Abstract
Background:
Complementary and Alternative Medicine (CAM) is widely used around the world to treat adverse effects derived from cancer treatment among children and young adults. Parents often seek CAM to restore and maintain the child’s physical and emotional condition during and after cancer treatment.
Objectives:
The objectives of this review were (i) to identify literature that investigates CAM use for treating adverse effects of conventional cancer treatment, (ii) to investigate the safety of the included CAM modalities, and (iii) to evaluate the quality of included studies.
Methods:
Five scientific research databases were used to identify observational, quasi-experimental, and qualitative studies from January 1990 to May 2021. Included studies investigated the use of CAM to treat adverse effects of cancer treatment in childhood cancer.
Results:
Fifteen studies were included in this review. Ten quasi-experimental, 3 observational studies (longitudinal/prospective), 2 qualitative studies, and 1 study with a quasi-experimental and qualitative arm were identified. Less than half (n = 6; 40%) of the studies included reported adverse effects for the CAM modality being studied. Among the studies that reported adverse effects, they were mostly considered as direct risk, as 13% reported mainly bleeding and bruising upon acupuncture treatment, and dizziness with yoga treatment. All adverse effects were assessed as minor and transient. CAM modalities identified for treating adverse effects of cancer treatment were alternative medical systems, manipulative and body-based therapies, biologically-based therapies, and mind-body therapies. CAM modalities were used to alleviate anxiety, pain, toxicity, prevent trauma, and improve health-related quality of life, functional mobility, and physical activity levels. All studies assessed scored 70% or above according to the Joanna Briggs Institute critical appraisal for study quality checklists.
Conclusion:
Most of the studies (58.3%) included in this review did not report adverse effects from CAM modalities used to treat adverse effects of cancer treatment in children and young adults. This lack of safety information is of concern because parents need to know whether the modality represents an extra burden or harm to the child. To improve awareness about safety in the field, a universal and uniform reporting system for adverse effects in CAM research is needed.
Keywords: systematic review, observational studies, qualitative studies, complementary and alternative medicine, risk, patient safety
Background
Cancer is the leading cause of death among children and adolescents in many countries. 1 The causes of childhood cancer are often unknown. 1 However, available data suggest that 10% of all children with cancer have genetic factors that predispose them to the disease. 2 The survival rate of childhood cancer has increased especially in the western world, where more than 80% of the children with cancer are cured. 1 The increase in survival rate is due to the accessibility of conventional care services and an improvement in therapy, including risk-adapted stratification. 1 In low-and middle-income countries (LMIC), the organization and delivery of health are poor due to the lack of resources, the cost of treatment, limited accessibility, and cultural health beliefs. All of the latter lead people to seek Complementary and Alternative Medicine (CAM) treatments. 3 Nevertheless, according to research, these modalities are not as effective as curative cancer treatments. It has previously been demonstrated that the overall 5-year survival rate with only CAM treatment of acute leukemia in children was 0%. 4 The most common types of childhood cancer are leukemias, brain cancers, lymphomas, and solid tumors. The delivery of health services depends on the understanding of what types of cancers and long-term effects of cancer treatment can be expected (ie, fatigue, cognitive difficulties, etc.). 5 Even though the survival rates from childhood cancer are increasing it is important to understand how to effectively decrease the burden of morbidities and incorporate supportive rehabilitation treatments that will increase and improve the well-being of children with cancer.
The combined use of CAM and conventional medicine in children undergoing cancer treatment is high in several countries.6-8 In Switzerland, Lüthi et al 7 reported that 69.3% of patients after diagnosis used CAM. CAM is defined as “a group of diverse medical health care systems, practices, and products that are not presently considered to be part of conventional medicine.” 9 If a non-mainstream approach is used together with conventional medicine, it is considered complementary. If a non-mainstream approach is used in place of conventional medicine it is considered alternative. 3 Integrated health brings conventional and complementary approaches together in a coordinated way. Integrative oncology is a patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments and aims to optimize health, quality of life, and clinical outcomes across the cancer care continuum. 10 CAM among pediatric patients is often used as part of supportive care as a way for parents to do everything possible for the child, to boost their immune system, improve their general well-being, and/or treat adverse effects of conventional therapy.11,12 CAM modalities most often used in pediatric oncology patients are herbal remedies, 9 homeopathy,7,8 diet, and nutrition. 9
CAM modalities are often considered to be natural and therefore safe, but patients may react unexpectedly to treatment that may cause harm. 13 It is therefore of significant importance to investigate the safety of these modalities when used to complement conventional medicine. Risk in medical science is defined as a measure of the probability and severity of adverse effects. 14 Risk in CAM can be divided into direct (related to interventions) and indirect (related to the setting effect) risk.15,16 Direct risk is related to the intervention, for example, harm caused by pharmacological products, medical treatments, and procedures. Direct risk is often described as adverse effects, adverse reactions, and adverse drug reactions. Adverse effects is a more suitable term to describe risk for most CAM modalities as they encompass physical and psychological complaints and are defined as all the unwanted or harmful reactions that result from medication or intervention regardless of their relation to the actual treatment.15,16 Indirect risk is related to the setting effects, such as the practitioner, rather than to the medicine. An example of indirect risk is a provider who overlooks serious symptoms and thereby causes a delay in necessary conventional treatment. 16
The adverse effects of cancer therapies can be burdensome to children undergoing cancer treatment as well as their parents, because apart from dealing with symptoms at the time of treatment, they have to endure the consequences of treatment for the rest of their lives. 17 Late and long-term effects are understood as long-lasting health problems following cancer treatment. 18 Some may develop during treatment and persist (long-term effects) such as fatigue, whereas others may develop many years later (late effects) such as secondary cancer and cardiovascular diseases. 19 Children have a developing body, and cancer treatments may have more or less strong adverse effects. 20 During growth children’s cells are dividing faster than adult cells. Cancer treatment such as chemotherapy and radiotherapy damages cancer cells as well as normal cells and this leads to adverse effects. For example, radiation treatment can slow the growth of bone and muscle in children causing serious effects. 21 Some of the adverse effects often reported are cough, drowsiness, fatigue, cognitive problems, and lack of energy. The most distressing symptoms reported by parents are lack of appetite, nausea, and pain, as well as psychological symptoms, such as feeling irritable and sad. 22
Although CAM modalities are widely used among pediatric cancer patients, CAM modalities are still under-investigated.23,24 Our research teams conducted a systematic review of RCTs in 2021. 25 The systematic review aimed to review the research literature to identify any CAM modalities used to treat adverse effects of conventional cancer treatment among children and young adults. The meta-analysis showed that CAM (including acupuncture and hypnosis) was effective in reducing chemotherapy induced nausea and vomiting (CINV) in children and young adults. The analysis demonstrated that only 29% of the studies included reported data on safety. 25 Many studies about CAM modalities (ie, acupressure, healing touch, massage, music therapy, reiki) investigate effectiveness, but they do not address or report safety events among the reviewed studies.26-28 In this review we want to investigate the safety of CAM modalities used to treat adverse effects of conventional cancer treatment in children and young adults. As observational and quasi-experimental studies are suitable to investigate adverse effects of an intervention, 29 we will investigate this using this methodology. Since many of these studies have a qualitative arm nested within the design, we decided to include qualitative studies as well. Therefore, the overall aim of this study is to gain more insight about CAM modalities used to treat adverse effects of conventional cancer treatment and their safety in real-life settings.
Aims
The aims of this systematic review were to evaluate the research literature to (i) to identify observational, quasi-experimental, and qualitative studies that investigate CAM modalities used for treating adverse effects of conventional cancer treatment, (ii) to investigate the safety of the included CAM modalities, and (iii) to investigate the quality of the included studies.
Methods
Results are reported according to the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) checklist (see Supplemental File). 30
The focus question was:
Are CAM modalities used in childhood cancer (to treat adverse effects of conventional cancer treatment) associated with adverse effects?
The PICOS format was used when searching for relevant articles, which included the following 4 parts:
Population: Children and young adults who were ever diagnosed with cancer and who used CAM to treat adverse effects of conventional cancer treatment (the pediatric population is considered 0-21 years old).
Intervention: Any CAM modality/All CAM modalities.
Comparison: Conventional medicine, usual care, waiting list, and other CAM modalities.
Outcome: Reduction/improvement of adverse effects of conventional cancer treatment, adverse events, adverse reactions, adverse drug reaction, harm, indirect/direct risks, risks factors, side effects, safety.
Types of Study: Prospective and retrospective studies, cohort studies, non-experimental studies, clinical studies, quasi-experimental studies, and qualitative studies.
A protocol for the systematic review was created, submitted, and registered by PROSPERO (CRD42022302788). Three authors (DCM, TS, GO) developed the search strategy and performed the searches. Eligible studies were searched in 5 electronic databases, central webpages, and journals were searched for eligible studies: AMED, CINAHL, EMBASE, PsycINFO, and MEDLINE/PubMed. According to the search methodology references of all included studies were hand-searched for additional eligible studies. A manual search was also performed in the gray literature.
Search Methods: Various combinations of controlled vocabulary/thesaurus terms (eg, MESH) and text words, adjusted for each database were used. The following controlled vocabulary/thesaurus terms were used: Exp neoplasms, exp complementary therapies, exp integrative medicine, alternative therapies, exp child, exp adolescent, exp young adult, exp infant, adverse effects. sf (subheading, fs), adverse event, side effects and adverse reactions, drug related side effects and adverse reactions, exp adverse drug reaction, reporting systems, exp cohort studies, exp qualitative studies, qualitative research, exp interview, exp observational study, exp nonexperimental studies.
