Abstract
Background:
Both tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are known to reduce the risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB). This study aimed to compare the difference in HCC risk reduction between TDF and ETV in treatment-naïve patients with CHB-related compensated cirrhosis.
Methods:
Patients with compensated cirrhosis initially treated with TDF or ETV at nine Chinese hospitals between June 2014 and March 2021 were enrolled in this retrospective study. The cumulative HCC incidence rates for the two drugs were compared for the entire cohort, and a subgroup analysis was performed according to the HCC risk scores. Propensity score matching (PSM) was used to control confounding biases.
Results:
The analysis included 1453 patients (TDF group, n = 188; ETV group, n = 1265). Ninety-five patients developed HCC, with a median follow-up period of 26.1 months. The 3-year HCC incidence was 2.0% in the TDF group and 7.5% in the ETV group (log-rank p = 0.005). TDF treatment was associated with a lower risk of HCC than ETV treatment [hazard ratio (HR) = 0.222, 95% confidence interval (CI), 0.070–0.702, p = 0.010] but was similar after PSM (HR = 0.483, 95% CI, 0.144–1.626, p = 0.240; log-rank p = 0.230). However, subgroup analysis showed that the cumulative HCC incidence was lower in the TDF group than in the ETV group among patients with a modified PAGE-B score (mPAGE-B) ⩾9, either before or after PSM (log-rank p = 0.048 and p = 0.023, respectively).
Conclusion:
Among patients with an mPAGE-B score ⩾9, TDF is associated with a lower HCC incidence than ETV in patients with CHB-related compensated cirrhosis.
Keywords: chronic hepatitis B, compensated cirrhosis, entecavir, hepatocellular carcinoma, modified PAGE-B score, risk scores, tenofovir disoproxil fumarate
Introduction
More than 250 million individuals worldwide are infected with hepatitis B virus (HBV); 20–30 million people have chronic hepatitis B (CHB) infection.1,2 Persistent HBV replication is a risk factor for CHB progression to cirrhosis and hepatocellular carcinoma (HCC). 3 Nucleoside/nucleotide analogue (NA) therapy decreases HCC incidence and HCC-related mortality in patients with CHB through suppression of viral replication.4,5 Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are recommended as first-line antiviral agents for patients with CHB.6,7 It is still unclear whether TDF or ETV is more effective in preventing HCC development in patients with CHB.8–16 Risk factors associated with HCC include age, sex, platelet counts (PLT), alanine aminotransferase (ALT) level, HBV DNA level, and HBV e antigen (HBeAg) status.17–22 Several HCC risk scores for patients with chronic hepatitis B based on clinical and laboratory parameters were proposed to predict the risk of developing HCC, including the modified guide with age, gender, HBV DNA, core promoter mutations and cirrhosis-HCC (GAG-HCC score), 17 the Chinese University-HCC score (CU-HCC score), 18 the PAGE-B score, 20 the modified PAGE-B score, 21 and the aMAP (age, male, albumin–bilirubin and platelet data) score. 22
As current HBV treatment guidelines do not show preference for a particular first-line NA in cirrhotic patients,6,7 the difference in HCC development between TDF and ETV treatment remains unknown. Our study aimed to compare the difference in HCC incidence between these two first-line agents in patients with CHB-related compensated cirrhosis and explore the benefit difference between TDF and ETV among patients with various HCC risk stratification scores.
Materials and methods
Study design and patients
This retrospective study included consecutive patients with CHB-related compensated cirrhosis initially treated with ETV or TDF for ⩾12 months at nine hospitals in mainland China between June 2014 and March 2021. The participating hospitals are listed in Supplementary Table 2. According to studies from Taiwan,12,13 the cumulative incidence of HCC in Chinese patients with cirrhosis treated with ETV over 3 years was approximately 11.3%–15.2%, and we estimated it to be 13.2%. Meanwhile, the 3-year incidence of HCC in patients treated with TDF was approximately 6.7%–7.6%, and we estimated it to be 7.2%. The actual ratio of ETV:TDF use by patients in China is approximately 6:1. We calculated that, for this study to have 80% power to detect a 5% relative difference between the TDF and ETV groups, at least 138 and 1106 patients would need to be enrolled in the TDF and ETV treatment groups, respectively, which is based on the log-rank (Lakatos) test, and there would have to be 226 events of HCC.
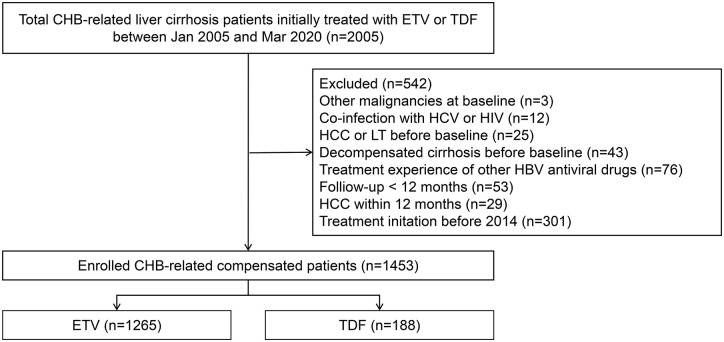
The inclusion criteria in this study were as follows: (1) HBsAg positivity for ⩾6 months; (2) ⩾18 years of age at therapy initiation; (3) initially treated with TDF 300 mg/day or ETV 0.5 mg/day; and (4) patients with compensated cirrhosis. Patients meeting any of the following criteria were excluded: (1) with evidence of other chronic liver disease, including other viral hepatitis (including hepatitis A, hepatitis C and hepatitis D), autoimmune hepatitis (AIH) and human immunodeficiency virus (HIV) infection; (2) history of HCC, decompensated cirrhosis, liver transplantation or other malignancies before NA treatment; (3) treatment experience with other HBV antiviral drugs; (4) HCC, decompensated cirrhosis or liver transplantation within 12 months, and (5) follow-up <12 months. The patients were categorised into TDF and ETV groups according to the treatment received (Figure 1).
