Abstract
Background:
Disease activity in the first years after a diagnosis of relapsing-remitting multiple sclerosis (RRMS) is a negative prognostic factor for long-term disability. Markers of both clinical and radiological responses to disease-modifying therapies (DMTs) are advocated.
Objective:
The objective of this study is to estimate the value of cerebrospinal fluid (CSF) inflammatory markers at the time of diagnosis in predicting the disease activity in treatment-naïve multiple sclerosis (MS) patients exposed to dimethyl fumarate (DMF).
Methods:
In total, 48 RRMS patients (31 females/17 males) treated with DMF after the diagnosis were included in this 2-year longitudinal study. All patients underwent a CSF examination, regular clinical and 3T magnetic resonance imaging (MRI) scans that included the assessment of white matter (WM) lesions, cortical lesions (CLs) and global cortical thickness. CSF levels of 10 pro-inflammatory markers – CXCL13 [chemokine (C-X-C motif) ligand 13 or B lymphocyte chemoattractant], CXCL12 (stromal cell-derived factor or C-X-C motif chemokine 12), tumour necrosis factor (TNF), APRIL (a proliferation-inducing ligand, or tumour necrosis factor ligand superfamily member 13), LIGHT (tumour necrosis factor ligand superfamily member 14 or tumour necrosis factor superfamily member 14), interferon (IFN) gamma, interleukin 12 (IL-12), osteopontin, sCD163 [soluble-CD163 (cluster of differentiation 163)] and Chitinase3-like1 – were assessed using immune-assay multiplex techniques. The combined three-domain status of ‘no evidence of disease activity’ (NEDA-3) was defined by no relapses, no disability worsening and no MRI activity, including CLs.
Results:
Twenty patients (42%) reached the NEDA-3 status; patients with disease activity showed higher CSF TNF (p = 0.009), osteopontin (p = 0.005), CXCL12 (p = 0.037), CXCL13 (p = 0.040) and IFN gamma levels (p = 0.019) compared with NEDA-3 patients. After applying a random forest approach, TNF and osteopontin revealed the most important variables associated with the NEDA-3 status. Six molecules that emerged at the random forest approach were added in a multivariate regression model with demographic, clinical and MRI measures of WM and grey matter damage as independent variables. TNF levels confirmed to be associated with the absence of disease activity: odds ratio (OR) = 0.25, CI% = 0.04–0.77.
Conclusion:
CSF inflammatory markers may provide prognostic information in predicting disease activity in the first years after DMF initiation. CSF TNF levels are a possible candidate in predicting treatment response, in addition to clinical, demographic and MRI variables.
Keywords: chemokines, cytokines, dimethyl fumarate, disease activity, osteopontin, relapsing multiple sclerosis, TNF
Introduction
Relapsing-remitting multiple sclerosis (RRMS) is characterized by the occurrence of new neurological symptoms with or without disability accumulation, followed in most cases by a slow accumulation of irreversible disability that defines the transition towards the progressive stage (secondary progressive multiple sclerosis, SPMS).1,2
Many efforts have been made to achieve an early diagnosis of multiple sclerosis (MS). 3 This implies the access for MS patients to disease-modifying therapies (DMTs), aimed to reduce disease activity in the first years after the diagnosis and positively affect patients’ long-term prognosis.4,5
Therefore, there is a prime need to identify clinical and magnetic resonance imaging (MRI) variables capable of predicting the MS treatment response with the final aim of developing a tailored, personalized therapeutic approach. Unfortunately, despite some cerebrospinal fluid (CSF) inflammatory markers have been suggested to predict the disease activity after 4 years of follow-up,6,7 no data are available about their possible predictive value of the treatment response in the first years of the disease course.
We selected a cohort of patients treated with dimethyl fumarate (DMF), a common first-line disease-modifying drug for the treatment of RRMS8,9 with the final aim to evaluate the association between the CSF inflammatory markers with treatment response.
Methods
Study population and design
In total, 48 treatment-naïve patients with RRMS, defined according to McDonald criteria, 3 that started DMF therapy at the standard dosage at the MS Centre of Verona University Hospital, were recruited to participate in this 2-year observational study.
Along with a diagnosis of RRMS, inclusion criteria were the absence of any other inflammatory disease, the availability of at least 1 ml of CSF obtained at diagnosis before any treatment and the completion of at least 2 years of follow-up.
