Abstract
Rationale:
Targeted cancer therapies have revolutionized the field of oncology by selecting for specific molecular pathways, thus improving overall clinical prognosis. However, many of these targeted treatments have been reported to have adverse kidney effects, including acute kidney injury, interstitial nephritis, and glomerular disease. Furthermore, some of these targeted therapies have also been found to cause an asymptomatic rise in serum creatinine levels through inhibition of active tubular secretion.
Presenting concerns:
A 79-year-old woman was being followed for stage 4 A2 chronic kidney disease secondary to type 2 diabetes and longstanding hypertension. She was diagnosed with invasive mammary carcinoma and was initiated on letrozole, an aromatase inhibitor, and palbociclib, a selective cyclin-dependent kinase inhibitor, was subsequently added. Prior to the initiation of her treatments, her baseline estimated glomerular filtration rate (eGFR) fluctuated between 25 and 28 mL/min/1.73 m2 over the previous year. After initiating palbociclib, her serum creatinine progressively increased, despite having well-controlled blood pressure and diabetes. In addition, there was no history of pre-renal events nor any sonographic evidence of obstruction. Within 7 months, her eGFR based on serum creatinine had decreased down to 12 mL/min/1.73 m2.
Interventions:
Given that there were no clinical or other biochemical changes suggestive of worsening renal function, a serum cystatin C was measured using an immunoturbidimetric assay, which was 1.71 mg/L and correlated with an eGFR of 33 mL/min/1.73 m2 based on the chronic kidney disease epidemiology collaboration (CKD-EPI) cystatin C equation (2012). This value was consistent with her previous baseline. Based on these findings, the significant decrease in eGFR measured by serum creatinine was attributed to the inhibitory effects of palbociclib on tubular creatinine secretion, rather than representing true kidney damage. Thus, a kidney biopsy was not performed in this context.
Outcomes:
Seven months later, a repeat serum cystatin C was repeated to assess for any worsening of the patient’s kidney function and revealed an eGFR of 35 mL/min/1.73 m2 based on the CKD-EPI cystatin C equation (2012), thus revealing stable kidney function and reinforcing the inhibitory effects of palbociclib on tubular creatinine secretion through its direct effects on kidney transporters.
Teaching points:
This case report and literature review acknowledges the importance of using alternative methods of assessing kidney function when patients are undergoing targeted cancer therapies known to affect tubular creatinine secretion, which include cyclin-dependent kinase 4/6 inhibitors, poly(adenosine diphosphate-ribose) polymerase inhibitors, tyrosine kinase inhibitors, and mesenchymal-epithelial transition inhibitors. The use of non–creatinine-based markers of glomerular filtration rate (GFR), such as cystatin C and nuclear renal scans, will allow for more accurate estimation of kidney function in the appropriate setting, thus avoiding invasive diagnostic tests and unnecessary adjustments of treatment plans. However, certain targeted cancer therapies have also been proven to cause true kidney injury; therefore, physicians must still maintain a high degree of suspicion and consider invasive investigations and/or cessation or reduction of treatments when alternative measurements of kidney function do not suggest an underestimation of GFR via serum creatinine.
Keywords: acute kidney injury, targeted cancer therapies, cystatin C, serum creatinine elevation, palbociclib
Abrégé
Contexte:
Les thérapies ciblées contre le cancer ont révolutionné le domaine de l’oncologie en sélectionnant des voies moléculaires spécifiques, ce qui améliore le pronostic clinique global. Il a toutefois été rapporté que plusieurs de ces traitements ciblés entraînent des effets indésirables sur les reins, notamment l’insuffisance rénale aiguë, la néphrite interstitielle et la glomérulonéphrite. Qui plus est, certains de ces traitements provoquent aussi une augmentation asymptomatique des taux de créatinine sérique en inhibant la sécrétion tubulaire active.
Présentation du cas:
Une femme de 79 ans était suivie pour une insuffisance rénale chronique de stade 4 A2 secondaire à un diabète de type 2 et à une hypertension de longue date. La patiente été diagnostiquée avec un carcinome mammaire invasif et a reçu du létrozole, un inhibiteur de l’aromatase. Un traitement au palbociclib, un inhibiteur sélectif de la kinase dépendante de la cycline, a été ajouté par la suite. Avant le début du traitement, le DFGe initial de la patiente avait fluctué de 25 à 28 ml/min/1,73 m2 lors de l’année précédente. Après avoir commencé le palbociclib, son taux de créatinine sérique a augmenté progressivement, malgré une pression artérielle et un diabète bien contrôlés. La patiente n’avait aucun antécédent d’événements pré-rénaux ni de preuves échographiques d’obstruction. En sept mois, son DFGe basé sur la créatinine sérique était passé à 12 ml/min/1,73 m2.
