Abstract
Management of chronic pain is one of the most difficult problems in modern practice. Grafted human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cells (hTERT-BMSCs) with inducible galanin (GAL) expression have been considered to be a potentially safe and controllable approach for the alleviation of chronic pain. Therefore, in this study, we aimed to assess the feasibility of hTERT-BMSCs/Tet-on/GAL cells secreting GAL under the transcriptional control of doxycycline (Dox) for controllable pain relief. After transplanted into the subarachnoid space of neuropathic rats induced by spared nerve injury of sciatic nerve, their analgesic actions were investigated by behavioral tests. The results showed that the pain-related behaviors, mechanical allodynia, and thermal hyperalgesia were significantly alleviated during 1 to 7 weeks after grafts of hTERT-BMSCs/Tet-on/GAL cells without motor incoordination. Importantly, these effects could be reversed by GAL receptor antagonist M35 and regulated by Dox induction as compared with control. Moreover, the GAL level in cerebrospinal fluid and spinal GAL receptor 1 (GalR1) expression were correlated with Dox administration, but not GAL receptor 2 (GalR2). Meanwhile, spinal protein kinase Mζ (PKMζ) expression was also inhibited significantly. Taken together, these data suggest that inducible release of GAL from transplanted cells was able to produce controllable pain relief in neuropathic rats via inhibiting the PKMζ activation and activating its GalR1 rather than GalR2. This provides a promising step toward a novel stem cell–based strategy for pain therapy.
Keywords: immortalized bone mesenchymal stromal cells, galanin, gene expression regulation, cellular therapy, pain relief
Introduction
Chronic neuropathic pain due to nerve injuries is an unpleasant, long-term sensory and emotional experience that affects millions of people. Current treatment options, such as physical, cognitive, pharmacological, and interventional approaches, are limited and unsatisfactory, and can even induce tolerance and unacceptable systemic side effects 1 . These unsatisfactory treatment effects are partially due to lack of knowledge concerning the molecular mechanisms underlying chronic pain development and maintenance. It has been reported that the diminished inhibitory neurotransmission in the superficial dorsal horn, particularly when there is an imbalance of excitatory and inhibitory systems, is likely the main mechanism underlying the induction and development of neuropathic pain following nerve injury2,3. Therefore, alternative methods targeting mechanisms of neuropathic pain are needed.
Galanin (GAL) is a neuropeptide of 29 or 30 (in humans) amino acids that is proteolytically processed from the peptide precursor preprogalanin. It is widely distributed in central and peripheral nervous system and has been shown to regulate numerous physiological and pathological processes through interactions with three G-protein-coupled receptors, GalR1 through GalR3 4 . There is evidence that high-level doses of GAL have antinociceptive effects. From behavioral studies, mice given exogenous GAL or transgenic mice overexpressing GAL showed diminished neuropathic pain-like behaviors after nerve injury5,6. However, because of short half-life in cerebrospinal fluid (CSF) 7 , a single intrathecal (i.t.) injection of GAL could only temporarily reverse pain, which may not be adequate to attenuate the chronic pain states.
Recently, the combination of gene transfer techniques and cell transplantation represents a promising approach to deliver antinociceptive molecules into the pain-processing centers for pain relief 8 . This cellular “biologic minipumps” could not only continuously deliver antinociceptive molecules, but also eliminate the problems associated with exogenous infusion pumps, such as lability of neurotrophins, infection at the catheter tip, and initial high dose of agents9,10. Although previous studies have demonstrated that astrocytes genetically modified by the rat preprogalanin gene could secrete higher levels of GAL in vitro and efficiently function to relieve neuropathic pain11,12, potential complications resulting from the continuous secretion of GAL appear inevitable. In addition, it is difficult to obtain primary neuronal cells from adult tissue and inevitable to face major ethical issues in clinical practice. The ideal therapeutic cells should be reproducible, safe, and controllable release of antinociceptive molecules. Therefore, studies have increasingly focused on the potential therapeutic effects of multipotent bone marrow mesenchymal stromal cells (BMSCs) transplantation for neurological diseases because of its easy harvest, immunoregulatory properties, and neuronal differentiation capability13,14. However, the life span of primary BMSCs is limited and difficult to obtain in large quantities 15 . Fortunately, these limitations have been overcome via introducing ectopic telomerase catalytic component (human telomerase reverse transcriptase, hTERT) and single inducible tetracycline-controlled gene expression system into BMSCs16,17. The hTERT–immortalized BMSCs with controllable GAL releases (hTERT-BMSCs/Tet-on/GAL) under the transcriptional control of doxycycline (Dox) have been established and appraised in our previous work 18 . The hTERT-BMSCs/Tet-on/GAL cells are not only easily manipulated, reproducible, and nontumorigenic, but also display high inducibility and controllable release of exogenous GAL in the presence of inducer Dox in vitro. Therefore, in this present study, we aimed to verify its feasibility for controllable pain relief on spared nerve injury (SNI)–induced neuropathic behavior and potential mechanisms.
Experimental Procedures
Animals and Cell Preparation
Adult male Sprague–Dawley rats (180–220 g) were obtained from the Experimental Animal Center of Sun Yat-Sen University. The rats were housed in cages at room temperature (20°C–25°C) under a 12-h light–dark cycle with free access to food and water. All animal procedures were performed during the light cycle and followed National Institutes of Health (NIH) guidelines.
The hTERT-BMSCs and hTERT-BMSCs//Tet-on/GAL cells have been established, identified, and cryopreserved in our department 18 . For transplantation, cells should be resuscitated and proliferated to near 70% confluence. Immediately before transplantation, these proliferated cells were gently dissociated from six-well culture plates with sterile 0.5 mM ethylenediaminetetraacetic acid (EDTA)/Dulbecco’s phosphate-buffered saline (DPBS) and pelleted by centrifugation, then resuspended in Ca2--Mg2- free Hank’s buffered saline solution. Viability and cell counts were assessed by trypan blue exclusion. An aliquot of 1 million cells was prepared immediately prior to each transplant to assure near 100% viability at the beginning of the experiment. Grafting was within 30 min of cell preparation.