Figure 1.
Flow diagram of the patient selection process. ETV, entecavir; HCC, hepatocellular carcinoma; LT, liver transplantation; TDF, tenofovir disoproxil fumarate.
The ethics committee of Ruijin Hospital approved the study (No. KY-2019-202) and waived the requirement for informed consent.
Data collection and definitions
Baseline data retrieved from the electronic medical records included age, sex, diabetes mellitus (DM), PLT, albumin (ALB), total bilirubin (TB), ALT, aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alpha-fetoprotein (AFP), HBeAg, serum HBV DNA levels, AST-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) index.
The presence of cirrhosis was defined as follows: (1) liver biopsy showing cirrhosis (Ishak score ⩾5 or Metavir score = 4); (2) liver stiffness measurement (LSM) using FibroScan (Echosens, Paris, France) ⩾12.0 kPa when TB was normal and ALT ⩽40 IU/ml, or LSM ⩾17.0 kPa when TB was normal and ALT <200 IU/ml; 23 (3) abdominal imaging results showing characteristic of cirrhosis (results showing coarse liver echotexture or nodular, parenchymal, or morphological abnormalities and signs of gastroesophageal varices); (4) APRI ⩾2.0; and/or (5) FIB-4 ⩾3.25.
Decompensated cirrhosis was defined as any of the following: Child–Pugh B/C (⩾7) or Child–Pugh A (5–6) accompanied by pleural effusion, ascites, oesophageal varices bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis or hepatorenal syndrome.
HCC was diagnosed based on histological evidence or typical radiological features, as follows: (1) liver/abdominal enhanced magnetic resonance (MR)/computed tomography (CT) suggestive of HCC, or (2) hepatic angiography lipiodol staining suggestive of HCC.
Diabetes mellitus (DM) was defined as follows: (1) exposure to any anti-diabetic agent; (2) fasting plasma glucose of ⩾ 7 mmol/l in two measurements or 11.1 mmol/l in one measurement; and/or (3) haemoglobin A1c ⩾6.5%. 24
HCC risk scores and cut-off values for risk stratification
Five predictive scores for the development of HBV-related HCC, including GAG-HCC score, 17 CU-HCC score, 18 PAGE-B score, 20 mPAGE-B score 21 and aMAP score, 22 were each calculated. Patients in this study were classified into low- or high-risk HCC groups according to the cut-off values of 82, 20, 10, 9 and 50 for the GAG-HCC, CU-HCC, PAGE-B, mPAGE-B and aMAP scores, respectively (Supplementary Table S1).
Outcomes and follow-up
The major outcome was HCC development. The secondary outcomes were liver transplantation and all-cause mortality.
The follow-up end point was the date of HCC diagnosis, liver transplantation or the last visit in the absence of HCC development. Patients lost to follow-up were censored at the last documented visit, and the last follow-up time was 1 March 2021.
Statistical analysis
Data were analysed using SAS (Version 9.4; SAS Institute Inc., Cary, NC, USA) and R 4.1.0 (R Foundation, Inc.; http://cran.r-project.org/). Continuous variables are expressed as mean ± standard deviation (SD) or as median [interquartile range (IQR)], as appropriate, whereas categorical variables are presented as number (percentage). Differences in continuous variables were examined for statistical significance using Student’s t test or the Kruskal–Wallis rank-sum test, depending on the distribution of the data.
Categorical variables were analysed using the Chi-square test or Fisher’s exact test. Factors associated with cumulative HCC incidence were identified using univariate and multivariate Cox regression analyses. All variables with a p value less than 0.1 under univariate Cox regression analysis entered the stepwise selection process and those with a p value less than 0.05 were retained. The hazard ratio (HR) and 95% confidence interval (CI) were calculated. A two-sided p value <0.05 was considered statistically significant.
TDF and ETV became available in mainland China in June 2014 and March 2006, respectively. Considering that TDF was approved more recently than ETV and that patients treated with ETV were relatively more likely to develop HCC, owing to the longer observation period, the initial follow-up period was initiated in June 2014, when TDF was available in mainland China. Propensity score matching (PSM) was performed to reduce significant differences in baseline characteristics between the two treatment groups. Factors with p < 0.1 in univariate logistic regression with treatment types (ETV/TDF) were identified as different baseline factors between the two groups and were incorporated into the PS model using 1:4 nearest-neighbour matching. The detailed results of the logistic regression used to apply the PS model are described in Supplementary Table S4. The predictive performance of the HCC risk scores was assessed by calculating Harrell’s c-index.
Results
Demographic characteristics
A total of 1453 patients with CHB-related compensated cirrhosis who were initially treated with TDF or ETV were included in the analysis (Figure 1). Median age was 46 years (IQR, 37–54) and 1049 (72.2%) were men. The TDF and ETV groups comprised 188 patients (12.9%) and 1265 patients (87.1%), respectively. TDF-treated patients were younger, less frequently diabetic and had a lower median FIB-4 score compared with ETV-treated patients (all p < 0.05; Table 1). The TDF group had higher median PLT, ALB, ALT, AST, and HBV DNA levels, as well as a higher proportion of HBeAg positivity, than the ETV group (all p < 0.05; Table 1). The five HCC risk scores in the TDF-treated patients were lower than those in the ETV-treated patients, which was reflected by different HCC risk scores (Table 1).