Intervention:
En absence de changements cliniques ou biochimiques suggérant une aggravation de la fonction rénale, la cystatine C sérique a été mesurée par dosage immunoturbidimétrique. Son taux (1,71 mg/L) correspondait à un DFGe de 33 ml/min/1,73 m2 obtenu par l’équation CKD-EPI (2012) pour la cystatine C; une valeur comparable à sa référence précédente. À la lumière de ces résultats, la chute significative du DFGe mesurée par la créatinine sérique a été attribuée aux effets inhibiteurs du palbociclib sur la sécrétion tubulaire de créatinine, plutôt qu’à une véritable atteinte rénale. C’est pourquoi une biopsie rénale n’a pas été effectuée.
Résultats:
Après sept mois, la mesure de la cystatine C sérique a été répétée pour évaluer une potentielle aggravation de la fonction rénale. Cette nouvelle mesure a révélé un DFGe de 35 ml/min/1,73 m2 obtenu avec l’équation CKD-EPI (2012) de la cystatine C, ce qui indique une fonction rénale stable et renforce le diagnostic retenu d’un effet inhibiteur du palbociclib sur la sécrétion tubulaire de créatinine dû à ses effets directs sur les transporteurs rénaux.
Enseignements tirés:
Ce rapport de cas et la revue de la littérature soulignent l’importance d’utiliser d’autres méthodes d’évaluation de la fonction rénale lorsque les patients suivent des traitements ciblés contre le cancer connus pour affecter la sécrétion tubulaire de créatinine, notamment les inhibiteurs de la kinase dépendante de la cycline (CDK4/6), les inhibiteurs de la polymérase poly-adénosine diphosphate ribose (PARP), les inhibiteurs de la tyrosine kinase (TK) et les inhibiteurs de la transition mésenchymateuse-épithéliale (TEM). L’utilisation de marqueurs du DFG autres que la créatinine, tels que la cystatine C et la scintigraphie rénale nucléaire, permettra une estimation plus précise de la fonction rénale dans le contexte approprié, évitant ainsi des tests diagnostiques invasifs et des ajustements inutiles des plans de traitement. On sait toutefois que certains traitements ciblés contre le cancer sont à l’origine de véritables lésions rénales. Par conséquent, les médecins doivent maintenir un haut degré de suspicion et envisager des examens invasifs et/ou l’arrêt ou la réduction des traitements lorsque d’autres mesures de la fonction rénale ne suggèrent pas une sous-estimation du DFG par la créatinine sérique.
Introduction
Targeted cancer therapies have revolutionized the field of oncology by selecting for specific molecular pathways for several types of cancers, thus improving overall clinical prognosis. However, many of these treatments have been reported to have adverse renal side effects, including acute kidney injury (AKI), interstitial nephritis, and glomerular disease. 1 Furthermore, some of these treatments have also been found to cause an asymptomatic rise in serum creatinine levels through inhibition of active tubular secretion. 2
Serum creatinine, an endogenous product of creatinine phosphate metabolism in skeletal muscles, is one of the most widely used biomarkers to assess kidney function in the clinical setting. 3 Creatinine is mainly filtered by the glomerulus, but approximately 10% to 40% of creatinine clearance relies on active tubular secretion mediated by multiple solute-carrier transporters, namely, organic cation transporter 2 (OCT2), multidrug and toxin extrusion protein (MATE) 1, and MATE2-K. 2 Organic cation transporter 2 is found on the basolateral membrane of the proximal tubule cells and is responsible for the uptake of cations from blood into cells, whereas MATE1 and MATE2-K are found on the apical membrane and mediate efflux of organic compounds, such as drugs and toxins, from cells to urine. 2 Hence, inhibition of these specific solute-carrier transporters can lead to a significant decrease in creatinine clearance, which will subsequently cause an increase in serum creatinine levels. Given that serum creatinine is greatly affected by biological variability and relies on tubular secretion, 4 alternative methods for assessing glomerular filtration rate (GFR), such as cystatin C and renal scans, in the clinical setting should be considered when specific therapies known to affect tubular creatinine secretion are being administered.