Chronic i.t. Catheters and Cell Transplantation
Rats were anesthetized and maintained with 1% to 3% sevoflurane in oxygen; a catheter (PE 10,o.d.0.61 mm) was inserted into the subarachnoid space between the L5 and L6 vertebrae, with its tip at the lumbar enlargement. The proper location of the catheter was tested by assessing sensory and motor blockade after i.t. injection of 7 μl of lidocaine (20 mg/ml) 19 . Following the i.t. catheter, rats were allowed to recover at 37° for 12 h, after which time, they were returned to the animal care facility and housed one per cage with chow and water ad libitum. For transplantation, viable cells (106) were injected into the subarachnoid space through catheter, the external portion of the chronic indwelling catheter was protected according to Milligan’s method 20 .
Establishment of SNI
The surgery to produce the SNI model of neuropathic pain was originally described by Decosterd and Woolf 21 . Briefly, 1 week after i.t. catheter, rats were anesthetized by 1% to 3% sevoflurane in oxygen, the skin on the lateral surface of the thigh was incised, and a section made directly through the biceps femoris muscle exposing the sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The common peroneal and the tibial nerves were tight-ligated with 5/0 silk and sectioned distal to the ligation, removing 2 to 4 mm of the distal nerve stump. Great care was taken to avoid any contact with or stretching of the intact sural nerve during the whole procedure. In sham-operated animals, the sciatic nerve branches were exposed but not lesioned. Animals that had undergone SNI surgery and demonstrated vigorous mechanical and thermal hypersensitivity effect of nerve injury were used for further cell transplantation.
Animal Grouping
For pain behavior tests, rats were randomly assigned to five groups (10 in each group). Group 1 received neither SNI nor injections and served as Sham group. Group 2 received SNI but not i.t. injections and served as the SNI alone. Group 3 received SNI as well as i.t. transplanted with hTERT-BMSCs cells. Groups 4 and 5 received SNI and i.t. with hTERT-BMSCs/Tet-on/GAL cells. All animals were administered Dox (2 mg/ml; Sigma, St. Louis, MO, USA) in drinking water ad libitum except for group 4. For motor coordination assessment, Sham and SNI rats with Dox drinking were subdivided into four groups (four in each) according to the different substance injected intrathecally, named as Sham + NS + Dox, Sham + hTERT-BMSCs/Tet-on/GAL + Dox, SNI + NS + Dox, and SNI + hTERT-BMSCs/Tet-on/GAL + Dox group, respectively. Here, NS referred to normal saline. In addition, for determination of GAL secretion levels from cells in CSF, additional animals grafted with hTERT-BMSCs/Tet-on/GAL cells were allocated into two groups (five in each group) according to different dose and way of Dox administration.
Motor Coordination Test
To exclude any further motor impairment that could possibly be generated by the i.t. grafts, the Rotarod Motor test was applied. This test consists of putting the rat on a rotary cylinder to measure the time of its equilibrium before falling. The cylinder is subdivided into four sections, allowing screening of four animals (one for each section) simultaneously. Below the cylinder, there is a platform in its turn in correspondence of the four sections, which is connected to a magnet activated from the fall of rat on the plate, and records the time of permanence on the cylinder (Rota Rod Treadmills ZH-300, Anhui Zheng Hua Biologic Apparatus Facilities Co., Ltd, China). After a period of adaptation, the spin speed gradually increased from 5 to 40 (rpm) for the maximum time of 5 min. On the same day, the animals were analyzed twice with an interval of 1 h. The residence time on the cylinder was expressed as latency to fall (s) 22 .
Thermal and Mechanical Stimulation Tests
All animals were examined before SNI and 8 weeks after SNI and transplants for hind paw withdrawal in response to the stimulation. Thermal hyperalgesia thresholds were measured according to a previous method 21 . Rats were placed on a preheated glass platform within a plastic chamber. After habituated for at least 2 h, the lateral plantar area was exposed to a beam of radiant heat through a transparent perspex surface (PL-200 thermal nociceptive stimulator, Chengdu Techman Software Co., Ltd, Chengdu, China). The withdrawal response latency to nociceptive heat stimulation and the duration of withdrawal after the heat stimulation were recorded to the nearest 0.5 s, with a cut-off time of 10 s. The heat stimulation was repeated three times at an interval of 5 to 10 min for each paw, and the mean was calculated.
Mechanical allodynia thresholds were measured using a Von Frey Hairs testing device (Aesthesio®, Ugo Basile, Varese, Italy) for evaluating cutaneous sensation levels. Rats were put in perspex enclosures placed on an elevated metal platform with a mesh-like open wire grid of square holes 5 × 5 mm2, and the plantar surface of hind paw was stimulated with a series of ascending forced von Frey filaments from 0 to a maximum of 25 g over a 20-s period. When the paw was unexpectedly touched, rats exhibited a paw withdrawal reflex. The force at which the animal withdrew its paw was recorded to the nearest 0.1 g 23 .
M35 Challenge
Two weeks after transplantation, five rats grafted with hTERT-BMSCs/Tet-on/GAL and Dox drinking (2 mg/ml) were injected intrathecally with M35 (3 μg/kg) 24 , a putative GAL receptor antagonist (Sigma, St. Louis, MO, USA), to determine whether the analgesic efficacy was acted through GAL receptors, while others in the same group were injected with NS as control. To rule out non-specific effects on pain processing of the antagonist, some rats in group 3 were also injected with the same dose of M35. Thermal hyperalgesia and mechanical allodynia were observed every 30 min until 90 min after injection.