All patients underwent periodical neurological evaluation, including the Expanded Disability Status Scale (EDSS) assessment, 10 every 6 months, with additional examinations in case of relapses. A relapse was defined as a worsening of neurological impairment or appearance of a new symptom or abnormality attributable to MS, lasting at least 24 h and preceded by the stability of at least 1 month. 11 All patients were scheduled to undergo a brain 3T-MRI after 3 (re-baseline), 12 and 24 months from DMF initiation. Adverse events, including the occurrence of lymphopenia, were recorded. Lymphopenia was defined in accordance with the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) as follows: grade 0 (⩾910 × 109/L), grade 1 (⩾800 × 109/L), grade 2 (<800–500 × 109/L), grade 3 (<500–200 × 109/L) and grade 4 (<200 × 109/L). The combined three-domain status of ‘no evidence of disease activity’ (NEDA-3) was defined by no evidence of relapses, MRI activity [new or enlarged white matter (WM) T2 hyperintense lesions, Gadolinium-enhancing lesions, Gd+] and 6-month confirmed disability progression (CDP), defined as an increase of ⩾1 point in EDSS. 12 The no appearance of cortical lesions (CLs) was included in the definition of NEDA-3. The local ethics committee of University of Verona approved the study, and informed consent was obtained from all the patients.
CSF protein analysis
CSF samples were obtained at the time of diagnosis, at least 2 months after the last relapse, according to Consensus Guidelines for CSF and Blood Biobanking. 13 After centrifugation, the supernatant and the cell pellet were stored separately at −80°C. The CSF analysis was optimized and performed by two independent investigators, blinded with respect to the patients’ clinical and MRI features. The concentrations (ng/ml/mgProt) of CXCL13 [chemokine (C-X-C motif) ligand 13 or B lymphocyte chemoattractant], CXCL12 (stromal cell-derived factor or C-X-C motif chemokine 12), tumour necrosis factor (TNF), APRIL (a proliferation-inducing ligand, or tumour necrosis factor ligand superfamily member 13), LIGHT (tumour necrosis factor ligand superfamily member 14 or tumour necrosis factor superfamily member 14), interferon (IFN) gamma, interleukin 12 (IL-12), osteopontin, sCD163 [soluble-CD163 (cluster of differentiation 163)] and Chitinase3-like1 were assessed using immune-assay multiplex techniques based on the Luminex technology (Bio-Plex-X200 System equipped with a magnetic workstation; BioRad, Hercules, CA, USA) according to previously published procedures.6,14 The presence of CSF oligoclonal bands (OCBs) was assessed in each patient.
MRI acquisition protocol
All 3T-MRI scans were acquired using a Philips Achieva 3T-MRI Scanner at the Neuroradiology Unit of the University Hospital of Verona. Manual quality check was carried out to exclude significant artefacts.
A standardized protocol 15 was employed to acquire the following sequences: (1) three-dimensional (3D)-T1 weighted (T1w) Turbo Field Echo (TFE) (repetition time, TR/echo time, TE = 8.4/3.7 ms, voxel size of 1 × 1 × 1 mm3, acquisition time of 5 h 51 min); (2) 3D-Double Inversion Recovery (DIR; TR/TE = 5500/292 ms; inversion times, TIs: TI1/TI2 = 525/2530 ms voxel size of 1 × 1 × 1 mm3; acquisition time of 10 h 49 min); (3) 3D-Fluid Attenuated Inversion Recovery (FLAIR; TR/TE = 5500/292 ms, TI = 1650 ms, voxel size of 1 × 1 × 1 mm3, acquisition time of 4 h 48 min); and (4) 3D-T1w TFE post-contrast with the same parameters of the pre-contrast sequence (TR/TE = 8.4/3.7 ms, voxel size of 1 × 1 × 1 mm3, acquisition time of 5 h 51 min).
MRI analysis
Lesion detection
The number of white matter lesions (WMLn) at baseline and new and enlarging WM lesions at the end of the study were assessed on FLAIR images by a neuroradiologist with extensive experience of MS (Pizzini). The number of total cortical lesions (CLn) and the new CLs were assessed on DIR images based on recent recommendations. 16 Owing to the suboptimal performance of the MRI in visualizing subpial lesions, the present analysis has taken into account mainly the intracortical and leukocortical lesions.