We will start by reporting a case of palbociclib-induced elevation in serum creatinine and then, based on a literature review, focus on classes of targeted therapies that have been reported to cause elevated levels of serum creatinine without evidence of true AKI.
Case Presentation
The patient was a 79-year-old woman who was followed for stage 4 A2 chronic kidney disease (CKD) secondary to type 2 diabetes and longstanding hypertension. Her medical history was significant for dyslipidemia, osteoporosis, iron deficiency anemia, and mild Alzheimer disease. Her home medications at this time included nifedipine, rosuvastatin, telmisartan, alogliptin, donepezil, calcium carbonate, and vitamin D3.
The patient initially presented with breast pain and a palpable lump, for which a mammography and breast ultrasound were done in March 2020. Imaging revealed an infiltrative right breast mass involving all 4 quadrants with suspicious right axillary lymph nodes. On pathology, she was found to have invasive mammary carcinoma, which was hormone receptor positive and human epidermal growth factor receptor 2 (HER2) negative with an unsatisfactory axillary pathology.
Given her age and comorbidities, she was not a candidate for surgery or neoadjuvant chemotherapy. Therefore, she was started on letrozole 2.5 mg orally daily in April 2020, an aromatase inhibitor, and palbociclib 125 mg orally daily, a selective cyclin-dependent kinase (CDK) inhibitor, was subsequently added in May 2020 due to a strong suspicion of axillary involvement.
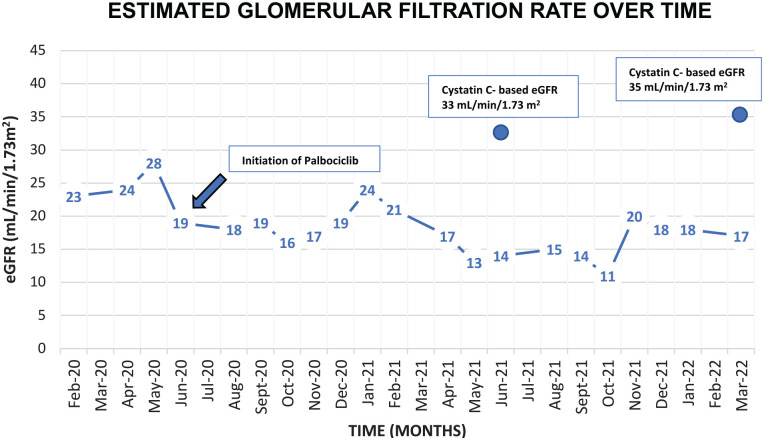
Prior to the initiation of letrozole and palbociclib, her baseline eGFR fluctuated between 25 and 28 mL/min/1.73 m2 over the previous year. After initiation of the latter breast cancer treatment, her serum creatinine progressively increased, despite having well-controlled blood pressure and diabetes. In addition, there was no history of pre-renal events nor any sonographic evidence of obstruction. By January 2021, her eGFR based on serum creatinine by CKD-EPI formula had decreased down to 12 mL/min/1.73 m2. Urine protein/creatinine ratio fluctuated between 1 and 2 g/g, which was not significantly different from baseline. Given that there were no clinical or other biochemical changes suggestive of worsening renal function, a serum cystatin C was measured using an immunoturbidimetric assay, which revealed an eGFR of 33 mL/min/1.73 m2 based on the CKD-EPI cystatin C equation (2012). 5 This value was consistent with her previous baseline. Based on these findings, the significant decrease in eGFR measured by serum creatinine over the past year was attributed to inhibitory effects of palbociclib on creatinine secretion, rather than representing true kidney damage. A kidney biopsy was not performed in this context since the cystatin C eGFR revealed stable kidney function (Figures 1 and 2). A serum cystatin C was measured 7 months later, which was found to be 1.65 mg/L and correlated with an eGFR of 35 mL/min/1.73 m2 based on the CKD-EPI cystatin C equation (2012). 5 Thus, the patient’s stable kidney function reinforces the inhibitory effects of palbociclib on tubular creatinine secretion by its direct actions on kidney transporters.
Figure 1.
Graph illustrating the changes in serum creatinine over time in relation to the initiation of palbociclib.
Figure 2.
Graph illustrating the changes in eGFR (CKD-EPI equation) over time in relation to the initiation of palbociclib.
Note. eGFR = estimated glomerular filtration rate; CKD-EPI = chronic kidney disease epidemiology collaboration.
