Abstract
Cirrhosis consists of two main stages: compensated (asymptomatic) and decompensated, the latter with a higher mortality. Variceal hemorrhage, together with ascites or encephalopathy, or both, are events that define cirrhosis decompensation and are driven by portal hypertension. The approach and management of patients with compensated cirrhosis has been mostly focused on preventing variceal hemorrhage in those who have high-risk varices on endoscopy. Recent studies suggest a paradigm shift aimed at preventing all decompensating events, not only variceal hemorrhage, in patients with cirrhosis and clinically significant portal hypertension identified via noninvasive measures such as liver stiffness and platelet count. In these patients, nonselective beta-blockers have been shown to prevent ascites (the most common decompensating event) and variceal growth. Variceal hemorrhage has a high mortality rate and even though advances in diagnostic approach and standard of care over the past decades have led to a decrease in mortality, it is still high with a 6-week mortality rate of 15–20%. Survival has improved with the preemptive placement of the transjugular intrahepatic portosystemic shunt in patients at high risk of failing standard therapy. In this review, we provide an overview of the pathophysiology and bases for therapy of portal hypertension and varices, the diagnostic approach and management of compensated cirrhosis with clinically significant portal hypertension, and the management of acute variceal hemorrhage as well as prevention strategies for variceal hemorrhage recurrence.
Keywords: Clinically significant portal hypertension, nonselective beta-blockers, portal hypertension, transjugular intrahepatic portosystemic shunt, variceal hemorrhage, varices
Risk stratification of cirrhosis
Cirrhosis is the end stage of chronic liver disease of any etiology and is divided into two distinct clinical stages: compensated and decompensated. 1 Decompensated cirrhosis is defined by the presence of a clinically evident decompensating event such as ascites, variceal hemorrhage (VH), or hepatic encephalopathy.
Compensated cirrhosis is the longer stage where these events have not occurred. 2 In the compensated stage, the median survival can exceed 12 years, while in the decompensated stage it is only 1.8 years. 3 If using the Child–Turcotte–Pugh (CTP) classification, CTP-A class are compensated, while those in CTP-B/C class are mostly decompensated. 4 A recent term, compensated advanced chronic liver disease (cACLD), has been proposed to include not only patients with a formal histological diagnosis of cirrhosis but those in whom noninvasive tests such as liver stiffness measurement (LSM) in a compensated patient would indicate the presence of portal hypertension and a higher risk of decompensation and death. Currently both terms, cACLD and compensated cirrhosis, are acceptable. 2
Portal hypertension is the main consequence of cirrhosis and the main driver of decompensation. It results from increased intrahepatic resistance and increased portal venous inflow. Portal hypertension is defined by a hepatic venous pressure gradient (HVPG) >5 mmHg. An HVPG > 10 mmHg is the strongest predictor of the development of varices 5 and of any decompensating event. 6 Therefore, this pressure threshold defines an entity called ‘clinically-significant portal hypertension (CSPH)’.
The HVPG is measured invasively, with a balloon catheter advanced through the jugular vein to the hepatic vein and measuring both a free pressure and a wedge pressure. The difference represents the pressure gradient between the portal vein and the free hepatic vein pressures and is the most utilized/validated way to estimate portal pressure. Given the limitations of HVPG being an invasive method, noninvasive assessment of CSPH using LSMs by transient elastography, platelet count, and spleen size or, or all, stiffness have been studied and validated. An LSM of >25 kPa or LSM between 20 and 25 kPa with platelet count <150 x103 can rule in CSPH (determined by HVPG) in most etiologies of cirrhosis. 7 The one exception is obese patients with NASH cirrhosis, where the positive predictive value of the model used was lower and therefore a different model including body mass index was used to construct a nomogram that can be applied in patients with obesity and NASH cirrhosis. In the same study, an LSM < 15 kPa in combination with platelet count >150 x103 can rule out CSPH in most cases. 7 In addition, for all etiologies, the presence of gastroesophageal varices on esophagogastroduodenoscopy (EGD) and the presence of portosystemic collaterals on cross-sectional imaging are both indicative of CSPH. 2 The usefulness of the noninvasive measures relies on being able to better triage and identify the population at risk of cirrhosis decompensation/death to target therapies aimed at preventing decompensation as well as avoiding unnecessary procedures such as EGD in very low-risk populations.