Cortical thickness evaluation
The average global cortical thickness was obtained from the 3D T1w sequence by applying FreeSurfer 17 image analysis suite (version 6.0; http://surfer.nmr.mgh.harvard.edu/). Topological defects in cortical surfaces due to WM and leukocortical lesions were corrected using a semi-automated procedure, including WM lesion segmentation and lesion filling.
Statistical analysis
Differences among groups (patients with and without disease activity) were initially assessed with Mann–Whitney test and chi-square/Fisher’s exact test when appropriate.
Random forest (RF) approach was used to obtain CSF markers associated with the NEDA-3 outcome using the minimal depth (MD) and the total number of trees (times a root). Lower the MD, higher was the variable predictive accuracy, while higher times a root measure, higher was the prediction power of the CSF marker. After having identified the most important CSF variables related to the NEDA-3 status, a multivariable logistic model, including also the CLn and global CTh (cortical thickness) as markers of Gray matter (GM) damage, was applied to assess the additional prognostic value of CSF inflammation to the clinical and MRI parameters collected at the time of diagnosis. CSF variables were log2-transformed to have an intuitive interpretation of odds ratio (OR): each unit in log base 2 (protein level) corresponds to a doubling in protein level.
Likelihood ratio tests (LRTs) were used to compare the goodness-of-fit hierarchical logistic models showing whether adding the CSF variables makes the model significantly more accurate. A p value <0.05 was considered statistically significant. Statistical analysis was performed by means of Prism 7.0 and R studio 3.5.3 version.
Results
Patient’s cohort
All patients completed the 2 years of follow-up. Demographic and clinical characteristics of the study population at baseline are reported in Table 1. At the end of follow-up, 41.7% (20/48) of patients remained free from disease activity (Table 1). Twelve patients (25%) experienced a relapse (7 in the first year and 5 in the second year), while the occurrence of new or enlarging T2 lesions, new CLs or Gd-enhancing lesions was evident in 50% of patients (24/48, 7 in the first year and 17 in the second year) and CDP occurred in 16.7% (8/48, all in the second year). A lower EDSS at the time of diagnosis characterized patients without disease activity (p = 0.002; Table 1).
Table 1.
Baseline demographic, clinical and MRI characteristics of the whole population and accordingly to disease activity at the 2-year follow-up.
| Total MS (n = 48) | 2-year EDA (n = 28) | 2-year NEDA (n = 20) | p value EDA vs NEDA | |
|---|---|---|---|---|
| Age, years | 34 ± 12.0 | 33 ± 12.4 | 36 ± 11.5 | 0.386 |
| Female sex, no. (%) | 31 (64.6) | 21 (72.4) | 10 (52.7) | 0.221 |
| EDSS score, median (range) | 2.0 (0.0–5.0) | 2.0 (0.0-–.0) | 1.0 (0.0–5.0) | 0.002 |
| WMLn, mean ± SD | 8.6 ± 4.9 | 8.0 ± 4.5 | 9.5 ± 5.5 | 0.390 |
| Spinal cord lesion number | 0.19 ± 0.53 | 0.21 ± 0.63 | 0.15 ± 0.37 | 0.999 |
| Gd+ lesions | 0.38 ± 0.64 | 0.36 ± 0.62 | 0.40 ± 0.68 | 0.915 |
| CLn, mean ± SD | 3.8 ± 4.5 | 4.6 ± 5.0 | 2.6 ± 3.3 | 0.299 |
| Global CTh (mm) | 2.17 ± 0.82 | 2.18 ± 0.80 | 2.10 ± 0.87 | 0.889 |
| CSF OCBs (yes/no) | 38/10 | 25/4 | 13/6 | 0.164 |
| Albumin CSF/serum | 5.23 ± 1.83 | 5.23 ± 1.6 | 5.22 ± 2.24 | 0.954 |
Patients with disease activity had an increased EDSS at the time of diagnosis. A p value <0.05 was considered significant.
