Abstract
Background:
About half of myasthenia gravis (MG) patients with purely ocular symptoms at onset progress to generalized myasthenia gravis (gMG).
Objectives:
To develop and validate a model to predict the generalization of MG at 6 months after disease onset in patients with ocular-onset myasthenia gravis (OoMG).
Methods:
Data of patients with OoMG were retrospectively collected from two tertiary hospitals in Germany and China. An accelerated failure time model was developed using the backward elimination method based on the German cohort to predict the generalization of OoMG. The model was then externally validated in the Chinese cohort, and its performance was assessed using Harrell’s C-index and calibration plots.
Results:
Four hundred and seventy-seven patients (275 from Germany and 202 from China) were eligible for inclusion. One hundred and three (37.5%) patients in the German cohort progressed from OoMG to gMG with a median follow-up time of 69 (32–116) months. The median time to generalization was 29 (16–71) months. The estimated cumulative probability of generalization was 30.5% [95% CI (confidence interval), 24.3–36.2%) at 5 years after disease onset. The final model, which was represented as a nomogram, included five clinical variables: sex, titer of anti-AChR antibody, status of anti-MuSK antibody, age at disease onset and the presence of other autoimmune disease. External validation of the model using the bootstrap showed a C-index of 0.670 (95% CI, 0.602–0.738). Calibration curves revealed moderate agreement of predicted and observed outcomes.
Conclusion:
The nomogram is a good predictor for generalization in patients with OoMG that can be used to inform of the individual generalization risk, which might improve the clinical decision-making.
Keywords: accelerated failure time model, generalization, generalized myasthenia gravis, ocular-onset myasthenia gravis, prediction model
Introduction
Myasthenia gravis (MG) is a rare autoimmune disease caused by autoantibodies binding to acetylcholine receptor (AChR), muscle-specific kinase (MuSK), lipoprotein receptor–related protein 4 (LRP4), or other related crucial proteins in the postsynaptic muscle membrane. 1 Typical symptoms include fluctuating weakness of skeletal muscles, which can be localized or generalized, and unusual fatigue, which is a feeling of tiredness or an experienced lack of energy not related to muscle weakness and often interferes with mental or physical activities. At disease onset, almost half of MG patients presented with pure ocular symptoms, for example, ptosis or diplopia, which are frequently defined as ocular-onset myasthenia gravis (OoMG). 2
In many cases, OoMG is visually disabling and results in a reduced quality of life.3,4 Both the disease course and response to treatment of OoMG differ individually. 2 More than half of patients with OoMG will develop generalized MG (gMG), mostly within 2–3 years after disease onset.5–11 A small randomized controlled trial of prednisone for OoMG 12 and a few retrospective studies5,10 have suggested that the usage of prednisone seems to be safe and useful to prevent the generalization of OoMG. Moreover, our previous study suggests that thymectomy before the generalization of OoMG might improve clinical outcomes compared with thymectomy only after generalization. 13 Thus, minimally invasive thymectomy might also be a treatment option for well-defined patients with OoMG, given the side effects of long-term treatment with prednisone. 14 However, since not all patients with OoMG will progress to gMG, a wait-and-see strategy appears to be justified hoping to achieve spontaneous remission and avoiding the ‘aggressive’ treatment with thymectomy only potentially improving the clinical outcome.
To overcome this problem of uncertainty, risk factors associated with the generalization of OoMG have been identified in order to tailor the treatment, aiming at protection of patients with a low risk of generalization from exposure to thymectomy and improving the clinical outcomes of those with a high risk.5–11 In the context of tailored treatment, a predictive tool of the development of secondary generalization for patients with OoMG could be helpful for making treatment decisions. A previous study has developed a scoring system based on a cohort of 101 patients to stratify patients with OoMG based on the risk of conversion to gMG. 15 This scoring system includes three independent variables: presence of one or more comorbidities, anti-AChR antibody status, and thymic hyperplasia which is often difficult to identify from imaging only. 16 Importantly, this study reveals that patients with OoMG might have different risk levels of conversion to gMG, and it is possible to stratify patients according to the generalization risk. Therefore, our study aimed to develop and externally validate a multivariable prediction model based on routine clinical variables to predict the development of secondary generalization in MG patients with purely ocular symptoms at onset.
