Abstract
Precision oncology has opened a new era in cancer treatment focused on targeting specific cellular pathways directly involved in tumorigenesis. The REarrangement during Transfection (RET) proto-oncogene is involved in the pathogenesis of various thyroid cancer subtypes. Mutations in RET give rise to both hereditary and sporadic medullary thyroid cancer (MTC). RET fusions are found in follicular cell-derived thyroid cancers (papillary, poorly differentiated, and anaplastic). Hence, drugs that block the RET tyrosine kinase receptor have been explored in the management of locally advanced or metastatic thyroid cancer. The multikinase inhibitors (MKIs) with nonselective RET inhibition are sorafenib, lenvatinib, vandetanib, cabozantinib, and sunitinib. Although the efficacy of these drugs varies, a major issue is the lack of specificity resulting in a higher rate of drug-related toxicities, leading to dose reduction, interruption, or discontinuation. Moreover, MKIs are subject to drug resistance by RET Val804 residue gatekeeper mutations. In phase I/II clinical studies, the highly selective first-generation RET inhibitors, selpercatinib and pralsetinib, demonstrate high efficacy in controlling disease even in the presence of gatekeeper mutations combined with greater tolerability. However, resistance mechanisms such as RET solvent front mutations (SFMs) have evolved in some patients, giving the need to develop the selective second-generation RET inhibitors. Although the approval of selpercatinib and pralsetinib in 2020 has profoundly benefited patients with RET-altered thyroid cancer, further research into optimal treatment strategies, mechanisms of drug resistance, long-term consequences of potent RET-inhibition, and development of more effective agents against emergent mutations are much needed.
Keywords: multikinase inhibitor, pralsetinib, RET-altered thyroid cancer, RET inhibitor, selpercatinib, thyroid cancer
Introduction
Precision oncology has impacted the therapeutic landscape by identifying targetable oncoproteins. Radioactive iodine (131I) is considered as the original molecular-targeted therapy against the sodium/iodide symporter expressed by thyroid follicular cells in the treatment of differentiated thyroid cancer (DTC) [papillary thyroid cancer (PTC), follicular thyroid cancer]. Its efficacy was described by Dr Seidlin, in 1946, when he successfully treated a patient with functional metastatic thyroid cancer with 131I. 1 In the early 2000s, based on the success of imatinib targeting the KIT kinase receptor in gastrointestinal stromal tumors, there was an exponential development of clinical studies on MKIs, primarily inhibiting vascular endothelial growth factor receptor (VEGFR), in various cancers including thyroid. 2 These trials led to the approval of cabozantinib and vandetanib for advanced and progressive medullary thyroid cancer (MTC) and lenvatinib and sorafenib for radioactive-iodine refractory (RAIR) progressive DTC.3 –6 These agents changed the paradigm of management of our patients with advanced thyroid cancer; however, dose-limiting toxicities associated with treatment would often lead to decreased efficacy and drug cessation.
With the advent of comprehensive next-generation sequencing (NGS) of tumors, identifying molecular drivers of tumorigenesis, there has been development of targeted therapies with greater efficacy and less potential off-target adverse events (AEs). Germline activating point mutations of REarrangement during Transfection (RET) are present in 25% of MTC cases and give rise to hereditary MTC presenting in the form of multiple endocrine neoplasia (MEN) syndromes types 2A and 2B, whereas 45% of sporadic MTC have a somatic RET mutation.7,8 Although BRAF V600E is the most common mutation identified in DTC (59.7%) and found in poorly differentiated thyroid cancer (PDTC, 33%) and anaplastic thyroid cancer (ATC, 45%), these follicular cell-derived thyroid cancers can harbor mutually exclusive fusions associated with RET (approximately 6–10% of PTC, 6% of PDTC, rarely in ATC), BRAF, NTRK, ALK, and PPARG.9 –11
In this review, we will describe the history of the RET proto-oncogene and its pathogenic role in various subtypes of thyroid cancer, summarize the characteristics of nonselective MKIs approved for thyroid cancer, note the development of novel and highly selective RET-inhibitors for RET-altered thyroid cancers, and finally emphasize emergent findings and unmet needs in the management of advanced thyroid cancer.
RET proto-oncogene
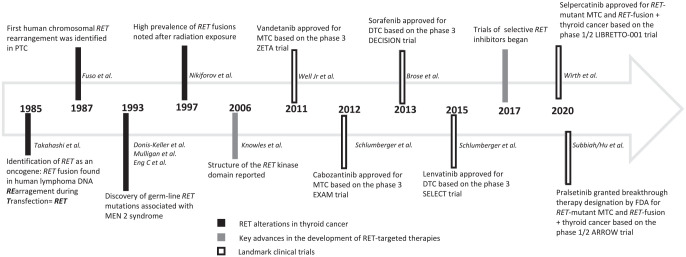
RET was isolated and cloned in 1985 by Takahashi et al. 12 from transformed mouse NIH/3T3 fibroblast cells which developed a DNA rearrangement with human T-cell lymphoma DNA during transfection (Figure 1). The RET proto-oncogene is located on the long arm of chromosome 10 (10q11.2) and encodes the RET tyrosine kinase transmembrane receptor which is a 170-kDa protein monomer.13–16
Figure 1.
RET proto-oncogene: historical background.
The RET receptor’s physiologic signaling process starts with the binding of growth factors to a coreceptor, which in turn causes RET dimerization and phosphorylation of the intracellular kinase domain. This leads to the activation of RAS/MAPK and PI3K/AKT pathways, involved in cell growth, proliferation, differentiation, survival, and migration.13,17
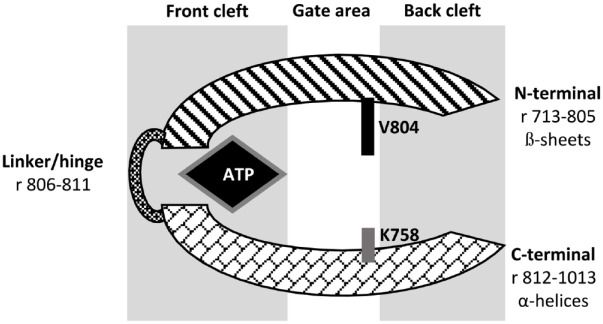
The RET receptor’s kinase domain consists of an N-terminal lobe (residues 713–805) and a C-terminal lobe (residues 812–1013) connected via a hinge/linker (residues 806–811). The N-terminal lobe consists of ß-sheets, whereas the C-terminal lobe contains α-helices. The catalytic cleft is located between the N-terminal lobe and the C-terminal lobe. It is divided into front cleft (containing the ATP binding site), gate area (preceding the hinge region), and back cleft. The catalytic cleft is the focus of kinase inhibitor development. ATP connects to the backbone of the hinge via hydrogen bonds. The size of the side chain of Val804 gatekeeper residue controls access to the catalytic pocket (Figure 2).18,19
Figure 2.
RET receptor kinase domain catalytic cleft.
r, residues; V804 is the gatekeeper residue.