Targeted Cancer Therapies and Inhibition of Tubular Creatinine Secretion
Based on the literature review, several classes of cancer treatments have been reported to cause elevations in creatinine levels secondary to decreased tubular secretion. The classes of targeted therapies include the following:
CDK 4/6 Inhibitors
Cyclin-dependent kinases 4 and 6 are crucial regulatory enzymes that mediate cell cycle transitions and ultimately cell division. 6 One of the key proteins in regulating cell division is the tumor suppressor retinoblastoma (Rb) protein, which determines the transition from G1 to S phase. 6 The CDK4/6-Rb axis has been an important target for several malignancies, but it has shown the most promise with metastatic hormone receptor–positive breast cancer. 7 Based on recent studies, estrogen has been proven to accelerate progression from G1 to S phase. 7 Therefore, selective inhibition of CDK 4/6 will cause cell cycle arrest, thus reducing breast tumor response and progression. 7
These novel CDK inhibitors, which include palbociclib, ribociclib, and abemaciclib, have been shown to inhibit renal tubular secretion without affecting the GFR. An important study done by Chappell et al assessed in vitro and clinical inhibition of renal transporters and its metabolites by using metformin as a substrate. 2 Metformin is excreted in urine through active tubular secretion, which is mediated by OCT2 uptake and MATE 1/2-K efflux. 2 Previous studies have also shown that coadministration of metformin with other OCT2 and/or MATE inhibitors has altered the pharmacokinetics of metformin. 2 Based on the study by Chappell et al, they demonstrated that abemaciclib inhibited OCT2, MATE1, and MATE2-K transporters in vitro by analyzing transporter interactions. 2 Clinically, abemaciclib decreased metformin clearance but did not affect renal function given that there were minimal changes in measured GFR with iohexol clearance and AKI biomarkers, such as kidney injury molecule 1 and neutrophil gelatinase-associated lipocalin. 2 Hence, this study strongly suggests that these increases in serum creatinine are caused by inhibition of transporters involved in tubular secretion.
A case report by Bonilla et al also documented the inhibitory effects of palbociclib on transporters mediating active tubular secretion, thus causing transient and reversible elevations in serum creatinine. 8 A 66-year-old woman with poorly differentiated invasive ductal carcinoma in the right breast was also treated with fulvestrant, a competitive estrogen receptor antagonist, and palbociclib for a year. She was referred to nephrology for increasing serum creatinine levels and a progressive decrease in eGFR. However, an eGFR calculated based on cystatin C levels revealed that her kidney function was at baseline for the past year.
Despite the known effects of CDK 4/6 inhibitors on renal transporters, physicians must still maintain a high degree of clinical suspicion for true AKI if alternative measurements of GFR do not suggest an underestimation of GFR via serum creatinine. Gupta et al presented a series of patients with biopsy-proven AKI associated with CDK 4/6 inhibitors. 9 In their series, 3 out of 6 patients had severe AKI with 1 patient requiring renal replacement therapy, and each had histopathologic evidence of AKI on biopsy, specifically acute tubular necrosis (ATN). 9 Therefore, it can be very challenging to differentiate between “pseudo-AKI” and true AKI when these therapies are being administered, thus simultaneous measurements of serum creatinine, eGFR based on cystatin C levels, and/or renal scans can help further clarify the clinical presentation and guide investigations and treatment.
Poly(Adenosine Diphosphate-Ribose) Polymerase (PARP) Inhibitors
Poly(ADP-ribose) polymerase is a multifunctional protein that plays a critical role in DNA repair and maintaining genome integrity. 10 Poly(ADP-ribose) polymerase inhibitors, which include olaparib, niraparib, and rucaparib, specifically target DNA cellular repair pathways and are primarily used in ovarian, metastatic breast, and endometrial cancers. 10 Poly(ADP-ribose) polymerase inhibitors prevent repair of DNA single-stranded breaks and stimulate the conversion of single-stranded breaks into double-stranded breaks. 11 Since cancer cells lack repair mechanisms for double-stranded breaks, namely, homologous recombination, the accumulation of double-stranded breaks eventually leads to cell death via apoptosis. 10 Therefore, PARP inhibitors have shown to be effective in BRCA mutations or cells defective in homologous recombination repair. 10
An important retrospective cohort study done by Molin et al described the inconsistencies between creatinine-based eGFR compared with calculated GFRs via imaging in patients taking PARP inhibitors. 12 There were 211 patients included in the study and those who had elevated serum creatinine levels (49% above baseline) underwent a renal scan to estimate GFR. In 63% of cases, it was found that there was a discrepancy between the creatinine-based eGFR and the calculated GFR from the renal scan. The eGFR from the renal scan was nearly identical to the patient’s baseline.