Epidemiology/natural history
Esophageal varices are present in 50–60% of patients with compensated cirrhosis and up to 85% in patients with decompensated cirrhosis. 8 Variceal size, red wale marks on varices, and advanced liver disease (CTP-B/C) are all risk factors for variceal hemorrhage (VH). 9 A first VH occurs at a rate of 10–15% per year, depending on the individual risk factors 10 and a recurrent VH at a rate of up to 60% per year. 11 Advancements from recent years in terms of treatment and prevention of VH have decreased the mortality from around 40% back in the 1980s 12 to a 6-week mortality of 15–20% in most recent years. 13 However, the most common decompensating event, and the one associated with a higher mortality in cirrhosis is ascites, and therefore the goals in the therapy of the patient with compensated cirrhosis have shifted from preventing VH to preventing decompensation (ascites, VH, and encephalopathy).
Pathophysiology and bases for therapy
Portal hypertension results mainly from two mechanisms: (1) increased intrahepatic resistance to portal flow and (2) increased portal venous flow (Figure 1). (1) The increased intrahepatic resistance to portal flow is the consequence of the combination of (1) a structural component secondary to regenerative nodules and fibrous tissue, which is responsible for 70% of the increased intrahepatic resistance and (2) a functional component secondary to endothelial dysfunction and decreased nitric oxide availability leading to increased hepatic vascular tone. 14 The structural component can be targeted by therapies directed at the underlying etiology of cirrhosis or antifibrotic agents. 15 The functional component can be targeted by therapies aimed at vasodilating the intrahepatic vasculature such as nitrates, α1 antagonists, and angiotensin-II blockers; 16 however, these therapies are limited by their systemic hypotensive effect. Statins have been proposed as a unique therapy, given they have both antifibrotic properties and improve endothelial dysfunction,15,17 without a hypotensive effect.
Figure 1.
Pathophysiology of portal hypertension and mechanism of action of various therapies used in the management of portal hypertension and variceal hemorrhage.
CSPH: clinical significant portal hypertension; EVL: endoscopic variceal ligation; HVPG: hepatic venous portal gradient; NSBB: nonselective beta-blockers; PH: portal hypertension; TIPS: transjugular intrahepatic portosystemic shunt; VH: variceal hemorrhage.
*Carvedilol has additional α-1 blockade effect.
Source: Created with BioRender.com.
The second mechanism driving portal hypertension is the increased venous portal flow. With portal hypertension, collaterals begin to develop between the portosystemic circulation, the most important being gastroesophageal varices due to their mortality rate when ruptured. Concomitant to the development of collaterals, there is splanchnic vasodilation that leads to increased blood flow in the gut and the portal system. Vasodilation also occurs in the systemic circulation creating an effective hypovolemia state and triggering the activation of the renin–angiotensin–aldosterone system, leading to sodium and water retention, and increased cardiac output creating overall a hyperdynamic circulatory state that maintains an increased venous portal inflow. 15 Therapies aimed at vasoconstricting the splanchnic circulation are the current mainstay of treatment for varices, VH, and portal hypertension. Non-selective beta-blockers (NSBBs) such as nadolol, propranolol, and carvedilol act mainly through β2 blocking effect leading to unopposed α1 vasoconstriction of the splanchnic circulation. In addition, β1 blocking effect leads to reduced cardiac output and reduced portal flow. 18 Carvedilol also has α1 blocking effect which generates intrahepatic vasodilation and is therefore preferred if a person can tolerate it in terms of systemic hypotension. 19 In the acute setting of VH, parenteral splanchnic vasoconstrictors are used: current available options include somatostatin and its analogs octreotide and vapreotide, and vasopressin and its analogue terlipressin. 18 Availability of these agents varies by country. In the United States, only octreotide is available.
In patients with decompensated cirrhosis, another approach to decreasing portal pressure is by creating a shunt that connects the high-pressure portal vein with the lower pressure hepatic vein bypassing the resistance of the liver. This is done by interventional radiologists using a transjugular approach. This shunt is therefore called ‘transjugular intrahepatic portosystemic shunt’ (TIPS). Because blood is shunted away from the liver, the main complication is hepatic encephalopathy that results from direct shunting of toxic metabolites from the portal to the systemic circulation. 20 Other less common complications include shunt dysfunction from occlusion (which has decreased since the widespread implementation of covered stents), 21 cardiac overload, development/worsening of pulmonary hypertension, 22 and liver failure. 23 Therefore, absolute contraindications to TIPS placement include congestive heart failure, severe pulmonary hypertension, multiple hepatic cysts, uncontrolled systemic infection or sepsis, and unrelieved biliary obstruction. 24 Once a patient has a TIPS, there is no need for other portal pressure-reducing therapies given that the HVPG significantly decreases and can even normalize, 25 although NSBB may be added when the post-TIPS target portal pressure gradient is not reached.
