brownstein A. J., Buonora M., Niikura K., Ho A., Correa da Rosa J., et al. (2015). Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience 285 34–46. 10.1016/j.neuroscience.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liang Y., Levran O., Randesi M., Yuferov V., Zhao C., et al. (2017). Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234 2259–2275. 10.1007/s00213-017-4657-y [DOI] [PMC free article] [PubMed] [Google Scholar]