Abstract
Intestinal strictures remain one of the most intractable and common complications of Crohn’s disease (CD). Approximately 70% of CD patients will develop fibrotic strictures after 10 years of CD diagnosis. Since specific antifibrotic therapies are unavailable, endoscopic balloon dilation and surgery remain the mainstay treatments despite a high recurrence rate. Besides, there are no reliable methods for accurately evaluating intestinal fibrosis. This is largely due to the fact that the mechanisms of initiation and propagation of intestinal fibrosis are poorly understood. There is growing evidence implying that the pathogenesis of stricturing CD involves the intricate interplay of factors including aberrant immune and nonimmune responses, host-microbiome dysbiosis, and genetic susceptibility. Currently, the progress on intestinal strictures has been fueled by the advent of novel techniques, such as single-cell sequencing, multi-omics, and artificial intelligence. Here, we perform a timely and comprehensive review of the substantial advances in intestinal strictures in 2021, aiming to provide prompt information regarding fibrosis and set the stage for the improvement of diagnosis, treatment, and prognosis of intestinal strictures.
Keywords: Crohn’s disease, diagnosis, fibrosis, intestinal strictures, pathogenesis, treatment
Introduction
Crohn’s disease (CD) is a chronic relapsing and remitting inflammatory disorder involving the whole gastrointestinal tract. 1 Approximately 70% of CD patients will develop stricturing or penetrating complications after 10 years of CD diagnosis. 2 A real-world study found that CD patients with fibrostenosis were at significantly higher risk for hospitalization, steroid dependency, repeated endoscopy, and surgery, as well as higher healthcare costs, compared with CD patients without fibrostenosis. 3 Therefore, intestinal strictures are becoming one of the most vexing and complex complications of CD.
Although anti-inflammatory therapies can alleviate inflammation and improve histological healing, they fall far short of a resolution for strictures. 4 For CD patients with complex complications, endoscopic balloon dilation (EBD) and surgery are currently available treatment options. 5 However, they are not ideal due to a high rate of postoperative recurrence and complications. 6 To date, there are still no licensed antifibrotic agents, 7 partly due to the poor understanding of the mechanisms of initiation and propagation of intestinal fibrosis. Specific predictors that are capable of identifying CD patients at a high risk of developing strictures are also lacking, which may account for the lack of timely treatment. 7 Given the large degree of overlap between the two types, distinguishing fibrosis-predominant strictures from inflammatory-predominant ones is also difficult using tools such as ultrasonic (US) examination, computed tomography, and magnetic resonance imaging (MRI) in clinical practice, 8 making the choice of treatment options difficult.5,9 Since intestinal strictures are a hot topic in both clinical research and basic science research, numerous studies have been designed to address these hurdles, and substantial progress has been achieved within 2021.
Here, we summarize a curated set of articles within the year 2021 that highlight cutting-edge advances in relation to the underlying mechanisms, diagnostic methods, as well as evolving therapeutic options for intestinal strictures, aiming to give researchers and clinicians more insight into the trend of intestinal strictures.
Search strategy
A literature review was conducted on the database of PubMed, Web of Science, and Embase for all relevant literatures published in the year 2021, supplemented by manually reviewing the references of the included studies and relevant review articles. The following search terms were used (all fields): (‘stenotic’ OR ‘stenosis’ OR ‘fibrosis’ OR ‘fibrotic’ OR ‘fibrostenotic’ OR ‘fibrostenosis’ OR ‘stricture’ OR ‘strictures’ OR ‘stricturing’ OR ‘strictured’ OR ‘constriction’ OR ‘constrictions’) AND (‘Crohn’s disease’ OR ‘inflammatory bowel disease’ OR ‘CD’ OR ‘IBD’) AND (Filters: from 1 January 2021 to 31 December 2021). References to the identified articles were also examined for additional studies meeting these criteria.
Mechanism of intestinal strictures
The pathogenesis of CD fibrosis is not completely understood, which is largely due to the lack of human-specific intestinal fibrosis models. Excitingly, in 2021, Estrada et al. 10 generated human intestinal organoids (HIOs) using induced pluripotent stem cells from CD patients with strictures, and epithelial and mesenchymal cells derived from HIOs under the stimulation of transforming growth factor beta (TGF-β) were able to replicate intestinal fibrotic responses. This model shed new insight on the exploration of mechanisms underlying intestinal fibrosis. Several novel findings are discussed in the following section, which is shown in Figure 1.
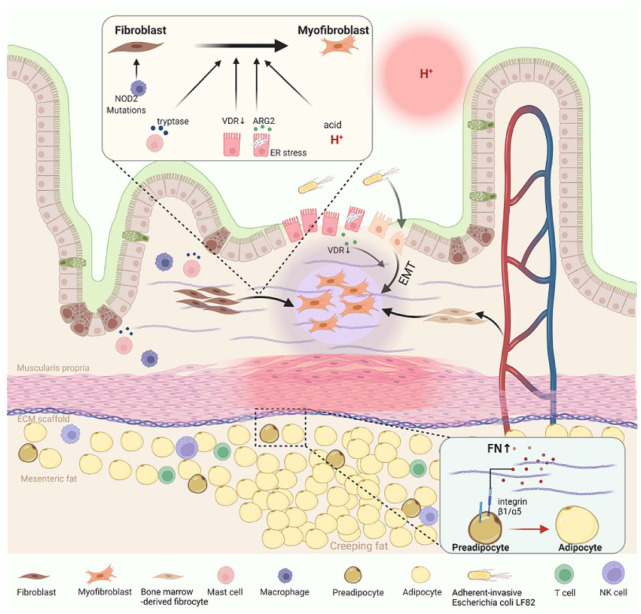
Figure 1.
Schematic diagram of novel mechanisms of intestinal stricture in 2021. NOD2 mutations caused dysregulated homeostasis of activated fibroblasts and macrophages. Tryptase released from MCs, VDR deficiency in epithelial cells, AGR2 secreted by epithelial cells under ER stress, and acidic pH facilitated differentiation of fibroblasts into myofibroblasts. VDR deficiency in epithelial cells also contributed to EMT. Bacteroides fragilis colonization and the increasement of circulating bone marrow-derived fibrocytes were correlated with fibrosis formation. FN which is secreted by HIMC mediated the migration of preadipocytes via the FN/α5β1 integrin signaling pathway, thus contributing to the formation of CrF.
AGR2, anterior gradient protein 2 homolog; CrF, creeping fat; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; ER stress, endoplasmic reticulum stress; FN, fibronectin; HIMC, human intestinal muscle cells; MCs, mast cells; NOD2, nucleotide-binding oligomerization domain 2; VDR, vitamin D receptor.
Immune and nonimmune cells
The complex interplay between immune cells and nonimmune stromal cells is a major driver of intestinal fibrosis. 11 In 2021, novel mechanisms related to mast cells (MCs), macrophages, and circulating bone marrow-derived fibrocytes in CD fibrosis have been unveiled.