In terms of local therapies for varices and VH, endoscopic variceal ligation (EVL) continues to be the main approach to control active bleeding. Rubber bands are placed around the varices in multiple sessions until they are obliterated. EVL has replaced the use of sclerotherapy that consisted in the injection of a sclerosant agent into varices. However, EVL and sclerotherapy are local, transient therapies and because they do not ameliorate portal hypertension, varices will recur and, more importantly, in compensated patients EVL/sclerotherapy will not prevent decompensation. Other local therapies that are used mostly in emergencies of a non-responding VH are balloon tamponade and expandable esophageal stents which are both used as a bridge therapy to a more long-term solution (TIPS or less frequently transplant). 26
Management of compensated cirrhosis with mild portal hypertension
Patients with compensated cirrhosis and mild portal hypertension (5–10 mmHg) are at a very low risk (if any) of decompensation as they have not yet developed the splanchnic vasodilation and hyperdynamic circulatory state. Consequently, therapies in this stage are limited to targeting intrahepatic structural resistance mostly addressing the underlying etiology of cirrhosis. NSBB and other vasoconstrictors do not play a role in mild portal hypertension.
Management of compensated cirrhosis with CSPH
NSBBs have been the main therapy for portal hypertension for the past 40 years; today, they still are, but the evidence and indications have changed and continue to progress with the years. Until very recently, guidance recommendations for using NSBB in patients with cirrhosis had the purpose of preventing VH in patients with cirrhosis and high risk of VH. These are defined as those with compensated cirrhosis and large varices or with small varices and red wale marks or patients with decompensated cirrhosis with varices of any size. 4 Endoscopic variceal ligation (EVL) is an alternative therapy to prevent VH in patients with medium/large varices and is the only therapy when NSBBs are not tolerated or are contraindicated. The main side effects of NSBB are fatigue, shortness of breath, and in patients with ascites, a decrease in renal perfusion pressure. 27 The combination of NSBB and EVL is not superior and can increase adverse effects.
Importantly, in the recent Baveno portal hypertension consensus conference, the recommendation was to consider the use of NSBB in patients with compensated cirrhosis and evidence of CSPH with the purpose of preventing cirrhosis decompensation (i.e. not only VH but also ascites). 28 This paradigm shift was mostly based on the results of the PREDESCI trial, a multicenter randomized trial studying the effect of NSBB in preventing decompensation of cirrhosis with portal hypertension. 29 In the study, 201 patients with cirrhosis and CSPH with none or small varices, were randomized to NSBB (propranolol 40–160 mg twice a day or carvedilol 6.25–25 mg daily) versus placebo. The results showed that the primary outcome, which was any decompensation (ascites, VH, hepatic encephalopathy) or death, was significantly lower in the NSBB group compared with the placebo group (17% versus 27%, HR 0.51, 95% CI 0.26–0.97). 29 The difference was mostly driven by ascites, which is usually the first and most common decompensation to occur. Progression to large varices (HR 0.60, 95% CI 0.30–1.21) was also lower in the NSBB group. In addition, carvedilol seemed to outperform propranolol with lower rates of ascites, death, and greater reduction in HVPG. The new paradigm would aim at using NSBB to prevent any decompensation rather than only VH and therefore patients who would benefit from carvedilol would be not only those with high-risk varices but also those with compensated cirrhosis and CSPH, which are at the highest risk of developing any decompensation. 6
In compensated patients who cannot receive NSBB, EGD should be performed and, in the presence of high-risk varices, EVL would be performed to prevent variceal hemorrhage (Figure 2 ).
Figure 2.
(a) and (b) Approach to compensated cirrhosis relying on less invasive strategies to identify patients with and without clinical significant portal hypertension (CSPH). Goal of patient with clinical significant portal hypertension now is the prevention of any decompensation. (c) Approach to patient presenting with ascites and no history of variceal hemorrhage.
CSPH, clinical significant portal hypertension; EVL, esophageal varices ligation; KPa, kilopascals; LVP, large-volume paracentesis; NSBB, nonselective beta-blocker; plt, platelets.
aSmall (<5 mm), no red signs, no CTP-C.
bLarge (>5 mm), red spot signs, CTP-C LSM: liver stiffness measurement.
*Based on the PREDISCI trial carvedilol outperformed propranolol.
Source: Created with BioRender.com.
Patients with compensated cirrhosis and mild portal hypertension do not require any specific therapy as they are unlikely to decompensate.
Another therapy that can potentially decrease risk of decompensation and continues to be studied are the statins. As mentioned above, statins, by increasing the bioavailability of nitric oxide in the intrahepatic circulation, will reduce intrahepatic resistance and, thereby, portal pressure. 30 A proof-of-concept study showed that a 1-month course of simvastatin in patients with cirrhosis and CSPH significantly reduced HVPG and improved liver perfusion. 31 The use of statins in patients with cirrhosis has been judicious given their potential for hepatotoxicity; however, studies have favored their safety in patients with chronic liver disease. 32 In a large retrospective propensity-matched cohort study, statin use was associated with a lower risk of decompensation, mainly VH and ascites, and a lower risk of death in patients with Hep C compensated cirrhosis. 33 The results of randomized controlled trials (RCTs) of statins in preventing decompensation in cirrhosis are eagerly awaited.