RET is important in the normal formation of the kidney, influencing the development of the Wolffian duct and ureteric bud epithelium and the proliferation, differentiation, and survival of neural crest cells. The importance of RET became evident in neonatal mice with a homozygous inactivating RET mutation that die soon after birth with renal agenesis and absence of enteric neurons in the digestive tract.20–22 RET signaling also plays a role in the regulation of hematopoietic cells and spermatogenesis.23,24 During adulthood, RET is mainly present in organs derived from neural crest cells. 25 Loss-of-function RET mutations in humans are associated with Hirschsprung disease, congenital malformations of the kidney and urinary tract, and congenital hypoventilation syndrome. 11
RET activating mutations
Germline mutations
MTC can be inherited in 25% of cases. Germline activating RET mutations occur in the hereditary MEN 2 syndrome, which is further classified based on genotype–phenotype correlation as MEN2A and MEN2B. 8
MEN2A is the most common type accounting for ~95% of MEN2 cases. It is characterized by MTC in all cases, pheochromocytoma (PHEO) in ~50% of cases, primary hyperparathyroidism ~20–30% of cases. About 95% of mutations in MEN2A occur in the cysteine-rich domain of RET extracellular region (codon 634 in exon 11 accounts for ~85% of cases). These mutations substitute cysteine with another amino acid and result in the formation of disulfide-bonded RET homodimers with subsequent ligand-independent constitutive activation of the kinase region.8,26
MEN2B corresponds to ~5% of MEN2 cases. It is the most aggressive type with early onset of MTC. It is characterized by MTC in all cases, PHEO in ~50% of cases, and unique physical features like ganglioneuromas and marfanoid habitus. About 95% of mutations in MEN2B occur in codon M918T of exon 16 which corresponds to the most aggressive form of disease. M918T increases the ATP binding affinity to RET monomers with resultant autophosphorylation without the need of dimerization.8,26 All patients with MTC should undergo genetic testing for a RET mutation, because 1–7% of apparently sporadic MTC are due to de novo germline mutations. 8
Somatic mutations
MTC can be sporadic in 75% of cases. Up to 45% of these patients have somatic-activating RET mutations. M918T is the most common reported RET somatic mutation, but somatic mutations have been identified involving codons 634, 804, and many others. Deletions and duplications in RET have also been found on NGS tumor testing. Mutually exclusive point mutations of RAS have been reported in sporadic MTC but with less frequency (approximately 15%). 7
RET rearrangements or fusions
In addition to activating germline and somatic mutations of RET, oncogenesis can be mediated by the development of a chromosomal rearrangement that encodes a protein that fuses the RET kinase domain to a protein partner harboring the dimerization domain. The molecular mechanism responsible for RET fusions is thought to be a mistake in repairing DNA double-strand breaks. Chromosomal breakpoints lead to the fusion of the 3′ sequence of RET encoding the kinase domain to the 5′ sequence of another upstream partner gene, encoding a dimerization and localizing domain, which in turn produces an active RET fusion protein.26–30 The malignant potential of RET fusion proteins is determined by two mechanisms: (1) ligand-independent constitutive proliferative signaling and (2) impaired RET inactivation by endocytosis and recruitment of membrane-associated ubiquitin ligases.31,32
The first human chromosomal RET rearrangement was identified in PTC by Fuso et al. in 1987. They noted a fusion of the RET tyrosine kinase domain with the 5′ terminal region of CCDC6. This chromosomal rearrangement was named RET-PTC1. 33 Although RET fusions genes identified with PTC were labeled historically in numeric order (e.g. RET-PTC1, RET-PTC2, RET-PTC3/4), the preferred current nomenclature includes the fusion-partner gene name (e.g. CCDC6-RET, PRKAR1A-RET, and NCOA4-RET). Multiple RET rearrangements have been described and are cell-specific somatic fusions; no germline RET fusions have been identified (Table 1).9,33–46 RET fusions are found in ~6–10% of PTC, 6% of PDTC and are less frequently in ATC.9,10,47,48 The prevalence of RET fusions is higher (approximately 60–80%) in radiation-induced thyroid cancer as evidenced after the Chernobyl nuclear accident and the atomic bomb in Japan.49–51 RET fusions are seen more often in children and young adults diagnosed with thyroid cancers.52–57
Table 1.
RET fusions.
| Study | RET/PTC type – historical nomenclature | Partner gene | Tumor type |
|---|---|---|---|
| Grieco et al. 33 | RET/PTC1 | CCDC6 | PTC, NSCLC, CRC |
| Bongarzone et al. 34 | RET/PTC2 | PRKAR1A | PTC |
| Santoro et al.
35
Fugazzola et al. 36 |
RET/PTC3 RET/PTC4 |
NCOA4 | PTC, NSCLC, CRC |
| Klugbauer et al. 37 | RET/PTC5 | GOLGAS | PTC |
| Klugbauer and Rabes 38 | RET/PTC6 | TRIM24 | PTC |
| Klugbauer and Rabes 38 | RET/PTC7 | TRIM33 | PTC, NSCLC |
| Nakata et al. 39 | ELKS-RET | ELKS | PTC |
| Salassidis et al. 40 | RET/PTC8 | KTN1 | PTC |
| Klugbauer et al. 41 | RET/PTC9 | RFG9 | PTC |
| Corvi et al. 42 | PCM1-RET | PCM1 | PTC |
| Saenko et al. 43 | ∆RFP-RET | TRIM27 | PTC |
| Ciampi et al. 44 | HOOK3-RET | HOOK3 | PTC |
| Cancer Genome Atlas Research Network 9 | ERC1-RET | ERC1 | PTC |
| Cancer Genome Atlas Research Network 9 | AKAP13-RET | AKAP13 | PTC |
| Cancer Genome Atlas Research Network 9 | TBL1XR1-RET | TBL1XR1 | PTC |
| Cancer Genome Atlas Research Network 9 | FKBP-RET | FKBP | PTC |
| Cancer Genome Atlas Research Network 9 | SPECC1L-RET | SPECC1L | PTC |
| Cancer Genome Atlas Research Network 9 | RET-ANK3 | ANK3 | PTC |
| Hamatani et al. 45 | ACBD5/RET | ACBD5 | PTC |
| Grubbs et al. 46 | MYH13-RET | MYH13 | MTC |
CRC, colorectal cancer; NSLC, non-small cell lung cancer; PTC, papillary thyroid cancer.
Testing strategies for RET
Germline RET testing is the standard of care for all patients diagnosed with MTC to evaluate for hereditary MTC in the context of MEN2 syndromes. This is regardless of personal or family history of other MEN2-related neoplasias or MTC, as 1–6% of apparently sporadic MTC will harbor a germline RET mutation. 8
Somatic testing for driving mutations in sporadic MTC will be able to identify targetable mutation of RET, clarify if the cancer harbors a resistance mechanism such as the gatekeeper 804, or identify a currently nontargetable mutation of RAS or others. Testing should be done with NGS, PCR, FISH, or liquid biopsy platforms that are validated. 58
RET inhibitors
As RET mutations and fusions result in improper RET kinase domain activation, the ATP binding pocket of the kinase domain is a key target for treatment of the associated cancers. MKIs function as ATP-competitive inhibitors and are categorized into five types: type I competes with ATP when the kinase is in its activated form (i.e. vandetanib, sunitinib, pazopanib); type II binds to the ATP binding site and an adjacent hydrophobic/allosteric site only available when the kinase is inactivated and maintains the inactive conformation of the kinase (i.e. cabozantinib, sorafenib, ponatinib); types III and IV are noncompetitive selective inhibitors that bind to an allosteric site distal to the ATP binding site and the hinge; and type V selectively and irreversibly binds to the active kinase site by forming covalent bonds.59,60
Nonselective multikinase inhibitors
MKIs have nonselective RET inhibition, targeting a spectrum of kinases besides RET often with greater potency. The primary therapeutic target of MKIs is VEGFR-2 to inhibit angiogenesis. The inhibition of VEGFR-2 has been implicated in many of the dose-limiting AEs of these agents, such as hypertension, thrombosis, hemorrhage, and problems with wound healing.61,62 The inferior pharmacokinetic properties contribute to less potent RET inhibition and nonselective targeting of other kinases facilitate drug-related AEs, which can in turn result in dose reduction, interruption, and discontinuation, which limit the efficacy of these drugs (Table 2). 27 Besides having off-target side effects which can be dose limiting, MKIs are ineffective against RET V804 gatekeeper mutations. 63
Table 2.