These text words were used: Neoplasm, leukemia, lymphoma/soft tissue sarcoma, pediatric cancer, pediatric oncology, integrative oncology, cancer treatment, childhood cancer, pediatric, palliative care, CAM modalities, CAM treatment, CAM, integrative medicine, complementary medicine, alternative medicine, unconventional medicine, spiritual healing/faith healing, children, child*, infant, adolescent, juvenile, pediatric, puberty, young adults, young person, teen*, childhood, toddler, side effects, safety, risks factors, harm, adverse reactions, indirect/direct risks, adverse drug reaction, symptom management, hopelessness, suffering (the search string from MEDLINE is attached as Supplemental Material).
Inclusion and Exclusion Criteria
The filters used were human, Danish, Dutch, English, German, Norwegian, Spanish, and Swedish. The searches had a limited period from January 1990 to May 2021. The inclusion comprised observational and qualitative studies that reported CAM modalities to treat adverse effects of cancer treatment among children and young adults. The search considered any adverse effects and CAM modalities. Studies including data on parents/caregivers of children with cancer and their health care providers were also included. Studies with children and young adults up to 21 years of age were included when this age group was described as part of the pediatric cancer population in the publication.
The studies excluded did not provide adverse effects from conventional cancer treatment, were not related to cancer or CAM, were not observational, quasi-experimental, and qualitative studies, were conducted among adults with cancer, or were in languages other than the ones previously stated.
Study Selection and Data Management
Endnote was used as the reference manager to upload the results and facilitate study selection, and a single data management file was produced of all references identified through the search process. Duplicates were removed and 2 authors screened the remaining references independently for inclusion using Rayyan web app 31 (DCM and TS). Reasons for excluding articles were documented. Neither of the review authors was blind to the journal titles, study authors, or institutions. A flowchart of the study selection and identification according to the (PRISMA-P) guidelines 32 was generated.
Control Interventions
The control interventions consisted of usual care, and other CAM modalities such as yoga, acupuncture, and art and music therapy.
Methodological Assessment of the Studies
Data from observational and quasi-experimental studies were validated and extracted according to 10 technical items 33 : Indication, sample size, baseline comparability, inclusion/exclusion criteria, intervention (treatment vs control), dropout, objectives, duration of treatment, main results, and funding (Table 1). The first and last authors (DCM and TS) extracted the data. Checklists used to critically appraise observational and quasi-experimental studies tend to concentrate on issues of external and internal validity, including items like comparability of subjects, details of intervention and outcome measures, statistical analysis, and funding.34-36 Thus, these recommended items are in line with those applied in this systematic review. Data from qualitative studies were validated and extracted according to the following 10 criteria: Population, method, design/analysis, setting, aim(s), participants, sample size, inclusion/exclusion criteria, duration of treatment, results, and funding. 37
Table 1.
Methodological Assessment of Quasi-Experimental Studies.
| Quasi-experimental studies (n = 10) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Indication | Participants | Criteria | Intervention | Dropout | Objectives | Duration of treatment (follow-up) | Main results | Funding | ||
| Sample size | Baseline comparability | Inclusion | Exclusion | Treatment vs control (n) | Treatment vs control (n) | ||||||
| Barrera et al 40 | Music therapy for anxiety | Hospitalized children with cancer (n = 70) | The participants differ regarding age and stages of cancer and treatment. These differences were accounted for in the statistical analysis. | Hospitalized children with cancer 0 to 17 y of age | NR | A music therapist was with the child for more than 15 min, engaging in calming interactive music experiences leading the child to other activities. About 1 to 3 sessions for each child ranging from 15 to 45 min. | n = 5 | Examine the general benefits of music therapy for hospitalized pediatric cancer patients | Depended on the length of time the child stayed at the hospital. Participants received no more than 3 music sessions. | Significant improvement in children’s rating of their feelings from pre to post music therapy (P < .01). However, the results varied with the age of the children. | Association of families of children with cancer, Toronto, Ontario; Hematology/Oncology Program, Hospital for Sick Children |
| Diorio et al 42 | The feasibility of a 3-wk yoga program for fatigue | Children receiving chemotherapy or HSCT (n = 22) | NA (a single group) | Inpatient children with AML, relapsed ALL, stages 3 and 4 Burkitt’s lymphoma; about to receive HSCT, 7 to 18 y old at enrollment, expected to be an inpatient for at least 3 wk after initiation of chemotherapy or conditioning | Unable to perform yoga; motor disability; cardiopulmonary symptoms; fractures; parents who do not understand English | Yoga, 3 times weekly (n = 11) | n = 11 | Determine the feasibility of individualized yoga for hospitalized children receiving chemotherapy | 3 wk | The yoga program was feasible. Qualitative feedback from both children and parents indicated the physical and psychological benefits of yoga. | No grant funding for this study |
| Favera-Scacco et al 43 | Art therapy for painful procedures during cancer treatment | Children with leukemia who underwent lumbar puncture or bone marrow aspiration (n = 49) | The children in the intervention group were younger than those in the control group | Children with leukemia who were candidates for lumbar puncture and bone marrow aspiration | NR | Art therapy (n = 32) vs usual care (n = 17). Art therapy consisted of: Introduction of a toy: a safe person; visual imagination; medical play; drawing; reading; dramatization | NR | Investigate art therapy as support (reduce stress and anxiety) for children with leukemia during painful procedures | NR | Art therapy can be a useful intervention that can prevent trauma and support children and parents during intrusive interventions | Supported by a grant from Regione Sicilia and MURST (40% grant) |
| Geyer et al 51 | Yoga to improve quality of life | Children and adolescents with oncological diagnoses (Edwin sarcoma, All, AML, Fanconi’s anemia [n = 6] parents/caregivers [n = 4]) | NA (a single group) | Children and adolescents with oncological diagnoses. Platelet counts more than 5000, absolute neutrophil count more than 200, and hemoglobin 8 to 10 g/dL | Comorbid diseases, or developmental disorders, in the induction phase of treatment | Therapeutic yoga (1 h) 5 consecutive weekly section | No dropout | To describe the effect of therapeutic yoga on child and parental reports of quality of life in children hospitalized with oncological diagnoses | 5 wk | Therapeutic yoga positively affected child’s perception of gross motor function (P = .016) | Lance Armstrong Foundation |
| Govardhan et al 52 | To establish the feasibility and therapeutic effect of yoga on pediatric brain tumor and to provide a foundation for the development of an RCT | Children and adolescents between 6 and 18 planned for either radiation or chemotherapy for brain tumors (n = 18) | NA (a single group) | Children and adolescents between 6 and 18 planned for either radiation or chemotherapy for brain tumors | Children with metastasis, developmental or intellectual disorders, and prior exposure to yoga | Individualized yoga for an hour at least 3 times a week over a 4-wk timeframe after conventional cancer treatment (n = 13) | NR | To establish the feasibility and therapeutic effect of yoga to address the effects of radiotherapy and chemotherapy in pediatric brain tumor | 4 wk | The yoga intervention was feasible. A significant difference was reported in respect to pain (P = .0001), relief in headache (P = .0005), increase in appetite (P = .0005), better sleep (P = .0003), and reduced fatigue (P = .007), and overall daily activity (P = .0018) | NR in publication |
| Hooke et al 53 | Yoga to improve fatigue, anxiety, balance, and sleep | Children and adolescents between 10 and 18 who completed therapy in the past 2 to 24 mo (n = 18) | NA (a single group) | Children and adolescents (10-18 y) who completed therapy in the past 2 to 24 mo for pediatric cancer, received chemo, radiotherapy, or if CNS tumor was treated with surgery, had not participated in a yoga class the previous 3 mo | Had an antecedent neurological, developmental, or genetic disorder before their cancer diagnoses | 45-min yoga classes, for 6 wk (n = 13) | n = 2 due to relapse | Determine if children and adolescents who were cancer survivors had less fatigue, better balance, and sleep quality, and less psychological distress compared with baseline measurements | 6 wk | The anxiety score decreased significantly among children (P = .04) but not for adolescents. The scores for fatigue, sleep, and balance showed no significant changes | Alex’s Lemonade Stand Foundation |
| n = 3 dropped out | |||||||||||
| Nilsson et al 47 | Non-immersive virtual reality (VR) for painful procedures | Children and adolescents with cancer (n = 42) | The groups did not differ at baseline | Children and adolescents 5 to 18 y old, who have undergone painful procedures at least once before | Children with cognitive impairment; children of parents who did not understand Swedish | Children played the virtual world game that started 1 to 5 min before the procedure and continued until the procedure was completed (n = 21) vs no game application (usual care) (n = 21) | n = 5 | Examine the effect of using non-immersive VR a 3 D display during a needle procedure on reported pain or distress of children/adolescents with cancer | For how long the needle procedure lasted | No statistical difference was found between the intervention group and the control group | Children’s Cancer Foundation at the Queen Silvia Children’s Hospital, the Sigurd and Elsa Goljes Foundation, the Federation of Swedish County Councils (VG-region), the Ebba Danelius Foundation, and the Wilhelm and Martina Lundgrens Foundation |
| Orsey et al 54 | This study had 2 objectives: (1) To assess the feasibility of the study and (2) To assess the efficacy of yoga intervention for pain management, fatigue, stress, anxiety, and overall QoL for pediatric cancer patients and their families | (1) Children undergoing cancer treatment (n = 20) parents (n = 20) and (2) Dyads (n = 22) | NA (a single group) | Children 8 to 18, with cognitive ability at least at 8-y old level undergoing cancer treatment, parents older than 18, parents and children physically able to do yoga. English speakers. (2) ability to attend at least 8 yoga sessions over 8 wk | NR | A weekly yoga intervention. Patients could pick from (a) bedside yoga (b) classroom yoga (c) chair yoga (n = 10) | 12 dyads withdrew (study 2) | (1) To study the feasibility of a yoga intervention (2) To test the efficacy of yoga intervention on the well-being of pediatric patients during active cancer treatment | 8-wk | Study (1) Demonstrated high levels of interest from patients and family members Study (2) Results trend toward improvement of QoL for patients and their parents | In CHIP Seed Grant Funding in Cancer Control |
| Thygeson et al 48 | Yoga for anxiety and distress | Children with cancer (n = 20); adolescents with cancer (n = 12); parents of children with cancer (n = 45) | NA | Children/adolescents 7 to 18 y old, who were hospitalized with cancer or blood disorders; spoke English; activity level appropriate for yoga; no previous yoga experience. Parents: Had a child with cancer, who spoke English, no previous experience with yoga. | NR | A total of (n = 49) participants: (n = 11) children; (n = 5) adolescents and (n = 33) parents received one yoga session | n = 28 | Explore the feasibility of a single yoga session for children/adolescents (and parents) hospitalized with cancer or other blood disorders | 45 min | Adolescents (P = .04) and parents (P < .01) experienced a significant decrease in anxiety scores. Children’s anxiety scores did not change from pre-class (P = .21). | No grant funding for this study |
| Wurz et al 49 | Yoga for physical benefits (health-related quality of life [HRQL]; physical fitness outcomes and physical activity levels [PAL]) | Children with cancer (n = 11) | NA (1 group only) | Children 5 y and older (5-17 y old); outpatient; limited previous yoga experience; not meeting the Canadian society for exercise physiology guidelines | NR | A total of (n = 8) received yoga 2 times weekly for 12 wk | n = 3 | Explore the feasibility and benefits of yoga for pediatric cancer patients | 12-wk Yoga intervention 2 times/weekly/60-min sessions | This 12-wk yoga program was feasible and provided preliminary evidence for the benefits of yoga (P = .02); Parent-reported HRQL (P = .03), functional mobility (P = .01), and total PAL (P = .02) | Grant from Canadian Institutes of Health Research; Alberta Children’s Hospital Research Institute; Psychosocial Oncology Research Training Program, University of Calgary |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplantation; NR, not reported; NA, not applicable.