Prevention of variceal bleeding in patients with ascites
In patients who present with ascites (i.e. already decompensated) but who have never bled from varices should have an upper endoscopy to determine the presence of high-risk varices. If these are present, guidelines recommend NSBB or EVL. 4 However, per the recent Baveno VII conference, NSBB would be preferable as they have been shown to prevent further decompensation and death in patients with ascites. If ascites is recurrent (>3 large-volume paracentesis within 1 year), TIPS placement should be considered and, if placed, neither NSBB nor EVL would be necessary 28 (Figure 2 ).
Management of acute variceal hemorrhage
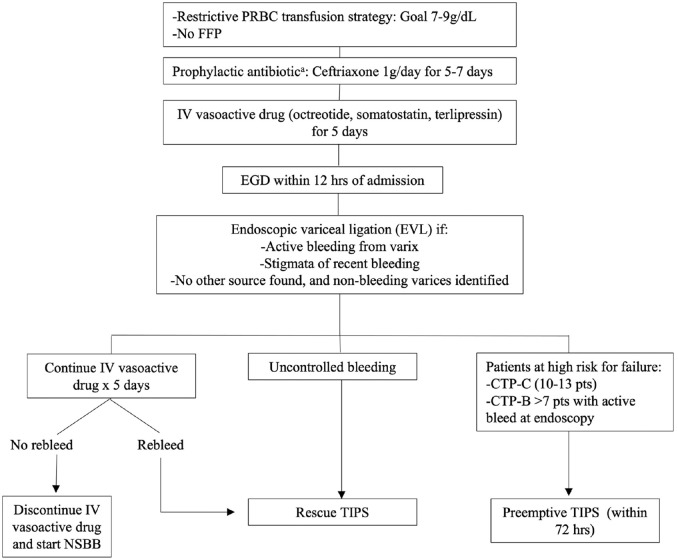
Acute VH is a life-threatening emergency which, despite therapeutic advances in the past decade, continues to have a high 6-week mortality rate of up to 20%. 34 Overall, the current approach is a multimodal strategy aiming to control the acute bleeding, prevent rebleeding, and decrease 6-week mortality rate, which is the main treatment outcome by consensus. The management consists of the following steps (Figure 3):
Figure 3.
Acute variceal hemorrhage management algorithm.
CTP, Child–Turcotte–Pugh; FFP, fresh frozen plasma; NSBB, nonselective beta-blockers; PRBC, packed red blood cells; TIPS, transjugular intrahepatic portosystemic shunt.
aBefore starting prophylactic antibiotic ideally perform diagnostic paracentesis to increase yield in the case of spontaneous bacterial peritonitis.
(a) Adequate resuscitation. Proper intravenous access and intubation in patients with massive hematemesis for airway protection.
(b) Transfusion of red blood cells using a restrictive strategy meaning initiating transfusion when hemoglobin is less than 7 g/dl and maintaining a goal of 7–9 g/dl. This approach had lower rebleeding and mortality rates, especially in those with CTP A and B when compared with a more liberal approach. 35
(c) Short-term administration of prophylactic antibiotics. RCTs have showed the use of prophylactic antibiotics that lead to significantly lower rates of infection, death, and early rebleeding.36,37 Intravenous ceftriaxone proved to be more effective than oral norfloxacin, 38 and given the higher rates of quinolone resistant organisms and discontinuation of norfloxacin use in the United States, IV ceftriaxone for 5–7 days is the recommended choice. It is important to note that an initial infection, especially spontaneous bacterial peritonitis, could have led to the VH in the first place, therefore ideally, diagnostic paracentesis should be done before administering prophylactic antibiotics.
(d) Intravenous infusion of splanchnic vasoactive medications such as octreotide, somatostatin, or terlipressin to be continued for 5 days. The use of these agents has been associated with lower 7-day all-cause mortality and lower transfusion requirements. 39 Any available agent can be used as no difference in controlled bleeding, rebleeding, or mortality rate was identified in a noninferiority trial. 40
(e) Transfusion of fresh frozen plasma is not recommended as it will not correct coagulopathy and may lead to volume overload and worsening of portal hypertension. 28
(f) IV proton pump inhibitors can be initiated when a patient presents with upper gastrointestinal bleeding. However, they should not be continued once a variceal source is identified, unless there is a strict indication to continue them. 28
(g) EGD must be done within the first 12 h of admission and once the patient is hemodynamically stable. EVL is indicated when the source of upper gastrointestinal hemorrhage is variceal, that is, in the presence of the following endoscopic findings: 4
Active bleeding from a varix.