Methods
Study design
This is a dual-center retrospective observational consecutive case series study, which was conducted in accordance with the Declaration of Helsinki and proved by the Ethics Committees of both Charite University Hospital Berlin (EA1/025/11) and the First Affiliated Hospital of Zhengzhou University (2020-KY-231). Written informed consent for the use of clinical data was waived for the Chinese cohort, and it was not necessary for the German cohort according to the § 25 of State Hospital Act of Berlin.
Study population
The German cohort was used to develop the multivariable prediction model, and the Chinese cohort was used for validation. The German cohort was retrieved from an MG database at the Integrated Center for Myasthenia Gravis in Charite, Berlin, in October 2019. Since the database was considered a non-thymectomy MG database, patients who had a mediastinal tumor on the imaging findings and those who underwent thymectomy were excluded at first hand. The Chinese cohort consists of patients who presented to the First Affiliated Hospital of Zhengzhou University for the diagnosis or further treatment of OoMG between January 2012 and January 2020. The exclusion criteria are as follows: unconfirmed diagnosis of OoMG, acceptance of treatment with thymectomy, missing data on the development of secondary generalization or time from disease onset to secondary generalization, follow-up time less than 6 months, and development of secondary generalization within 6 months after disease onset.
In this study, a confirmed diagnosis of OoMG was made according to typical symptoms (e.g. ptosis and/or diplopia) and one or more positive findings from the following examinations: seropositivity for related autoantibodies (e.g. anti-AChR antibody titer >0.45 nM or antibody against muscle-specific tyrosine kinase [MuSK] > 0.4 U/ml), electrophysiologic tests (e.g. repetitive nerve stimulation electromyography and/or single-fiber electromyography), and Tensilon test. Computed tomography of the chest was routinely used to screen for thymic epithelial tumor once a diagnosis of MG was confirmed.
Outcome and predictors
The primary outcome measures were the development of secondary generalization and time to generalization after disease onset. The development of secondary generalization was evaluated and confirmed by the neurologists at each center according to the first presence of generalized symptoms, such as arm weakness, leg weakness, speaking disorder, chewing problems, and respiratory shortness, after excluding other differential diagnoses. Time to generalization was defined as the duration from symptoms onset to the development of generalized symptoms.
Several routine clinical variables were selected according to the clinical experience and potential predictors reported in the previous studies.5–11 Sex, age at disease onset, titer of anti-AChR antibody, status of anti-MuSK antibody test, status of anti-Titin antibody test, response to Tensilon test, status of repetitive nerve stimulation electromyography test, presence of other autoimmune disease (OAID) and corticosteroids use within 6 months after disease onset were collected from the hospital information system. Most patients had tested the anti-AChR antibody test for several times, but only the earliest available titer before generalization was chosen for analyses. Anti-MuSK antibody titer over 0.4 U/ml was defined as positive, otherwise negative. In terms of repetitive nerve stimulation electromyography, positive findings were defined as decrease in the amplitude not less than 10%. The use of corticosteroids was defined as any record of corticosteroids agents (prednisolone, prednisone, and methylprednisolone) use for the treatment of OoMG from the hospital information system. Since we did not document data of time to diagnosis and it is frequently reported to be 3 to 5 months,15,17 we set time 0 as 6 months after disease onset. Therefore, early corticosteroids use is defined as requirement for corticosteroids in the first 6 months, and the model can be applied at 6 months after disease onset.
Missing data
Variables with more than 20% missing data were excluded for analysis, such as status of anti-Titin antibody test, response to Tensilon test, and status of repetitive nerve stimulation electromyography test in our case. 18 Remaining missing data were handled by multiple imputation using ‘mice’ package; five complete databases were created after imputation, but only the median values of the imputed ones were chosen for final analyses.