Kinetics Analysis of GAL Secretion Under the Dox Induction In Vivo
To determine the secretion levels of GAL peptide in the grafted animals, 1 week after hTERT-BMSCs/Tet-on/GAL cells transplantation, the grafted rats were administered higher dose (2 mg/ml) or lower dose (1 mg/ml) of Dox in drinking water at different predetermined times after transplantation. Every week, 20-ul CSF samples were collected through the polyethylene catheter placed in the animals’ subarachnoid space, and equal amounts of artificial CSF prepared as described before were injected to substitute 10 . The GAL levels in CSF were assayed via GAL enzyme-linked immunosorbent assay (ELISA) kit (JM-E1001) according to the manufacturer’s instructions (TSZ Biological Trade Co. Ltd, NJ, USA) as described before 18 . Briefly, the CSF from the grafted animals was collected and centrifuged at 1,000 rpm for 5 min at 4°C, and subsequently incubated on a microplate precoated with a rat GAL monoclonal antibody for 45 min at 37°C (five wells for each). After the second antibody conjugated with horseradish peroxidase (HRP) was added and bound to the captured GAL, the HRP substrate TMB (tetramethylbenzidine) was added to the wells. The OD450 was measured to generate a standard curve and calculate the GAL concentration using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The secretion levels were standardized and expressed in µg/ml of supernatant.
Determination of Lumbar Spinal GalR1, GalR2, and p-PKMζ Expression
Spinal dorsal horn (SDH) is an important part of mediating nociceptive signaling and central sensitization 25 . It has also been well recognized that nerve lesion induces change of gene and protein expression of neuropeptides and receptors in SDH corresponding to neuropathic pain induction 26 . Thus, the GalR1, GalR2, and phosphor-protein kinase Mζ (p-PKMζ) expression levels in SDH were analyzed by Western blot assay 8 weeks following nociceptive stimulation tests. In short, rats were sacrificed with 5% isoflurane anesthesia, and the lumbar spinal cord segments (L4-6) (100 mg) in each group were harvested. The samples were homogenized in 10 mM Tris-HCl buffer (pH 7.4) with protease inhibitors, and then centrifuged at 15,000 × g for 20 min, the supernatant were collected for Western blot assay. After determining the protein concentrations of the supernatants, 50 mg protein of each sample was loaded onto the 10% SDS (sodium dodecyl sulfate) gel, separated by electrophoresis and transferred to nitrocellulose membrane. The membranes were blocked in blocking buffer (5% nonfat milk) for 2 h at room temperature, and then incubated with rabbit anti-p-PKMζ (cat# CG1453) (1:400, Cell Applications Inc., San Diego, CA, USA), rabbit anti-GalR1 (cat# sc-16216) (1:500, Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-GalR2 (cat# sc-16219) (1:500, Santa Cruz Biotechnology, Dallas, TX, USA) or rabbit anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (cat#TA-08) (1:500, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., China) overnight at 4°C. After being washed three times for 10 min with washing solution, an HRP conjugated goat anti-rabbit antibody (1:300, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) was added as the secondary antibody, and the blots were developed and visualized by enhanced chemiluminescence of Western blotting detection kit on light sensitive film. For densitometric analysis, the blots were scanned and quantified with GeneSnap V6.05 software (England). The protein levels were expressed as the ratio of GalR1, GalR2, and p-PKMζ to internal control GAPDH. Each sample was measured three times, and the average value was taken for statistical analysis.
Statistics
The data are expressed as the mean ± SEM and analyzed using SPSS 11.5 for Windows (Chicago, IL, USA). The normal distribution of all data was verified. If the normality test is fail, the data were analyzed with non-parametric test. If the normality test is pass, the two-way repeated-measures analysis of variance (ANOVA) was used for the data (behavioral test data), multiple comparisons were made between the groups, and a simple effect test was made on the two within-subject factors (time and groups) or analyze the data by one-way ANOVA (the level of Gal, GalR1, GalR2, and p-PKMζ). Student’s two-tailed t test was used for analysis of two independent samples. A P < 0.05 was considered statistically significant.
Ethical Approval
This study was approved by the Regional Ethical Review Boards in the First Affiliated Hospital of Sun Yat-Sen University.
Results
Behavioral Response to Thermal and Mechanical Stimulation After Intrathecal Transplantation of Cells Into SNI Rats
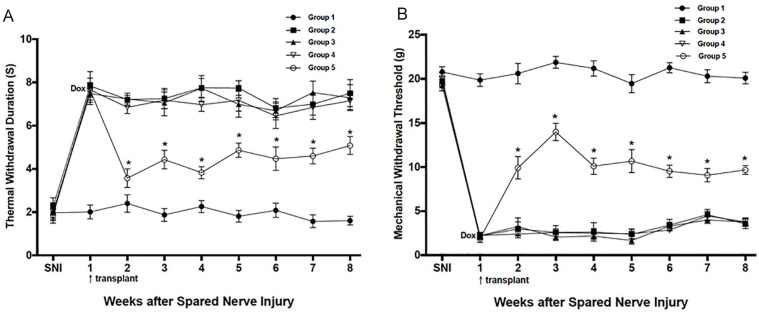
The effects of transplanted cells on thermal hyperalgesia and mechanical tactile allodynia after SNI are shown in Fig. 1A, B. No abnormal behavioral change to the heat and mechanical stimulus occurred in Sham group, and no decrease in latency of withdrawal to the radiant heat stimulus (no heat allodynia) was found in all groups during the whole observation period (data not shown). In contrast, 1 week after SNI surgery, the animals in groups 2, 3, 4, and 5 refrained from weight bearing on the affected paw and modified their stance accordingly, with eversion of the foot. In addition, the duration of withdrawal in response to the heat stimulus in the ligated hind paw increased significantly over the baseline values; at the same time, a significant and vigorous hypersensitivity to innocuous mechanical von Frey filament stimulation was also seen after the same lesion and reached a peak at about 2 weeks after SNI, which did not resolve throughout the 8 weeks following the operation. However, as compared with other groups, the marked hyper-responsiveness on thermal hyperalgesia and mechanical allodynia of animals with Dox induction in group 5 were attenuated significantly 1 week after cells grafted and persisted for at least 7 weeks. Meanwhile, their abnormal stance was relieved and ameliorated a little after cells transplantation. In contrast, transplantation of control cells had no effect on the hypersensitivity to stimulation whether Dox was given or not.