Subsequently, a similar retrospective study was done by Bruin et al who identified patients with elevated serum creatinine levels undergoing treatment with olaparib and wanted to demonstrate if these increases in creatinine truly correlated with a decline in GFR by comparing on/off treatment values. 13 A total of 66 patients were included in the study. Participants who were treated with olaparib were found to have a 14% increase in median creatinine and a 13% decrease in creatinine-derived eGFR. However, there was no substantial effect on the median cystatin C levels and cystatin C–derived eGFR.
Tyrosine Kinase Inhibitors (TKIs)
Tyrosine kinases are enzymes that phosphorylate substrate enzymes, which help regulate signal transduction signals, leading to downstream cellular changes, including cell growth, migration, differentiation, apoptosis, and death. 14 However, continuous activation or inhibition of these transduction signals can lead to dysregulation in signal cascades and cause various pathologies, such as malignancy. 14 Therefore, TKIs are a broad class of drugs that can affect several molecular pathways by preventing the activity of these dysfunctional or mutated tyrosine kinases, thus preventing tumor growth and/or progression of disease. 14 There are various classes of TKIs, such as anaplastic lymphoma kinase (ALK) inhibitors, Fusion of breakpoint cluster region and Abelson (BCR-ABL) inhibitors, epidermal growth factor receptor (EGFR) inhibitors, and vascular endothelial growth factor (VEGF) inhibitors.
Anaplastic lymphoma kinase inhibitors, such as crizotinib, ceritinib, and alectinib, are primarily used in the treatment of advanced non–small cell lung cancer (NSCLC) in which around 3% to 7% of patients are positive for the ALK gene rearrangement or the fusion with echinoderm microtubule–associated protein-like 4. 15 A recent study done by Arakawa et al evaluated the in vitro inhibitory effects of crizotinib on OCT2 by directly measuring the creatinine uptake by OCT2 and demonstrated that crizotinib inhibited creatine uptake by OCT2 in a competitive and substrate-dependent manner. 16 Therefore, at clinically relevant concentrations, crizotinib can cause a rise in serum creatinine levels without representing true kidney injury through its direct actions on renal transporters. 16 Nevertheless, recent case reports and studies have indicated that crizotinib can also cause biopsy-proven kidney injury. Gastaud et al reported a case of crizotinib treatment for a locally advanced NSCLC in a 49-year-old patient with previously normal kidney function. 17 Within 3 weeks of initiating therapy, there was a noticeable rise in serum creatinine and crizotinib discontinuation resulted in improvement of kidney function within 8 days. 17 However, restarting the therapy led to a decline in kidney function associated with proteinuria, macroscopic hematuria, and kidney biopsy–proven ATN. 17 Similarly, Izzedine et al also reported a case of progressive and unexplained worsening renal function in the setting of crizotinib treatment, where a kidney biopsy revealed acute tubular injury and renal arteriolar myocyte vacuolization. 18 Hence, in rare cases, ALK inhibitors have the potential to cause true kidney damage and the changes in serum creatinine levels need to be interpreted judiciously. In cases where there is concern for kidney injury, a third-generation ALK inhibitor, such as lorlatinib, can also be used for the treatment of NSCLC as it has not been reported to cause elevations in serum creatinine and has been shown to be safe in patients with mild to moderate renal impairment based on a phase I study done by Lin et al. 19
Furthermore, an important study done by Omote et al investigated the inhibitory effects of different classes of TKIs on MATE1-mediated transport of creatinine. 20 The TKIs that were being evaluated include crizotinib (ALK inhibitor), gefitinib (EGFR inhibitor), imatinib (BCR-ABL inhibitor), pazopanib (VEGF inhibitor), sorafenib (VEGF inhibitor), and sunitinib (VEGF inhibitor). 20 By evaluating the uptake of creatinine by various cell lines and kidney slices, the authors concluded that crizotinib inhibited MATE1-overexpressing cells and the inhibitory effects increased with incubation time. 20 In addition, they found that gefitinib, imatinib, pazopanib, sorafenib, and sunitinib also inhibited MATE1-mediated creatinine uptake and all these TKIs except for pazopanib inhibited OCT2-mediated creatinine uptake. 20 Finally, they observed that crizotinib and imatinib, in particular, have the potential to increase serum creatinine by more than 10% in the clinical setting, which further reinforces the degree of caution that needs to be exerted when evaluating kidney function based on serum creatinine levels and the use of non–creatinine-based measures of GFR will help clarify the clinical picture. 20
In addition, tucatinib is a TKI that is selective for HER2 and used for the treatment of HER2-positive metastatic breast cancer. 21 Based on the literature, Topletz-Erikson et al studied the effects of tucatinib on kidney function by evaluating metformin pharmacokinetics and demonstrated that tucatinib affected tubular secretion through its direct inhibition of specific renal transporters.