Table 1.
Demographic characteristics of the entire cohort before and after propensity score matching (PSM).
| Characteristic | Before PSM matching | After PSM matching | ||||
|---|---|---|---|---|---|---|
| ETV (n = 1265) | TDF (n = 188) | p | ETV (n = 553) | TDF (n = 160) | p | |
| Age, years, median (IQR) | 47 (38–55) | 37 (31–46) | <0.001 | 40 (33–48) | 38 (31.5–46) | 0.054 |
| Male sex, n (%) | 918 (72.6%) | 131 (69.7%) | 0.410 | 406 (73.4%) | 119 (74.4%) | 0.809 |
| Diabetes, n (%) | 79 (6.3%) | 4 (2.1%) | 0.023 | 13 (2.4%) | 3 (1.8%) | 0.956 |
| PLT, 109/l, median (IQR) | 115 (78–151) | 135 (102–174) | <0.001 | 128 (96–164) | 133 (100.5–162) | 0.723 |
| ALB, g/l, median (IQR) | 42 (38–45) | 43 (40–46) | 0.019 | 43 (39–46) | 43 (40–46) | 0.524 |
| TB, µmol/l, median (IQR) | 16.4 (12.2–23.1) | 17.0 (11.5–23.7) | 0.949 | 15.6 (11.3–22.1) | 17.0 (11.5–23.9) | 0.214 |
| ALT, IU/l, median (IQR) | 46 (31–78) | 57.5 (34–153) | <0.001 | 50 (32–91) | 52.5 (34–138) | 0.119 |
| AST, IU/l, median (IQR) | 43 (30–69) | 50 (32–118.5) | 0.001 | 44 (30–77) | 45.5 (31.5–100) | 0.139 |
| AFP, ng/ml, median (IQR) | 6.2 (3.4–16.9) | 5.3 (2.9–14.1) | 0.041 | 5.9 (3.3–16.7) | 4.8 (2.9–14.1) | 0.109 |
| HBeAg–positive, n (%) | 509 (42.2%) | 103 (58.2%) | <0.001 | 295 (53.4%) | 92 (57.5%) | 0.353 |
| HBV DNA (log10 IU/ml), median (IQR) | 5.08 (3.60–6.26) | 5.36 (4.09–6.90) | 0.001 | 5.29 (3.81–6.49) | 5.23 (4.04–6.82) | 0.274 |
| APRI, median (IQR) | 1.06 (0.59–2.07) | 1.08 (0.57–2.56) | 0.350 | 0.92 (0.54–2.06) | 1.00 (0.57–2.22) | 0.220 |
| FIB-4 index, median (IQR) | 2.83 (1.58, 5.06) | 2.03 (1.46, 3.32) | <0.001 | 2.15 (1.25–3.72) | 2.00 (1.46–3.26) | 0.720 |
| GAG-HCC, median (IQR) | 94.7 (86.5–102.4) | 86.3 (79.5–96.7) | <0.001 | 89.1 (82.6–96.6) | 87.6 (80.6–97.5) | 0.192 |
| CU-HCC, median (IQR) | 19.0 (16.0–22.0) | 19.0 (16.0–20.5) | 0.101 | 19 (16–20.5) | 19 (16–20.5) | 0.956 |
| REACH-B, median (IQR) | 10 (8–12) | 9 (8–11) | 0.004 | 10 (7–11) | 10 (8–11) | 0.978 |
| PAGE-B, median (IQR) | 16 (13–18) | 14 (10–16) | <0.001 | 14 (12–16) | 14 (10–17) | 0.537 |
| mPAGE-B, median (IQR) | 10 (8–12) | 8 (6–10.5) | <0.001 | 9 (7–11) | 8 (6–11) | 0.117 |
| aMAP, median (IQR) | 56.5 (51.3–61.4) | 51.7 (46.4–57.3) | <0.001 | 53.3 (48.4–57.9) | 52.7 (46.8–57.5) | 0.181 |
AFP, alpha-fetoprotein; ALB, albumin; aMAP, age, male, albumin–bilirubin and platelet data; ALT, alanine transaminase; APRI, AST-to-platelet ratio index; AST, aspartate transaminase; CU-HCC, Chinese University-HCC; ETV, entecavir; FIB-4, fibrosis-4; GAG-HCC, guide with age, gender, HBV DNA, core promoter mutations and cirrhosis-HCC; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; IQR, interquartile range; PLT, platelet count; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B; TB, total bilirubin; TDF, tenofovir disoproxil fumarate.
Clinical outcomes and cumulative HCC incidence in the entire cohort
Among the entire cohort, 95 (6.54%) patients, 92 in the ETV group and 3 in the TDF group, developed HCC during a median follow-up duration of 26.1 months (IQR, 16.7–44.3 months). One ETV-treated patient died of pulmonary carcinoma, and no patients underwent liver transplantation. The 3-year HCC incidence was 2.0% (95% CI, 0.9%–3.1%) and 7.5% (95% CI, 6.6%–8.5%) in the TDF and ETV groups, respectively. TDF treatment was associated with a lower HCC risk than ETV (HR = 0.22; 95% CI, 0.070–0.702; p = 0.010), and the p value for the log-rank test was 0.005 in the entire cohort (Figure 2(a)).
Figure 2.