For methodological assessment, the included studies were exported to the System for the Unified Management, Assessment and Review of Information (SUMARI software program, Joanna Briggs Institute (JBI)) 38 for critical appraisal of study quality. Two reviewers (DCM, TS) independently assessed the methodological quality of included articles using the critical appraisal checklists in SUMARI (checklist for quasi-experimental studies and qualitative research).
A meta-analysis could not be performed because the safety data in the studies was not reported consistently. As it was not possible to conduct a meta-analysis, the research group conducted a descriptive synthesis of the studies.
Results
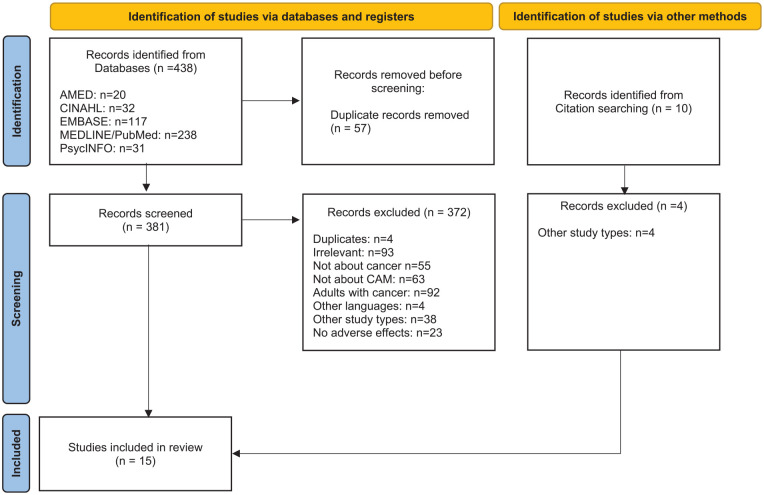
A total of 448 hits were identified. Twenty hits were identified in AMED, 32 in CINAHL, 117 in EMBASE, 238 in MEDLINE/PubMed, and 31 in PsycINFO. A total of 5 studies were identified after searches in reference lists. A total of 57 were excluded from further examination because they were duplicates and a total of 386 studies were included for further screening. Ten studies were identified from citation searching. Three hundred and seventy-six studies were excluded for the following reasons: 4 were duplicates, 93 were irrelevant (according to the criteria), 55 were not about cancer, 63 were not about CAM, 92 were about adults with cancer, 4 were written in languages other than the ones stated above, 42 were other study types, 22 were not about adverse effects of cancer treatment (Figure 1). A total of 1539-53 studies were included in this review, 10 quasi-experimental39,41,42,46-48,50-53 (Table 1), 3 observational studies40,44,45 (Table 2), and 2 qualitative studies43,49 (Table 3).
Figure 1.
Flow chart of the selection process of included studies.
Table 2.
Methodological Assessment of Observational Studies.
| Observational studies (n = 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Indication | Participants | Criteria | Intervention | Dropout | Objectives | Duration of treatment (follow-up) | Main results | Funding | ||
| Sample size | Baseline comparability | Inclusion | Exclusion | Treatment vs control (n) | Treatment vs control (n) | ||||||
| Chokshi et al 40 | Acupuncture for symptom management | Children receiving cancer treatment (n = 90) | Differences in age of participants accepting acupuncture compared to those who did not. The ethnicity of patients/parents was significantly associated with acceptance of acupuncture. | Acupuncture naive children/adolescents undergoing treatment for cancer at Columbia University Medical Center | Children who had previous experience with acupuncture and children with planned treatment protocols less than 6 mo | Individualized acupuncture (n = 49) vs CAM modalities (massage, yoga, meditation, nutrition counseling) (n = 41) | NR | Evaluate the use of acupuncture as a component of existing supportive care regimens among children and adolescents undergoing treatment for cancer | 6 mo | Acupuncture was more likely than other CAM modalities to be used for gastrointestinal/constitutional symptoms (P > .0001), lack of energy (P = .0001), and pain (P = .001) | NR in publication |
| Kennedy et al 44 | Antioxidants supplements (vitamin E; carotenoid; betacaroten; vitamin A) for inadequate plasma antioxidant concentrations | Children and adolescents with ALL (n = 103) | NA (1 single group) | Children and adolescents 1 to 21 y old with newly diagnosed ALL | NR | Received vitamin E; carotenoid; betacaroten; vitamin A (n = 100) | Timepoint 1: n = 3; timepoint 2: n = 16, timepoint 3: n = 16 | Investigate whether patients with sufficient antioxidant intakes while undergoing chemotherapy will have better tolerance to the treatment and experience fewer treatment-related adverse effects than those with insufficient antioxidant intakes | 6 mo | Greater vitamin C intake was associated with fewer therapy delays, less toxicity, and fewer days in hospital. Greater vitamin E intake was associated with a lower incidence of infection. Greater betacaroten intake was associated with a decrease in toxicity. Lower intakes of antioxidants were associated with increase in adverse side effects of chemotherapy. | Supported by a grant from the American Institute for Cancer Research, The Lener and Schwartz Family, and the American Cancer Society |
| Medina Córdoba and Pérez Villa 45 | Non-pharmacological measures (touch, play, music) for pain | Children with ALL (n = 35) | NA (1 group only) | Children <18 y old diagnosed with ALL | Children without clinical information on variables of interest; parents who show lack of interest | Touch; encourage children; explaining the procedures; music; play; video games; movies; thought replacement; images; drawing; comparing pain; breathing; massage; muscle contraction; relaxation images; TV; hot and cold applications (n = 35) | No dropout | Evaluate non-pharmacological measures to treat pain for children with ALL | As long as the patient was in the hospital, the average stay was 7 d | Music was the one factor that significantly improved pain (P = .01) | NR in publication |
Abbreviations: ALL, acute lymphoblastic leukemia; NR, not reported; NA, not applicable.
Table 3.