Mesenchymal-Epithelial Transition (MET) Inhibitors
Mesenchymal-epithelial transition is a proto-oncogene that encodes a transmembrane receptor tyrosine kinase that can be activated by an endogenous ligand or hepatocyte growth factor. 22 Its activation ultimately triggers downstream signaling pathways involved in cellular activities. 22 Therefore, a MET mutation, amplification, or overexpression can lead to dysregulation of cellular proliferation, apoptosis, and migration. 22 These alterations in the MET gene are thought to be one of the underlying mechanisms involved in the development of NSCLC. 23
Based on the current literature, there is only one case report that has documented the asymptomatic rise in creatinine levels associated with capmatinib treatment. Capmatinib is a selective type Ib MET inhibitor that is specifically used to treat NSCLC with MET exon-14 skipping mutations. 24 Mohan and Herrmann 24 reported a case of an 84-year-old man who was treated for NSCLC with capmatinib. He had a significant increase in serum creatinine levels while on treatment; therefore, the treatment was stopped and resumed multiple times, with notable increases in serum creatinine when therapy was resumed. However, further investigations with cystatin C and renal iothalamate clearance demonstrated that there were no significant changes in calculated and measured GFR, respectively, thus suggesting an alteration in creatinine secretion rather than true changes in kidney function.
Clinical Implications
Even though serum creatinine is routinely used to estimate GFR and to monitor for AKI or CKD, targeted therapies that affect the activity of renal transporters can lead to changes in serum creatinine that are not representative of “true” kidney damage (Table 1). Since it can be very challenging to distinguish between “pseudo-AKI” and true kidney injury, physicians should interpret elevations in serum creatinine with caution and use alternative measures of eGFR to guide further investigations and treatments.
Table 1.
Summary of Targeted Cancer Therapies Known to Cause an Asymptomatic Rise in Serum Creatinine Levels Through Inhibition of Active Tubular Secretion.
| Classes of targeted cancer therapies | Examples | Target cancers |
|---|---|---|
| CDK 4/6 inhibitors | Palbociclib, ribociclib, abemaciclib | Metastatic HR-positive and HER2-negative breast cancer |
| PARP inhibitors | Olaparib, niraparib, rucaparib | Ovarian cancer (BRCA1/2 +), metastatic breast cancer (BRCA1/2 +), endometrial cancer |
| Tyrosine kinase inhibitors 1. ALK inhibitors 2. BCR-ABL inhibitors 3. EGFR inhibitors 4. VEGFR inhibitors 5. HER2 inhibitors |
1. Crizotinib, alectinib, ceritinib 2. Imatinib 3. Gefitinib 4. Pazopanib, sunitinib, sorafenib 5. Tucatinib |
1. NSCLC 2. CML, ALL, and GIST. 3. Metastatic NSCLC 4. RCC, soft tissue sarcomas, GIST, HCC, and thyroid cancer 5. Advanced or metastatic HER2-positive breast cancer |
| MET inhibitors | Capmatinib | NSCLC |
Note. CDK = cyclin-dependent kinase; HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; PARP = poly(adenosine diphosphate-ribose) polymerase; ALK = anaplastic lymphoma kinase; BCR-ABL = fusion of breakpoint cluster region and Abelson; EGFR = epidermal growth factor receptor; VEGFR = vascular endothelial growth factor receptor; NSCLC = non–small cell lung cancer; CML = chronic myelogenous leukemia; ALL = acute lymphocytic leukemia; GIST = gastrointestinal stromal tumor; RCC = renal cell carcinoma; HCC = hepatocellular carcinoma; MET = mesenchymal-epithelial transition.