Cumulative incidences of hepatocellular carcinoma in tenofovir disoproxil fumarate (TDF) versus entecavir (ETV): (a) entire cohort and (b) entire cohort after propensity score matching (PSM).
Cox regression analysis of factors associated with HCC in the entire cohort
Other factors associated with HCC risk in the entire cohort included age (HR = 1.060; 95% CI, 1.050–1.080; p < 0.001), DM (HR = 3.870; 95% CI, 2.360–6.350; p < 0.001), PLT (HR = 0.989; 95% CI, 0.984–0.994; p < 0.001), ALB (HR = 0.936; 95% CI, 0.905–0.968; P < 0.001), TB (HR = 1.010; 95% CI, 1.000–1.010; p = 0.007), and ALT (HR = 0.997; 95% CI, 0.994–1.000; p = 0.026) (Supplementary Table S3). Furthermore, age (HR = 1.050; 95% CI, 1.030–1.069; p < 0.001), DM (HR = 2.698; 95% CI, 1.609–4.526; p < 0.001), PLT (HR = 0.994; 95% CI, 0.989–0.999; p = 0.026), TB (HR = 1.014; 95% CI, 1.007–1.022; p < 0.001), and ALT (HR = 0.995; 95% CI, 0.991–0.999; p = 0.008) were independently associated with HCC development in the entire cohort (Supplementary Table 3).
Comparison of effect of TDF and ETV treatment on HCC development risk after PSM
According to the results of logistic regression using ETV or TDF as dependent variables (Supplementary Table S4), age, sex, DM, PLT, ALT, AST, HBeAg status, HBV DNA, and FIB-4 index were calculated in the PS model for the entire cohort. The mPAGE-B score has also been matched since it showed the best predictive performance for HCC among six scoring systems in this cohort (Harrell’s c-index, 0.770; 95% CI, 0.722–0.817) (Table 2). After PSM, 160 TDF-treated and 553 ETV-treated patients were included. There was no significant difference in demographic characteristics between the two groups after PSM (all p > 0.05; Table 1). TDF treatment had a lower HCC development risk trend than ETV treatment, but the difference was not significant (HR = 0.48; 95% CI, 0.144–1.626; p = 0.240), and the p value for the log-rank test was 0.229 after PSM (Figure 2(b)).
Table 2.
Predictive performance of HCC risk scores at baseline in the entire cohort.
| HCC risk score | Harrell’s c-index | 95% CI |
|---|---|---|
| GAG-HCC | 0.735 | 0.689–0.781 |
| CU-HCC | 0.666 | 0.605–0.728 |
| PAGE-B | 0.723 | 0.664–0.782 |
| mPAGE-B | 0.770 | 0.722–0.817 |
| aMAP | 0.740 | 0.690–0.790 |
aMAP, age, male, albumin–bilirubin and platelet data; CI, confidence interval; CU-HCC, Chinese University-HCC; GAG-HCC, guide with age, gender, HBV DNA, core promoter mutations and cirrhosis-HCC.
Sensitivity analysis
The HCC risk between the TDF and ETV groups was compared in different HCC risk score subgroups (Figure 3). The results showed that TDF treatment was associated with lower HCC occurrence in high-risk patients with a GAG score ⩾82, PAGE-B score ⩾10, and aMAP score ⩾50 when compared with ETV treatment (all p < 0.05) (Figure 3). The cumulative HCC incidences were lower in the TDF group than in the ETV group among patients with high HCC risk (GAG-HCC score ⩾82 subgroup, CU-HCC score ⩾20 subgroup, PAGE-B score ⩾10 subgroup, mPAGE-B score ⩾9 subgroup, and aMAP ⩾50 subgroup) (all log-rank p < 0.05) (Figures 4 and 5(b)). In contrast, the differences in HCC development between TDF and ETV treatment did not exist in low-risk subgroups according to various risk score models (all p > 0.05) (Figures 3, 4, and 5(a)).
Figure 3.
Hepatocellular carcinoma incidence for tenofovir disoproxil fumarate (TDF) versus entecavir (ETV) in subgroups.
Figure 4.
Cumulative incidences of hepatocellular carcinoma in tenofovir disoproxil fumarate (TDF) versus entecavir (ETV) in subgroups: (a) GAG <82, (b) GAG ⩾82, (c) CU-HCC <20, (d) CU-HCC >20, (e) PAGE-B <10, (f) PAGE-B ⩾10, (g) aMAP <50 and (h) aMAP ⩾50.
Figure 5.

Cumulative incidences of hepatocellular carcinoma in tenofovir disoproxil fumarate (TDF) versus entecavir (ETV): (a) mPAGE-B score <9, (b) mPAGE-B score ⩾9 before PSM and (c) mPAGE-B score ⩾9 after PSM.
Subgroup analysis of HCC risk based on mPAGE-B score
Since the mPAGE-B score showed the best predictive performance of HCC in this cohort of five scoring systems (Table 2), we stratified all patients into high- and low-risk groups: patients with mPAGE-B score ⩾9 (high HCC risk) and others with mPAGE-B score <9 (low HCC risk). The difference in cumulative HCC incidence between TDF and ETV was significant among patients with mPAGE-B score ⩾9 (log-rank p = 0.048) but was not different among patients with mPAGE-B score <9 (log-rank p = 0.303) (Figure 5(a) and (b)).