Statistical analyses
All the analyses were performed using R statistical software version 3.6.3. 19 Categorical variables were shown as numbers and frequencies. Continuous variables were shown as mean ± SD if normally distributed or median (interquartile range, IQR) if not. Normal distribution was tested using Shapiro–Wilk test. Regarding the sample size, the number of variables was selected in accordance with the rule of at least 10 events per variable. 18 The possible non-linear effect of the clinical variables on the secondary generalization was explored using restricted cubic splines. Splines were prepared with knot placement based on the Akaike information criterion (AIC). All interactions with age at onset and anti-AChR antibody titer were explored. Time-varying effect was examined using survival analysis and generalized additive model with ‘survival’, ‘rms’, ‘pammtools’, ‘visreg’, and ‘mgcv’ packages18,20–23 and illustrated as three-dimensional perspective plots and contour maps. Since the proportional hazard assumption was violated, a log-normal accelerated failure time (AFT) model was used instead of a Cox proportional hazard regression model. In AFT models, predictors act multiplicatively on the time to event or additively on the log time to event, the exponentiated coefficients are represented as time ratios (TRs). A TR larger than 1 indicates a prolongation of the time to event, while a TR smaller than 1 implies a shortening of the time to event. Therefore, the median time to event would be halved if the regression coefficient of a binary variable equals to log(0.5).
Independent variables were selected using the backward elimination method based on the AIC. A redundancy analysis was then performed to delete any variables that could be predicted by the remaining ones. The adequacy of the log-normal AFT model was checked by overlaying the Kaplan–Meier estimates of the distribution of the residuals with the theoretical Gaussian one. The independent variables were integrated in a nomogram to represent the final model. The model performance was evaluated by calibration and discrimination. The calibration curves were plotted using bootstrap validation with 400 bootstrap replications and 60 patients per group, in order to show the relationship between the predicted outcome probabilities and the observed outcome probabilities. The discrimination was evaluated using Harrell’s C-index (also named concordance statistic).
Results
Patients and clinical characteristics
A study flowchart briefly summarizes the recruitment of patients (Figure 1). Regarding the German cohort, out of 498 patients 275 (among them 37.8% were female) with a median age at disease onset of 61 (47–71) years who initially presented with purely ocular symptoms were included for further analyses. In terms of the Chinese cohort, 202 out of 587 patients (46% female) with a median age of 49 (27, 61) years were eligible for inclusion (Table 1). In the Chinese cohort, the median age at disease onset is lower as compared with the German cohort (Table 1). Notably, less than 10% patients started using corticosteroids within 6 months after disease onset in the German cohort, while about 40% did so in the Chinese cohort.
Figure 1.
Flowchart of patient selection.
Table 1.
Baseline characteristics.
| Characteristic | German cohort (n = 275) | Chinese cohort (n = 202) |
|---|---|---|
| Sex, n (%) | ||
| Male | 171 (62.2%) | 109 (54.0%) |
| Female | 104 (37.8%) | 93 (46.0%) |
| Anti-AChR Ab titer [median (IQR)], nmol/L a | 2.20 [0.42, 10.00] | 1.96 [0.26, 8.62] |
| Anti-MuSK Ab, n (%) | ||
| Negative | 251 (91.3%) | 140 (69.3%) |
| Positive | 2 (0.7%) | 2 (1.0%) |
| Missing data | 22 (8%) | 60 (29.7%) |
| OAID | ||
| No | 236 (85.8%) | 173 (85.6%) |
| Yes | 39 (14.2%) | 29 (14.4%) |
| Age at disease onset [median (IQR)], years | 61.0 [47.0, 71.0] | 49.0 [27.0, 61.0] |
| Early corticosteroids use, n (%) | ||
| No | 249 (90.5%) | 122 (60.4%) |
| Yes | 24 (8.7%) | 80 (39.6%) |
| Missing data | 2 (0.7%) | 0 (0%) |
Anti-AChR Ab, antibody against acetylcholine receptor; Anti-MuSK Ab, antibody against muscle-specific tyrosine kinase; IQR, interquartile range; OAID, other autoimmune disease.
Fifty patients had missing data regarding anti-AChR Ab titer, including 26 (9.5%) patients in the German cohort and 24 (11.9%) patients in the Chinese cohort.