MCs, mostly located at host–environment interfaces (e.g. gastrointestinal tract), are the first defense cells to interact with allergens, invading pathogens and other dangerous antigens.12,13 Substantial evidence showed that MCs played a crucial role in fibrotic diseases. 14 However, their explicit effects on CD fibrosis remain unknown. Liu et al. 15 first found that MCs increasingly infiltrated fibrotic intestines of both CD patients and colitis mice compared to the corresponding controls. MCs were able to activate fibroblasts through releasing tryptase and then facilitate fibroblasts to differentiate into myofibroblasts via PAR-2/Akt/mTOR pathway. 15 In vivo, the authors further reported that the deficiency of MCs in mice could significantly alleviate dextran sulfate sodium (DSS)-induced intestinal fibrosis, while once MCs were reconstructed in MC-deficient mice, the degree of intestinal fibrosis tended to reappear. 15 Furthermore, inhibiting tryptase released from MCs conferred resistance to intestinal fibrosis, which was accompanied by decreased expression and deposition of extracellular matrix (ECM) proteins. 15 Therefore, targeting MCs may represent a potential target for intestinal fibrosis.
Macrophages, especially tissue-resident macrophages, are actively involved in intestinal fibrosis. 16 Dharmasiri et al. 17 analyzed the whole transcriptome of macrophages purified from the intestines of CD, ulcerative colitis (UC), and healthy individuals via RNA sequencing technology, and found that fibrotic-related genes were significantly upregulated in CD patients compared with UC patients and healthy controls. Interestingly, the expression of M2 signature genes, fibrotic genes, and TGF-β1 were enriched in macrophages from CD rather than from UC, which indicated that subsets of intestinal macrophages might be associated with CD fibrosis. 17
Circulating fibrocytes are bone marrow-derived cells characterized by carrying both hematopoietic markers (CD34) and fibroblastic markers (collagen1), which can be recruited into injured tissue and involved in fibrosis formation.18,19 In line with this, Ueno et al. 20 demonstrated that the number of fibrocytes was significantly increased in plasma and fibrostenotic small bowel (SB) of CD patients, compared with that of non-fibrostenotic CD patients. Meanwhile, 10 plasma proteins, associated with fibrogenesis or fibrocyte development, were significantly increased in fibrostenotic CD patients. 20 It was assumed that the unique plasma protein profile from fibrostenotic CD patients might predispose human peripheral blood mononuclear cells to transform into fibrocyte-like cells. 20 Besides, within a 30-month follow-up, the increment of circulating fibrocytes was correlated with the subsequent escalation of medical therapy, endoscopic dilation, or surgery. These results suggest that circulating fibrocytes might serve as a disease predictor and provide a new route toward intestinal fibrostenosis. 20
Molecules and signaling pathways
Molecules act as the mediators of cells and play a key role in intestinal fibrosis. 21 In the year 2021, several novel molecules have been discovered and are considered as new therapeutic targets for intestinal fibrosis, including Yes-associated protein and transcriptional coactivator with PDZ-binding motif (YAP/TAZ), 22 tumor necrosis factor-like ligand 1A (TL1A), 23 anterior gradient protein 2 homolog (AGR2), 24 pH-sensing G protein-coupled receptor 4 (GPR4), 25 miR-155, 26 human nuclear receptor 4A 1 (NR4A1), 27 and vitamin D receptor (VDR). 28
The activation and proliferation of intestinal fibroblasts is the hallmark of fibrosis, although its mechanism has not been fully elucidated. YAP/TAZ, transcription coactivators and effectors of Hippo pathway, 29 were found to be highly expressed and activated in fibroblasts isolated from stenotic intestines of CD patients and DSS-induced chronic colitis murine models. 24 In addition, the YAP/TAZ activation was associated with fibroblast activation and a high risk of intestinal obstruction of CD. 22 Knockdown of YAP/TAZ in fibroblasts isolated from the fibrostenotic intestines resulted in decreased expression of profibrotic-related genes and proliferation of fibroblasts, indicating that YAP/TAZ were critical activators of intestinal fibroblasts. 22 It is known that YAP/TAZ activation is dependent on Rho/Rho-associated coiled-coil-containing protein kinase (ROCK) signaling pathway. 22 In vivo, silencing of YAP/TAZ with ROCK1 inhibitor alleviated intestinal fibrosis, revealing the profibrotic effect of ROCK1-YAP/TAZ axis in intestinal fibroblasts [Figure 2(a)]. 22 Thus, YAP/TAZ in fibroblasts may offer potential therapeutic strategies for intestinal fibrosis. Other promising molecules that target fibroblasts or myofibroblasts include GPR4 and miR-155.25,26 GPR4/Gα 12/13 signaling was involved in the initiation of myofibroblast activation, and this effect could be reversed by the inhibition of GPR4. 25 MiR-155 was demonstrated to directly target HMG-box transcription factor 1 and then activate Wnt/β-catenin signaling pathway, leading to intestinal fibrosis [Figure 2(b)]. 26 Amamou et al. 30 reported that a high-salt diet also contributed to the activation of colon fibroblasts and intestinal fibrosis. The authors observed a significantly increased expression of fibrosis markers (e.g. colon MMP-9, plasmatic MMP-9, and MMP-2) in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice with a high-salt diet as well as ECM-associated proteins [e.g. α-smooth muscle actin (α-SMA), CTGF, Col1a1, Col3a1] and MMP-2 in fibroblasts treated with NaCl. 30
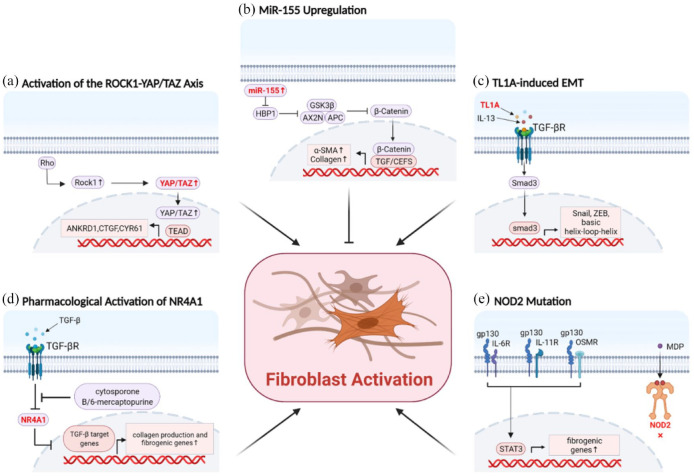
Figure 2.
Novel molecules are involved in activation of intestinal fibroblasts. (a) YAP/TAZ activation was dependent on Rho/ROCK signaling pathway, and increased the expression of profibrotic-related genes and proliferation of fibroblasts. (b) The upregulation of miR-155 targeted HBP1 and then activated the Wnt/βcatenin signaling pathway, leading to the activation of fibroblasts. (c) TL1A accompanied with IL-13 induced EMT and activated fibroblasts via TGF-β1/Smad3 pathway. (d) The pharmacological activation of NR4A1 with cytosporone B or 6-mercaptopurine attenuated TGF-β1-induced fibrosis in myofibroblasts. (e) Gp130 family- and STAT3-related genes were highly enriched in NOD2-mutated fibroblasts and macrophages, which led to the differentiation of macrophages into activated fibroblasts.