Serum creatinine concentration may not be a consistently reliable endogenous marker to assess kidney function in certain clinical settings. In fact, it can be affected by several patient factors unrelated to kidney function, including age, sex, nutritional status, total muscle mass, and tubular secretion. 3 In the context of malignancy, tumor growth is typically associated with a significant decrease in body mass, which may be exacerbated by chemotherapy. 3 Hence, these fluctuations in body weight can result in significant creatinine variations. As the CKD-EPI equation relies on steady-state measurements of serum creatinine, variations in creatinine levels can introduce errors when estimating kidney function. 3 In addition, levels of creatinine do not rise until >50% of active nephrons (GFR <40 mL/min/1.73 m2) are damaged, which can lead to inaccurate staging of CKD and false negatives. 3
In contrast, cystatin C, a cysteine proteinase inhibitor produced by all nucleated cells, has proven to be a reliable alternative method of measuring GFR. 3 Cystatin C is freely filtered by the glomerulus, completed reabsorbed and metabolized by proximal tubular cells. 3 It is not secreted by kidney transporters and not altered by specific patient factors, compared with creatinine. 4 However, cystatin C can be affected by hyperthyroidism, and underlying inflammation. 3 Vermassen et al suggested that cystatin C levels can be affected by certain targeted therapies due to cathepsin D–mediated proteolysis. 25 Cathepsin D, a lysosomal protease, is released from the tumor during lysis and is inhibited by cystatin C, a protease inhibitor, thus causing decreased concentrations of cystatin C. 25 Even though cystatin C has proven to be a reliable endogenous marker that can be routinely used to better estimate GFR, further research needs to determine its role in monitoring kidney function in oncology patients. 25
In regions where endogenous markers, such as cystatin C, are not available, there are also exogeneous biomarkers used to measure and estimate GFR, including iohexol clearance and nuclear renal scans, respectively.
Conclusion
Falsely elevated levels of serum creatinine may have serious implications for clinical management and can lead to unnecessary investigations or inappropriate clinical changes. Serum creatinine elevation and a corresponding decrease in eGFR could be misinterpreted as kidney dysfunction, which could lead to inappropriate dose adjustments or premature cessation of important cancer therapies. Dose adjustments made based on a reduced eGFR can lead to underdosing of patients and inappropriate treatment of their diseases. In addition, multiple drug-to-drug interactions can occur with inhibition of transporter-related tubular secretion; therefore, awareness of these interactions may limit therapeutic options.
In numerous cases, increased serum creatinine levels without a clear underlying etiology may lead to unnecessary invasive investigations, such as a kidney biopsy or early initiation of dialysis. These invasive interventions can have detrimental effects on patient outcomes, quality of life, and overall prognosis. By using non–creatinine-based methods to measure or calculate GFR, we can have a more accurate assessment of kidney function to guide management and treatment plans. However, given that certain therapies have been shown to cause true kidney injury and dysfunction, physicians must judiciously interpret laboratory changes and consider invasive investigations, such as a kidney biopsy, and/or cessation or reduction of therapies when alternative measurements of kidney function do not suggest an underestimation of GFR via serum creatinine.
In conclusion, serum creatinine is widely used in the clinical setting as an endogenous marker to eGFR. However, various targeted cancer treatments have been shown to cause misleading elevations in serum creatinine levels due to inhibition of active tubular secretion. These classes of targeted cancer therapies include CDK inhibitors, PARP inhibitors, TKIs, and MET inhibitors. Therefore, it is imperative to use other endogenous or exogenous markers, such as cystatin C or nuclear renal scans, to better estimate kidney function in these specific circumstances and determine if invasive investigations or treatments may be required if there is a possibility of true kidney injury. Further research is needed to clarify the role of cystatin C in oncology patients being treated with targeted cancer therapies.