Outcome
In the German cohort, 37.5% of patients developed a gMG with a median follow-up time of 69 (32–116) months. In the 103 patients who developed secondary generalization, the median time to generalization was 29 (16–71) months. The estimated cumulative probabilities of generalization were 18.5% [95% CI (confidence interval), 13.6–23.1%], 30.5% (95% CI, 24.3–36.2%) and 48.5% (95% CI, 39.7–56.1%) at 2, 5, and 10 years after disease onset, respectively. Supplemental eTable 1 summarizes the generalization outcome in the whole cohort, the German cohort, and the Chinese cohort. The estimated cumulative generalization rates at different time points were calculated using the Kaplan–Meier method in the whole cohort (Figure 2(a)), the German cohort (Figure 2(b)), and the Chinese cohort (Figure 2(c)), respectively.
Figure 2.
Kaplan–Meier curves for cumulative generalization probability in the whole cohort (a), the German cohort (b), and the Chinese cohort (c).
Model development
A non-linear effect was not identified, but non-proportionality of hazards was found in age at onset (p = 0.021), not in titer of anti-AChR antibody (p = 0.323). Moreover, Somers’ Dxy rank correlation between the potential predictors and original survival time showed that age at disease onset was closely correlated with generalization-free survival (Supplemental eFigure 1). The three-dimensional hazard ratio surface and the contour map for age at onset [Figure 3(a) and (b)] and titer of anti-AChR antibody [Figure 3(c) and (d)] were shown in Figure 3. Interestingly, the overall effect slightly increased within the first 2–3 years after disease onset and then gradually decreased over time. The prognostic effects of age at onset [Figure 3(a) and (b)] and anti-AChR antibody titer [Figure 3(c) and (d)] were diluted if a patient survived long enough without developing generalization of OoMG. The non-proportionality of hazards consists of longer time being associated with a lower risk of generalization, but about 4 years after disease onset, the risk of generalization started to increase for patients with an age at disease onset older than 75 years.
Figure 3.
The three-dimensional hazard ratio surface plots and the contour maps demonstrating time-varying effects of age at onset and anti-AChR Ab titer. (a) The three-dimensional surface showed the relations between any two variables of time, age at onset, and hazard ratio for generalization-free survival on the x-, y-, and z-axes. (b) The contour map uses a color gradient to visualize the effect of time and age at onset on the hazard ratio, where the dark shades of red imply an increase and the darker shades of blue denote a decrease in the hazard ratio. (c) The three-dimensional surface plot for anti-AChR Ab titer. (d) The contour map for anti-AChR Ab titer.
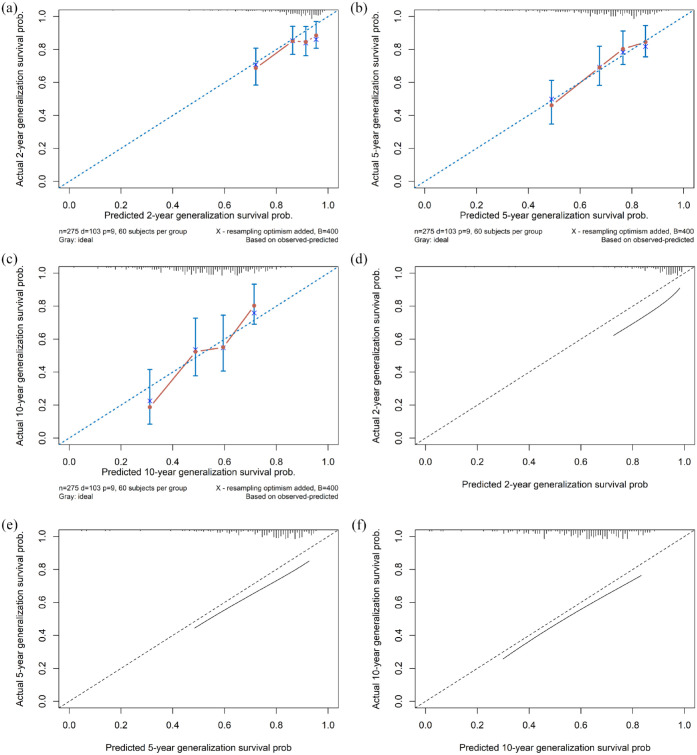
The log-normal AFT model adequately fitted the data with respect to the selected potential predictors (Supplemental eFigure 2). Five variables, sex, titer of anti-AChR antibody, status of anti-MuSK antibody, age at disease onset, and OAID, were independently associated with the development of secondary generalization according to the change of AIC. No redundancy was detected among the variables. Table 2 and Figure 4 summarize the estimated TRs of secondary generalization for default settings of predictors. An increase in anti-AChR Ab titer from 0.43 to 9.95 nM shortened the time to generalization by about 7%. Besides, a change in age at onset from 47 to 71 shortened the median generalization-free survival time by 17%. Internal validation using the bootstrap showed an adequate calibration [Figure 5(a)–(c)] and a moderate discriminative ability with a C-index of 0.667 (95% CI, 0.594–0.713). Furthermore, R2 = 0.127 shows that the study model can explain 12.7% of the variation in the outcome.