CLn, cortical lesion number; CSF, cerebrospinal fluid; CTh, cortical thickness; EDA, evidence of disease activity; EDSS, Expanded Disability Status Scale; Gd, gadolinium; MRI, magnetic resonance imaging; MS, multiple sclerosis; NEDA, no evidence of disease activity; OCBs, oligoclonal bands; SD, standard deviation; WMLn, number of white matter lesions.
Bold is statistically significant.
No severe adverse drug reactions leading to discontinuation were reported; moderate flushing and/or gastrointestinal symptoms occurred in 21/48 (43.8%) patients; no patients developed sustained grade 3 or 4 lymphopenia.
CSF markers of disease activity
The univariate analysis revealed that levels of TNF (p = 0.009), osteopontin (p = 0.005), IFN gamma (p = 0.019), CXCL12 (p = 0.037) and CXCL13 (p = 0.04) were significantly increased in patients with disease activity compared with those without signs or symptoms of disease activity (NEDA-3; Table 2).
Table 2.
CSF cytokine and chemokine levels in the whole population and accordingly to disease activity at the 2-year follow-up.
| Total MS (n = 48) | 2-year EDA (n = 29) | 2-year NEDA (n = 19) | p value | |
|---|---|---|---|---|
| TNF | 44.4 ± 43.3 | 62.2 ± 46.8 | 19.4 ± 16.7 | 0.009 |
| Osteopontin | 72,085.9 ± 81,174.0 | 94,224.2 ± 77,068.8 | 41,092.3 ± 76,244.0 | 0.005 |
| IFN gamma | 24.2 ± 34.0 | 35.3 ± 39.8 | 8.6 ± 9.0 | 0.019 |
| CXCL12 | 2974.7 ± 3222.5 | 3886.6 ± 3792.4 | 1698.0 ± 1245.6 | 0.037 |
| CXCL13 | 10.0 ± 10.9 | 12.6 ± 12.1 | 6.4 ± 7.4 | 0.040 |
| LIGHT | 329.3 ± 442.2 | 400.1 ± 476.5 | 230.4 ± 362.7 | 0.077 |
| IL-12 | 21.6 ± 29.92 | 31.2 ± 34.8 | 8.2 ± 10.3 | 0.091 |
| Chitinase3-like1 | 52,109.1 ± 44,537.3 | 54,085.3 ± 42,411.5 | 49,342.4 ± 47,351.4 | 0.514 |
| APRIL | 46,734.3 ± 65,301.4 | 3512.8 ± 36,403.3 | 62,724.4 ± 90,189.5 | 0.626 |
| sCD163 | 51,520.1 ± 40,499 | 47,366.7 ± 21,395.6 | 57,334.8 ± 57,633.7 | 0.860 |
Patients with disease activity had increased CSF values of CXCL13, CXCL12, TNF, IFN gamma and osteopontin. Values are expressed as ng/ml/mgProt; mean ± SD are reported. A p value <0.05 was considered significant.
APRIL, a proliferation-inducing ligand, or tumour necrosis factor ligand superfamily member 13; CSF, cerebrospinal fluid; CXCL12, stromal cell-derived factor or C-X-C motif chemokine 12; CXCL13, chemokine (C-X-C motif) ligand 13 or B lymphocyte chemoattractant; EDA, evidence of disease activity; IFN, interferon; IL-12, interleukin 12; LIGHT, tumour necrosis factor ligand superfamily member 14 or tumour necrosis factor superfamily member 14; MS, multiple sclerosis; NEDA, no evidence of disease activity; sCD163, soluble-CD163 (cluster of differentiation 163); TNF, tumour necrosis factor.
Bold is statistically significant.