Methodological Assessment of Qualitative Studies.
| Qualitative interview studies (n = 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Population | Method | Design/analysis | Setting | Aim(s) | Participant(s) | Sample size | Inclusion/exclusion criteria | Duration of treatment (follow-up) | Results | Funding |
| Clerici et al 49 | Magic tricks as support to psychological approaches for children with cancer | Psychological interviews with pediatric patients | Qualitative design. Descriptive study of consultations with patients. | Hospital setting | Describe illusionist techniques (magic), suggest utility, and arouse interest | Children who underwent cancer treatment younger than 10 y of age | n = 30 | Children’s difficulty adapting to disease and treatment; relational problems with the hospital team; stressful treatments; problems with compliance to treatment, emotional distress; adaptation problems in survivors; terminal disease; relational problems in the family; psychological problems; psychopathologic conditions | NR | The use of magic in the context of pediatric oncology can represent a useful resource as a complement to traditional psychological support approaches | This study did not receive any funding |
| Hu et al 43 | Childhood cancer and acupressure for well-being and positive emotions | Semi-structured interviews lasting 60 to 90 min, and participant observation of the acupuncture sessions | Qualitative design. Interviews were transcribed verbatim. Data were analyzed using grounded theory symbolic (interactionism and phenomenology). | This study was nested within a clinical trial testing the effect of acupressure on children being treated for cancer | Explore whether and how acupressure, when provided by a professional or trained caregiver, was perceived as eliciting a sense of well-being | Acupressure providers (n = 3); primary caregivers of children with cancer (n = 13) | Total sample (n = 16) | Caregivers of children 5 to 21 y of age receiving hospital-based cancer treatment, who have demonstrated engagement in the acupuncture intervention were asked to participate. Caregivers who had a minimal interest were not asked. | NR | Acupressure brought symptom relief, physical relaxation, and comfort to the child and caregiver | Patient-centered outcomes research institute: Pierre’s birthday fund; the National cancer institute: the National center for complementary and alternative medicine |
| Nilsson et al 46 * (qualitative arm) | Non-immersive virtual reality (VR) for painful procedures | Semi-structured interviews with 21 children and adolescents following the completion of the intervention. Median time for the interviews was 8.5 min | Qualitative design. Interviews were transcribed verbatim and analyzed using a qualitative content analysis. | This was a qualitative arm nested within a clinical trial testing VR for painful procedures | Examine the participants’ response to the use of VP equipment during painful procedures | Children and adolescents with cancer (n = 21) | Total sample (n = 21) | Children and adolescents 5 to 18 y old, who have undergone painful procedures at least once before | During needle procedures | These interviews showed that non-immersive VR was a positive experience for children undergoing painful procedures | Children’s Cancer Foundation at the Queen Silvia Children’s Hospital, the Sigurd and Elsa Goljes Foundation, the Federation of Swedish County Councils (VG-region), the Ebba Danelius Foundation, and the Wilhelm and Martina Lundgrens Foundation |
Abbreviation: NR: not reported.
All of the included studies were written in English except one written in Spanish. 45 Detailed characteristics of the included studies are presented in Tables 1 to 3. Sample size refers to the total number of participants in the study. In the participant group, n refers to the number of participants who received the treatment or control intervention, respectively. Dropout refers to the number of participants who left the study before completion. Six studies39,42,44,47,48,53 did not report exclusion criteria. Three studies40,42,51 did not report a dropout. In addition, Favera-Scacco et al 42 did not report the duration of intervention. Nine (n = 9, 60%) of the 15 studies stated that they received financial support39,42-44,46,48,50,52,53 3 studies (n = 3) reported that they did not receive financial support.41,47,49 Three (n = 3, 20%)40,45,51 of the 15 studies did not report sources of funding (Table 1).
Safety of CAM Modalities for Interventions
Adverse effects were recorded as reported in the included studies. This means that 1 study participant could experience and report several adverse effects. Six studies (n = 6, 40%),40,41,44,47,48,51 reported data on adverse effects (Table 4). Across yoga studies,41,47,48,49-53 only 1 case of dizziness 47 was reported among 49 participants (2%). The other 4 studies41,44,48,51 that reported safety data, reported that the participants did not experience any adverse effects of the yoga programs. Choksi et al 40 reported 15 cases of bleeding with acupuncture treatment (out of 252 sessions, 6%) and 5 cases of bruising (2%). They reported no increase in acute or delayed adverse effects in patients with and without thrombocytopenia (P = .189) or neutropenia (P = .497). Kennedy et al 44 reported no adverse effects of antioxidant supplementation. Among the studies that reported safety data, events were reported as adverse effects,40,47 which are considered direct risks. None of the studies reported events considered as indirect risks.
Table 4.
Safety Assessment of the Included Studies (n = 15).
| Quasi-experimental studies (n = 10) | |||||
|---|---|---|---|---|---|
| Study ID | Objectives | Reported adverse effects (yes/no) | Types of adverse effects | Direct risk | Indirect risk |
| Barrera et al 39 | Examine the general benefits of music therapy for hospitalized pediatric cancer patients | No | NR | NR | NR |
| Diorio et al 41 | Determine the feasibility of individualized yoga for hospitalized children receiving chemotherapy | Yes | No adverse effects were experienced by the participants | NR | NR |
| Favera-Scacco et al 42 | Investigate art therapy as support (reduce stress and anxiety) for children with leukemia during painful procedures | No | NR | NR | NR |
| Geyer et al 50 | To describe the effect of therapeutic yoga on child and parental reports of quality of life in children hospitalized with oncological diagnoses | No | NR | NR | NR |
| Govardhan et al 51 | To establish the feasibility and therapeutic effect of yoga to address the effects of radiotherapy and chemotherapy in pediatric brain tumor | Yes | No adverse effects were experienced by the participants | NR | NR |
| Hooke et al 52 | Determine if children and adolescents who were cancer survivors and participated in the intervention had less fatigue. Improve balance, improve sleep quality, and less psychological distress compared with baseline measurements. | No | NR | NR | NR |
| Nilsson et al 46 | Examine the effect of using non-immersive VR a 3 D display during a needle procedure on reported pain or distress of children/adolescents with cancer | No | NR | NR | NR |
| Orsey et al 53 | To study the feasibility of a yoga intervention. To test the efficacy of yoga intervention on the well-being of pediatric patients during active cancer treatment. | No | NR | NR | NR |
| Thygeson et al 47 | Explore the feasibility of a single yoga session for children/adolescents (and parents) hospitalized with cancer or other blood disorders | Yes | One case of dizziness (child) | Yes | NR |
| Wurz et al 48 | Explore the feasibility and benefits of yoga for pediatric cancer patients | Yes | No participants experienced adverse effects from the yoga program | NR | NR |
| Longitudinal/prospective observational studies (n = 3) | |||||
| Chokshi et al 40 | Evaluate the use of acupuncture as a component of existing supportive care regimens among children and adolescents undergoing treatment for cancer | Yes | 15 cases of bleeding (out of 252 sessions); 5 cases of Grade I bruising; No increase in acute or delayed adverse effects in patients with and without thrombocytopenia (P = .189) or neutropenia (P = .497). No serious events were reported. | Yes | NR |
| Kennedy et al 44 | Investigate whether patients with sufficient antioxidant intakes while undergoing chemotherapy will have better tolerance to the treatment and experience fewer treatment-related adverse effects than those with insufficient antioxidant intakes | Yes | Supplementation was not associated with adverse effects at any of the time points | NR | NR |
| Medina Córdoba and Pérez Villa 45 | Evaluate non-pharmacological measures to treat pain for children with ALL | No | NR | NR | NR |
| Qualitative interview studies (n = 3) | |||||
| Study ID | Population | Reported adverse effects (yes/no) | Types of adverse effects | ||
| Clerici et al 49 | To describe illusionist techniques (magic), suggest utility, and arouse interest | NR | NR | NR | NR |
| Hu et al 43 | Explore whether and how acupressure, when provided by a professional or trained caregiver, was perceived as eliciting a sense of well-being | NR | NR | NR | NR |
| Nilsson et al 46 (qualitative arm) | Examine participants’ response to the use of VR equipment during painful procedures | NR | NR | NR | NR |
Abbreviations: ALL, acute lymphoblastic leukemia; VR, virtual reality; NR, not recorded in manuscript.
In summary: Safety data is underreported as 60% of the studies did not collect data on safety. All the adverse effects reported were associated to direct risks. The events were assessed by the researchers as minor and transient. No serious adverse effects were noted for acupuncture, yoga, and antioxidant supplements.
CAM Modalities
The results of the literature search indicated that the existing observational and qualitative studies about the use of CAM modalities to alleviate the adverse effects of cancer treatment in children and young adults can be divided into 4 main areas: Alternative medical systems; manipulative and body-based therapies, mind-body therapies, and biologically-based therapies. These areas are in line with the National Institute of Health’s National Center for Complementary and Integrative Health, which organizes CAM into the following categories: biologically-based therapies, mind-body therapies, manipulative and body-based therapies, energy therapies, alternative medical systems, and lifestyle therapies. 54
Alternative medical systems (acupuncture)
Two studies investigated acupuncture. One study investigated the use and safety of acupuncture among children receiving cancer treatment at Columbia Medical Center, USA, 40 and another delineated the use of acupuncture for symptom management and general well-being 43 among hospitalized children. The latter was a qualitative study nested within a clinical acupuncture trial. Chokshi et al 40 looked at individualized needle acupuncture and reported that 54% of the children preferred acupuncture for symptom management compared to other complementary therapies such as massage, yoga, meditation, or nutrition counseling. They received a median of 4 treatment sessions/acupuncture was more likely to be used for gastrointestinal and constitutional symptoms including drowsiness (P < .0001), lack of energy (P = .0001), and pain (P = .001). Hu et al 43 investigated acupressure together with therapeutic touch, and qualitative data were obtained through semi-structured interviews with caregivers and acupuncturists. According to these participants, acupressure brought symptom relief (ie, pain, nausea, etc.), physical relaxation, and comforting touch to the child as well as to the parents.
In summary: Acupuncture studies report through statistical and/or analytical data beneficial outcomes for children with cancer for symptom management. A meta-analysis was not conducted because the studies presented incomparable outcomes and the reported data was inadequate to conduct a meta-analysis.