The baseline characteristics of TDF- and ETV-treated patients with mPAGE scores ⩾9 were described (Table 3), including 86 patients (9.4%) in the TDF group and 829 patients (90.6%) in the ETV group. In this population, 88 (9.6%) patients developed HCC (3 in the TDF group and 85 in the ETV group). To validate the difference in cumulative HCC incidence between the two drugs, age, sex, HBeAg, and mPAGE-B scores were matched in patients with an mPAGE-B score ⩾9 based on the results of logistic regression using ETV or TDF as dependent variables (Supplementary Table S4). After the 1:4 PS-matched analysis, the baseline characteristics of the ETV and TDF groups were described, and all baseline characteristics were comparable between the groups (all p > .05; Table 3). The cumulative HCC incidence was significantly lower in the TDF-treated patients than in the ETV-treated patients after PSM [log-rank p = 0.023; Figure 5(c)]. Cox regression analysis showed that TDF treatment was associated with a significantly lower HCC risk after PSM (HR = 0.277; 95% CI, 0.085–0.903; p = 0.033) in patients with an mPAGE-B score ⩾9.
Table 3.
Demographic characteristics of patients with mPAGE score ⩾9 before and after propensity score matching (PSM).
| Characteristic | Before PSM matching | After PSM matching | ||||
|---|---|---|---|---|---|---|
| ETV (n = 829) | TDF (n = 86) | p | ETV (n = 292) | TDF (n = 79) | p | |
| Age, years, median (IQR) | 51 (44–58) | 46.5 (42–52) | <0.001 | 46 (41–51) | 46 (41.5–51) | 0.865 |
| Male sex, n (%) | 602 (72.6%) | 73 (84.9%) | 0.014 | 258 (88.4%) | 70 (88.6%) | 0.951 |
| Diabetes, n (%) | 67 (8.1%) | 4 (4.7%) | 0.255 | 26 (9.0%) | 3 (3.8%) | 0.130 |
| PLT, 109/l, median (IQR) | 99 (68–131) | 115.5 (77–138) | 0.070 | 104 (68–132) | 115 (77.5–138) | 0.247 |
| ALB, g/l, median (IQR) | 41 (36–44) | 42 (37–45) | 0.070 | 41 (37–45) | 42 (37.5–44.5) | 0.858 |
| TB, µmol/l, median (IQR) | 17.2 (12.9–25.5) | 19.0 (13.1–26.4) | 0.457 | 17.8 (13.4–25.4) | 18.9 (13.1–26.8) | 0.611 |
| ALT, IU/l, median (IQR) | 46 (31–79) | 51.5 (33–114) | 0.101 | 46 (32–76) | 51 (34– 113.5) | 0.185 |
| AST, IU/l, median (IQR) | 46 (32–74) | 50 (32–85) | 0.361 | 43.5 (32–69) | 50 (33.5–82) | 0.229 |
| AFP, ng/ml, median (IQR) | 6.8 (3.6–21.8) | 9.6 (3.3–18.8) | 0.885 | 7.9 (3.9–29.1) | 9.2 (3.3–20.2) | 0.365 |
| HBeAg-positive, n (%) | 298 (37.3%) | 43 (54.4%) | 0.003 | 143 (49.0%) | 43 (54.4%) | 0.389 |
| HBV DNA (log10 IU/ml), median (IQR) | 5.09 (3.62–6.18) | 5.18 (4.02–6.14) | 0.199 | 4.89 (3.62–6.20) | 5.20 (4.08–6.19) | 0.097 |
| APRI, median (IQR) | 1.31 (0.76–2.36) | 1.28 (0.76–2.36) | 0.874 | 1.22 (0.69–2.31) | 1.28 (0.77–2.18) | 0.760 |
| FIB-4 index, median (IQR) | 3.64 (2.37–6.13) | 3.06 (1.93–4.68) | 0.017 | 3.08 (1.94–5.27) | 2.88 (1.91–4.53) | 0.545 |
| GAG-HCC, median (IQR) | 98.7 (92.73–105.0) | 97.2 (91.0–101.7) | 0.046 | 97.0 (91.9–102.3) | 97.3 (91.5–101.5) | 0.835 |
| CU-HCC, median (IQR) | 19.0 (17.5–23.5) | 19.0 (16.5–20.5) | 0.083 | 19.0 (16.5–22.0) | 19.0 (16.5–20.5) | 0.897 |
| REACH-B, median (IQR) | 11 (9–13) | 11 (10–13) | 0.361 | 11 (9–12) | 11 (10–13) | 0.205 |
| PAGE-B, median (IQR) | 17 (15–19) | 17 (16–19) | 0.309 | 17 (16–19) | 17 (16–19) | 0.215 |
| mPAGE-B, median (IQR) | 11 (10–13) | 11 (10–12) | 0.004 | 11 (10–12) | 11 (9–12) | 0.282 |
| aMAP, median (IQR) | 59.4 (55.8–63.4) | 57.6 (54.7–61.6) | 0.021 | 58.0 (55.1–61.9) | 57.5 (54.7–61.5) | 0.376 |
AFP, alpha-fetoprotein; ALB, albumin; aMAP, aMAP, age, male, albumin–bilirubin and platelet data; APRI, AST-to-platelet ratio index; ALT, alanine transaminase; AST, aspartate transaminase; CU-HCC, Chinese University-HCC; ETV, entecavir; FIB-4, fibrosis-4; GAG-HCC, guide with age, gender, HBV DNA, core promoter mutations and cirrhosis-HCC; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; IQR, interquartile range; PLT, platelet count; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B; TB, total bilirubin; TDF, tenofovir disoproxil fumarate.