RF analysis
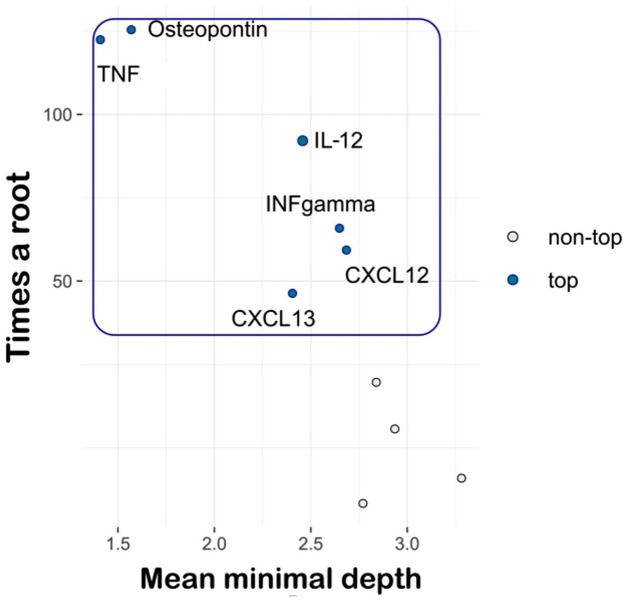
After applying the RF approach, by using MD and times a root measures, six CSF molecules (TNF, osteopontin, IFN gamma, IL-12, CXCL13 and CXCL12) were selected as the most important variables associated with disease activity. Among these, TNF and osteopontin provided the best performance (Figure 1).
Figure 1.
Random forest approach.
TNF, tumour necrosis factor; IL-12, interleukin 12, IFN, interferon; stromal cell-derived factor or C-X-C motif chemokine 12; CXCL13, chemokine (C-X-C motif) ligand 13 or B lymphocyte chemoattractant; NEDA-3, three-domain status of no evidence of disease activity.
Multiway importance plot: TNF, osteopontin, IL-12, IFN gamma, CXCL13 and CXCL12 were selected as the most important variables associated with the NEDA-3 status.
Multivariate logistic regression analysis
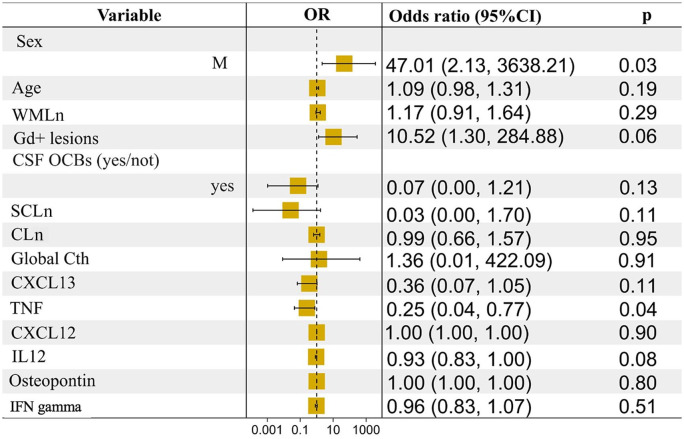
We then added the CSF markers that emerged with the RF approach in a logistic regression model that included age, sex, EDSS, WMLn, number of spinal cord lesions and Gd-enhancing lesions but also CLn and CTh (as markers of GM damage). Still, TNF levels were confirmed to be significantly reduced in patients without any sign or symptom of disease activity (OR = 0.25, CI% = 0.04–0.77; Figure 2).
Figure 2.
Regression analysis: clinical, demographic, MRI and CSF measures at study entry and NEDA-3 status after 2 years.
Male sex and lower levels of TNF were associated with NEDA-3 status at the end of follow-up when including measures of grey matter damage in the model.
95% CI, 95% confidence interval; CLn, cortical lesion number; CSF, cerebrospinal fluid; CTh, cortical thickness; Gd, gadolinium; MRI, magnetic resonance imaging; NEDA-3, three-domain status of no evidence of disease activity; OCBs, oligoclonal bands; OR, odds ratio; SCLn, spinal cord lesion number; TNF, tumour necrosis factor; WMLn, number of white matter lesions.
The LRT approach showed that this latter model was significantly more accurate than the one with only clinical, demographic and MRI variables at T0 (not shown, p < 0.001), suggesting an additional prognostic value of CSF markers.
Discussion
We herein provided evidence that the CSF inflammatory profile and especially the levels of TNF and osteopontin are associated with disease activity in early RRMS patients treated with DMF. We also suggest that testing for such markers could help clinicians in a more accurate treatment personalization highlighting patients who will be possible responders to DMF.
Notably, an early and proper introduction of a DMT to prevent disease activity in the first years after a diagnosis of MS, and possibly reduce the long-term disability accumulation, is mandatory.5,18 Although many treatments for RRMS, each with a different mechanism of action and target, have been developed, 19 a real personalized approach, based on individual biological characteristics, is still an unmet need. 20
In this context, we recruited a cohort of treatment-naïve patients at the time of diagnosis, avoiding biases due to different drug exposure or disease duration, and we evaluated the disease activity early after the exposure to a commonly adopted typical first-line therapy.