Footnotes
Ethical Approval: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Emilie Trinh  https://orcid.org/0000-0001-8479-6656
https://orcid.org/0000-0001-8479-6656
References
- 1. Tolaney S, Lam A, Mukundan S, et al. Abstract P6-15-01: analysis of renal function in MONARCH 1—a phase 2 study of abemaciclib, a CDK4 & 6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for metastatic breast cancer (MBC). Cancer Research. 2017;77:P6-P15. doi: 10.1158/1538-7445.Sabcs16-p6-15-01. [DOI] [Google Scholar]
- 2. Chappell JC, Turner PK, Pak YA, Bacon J, Chiang AY, Royalty J, et al. Abemaciclib inhibits renal tubular secretion without changing glomerular filtration rate. Clin Pharmacol Ther. 2019;105(5):1187-1195. doi: 10.1002/cpt.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem. 2015;68:57-69. doi: 10.1016/bs.acc.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 4. Kar S, Paglialunga S, Islam R. Cystatin C is a more reliable biomarker for determining eGFR to support drug development studies. J Clin Pharmacol. 2018;58(10):1239-1247. doi: 10.1002/jcph.1132. [DOI] [PubMed] [Google Scholar]
- 5. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20-29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471-475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spring LM, Wander SA, Zangardi M, et al. CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep. 2019;21:25. doi: 10.1007/s11912-019-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonilla M, Bashir KA, Jhaveri KD. An elevated serum creatinine in a patient receiving palbociclib. Journal of Onco-Nephrology 2021; 5: 133–135. doi: 10.1177/23993693211021420. [DOI] [Google Scholar]
- 9. Gupta S, Caza T, Herrmann SM, et al. Clinicopathologic features of acute kidney injury associated with CDK4/6 inhibitors. Kidney Int Rep. 2021;7:618-623. doi: 10.1016/j.ekir.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rose M, Burgess JT, O’Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8:564601. doi: 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng F, Zhang Y, Chen S, Weng X, Rao Y, Fang H. Mechanism and current progress of Poly ADP-ribose polymerase (PARP) inhibitors in the treatment of ovarian cancer. Biomed Pharmacother. 2020;123:109661. doi: 10.1016/j.biopha.2019.109661. [DOI] [PubMed] [Google Scholar]
- 12. Zibetti Dal Molin G, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer. 2020;30(1):89-93. doi: 10.1136/ijgc-2019-000714. [DOI] [PubMed] [Google Scholar]
- 13. Bruin MAC, Korse CM, van Wijnen B, et al. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur J Clin Pharmacol. 2021;77:179-188. doi: 10.1007/s00228-020-03070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors: a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10(5):470-481. [DOI] [PubMed] [Google Scholar]
- 15. Bonilla M, Jhaveri KD, Izzedine H. Anaplastic lymphoma kinase inhibitors and their effect on the kidney Canada [published online ahead of print February 26, 2022]. Clinical Kidney Journal. doi: 10.1093/ckj/sfac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arakawa H, Omote S, Tamai I. Inhibitory effect of crizotinib on creatinine uptake by renal secretory transporter OCT2. J Pharm Sci. 2017;106(9):2899-2903. doi: 10.1016/j.xphs.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 17. Gastaud L, Ambrosetti D, Otto J, Marquette CH, Coutts M, Hofman P, et al. Acute kidney injury following crizotinib administration for non-small-cell lung carcinoma. Lung Cancer. 2013;82(2):362-364. doi: 10.1016/j.lungcan.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 18. Izzedine H, Brocheriou I, Amoura Z, et al. Acute tubular injury and renal arterial myocyte vacuolization following crizotinib administration. Kidney Int Rep. 2020;6:526-528. doi: 10.1016/j.ekir.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin S, Gong J, Canas GC, et al. A Phase I study to evaluate the pharmacokinetics and safety of lorlatinib in adults with mild, moderate, and severe renal impairment. Eur J Drug Metab Pharmacokinet. 2022;47:220245. doi: 10.1007/s13318-021-00747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Omote S, Matsuoka N, Arakawa H, et al. Effect of tyrosine kinase inhibitors on renal handling of creatinine by MATE1. Sci Rep. 2018;8:9237. doi: 10.1038/s41598-018-27672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Topletz-Erickson AR, Lee AJ, Mayor JG, Rustia EL, Abdulrasool LI, Wise AL, et al. Tucatinib inhibits renal transporters OCT2 and MATE without impacting renal function in healthy subjects. J Clin Pharmacol. 2021;61(4):461-471. doi: 10.1002/jcph.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Mello RA, Neves NM, Amaral GA, et al. The role of MET inhibitor therapies in the treatment of advanced non-small cell lung cancer. J Clin Med. 2020;9:1918. doi: 10.3390/jcm9061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: will expectations finally be MET. J Thorac Oncol. 2017;12(1):15-26. doi: 10.1016/j.jtho.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohan A, Herrmann SM. Capmatinib-induced pseudo-acute kidney injury: a case report. Am J Kidney Dis. 2021;79:120-124. doi: 10.1053/j.ajkd.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 25. Vermassen T, Geboes K, De Man M, et al. Neither creatinine- nor cystatin C-estimated glomerular filtration rate is optimal in oncology patients treated with targeted agents. Nephrol Dial Transplant. 2018;33:402-408. doi: 10.1093/ndt/gfx063. [DOI] [PubMed] [Google Scholar]