Stigmata of recent bleeding (white nipple or clot on a varix).
If there is no other source of bleeding and nonbleeding varices are identified:
(a) If after EGD with EVL the bleeding is controlled, IV vasoactive agents should be continued for 2–5 days and upon their discontinuation the patient should be started on NSBB, titrated to HR 55–60 and SBP > 90 mmHg. Once IV vasoactive agents are discontinued, IV prophylactic antibiotic can also be discontinued.
(b) If during EGD the bleeding cannot be controlled, a self-expandable metal stent or a balloon tamponade can be used as a bridge to rescue TIPS. Self-expandable esophageal stents have greater efficacy and less complications than balloon tamponade and can remain in place longer than balloon tamponade (7 days versus 24 h). 41
(c) If after EGD with EVL the patient has a recurrent VH while still hospitalized, a rescue TIPS should be performed. Patients that are at a higher risk for 5-day failure (rebleeding or death) and who would therefore require a rescue TIPS are those in CTP-C. 42 However, rescue TIPS in these patients is associated with a high mortality.
(d) A preemptive TIPS (pTIPS), that is, a TIPS placed before the patient rebleeds ( ‘fails’) and requires a rescue TIPS has been shown to be associated with an improved survival in a select group of patients at a high risk of failure. The definition of these patients is still fluid. An initial randomized trial in mostly alcohol- and HCV-associated cirrhosis, showed pTIPS done within 72 h of admission, in patients with CTP-C (10–13 pts) and CTP-B with active bleeding during endoscopy had a 25% absolute risk reduction in mortality 43 A subsequent RCT and a recent meta-analysis also supported the survival benefit of pTIPS in patients CTP-C (10–13 pts) and in patients CTP-B > 7 who were actively bleeding at endoscopy.44,45 As such, risk stratification using CTP score is very important as it will affect the therapeutic decisions. A limitation overall from all pTIPS studies has been the need to place TIPS within 72 h of admission, which is complicated in many centers and the exclusion of an important proportion of patients commonly seen in the clinical setting including those older than 70–75 years, with heart failure, CTP-C (14–15 pts) creatinine above 2.5 mg/dl, hepatocellular carcinoma beyond Milan among other exclusion criteria.
Prevention of variceal hemorrhage recurrence
After patients survive a first episode of VH, the 1-year risk of a recurrent VH can be as high as 60%. The current recommendation for the prevention of VH recurrence is to combine both NSBB and EVL as the combination has resulted in reduced rebleeding compared with either treatment alone. 11 Initially studies used NSBB plus isosorbide-mononitrate given the greater reduction in HVPG; however, a meta-analysis showed the combination was associated with more side effects and was no different in terms of mortality or rebleeding compared with NSBB alone. 46 When comparing combination therapy versus NSBB or EVL alone, the difference in rebleeding was much more significant compared with EVL alone than with NSBB alone, suggesting the stronger effect in the combination therapy is driven by the NSBB. An individual patient meta-analysis which risk-stratified the patients found that combination therapy also had a reduced mortality rate compared with EVL alone in patients with CTP-B/C. 47 The same meta-analysis confirmed the essential role of NSBB in the combination therapy. Because NSBB may be associated with a decrease in blood pressure and in renal perfusion pressure in decompensated patients, particularly those with refractory ascites, NSBB should be used cautiously and NSBB dose-reduced or discontinued if MAP < 65 mmHg or SBP < 90 mmHg.27,28
TIPS is indicated in patients who experience variceal rebleeding while on NSBB + EVL, that is, it is second-line therapy. TIPS could be considered as first-line therapy in the prevention of rebleeding in patients who also have recurrent ascites (>3 large-volume paracentesis within 1 year) 4,28 or in patients who cannot tolerate or have a contraindication to NSBB (and would be left on EVL alone).
Patients who had TIPS done during hospitalization for VH (either rescue or pTIPS) should not receive NSBB nor EVL for the prevention of recurrent VH, as the ultimate portal pressure-reducing method (TIPS) is already in place.
Gastric varices
Epidemiology and classification
Gastric varices (GV) are present in approximately 20% of patients with cirrhosis. Sarin’s classification is the most common classification used and divides GV into four main types based on localization: 48
(a) Gastroesophageal varices type 1 (GOV1) are esophageal varices that extend to the lesser curvature of the stomach and are the most common type (75% of GV);
(b) Gastroesophageal varices type 2 (GOV2) are esophageal varices that extend to the fundus of the stomach;
(c) Isolated GV type 1 (IGV1) are localized in the fundus of the stomach;
(d) Isolated GV type 2 (IGV2) are located somewhere else in the stomach (the least frequent).