EMT, epithelial-to-mesenchymal transition; HBP1, HMG-box transcription factor 1; IL, interleukin; NOD2, nucleotide-binding oligomerization domain 2; NR4A1, nuclear receptor 4 A1; ROCK, Rho-associated coiled-coil-containing protein kinase; STAT3, signal transducer and activator of transcription 3; TAZ, transcriptional coactivator with PDZ-binding motif; TGF-β1, transforming growth factor beta 1; TL1A, tumor necrosis factor-like ligand 1A; YAP, Yes-associated protein; α-SMA, α-smooth muscle actin.
Other molecules that should be mentioned here are TL1A and AGR2. TL1A has been previously reported to participate in intestinal inflammation and fibrosis, but the underlying mechanism remains unclear. 23 A study in 2021 found that the expression of TL1A in intestines of CD patients was almost twice as high as that in healthy individuals. 31 The expression of TL1A was negatively associated with E-cadherin expression, whereas positively associated with β-catenin and α-SMA expression, implying that TL1A might be involved in the process of epithelial-to-mesenchymal transition (EMT). 31 Indeed, evidence from both CD patients and a DSS-induced murine model indicated that TL1A could induce EMT through interleukin (IL)-13 and TGF-β1/Smad3 pathway [Figure 2(c)]. 31 Another interesting molecule AGR2 was identified via secretome profile of epithelium in ileal CD strictures. 24 AGR2 and binding immunoglobulin protein were found to be increasingly expressed in the epithelium, especially under endoplasmic reticulum stress. 24 The enhanced expression of AGR2 was capable to trigger the differentiation of fibroblasts into myofibroblasts, thus promoting intestinal fibrosis. 24
In contrast to the aforementioned molecules that have profibrotic property, NR4A1 can dampen fibrogenic signaling in nonintestinal systems, and protect against fibrosis. 32 Pulakazhi Venu et al. 27 added new evidence to support the antifibrotic effect of NR4A1 in intestine system using a Crohn’s-like ileitis murine model. They demonstrated that pharmacological activation of NR4A1 with cytosporone B or 6-mercaptopurine could decrease the deposition of ECM and proliferation of myofibroblasts, ultimately preventing fibrosis. Besides, NR4A1 activation was found to be able to attenuate TGF-β1-induced proliferation, collagen production, and fibrogenic gene expression in human intestinal fibroblasts [Figure 2(d)]. 27 Similarly, VDR activation or VD supplement also contributed to alleviating intestinal fibrosis. 28
Gut microbiota
Gut microbiota is an indispensable driver in intestinal fibrogenesis.1,33,34 Becker et al. 35 found that Bacteroides fragilis, a gram-negative commensal of the phylum Bacteroidetes, was associated with stricturing disease behavior and severe disease activity in CD patients. However, a deeper understanding of B. fragilis in CD fibrosis needs to be clarified in further investigations. Another gram-negative proteobacterium called Adherent-invasive Escherichia coli (AIEC) has been observed abundantly in CD patients and has been proved to cause intestinal fibrosis in a murine model.36,37 Using a DSS-induced murine model, Chokr et al. 38 found that LF82, the prototype AIEC strain, was capable to enhance the deposition of collagen and mRNA expression of profibrotic-related genes (e.g. Col1a1, Col3a1, and Fn1), thus exacerbating intestinal fibrosis. In vitro, LF82 was able to induce intestinal epithelial cells to undergo EMT in response to TGF-β1 stimulation. 38
Creeping fat
Creeping fat (CrF) is referred to as the characteristic expansion of mesenteric tissue at the inflamed intestinal sites in CD patients, which was originally described by Burril B. Crohn in 1932. 39 However, there is a paucity of information on the relationship between CrF and intestinal fibrosis. Ha et al. 40 reported a gut microbiota-dependent mechanism that drove the formation of CrF in CD in 2020. In 2021, our team revealed another novel mechanism of CrF formation. 41 We first observed an inflammatory ECM scaffold produced by muscularis propria (MP) cells, which was positioned between CrF and MP exclusively in strictured intestines resected from CD patients. 41 Under the speculation that the source of ECM scaffold was MP cells rather than CrF, human intestinal muscle cells (HIMCs) isolated from MP tissue pretreated with TGF-β1 were further studied. 41 The results showed that only fibronectin (FN) and decorin changed significantly among proteins secreted from HIMC, which was in line with the immunofluorescence staining result of ECM scaffold in stricturing CD. 41 We further identified that FN mediated the migration of preadipocytes via FN/α5β1 integrin signaling pathway, thus contributing to the formation of CrF.41,42
One big obstacle to studying CrF is the lack of ideal animal models to mimic intestinal CrF. Remarkably, a new animal model via repeated colonic biopsy was established in 2021. 43 After four cycles of biopsy injuries (once per week), the penetrating fibrosis involving all layers of the murine colon and the expansion of mesenteric fat appeared, which was morphologically similar to CD patients with strictures. 43 Additionally, transcriptomic analysis revealed that gene expression in repeated colonic biopsy could recapitulate human CD. 43 The novel animal model thus paves a new avenue to study CrF in the future.
Host genetic susceptibility
Nucleotide-binding oligomerization domain 2 (NOD2) is the first identified gene conferring susceptibility to ileal CD, and its mutation is related to the increased risk of intestinal strictures. 44 However, the underlying mechanisms through which NOD2 drives fibrosis formation remain elusive. 6 Nayar et al. 45 first unveiled how NOD2 deficiencies predisposed CD patients to develop a stricturing phenotype [Figure 2(e)]. They defined a unique activated fibroblast population characterized by CD14 and PDGFRA expression via single-cell RNA sequencing of inflamed ileum from patients with CD. 45 Loss of NOD2 was found to lead to dysregulated homeostasis of activated fibroblasts and macrophages. 45 Especially, Gp130 family (e.g. IL-6, IL-11, and OSM) and signal transducer and activator of transcription 3 regulation were demonstrated to be highly enriched in these activated fibroblasts and macrophages. 45 Blocking gp130 was then found to ameliorate the activated myeloid-stromal niche, which might benefit CD patients refractory to antitumor necrosis factors (TNF) therapy. 45 This study sheds insight on NOD2-driven fibrosis in CD, and highlights that gp130 blockade may be an alternative for CD patients with anti-TNF treatment failure.
Assessment of intestinal strictures
Identification and quantification of intestinal strictures is critical to accurate the diagnosis of patients with CD and to tailor the individualized treatment strategies. Histologically, it has been well documented that CD strictures are characterized by the hypertrophy and hyperplasia of intestinal smooth muscle.46,47 However, a recent study has identified a novel histopathological phenotype in a subset of CD patients with ileal strictures, in which part of strictures were due to mural constriction rather than due to fibromuscular hypertrophy. 48 CD patients who presented with constrictive ileal strictures are featured by earlier onset, frequent multiplicity, and little fibromuscular mural expansion, which are pathologically and clinically distinct from those with hypertrophic strictures. 48 In addition, patients with constrictive strictures manifest as multiple strictures, and have significantly shorter surgery-free intervals than those with hypertrophic strictures. 48 This study provides evidence that intestinal strictures are truly heterogeneous entities. Thus, establishing a detailed diagnosis and treatment criteria for intestinal fibrosis is an urgent requirement. In 2021, several novel assessment and prediction models for intestinal fibrosis have been proposed and some key information is displayed in Table 1. Based on the characteristics of the above models and the area under the receiver operating characteristic curve (AUC) value, radiomic model (RM) and deep learning network displayed excellent diagnostic performances for the identification and quantification of intestinal stenosis, while blood protein and serologic markers showed a fair predictive ability.