Common adverse events (AEs) associated with tyrosine kinase inhibitors approved for thyroid cancer, in order of frequency. For further description of grading of AEs, recommend referring to the published trial results.4–6,64–66.
| Sorafenib | Lenvatinib | Vandetanib | Cabozantinib | Selpercatinib | Pralsetinib |
|---|---|---|---|---|---|
| Hand foot skin (76%) | Hypertension (68%) | Diarrhea (57%) | Diarrhea (63%) | Dry mouth (39%) | Leukopenia (34%) |
| Diarrhea (69%) | Diarrhea (59%) | Rash (53%) | Stomatitis (51%) | Hypertension (30%) | Neutropenia (34%) |
| Alopecia (67%) | Fatigue (59%) | Dermatitis acneiform/acne (35%) | Hand foot skin (50%) | Increased AST (28%) | Increased AST (34%) |
| Rash/desquamation (50%) | Decreased appetite (50%) | Nausea (33%) | Weight loss (48%) | Increased ALT (26%) | Hypertension (33%) |
| Fatigue (50%) | Weight loss (46%) | Hypertension (33%) | Decreased appetite (46%) | Fatigue (26%) | Anemia (29%) |
| Weight loss (47%) | Nausea (41%) | Headache (26%) | Nausea (43%) | Peripheral edema (18%) | Constipation (28%) |
| Hypertension (41%) | Stomatitis (36%) | Fatigue (24%) | Fatigue (41%) | Diarrhea (17%) | Asthenia (26%) |
| Anorexia (32%) | Hand foot skin (32%) | Decreased appetite (21%) | Dysgeusia (34%) | Constipation (16%) | Increased ALT (23%) |
| Oral mucositis (23%) | Proteinuria (31%) | Abdominal pain (21%) | Hair color changes (34%) | Nausea (15%) | Hyperphosphatemia (22%) |
| Pruritus (21%) | Vomiting (28%) | Dry skin (15%) | Hypertension (33%) | Increased creatinine (14%) | Lymphopenia (20%) |
| Nausea (21%) | Headache (28%) | Vomiting (15%) | Constipation (27%) | Headache (13%) | Increased creatinine (18%) |
| Headache (18%) | Dysphonia (24%) | QT prolongation (14%) | Abdominal pain (27%) | QT prolongation (13%) | Muscle/joint pain (18%) |
| Cough (15%) | Arthralgia (18%) | Photosensitivity reaction (13%) | Vomiting (24%) | Dysgeusia (16%) | |
| Constipation (15%) | Dysgeusia (17%) | Hypocalcemia (11%) | Dysphonia (20%) | Diarrhea (16%) | |
| Low platelets (15%) | |||||
| Edema (15%) | |||||
| Headache (13%) | |||||
| Dry mouth (12%) |
Approved nonselective MKIs for DTC: sorafenib and lenvatinib
Sorafenib and lenvatinib are MKIs with strong VEGFR blockade. DECISION (NCT00984282 for sorafenib) and SELECT (NCT01321554 for lenvatinib) were the phase III clinical trials that led to approval by the US Food and Drug Administration (FDA) and by the European Union European Medical Agency (EMA) for the treatment of locally recurrent or metastatic progressive RAIR DTC. Keeping in mind that these clinical trials are not comparable to each other, sorafenib showed an increase of 5 months and lenvatinib of 14.7 months in the median progression-free survival (PFS) compared with placebo (primary end point in both trials). Sorafenib showed an overall response rate (ORR) (all PRs) of 12.2% and lenvatinib of 64.8% (CR 1.5% and PR 63.2%). There was no difference in overall survival (OS) between the sorafenib and placebo groups; however, this was confounded by the fact that 71.4% of the placebo-randomized patients crossed over to sorafenib treatment on disease progression. An updated survival analysis found that lenvatinib led to an increase in OS – median OS not reached after 34 months of treatment in the lenvatinib group compared with 19.1 months in the placebo crossover arm (HR = 0.53; p = 0.0051). 67
In general, the most common AEs to both drugs were hypertension, diarrhea, skin/hair/mucous membranes alterations [hand–foot–skin reaction (HFSR), rash, desquamation, alopecia, mucositis], fatigue, decreased appetite, weight loss, and nausea. Common reported laboratory abnormalities were elevation in serum thyroid-stimulating hormone (TSH), hypocalcemia, and proteinuria. AEs prompted dose interruptions (66.2%), reductions (64.3%), or withdrawals (18.8%) in patients receiving sorafenib. HFSR was the most common reason for these to occur. Lenvatinib led to a dose interruption, reduction, and discontinuation in 82.4%, 67.8%, and 14.2% of patients, respectively. Asthenia and hypertension were the most frequent AEs leading to dose discontinuation (each in 1.1% of patients); while diarrhea (22.6%), hypertension (19.9%), proteinuria (18.8%), and decreased appetite (18.0%) were the most common reasons for lenvatinib treatment interruption or reduction.4,64
Approved nonselective MKIs for MTC: vandetanib and cabozantinib
Vandetanib and cabozantinib are MKIs with predominant VEGFR blockade; however, vandetanib also inhibits epidermal growth factor receptor (EGFR) while cabozantinib targets mesenchymal epithelial transition factor (MET). ZETA (NCT00410761 for vandetanib) and EXAM (NCT00704730 for cabozantinib) were the phase III clinical trials that led to approval by the FDA and EMA for the treatment of progressive, unresectable, locally advanced, or metastatic MTC, regardless of tumor genotype.5,6 Vandetanib showed an increase of 11.2 months and cabozantinib of 7.2 months in median PFS compared with placebo. Vandetanib showed an ORR of 45% (all PRs) and cabozantinib of 28% (all PRs). Responses were seen regardless of RET mutation status; however, a subgroup analysis in the vandetanib trial showed a PFS statistical benefit in MTC harboring the somatic RET M918T mutation compared with placebo; and a similar observation was appreciated with cabozantinib in germline/somatic RET M918T mutation. The cabozantinib trial also suggested a PFS benefit in RAS-mutated MTC when compared with placebo but this was not statistically significant due to the small number of patients analyzed. Both trials are not comparable to each other, and it is important to highlight that the cabozantinib trial required radiological progression before enrollment, whereas the vandetanib study did not. Both drugs led to a biochemical response rate of calcitonin reduction in more than 45% from baseline with a similar trend in carcinoembryonic antigen (CEA). At the study cut-off date, the median OS calculation for vandetanib was not reached; however, it is likely to be confounded due to cross-over from placebo to the active treatment in progressing patients. There was a 5.5 month improvement in median OS with cabozantinib over placebo (26.6 versus 21.1 months) that was not statistically significant. 68 Patients receiving placebo were not allowed to cross over to cabozantinib at the time of progression in the trial.
In general, the most common AEs to both drugs were diarrhea, stomatitis, rash, palmar-plantar erythrodysesthesia (PPE), decreased weight and appetite, nausea, vomiting, abdominal pain, fatigue, hypertension, and headache. Potential laboratory abnormalities consisted of elevated liver function tests (LFTs), electrolyte disorders (hypocalcemia, hypophosphatemia, hypomagnesemia, hypokalemia, hyponatremia), alterations in complete blood count (lymphopenia, neutropenia, thrombocytopenia), proteinuria, and elevated TSH. AEs prompting dose reductions or withdrawals occurred in 35% and 12% of patients receiving vandetanib, respectively. Asthenia (1.7%) and rash (1.3%) were the most frequent AEs leading to discontinuation of vandetanib. QTc prolongation was present in 8% of patients receiving vandetanib. Five patients on vandetanib had AEs leading to death (aspiration pneumonia, respiratory arrest, respiratory failure, staphylococcal sepsis, and arrhythmia with cardiac failure). 5 A Risk Evaluation and Mitigation Strategy (REMS) is required to prescribe vandetanib due to its black box warning indicating the possibility of QT prolongation, torsade de pointes or sudden death. Cabozantinib had dose reduction, interruption, and discontinuation rates of 79%, 65%, and 16% of patients, respectively. Cabozantinib led to rare but serious AEs associated with its VEGF pathway inhibition (perforation, fistula, hemorrhage, thrombosis, impaired wound healing, osteonecrosis, and HFSR). 6 Cabozantinib has a black box warning listing gastrointestinal perforations, fistulas, and hemorrhage (including hemoptysis and gastrointestinal hemorrhage); therefore, it should be used with caution or avoided in patients with a history of radiation to the neck or mediastinum and injury to the respiratory or gastrointestinal mucosa (i.e. diverticulitis, inflammatory bowel disease, active peptic ulcer disease). In addition, tumor invasion of the trachea, bronchi, and esophagus increases the risk of fistula formation. Tumors that encase major blood vessels or invade the GI mucosa are associated with a high risk of bleeding; whereas conditions known to cause acute surgical abdomen such as cholecystitis and appendicitis are at increased risk of organ perforation.69,70 Clinically relevant QTc prolongation >500 ms was not appreciated in the cabozantinib phase III trial, as it was with vandetanib.6,71
Selective RET inhibitors
These are small molecule, ATP-competitive, potent, and highly selective RET inhibitors designed to overcome gatekeeper RET mutations and associated with less toxicity, dose reductions, and treatment discontinuations (Table 3). 72
Table 3.