Table 2.
Estimated survival time ratios for default settings of predictors in the final AFT model to predict generalization in patients with OMG.
| Variable | TR (95% CI) | p value | ∆AIC a |
|---|---|---|---|
| Anti-AChR Ab titer, nM, 0.43 vs. 9.95 | 0.93 (0.56–1.56) | 0.1108 | –1.8 |
| Age at onset, years, 47 vs. 71 | 0.83 (0.44–1.54) | 0.1675 | –7.4 |
| Sex, male vs. female | 0.48 (0.31–0.75) | <0.0001 | –8.6 |
| Anti-MuSK Ab, negative vs. positive | 0.06 (0.01–0.48) | 0.0075 | –5.1 |
| OAID, no vs. yes | 0.64 (0.36–1.13) | 0.1245 | –0.3 |
AFT, accelerated failure time; ∆AIC, change in Akaike information criterion; Anti-AChR Ab titer, antibody titer against acetylcholine receptor; Anti-MuSK Ab, antibody against muscle-specific tyrosine kinase; CI, confidence interval; OAID, other autoimmune disease; TR, time ratio.
Note that the default values of the continuous predictors were lower quartiles and upper quartiles. TRs are derived from a multivariable log-normal AFT model and represent its exponentiated coefficients. TR < 1 means that a change in the value of the covariate is associated with shorter generalization-free survival, and TR > 1 indicates that a change in the value of the covariate is associated with longer generalization-free survival. For example, when anti-AChR Ab titer changes from 0.43nM to 9.95 nM, median generalization-free survival time decreases by about 7%; when age at disease onset changes from 47 to 71, median generalization-free survival time decreases by 17%.
A negative value implies that the variable improves the model and should not be eliminated.
Figure 4.
Visualization of the estimated survival time ratios for default settings of predictors in the final AFT model.
AFT, accelerated failure time; Anti-AChR Ab titer, antibody titer against acetylcholine receptor; Anti-MuSK Ab, antibody against muscle-specific tyrosine kinase; OAID, other autoimmune disease; TR, time ratio.
Note that the default values of the continuous predictors were lower quartiles and upper quartiles. TRs are derived from a multivariable log-normal AFT model and represent its exponentiated coefficients. TR < 1 means that a change in the value of the covariate is associated with shorter generalization-free survival, and TR > 1 indicates that a change in the value of the covariate is associated with longer generalization-free survival. For example, when anti-AChR Ab titer changes from 0.43 to 9.95 nM, median generalization-free survival time decreases by about 7%; when age at disease onset changes from 47 to 71, median generalization-free survival time decreases by 17%.
Figure 5.
Internal and external validation of calibration curves. Internal bootstrap validation for predicting generalization within 2 year (a), 5 years (b), and 10 years (c) after disease onset. Red dots represent calibration accuracy, blue vertical bars show 95% CIs, and the blue diagonal dotted line depicts the ideal relationship. External validation for predicting generalization within 2 year (d), 5 years (e), and 10 years (f).