Figure 1.
Behavioral changes in response to noxious thermal (A) and mechanical stimulation (B) before and after SNI and cell transplantation. Animals were either left unoperated Sham (group 1 [-●-]), given the SNI (group 2[-◼-]), or SNI as well as cell transplantation with hTERT-BMSCs cells (group 3[-▲-]) or hTERT-BMSCs/Tet-on/GAL cells (group 4[-▽-] and group 5[-◯-]) 1 week following the SNI. All animals were administered Dox except for group 4. The data reported are mean ± SEM. GAL: galanin; hTERT-BMSC: human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cell; SNI: spared nerve injury. Asterisks (*) indicate the animals in group 5 that differed significantly from the other groups at each time point (P < 0.05).
Grafts on Motor Coordination and Daily Activities
The results showed the motor balance function of rats in the Sham + NS and Sham + hTERT-BMSCs/Tet-on/GAL group was not affected, reaching 30 s almost. Although the residence time of rats in SNI + NS and SNI + hTERT-BMSCs/Tet-on/GAL group was markedly shorter than that in Sham group after NS or cell injection, there was no significant difference between these two groups, indicating that cell transplantation had no effect on the motor balance and coordination of rats, once intrathecally injected (Fig. 2). Moreover, no autotomy or body weight loss was observed; the animals’ grooming, sleep–wake cycles, and social interaction with other rats in the cage were not obviously affected after cells grafted.
Figure 2.

The effects of cell transplantation on performance in the Rotarod test. Sham and SNI animals were either injected NS (Sham + NS[-●-], SNI + NS[-▲-]) or hTERT-BMSCs/Tet-on/GAL cells (Sham + hTERT-BMSCs/Tet-on/GAL[-◼-], SNI + hTERT-BMSCs/Tet-on/GAL group[-◯-]). Intrathecally administration of cells (106) to Sham or SNI rats had no effects on motor function when compared with vehicle (NS) treated rats. Values are expressed as mean ± SEM of the latency (s; n = 4 rats/group). GAL: galanin; hTERT-BMSC: human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cells; NS: normal saline; SNI: spared nerve injury.
Effects of M35 on Antinociceptive Effects of GAL Secreted From Grafts
The antinociceptive effects of group 5 were antagonized by an i.t. injection of M35 2 weeks after SNI and transplantation, while no effect occurred with NS administration. The reversal effects of M35 were temporary and behavior returned to pre-M35 levels within 90 min after injection (Fig. 3A, B). No animal in group 3 showed further abnormal sensory or motor dysfunction after i.t. administration of M35.
Figure 3.
The effects of M35 on thermal withdrawal duration (A) and mechanical withdrawal threshold (B). SNI animals grafted with hTERT-BMSCs ([-▲-]) or hTERT-BMSCs/Tet-on/GAL ([-◼-]) cells were intrathecally injected with M35 2 weeks after transplantation, while other SNI animals grafted with hTERT-BMSCs/Tet-on/GAL cell were injected with NS as control ([-●-]). Values are expressed as mean ± SEM (n = 5 rats/group). GAL: galanin; hTERT-BMSC: human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cell; NS: normal saline; SNI: spared nerve injury. Asterisks (*) indicate the animals in that group differed significantly from the other two groups at these time points (P < 0.05).
Regulation of GAL Expression in CSF by Dox In Vivo
To determine the fluctuating expression of GAL and regulation efficacy of Dox in hTERT-BMSCs/Tet-on/GAL-grafted animals, Dox was administered and eliminated from drinking water over the course of 8 weeks. Similar levels of early leakiness were observed in all animals (lower dose group: 3.72 ± 0.69 μg/ml; higher dose group: 4.11 ± 0.21 μg/ml) post-grafting. Animals in the higher dose of Dox had an significant increase in GAL levels when 2 mg/ml Dox was administered to their drinking water. The GAL expression reached a maximum level on the second week after Dox induction (15.00 ± 1.41 μg/ml), but dropped to that of controls after Dox was withdrawn. In contrast, when animals with lower dose of Dox were subsequently exposed to an intermediate dose of Dox (1 mg/ml), GAL levels in CSF also showed the same expression characteristics, but the peak level (7.87 ± 0.27 μg/ml) was less than that of higher dose (Fig. 4).
Figure 4.

Regulation of GAL secretion with different dose of Dox treatment. Some rats in the SNI + hTERT-BMSCs/Tet-on/GAL + Dox (2 mg/ml) group(-◼-) were administered Dox (2 mg/ml) in drinking water from the first to the fourth week after cell transplantation and then removed, while other rats in the SNI + hTERT-BMSCs/Tet-on/GAL + Dox (1 mg/ml) group (-●-) were administered with Dox (1 mg/ml) from the fourth to the sixth week after transplantation and then withdrawal. All animals were observed for 8 weeks. Values are expressed as mean ± SEM (µg/ml in CSF, n = 5 rats/group). CSF: cerebrospinal fluid; GAL: galanin; hTERT-BMSC: human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cell; SNI: spared nerve injury. Asterisks (*) indicate the animals in higher dose of Dox differed significantly from those in lower dose at these time points (P < 0.05).
Western Blot for GalR1, GalR2, and p-PKMζ Expression in Lumbar SDH
The results showed that GalR1 protein levels increased markedly in spinal cord 8 weeks after SNI injury as compared with that in sham-operated control animals, while the GalR1 levels in group 5 were significantly higher than that in other groups (P < 0.01) (Fig. 5A). GalR2 levels also increased apparently after SNI injury as compared with that in Sham group at the same experimental time point (Fig. 5B), but GalR2 levels in groups 3, 4, and 5 were slightly lower than that in group 2 (P < 0.05); no significant difference was found among them. In addition, the level of p-PKMζ expression in spinal cord was significantly higher after SNI than that in sham-operated control group (P < 0.01), while p-PKMζ levels in group 5 showed significantly lower than that in other groups except for Sham group (Fig. 5C). These results implied that GAL released from transplanted cells under the Dox induction could produce antinociceptive effect by inhibiting PKMζ-mediated central sensitization via activating inhibitory receptor GalR1 but not GalR2.