Mind-body therapies (art, music, and imagination therapy)
Five studies (n = 5)39,42,45,46,49 investigated different CAM modalities for supportive care in pediatric cancer patients. Three of these studies investigated CAM modalities for pain and painful procedures during cancer treatment.42,45,46 One study 39 investigated music therapy to decrease anxiety and increase support and finally, one study 49 used magic techniques (illusionism) as a support resource for children with cancer. Nilsson et al 46 used a virtual reality device for needle-related pain and reported no statistically significant difference between the intervention and control group regarding pain and distress during and after the procedures. No statistical difference was found in heart rate during the procedure between the groups. In a qualitative arm, nested within this study, the participants reported that the virtual device was a positive experience. Medina Córdoba and Perez Villa 47 investigated non-pharmacological measures such as therapeutic touch, play, and music for painful procedures in children with acute lymphoblastic leukemia (ALL). They found that music therapy was the only modality that significantly improved pain (P = .01) for painful procedures. Favara-Scacco et al 42 investigated art therapy (visual imagination, play, drawing, and dramatization) for children with ALL who underwent lumbar puncture and bone marrow transplantation. Compared to the control group, children who used art therapy exhibited collaborative behavior before the procedure. The modality was shown to be a useful intervention, and parents declared that they were better able to manage the painful procedures when art therapy was offered to the child. Barrera et al 39 investigated music therapy for children hospitalized with cancer. In a pre-and post-design, they reported a significant improvement in children’s feelings from pre to post music therapy (P < .01). There was also a significant main effect of engagement, indicating that actively engaged children had higher scores than the passive children (P < .01). However, the results varied with the age of the child. In a qualitative design, Clerici et al 49 explored the use of magic tricks as support to psychological approaches in consultations with hospitalized children. Based on these data, they suggested the use of magic tricks to be helpful in providing support for communication and relations, as well as for compliance and rehabilitation for children with cancer.
In summary: Art, music, and imaginary modalities studies report beneficial support for children with cancer through statistical and narrative results.
Mind-body therapies (yoga)
Seven studies (n = 7) investigated the benefits of yoga41,47,48,50-53 for children with cancer. Diorio et al 41 investigated the feasibility of a 3-week yoga program for children who were receiving intensive chemotherapy. In addition, they investigated whether yoga could be a useful intervention for cancer-related fatigue. They found that yoga was feasible, as 10/11 participants met the threshold for feasibility. Feedback from parents and children indicated the physical and psychological benefits of yoga. Thygeson et al 47 looked at yoga for distress and anxiety and investigated whether 1 yoga session could offer benefits to children and their parents in an outpatient oncology unit. Children with a normal anxiety score pre-class did not change (P = .21). Parents (P < .01) and adolescents (P = .04) experienced a significant decrease in anxiety scores after the yoga session. Wurz et al 48 investigated the feasibility and benefits of a 12-week yoga program. The program was feasible and indicated significant improvement for patients (P = .02), and parents reported improved health according to the health-related quality of life (HRQL) scale (P = .03), functional mobility (P = .01), total physical fitness outcomes and physical activity (PAL) (P = .02) pre- to post-intervention. Geyer et al 50 described the effect of therapeutic yoga on child and parents. The study reported quality of life in children hospitalized with oncological diagnoses. Therapeutic yoga had a positive effect on a child’s perception of gross motor functioning (P = .016). Govardhan et al 51 wanted to establish the feasibility and therapeutic effect of yoga to address the effects of radiotherapy and chemotherapy in pediatric brain tumors. The feasibility of the yoga intervention was established. The median sessions attended were 16 of 20. Significant difference was reported in respect to pain (P = .0001), relief in headache (P = .0005), increase in appetite (P = .0005), better sleep (P = .0003), reduced fatigue (P = .007), and overall daily activity (P = .0018). Hooke et al 52 sought to explore the feasibility and benefits of a 6-session weekly yoga intervention for pediatric cancer survivors who completed therapy in the past 2 to 24 months. About 72% of the participants enrolled completed the study, establishing the feasibility of the study. After the 6-week yoga intervention, most of the symptoms measured (balance, fatigue, and sleep) remained unchanged. Anxiety scores had a significant (P = .04) decrease after the yoga intervention. Orsey et al 53 determined the feasibility and preliminary efficacy of a yoga intervention for pediatric cancer patients in active treatment and their families. The study reported significant improvement in measures of emotional (P = .03) and social function (P = .03) and the total score (P = .006). Furthermore, among parents, the mental health composite score significantly (P < .05) increased post-intervention.
In summary: The studies report that yoga programs were feasible through both narrative and statistical results, and both parents and children indicated physical and psychological benefits of yoga.
Biologically-based therapies
One study investigated biological therapies. Kennedy et al 44 investigated whether patients with sufficient antioxidant intakes while undergoing chemotherapy would have better tolerance to the treatment and experienced fewer treatment-related adverse effects than those with insufficient antioxidant intakes. The researchers found that lower intakes of antioxidants were associated with increases in adverse effects of chemotherapy. Participants were classified as having adequate or inadequate nutrient plasma concentrations as compared with clinical chemistry standards for vitamins A, C, and E.
Methodological Quality of Studies
The Joanna Briggs Institute’s quasi-experimental study appraisal checklist was used to assess the quality of the quasi-experimental studies, the cohort studies checklist was used for the observational studies (longitudinal and prospective), and the checklist for qualitative research was used for the interview studies. All studies scored above 70% (Tables 5-). One study (n = 1) 46 met the criteria for checking every item (9 out of 9 items for quasi-experimental studies and 10 out of 10 items for qualitative studies). Eight studies (n = 8)39,41,47,48,50-53 addressed 8 out of 9 items (Table 5). For the observational studies, 1 study addressed 9, 44 another 8, 45 and another 7 40 of the 11 items for cohort studies (Table 6). Two qualitative studies43,49 addressed 8 and 9 out of 10 items respectively and finally, 1 study 42 addressed 7 out of 9 items (Table 7).
Table 5.
Quasi-Experimental Studies Appraisal.
| Citation | Q1. Is it clear in the study what is the “cause” and what is the “effect” (ie, there is no confusion about which variable comes first)? | Q2. Were the participants included in any comparisons similar? | Q3. Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? | Q4. Was there a control group? | Q5. Were there multiple measurements of the outcome both pre and post the intervention/exposure? | Q6. Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed? | Q7. Were the outcomes of participants included in any comparisons measured in the same way? | Q8. Were outcomes measured in a reliable way? | Q9. Was appropriate statistical analysis used? | % |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrera et al 39 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Diorio et al 41 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Favara-Scacco et al 42 | Y | Y | Y | Y | Y | Y | Y | U | U | 78 |
| Nilsson et al 46 | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Geyer et al 50 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Govardhan et al 51 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Hooke et al 52 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Orsey et al 53 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
| Thygeson et al 47 | Y | Y | Y | N | Y | Y | Y | Y | N | 89 |
| Wurz et al 48 | Y | Y | Y | N | Y | Y | Y | Y | Y | 89 |
Abbreviations: Y, Yes; N, No; U, unclear.
Table 6.
Longitudinal/Prospective Observational Studies Appraisal.
| Citation | Q1. Were the 2 groups similar and recruited from the same population? | Q2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Q3. Was the exposure measured in a valid and reliable way? | Q4. Were confounding factors identified? | Q5. Were strategies to deal with confounding factors stated? | Q6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Q7. Were the outcomes measured in a valid and reliable way? | Q8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Q9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Q10. Were strategies to address incomplete follow up utilized? | Q11. Was appropriate statistical analysis used? | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chokshi et al 40 | U | Y | Y | N | N | Y | Y | Y | Y | NA | Y | 70 |
| Kennedy et al 44 | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | 81 |
| Medina Córdoba and Pérez Villa 45 | Y | Y | Y | N | N | Y | Y | Y | Y | NA | Y | 80 |
Abbreviations: Y, yes; N, no; NA, not applicable; U, unclear.
Table 7.
Qualitative Studies Appraisal.
| Citation | Q1. Is there congruity between the stated philosophical perspective and the research methodology? | Q2. Is there congruity between the research methodology and the research question or objectives? | Q3. Is there congruity between the research methodology and the methods used to collect data? | Q4. Is there congruity between the research methodology and the representation and analysis of data? | Q5. Is there congruity between the research methodology and the interpretation of results? | Q6. Is there a statement locating the researcher culturally or theoretically? | Q7. Is the influence of the researcher on the research, and vice-versa, addressed? | Q8. Are participants, and their voices, adequately represented? | Q9. Is the research ethical according to current criteria or, for recent studies, and is there evidence of ethical approval by an appropriate body? | Q10. Do the conclusions drawn in the research report flow from the analysis, or interpretation, of the data? | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clerici et al 49 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 90 |
| Hu et al 43 | Y | Y | Y | Y | Y | U | Y | Y | U | Y | 80 |
Abbreviations: Y, Yes; N, No; U, unclear.
In summary: According to the SUMMARI software program from Joanna Briggs Institute, the score for the methodological quality of most (n = 15) of the included studies was 70% and above. One study (n = 1) 46 obtained a total score of 100% and 13 studies (n = 13)39,41-45,47-53 obtained scores between 75% and 90%. One study 40 obtain a score of 70%.