Table 1.
Novel assessment and prediction models for intestinal strictures in CD.
| Model | Equipment | Parameters | Functions | AUC value | Accuracy | Sensitivity | Specificity | Limitations | References |
|---|---|---|---|---|---|---|---|---|---|
| RM | CTE | Busynessoriginal, busynesscoif3r05, busynessdb1r05, busynessdb1r15 | Differentiate moderate–severe from none–mild intestinal fibrosis | 0.888 | 0.857 | 0.815 | 0.939 | Relatively small sample sizes; time-consuming for manual volume of interest segmentation; not suitable for intestinal strictures with blurred contour | Li et al. 49 |
| State-of-the-art deep learning network | CE | Strictures images, normal mucosa images, and ulcer images | Identify intestinal strictures | 0.989 | 0.935 | 0.92 | 0.89 | A retrospective analysis; lack of strictures that were failed to pass CE or visible on cross-sectional images; lack of dynamic time-lapse movement images | Klang et al. 50 |
| Blood protein and serologic markers | – | Four plasma proteins (IL7, MMP10, IL12B, and CCL11) and two serologic markers (LnASCA IgA and LnCbir) | Predict the risk of stricturing complications | 0.68 | – | – | – | Relatively small sample sizes; no specific timepoints to diagnosis complications; no comparable external validation cohort | Ungaro et al. 51 |
The accuracy, sensitivity, and specificity were calculated from the receiver operating characteristic curve according to the Youden index.
AUC, area under the receiver operating characteristic curve; CD, Crohn’s disease; CE, capsule endoscopy; CTE, computed tomography enterography; RM, radiomic model.
Cross-sectional imaging modality
Cross-sectional imaging modalities, including magnetic resonance enterography, computed tomography enterography (CTE), and US, are widely used to evaluate transmural fibrosis. 11 However, currently, there are no reliable and widely accepted methods for determining intestinal fibrosis in CD. 49 Small-bowel stenosis (SBS) in CD was usually defined as luminal narrowing with upstream bowel dilation on cross-sectional imaging modalities. 52 However, Stocker et al. 53 argued that luminal stenosis was sufficient for diagnosing SBS, because it had higher accuracy and sensitivity compared with the combination of luminal stenosis and upstream dilation. In the 2021 international consensus, the value of upstream dilation in histopathologically diagnosing strictures remained an unsolved issue. 54
Recently, US has gained much attention in distinguishing fibrosis because of its noninvasive and nonradiating nature. 55 Bhatnagar et al. 56 examined the association between sonographic features and histopathological scores, and revealed that submucosal layer echogenicity, clarity, and mucosal layer thickness were associated with intestinal fibrosis. Besides, the changes in intestinal tissue stiffness were positively correlated with the severity of fibrosis, with a correlation coefficient of 0.73. 57 A systematic review including 11 studies showed that intestinal ultrasound elastography (UE) was able to evaluate intestinal strictures and differentiate inflammation from fibrosis phenotype in CD patients. 58 UE is emerging as a feasible technique to estimate tissue stiffness. 59
Magnetic resonance elastography (MRE) is also a technique to evaluate tissue stiffness. 60 In 2021, the ability of MRE to detect intestinal fibrosis in CD patients was first evaluated. Results from a pilot study showed that the intestinal stiffness value measured by MRE was proportional to the degree of fibrosis in CD patients. 61 The value of bowel stiffness over 3.57 kPa achieved a good prediction for hospitalization and surgical resection, with AUC of 0.82. 61 MRE may act as a predictor to estimate the risk and severity of intestinal fibrosis in CD patients. In addition, MR enterography was first used to measure terminal ileum motility in pediatric CD patients with standard free-breathing techniques in 2021. 62 Terminal ileum motility was found to be negatively associated with CD activity, which might be a candidate biomarker for monitoring disease progression and treatment efficacy.62,63
Radiomics is a popular form of radiologic imaging analysis aiming to extract high-dimensional imaging features to reveal subtle disease characteristics. 64 Li et al. 49 developed a novel CTE-based RM to assess intestinal fibrosis in CD patients, which substantially improved the quantitation of CTE in intestinal fibrosis. Results showed that RM had an excellent discriminatory ability for identifying moderate–severe and none–mild intestinal fibrosis with the AUC value of 0.888. 49 Subsequently validated in three different centers, RM achieved consistently high AUC values ranging from 0.724 to 0.816, 49 superior to even experienced radiologists in terms of the predictive ability for intestinal fibrosis. 49
Endoscopy
Endoscopy is an invasive method that allows direct visualization of the surface of the affected intestine; however, it fails to evaluate transmural fibrosis. 65 A multicenter study in 2021 showed that the frequency of endoscopic diagnosis was significantly increased in CD patients with SB strictures, but its diagnostic yield did not change over time. 66 Integrating artificial intelligence (AI) technology with endoscopy may be a solution to this problem. Studies showed that AI was faster and more accurate when detecting crucial subtle lesions compared with an endoscopist, although they had a comparable diagnostic performance to some extent. 67 In addition, the application of AI to capsule endoscopy (CE) provides a new means for assessment of SB and colonic lesions, 68 especially for non-obstructed patients with SBS. 50 Notably, a state-of-the-art deep learning network had a high accuracy (93.5%) in detecting stenosis and an excellent discrimination between strictures and ulcers (AUC=0.942) on CE images. 50
Biomarkers
Up till now, specific or reliable markers to predict CD strictures are still unavailable.69,70 Prentice et al. 71 first investigated a novel approach to diagnose and manage CD patients with strictures. They found that fecal calprotectin, together with US, was able to assess strictures as well as monitor treatment response in pregnant CD patients. 71 Additionally, Ungaro et al. 51 performed a prospective inception cohort with 265 pediatric CD patients to assess the association between novel blood protein biomarkers and stricturing complication. Four plasma proteins (IL7, MMP10, IL12B, and CCL11) and two serologic markers (LnASCA IgA and LnCbir) were identified, and then were pooled to predict the risk of stricturing complication with the AUC value of 0.68. 51 However, there is still a long distance to go in identifying validated and accurate predictive biomarkers for intestinal strictures.
Treatment of intestinal strictures
The common methods to relieve symptoms related to intestinal strictures include drug therapy, endoscopic therapy, and surgical treatment. In 2021, several studies were put forward to evaluate the efficiency of available management strategies and to explore potential novel antifibrotic therapies.