Discussion
Current treatment guidelines do not indicate a preference for ETV or TDF in patients with cirrhosis.6,7 This multicentre, retrospective study of treatment-naïve patients with CHB-related compensated cirrhosis compared HCC development between those initially treated with TDF or ETV. We found that there was no difference in the risk of HCC development between CHB patients and compensated patients with cirrhosis. However, among those with high HCC risk scores (e.g. an mPAGE-B score ⩾9), TDF-treated patients had a significantly lower HCC incidence than ETV-treated patients.
Several studies with treatment-naïve patients with CHB-related compensated cirrhosis as well as a cirrhotic subgroup found no difference in HCC risk between TDF and ETV therapy.9–14 In contrast, Choi’s study included 2914 pairs after baseline characteristics matching and found that TDF treatment was associated with a significantly lower risk of HCC in cirrhotic patients by multivariable analysis (HR = 0.64; 95% CI, 0.43–0.95; p = 0.03). 15
A series of studies have compared the difference of efficacy and safety between the two first-line drugs in compensated cirrhotic populations, but few have focused on that in the context of HCC development according to risk stratification. Our study collected 1493 treatment-naïve patients with CHB-related compensated cirrhosis and found that TDF treatment was not associated with lower HCC incidence compared with ETV treatment among all patients by multivariate analysis and PS-matched analysis, which was similar to most previous studies.9–14 After comparing the baseline characteristics of our study (before PSM) and Choi’s study, 15 we found that the HCC risk score in the cirrhotic subgroup from Choi’s cohort was similar or higher than that in our study. Although studies with negative results9–14 did not evaluate HCC risk scores for each subject, the baseline platelet counts in CHB-related cirrhosis patients in these studies were much higher than those in our study. There is no doubt that low platelet count is a high-weight factor in several HCC scoring systems20–22 and is significantly associated with severe liver cirrhosis and HCC development.
Meanwhile, a recent study demonstrated that TDF-treated patients had a lower risk of HCC than ETV-treated patients among those with decompensated cirrhosis (log-rank p = 0.042 after PSM). 16 This is consistent with our results, as the risk of HCC development varies with the severity of the disease among patients with compensated cirrhosis. Our findings suggested that TDF had more benefits than ETV in reducing the risk of HCC in both before and after PSM populations with high-risk HCC, as assessed by the mPAGE-B score. Moreover, two recent retrospective studies that compared the recurrence rate of HCC after curative liver resection treatment between ETV and TDF found that treatment with TDF was significantly associated with lower risk of late HCC recurrence compared with ETV therapy.25,26 It is generally accepted that most early cases of recurrence, within 2 years, of hepatectomy result from the dissemination of the primary tumour, whereas most late cases of recurrence, after 2 years, of hepatectomy stem from the de novo recurrence of tumours spontaneously arising in the remaining liver. 27 These findings suggest that the difference in recurrence rates between the two groups may originate from their different preventive effects on de novo HCC recurrence. Besides, most patients with HCC not only developed HCC from cirrhosis but also were in the population with a high risk for HCC. These studies on individuals with HCC reinforce our conclusion. All these above could be understood as the strength of the relationship increasing with increased disease severity.
The mechanisms underlying this significant difference remain unclear. Previous studies reported that the additional administration of nucleotide analogues represented by TDF could increase serum interferon (IFN)-λ3 levels, which would promote IFN gene expression and suppress tumour growth compared with nucleoside analogues represented by ETV.28,29 The underlying mechanisms need to be explored further.
Our study not only compares the two drugs in terms of the reduction in HCC risk among cirrhotic patients undergoing antiviral treatment but also reveals those who require particular attention. It was found that age, DM, PLT, TB level, and ALT level were independently associated with HCC in patients with HBV-related cirrhosis, which is a finding that is in agreement with previous studies.30–32 An increasing number of studies have exhibited an association between DM and HCC,33,34 although the exact mechanism underlying this association is incompletely understood. 35 Moreover, a recent study reported that patients with DM were less likely to have regression of cirrhosis after NA therapy. 36 Our data further indicate that DM is becoming a major outcome and determinant of CHB-related cirrhosis in the era of antiviral therapy.
The overall incidence of HCC in this study (6.4%, 95 of 1453 patients) was relatively lower than that in previous studies, which might be due to the exclusion of patients with HCC development within 12 months after treatment initiation. Compared with the exclusion of patients who developed HCC within 6 months,8–10,12,15,16 we set more stringent exclusion criteria to exclude micro-HCC that had occurred prior to NA treatment. The other strength of this study is that it included a multicentre cohort involving nine hospitals. In addition, considering that the difference in the follow-up time between the two drugs resulted in different levels of HCC risk, a consistent treatment commencement time was implemented to make the two groups more comparable. The sensitivity analysis and detailed subgroup analysis confirmed the reliability of the conclusions.
Our study has several limitations. First, this retrospective study was subject to selection bias and confounding, as in other observational studies. PSM was used to minimise baseline confounding between the two groups. Second, the other limitations were the considerable difference in sample sizes between the two groups and the short follow-up time. Due to the short launch-to-market time and high price of TDF in China, TDF has a relatively poor level of availability and affordability compared with ETV. At that time, TDF was mainly used for maternal and infant medicine. Moreover, elderly patients with CHB-related cirrhosis are more likely to avoid TDF due to their high renal toxicity and osteoporosis risks.6,7 Third, data on other factors associated with the development of HCC were also lacking, including family history, HBV genotypes and HBsAg levels.37,38 Most CHB patients in China are infected with HBV genotypes B and C, which are different from the genotypes that infect Western populations. 38 The risk of HCC varies with HBV genotype; therefore, the conclusion needs to be further validated in other populations. Finally, as metabolic factors, including DM, obesity, and hypertension, influence HCC development, 39 future studies should account for them.