CIs, confidence intervals.
Model validation
The model was then externally validated in a Chinese cohort. The calibration curves showed a good agreement between the predicted outcome probabilities and the observed outcome frequencies [Figure 5(d)–(f)]. Likewise, the model had a moderate discriminative ability which was indicated by a C-index of 0.670 (95% CI, 0.602–0.738). Figure 6 shows a nomogram which graphically represents the final model. The underlying equation of the model (Supplementary 1) with a detailed example (Supplemental eFigure 3) of its use is presented in the supplementary data.
Figure 6.
Nomogram for predicting median generalization-free survival time and 2-, 5-, and 10-year generalization probability, based on the final AFT model.
AFT, accelerated failure time; Anti-AChR Ab titer, antibody titer against acetylcholine receptor; Anti-MuSK Ab, antibody against muscle-specific tyrosine kinase; OAID, other autoimmune disease; TR, time ratio.
Discussion
To our knowledge, this is the largest series published to date aiming at prediction of the individual risk for secondary generalization of OoMG at 6 months after disease onset. A multivariable prediction model was developed based on 275 patients from a German cohort and then validated in a Chinese cohort according to the TRIPOD reporting guideline. 24 The predictors, including sex, titer of anti-AChR antibody, status of anti-MuSK antibody, age at disease onset, and the presence of OAID, are routinely available in the clinical practice. The study model showed an adequate calibration and a moderate discrimination at both strong internal validation and external validation. A nomogram was proposed to graphically represent the study model and make it easy to use at the bedside.
The study model was specifically designed to be used in the clinical practice with the aim of better informing the risk of generalization for nonsurgical patients with OoMG, thereby potentially improving the clinical decision-making process. Thus, only variables which are widely used in clinical practice and frequently reported to be associated with the development of generalization were included for analyses.5–11 They are all potential predictors of generalization, but their combined predictive ability has not been assessed before. Although several studies have shed light on the association between thymic hyperplasia and the development of generalization of OoMG,15,25 we did not include thymic hyperplasia as a potential variable, because it is, at least for some, difficult to differentiate lymphoid follicular hyperplasia from true thymic hyperplasia, normal thymus, and thymic involution. 16 Moreover, it is worth mentioning the application criteria of this study model: a confirmed diagnosis of OoMG according to the diagnosis criteria of the study, at 6 months after disease onset, no surgical treatment (thymectomy) and no suspicious mediastinal lesion on the imaging studies.
In the whole cohort, with a median follow-up time of 60 (32–97.5) months, 165 of 477 (34.6%) patients developed secondary generalization, which is comparable with that reported in the previous studies (9.4–73.7%).7,8,10,11,26 The reported rate of generalization in patients with OoMG varies significantly among different studies, mainly because many factors could influence its occurrence, including, but not limited to, patient characteristics, thymic histopathology, medications, and physical and emotional stress.6–11 The rate of generalization in the Chinese cohort (30.7%) was slightly lower than that in the German cohort (37.5%), and the median time to generalization in the Chinese cohort (18, IQR, 11–38, months) was also shorter than that in the German cohort (29, IQR, 16–71, months). A previous study also indicated the existence of ethnicity-based differences in MG. 27 As to the non-linear effect of age at onset, especially in patients older than 75 years, the effect was only diluted temporally after the first 2–3 years and later started to increase at about 4 years after disease onset. This sheds light on the importance of the predictive value of age at onset over time and seems to be in line with the conversion distribution in this population (Supplemental eFigure 4). The conversion percent was large within the first 2–3 years after disease onset, and got smaller shortly afterwards, but in patients older than 75 years at onset, the conversion percent was also large at the sixth year after disease onset. Besides, comorbid autoimmune diseases are not uncommon in patients with MG, the most common ones were Graves’ disease, Hashimoto’s disease, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, type I diabetes, pernicious anemia, Sjoegren’s syndrome, Crohn’s disease, and ulcerative colitis. 28 About 15% of patients in our study have OAIDs, which is in line with the data of previous studies.29,30 A predictive role of comorbid autoimmune disease in the development of generalization of OoMG was also observed in our study, similar to the findings of a previous study. 