Medical therapy
Alleviating or even reversing intestinal fibrosis is a tough challenge in the management of intestinal strictures. 72 Immunosuppressants and biological agents have no direct effect on fibrosis, although these are associated with a 96% and 94% reduction of stricture development, respectively.1,73
Anti-TNF therapy still remains the backbone of treatment in fibrostenotic CD, 74 of which effect on strictures has been further investigated in 2021. A real-world study including 59 patients with stricturing CD showed that 88% of patients achieved clinical improvement when receiving TNF induction therapy with a median duration of 14 months. 75 Among them, 69% of patients at 1 year, 51% at 2 years, and 28% at 5 years had reached an overall cumulative treatment success, defined as no escalation or withdrawal of medical therapy, endoscopic dilation, or surgery. 75 The short- and long-term results of this real-world study were similar to a multicenter, prospective, and observational cohort (CREOLE) study. 76 However, 97 CD patients with SB strictures in the CREOLE study exclusively received adalimumab, whereas 59 CD patients with SB and large bowel strictures in the real-world study received either infliximab or adalimumab.75,76 Besides, 64.4% of patients in the real-world study had multiple strictures, whereas 71% of patients in the CREOLE study had a single stricture, which may be one potential reason for the lower surgery rate in the former study (11.8% within 5 years versus 49.3% within 4 years).75,76 A systematic review including 25 studies also demonstrated that anti-TNF therapy was effective, with 50% of patients free from surgery within 4 years of follow-up. 77 These results suggest that anti-TNF therapy is at least beneficial to stricturing CD in the short term.
Schulberg et al. 78 first performed a randomized controlled trial evaluating anti-TNF treatment in 123 CD patients with strictures. The results showed that patients receiving intensive high-dose adalimumab with later dose escalation plus thiopurine had slight improvements in obstructive symptom score, morphological stricture changes (such as bowel wall thickness in US), and higher reduction of inflammatory index (such as Crohn’s Disease Activity Index, MRI Index of Activity score, fecal calprotectin, and C-reactive protein), compared with standard adalimumab monotherapy. 78 However, there were no statistically significant differences between the two groups. 78 Thus, well-designed studies are needed to support the utilization of anti-TNF escalation plus immunosuppressives in CD strictures.
In addition to novel biologics, a number of new potential antifibrosis agents have been proven to be feasible in fibroblasts and intestinal fibrosis murine models. For example, ABT-263, a mimic of B-cell lymphoma-2 homology 3, was able to reduce the expression of fibrotic-related genes and proteins. 79 Both in vivo and in vitro experiments showed that ABT-263 increased the susceptibility of myofibroblasts to apoptosis. 79 Another study showed that oral administration of hydroxamate of betulinic acid (HBA), an inhibitor of hypoxia-inducing factor, displayed a significant reduction of fibrotic and inflammatory-related markers in colitis murine. 80 HBA could improve the integrity of epithelial barrier and promote the process of wound healing, leading to alleviation of colonic inflammation and fibrosis. 80
Endoscopic management
EBD is a safe and effective therapy for CD patients with de novo or anastomotic strictures.77,81,82 Takeda et al. 83 suggested that for patients with stenotic CD who received anti-TNF as maintenance therapy, EBD was also effective and safe, with surgery-free rates of 81.1% and 73.5% after 3-year and 5-year follow-up, respectively. Several risk factors for patients who are prone to undergo surgery after EBD were identified in recent studies including age less than 50 years, SB lesions, obstructive symptoms or biologic treatment at the time of EBD, or no history of perianal involvement.84,85 As for duodenal CD-associated strictures, serial dilations have much longer surgery-free time and recurrence-free survival than nonserial dilation. 81 Another study reported that CD patients with SBS who underwent double-balloon endoscopy had an 80% surgery-free rate within 25 months after initial dilation. 86 Of note, the mean intervention-free period can extend to 54 months for CD patients with asymptomatic SBS who had strictures in ileocecal valve or had no elemental diets. 87
Since the recurrence rate of EBD is high up to 50% during follow-up, 88 a self-expandable metal stent with antimigration and removable features may be an alternative option.89,90 A nationwide cohort study included 46 CD patients with partially covered self-expandable stents and found an obstruction-free rate of 58.7%, and a low migration rate of 6.5% with no adverse events could be achieved at 26 months. 91 However, given the small sample size, further studies are needed to investigate its effectiveness.
Surgical management
CD patients refractory to medical or endoscopic treatment, or with complex complications (strictures, abscesses, and fistulae), would eventually need surgical interventions such as strictureplasty (SXP) and bowel resection.1,92 Bislenghi et al. 93 conducted a meta-analysis including 26 studies aiming to explore the efficacy of conventional (short and intermediate procedures such as Heineke–Mikulicz and Finney) and nonconventional (long entero-enterostomies such as Michelassi) SXP. Results showed a comparatively low rate of postoperative complications (15.5% versus 19.2%) and surgical recurrence (26.9% versus 18.2%) for the two types of surgical therapies during median follow-up periods of 65.3 and 96.3 months, respectively, suggesting that both techniques were feasible. 93 Considering the significant heterogeneity among the included studies, the conclusion should however be further verified. For the first time, a prospective cohort study investigated the safety and feasibility of conventional or nonconventional SXP in combination with laparoscopy for complicated CD. 94 They reported that both conventional and nonconventional SXP were safe and feasible by laparoscopy with low morbidity rates. 94 Of note, SXP is not recommended for patients with dysplasia or cancer, or colonic strictures. 95 On these occasions, bowel resection is an alternative option. Recently, a study recruited 5158 patients with CD to compare the safety of open surgery with laparoscopic and robotic-assisted ileocecal resection. 96 Results showed that laparoscopic and robotic-assisted surgery displayed lower incidences of reoperation, wound infection, and anastomotic leaks than open surgery.
Future outlook
In 2021, a remarkable progress has been achieved in CD-related intestinal strictures in the fields of mechanism, disease assessment, and therapeutic management. However, there are still many issues needed to be addressed. First, the pathogenesis of stricturing CD has not been fully understood yet. Novel techniques such as multi-omics and single-cell sequencing may open up a new research direction for in-depth study on the pathogenesis of fibrosis, which may fuel the exploration of fibrosis mechanisms. Besides, novel animal models that mimic molecular features of intestinal fibrosis in CD have been successfully established, which allow for investigating the pathogenesis of intestinal strictures in more detail. Second, there is a dire need for the development of specific predictors and diagnostic approaches to identifying strictures. Medical imaging combined with AI may provide a more precise approach for assessing CD fibrosis. More studies are needed to investigate the efficiency of AI-assisted medical imaging in the future. Lastly, in parallel with our increased understanding of the underlying mechanisms of intestinal strictures, growing potential antifibrotic agents have been developed and investigated in experimental models. However, none of them have been investigated in clinical trials, partly due to the lack of definitive clinical endpoints that are of pivotal importance to guide clinical trials. Excitingly, the Stenosis Therapy and Anti-Fibrotic Research consortium is concentrating on establishing the standardized definitions of endpoints for management of stricturing CD and has achieved some progress. All progress described above in intestinal strictures will be gradually carried forward, and will be put into use from bench to bedside in the not too distant future.
Acknowledgments
All figures are created with BioRender.com.
Footnotes
Author contributions: Xiaoxuan Lin: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Yu Wang: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Zishan Liu: Investigation; Writing – review & editing.
Sinan Lin: Investigation; Writing – review & editing.