Figure 5.
Western blotting assays for lumbar spinal GalR1, GalR2, and p-PKMζ expression. Eight weeks after SNI, the protein level of GalR1 increased dramatically than that of the sham-operated animals, while it was significantly higher in those rats transplanted with hTERT-BMSCs/Tet-on/GAL cells and Dox induction (group 5) (*P < 0.05) (A). GalR2 levels also increased obviously after SNI than that in the group 1 (*P < 0.05), but the increase degree was slighter in groups 3, 4, and 5 with cell transplantation, and there was no significant difference among them regardless of Dox given (**P > 0.05) (B). The level of p-PKMζ expression in spinal cord was also significantly higher when compared with sham-operated animals (*P < 0.01), but the increment of which was obviously smaller in group 5, there was no significant difference among groups 2, 3, and 4 (**P > 0.05) (C). Samples in different lanes starting from the left represented as lane 1: Sham + Dox; lane 2: SNI + Dox; lane 3: SNI + hTERT-BMSCs + Dox; lane 4: SNI + hTERT-BMSCs/Tet-on/GAL; lane 5: SNI + hTERT-BMSCs/Tet-on/GAL + Dox. The columns represent the densitometric quantitation of immunoreactive protein expressed relative to control GAPDH and plotted as a histogram. GAL: galanin; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; hTERT-BMSC: human telomerase reverse transcriptase–immortalized bone marrow mesenchymal stromal cell; p-PKMζ: phosphor-protein kinase Mζ; SNI: spared nerve injury.
Discussion
The current treatment options for neuropathic pain due to nerve injuries are limited and largely unsatisfactory. The combination of ex vivo gene transfer and stem cell–based transplantation is becoming a promising strategy for the treatment of neurodegenerative diseases and traumatic injuries. The use of cell lines as vehicles to deliver controllable antinociceptive molecules, such as GAL, into the pain-processing centers to balance the excitatory and inhibitory systems represents a newly developed technique for pain therapy. In our previous study, using a single Tet-on inducible lentivirus system, a hTERT-BMSCs/Tet-on/GAL cell line with controllable GAL release was constructed and displayed low background activity coupled with high inducibility in the presence of inducer Dox in a dose-dependent manner in vitro 18 . Therefore, in this present study, it is confirmed that subarachnoid transplantation of hTERT-BMSCs/Tet-on/GAL cells into SNI-induced neuropathic rats could produce controllable pain relief. This strategy is the first promising step toward a novel hTERT-BMSCs-based “regulable biological minipumps” for pain therapy.
Neuropathic pain caused by a lesion or disease of the somatosensory nervous system is a common chronic pain condition, which is often characterized by hyperalgesia and allodynia to mechanical and thermal stimuli, as well as spontaneous pain 27 . The advance in the treatment of pain has been greatly benefited from the development of animal models that reflect the same element of pain syndromes in human. Several attempts have been made to model partial nerve injury, such as the Bennett chronic constriction injury model and the Chung spinal nerve ligation model. However, the degree of damage in these cases is inherently difficult to reproduce leading to some variability in the number of responders and their behavior 28 . In addition, although most animal models find a reduction in the withdrawal latency to heat stimulus, this is not a typical feature in patients with neuropathic pain and is generally considered to indicate a change in sensitivity of peripheral nociceptor rather than a change in the overall excitability of the somatosensory system 29 . Fortunately, an alternative approach, the SNI model, was recently developed by Decosterd and Woolf 21 . This surgical procedure is easy to perform and reproduce, involving a lesion of two of the three terminal branches of the sciatic nerve (tibial and common peroneal nerves, leaving the sural nerve intact), which enables investigation of changes in both injured primary sensory neurons and neighboring intact sensory neurons, and their contribution to the pathophysiology of neuropathic pain. The constellation of symptoms, mechanical allodynia, mechanical hyperalgesia, no change in thermal heat threshold, and a hyper-responsiveness to a supra-threshold heat stimulus found in SNI model, is representative of many of the symptoms encountered in human patients with neuropathic pain 30 . Thus, the SNI animal model was introduced in the present study; it has been proved to be robust, with substantial and prolonged changes in mechanical sensitivity and thermal responsiveness developed within the first weeks after the insult and preserved for at least 2 months, which could provide a useful model for identifying the efficacy of this new treatment method.
It has been demonstrated that neuropeptide GAL is upregulated after injury in the SDH where it plays a predominantly antinociceptive and gatekeeper role in the inhibition of neuropathic pain5,26. Because of the relationship of the preprogalanin gene to GAL production and the activation of GAL through its receptors in the central nervous system (CNS) 31 , we introduced the GAL gene into hTERT-BMSCs as a potential treatment for chronic neuropathic pain and found GAL released from the grafted genetically modified BMSCs in subarachnoid space has a similar profile to endogenous GAL and attenuates chronic neuropathic pain. When animals grafted with hTERT-BMSCs/Tet-on/GAL cells were administrated with Dox in their drinking water, the analgesia effect was significantly better than that without Dox given, the effect lasted over a 7-week period in a Dox-controllable manner. Kinetic tests on GAL secretion from hTERT-BMSCs/Tet-on/GAL cells also showed the preprogalanin gene activation could be switched on and off under the transcriptional control of an inducer Dox in vitro. Moreover, to exclude any further motor coordination impairment that could possibly be generated by cells injection, we applied the Rotarod test to compare SNI + hTERT-BMSCs/Tet-on/GAL with SNI + NS treated rats. Although SNI rats proved to be less able to walk and shorter residence time in respect to Sham rats, there is no significant difference in motor coordination outcome for these SNI rats with or without transplant (Fig. 3), implying that neither the administration procedure nor stem cells injection modified motor coordination.