Efficacy of selective RET inhibitors phase I/II clinical trials.
| Agents | References | Trial design (name) | Subjects (n)/ cancer type | ORR
a
n (%) |
CR n (%) |
PR n (%) |
SD n (%) |
PFS (at 1 year) |
OS (at 1 year) |
|---|---|---|---|---|---|---|---|---|---|
| Selpercatinib | Wirth et al. | Phase I/II trial (LIBRETO-001) |
55 RET + MTC previously treated b | 38 (69%) | 5 (9%) | 33 (60%) | 14 (25%) | 82% | NR |
| 88 RET + MTC treatment naive | 64 (72%) | 10 (11%) | 54 (61%) | 20 (23%) | 92% | NR | |||
| 19 RET fusion + thyroid cancer c | 15 (79%) | 1 (5%) | 14 (74%) | 4 (21%) | 64% | NR | |||
| Pralsetinib | Subbiah/Hu et al. | Phase I/II trial (ARROW) |
55 RET + MTC previously treated b | 33 (60%) | 1 (2%) | 32 (58%) | 18 (33%) | 75% | 89% |
| 21 RET + MTC treatment naïve | 15 (71%) | 1 (5%) | 14 (67%) | 6 (29%) | 91% | 91% | |||
| 9 RET fusion + thyroid cancer d | 8 (89%) | 0 | 8 (89%) | 0 | 81% | 91% |
CR, complete response; MTC, medullary thyroid cancer; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR partial response; SD, stable disease.
Primary outcome.
With vandetanib and cabozantinib.
Thirteen papillary thyroid cancer (PTC), three poorly differentiated thyroid carcinoma (PDTC), two anaplastic thyroid carcinoma, one Hurthle cell carcinoma.
Ten PTC, one PDTC (9/11 response-evaluable patients).
Selpercatinib
The safety and efficacy of selpercatinib (formerly known as LOXO-292) was evaluated in an international, multicenter, open-label, phase I/II trial known as LIBRETTO-001 (NTC03157128). 65 A total of 531 patients (⩾12 years old) with any locally advanced or metastatic solid tumor type harboring an activating REt alteration were enrolled in the study, out of which 162 patients had a RET-altered thyroid cancer. In the thyroid cancer population, 55 patients had RET-mutant MTC previously treated with vandetanib and cabozantinib, 88 patients had RET-mutant MTC not previously treated with vandetanib or cabozantinib, and 19 patients had RET fusion-positive previously treated non-MTC (13 PTC, 3 PDTC, 2 ATC, 1 Hurthle cell). RET M918T mutation and CCDC6-RET fusion were the most common REt alterations; in addition, patients with the acquired gatekeeper-resistance mutation RET V804 were included in the study.
Eligible patients had advanced disease that have progressed following prior treatment or had no acceptable alternative treatment options. In addition, eligible RET fusion-positive thyroid cancer patients were defined as having RAIR disease (except for ATC in which radioiodine is not used) and had received at least one previous systemic therapy other than radioiodine. It is important to note that radiographic tumor progression was not a definite inclusion criterion, and patients with progressive disease on study drug could continue selpercatinib if they were receiving clinical benefit overall.
Phase I was the dose-escalation portion of the study and determined the recommended phase II dose of 160 mg twice daily by mouth. RET-mutant MTCs previously treated with vandetanib and cabozantinib had an ORR (primary endpoint) of 69% [CR 9% (n = 5), PR 60% (n = 33), SD 25% (n = 14)]; and a 1-year PFS of 82%. RET-mutant MTCs not previously treated with vandetanib or cabozantinib had an ORR of 73% [(CR 11% (n = 10), PR 61% (n = 54), SD 23% (n = 20)]; and a 1-year PFS of 92% (95% CI, 82–97). RET fusion-positive non-MTC patients had an ORR of 79% with activity seen across all histologic types [CR 5% (n = 1), PR 74% (n = 14)] and a 1-year PFS of 64%. Patients with RET-mutant previously treated MTC had a biochemical response of 91% (54 patients evaluated) regarding calcitonin (median time to biochemical response = 0.5 months) and 66% (53 patients evaluated) regarding CEA (median time to biochemical response = 1.8 months). The efficacy of selpercatinib treatment was observed regardless of the number of previous MKI therapies received, radioiodine treatments, or type of RET mutation/fusion. Notably, responses were observed in 3 of the 8 MTC patients with RET V804 gatekeeper mutations.
The most common treatment-related adverse events (TRAEs) were dry mouth, hypertension, fatigue, peripheral edema, diarrhea, constipation, nausea, headache, QTc prolongation, rash, vomiting, abdominal distention, dizziness, arthralgia, increased weight, abdominal pain, cough, and back pain. Laboratory abnormalities consisted of elevated LFTs, increased serum creatinine, elevated TSH, and electrolyte abnormalities (hypocalcemia, hyponatremia). Most TRAEs were grade 1 or 2; however, the most common grade 3 or 4 AE was hypertension (12%) followed by increased LFTs, diarrhea, and prolonged QTc. The investigators deemed all grade 5 AEs (hemoptysis, postprocedure hemorrhage, sepsis, cardiac failure, and cardiac arrest) to be unrelated to selpercatinib. One patient with MTC developed grade 3 tumor lysis syndrome. Of the total 531 cohort of patients who received selpercatinib, 160 (30%) had a dose reduction and 12 (2%) had drug discontinued due to TRAEs.
Based on results from the phase I/II LIBRETTO-001 trial, selpercatinib was approved by the FDA in May 2020 and by the EMA in February 2021 for the treatment of advanced or metastatic RET fusion-positive non-small cell lung cancer (NSCLC), RET fusion-positive RAIR-thyroid cancer, and RET-mutant MTC in adults and children ⩾ 12 years old. The treatment dose is 160 mg oral twice daily or a reduced dose of 120 mg twice daily in patients who weigh <50 kg. 73 A large, international phase III trial comparing selpercatinib with standard of care (based on investigator’s choice of cabozantinib or vandetanib) is actively enrolling RET-mutated, treatment naïve MTC patients with the clinically meaningful primary outcome of treatment failure-free survival (NCT04211337).
Pralsetinib
Subbiah et al. 74 reported that the antitumor activity of pralsetinib (formerly known as BLU-667) was ⩾10-fold than vandetanib and cabozantinib in preclinical models harboring different RET oncogenic alterations (KIF5B-RET, CCDC6-RET, RET C634W, RET M918T, and gatekeeper mutations RET V804L/M/E) with comparatively less activity against VEGFR-2.