GOV2 and IGV1 are usually referred to as cardiofundal varices (CFV). While patients with GOV1 varices should be treated in the same manner as described above for esophageal varices, patients with CFV require a different management. Bleeding from CFV is more severe (higher transfusion requirements and higher mortality) compared with bleeding from esophageal/GOV-1 varices) and the vascular anatomy is often different than that of esophageal/GOV1 varices.48,49
Because bleeding from CFV is rare, the evidence supporting recommendations for their management is less and of lower quality compared with evidence for esophageal varices because trials are fewer, sample size is usually small, and there is more vascular heterogeneity.
Prevention of GFV hemorrhage
There is only one RCT of 89 patients comparing therapies to prevent a first variceal bleed or death. The results showed injection of cyanoacrylate had lower probability of bleeding (13%) compared with NSBB (28%) and observation (45%). There was no difference in survival between injection of cyanoacrylate and NSBB. 50 Based on the latest Baveno VII consensus, NSBB would be indicated in these patients to prevent any decompensation28,50. Further studies are needed comparing other types of therapies in addition to NSBB. Currently, there is no indication for balloon-occluded retrograde (antegrade) transvenous obliteration (BRTO or BATO) or TIPS for the prevention of a first GFV hemorrhage. 28
Management of acute variceal hemorrhage and prevention of rebleeding
Prior to diagnostic endoscopy, the initial management of GV hemorrhage is the same as the management of esophageal VH in terms of resuscitation, IV splanchnic vasoconstrictors, and initiation of antibiotic. Endoscopic management does differ because EVL can sometimes be challenging with GFV given their larger size and the thicker gastric mucosa that makes the complete suction of the varix into the ligator difficult and increases the chance of post banding ulcers and severe hemorrhage. Therefore, placement of gastric compression balloons (Sengstaken–Blakemore or Linton–Nachlas tubes) and endoscopic injection of cyanoacrylate glue are temporizing therapies. The most feared complication of cyanoacrylate injection is glue embolization leading to pulmonary embolism or stroke. In the largest series of endoscopic injection of cyanoacrylate to date, embolization rate was reported to be 0.7%. 51 In the United States, injection of cyanoacrylate is not approved by the Food and Drug Administration (FDA) for the treatment of CFV hemorrhage; however, in clinical practice it is routinely used. 52
Other efficient endoscopic temporizing methods for bleeding CFV include endoscopic ultrasound with combined cyanoacrylate injection and coil insertion. Relatively large case series have demonstrated its safety and efficacy 53 and potential benefits include real-time assessment of Doppler flow and possibly reduced risk of embolization; however, there are no studies directly comparing it with standard endoscopic injection of cyanoacrylate. 52 These temporizing therapies vary in clinical practice depending on resources, specialists availability, and training at different centers.
Ideally, prior to definitive therapy, all patients with CFV should have abdominal imaging (preferably contrast-enhanced CT or MRI) to determine vascular anatomy and the presence (or not) of spleno/gastro-renal shunts which will guide further therapy.28,52
Definitive therapies for bleeding CFV consist of interventional radiology procedures such as TIPS and retrograde transvenous venous obliteration (RTO).
TIPS has been shown to be very effective for initial hemostasis of GV hemorrhage with success rates of >90%. 54 TIPS can be combined with variceal embolization to control bleeding or to reduce the risk of recurrent bleeding, especially in patients where portal flow remains diverted to collaterals. 28 RTO is a procedure that can treat fundal varices with a large gastro-splenorenal collateral. 55 It consists of retrograde cannulation of the left renal vein via the jugular or femoral vein and balloon occlusion (BRTO) with slow infusion of an sclerosant agent to obliterate the gastro-splenorenal collateral and the fundal varices 56 or occlusion using a vascular plug (PARTO) or coils (CARTO). The theoretical advantage over TIPS is that RTO, by occluding a large portosystemic shunt, has no increased risk for worsening hepatic encephalopathy and, in fact, may improve existing encephalopathy.28,57,58 However, RTO does have a risk of increased portal pressure that may worsen ascites or bleeding from esophageal varices. As such, some centers will combine both and place a TIPS after BRTO to compensate for the increase in portal pressure after RTO. 59 In patients who have bled from CFV, TIPS or BRTO are recommended by the AASLD as first-line treatments to prevent rebleeding.4,55,56 Currently there are no RCT comparing BRTO with other therapies and overall, there is insufficient high-quality data comparing the different therapies for GV hemorrhage.