Sensory hypersensitivity followed by peripheral nerve injury is caused by the hyper C-fiber activity and sensitization of CNS, especially in SDH27,28. The superficial layers of SDH receives strong input from thin primary afferent fibers and is involved in nociception, pain, temperature sensing, and other experiences5,30. After i.t. transplantation, GAL secreted from the grafted cells mainly diffuses directly into the superficial neurons in the SDH. Although the fate of transplanted cells in the i.t. space was not detected in our present study, the long-term analgesia effects and fluctuating secretion of GAL by daily Dox administration from cell-grafted animals suggest transplanted hTERT-BMSCs/Tet-on/GAL cells may maintain survival and continuous secretion of GAL peptide for a long time.
Recently, a modified version of chimeric peptide M35 (galanin[1-13]-Gln14-bradykinin [2-9] amide) was reported as a novel GalR1-selective ligand, where a glutamine introduced at position 14 contributes to the molecule’s observed 25-fold selectivity for GalR1 over GalR24. Although M35 is a full agonist in vitro, specifically in cell lines expressing individual GAL receptor subtypes, it is only in vivo that acts as antagonists in many experimental models such as the flexor reflex and chronic constriction injury rat24,32. In the absence of endogenous GAL in the GAL-knockout mouse, M35 has an agonistic effect, whereas in the presence of GAL, its agonistic activity is masked and it acts as an antagonist 33 . Therefore, as found in group 5, animals had a significant increase of GAL levels in CSF after hTERT-BMSCs/Tet-on/GAL cells grafted and displayed pronounced analgesic effects under the Dox induction, that were partially reversed by the i.t. injection of M35, further implying that these antihyperalgesic responses are mainly mediated by the exogenous GAL release from grafted cells.
Extensive research has examined the role of GAL and its receptors in the regulation of pain processing. In the rat spinal cord, many intrinsic neurons in laminae I and II express GalR1-R mRNA, with some neurons in laminae III to V, whereas only a few neurons express GalR2 mRNA in the dorsal horn 34 . Studies have shown that nerve injury induces complex plasticity of Gal, GalR1, and GalR2 expression in DRG and SDH neurons, which play a crucial role in neuropathic pain and nerve regeneration35,36. Consistent with these results, in our previous study, SNI caused a significantly upregulation of endogenous GAL in SDH; this change might be a compensatory effect, indicating that GAL upregulation after peripheral nerve injury could be considered as a transform of normal neuronal sensory conduction state to a protection and regeneration one 12 . Besides the dramatic change and important role of GAL after nerve injury, another very interesting question is whether and how GAL receptors change in this condition. Although the precise actions of GAL and its receptor subtypes in nociception and the exact sites of action have not yet been fully clarified, more and more evidence indicates that GalR1 may mediate the analgesic response in chronic pain, while GalR2 mediates the hyperalgesic effect37,38. In our present study, GalR1 and GalR2 expression was upregulated in lumbar spinal cord after SNI injury at the same experimental time point, whereby GalR1 expression levels in SNI animals with hTERT-BMSCs/Tet-on/GAL cells injected under Dox induction (group 5) showed significant higher as compared with other control groups. GalR1 has been confirmed to predominately locate in postsynaptic membrane 39 . The effect of GalR1 increasing by exogenous GAL may be an activator/receptor positive feedback just like the other receptor adaptive reaction. Except for inhibition of active neuropeptide release, activation of GalR1 may also reduce excitability of glutamatergic interneurons in SDH 40 . The inhibitory role perhaps mainly results from inhibition of cAMP production mediated by Gi-coupled GalR1 on intrinsic dorsal horn neurons. In contrast to GalR1, only couple to Gi protein, GalR2 can also activate Gq/11 protein leading to more complex functions 41 . Many studies pay close attention to neuroprotective and neuronal trophic effect of GAL conducted by GalR2 42 . In our present study, the GalR2 levels in the spinal cord were also upregulated, which may make the increasing GAL acts as neurotrophy more effective after SNI. However, compared with SNI alone group, the levels of GalR2 expression decreased in SNI animals after cells transplant, and the GalR2 level of animals in group 5 just showed slightly lower than that in these groups even if under Dox induction, indicating that inhibiting excitatory receptor GalR2 may play a little antinociceptive effect in terms of pain behavioral changes. In short, the upregulation GalR1 in SDH makes this effect more potent after nerve injury, emphasizing the antinociceptive effects of GAL released inducibly from hTERT-BMSCs/Tet-on/GAL cells on pain may be mainly mediated through GalR1 rather than GalR2. However, its exact downstream molecular mechanism is unclear.
Intracellular protein kinases play a significant role in the processing and development of chronic pain, and late long-term potentiation (L-LTP) in nociceptive spinal pathways shares several common features with hyperalgesia and cellular mechanism of pain amplification in acute and chronic pain 43 . Protein kinase Mζ (PKMζ), an atypical protein kinase C, is an essential substrate of the L-LTP underlying central sensitization, which is also considered as one mechanism for memory formation; PKMζ lacks a regulatory region and has the potential to maintain autonomous activity over extended periods of time once it is translated and phosphorylated by phosphoinositide-dependent protein kinase 1 44 . Protein kinase Mζ was first recognized as a constitutively active kinase that may play a role in the maintenance of L-LTP. These factors represent the molecular engine and substrate for the maintenance of L-LTP and long-term pain memory 45 . Although there are some differences between memory reconsolidation and pain maintenance, it is possible that these processes share at least some of the same underlying cellular and molecular mechanisms46,47. However, the potential contributions of exogenous GAL to PKMζ in chronic pain state are not known. To this end, we conducted this experiment to detect the possible variation of spinal p-PKMζ to SNI and hTERT-BMSCs/Tet-on/GAL grafts. Western blot assay data showed that the spinal p-PKMζ protein expression level was increased significantly in the SNI animals 8 weeks later, while its levels in animals grafted with hTERT-BMSCs/Tet-on/GAL cells and Dox induction (group 5) showed significantly lower than that in other control groups. These implied the sustaining hypersensitivity after SNI insult paradigm may be paralleled with the spinal PKMζ phosphorylation. Galanin released from the transplanted cells under the Dox induction could produce antinociception induced by GalR1 via inhibiting PKMζ signaling pathway in the SDH of rats with neuropathic pain. These data offer a new point of view on the antinociceptive effects of GAL and its receptor signaling system.