Our results are in line with the idea that CSF inflammatory markers could reflect chronic intrathecal processes that occur since early disease phases, 14 and that an early assessment of inflammatory markers could contribute to a better stratification of patients at higher risk of disease activity in the short- and long-term follow-up.6,7,21 Since included in the recent revision of diagnostic criteria and available for all patients, we decided to include the occurrence of new CLs in our definition of NEDA-3. This makes our percentage of cases that showed disease activity not easily comparable with the previously reported results22,23 but possibly makes more accurate our identification of treatment responders.
Globally, the extent of intrathecal inflammation was significantly increased in patients with disease activity, with particular regard to TNF, osteopontin, IFN gamma, the B-cell chemoattractants CXCL12 and CXCL13, all molecules that have been previously associated with disease activity and early disability progression.6,24 Due to complex relationships between CSF variables, with a high correlation between each other, we considered the RF as the most suitable approach in order to improve the data interpretability. In particular, by RF, we computed how much each variable contributes to the NEDA outcome using two different stable measures – that is, MD and times a root – which are based on the topology and the construction of the model.
The result of our RF approach is in line with several previous published data. Increasing evidence from in vitro and in vivo studies points to a crucial role of TNF signalling in mediating both WM and GM pathology.25–28 CSF TNF levels in progressive MS patients have been linked to altered synaptic transmission, with exacerbation of glutamatergic transmission and neuronal damage when incubating CSF with corticostriatal slices.29,30 Recently, persistent expression of CSF TNF along with IFN gamma has been described as a potent inducer of meningeal inflammation and subpial demyelination in the myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) model. 27 Nevertheless, so far, targeting TNF and its receptors did not provide satisfactory results in clinical trials. This is possibly due to the inadequate therapeutic levels of anti-TNF reaching the central nervous system (CNS) or to the dual intrinsic nature of TNF signalling that involves soluble and membrane-bound isoforms and two receptors, thus leading to both pro- and anti-inflammatory downstream signals.30,31 In particular, a shift towards the TNF/TNFR1 and RIPK3-mediated signalling have been recently found associated with GM demyelination and neurodegeneration, 25 being involved in neuronal necroptosis in the cortex of post-mortem MS cases, 32 thus suggesting new possible pathogenetic mechanisms and targets related to the TNF-related downstream cascades. Notably, when adding to our model MRI measures of CLs and global CTh, the association of CSF TNF levels with the EDA status was confirmed, suggesting an additional in vivo value of CSF assessment also when considering MRI measures related to focal and diffuse GM damage. Among others, TNF was the only marker exerting a prognostic effect when inserted in the multivariate model, including baseline features.
Along with TNF levels, osteopontin emerged after the RF approach as the most important protein linked to disease activity. Notably, osteopontin has been suggested to be locally produced by CNS residents and infiltrating cells, further underlying the crucial role of intrathecal processes in MS-related pathology. This involves the intrathecal release of the molecule by endothelial cells, microglial cells, macrophages and dendritic cells, and the survival of myelin-reactive T cell with subsequent relapses and early progression of disability.33,34 Along with its strong association with disease activity, osteopontin has also been associated with progressive MS course from the onset, 24 further underlying its contribution to pathological processes that ultimately lead to MS-related disability accumulation.
Our work is limited by the low sample size and the absence of a validation cohort that prevent us from drawing conclusions, particularly regarding the application of these markers in predicting MS response to other treatments. Furthermore, we assessed the treatment response only through the NEDA-3 status. Although this is commonly adopted in clinical practice, 12 it is mainly weighted on neuroinflammation and focal demyelination parameters, thus reflecting only clinical and subclinical WM inflammatory activity, whose long-term prognostic value on disability accumulation remains debated.35,36 This is why we decided to include our definition of disease activity also the appearance of new CLs; nevertheless, further MRI measures including brain and spinal cord atrophy or assessment of chronic lesion activity37–41 as well as assessment of fluid markers of neurodegeneration 42 would provide additional prognostic information. 43 However, whether evaluating inflammatory markers at the time of diagnosis will provide additional information on long-term disability remains to be fully elucidated. With the above limitations, our study confirms the importance of studying the CSF inflammatory profile at diagnosis to identify the most suitable patients for a tailored and proper approach with a first-line DMT such as DMF
Footnotes
Author contributions.: Damiano Marastoni: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Anna I. Pisani: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Gianmarco Schiavi: Data curation; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Valentina Mazziotti: Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Marco Castellaro: Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft; Writing – review & editing.