In conclusion, the management of varices and VH in patients with cirrhosis continues to be of extreme importance due to the ongoing high mortality associated with VH. Risk stratification is essential to decide on the best preventive/therapeutic approach. Studies from the past decades have allowed us to reach the current recommendations that not only include management of variceal hemorrhage but also prevention of the development, not only of VH, but also of ascites, the most common event complicating portal hypertension. Ongoing and future studies will further characterize populations at risk, increasingly relying on noninvasive methods and leading to optimized management to better target specific populations.
Footnotes
Ethics approval and consent to participate: Not applicable
Consent for publication: The authors provide consent for publication.
Author contribution(s): Maria P. Diaz-Soto: Conceptualization; Writing – original draft.
Guadalupe Garcia-Tsao: Conceptualization; Writing – original draft; Writing – review & editing.
ORCID iDs: Maria P. Diaz-Soto  https://orcid.org/0000-0003-3713-8030
https://orcid.org/0000-0003-3713-8030
Guadalupe Garcia-Tsao  https://orcid.org/0000-0002-6175-8216
https://orcid.org/0000-0002-6175-8216
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable
Contributor Information
Maria P. Diaz-Soto, Hospital Medicine Unit, Massachusetts General Hospital, Boston, MA, USA
Guadalupe Garcia-Tsao, Section of Digestive Diseases, Yale School of Medicine, PO Box 208056, 333 Cedar Street, LMP 1080, New Haven, CT 06520-8019, USA; Section of Digestive Diseases, VA-Connecticut Healthcare System, West Haven, CT, USA.
References
- 1. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44: 217–231. [DOI] [PubMed] [Google Scholar]
- 2. de Franchis R. and Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop – stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63: 743–752. [DOI] [PubMed] [Google Scholar]
- 3. D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014; 39: 1180–1193. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 5. Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353: 2254–2261. [DOI] [PubMed] [Google Scholar]
- 6. Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133: 481–488. [DOI] [PubMed] [Google Scholar]
- 7. Pons M, Augustin S, Scheiner B, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol 2021; 116: 723–732. [DOI] [PubMed] [Google Scholar]
- 8. Kovalak M, Lake J, Mattek N, et al. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc 2007; 65: 82–88. [DOI] [PubMed] [Google Scholar]
- 9. North Italian Endoscopic Club for the S and Treatment of Esophageal V. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices: a prospective multicenter study. N Engl J Med 1988; 319: 983–989. [DOI] [PubMed] [Google Scholar]
- 10. D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999; 19: 475–505. [DOI] [PubMed] [Google Scholar]
- 11. Bosch J, Garcia-Pagan JC. Prevention of variceal rebleeding. Lancet 2003; 361: 952–954. [DOI] [PubMed] [Google Scholar]
- 12. Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology 1981; 80: 800–809. [PubMed] [Google Scholar]
- 13. Reverter E, Tandon P, Augustin S, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology 2014; 146: 412–419. [DOI] [PubMed] [Google Scholar]
- 14. Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol 2007; 46: 927–934. [DOI] [PubMed] [Google Scholar]
- 15. Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol 2015; 62: S121–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhathal PS, Grossman HJ. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol 1985; 1: 325–337. [DOI] [PubMed] [Google Scholar]
- 17. Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol 2007; 46: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 18. Bosch J, Berzigotti A, Garcia-Pagan JC, et al. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol 2008; 48: S68–S92. [DOI] [PubMed] [Google Scholar]
- 19. Sinagra E, Perricone G, D’Amico M, et al. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther 2014; 39: 557–568. [DOI] [PubMed] [Google Scholar]
- 20. Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol 2008; 103: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 21. Bureau C, Garcia Pagan JC, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int 2007; 27: 742–747. [DOI] [PubMed] [Google Scholar]
- 22. Wannhoff A, Hippchen T, Weiss CS, et al. Cardiac volume overload and pulmonary hypertension in long-term follow-up of patients with a transjugular intrahepatic portosystemic shunt. Aliment Pharmacol Ther 2016; 43: 955–965. [DOI] [PubMed] [Google Scholar]
- 23. Luca A, Miraglia R, Maruzzelli L, et al. Early liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end-stage liver disease score of 12 or less: incidence, outcome, and prognostic factors. Radiology 2016; 280: 622–629. [DOI] [PubMed] [Google Scholar]
- 24. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51: 306. [DOI] [PubMed] [Google Scholar]
- 25. Allaire M, Walter A, Sutter O, et al. TIPS for management of portal-hypertension-related complications in patients with cirrhosis. Clin Res Hepatol Gastroenterol 2020; 44: 249–263. [DOI] [PubMed] [Google Scholar]
- 26. Hubmann R, Bodlaj G, Czompo M, et al. The use of self-expanding metal stents to treat acute esophageal variceal bleeding. Endoscopy 2006; 38: 896–901. [DOI] [PubMed] [Google Scholar]