Jinyu Tan: Investigation; Writing – review & editing.
Jinshen He: Methodology; Writing – review & editing.
Fan Hu: Investigation; Writing – review & editing.
Xiaomin Wu: Investigation; Writing – review & editing.
Subrata Ghosh: Conceptualization; Writing – review & editing.
Minhu Chen: Supervision; Writing – review & editing.
Fen Liu: Data curation; Investigation; Writing – review & editing.
Ren Mao: Conceptualization; Supervision; Validation; Writing – review & editing.
ORCID iDs: Xiaoxuan Lin  https://orcid.org/0000-0002-4668-9101
https://orcid.org/0000-0002-4668-9101
Subrata Ghosh  https://orcid.org/0000-0002-1713-7797
https://orcid.org/0000-0002-1713-7797
Minhu Chen  https://orcid.org/0000-0001-9925-135X
https://orcid.org/0000-0001-9925-135X
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC grant No 81970483, and 82170537 to Ren Mao).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Xiaoxuan Lin, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Yu Wang, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Zishan Liu, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Sinan Lin, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Jinyu Tan, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Jinshen He, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Fan Hu, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Xiaomin Wu, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Subrata Ghosh, APC Microbiome Ireland, University College Cork, Cork, Ireland.
Minhu Chen, Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Fen Liu, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, 58 Zhongshan Road 2nd, Guangzhou 510080, People’s Republic of China.
Ren Mao, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, 58 Zhongshan Road 2nd, Guangzhou 510080, People’s Republic of China; Department of Gastroenterology, Huidong People’s Hospital, Huizhou 516399, China.
References
- 1. Sleiman J, El Ouali S, Qazi T, et al. Prevention and treatment of stricturing Crohn’s disease - perspectives and challenges. Expert Rev Gastroenterol Hepatol 2021; 15: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mager R, Roda G, Shalaby MK, et al. Fibrotic strictures in Crohn’s disease: mechanisms and predictive factors. Curr Drug Targets 2021; 22: 241–251. [DOI] [PubMed] [Google Scholar]
- 3. Dehghan M, Wong G, Neuberger E, et al. Worse outcomes and higher costs of care in fibrostenotic Crohn’s disease: a real-world propensity-matched analysis in the USA. BMJ Open Gastroenterol 2021; 8: e000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iwata K, Mikami Y, Kato M, et al. Pathogenesis and management of gastrointestinal inflammation and fibrosis: from inflammatory bowel diseases to endoscopic surgery. Inflamm Regen 2021; 41: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tavares de Sousa H, Gullo I, Castelli C, et al. Ileal Crohn’s disease exhibits similar transmural fibrosis irrespective of phenotype. Clin Transl Gastroenterol 2021; 12: e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmoyer CJ, Saidman J, Bohl JL, et al. The pathogenesis and clinical management of stricturing Crohn disease. Inflamm Bowel Dis 2021; 27: 1839–1852. [DOI] [PubMed] [Google Scholar]
- 7. Bamias G, Pizarro TT, Cominelli F. Immunological regulation of intestinal fibrosis in inflammatory bowel disease. Inflamm Bowel Dis 2022; 28: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D’Haens G, Rieder F, Feagan BG, et al. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology 2022; 162: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narula N, Wong ECL, Dulai PS, et al. Outcomes of passable and non-passable strictures in clinical trials of Crohn’s disease: a post-hoc analysis. J Crohns Colitis 2021; 15: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 10. Estrada HQ, Patel S, Rabizadeh S, et al. Development of a personalized intestinal fibrosis model using human intestinal organoids derived from induced pluripotent stem cells. Inflamm Bowel Dis 2022; 28: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Lin S, Brown JM, et al. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev 2021; 302: 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamey FK, Lau WWY, Kucinski I, et al. Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development. Allergy 2021; 76: 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu M, Song XT, Liu B, et al. The emerging role of mast cells in response to fungal infection. Front Immunol 2021; 12: 688659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradding P, Pejler G. The controversial role of mast cells in fibrosis. Immunol Rev 2018; 282: 198–231. [DOI] [PubMed] [Google Scholar]
- 15. Liu B, Yang MQ, Yu TY, et al. Mast cell tryptase promotes inflammatory bowel disease-induced intestinal fibrosis. Inflamm Bowel Dis 2021; 27: 242–255. [DOI] [PubMed] [Google Scholar]
- 16. Lis-Lopez L, Bauset C, Seco-Cervera M, et al. Is the macrophage phenotype determinant for fibrosis development? Biomedicines 2021; 9: 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dharmasiri S, Garrido-Martin EM, Harris RJ, et al. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and Crohn disease. Inflamm Bowel Dis 2021; 27: 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mifflin RC, Pinchuk IV, Saada JI, et al. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol 2011; 300: G684–G696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn’s disease. Dig Dis Sci 2014; 59: 760–768. [DOI] [PubMed] [Google Scholar]
- 20. Ueno A, Jijon HB, Peng R, et al. Association of circulating fibrocytes with fibrostenotic small bowel Crohn’s disease. Inflamm Bowel Dis 2022; 28: 246–258. [DOI] [PubMed] [Google Scholar]
- 21. Elias M, Zhao S, Le HT, et al. IL-36 in chronic inflammation and fibrosis - bridging the gap? J Clin Invest 2021; 131: e144336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ou W, Xu W, Liu F, et al. Increased expression of yes-associated protein/YAP and transcriptional coactivator with PDZ-binding motif/TAZ activates intestinal fibroblasts to promote intestinal obstruction in Crohn’s disease. EBioMedicine 2021; 69: 103452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang M, Jia W, Wang D, et al. Effects and mechanism of constitutive TL1A expression on intestinal mucosal barrier in DSS-induced colitis. Dig Dis Sci 2019; 64: 1844–1856. [DOI] [PubMed] [Google Scholar]
- 24. Vieujean S, Hu S, Bequet E, et al. Potential role of epithelial endoplasmic reticulum stress and anterior gradient protein 2 homologue in Crohn’s disease fibrosis. J Crohns Colitis 2021; 15: 1737–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weder B, Schefer F, van Haaften WT, et al. New therapeutic approach for intestinal fibrosis through inhibition of pH-sensing receptor GPR4. Inflamm Bowel Dis 2022; 28: 109–125. [DOI] [PubMed] [Google Scholar]
- 26. Li N, Ouyang Y, Xu X, et al. MiR-155 promotes colitis-associated intestinal fibrosis by targeting HBP1/Wnt/beta-catenin signalling pathway. J Cell Mol Med 2021; 25: 4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pulakazhi Venu VK, Alston L, Iftinca M, et al. Nr4A1 modulates inflammation-associated intestinal fibrosis and dampens fibrogenic signaling in myofibroblasts. Am J Physiol Gastrointest Liver Physiol 2021; 321: G280–G297. [DOI] [PubMed] [Google Scholar]