Our findings may contribute to better management of patients with CHB-related cirrhosis, especially those at a high risk of HCC. Previous studies have shown that PEG-IFN can reduce the risk of HCC and have also attempted to use PEG-IFN to reduce the risk of HCC in patients with high HCC risk scores. 40 However, most patients with cirrhosis cannot tolerate PEG-IFN well, and the incidence of discontinuation is high. Our data demonstrated that TDF was superior to ETV in reducing HBV-related HCC in patients with CHB-related compensated cirrhosis at high HCC risk scores, especially who are intolerant of and ineligible for PEG-IFN. This will provide important insights into clinical practice. Future prospective studies with larger sample sizes and longer follow-up periods are needed to confirm our findings.
Conclusion
Our results suggest that there was no difference in the risk of HCC in treatment-naïve patients with CHB-related compensated cirrhosis between TDF and ETV as initial treatment. Among those with high HCC risk scores, especially those with an mPAGE-B score ⩾9, TDF was associated with a lower HCC incidence than ETV. Therefore, TDF may be a better choice than ETV in such a population, and this is a finding that may improve the management of patients with CHB-related cirrhosis to reduce their HCC risk.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221102791 for Tenofovir is superior to entecavir in reducing HCC for patients with HBV-related compensated cirrhosis at high HCC risk scores by Yan Huang, Lichang Chen, Rui Huang, Chuanwu Zhu, Jia Shang, Yunsong Qian, Jianqi Lian, Longgen Liu, Jianning Jiang, Chenghai Liu, Honglian Gui and Qing Xie in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors acknowledge the following investigators who contributed to the data collection in this study: Zhimin Zhao (Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai), Chao Wu (Department of Infectious Disease, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing), Yueping Zhu (Department of Infectious Diseases, the Affiliated Infectious Diseases Hospital of Soochow University, Suzhou), Peipei Ren (Department of Infectious Diseases, Henan Provincial People’s Hospital, Zhengzhou), Jian Wang (Department of Hepatology, HwaMei Hospital, University Of Chinese Academy Of Sciences, Ningbo) and Linxu Wang (Department of Infectious Diseases, Tangdu Hospital, The Fourth Military Medical University, Xian). They would like to thank Editage (www.editage.cn) for the English-language editing.
Footnotes
Ethics approval and consent to participate: The Ruijin Hospital Ethics Committee at the Shanghai Jiao Tong University School of Medicine approved this study (No. KY-2019-202). The requirement for informed patient consent was waived because of the retrospective study design.
Consent for publication: Consent for publication was wavied by the Committee considering the retrospective design.
Author contributions: Yan Huang: Data curation; Methodology; Writing – original draft; Writing – review & editing.
Lichang Chen: Methodology; Validation.
Rui Huang: Data curation; Validation.
Chuanwu Zhu: Data curation.
Jia Shang: Data curation.
Yunsong Qian: Data curation.
Jianqi Lian: Data curation.
Longgen Liu: Data curation.
Jianning Jiang: Data curation.
Chenghai Liu: Data curation; Methodology; Validation.
Honglian Gui: Data curation; Funding acquisition; Supervision; Writing – review & editing.
Qing Xie: Funding acquisition; Methodology; Supervision; Validation; Writing – review & editing.
Availability of data and materials: The datasets generated and analysed during the present study are available from the corresponding author upon reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by supported by Gilead Science, the National Natural Science Foundation of China (No. 82070604, No. 81770587 and No. 81800508), Key Projects in the National Science & Technology Pillar Program during the Thirteenth Five-year Plan Period (2017ZX10203201-008, 2018ZX09201016-003-001, and 2017ZX10202202-005-004), the Shanghai Municipal Key Clinical Specialty (shslczdzk01103) and the Shanghai Ruijin Hospital Three-Year Plan of the Clinical Skills and Innovations (2018CR005). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Prof. Xie received research funding from Gilead Sciences. None of the other authors have served as a speaker, a consultant, or member of an advisory board for any of the organisations funding this work, and none of the other authors have received research funding from any of the organisations funding this work.
ORCID iD: Qing Xie  https://orcid.org/0000-0002-2582-8803
https://orcid.org/0000-0002-2582-8803
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yan Huang, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Lichang Chen, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Rui Huang, Department of Infectious Disease, Nanjing Drum Tower Hospital, Clinical College of Nanjing Medical University, Nanjing, China.
Chuanwu Zhu, Department of Infectious Diseases, The Affiliated Infectious Diseases Hospital of Soochow University, Suzhou, China.
Jia Shang, Department of Infectious Diseases, Henan Provincial People’s Hospital, Zhengzhou, China.
Yunsong Qian, Department of Hepatology, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, China.
Jianqi Lian, Department of Infectious Diseases, Tangdu Hospital, The Fourth Military Medical University, Xian, China.
Longgen Liu, Department of Infectious Diseases, The Third People’s Hospital of Changzhou, Changzhou, China.