ARROW is an ongoing, international, multicenter, open-label phase I/II clinical trial (NCT03037385) evaluating pralsetinib in patients with unresectable, locally advanced, or metastatic RET-driven solid cancers. Patients ⩾ 18 years old with both kinase inhibitor-naive and kinase inhibitor-refractory disease, as well as any number of prior therapies, were eligible. The phase I dose-escalation study recommended the phase II dose of 400 mg oral once daily. The phase II portion enrolled the following thyroid cancer cohorts: (1) RET-mutant MTC and (2) RET-fusion-positive thyroid cancer. For inclusion in the MTC group, patients were required to have disease progression within 14 months before enrollment. All subjects required measurable disease at the time of enrollment and a confirmed pathogenic RET mutation. The primary outcomes from phase II were ORR and safety. Most secondary outcomes at the time of data cut-off were not reached and included median duration of response (DoR), clinical benefit rate, disease control rate (DCR), PFS, and OS. 66
The RET-mutant MTC cohort consisted of a total of 84 patients with mostly sporadic disease and harboring different activating mutations: 58% M918T, 31% cysteine- rich domains, 7% V804L/M (including 3 of whom also had a coincident M918T mutation), and 7% other mutations. Of these, 55 response-evaluable patients (REP) were previously treated with cabozantinib and vandetanib and 21 REP were treatment-naïve.
In RET-mutant MTC patients previously treated with cabozantinib and vandetanib, the ORR was 60% (n = 33/55; 95% CI = 46–73) with 2% CR (n = 1/55). Median time to first response was 3.7 months. The median DoR was not reached with median follow-up of 11.2 months. The estimated ongoing response at 6 months was 96% and at 12 months was 92%. The estimated 1-year PFS was 75% after a median follow-up of 14.9 months. The estimated 1-year OS was 89% after median follow-up of 16.5 months. 66
Among treatment naïve RET-mutant MTC patients, the ORR was 71% (n = 15/21; 95% CI = 48–89) with 5% CR (n = 1/21). Median time to first response was 5.6 months. The median DoR was not reached with median follow-up of 10.8 months. The estimated ongoing response at 6 months was 93% and at 12 months was 84%. The estimated 1-year PFS was 81% after a median follow-up of 15.1 months. The estimated 1-year OS was 91% after median follow-up of 18.5 months. 66
Responses were observed regardless of the RET mutation in both MTC cohorts, including gatekeeper mutations V804L/M. Disease-related diarrhea resolved in 14/15 patients by the end of the second cycle. Biochemical response rates of calcitonin and CEA were 87% (n = 72/83) and 66% (n = 52/79), respectively. 66
The RET-fusion positive thyroid cancer cohort included 10 PTC and 1 PDTC. RET-fusion partners included CCDC6 (6, 55%), NCOA4 (2, 18%), and other (3, 27%). Patients with RAIR disease and any prior systemic therapy were allowed in the study. Of the 9 REP, the ORR was 89% (n = 8/9; 95% CI = 52–100) with all PR. Median time to first response was 1.9 months. The median DOR was not reached with a median follow-up of 9.5 months. The estimated ongoing response at 6 months was 100% and at 12 months was 86%. The estimated 1-year PFS was 81% after a median follow-up of 12.9 months. The estimated 1-year OS was 91% after median follow-up of 15.8 months. Responses were observed across fusion genotypes. 66
The ARROW study reported safety for the total 142 patients with RET-altered thyroid cancer who initiated pralsetinib at the recommended phase II dose of 400 mg PO daily. The most common TRAEs were anemia, musculoskeletal pain, constipation, elevated AST, and hypertension. Common grade 3 and above AEs were hypertension and blood cell alterations (neutropenia, lymphopenia, and anemia). The most frequent serious TRAE was pneumonitis in 4% (n = 5). Dose reduction owing to TRAEs occurred in 46% (n = 66), dose interruptions in 54% (n = 76), and treatment discontinuation in 4% (n = 5 due to anemia in 2 patients, pneumonia in 1, elevated CPK in 1, and ARDS and pneumonitis in 1). One patient died after developing interstitial pneumonitis considered a TRAE who succumbed due to Pneumocystis jirovecii pneumonia. QTc prolongation was seen in 7 patients, mostly grade 1–2 with one being grade 3. 66
Based on the data from the phase I/II ARROW study, the FDA granted breakthrough therapy designation to pralsetinib in September 2020 for the treatment of adults with metastatic RET fusion-positive NSCLC. In December 2020, the FDA approved pralsetinib for the treatment of adults and pediatric patients ⩾12 years old with advanced or metastatic RET-mutant MTC who require systemic therapy, or with advanced or metastatic RET fusion-positive nonmedullary RAIR thyroid cancer who require systemic therapy. The initial treatment dose for both adults and children ⩾12 years old is 400 mg oral once daily. The ARROW study is ongoing and continues to enroll patients in the non-MTC cohort and other solid tumors (excluding NSCLC) with RET-alterations with an estimated primary completion date of December 2021. 66
Both selpercatinib and pralsetinib seem equally effective for these patient populations. The only clearly distinguishable features are the difference in dosing interval (selpercatinib given twice a day; pralsetinib given once a day) and that selpercatinib can be dissolved per specified instructions.
TPX-0046
TPX-0046 is a novel, potent, and selective inhibitor of both RET and SRC with a rigid macrocyclic structure unlike other RET inhibitors which makes it active against various mutations, especially the emergent SFM RET G810 that conveys resistance to selpercatinib and pralsetinib (discussed later in this article). Its potency was evidenced in vitro with cell culture proliferation assays where at low nanomolar levels, TPX-0046 inhibited wild-type RET, many mutated RET receptors, SRC, but spared VEGFR2. TPX-0046 was also able to overcome the SFM G810R at a mean IC50 of 17 nM compared with selpercatinib and pralsetinib which have IC50s >500 nM. In addition, TPX-0046 showed robust anti-tumor efficacy with in vivo cell-derived and patient-derived xenograft RET-driven tumor models. 75 However, TPX-0046 does not target the gatekeeper V804, which limits its effectiveness in some patients with MTC especially if they harbor both gatekeeper and SFMs. It is currently undergoing a phase I/II clinical trial (NCT04161391) in adult subjects (⩾18 years old) with advanced, progressive, or metastatic solid tumors harboring RET fusions or mutations.
BOS172738
BOS172738 is a small molecule RET inhibitor that has demonstrated robust low nanomolar potency (kd ⩽1 nM) against wild-type RET and fusion and mutated protein receptors including M918T, V804L, and V804M, while keeping approximately 300-fold selectivity against VEGFR2. BOS172738 produced durable tumor regression and tumor growth inhibition at similar or lower IC50 concentrations compared with ponatinib in preclinical studies.76,77 It is currently undergoing a phase I (NCT03780517), open label, multicenter, dose escalation study to evaluate safety, tolerability, pharmacokinetics, ad pharmacodynamics in adult patients (⩾18 yo) with advanced solid tumors with REt alterations.
TAS0953/HM06
TAS0953/HM06 is a selective RET inhibitor undergoing a phase I/II clinical trial (MARGARET study) (NCT04683250) in adult patients (⩾18 years old) with advanced, progressive, or metastatic RET-altered solid tumors with or without prior MKI therapy. Phase I aims to recommend the maximum tolerated dose and dose expansion whereas phase II will determine ORR as primary outcome.
Mechanisms of resistance to RET inhibitors
Resistance mechanisms are usually first identified in preclinical studies followed by patient studies, and later overcome with the discovery of new drugs. However, with the accessibility and wider use of NGS of progressing tumors and liquid biopsy, real-time identification of emergent mutations in patients that progress on treatments can offer insight into possible resistance mechanisms optimizing further treatment planning. Acquired resistance to RET inhibitors takes place via two different mechanisms: (1) on-target mutations (target modification) that prevent drug binding and (2) activated alternative mechanisms which bypass the targeted kinase (bypass signaling).