Discussion
As cancer survival among children increases, it is important to assess different methods to alleviate the adverse effects derived from cancer treatment and thereby lessen the burden on children, young adults, and their families. Hence, we performed this present review and found that no serious adverse effects from the CAM treatments were reported among the studies included in this review, but less than half of the studies reported adverse effects, which is a threat to patient safety. However, all included studies had critical appraisal scores above 70% according to the JBI SUMMARI tool criteria. CAM modalities were used with the purpose to alleviate anxiety, pain, toxicity, prevent trauma, and improving HRQL, functional mobility and physical activity levels. Both children and parents reported physical and physiological benefits such as a decrease in anxiety from acupuncture and yoga.
Safety
In the hierarchy of study designs, observational studies are categorized methodologically at an intermediate level, and randomized controlled trials (RCTs) as the studies with the gold standard methodology. 55 Although RCTs are leading in evidence-building, it is important to acknowledge the contributions that results from observational studies can provide to the healthcare field. 55 Unlike RCTs, observational studies are less restrictive of the sample of patients selected, the intervention delivered, or the outcome(s) measured; hence contributing to the generalizability of the study. 55 Observational studies also identify serious uncommon harms and longtime effects of medical interventions 56 as they are often conducted for longer periods and are in real-life settings. 57 In contrast to conventional medicine, CAM therapies have no regulatory gatekeeper controlling their therapeutic quality, safety, efficacy, and effectiveness before they are marketed. Thus, many CAM modalities were traditionally and widespread in use before they were investigated or regulated. In addition, CAM modalities are often provided as an integrated “whole system” of care (ie, Ayurveda), without careful consideration of safety issues. 58 Even though the results of this review show minor adverse effects to CAM treatments, the results are in line with literature that shows that adverse effects are seldom reported in studies with CAM.59,60 Natural remedies are often perceived as safe; however, that is not always the case because they might interact negatively with conventional cancer treatment. 61 In an evaluation of the safety of CAM trials, Tuner et al 59 reported that more than half of the trials in their review had inadequate reporting of safety data. According to the literature, 62 parents do not want to use modalities that add further suffering to their child. Safety information is therefore of high importance for parents as they want to avoid CAM modalities that have known adverse effects. 12
In contrast, other studies have reported that adverse effects in acupuncture 63 and homeopathy 64 are commonly reported. The report of adverse effects among these modalities could be attributed to well-established reporting guidelines such as the Standard for Reporting Interventions in Controlled Trials of Acupuncture (STRICTA) guidelines 65 and Consolidated Standards of Reporting Trials (CONSORT) for herbal medicine. 66
The lack of regulation for CAM modalities and products as well as the lack of a standardized reporting system for the field as a whole, make it difficult to compare studies on safety. Given the substantial use of CAM worldwide, it is important to have accurate information on the safety of such treatments and modalities. Fønnebø et al 58 proposed a research strategy for CAM that accounts for the lack of regulation of CAM in western countries. The strategy proposes to (1) look at the context, paradigms, philosophical understanding; (2) assess the safety status; (3) examine the effectiveness of the treatment; (4) assess the efficacy; and (5) understand the biological mechanism of the treatment. 58 According to this strategy, it is important to investigate safety before the effect of a modality. Deng et al 67 also highlight the importance of examining the safety and efficacy of different CAM modalities. In this clinical practice guidelines for integrative oncology, the researchers make recommendations based on a risk versus efficacy evaluation. If a CAM modality is considered safe and efficacious the modality should be recommended. If the modality is considered safe but the evidence for efficacy is inconclusive, the modality should be recommended, however, effectiveness should be closely monitored. If the modality is efficacious, but the evidence for safety is inconclusive, the modality should be recommended, but the safety should be closely monitored. Lastly, if the modality is not efficacious and is connected with serious risks, the modality should be avoided. Research strategies and recommendations guidelines such as the ones provided by Fønnebø et al and Deng et al should be adopted and implemented throughout the different CAM modalities for research and clinical practice.
It is essential to extend the existing guidelines in journals and study appraisal checklists to encourage appropriate standardized reporting of adverse effects of CAM studies. STRICTA guidelines, for example, include in their checklist the reporting of harms. 65 Such reporting will improve the quality of the research and provide a greater understanding of the safety of CAM treatments and products.
CAM Modalities
Twelve39,41,42,45-53 out of the 12 studies reviewed in this article were related to mind and body practices. All of the studies reported beneficial results from CAM treatments for physical and emotional symptoms derived from cancer treatment. Existing literature is consistent with the results of this review.68-77 Several studies have reported promising results of yoga among pediatric patients69-71,73 as well adult cancer patients.72,74,75 For example, Mandanmohan et al 76 reported that yoga training among children produced significant gains in muscle strength. Five 39,42,45,46,49 of the studies reported in this review examined the effects of art and music therapy among pediatric patients undergoing cancer treatment. Most of the studies demonstrated that art-music therapy and magic tricks had a positive effect on symptoms such as pain, anxiety, engagement, support, and communication. This is in line with other studies that found art and music therapy beneficial for children with cancer.26-28,78-83 Acupuncture was used in 2 studies40,43 included in this review. Existing acupuncture literature among children 84 and adults85,86 with cancer is consistent with the findings of this review. In a systematic review, Jindal et al 87 reported that acupuncture was used to treat gastrointestinal disorders and pain in children. 87 One study 44 included in this review accessed the association of antioxidant intake and increases in the adverse effects of chemotherapy in children. Different vitamins were attributed different benefits. The use of different vitamins such as vitamin D deficiency has shown an association with oral mucositis in pediatric patients but the effects of vitamins to treat adverse events of cancer in children are still inconclusive.44,88,89 More research with a rigorous design (RCTs), is needed to confirm these results before recommendations for clinical practice.
Limitations
This review should be understood considering its limitations. Among the limitations of this review are that the studies included were not homogenous regarding study design, participants, intervention, control, and outcome measures therefore making it impossible for meta-analysis to access the safety of the modalities used to treat adverse effects caused by cancer treatment in children. Another limitation is the size of the studies; most of the studies presented had small samples affecting the generalizability of the results. CAM is a field that encapsulates many modalities and not all of them are presented in this review. Generally, many CAM modalities are under-researched, especially among this population. Efforts have been made to retrieve all observational, quasi-experimental, and qualitative studies of interest, but it is impossible to be entirely certain that all potentially eligible studies have been found. The literature was searched in several databases, but it is possible studies were overlooked. Limiting the studies to the languages stated in the methods could also have led us to miss some relevant papers. Another limitation is that there are 2 articles43,44 where participants older than 18 years were included. The results reported in this review therefore to some extent also represent young adults with cancer. Although this review has limitations, those are counteracted by carefully implementing the search methods by a research librarian and by assessing the methodological quality of the articles with the use of critical appraisal tools. Although we used well-known critical appraisal tools it is possible that other tools can provide different results from the ones presented in this review.
Implication for Practice
The review indicates that CAM modalities such as mind and body treatments are being used in the management of symptoms from cancer treatment such as anxiety, yet they lack appropriate reporting for adverse effects. The latter finding should be used to promote further research and pilot tests related not only to safety but also to other aspects such as dosage for different CAM modalities used among children and young adults with cancer.
Implication for Research
Unlike conventional medicine, CAM is evaluated holistically. Hence, research should focus on the different aspects of treatment and implementation. 58 Symptoms of distress among children and young adults undergoing cancer treatment are high. 90 Symptoms do not often present themselves individually but as clusters. A symptom cluster is defined as 2 or more symptoms that occur together and are related to each other. 91 CAM modalities (ie, massage and reiki) have shown possible effectiveness on cluster symptom management 27 and could be considered more often to treat symptom clusters that conventional medicine has difficulty treating such as feeling nervous, sad, and lacking energy. 27 Furthermore, quality assessment and peer review tools should be modified to encourage adequate reporting of harmful events for CAM studies. Also, due to their comprehensive nature, more RCTs, as well as observational, quasi-experimental, and qualitative studies, should be implemented to enhance our understanding of the effect, effectiveness, and safety of CAM treatments.
Conclusion
This review demonstrates that the majority of the studies of CAM use in pediatric cancer lack proper reporting of safety. It is therefore important to encourage CAM researchers to record and report adverse effects of interventions. This is particularly important in pediatric oncology where parents do not want to add any unnecessary burden to the child and need adequate safety information on CAM.
Supplemental Material
Supplemental material, sj-pdf-1-ict-10.1177_15347354221105563 for Safety of Complementary and Alternative Medicine (CAM) treatment among children and young adults who suffer from adverse effects of conventional cancer treatment: A systematic review by Dana C. Mora, Agnete E. Kristoffersen, Grete Overvåg, Miek C. Jong, Marit Mentink, Jianping Liu and Trine Stub in Integrative Cancer Therapies
Footnotes
Author Contributions: TS: Conceived the study. Together with DCM, they performed the searches, selected studies for inclusion and collected study data, assessed the studies for risk of bias (methodological assessment) and performed the methodological assessment of the studies according to the JBI methodology, and drafted the manuscript. GO: Developed the search strategy and performed the searches together with TS and DCM. AEK, GO, MJ, MM, JPL: Contributed with intellectual content and reviewed subsequent versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Ekhagastiftelsen (2020-76) and NAFKAM. The publication charges for this article have been funded by UIT The Arctic University of Norway.
ORCID iD: Dana C. Mora  https://orcid.org/0000-0003-1209-3526
https://orcid.org/0000-0003-1209-3526
Supplemental Material: Supplemental material for this article is available online.