Despite significant differences in GAL secretion from transfected cells observed in the presence and absence of Dox, some weak residual gene expression or leakiness in this system still remained in the off state in both vitro 18 and vivo experiments, reflecting the unexpected translation in transduced cells in the absence of Dox. But fortunately, there was a sensitive and effective induction of expression upon Dox administration observed in this ex vivo study. Leakiness of the Tet system is an often-reported phenomenon that could limit its use in certain applications. Although our findings show significant differences in GAL secretion and analgesic effects of hTERT-BMSCs/Tet-on/GAL cells in the presence and absence of Dox, further research is necessary to improve the system. In addition, the action site of injected cells (cord vs brain stem) and deleterious effects, such as chronic arachnoiditis, in transplanted animals after longer periods of time should be included in the scope of future studies. Furthermore, we also believe that tracking the cells post-implantation is very important. Knowing the fate of the transplanted cells, how they distribute, and their survival/grafting rate may have a major impact not only for this study but can also provide valuable insight into the potential use for multiple applications.
Conclusion
Taken together, using a single tetracycline-inducible lentivirus delivery system, hTERT-BMSCs/Tet-on/GAL cells have been engineered, which can release GAL under the control of an inducer Dox in a dose-dependent manner. When grafted into the SNI neuropathic rats, these inducible GAL-producing cells produced significantly controllable pain relief via activation of GalR1 but not GalR2, and inhibition of PKMζ activation mediated central sensitization as well, suggesting transplantation of such BMSCs-based “biological minipumps’’ for GAL expression regulated by a Tet-on system within the CNS may be developed as an adjunct to currently used therapies for pain, but further research is necessary to improve this promising technique for pre-clinical application.
Acknowledgments
All authors would like to thank Cyagen Biosciences (Guangzhou, China) for the construction of the lentiviral vectors and creation of the hTERT-BMSCs. We are also grateful to Prof. David Y.B. Deng (Laboratory of Research Center for Translational Medicine, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China) for his technical and administrative supports.
Footnotes
Ethical Approval: This study was approved by the Administration Committee of Experimental Animals in the First Affiliated Hospital of Sun Yat-Sen University, Guangdong Province, China (permit number 2013-A-001).
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care and Use guidelines of Sun Yat-Sen University, China, and approved by the Administrative Committee of Experimental Animals, Guangdong Province, China.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (grant number 81171468) and Research Fund for the Doctoral Program of Higher Education of China (grant number 20080558112).
ORCID iD: Ke An  https://orcid.org/0000-0001-8369-6557
https://orcid.org/0000-0001-8369-6557
References
- 1. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl): S22–32. [DOI] [PubMed] [Google Scholar]
- 2. Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28(5):633–38. [DOI] [PubMed] [Google Scholar]
- 3. Niederberger E, Kühlein H, Geisslinger G. Update on the pathobiology of neuropathic pain. Expert Rev Proteomics. 2008;5(6):799–818. [DOI] [PubMed] [Google Scholar]
- 4. Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007; 115(2):177–207. [DOI] [PubMed] [Google Scholar]
- 5. Wynick D, Thompson SW, McMahon SB. The role of galanin as a multi-functional neuropeptide in the nervous system. Curr Opin Pharmacol. 2001;1(1):73–77. [DOI] [PubMed] [Google Scholar]
- 6. Wiesenfeld-Hallin Z, Xu XJ. Neuropeptides in neuropathic and inflammatory pain with special emphasis on cholecystokinin and galanin. Eur J Pharmacol. 2001;429:49–59. [DOI] [PubMed] [Google Scholar]
- 7. Bedecs K, Langel U, Bartfai T. Metabolism of galanin and galanin (1–16) in isolated cerebrospinal fluid and spinal cord membranes from rat. Neuropeptides. 1995;29(3):137–43. [DOI] [PubMed] [Google Scholar]
- 8. Czech KA, Sagen J. Update on cellular transplantation into the CNS as a novel therapy for chronic pain. Prog Neurobiol. 1995;46(5):507–29. [DOI] [PubMed] [Google Scholar]
- 9. Eaton MJ. Emerging cell and molecular strategies for the study and treatment of painful peripheral neuropathies. J Peripher Nerv Syst. 2000;5(2):59–74. [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Tian XB, An K, Yang H, Tian YK. Lumbar transplantation of immortalized enkephalin-expressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2008;12(4):525–33. [DOI] [PubMed] [Google Scholar]
- 11. An K, Tian Y, Yang H, Gao F, Wang P. Immortalized rat astrocyte strain genetically modified by rat preprogalanin gene. J Huazhong Univ Sci Technolog Med Sci. 2005;25(2):144–46, 197. [DOI] [PubMed] [Google Scholar]
- 12. An K, Xu Y, Yang H, Shu HH, Xiang HB, Tian YK. Subarachnoid transplantation of immortalized galanin-overexpressing astrocytes attenuates chronic neuropathic pain. Eur J Pain. 2010;14(6):595–601. [DOI] [PubMed] [Google Scholar]
- 13. Phinney DG, Isakova IA. Mesenchymal stem cells as cellular vectors for pediatric neurological disorders. Brain Res. 2014;1573:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Y, Zhang D, Li H, Long R, Sun Q. Intrathecal administration of human bone marrow mesenchymal stem cells genetically modified with human proenkephalin gene decrease nociceptive pain in neuropathic rats. Mol Pain. 2017;13:1744806917701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Hikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592–96. [DOI] [PubMed] [Google Scholar]