Target modification
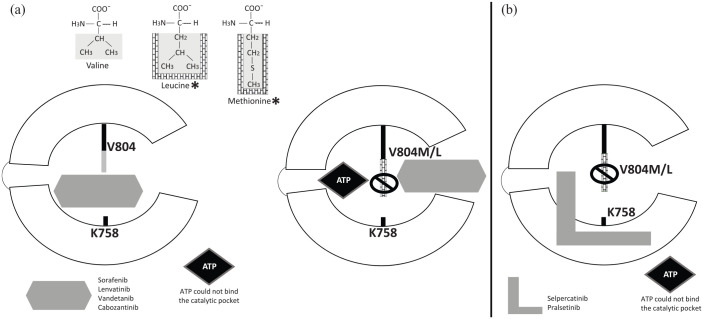
The presence of the gatekeeper RET V804M/L mutations will convey resistance to MKIs with low selectivity for RET (Table 4).27,63,78–81 This takes place because MKIs bind to the front and back clefts of the RET kinase catalytic pocket by inserting through the V804 gate residue that separates the two clefts. V804 mutants with bulkier leucine or methionine side chains prevent insertion of the MKIs between the front and back clefts (Figure 3(a)).18,63 RET gatekeeper mutations V804M/L have been reported in NSCLC and MTC.78,80,82,83 Another point mutation of RET S904F in the activation loop reduces vandetanib drug binding. 84
Table 4.
Mechanisms of resistance and IC50 (μM) for each drug.
| Mutation status | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| Lenvatinib | Vandetanib | Cabozantinib | Selpercatinib | Pralsetinib | ||
| RET wild type | 0.19 | 0.1 | 0.0098 | 0.0004 | 0.0004 | |
| M918T | 1.42 | 1.83 | 1.57 | 0.009 | 0.001 | |
| Gatekeeper | V804L | 10.60 | 6.10 | 3.22 | 0.0172 | 0.0018 |
| V804M | 5.42 | 5.83 | 4.26 | 0.0559 | 0.0168 | |
| Solvent front | G810A | 0.11 | 2.76 | 0.22 | – | – |
| G810R | – | – | – | 2.744 | 2.650 | |
| G810S | 0.67 | 5.47 | 1.05 | 0.8802 | 0.3906 | |
| G810C | – | – | – | 1.227 | 0.6417 | |
| Fusion | CCDC6-RET | – | 0.02 | 0.034 | 0.01 | 0.00045 |
| VEGFR2 | 0.004 | 0.04 | 0.000035 | 0.1 | 0.035 | |
IC50 concentration causes 50% inhibition of growth.
The values are mean.
Gray: resistant; black: nonresistant.
Figure 3.
Mechanism of resistance to RET inhibition: Target modification V804M/L gatekeeper mutation. RET tyrosine kinase domain catalytic cleft as shown in Figure 2. (a) Asterisks indicate structural differences of leucine and methionine with bulkier side chains compared with nonmutant valine residue. The bulkier side chains (in brick wall) prevent multitargeted tyrosine kinase inhibitors (sorafenib, lenvatinib, vandetanib, cabozantinib) to communicate between the front and back clefts of the RET receptor kinase domain catalytic cleft. (b) Selpercatinib and pralsetinib can overcome gatekeeper mutations as they bind to the front and back clefts of the RET kinase domain catalytic pocket without going through the gate between V804 and K758. Instead, they pass around the gate wall K758 residue to access the back pocket.
The selective RET inhibitors were designed to overcome gatekeeper mutations. Both selpercatinib and pralsetinib bind to the front and back clefts of the RET kinase catalytic pocket without going through the gate between V804 and K758. Instead, they pass around the gate wall K758 residue to access the back pocket (Figure 3(b)). In this way, gatekeeper mutations do not disrupt their binding mode, but they remain vulnerable to several identified nongatekeeper mutations.63,72,74
In addition, several emergent nongatekeeper mutations have been identified recently. The RET G810 residue is located at the C-lobe solvent front side. Mutations at this site are known as SFMs, where glycine (which has a smaller side chain) is replaced with aminoacids like alanine, cysteine, serine, arginine, or valine (G810A/C/S/R/V, which have larger side chains that prevent drug binding).63,85 A resistant SFM was first described in a KIF5B-RET-fusion NSCLC patient who initially responded to selpercatinib on study but then progressed correlating with detectable circulating tumor DNA for G810R/S/C. 86 In addition, a MTC patient with dual mutations of RET M918T and V804M had initial durable response to selpercatinib but then progressed with demonstration of rising levels of cell-free DNA for M918T, V804M as well as G810C/S mutations. 63 Acquired SFMs G810C/S were also reported in CCDC6-RET fusion NSCLC patients at the time of progression while on pralsetinib. 87
In addition to RET gatekeeper and SFMs, Subbiah et al. 63 identified additional mutations affecting other portions of the RET receptor which contributed to diminished efficacy of selective RET-inhibitors. The RET Y806 residue is located at the hinge site of the kinase domain where the hydrophobic side chain of tyrosine allows van der Waals interactions with selpercatinib and pralsetinib rings. These interactions would be interrupted if cysteine or asparagine are substituted in for tyrosine (Y806C/N) as they have nonhydrophobic, shorter side chains. In addition, selpercatinib and pralsetinib interact with the side chain V738 located on the ß2 strand of N-lobe in the front pocket. This interaction would be lost if V738 was substituted with the shorter side chain of alanine (V738A). The IC50s of both selpercatinib and pralsetinib against RET Y806 hinge and V738A mutations were approximately over 150 nM. 63
Second-generation selective RET inhibitors are being developed with the aim to overcome evolving mutations. Although TPX-0046 has the benefit of overcoming SFMs, it is not effective against the gatekeeper V804 mutations. 75
Bypass signaling
RET-altered tumors can develop escape mechanisms to drug receptor inhibition by activating oncogenic alternative or downstream pathways independent of RET activation. Preclinical and clinical data on RET-fusion NSCLC have shown that RET-inhibition can be overcome through oncogenic activation of MET, EGF, and RAS.88–91 Coincident activating BRAF, KRAS, and NRAS mutations have been demonstrated in RET-altered PTCs, although it is hard to assess whether they are present in the same cell or represent different clones of cells within the same tumor.92,93 Hu et al. 94 reported the emergence of KRAS pan G12/G13 bypass mutation alone or combined with RET gatekeeper mutation V804M in sporadic MTC patients with progressive disease after a minimum of 6-month treatment with cabozantinib and vandetanib. A targeted drug combination therapy such as a selective kinase inhibitor with a MEK-inhibitor (for patients with RAS bypass mutations) or an m-TOR inhibitor (for inhibition of the PI3K/AKT pathway) could potentially be used to overcome bypass signaling.95–97
Uncertainty of RET inhibition on nontumor cells
The effects of long-term RET inhibition on normal tissues are not completely understood but remain as potential risks that should be carefully considered. For instance, potential linear growth and development retardation in children with open epiphyses remain uncertain as well as the effects on fertility. There is animal toxicity data showing abnormal bone growth and tooth dysplasia and discoloration in rats and minipigs, hence the suggestion to monitor open growth plates in adolescent patients. 98 The selective RET-inhibitors, pralsetinib, and selpercatinib, are known to have good penetration into the central nervous system (CNS) which is clinically beneficial for patients with CNS metastases; however, there is concern of neuron disruption, particularly dopaminergic neurons where GFL-RET signals are important for survival. The inhibition of RET signaling could also potentially affect hematopoietic cells with a negative impact on immune responses as seen in animal models. 99 Since these medications are relatively new for use in RET-altered cancer patients, long-term monitoring will help clarify potential repercussions on normal physiology from decreased RET activity.