References
- 1. WHO. World Health Organization childhood cancer. Accessed September 7, 2021. https://www.who.int/news-room/fact-sheets/detail/cancer-in-children
- 2. Zhang J, Nichols KE, Downing JR. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2016;374:2336-2346. [DOI] [PubMed] [Google Scholar]
- 3. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? May 10, 2021. Updated 2018. Accessed May 10, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- 4. Chotsampancharoen T, Sripornsawan P, Duangchu S, Wongchanchailert M, McNeil E. Survival outcome of alternative medicine treatment for newly diagnosed acute leukemia in children. Acta Haematol. 2018;140:203-208. [DOI] [PubMed] [Google Scholar]
- 5. Bhakta N, Force LM, Allemani C, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20:e42-e53. [DOI] [PubMed] [Google Scholar]
- 6. Ladas EJ. Integrative medicine in childhood cancer. J Altern Complement Med. 2018;24:910-915. [DOI] [PubMed] [Google Scholar]
- 7. Lüthi E, Diezi M, Danon N, et al. Complementary and alternative medicine use by pediatric oncology patients before, during, and after treatment. BMC Complement Med Ther. 2021;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuzak TJ, Boňková J, Careddu D, et al. Use of complementary and alternative medicine by children in Europe: published data and expert perspectives. Complement Ther Med. 2013;21(Suppl 1):S34-S47. [DOI] [PubMed] [Google Scholar]
- 9. Bishop FL, Prescott P, Chan YK, Saville J, von Elm E, Lewith GT. Prevalence of complementary medicine use in pediatric cancer: a systematic review. Pediatrics. 2010;125:768-776. [DOI] [PubMed] [Google Scholar]
- 10. Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. J Natl Canc Inst Monogr. 2017; 57:3-8. doi: 10.1093/jncimonographs/lgx012 [DOI] [PubMed] [Google Scholar]
- 11. Diorio C, Lam CG, Ladas EJ, et al. Global use of traditional and complementary medicine in childhood cancer: a systematic review. J Glob Oncol. 2017;3:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stub T, Kristoffersen AE, Overvåg G, Jong MC. An integrative review on the information and communication needs of parents of children with cancer regarding the use of complementary and alternative medicine. BMC Complement Med Ther. 2020;20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacLennan AH, Myers SP, Taylor AW. The continuing use of complementary and alternative medicine in South Australia: costs and beliefs in 2004. Med J Aust. 2006;184:27-31. [DOI] [PubMed] [Google Scholar]
- 14. Lowrance WW. Of acceptable risk: science and the determination of safety. William Kaufmann; 1976. [Google Scholar]
- 15. Stub T. Safety of treatment provided by homeopaths homeopathic aggravations, adverse effects and risk assessment. [PhD thesis]. UiT The Arctic University of Norway; 2013. [Google Scholar]
- 16. Stub T, Salamonsen A, Kristoffersen A, Musial F. How to handle worsening of condition during treatment – risk assessment in homeopathic practice. Forsch Komplementmed. 2015;22:30-35. [DOI] [PubMed] [Google Scholar]
- 17. Barnekreftforeningen. English: children’s cancer society. Childhood Cancer. July 23, 2021. Accessed July 22, 2021. https://www.barnekreftforeningen.no/barnekreft
- 18. The Norwegian Cancer Society. Senskader (English: late effects) the Norwegian Cancer Society. September 7, 2021. Accessed September 7, 2021. https://kreftforeningen.no/om-kreft/senskader/
- 19. Helsedirektoratet. The Norwegian Directorate of Health. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft hos barn. September 7, 2021. Accessed September 7, 2021. https://www.helsedirektoratet.no/retningslinjer/kreft-hos-barn-handlingsprogram/Nasjonalt%20handlingsprogram%20med%20retningslinjer%20for%20diagnostikk,%20behandling%20og%20oppf%C3%B8lging%20av%20kreft%20hos%20barn.pdf/_/attachment/inline/bb5802ba-8f82-494f-aeb8-0abf0e59382d:51e8734fdfed4f99153c96b98fb775d6f2d59341/Nasjonalt%20handlingsprogram%20med%20retningslinjer%20for%20diagnostikk,%20behandling%20og%20oppf%C3%B8lging%20av%20kreft%20hos%20barn.pdf [Google Scholar]
- 20. Lissauer TCGP. English: Pedriatrics. FADL’s Forlag; 2016. [Google Scholar]
- 21. Bottomley SJ, Kassner E. Late effects of childhood cancer therapy. J Pediatr Nurs. 2003;18:126-133. [DOI] [PubMed] [Google Scholar]
- 22. Hedén L, Pöder U, von Essen L, Ljungman G. Parents’ perceptions of their child’s symptom burden during and after cancer treatment. J Pain Symptom Manag. 2013;46:366-375. [DOI] [PubMed] [Google Scholar]
- 23. Joseph PD, Craig JC, Caldwell PH. Clinical trials in children. Br J Clin Pharmacol. 2015;79:357-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bond MC, Pritchard S. Understanding clinical trials in childhood cancer. Paediatr Child Health. 2006;11:148-150. [PMC free article] [PubMed] [Google Scholar]
- 25. Mora DC, Overvåg G, Jong MC, et al. Complementary and alternative medicine modalities used to treat adverse effects of anti-cancer treatment among children and young adults: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Med Ther. 2022;22:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nunes MDR, Bomfim E, Olson K, et al. Interventions minimizing fatigue in children/adolescents with cancer: an integrative review. J Child Health Care. 2018;22:186-204. [DOI] [PubMed] [Google Scholar]
- 27. Lopes-Júnior LC, Urbano IR, Schuab SIPDC, Pessanha RM, Rosa GS, Lima RAGD. Effectiveness of complementary therapies for the management of symptom clusters in palliative care in pediatric oncology: a systematic review. Rev Esc Enferm USP. 2021;55:1-14 [DOI] [PubMed] [Google Scholar]
- 28. Lopes-Júnior LC, Bomfim EO, Nascimento LC, Nunes MD, Pereira-da-Silva G, Lima RA. Non-pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: an integrative review. Eur J Cancer Care. 2016;25:921-935. [DOI] [PubMed] [Google Scholar]
- 29. Vandenbroucke JP. When are observational studies as credible as randomised trials?. Lancet. 2004;363:1728-1731. [DOI] [PubMed] [Google Scholar]
- 30. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. A E. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;74:790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [DOI] [PubMed] [Google Scholar]
- 34. Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol. 2006;59:765-769. [DOI] [PubMed] [Google Scholar]
- 35. Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63:1061-1070. [DOI] [PubMed] [Google Scholar]
- 36. Waddington H, Aloe AM, Becker BJ, et al. Quasi-experimental study designs series-paper 6: risk of bias assessment. J Clin Epidemiol. 2017;89:43-52. [DOI] [PubMed] [Google Scholar]
- 37. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2008. [Google Scholar]
- 38. Piper C. System for the unified management, assessment, and review of information (SUMARI). J Med Libr Assoc. 2019;107:634-636. [Google Scholar]
- 39. Barrera ME, Rykov MH, Doyle SL. The effects of interactive music therapy on hospitalized children with cancer: a pilot study. Psychooncology. 2002;11:379-388. [DOI] [PubMed] [Google Scholar]
- 40. Chokshi SK, Ladas EJ, Taromina K, et al. Predictors of acupuncture use among children and adolescents with cancer. Pediatr Blood Cancer. 2017;64:e26424. doi: 10.1002/pbc.26424 [DOI] [PubMed] [Google Scholar]
- 41. Diorio C, Schechter T, Lee M, et al. A pilot study to evaluate the feasibility of individualized yoga for inpatient children receiving intensive chemotherapy. BMC Complement Altern Med. 2015;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Favara-Scacco C, Smirne G, Schilirò G, Di Cataldo A. Art therapy as support for children with leukemia during painful procedures. Med Pediatr Oncol. 2001;36:474-480. [DOI] [PubMed] [Google Scholar]
- 43. Hu H, Shear D, Thakkar R, et al. Acupressure and therapeutic touch in childhood cancer to promote subjective and intersubjective experiences of well-being during curative treatment. Glob Adv Health Med. 2019;8:2164956119880143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kennedy DD, Tucker KL, Ladas ED, Rheingold SR, Blumberg J, Kelly KM. Low antioxidant vitamin intakes are associated with increases in adverse effects of chemotherapy in children with acute lymphoblastic leukemia. Am J Clin Nutr. 2004;79:1029-1036. [DOI] [PubMed] [Google Scholar]
- 45. Medina Córdoba CA, Pérez Villa M. Medidas no farmacológicas implementadas por las enfermeras para el dolor de niños con Leucemia Linfocítica Aguda. Index de Enfermería. 2019;28:46-50. [Google Scholar]
- 46. Nilsson S, Finnström B, Kokinsky E, Enskär K. The use of virtual reality for needle-related procedural pain and distress in children and adolescents in a paediatric oncology unit. Eur J Oncol Nurs. 2009;13:102-109. [DOI] [PubMed] [Google Scholar]
- 47. Thygeson MV, Hooke MC, Clapsaddle J, Robbins A, Moquist K. Peaceful play yoga: serenity and balance for children with cancer and their parents. J Pediatr Oncol Nurs. 2010;27:276-284. [DOI] [PubMed] [Google Scholar]