- 28. Yu M, Wu H, Wang J, et al. Vitamin D receptor inhibits EMT via regulation of the epithelial mitochondrial function in intestinal fibrosis. J Biol Chem 2021; 296: 100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heng BC, Zhang X, Aubel D, et al. An overview of signaling pathways regulating YAP/TAZ activity. Cell Mol Life Sci 2021; 78: 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amamou A, Rouland M, Yaker L, et al. Dietary salt exacerbates intestinal fibrosis in chronic TNBS colitis via fibroblasts activation. Sci Rep 2021; 11: 15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wenxiu J, Mingyue Y, Fei H, et al. Effect and mechanism of TL1A expression on epithelial-mesenchymal transition during chronic colitis-related intestinal fibrosis. Mediators Inflamm 2021; 2021: 5927064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med 2015; 21: 150–158. [DOI] [PubMed] [Google Scholar]
- 33. Caparros E, Wiest R, Scharl M, et al. Dysbiotic microbiota interactions in Crohn’s disease. Gut Microbes 2021; 13: 1949096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhan S, Li N, Liu C, et al. Intestinal fibrosis and gut microbiota: clues from other organs. Front Microbiol 2021; 12: 694967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Becker HEF, Jamin C, Bervoets L, et al. Higher prevalence of bacteroides fragilis in Crohn’s disease exacerbations and strain-dependent increase of epithelial resistance. Front Microbiol 2021; 12: 598232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020; 16: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Dejanovic D, Zangara MT, et al. Mouse models of intestinal fibrosis. Methods Mol Biol 2021; 2299: 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chokr D, Cornu M, Neut C, et al. Adherent invasive Escherichia coli (AIEC) strain LF82, but not Candida albicans, plays a profibrogenic role in the intestine. Gut Pathog 2021; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suau R, Pardina E, Domenech E, et al. The complex relationship between microbiota, immune response and creeping fat in Crohn’s disease. J Crohns Colitis 2022; 16: 472–489. [DOI] [PubMed] [Google Scholar]
- 40. Ha CWY, Martin A, Sepich-Poore GD, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 2020; 183: 666–683.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mao R, Doyon G, Gordon IO, et al. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn’s disease. Gut 2022; 71: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kennelly TM, Li Y, Cao Y, et al. Distinct binding interactions of alpha5beta1-integrin and proteoglycans with fibronectin. Biophys J 2019; 117: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong S, Whitehurst CE, Li L, et al. Reverse translation approach generates a signature of penetrating fibrosis in Crohn’s disease that is associated with anti-TNF response. Gut. Epub ahead of print 14 July 2021. DOI: 10.1136/gutjnl-2020-323405. [DOI] [PubMed] [Google Scholar]
- 44. Ashton JJ, Mossotto E, Stafford IS, et al. Genetic sequencing of pediatric patients identifies mutations in monogenic inflammatory bowel disease genes that translate to distinct clinical phenotypes. Clin Transl Gastroenterol 2020; 11: e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nayar S, Morrison JK, Giri M, et al. A myeloid-stromal niche and gp130 rescue in NOD2-driven Crohn’s disease. Nature 2021; 593: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. D’Alessio S, Ungaro F, Noviello D, et al. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol 2022; 19: 169–184. [DOI] [PubMed] [Google Scholar]
- 47. Chen W, Lu C, Hirota C, et al. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017; 11: 92–104. [DOI] [PubMed] [Google Scholar]
- 48. Liu Q, Zhang X, Ko HM, et al. Constrictive and hypertrophic strictures in Ileal Crohn’s disease. Clin Gastroenterol Hepatol 2022; 20: e1292–e1304. [DOI] [PubMed] [Google Scholar]
- 49. Li X, Liang D, Meng J, et al. Development and validation of a novel computed-tomography enterography radiomic approach for characterization of intestinal fibrosis in Crohn’s disease. Gastroenterology 2021; 160: 2303–2316.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klang E, Grinman A, Soffer S, et al. Automated detection of Crohn’s disease intestinal strictures on capsule endoscopy images using deep neural networks. J Crohns Colitis 2021; 15: 749–756. [DOI] [PubMed] [Google Scholar]
- 51. Ungaro RC, Hu L, Ji J, et al. Machine learning identifies novel blood protein predictors of penetrating and stricturing complications in newly diagnosed paediatric Crohn’s disease. Aliment Pharmacol Ther 2021; 53: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bruining DH, Zimmermann EM, Loftus EV, Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology 2018; 154: 1172–1194. [DOI] [PubMed] [Google Scholar]
- 53. Stocker D, King MJ, El Homsi M, et al. Luminal narrowing alone allows an accurate diagnosis of Crohn’s disease small bowel strictures at cross-sectional imaging. J Crohns Colitis 2021; 15: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 54. Gordon IO, Bettenworth D, Bokemeyer A, et al. International consensus to standardise histopathological scoring for small bowel strictures in Crohn’s disease. Gut 2022; 71: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferretti F, Cannatelli R, Ardizzone S, et al. Ultrasonographic evaluation of intestinal fibrosis and inflammation in Crohn’s disease. The state of the art. Front Pharmacol 2021; 12: 679924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhatnagar G, Rodriguez-Justo M, Higginson A, et al. Inflammation and fibrosis in Crohn’s disease: location-matched histological correlation of small bowel ultrasound features. Abdom Radiol (NY) 2021; 46: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhao J, Liao D, Wilkens R, et al. Bowel stiffness associated with histopathologic scoring of stenosis in patients with Crohn’s disease. Acta Biomater 2021; 130: 332–342. [DOI] [PubMed] [Google Scholar]
- 58. Slosarz D, Poniewierka E, Neubauer K, et al. Ultrasound elastography in the assessment of the intestinal changes in inflammatory bowel disease-systematic review. J Clin Med 2021; 10: 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gabbiadini R, Zacharopoulou E, Furfaro F, et al. Application of ultrasound elastography for assessing intestinal fibrosis in inflammatory bowel disease: fiction or reality? Curr Drug Targets 2021; 22: 347–55. [DOI] [PubMed] [Google Scholar]
- 60. Poterucha JT, Johnson JN, Qureshi MY, et al. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc 2015; 90: 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Avila F, Caron B, Hossu G, et al. Magnetic resonance elastography for assessing fibrosis in patients with Crohn’s disease: a pilot study. Dig Dis Sci. Epub ahead of print 21 November 2021. DOI: 10.1007/s10620-021-07311-9. [DOI] [PubMed] [Google Scholar]
- 62. Cococcioni L, Fitzke H, Menys A, et al. Quantitative assessment of terminal ileum motility on MR enterography in Crohn disease: a feasibility study in children. Eur Radiol 2021; 31: 775–784. [DOI] [PubMed] [Google Scholar]
- 63. Arkko A, Kaseva T, Salli E, et al. Automatic detection of Crohn’s disease using quantified motility in magnetic resonance enterography: initial experiences. Clin Radiol 2022; 77: 96–103. [DOI] [PubMed] [Google Scholar]