Conclusion and further directions
Precision oncology in the treatment of RET-dependent cancers is an evolving field with promising outcomes with the development of highly selective and potent RET inhibitors. The approval of selpercatinib and pralsetinib in 2020 based on phase I/II trials demonstrating high response rates in both RET-mutated MTC (previously treated and kinase inhibitor-naïve) and RET-fusion non-MTC balanced with fewer off-target side effects gives rise to an optimistic new phase in the management of these rare cancers. However, the development of on-target or bypass resistance mechanisms likely will become more common with wider use of RET inhibitors. Further research and development of potent RET inhibitors with broader coverage of known (gatekeeper and SFMs) and potential resistance mechanisms are much needed. In addition, exploration of combination therapies should be undertaken to optimally target-activated intracellular pathways. Advanced thyroid cancers with RAS or other nontargetable mutations can be treated with the approved nonselective MKIs, but as discussed in this review, dose-limiting toxicities often limit their effectiveness. More effective treatments are much needed for such patients with targetable mutations. The potential long-term repercussions of potently inhibiting normal RET physiology of other cells remain incompletely understood and should not be dismissed. It is tempting to consider implementing a highly effective and well-tolerated drug earlier on in a patient’s care if there is biochemical progression without significantly burdensome or progressive structural disease. However, unless clinical trials demonstrate oncologic benefit of this treatment paradigm and long-term risk of RET inhibition is found to be minimal, use of RET inhibitors for advanced RET-altered thyroid cancer should remain reserved for patients with structurally progressive disease not amenable to other treatments.
Footnotes
Author contribution(s): Danica M. Vodopivec: Conceptualization; Data curation; Investigation; Methodology; Resources; Visualization; Writing – original draft.
Mimi I. Hu: Conceptualization; Data curation; Investigation; Methodology; Resources; Supervision; Validation; Visualization; Writing – review & editing.
ORCID iD: Danica M. Vodopivec  https://orcid.org/0000-0001-6605-4667
https://orcid.org/0000-0001-6605-4667
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Danica M. Vodopivec, Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center, Houston, TX 77030-4000, USA.
Mimi I. Hu, Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
References
- 1. Seidlin SM, Marinelli LD, Oshry E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J Am Med Assoc 1946; 132: 838–847. [DOI] [PubMed] [Google Scholar]
- 2. Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1084–1086. [DOI] [PubMed] [Google Scholar]
- 3. Worden F, Fassnacht M, Shi Y, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer 2015; 22: 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372: 621–630. [DOI] [PubMed] [Google Scholar]
- 5. Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. JCO 2012; 30: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. JCO 2013; 31: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. COSMIC, https://cancer.sanger.ac.uk/cosmic
- 8. Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25: 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016; 126: 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018; 15: 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985; 42: 581–588. [DOI] [PubMed] [Google Scholar]
- 13. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005; 16: 441–467. [DOI] [PubMed] [Google Scholar]
- 14. Anders J, Kjær S, Ibáñez CF. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem 2001; 276: 35808–35817. [DOI] [PubMed] [Google Scholar]
- 15. Ibáñez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol 2013; 5: a009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi M, Asai N, Iwashita T, et al. Characterization of the ret proto-oncogene products expressed in mouse L cells. Oncogene 1993; 8: 2925–2929. [PubMed] [Google Scholar]
- 17. Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 1996; 85: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 18. Knowles PP, Murray-Rust J, Kjær S, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem 2006; 281: 33577–33587. [DOI] [PubMed] [Google Scholar]
- 19. Linden OPJ, van Kooistra AJ, Leurs R, et al. KLIFS: a knowledge-based structural database to navigate kinase–ligand interaction space. J Med Chem 2014; 57: 249–277. [DOI] [PubMed] [Google Scholar]
- 20. Schuchardt A, D’Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994; 367: 380–383. [DOI] [PubMed] [Google Scholar]
- 21. Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell 2005; 8: 65–74. [DOI] [PubMed] [Google Scholar]
- 22. Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 1993; 119: 1005–1017. [DOI] [PubMed] [Google Scholar]
- 23. Jain S, Naughton CK, Yang M, et al. Mice expressing a dominant-negative RET mutation phenocopy human Hirschsprung disease and delineate a direct role of RET in spermatogenesis. Development 2004; 131: 5503–5513. [DOI] [PubMed] [Google Scholar]
- 24. Fonseca-Pereira D, Arroz-Madeira S, Rodrigues-Campos M, et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 2014; 514: 98–101. [DOI] [PubMed] [Google Scholar]
- 25. Takaya K, Yoshimasa T, Arai H, et al. Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J Mol Med (Berl) 1996; 74: 617–621. [DOI] [PubMed] [Google Scholar]
- 26. Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 2016; 12: 192–202. [DOI] [PubMed] [Google Scholar]
- 27. Subbiah V, Yang D, Velcheti V, et al. State-of-the-art strategies for targeting RET -dependent cancers. JCO 2020; 38: 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santoro M, Carlomagno F. Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol 2013; 5: a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol 2006; 155: 645–653. [DOI] [PubMed] [Google Scholar]
- 30. Greco A, Borrello MG, Miranda C, et al. Molecular pathology of differentiated thyroid cancer. Quart J Nucl Med Mol Imag 2009; 53: 440–453. [PubMed] [Google Scholar]
- 31. Richardson DS, Gujral TS, Peng S, et al. Transcript level modulates the inherent oncogenicity of RET/PTC oncoproteins. Cancer Res 2009; 69: 4861–4869. [DOI] [PubMed] [Google Scholar]
- 32. Hyndman BD, Crupi MJF, Peng S, et al. Differential recruitment of E3 ubiquitin ligase complexes regulates RET isoform internalization. J Cell Sci 2017; 130: 3282–3296. [DOI] [PubMed] [Google Scholar]
- 33. Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990; 60: 557–563. [DOI] [PubMed] [Google Scholar]
- 34. Bongarzone I, Monzini N, Borrello MG, et al. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RI alpha of cyclic AMP-dependent protein kinase A. Mol Cell Biol 1993; 13: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santoro M, Dathan NA, Berlingieri MT, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene 1994; 9: 509–516. [PubMed] [Google Scholar]
- 36. Fugazzola L, Pierotti MA, Vigano E, et al. Molecular and biochemical analysis of RET/PTC4, a novel oncogenic rearrangement between RET and ELE1 genes, in a post-Chernobyl papillary thyroid cancer. Oncogene 1996; 13: 1093–1097. [PubMed] [Google Scholar]
- 37. Klugbauer S, Demidchik EP, Lengfelder E, et al. Detection of a novel type of RET rearrangement (PTC5) in thyroid carcinomas after chernobyl and analysis of the involved RET-fused gene RFG5. Cancer Res 1998; 58: 198–203. [PubMed] [Google Scholar]
- 38. Klugbauer S, Rabes HM. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene 1999; 18: 4388–4393. [DOI] [PubMed] [Google Scholar]
- 39. Nakata T, Kitamura Y, Shimizu K, et al. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11; p13) in a papillary thyroid carcinoma. Genes Chromosomes Cancer 1999; 25: 97–103. [DOI] [PubMed] [Google Scholar]
- 40. Salassidis K, Bruch J, Zitzelsberger H, et al. Translocation t(10;14)(q11.2:q22.1) fusing the kinetin to the RET gene creates a novel rearranged form (PTC8) of the RET proto-oncogene in radiation-induced childhood papillary thyroid carcinoma. Cancer Res 2000; 60: 2786–2789. [PubMed] [Google Scholar]
- 41. Klugbauer S, Jauch A, Lengfelder E, et al. A novel type of RET rearrangement (PTC8) in childhood papillary thyroid carcinomas and characterization of the involved gene (RFG8). Cancer Res 2000; 60: 7028–7032. [PubMed] [Google Scholar]
- 42. Corvi R, Berger N, Balczon R, et al. RET/PCM-1: a novel fusion gene in papillary thyroid carcinoma. Oncogene 2000; 19: 4236–4242. [DOI] [PubMed] [Google Scholar]
- 43. Saenko V, Rogounovitch T, Shimizu-Yoshida Y, et al. Novel tumorigenic rearrangement, Δrfp/ret, in a papillary thyroid carcinoma from externally irradiated patient. Mutat Res 2003; 527: 81–90. [DOI] [PubMed] [Google Scholar]
- 44. Ciampi R, Giordano TJ, Wikenheiser-Brokamp K, et al. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocr Relat Cancer 2007; 14: 445–452. [DOI] [PubMed] [Google Scholar]