Agnese Tamanti: Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Writing – original draft; Writing – review & editing.
Francesca Bosello: Data curation; Investigation; Methodology; Software; Validation; Visualization; Writing – review & editing.
Francesco Crescenzo: Data curation; Investigation; Methodology; Writing – review & editing.
Giuseppe K. Ricciardi: Formal analysis; Methodology; Software; Validation; Writing – review & editing.
Stefania Montemezzi: Data curation; Formal analysis; Methodology; Software; Validation; Writing – review & editing.
Francesca B. Pizzini: Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – review & editing.
Massimiliano Calabrese: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DM received honoraria for research or speaking and funds for travel from Biogen Idec, Roche, Sanofi-Genzyme and Novartis. AIP, GMS, VM, MCas, AT, FB, FC, SM, FBP and GKR: no disclosures relevant to the manuscript. MC received honoraria for research or speaking and funds for travel from Roche, Sanofi-Genzyme, Merck-Serono, Biogen Idec, Teva and Novartis.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Prof. Calabrese was supported by the GR-2013-02-223553 grant from the Italian Ministry of Health.
Ethics statement and informed consent: The Ethics Committee of the University of Verona approved the present study (Protocol Number 66418). All participants provided written informed consent to the study.
ORCID iD: Damiano Marastoni  https://orcid.org/0000-0003-0358-9431
https://orcid.org/0000-0003-0358-9431
Data availability: De-identified data will be shared on request from a qualified investigator.
Contributor Information
Damiano Marastoni, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Anna I. Pisani, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Gianmarco Schiavi, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Valentina Mazziotti, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Marco Castellaro, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Agnese Tamanti, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Francesca Bosello, Department of Neurosciences, Biomedicine and Movement Sciences, Eye Clinic, Ocular Immunology and Neuroophthalmology Service, AOUI-University of Verona, Verona, Italy.
Francesco Crescenzo, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Giuseppe K. Ricciardi, Neuroradiology & Radiology Units, Integrated University Hospital of Verona, Verona, Italy
Stefania Montemezzi, Neuroradiology & Radiology Units, Integrated University Hospital of Verona, Verona, Italy.
Francesca B. Pizzini, Radiology, Department of Diagnostic and Public Health, Integrated University Hospital of Verona, Verona, Italy
Massimiliano Calabrese, Neurology B, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Policlinico ‘G.B. Rossi’ Borgo Roma, Piazzale L. A. Scuro, 10, 37134 Verona, Italy.
References
- 1. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 2. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 4. Scalfari A, Romualdi C, Nicholas RS, et al. The cortical damage, early relapses, and onset of the progressive phase in multiple sclerosis. Neurology 2018; 90: e2099–e2106. [DOI] [PubMed] [Google Scholar]
- 5. Amato MP, Fonderico M, Portaccio E, et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain 2020; 143: 3013–3024. [DOI] [PubMed] [Google Scholar]
- 6. Magliozzi R, Scalfari A, Pisani AI, et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann Neurol 2020; 88: 562–573. [DOI] [PubMed] [Google Scholar]
- 7. Comabella M, Sastre-Garriga J, Borras E, et al. CSF chitinase 3-like 2 is associated with long-term disability progression in patients with progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. Epub ahead of print 8 September 2021. DOI: 10.1212/NXI.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134: 678–692. [DOI] [PubMed] [Google Scholar]
- 9. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 10. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 11. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13: 227–231. [DOI] [PubMed] [Google Scholar]
- 12. Giovannoni G, Tomic D, Bright JR, et al. ‘No evident disease activity’: the use of combined assessments in the management of patients with multiple sclerosis. Multi Scler 2017; 23: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009; 73: 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magliozzi R, Howell OW, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 2018; 83: 739–755. [DOI] [PubMed] [Google Scholar]
- 15. Calabrese M, Castellaro M. Cortical gray matter MR imaging in multiple sclerosis. Neuroimaging Clinics of North America 2017; 27: 301–312. [DOI] [PubMed] [Google Scholar]