- 27. Tellez L, Ibanez-Samaniego L, Perez Del Villar C, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol 2020; 73: 1404–1414. [DOI] [PubMed] [Google Scholar]
- 28. de Franchis R, Bosch J, Garcia- Tsao G, et al. Baveno VII – Renewing consensus in portal hypertension: report of the Baveno VII Consensus Workshop – personalized care in portal hypertension. J Hepatol 2022; 76: 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villanueva C, Albillos A, Genesca J, et al. Beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2019; 393: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 30. Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology 2004; 126: 749–755. [DOI] [PubMed] [Google Scholar]
- 31. Abraldes JG, Albillos A, Banares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 2009; 136: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 32. Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007; 46: 1453–1463. [DOI] [PubMed] [Google Scholar]
- 33. Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology 2016; 150: 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ardevol A, Ibanez-Sanz G, Profitos J, et al. Survival of patients with cirrhosis and acute peptic ulcer bleeding compared with variceal bleeding using current first-line therapies. Hepatology 2018; 67: 1458–1471. [DOI] [PubMed] [Google Scholar]
- 35. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368: 11–21. [DOI] [PubMed] [Google Scholar]
- 36. Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology 1999; 29: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 37. Hou MC, Lin HC, Liu TT, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology 2004; 39: 746–753. [DOI] [PubMed] [Google Scholar]
- 38. Fernandez J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131: 1049–1056; quiz 1285. [DOI] [PubMed] [Google Scholar]
- 39. Wells M, Chande N, Adams P, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther 2012; 35: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 40. Seo YS, Park SY, Kim MY, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology 2014; 60: 954–963. [DOI] [PubMed] [Google Scholar]
- 41. Escorsell A, Pavel O, Cardenas A, et al. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: a multicenter randomized, controlled trial. Hepatology 2016; 63: 1957–1967. [DOI] [PubMed] [Google Scholar]
- 42. Amitrano L, Guardascione MA, Manguso F, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol 2012; 107: 1872–1878. [DOI] [PubMed] [Google Scholar]
- 43. Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362: 2370–2379. [DOI] [PubMed] [Google Scholar]
- 44. Nicoara-Farcau O, Han G, Rudler M, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: a meta-analysis of individual patient data. Gastroenterology 2021; 160: 193.e110–205.e. [DOI] [PubMed] [Google Scholar]
- 45. Lv Y, Yang Z, Liu L, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol 2019; 4: 587–598. [DOI] [PubMed] [Google Scholar]
- 46. Gluud LL, Langholz E, Krag A. Meta-analysis: isosorbide-mononitrate alone or with either beta-blockers or endoscopic therapy for the management of oesophageal varices. Aliment Pharmacol Ther 2010; 32: 859–871. [DOI] [PubMed] [Google Scholar]
- 47. Albillos A, Zamora J, Martinez J, et al. Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis. Hepatology 2017; 66: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992; 16: 1343–1349. [DOI] [PubMed] [Google Scholar]
- 49. Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology 2004; 126: 1175–1189. [DOI] [PubMed] [Google Scholar]
- 50. Mishra SR, Sharma BC, Kumar A, et al. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol 2011; 54: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 51. Cheng LF, Wang ZQ, Li CZ, et al. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol 2010; 8: 760–766. [DOI] [PubMed] [Google Scholar]
- 52. Henry Z, Patel K, Patton H, et al. AGA clinical practice update on management of bleeding gastric varices: expert review. Clin Gastroenterol Hepatol 2021; 19: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 53. Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. Gastrointest Endosc 2016; 83: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 54. Chau TN, Patch D, Chan YW, et al. ‘Salvage’ transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology 1998; 114: 981–987. [DOI] [PubMed] [Google Scholar]
- 55. Tsauo J, Noh SY, Shin JH, et al. Retrograde transvenous obliteration for the prevention of variceal rebleeding in patients with hepatocellular carcinoma: a multicentre retrospective study. Clin Radiol 2021; 76: 681–687. [DOI] [PubMed] [Google Scholar]
- 56. Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol 2012; 29: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Imai Y, Nakazawa M, Ando S, et al. Long-term outcome of 154 patients receiving balloon-occluded retrograde transvenous obliteration for gastric fundal varices. J Gastroenterol Hepatol 2016; 31: 1844–1850. [DOI] [PubMed] [Google Scholar]
- 58. Lee SJ, Kim SU, Kim MD, et al. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol 2017; 32: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 59. Saad WE, Wagner CC, Lippert A, et al. Protective value of TIPS against the development of hydrothorax/ascites and upper gastrointestinal bleeding after balloon-occluded retrograde transvenous obliteration (BRTO). Am J Gastroenterol 2013; 108: 1612–1619. [DOI] [PubMed] [Google Scholar]