- 48. Wurz A, Chamorro-Vina C, Guilcher GM, Schulte F, Culos-Reed SN. The feasibility and benefits of a 12-week yoga intervention for pediatric cancer out-patients. Pediatr Blood Cancer. 2014;61:1828-1834. [DOI] [PubMed] [Google Scholar]
- 49. Clerici CA, Pagani Bagliacca E, Silva M, et al. Illusionist techniques as a complement to psychological support for children with cancer. Tumori J. 2021;107:171-174. [DOI] [PubMed] [Google Scholar]
- 50. Geyer R, Lyons A, Amazeen L, Alishio L, Cooks L. Feasibility study: the effect of therapeutic yoga on quality of life in children hospitalized with cancer. Pediatr Phys Ther. 2011;23:375-379. [DOI] [PubMed] [Google Scholar]
- 51. Govardhan H, Nelson N, Khaleel I, et al. Effect of yoga on the symptoms response in pediatric brain tumor in-patients undergoing chemo and radiotherapy. Oncol Radiother. 2019;1:34-38. [Google Scholar]
- 52. Hooke MC, Gilchrist L, Foster L, Langevin M, Lee J. Yoga for children and adolescents after completing cancer treatment. J Pediatr Oncol Nurs. 2016;33:64-73. [DOI] [PubMed] [Google Scholar]
- 53. Orsey AD, Park CL, Pulaski R, Shankar NL, Popp JM, Wakefield D. Results of a pilot yoga intervention to improve pediatric cancer patients’ quality of life and physical activity and parents’ well-being. Rehabil Oncol. 2017;35:15-23. [Google Scholar]
- 54. Bondurant S, Anastasi J, Berman B, et al. Complementary and Alternative Medicine in the United States. Institute of Medicine, National Academies Press; 2005. [PubMed] [Google Scholar]
- 55. Concato J. Observational versus experimental studies: what’s the evidence for a hierarchy? NeuroRx. 2004;1:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Papanikolaou PN, Christidi GD, Ioannidis JP. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006;174:635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stub T, Kristoffersen AE, Overvåg G, Jong MC, Musial F, Liu J. Adverse effects in homeopathy: a systematic review and meta-analysis of observational studies. Explore. 2022;18:114-128. [DOI] [PubMed] [Google Scholar]
- 58. Fønnebø V, Grimsgaard S, Walach H, et al. Researching complementary and alternative treatments – the gatekeepers are not at home. BMC Med Res Methodol. 2007;7:7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Turner L-A, Singh K, Garritty C, et al. An evaluation of the completeness of safety reporting in reports of complementary and alternative medicine trials. BMC Complement Altern Med. 2011;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karkhaneh M, Zorzela L, Jou H, Funabashi M, Dryden T, Vohra S. Adverse events associated with paediatric massage therapy: a systematic review. BMJ Paediatr Open. 2020;4:e000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Myers SP, Cheras PA. The other side of the coin: safety of complementary and alternative medicine. Med J Aust. 2004;181:222-225. [PubMed] [Google Scholar]
- 62. Stub T, Quandt SA, Kristoffersen AE, Jong MC, Arcury TA. Communication and information needs about complementary and alternative medicine: a qualitative study of parents of children with cancer. BMC Complement Med Ther. 2021;21:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adams D, Cheng F, Jou H, Aung S, Yasui Y, Vohra S. The safety of pediatric acupuncture: a systematic review. Pediatrics. 2011;128:e1575-e1587. [DOI] [PubMed] [Google Scholar]
- 64. Stub T, Musial F, Kristoffersen AA, Alræk T, Liu J. Adverse effects of homeopathy, what do we know? A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2016;26:146-163. [DOI] [PubMed] [Google Scholar]
- 65. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for reporting interventions in clinical trials of acupuncture (Stricta): extending the CONSORT statement. PLoS Med. 2010;7:e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364-367. [DOI] [PubMed] [Google Scholar]
- 67. Deng GE, Frenkel M, Cohen L, et al. Evidence-based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol. 2009;7:85-120. [PubMed] [Google Scholar]
- 68. Kanitz JL, Camus ME, Seifert G. Keeping the balance—an overview of mind-body therapies in pediatric oncology. Complement Ther Med. 2013;21(Suppl 1):S20-S25. [DOI] [PubMed] [Google Scholar]
- 69. Birdee GS, Yeh GY, Wayne PM, Phillips RS, Davis RB, Gardiner P. Clinical applications of yoga for the pediatric population: a systematic review. Acad Pediatr. 2009;9:212-220.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Galantino ML, Galbavy R, Quinn L. Therapeutic effects of yoga for children: a systematic review of the literature. Pediatr Phys Ther. 2008;20:66-80. [DOI] [PubMed] [Google Scholar]
- 71. Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260. [DOI] [PubMed] [Google Scholar]
- 73. Rheingans JI. A systematic review of nonpharmacologic adjunctive therapies for symptom management in children with cancer: review systematic review. J Pediatr Oncol Nurs. 2007;24:81-94. [DOI] [PubMed] [Google Scholar]
- 74. Michalsen A, Grossman P, Acil A, et al. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. Med Sci Monit. 2005;11:CR555-CR561. [PubMed] [Google Scholar]
- 75. Mustian KM, Palesh O, Sprod L, et al. Effect of YOCAS yoga on sleep, fatigue, and quality of life: a URCC CCOP randomized, controlled clinical trial among 410 cancer survivors. J Clin Oncol. 2010;28:9013-9013. [Google Scholar]
- 76. Mandanmohan M, Jatiya L, Udupa K, Bhavanani AB. Effect of yoga training on handgrip, respiratory pressures and pulmonary function. Indian J Physiol Pharmacol. 2003;47:387-392. [PubMed] [Google Scholar]
- 77. Juangpanich U, Onbunreang J, Khansorn T, Lunlud J, Vatanasapt P. Effect of music therapy on anxiety and pain in cancer patients. J Nurses’ Assoc Thailand, North-Eastern Div. 2012;30:46-52. [Google Scholar]
- 78. Nguyen TN, Nilsson S, Hellstrom AL, Bengtson A. Music therapy to reduce pain and anxiety in children with cancer undergoing lumbar puncture: a randomized clinical trial: randomized controlled trial. J Pediatr Oncol Nurs. 2010;27:146-155. [DOI] [PubMed] [Google Scholar]
- 79. Velez-Florez G, Velez-Florez MC, Mantilla-Rivas JO, Patarroyo-Rodríguez L, Borrero-León R, Rodríguez-León S. Mind-body therapies in childhood cancer. Curr Psychiatry Rep. 2018;20:58. [DOI] [PubMed] [Google Scholar]
- 80. Facchini M, Ruini C. The role of music therapy in the treatment of children with cancer: a systematic review of literature. Complement Ther Clin Pract. 2021;42:101289. [DOI] [PubMed] [Google Scholar]
- 81. Abdulah DM, Abdulla BMO. Effectiveness of group art therapy on quality of life in paediatric patients with cancer: a randomized controlled trial. Complement Ther Med. 2018;41:180-185. [DOI] [PubMed] [Google Scholar]
- 82. Aguilar BA. The efficacy of art therapy in pediatric oncology patients: an integrative literature review. J Pediatr Nurs. 2017;36:173-178. [DOI] [PubMed] [Google Scholar]
- 83. Lopes-Junior LC, Silveira DSC, Olson K, et al. Clown intervention on psychological stress and fatigue in pediatric patients with cancer undergoing chemotherapy. Cancer Nurs. 2020;43:290-299. [DOI] [PubMed] [Google Scholar]
- 84. Flank J, Robinson PD, Holdsworth M, et al. Guideline for the treatment of breakthrough and the prevention of refractory chemotherapy-induced nausea and vomiting in children with cancer. Pediatr Blood Cancer. 2016;63:1144-1151. [DOI] [PubMed] [Google Scholar]
- 85. Tsukinoki R, Murakami Y. Non-communicable disease epidemic: epidemiology in action (EuroEpi 2013 and NordicEpi 2013): Aarhus, Denmark from 11 August to 14 August 2013. Eur J Epidemiol. 2013;28:1-270. [DOI] [PubMed] [Google Scholar]
- 86. O’Regan D, Filshie J. Acupuncture and cancer. Auton Neurosci. 2010;157:96-100. [DOI] [PubMed] [Google Scholar]
- 87. Jindal V, Ge A, Mansky PJ. Safety and efficacy of acupuncture in children: a review of the evidence. J Pediatr Hematol Oncol. 2008;30:431-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Diorio C, Kelly KM, Afungchwi GM, Ladas EJ, Marjerrison S. Nutritional traditional and complementary medicine strategies in pediatric cancer: a narrative review. Pediatr Blood Cancer. 2020;67(Suppl 3):e28324. [DOI] [PubMed] [Google Scholar]
- 89. Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: a systematic review. J Clin Oncol. 2004;22:517-528. [DOI] [PubMed] [Google Scholar]
- 90. Williamson Lewis R, Effinger KE, Wasilewski-Masker K, Mertens A, Xiao C. Self-reported late effect symptom clusters among young pediatric cancer survivors. Support Care Cancer. 2021;29:8077-8087. [DOI] [PubMed] [Google Scholar]
- 91. Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28:270-282; quiz 283-284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ict-10.1177_15347354221105563 for Safety of Complementary and Alternative Medicine (CAM) treatment among children and young adults who suffer from adverse effects of conventional cancer treatment: A systematic review by Dana C. Mora, Agnete E. Kristoffersen, Grete Overvåg, Miek C. Jong, Marit Mentink, Jianping Liu and Trine Stub in Integrative Cancer Therapies