- 17. Barde I, Zanta-Boussif MA, Paisant S, Leboeuf M, Rameau P, Delenda C, Danos O. Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector. Mol Ther. 2006;13(2):382–90. [DOI] [PubMed] [Google Scholar]
- 18. An K, Liu HP, Zhong XL, Deng DYB, Zhang JJ, Liu ZH. hTERT-immortalized bone mesenchymal stromal cells expressing rat galanin via a single tetracycline-inducible lentivirus system. Stem Cells Int. 2017;2017:6082684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Størkson RV, Kjørsvik A, Tjølsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65(2):167–72. [DOI] [PubMed] [Google Scholar]
- 20. Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. [DOI] [PubMed] [Google Scholar]
- 21. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2): 149–58. [DOI] [PubMed] [Google Scholar]
- 22. Siniscalco D, Giordano C, Galderisi U, Luongo L, de Novellis V, Rossi F, Maione S. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villetti G, Bergamaschi M, Bassani F, Bolzoni PT, Maiorino M, Pietra C, Rondelli I, Chamiot-Clerc P, Simonato M, Barbieri M. Antinociceptive activity of the N-methyl-D-aspartate receptor antagonist N-(2-Indanyl)-glycinamide hydrochloride (CHF3381) in experimental models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:804–14. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Hökfelt T. Effect of intrathecal galanin and its putative antagonist M35 on pain behavior in a neuropathic pain model. Brain Res. 2000;886:67–72. [DOI] [PubMed] [Google Scholar]
- 25. Jarvis MF, Boyce- Rustay JM. Neuropathic pain: models and mechanisms. Curr Pharm Des. 2009;15(15):1711–16. [DOI] [PubMed] [Google Scholar]
- 26. Xu X, Yang X, Zhang P, Chen X, Liu H, Li Z. Effects of exogenous galanin on neuropathic pain state and change of galanin and its receptors in DRG and SDH after sciatic nerve-pinch injury in rat. PLoS ONE. 2012;7(5):e37621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. [DOI] [PubMed] [Google Scholar]
- 28. Abboud C, Duveau A, Bouali-Benazzouz R, Massé K, Mattar J, Brochoire L, Fossat P, Boué-Grabot E, Hleihel W, Landry M. Animal models of pain: diversity and benefits. J Neurosci Methods. 2021;348:108997. [DOI] [PubMed] [Google Scholar]
- 29. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erichsen HK, Blackburn-Munro G. Pharmacological characterization of the spared nerve injury model of neuropathic pain. Pain. 2002;98:151–61. [DOI] [PubMed] [Google Scholar]
- 31. Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hökfelt T, Kofler B. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev. 2015;67(1):118–75. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Gao Y, Li CY, Dong W, Dong Y, Li MN, Liu Y-N, Xu S-L. Galanin receptor 1 plays an antinociceptive effect via inhibiting PKA activation in the nucleus accumbens of rats with neuropathic pain. Physiol Res. 2019;68(3):511–18. [DOI] [PubMed] [Google Scholar]
- 33. Mahoney SA, Hosking R, Wynick D. The galanin antagonist M35 has intrinsic agonistic activity in the dorsal root ganglion. NeuroReport. 2003;14(12):1649–52. [DOI] [PubMed] [Google Scholar]
- 34. Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hökfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98(17):9960–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coronel MF, Brumovsky PR, Hökfelt T, Villar MJ. Differential galanin upregulation in dorsal root ganglia and spinal cord after graded single ligature nerve constriction of the rat sciatic nerve. J Chem Neuroanat. 2008;35(1):94–100. [DOI] [PubMed] [Google Scholar]
- 36. Hobson SA, Bacon A, Elliot-Hunt CR, Holmes FE, Kerr NC, Pope R, Vanderplank P, Wynick D. Galanin acts as a trophic factor to the central and peripheral nervous systems. Exp Suppl. 2010;102:25–38. [DOI] [PubMed] [Google Scholar]
- 37. Liu HX, Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23(10):468–74. [DOI] [PubMed] [Google Scholar]
- 38. Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci. 2008;65:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alier KA, Chen Y, Sollenberg UE, Langel Ü, Smith PA. Selective stimulation of GalR1 and GalR2 in rat substantia gelatinosa reveals a cellular basis for the anti- and pro-nociceptive actions of galanin. Pain. 2008;137(1):138–46. [DOI] [PubMed] [Google Scholar]
- 40. Landry M, Bouali-Benazzouz R, André C, Shi TJ, Léger C, Nagy F, Hökfelt T. Galanin receptor 1 is expressed in a subpopulation of glutamatergic interneurons in the dorsal horn of the rat spinal cord. J Comp Neurol. 2006;499(3):391–403. [DOI] [PubMed] [Google Scholar]
- 41. Wittau N, Grosse R, Kalkbrenner F, Gohla A, Schultz G, Gudermann T. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene. 2000;19(37):4199–4209. [DOI] [PubMed] [Google Scholar]
- 42. Abbosh C, Lawkowski A, Zaben M, Gray W. GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors. J Neurochem. 2011;117(3):425–36. [DOI] [PubMed] [Google Scholar]
- 43. Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol. 2013;74(5): 630–36. [DOI] [PubMed] [Google Scholar]
- 44. Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12(1):9–15. [DOI] [PubMed] [Google Scholar]
- 45. Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008; 169:27–40. [DOI] [PubMed] [Google Scholar]
- 46. Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park S-W, Shim J, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330(6009):1400–404. [DOI] [PubMed] [Google Scholar]
- 47. An K, Zhen C, Liu ZH, Zhao Q, Liu HP, Zhong XL, Huang WQ. Spinal protein kinase Mζ contributes to the maintenance of peripheral inflammation-primed persistent nociceptive sensitization after plantar incision. Eur J Pain. 2015;19(1):39–47. [DOI] [PubMed] [Google Scholar]