- 16. Geurts JJ, Calabrese M, Fisher E, et al. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol 2012; 11: 1082–1092. [DOI] [PubMed] [Google Scholar]
- 17. Fischl B. FreeSurfer. NeuroImage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019; 76: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derfuss T, Mehling M, Papadopoulou A, et al. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol 2020; 19: 336–347. [DOI] [PubMed] [Google Scholar]
- 20. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 2019; 15: 287–300. [DOI] [PubMed] [Google Scholar]
- 21. Ferraro D, Galli V, Vitetta F, et al. Cerebrospinal fluid CXCL13 in clinically isolated syndrome patients: association with oligoclonal IgM bands and prediction of multiple sclerosis diagnosis. J Neuroimmunol 2015; 283: 64–69. [DOI] [PubMed] [Google Scholar]
- 22. Prosperini L, Lucchini M, Haggiag S, et al. Fingolimod vs dimethyl fumarate in multiple sclerosis a real-world propensity score-matched study. Neurology 2018; 91: E153–E161. [DOI] [PubMed] [Google Scholar]
- 23. Havrdova E, Giovannoni G, Gold R, et al. Effect of delayed-release dimethyl fumarate on no evidence of disease activity in relapsing-remitting multiple sclerosis: integrated analysis of the phase III DEFINE and CONFIRM studies. Eur J Neurol 2017; 24: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marastoni D, Magliozzi R, Bolzan A, et al. CSF Levels of CXCL12 and osteopontin as early markers of primary progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflammation 2021; 8: e1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magliozzi R, Howell OW, Durrenberger P, et al. Meningeal inflammation changes the balance of TNF signalling in cortical grey matter in multiple sclerosis. J Neuroinflammation 2019; 16: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veroni C, Serafini B, Rosicarelli B, et al. Connecting immune cell infiltration to the multitasking microglia response and TNF receptor 2 induction in the multiple sclerosis brain. Front Cell Neurosci 2020; 14: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James RE, Schalks R, Browne E, et al. Persistent elevation of intrathecal pro-inflammatory cytokines leads to multiple sclerosis-like cortical demyelination and neurodegeneration. Acta Neuropathol Commun 2020; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magliozzi R, Pezzini F, Pucci M, et al. Changes in cerebrospinal fluid balance of TNF and TNF receptors in naïve multiple sclerosis patients: early involvement in compartmentalised intrathecal inflammation. Cells 2021; 10: 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossi S, Motta C, Studer V, et al. Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Mult Scler 2014; 20: 304–312. [DOI] [PubMed] [Google Scholar]
- 30. Fresegna D, Bullitta S, Musella A, et al. Re-examining the role of TNF in MS pathogenesis and therapy. Cells 2020; 9: 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suvannavejh GC, Lee HO, Padilla J, et al. Divergent roles for p55 and p75 tumor necrosis factor receptors in the pathogenesis of MOG35-55-induced experimental autoimmune encephalomyelitis. Cell Immunol 2000; 205: 24–33. [DOI] [PubMed] [Google Scholar]
- 32. Picon C, Jayaraman A, James R, et al. Neuron-specific activation of necroptosis signaling in multiple sclerosis cortical grey matter. Acta Neuropathol 2021; 141: 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001; 294: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 34. Hur EM, Youssef S, Haws ME, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol 2007; 8: 74–83. [DOI] [PubMed] [Google Scholar]
- 35. Prosperini L, Ruggieri S, Haggiag S, et al. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. Epub ahead of print 9 August 2021. DOI: 10.1212/NXI.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 37. Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012; 135: 2952–2961. [DOI] [PubMed] [Google Scholar]
- 38. Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology 2013; 81: 1759–1767. [DOI] [PubMed] [Google Scholar]
- 39. Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016; 22: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haider L, Prados F, Chung K, et al. Cortical involvement determines impairment 30 years after a clinically isolated syndrome. Brain 2021; 144: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 2019; 76: 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: E1007–E1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021. MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 2021; 20: 653–670. [DOI] [PubMed] [Google Scholar]