Jianning Jiang, Department of Infectious Diseases, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Chenghai Liu, Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Honglian Gui, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Qing Xie, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
References
- 1. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3: 383–403. [DOI] [PubMed] [Google Scholar]
- 2. Liu J, Liang W, Jing W, et al. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 2019; 97: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130: 678–686. [DOI] [PubMed] [Google Scholar]
- 4. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351: 1521–1531. [DOI] [PubMed] [Google Scholar]
- 5. Lim YS, Han S, Heo NY, et al. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology 2014; 147: 152–161. [DOI] [PubMed] [Google Scholar]
- 6. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis (Hoboken) 2018; 12: 33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67: 370–398. [DOI] [PubMed] [Google Scholar]
- 8. Yip TC, Wong VW, Chan HL, et al. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology 2020; 158: 215–225. [DOI] [PubMed] [Google Scholar]
- 9. Kim SU, Seo YS, Lee HA, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naive chronic hepatitis B in South Korea. J Hepatol 2019; 71: 456–464. [DOI] [PubMed] [Google Scholar]
- 10. Lee SW, Kwon JH, Lee HL, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naive patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut 2020; 69: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim BG, Park NH, Lee SB, et al. Mortality, liver transplantation and hepatic complications in patients with treatment-naive chronic hepatitis B treated with entecavir vs tenofovir. J Viral Hepat 2018; 25: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 12. Hu TH, Yueh-Hsia CS, Tseng PL, et al. Five-year comparative risk of hepatocellular carcinoma development under entecavir or tenofovir treatment-naive patients with chronic hepatitis B-related compensated cirrhosis in Taiwan. Aliment Pharmacol Ther 2020; 52: 1695–1706. [DOI] [PubMed] [Google Scholar]
- 13. Chen CH, Chen CY, Wang JH, et al. Comparison of incidence of hepatocellular carcinoma between chronic hepatitis B patients with cirrhosis treated with entecavir or tenofovir in Taiwan – a retrospective study. Am J Cancer Res 2020; 10: 3882–3895. [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu YC, Wong GL, Chen CH, et al. Tenofovir versus entecavir for hepatocellular carcinoma prevention in an international consortium of chronic hepatitis B. Am J Gastroenterol 2020; 115: 271–280. [DOI] [PubMed] [Google Scholar]
- 15. Choi J, Kim HJ, Lee J, et al. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol 2019; 5: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang TS, Yang YH, Chen WM, et al. Long-term risk of primary liver cancers in entecavir versus tenofovir treatment for chronic hepatitis B. Sci Rep 2021; 11: 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010; 28: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 18. Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009; 50: 80–88. [DOI] [PubMed] [Google Scholar]
- 19. Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011; 12: 568–574. [DOI] [PubMed] [Google Scholar]
- 20. Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 2016; 64: 800–806. [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Kim YD, Lee M, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 2018; 69: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 22. Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020; 73: 1368–1378. [DOI] [PubMed] [Google Scholar]
- 23. Chinese Foundation for Hepatitis Prevention and Control. Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update. Zhonghua Gan Zang Bing Za Zhi 2019; 27: 182–191. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi J, Jo C, Lim YS. Tenofovir versus entecavir on recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. Hepatology 2021; 73: 661–673. [DOI] [PubMed] [Google Scholar]
- 26. Tsai MC, Wang CC, Lee WC, et al. Tenofovir is superior to entecavir on tertiary prevention for Bclc stage 0/a hepatocellular carcinoma after curative resection. Liver Cancer 2022; 11: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen YJ, Yeh SH, Chen JT, et al. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology 2000; 119: 431–440. [DOI] [PubMed] [Google Scholar]
- 28. Murata K, Asano M, Matsumoto A, et al. Induction of IFN-lambda3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut 2018; 67: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sato A, Ohtsuki M, Hata M, et al. Antitumor activity of IFN-lambda in murine tumor models. J Immunol 2006; 176: 7686–7694. [DOI] [PubMed] [Google Scholar]
- 30. El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4: 369–380. [DOI] [PubMed] [Google Scholar]
- 31. Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 2008; 135: 111–121. [DOI] [PubMed] [Google Scholar]
- 32. Siddique A, Kowdley KV. Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis 2011; 15: 281–296, vii–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381: 468–475. [DOI] [PubMed] [Google Scholar]
- 34. Yang HI, Sherman M, Su J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol 2010; 28: 2437–2444. [DOI] [PubMed] [Google Scholar]
- 35. Kao JH. Hepatitis B viral genotypes: clinical relevance and molecular characteristics. J Gastroenterol Hepatol 2002; 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 36. Gui H, Huang Y, Zhao G, et al. External validation of aMAP hepatocellular carcinoma risk score in patients with chronic hepatitis B-related cirrhosis receiving ETV or TDF therapy. Front Med (Lausanne) 2021; 8: 677920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ren P, Cao Z, Mo R, et al. Interferon-based treatment is superior to nucleos(t)ide analog in reducing HBV-related hepatocellular carcinoma for chronic hepatitis B patients at high risk. Expert Opin Biol Ther 2018; 18: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 38. Hsu YC, Wu CY, Lane HY, et al. Determinants of hepatocellular carcinoma in cirrhotic patients treated with nucleos(T)ide analogues for chronic hepatitis B. J Antimicrob Chemother 2014; 69: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 39. Flemming JA, Yang JD, Vittinghoff E, et al. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer 2014; 120: 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu YC, Ho HJ, Lee TY, et al. Temporal trend and risk determinants of hepatocellular carcinoma in chronic hepatitis B patients on entecavir or tenofovir. J Viral Hepat 2018; 25: 543–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221102791 for Tenofovir is superior to entecavir in reducing HCC for patients with HBV-related compensated cirrhosis at high HCC risk scores by Yan Huang, Lichang Chen, Rui Huang, Chuanwu Zhu, Jia Shang, Yunsong Qian, Jianqi Lian, Longgen Liu, Jianning Jiang, Chenghai Liu, Honglian Gui and Qing Xie in Therapeutic Advances in Chronic Disease