- 64. Tomaszewski MR, Gillies RJ. The biological meaning of radiomic features. Radiology 2021; 298: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bufman H, Eliakim R, Tau N, et al. Magnetic resonance enterography in Crohn’s disease patients: current state of the art and future perspectives. Expert Rev Med Devices 2021; 18: 657–667. [DOI] [PubMed] [Google Scholar]
- 66. Jeon SR, Kim JO, Byeon JS, et al. Enteroscopy in Crohn’s disease: are there any changes in role or outcomes over time? A KASID multicenter study. Gut Liver 2021; 15: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bang CS, Lee JJ, Baik GH. Computer-aided diagnosis of gastrointestinal ulcer and hemorrhage using wireless capsule endoscopy: systematic review and diagnostic test accuracy meta-analysis. J Med Internet Res 2021; 23: e33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chahal D, Byrne MF. A primer on artificial intelligence and its application to endoscopy. Gastrointest Endosc 2020; 92: 813–820.e4. [DOI] [PubMed] [Google Scholar]
- 69. Wu J, Lubman DM, Kugathasan S, et al. Serum protein biomarkers of fibrosis aid in risk stratification of future stricturing complications in pediatric Crohn’s disease. Am J Gastroenterol 2019; 114: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steiner CA, Berinstein JA, Louissaint J, et al. Biomarkers for the prediction and diagnosis of fibrostenosing Crohn’s disease: a systematic review. Clin Gastroenterol Hepatol 2022; 20: 817–846.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Prentice R, Wright EK, Flanagan E, et al. The use of fecal calprotectin and intestinal ultrasound in the evaluation and management of stricturing Crohn’s disease in pregnancy. Inflamm Bowel Dis 2022; 28: e13–e16. [DOI] [PubMed] [Google Scholar]
- 72. Li Y, Chen J, Bolinger AA, et al. Target-based small molecule drug discovery towards novel therapeutics for inflammatory bowel diseases. Inflamm Bowel Dis 2021; 27(Suppl 2): S38–S62. [DOI] [PubMed] [Google Scholar]
- 73. Lawrence LS, Heider A, Singer AAM, et al. Granulomas in diagnostic biopsies associated with high risk of Crohn’s complications-but may be preventable. Inflamm Bowel Dis 2022; 28: 523–530. [DOI] [PubMed] [Google Scholar]
- 74. Abdulla M, Chew TS. Molecular targets and the use of biologics in the management of small bowel fibrosis in inflammatory bowel disease. Curr Opin Gastroenterol 2021; 37: 275–283. [DOI] [PubMed] [Google Scholar]
- 75. Vuyyuru SK, Kante B, Kumar P, et al. Real world analysis on the efficacy and safety of anti-tumor necrosis factor therapy in patients with stricturing Crohn’s disease. Sci Rep 2021; 11: 11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schulberg JD, Wright EK, Holt BA, et al. Efficacy of drug and endoscopic treatment of Crohn’s disease strictures: a systematic review. J Gastroenterol Hepatol 2021; 36: 344–361. [DOI] [PubMed] [Google Scholar]
- 78. Schulberg JD, Wright EK, Holt BA, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn’s disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol 2022; 7: 318–331. [DOI] [PubMed] [Google Scholar]
- 79. Johnson LA, Rodansky ES, Tran A, et al. Effect of ABT-263 on intestinal fibrosis in human myofibroblasts, human intestinal organoids, and the mouse salmonella typhimurium model. Inflamm Bowel Dis 2022; 28: 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prados ME, Garcia-Martin A, Unciti-Broceta JD, et al. Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease. Acta Pharmacol Sin 2021; 42: 1124–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang J, Li Y, Diao Y, et al. Long-term outcome of endoscopic balloon dilation for duodenal Crohn’s disease-associated strictures. Dig Dis Sci 2021; 66: 3570–3577. [DOI] [PubMed] [Google Scholar]
- 82. McSorley B, Cina RA, Jump C, et al. Endoscopic balloon dilation for management of stricturing Crohn’s disease in children. World J Gastrointest Endosc 2021; 13: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Takeda T, Kishi M, Takatsu N, et al. Long-term outcomes of endoscopic balloon dilation for intestinal strictures in patients with Crohn’s disease during maintenance treatment with anti-tumor necrosis factor alpha antibodies. Dig Endosc 2022; 34: 517–525. [DOI] [PubMed] [Google Scholar]
- 84. Lee HS, Chiorean MV, Boden E, et al. Usefulness of fluoroscopy for endoscopic balloon dilation of Crohn’s disease-related strictures. Dig Dis Sci 2022; 67: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 85. Sivasailam B, Manski S, Wentz A, et al. Presence of obstructive symptoms and absence of perianal Crohn disease is predictive of surgery after endoscopic balloon dilation. Inflamm Bowel Dis 2021; 27: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 86. Halloran BP, Jamil LH, Lo SK, et al. Double-balloon endoscopy in crohn disease: a tertiary referral center experience. Inflamm Bowel Dis 2021; 27: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 87. Hayashi Y, Takabayashi K, Hosoe N, et al. Predictors of necessity for endoscopic balloon dilatation in patients with Crohn’s disease-related small bowel stenosis. Ann Med 2021; 53: 2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bettenworth D, Bokemeyer A, Kou L, et al. Systematic review with meta-analysis: efficacy of balloon-assisted enteroscopy for dilation of small bowel Crohn’s disease strictures. Aliment Pharmacol Ther 2020; 52: 1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marcellier G, Lorenzo D, Hedjoudje A, et al. First treatment of Crohn’s disease refractory anastomotic stricture with a lumen-apposing metallic stent suitable for colonoscopy. Endoscopy. Epub ahead of print 8 September 2021. DOI: 10.1055/a-1559-1279. [DOI] [PubMed] [Google Scholar]
- 90. Hedenstrom P, Stotzer PO. Endoscopic treatment of Crohn-related strictures with a self-expandable stent compared with balloon dilation: a prospective, randomised, controlled study. BMJ Open Gastroenterol 2021; 8: e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Attar A, Branche J, Coron E, et al. An anti-migration self-expandable and removable metal stent for Crohn’s disease strictures: a nationwide study from GETAID and SFED. J Crohns Colitis 2021; 15: 521–528. [DOI] [PubMed] [Google Scholar]
- 92. Kavalukas SL, Scheurlen KM, Galandiuk S. State-of-the-art surgery for Crohn’s disease: part I-small intestine/ileal disease. Langenbecks Arch Surg. Epub ahead of print 4 November 2021. DOI: 10.1007/s00423-021-02324-4. [DOI] [Google Scholar]
- 93. Bislenghi G, Sucameli F, Fieuws S, et al. Non-conventional versus conventional strictureplasties for Crohn’s disease. A systematic review and meta-analysis of treatment outcomes. J Crohns Colitis 2022; 16: 319–330. [DOI] [PubMed] [Google Scholar]
- 94. Sampietro GM, Colombo F, Frontali A, et al. Strictureplasties performed by laparoscopic approach for complicated Crohn’s disease. A prospective, observational, cohort study. Dig Liver Dis 2021; 53: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 95. Fumery M, Yzet C, Chatelain D, et al. Colonic strictures in inflammatory bowel disease: epidemiology, complications, and management. J Crohns Colitis 2021; 15: 1766–1773. [DOI] [PubMed] [Google Scholar]
- 96. Hota S, Parascandola S, Smith S, et al. Robotic and laparoscopic surgical techniques in patients with Crohn’s disease. Surg Endosc 2021; 35: 4602–4608. [DOI] [PubMed] [Google Scholar]