- 45. Hamatani K, Eguchi H, Koyama K, et al. A novel RET rearrangement (ACBD5/RET) by pericentric inversion, inv(10)(p12.1; q11.2), in papillary thyroid cancer from an atomic bomb survivor exposed to high-dose radiation. Oncol Rep 2014; 32: 1809–1814. [DOI] [PubMed] [Google Scholar]
- 46. Grubbs EG, Ng PK, Bui J, et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab 2015; 100: 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 2008; 93: 3106–3116. [DOI] [PubMed] [Google Scholar]
- 48. Pozdeyev N, Gay LM, Sokol ES, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 2018; 24: 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest 2013; 123: 4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res 2000; 6: 1093–1103. [PubMed] [Google Scholar]
- 51. Hamatani K, Eguchi H, Ito R, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 2008; 68: 7176–7182. [DOI] [PubMed] [Google Scholar]
- 52. Elisei R, Romei C, Vorontsova T, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab 2001; 86: 3211–3216. [DOI] [PubMed] [Google Scholar]
- 53. Nikiforov YE, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997; 57: 1690–1694. [PubMed] [Google Scholar]
- 54. Fenton CL, Lukes Y, Nicholson D, et al. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab 2000; 85: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 55. Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens) 2007; 6: 200–209. [PubMed] [Google Scholar]
- 56. Vanden Borre P, Schrock AB, Anderson PM, et al. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations, including kinase fusions. Oncologist 2017; 22: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Su X, Li Z, He C, et al. Radiation exposure, young age, and female gender are associated with high prevalence of RET/PTC1 and RET/PTC3 in papillary thyroid cancer: a meta-analysis. Oncotarget 2016; 7: 16716–16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Belli C, Penault-Llorca F, Ladanyi M, et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol 2021; 32: 337–350. [DOI] [PubMed] [Google Scholar]
- 59. De Falco V, Carlomagno F, Li HY, et al. The molecular basis for RET tyrosine-kinase inhibitors in thyroid cancer. Best Pract Res Clin Endocrinol Metab 2017; 31: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pottier C, Fresnais M, Gilon M, et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers (Basel) 2020; 12: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu B, Ding F, Liu Y, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget 2016; 7: 67661–67673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang X, Shao Y, Wang K. Incidence and risk of hypertension associated with cabozantinib in cancer patients: a systematic review and meta-analysis. Expert Rev Clin Pharmacol 2016; 9: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 63. Subbiah V, Shen T, Terzyan SS, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol 2021; 32: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014; 384: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020; 383: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021; 9: 491–501. [DOI] [PubMed] [Google Scholar]
- 67. Guo M, Sherman S, Wirth L, et al. 2805 Overall survival gain with lenvatinib vs. Placebo in radioactive iodine refractory differentiated thyroid cancer (RR-DTC): an updated analysis. Eur J Cancer 2015; 51: S559. [Google Scholar]
- 68. Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol 2017; 28: 2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cabanillas ME, Hu MI, Jimenez C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: to treat or not to treat – and with which drug – those are the questions. J Clin Endocrinol Metab 2014; 99: 4390–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maciel LMZ, Magalhães PKR. Medullary thyroid carcinoma – adverse events during systemic treatment: risk-benefit ratio. Arch Endocrinol Metab 2017; 61: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miles DR, Lacy SA, Wada DR, et al. Assessment of cabozantinib treatment on QT interval in a phase 3 study in medullary thyroid cancer: evaluation of indirect QT effects mediated through treatment-induced changes in serum electrolytes. Cancer Chemother Pharmacol 2017; 80: 295–306. [DOI] [PubMed] [Google Scholar]
- 72. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018; 29: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Markham A. Selpercatinib: first approval. Drugs 2020; 80: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET -driven cancers. Cancer Discov 2018; 8: 836–849. [DOI] [PubMed] [Google Scholar]
- 75. Drilon A, Rogers E, Zhai D, et al. 506P – TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann Oncol 2019; 30: v190–v191. [Google Scholar]
- 76. Keegan M, Wilcoxen K, Ho PT. Abstract 2199: BOS172738: a novel highly potent and selective RET kinase inhibitor in Phase 1 clinical development. Cancer Res 2019; 79: 2199–2199. [Google Scholar]
- 77. Schoffski P, Aftimos PG, Massard C, et al. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non-small cell lung cancer and medullary thyroid cancer. JCO 2019; 37: TPS3162–TPS3162. [Google Scholar]
- 78. Liu X, Shen T, Mooers BHM, et al. Drug resistance profiles of mutations in the RET kinase domain. Br J Pharmacol 2018; 175: 3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fancelli S, Caliman E, Mazzoni F, et al. Chasing the target: new phenomena of resistance to novel selective RET inhibitors in lung cancer. Updated evidence and future perspectives. Cancers (Basel) 2021; 13: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004; 23: 6056–6063. [DOI] [PubMed] [Google Scholar]
- 81. Bentzien F, Zuzow M, Heald N, et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid 2013; 23: 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang Q, Schneeberger VE, Luetteke N, et al. Preclinical modeling of KIF5B-RET fusion lung adenocarcinoma. Mol Cancer Ther 2016; 15: 2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wirth LJ, Kohno T, Udagawa H, et al. Emergence of V804M resistance gatekeeper mutation in sporadic medullary thyroid carcinoma patients treated with TKI tyrosine kinase inhibitors. Thyroid 2017; 27: A168. [Google Scholar]
- 84. Nakaoku T, Kohno T, Araki M, et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun 2018; 9: 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Terzyan SS, Shen T, Liu X, et al. Structural basis of resistance of mutant RET protein-tyrosine kinase to its inhibitors nintedanib and vandetanib. J Biolog Chem 2019; 294: 10428–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Solomon BJ, Tan L, Lin JJ, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol 2020; 15: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gainor J, Curigliano G, Doebele RC, et al.OA05.02 analysis of resistance mechanisms to pralsetinib in patients with ret fusion-positive non-small cell lung cancer (NSCLC) from the ARROW study. J Thorac Oncol 2021; 16: S5. [Google Scholar]
- 88. Chang H, Sung JH, Moon SU, et al. EGF induced RET inhibitor resistance in CCDC6-RET Lung cancer cells. Yonsei Med J 2017; 58: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Plenker D, Riedel M, Brägelmann J, et al. Drugging the catalytically inactive state of RET kinase in RET-rearranged tumors. Sci Transl Med 2017; 9: eaah6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-inhibition in RET-rearranged NSCLC is mediated by reactivation of RAS/MAPK signaling. Mol Cancer Ther 2017; 16: 1623–1633, https://mct.aacrjournals.org/content/16/8/1623.long (accessed 30 March 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov 2018; 8: 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guerra A, Zeppa P, Bifulco M, et al. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid 2014; 24: 254–259. [DOI] [PubMed] [Google Scholar]
- 93. Zou M, Baitei EY, Alzahrani AS, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid 2014; 24: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hu MI, Cote GJ, Hai T, et al. Emergence of resistance-associated mutations of RET V804M and KRAS in medullary thyroid carcinoma (MTC) patients treated with tyrosine kinase inhibitors (TKI) cabozantinib and vandetanib. Thyroid 2019; 29: 10. [Google Scholar]
- 95. Koh YW, Shah MH, Agarwal K, et al. Sorafenib and Mek inhibition is synergistic in medullary thyroid carcinoma in vitro. Endocr Relat Cancer 2012; 19: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Heilmann AM, Subbiah V, Wang K, et al. Comprehensive genomic profiling of clinically advanced medullary thyroid carcinoma. Oncology 2016; 90: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gild ML, Landa I, Ryder M, et al. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr Relat Cancer 2013; 20: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. RETEVMO_selpercatinib capsule. Eli Lilly and Company, https://uspl.lilly.com/retevmo/retevmo.html#s49 [Google Scholar]
- 99. Mulligan LM. GDNF and the RET receptor in cancer: new insights and therapeutic potential. Front Physiol 2019; 9: 1873. [DOI] [PMC free article] [PubMed] [Google Scholar]