15 Interestingly, the usage of corticosteroids within the first 6 months was not independently associated with the development of generalization. This is contrary to our common knowledge that the usage of corticosteroids might reduce the rate of generalization of OoMG.8,10,26 It is possibly because the number of patients (24/275) who started using corticosteroids agents in the first 6 months was small, and the non-users here also included patients who started using corticosteroids agents after 6 months from disease onset. Also, data on the usage of non-steroid immunosuppressants within the 6 months after disease onset were not included for analyses, which might influence the impact of the usage of corticosteroids agents and introduce bias to the prediction model. However, a cut-off timepoint, at 6 months for the study, had to be chosen so that the prediction model can be applied at this time point. Not surprisingly, post hoc analyses using Kaplan–Meier method showed that OoMG patients who received treatment with corticosteroids, regardless of the start time, had a lower cumulative generalization probability than those who did not (Supplemental eFigure 5). Although positivity for anti-MuSK antibody was significantly associated with the generalization of OoMG, thymectomy is not likely to benefit these patients according to the current evidence from previous studies.31,32
The most important limitation of the study lies in the retrospective study design. Patients who were lost to follow-up, those with missing data of secondary generalization, those who had a mediastinal tumor on the imaging findings, and those who underwent thymectomy were excluded for analyses, which might introduce selection bias. Besides, some potential predictors were not included for analyses because they were either hardly available for a retrospective review, for example, use of cautionary drugs for MG, mood fluctuations, infections and surgery or trauma, 6 or only tested in a small amount of patients, for example, status of repetitive nerve stimulation electromyography test and status of anti-Titin antibody test. 11 Also, the earliest available titer of anti-AChR antibody were collected, but the exact tested time point (disease duration) and antibody test assay might vary individually, which makes the interpretation difficult.
The study has several additional limitations. First, the model’s usefulness is probably limited. The study model was developed based on a small range of all patients in Germany and then validated in a small cohort from China. Besides, we only included patients who met the OoMG diagnosis criteria of the study. Second, given the complexity and variety of the disease course, further external validation with prospective database should be performed to validate or even update our model. Third, the number of patients with MuSK antibody is pretty small due to its low incidence, which might introduce bias to the prediction model. Since the underlying pathogenetic mechanisms of the generalization might not be fundamentally associated with and totally reflected by the routine clinical variables, further research should explore the role of genomic data modeling in predicting the development of secondary generalization. The genetic and clinical characteristics of MG might vary by geographic location and ethnic origin.33–35 The performance of the prediction model is fair, but not excellent, perhaps due to genetic factors. A recent meta-analysis showed that the HLA-DRB1 07 allele might be a risk factor for Asian patients with late-onset MG but not in Caucasian patients. 35 On the contrary, HLA-DRB1 04 and 14 alleles were associated with late-onset MG in Caucasian patients, but not in Asian patients. 35 As to non-HLA genes, PTPN22, CTLA4, and FOXP3 were frequently reported to be associated with MG.36–38 A previous meta-analysis showed that PTPN22 R620 W polymorphism is associated with MG risk in Caucasian patients in a geography-dependent manner. 39
The study model has several practical applications. First, it could be used to stratify patients with OoMG according to the generalization risk. This might be useful for patient enrollment strategy in clinical trials. Targeted selection OoMG patients with a high risk of generalization is efficient and cost-effective. Second, the study model could move a step forward to precision medicine in the treatment of OoMG. Because it assigns each patient, a specific value of time to generalization and probabilities of developing secondary generalization at different time points. Patients with a high risk of generalization have a short time to generalization and high probability of developing generalization. These patients could be offered more ‘aggressive’ treatment to prevent the development of generalization, for example, corticosteroids agents and/or thymectomy. Third, the study model might facilitate the application of the early fast-acting treatment strategy for the treatment of OoMG, which might improve the clinical outcomes. 40 Moreover, a previous study also demonstrated that early thymectomy is more likely to offer patients with OoMG better clinical outcomes. 14
Given the lack of practical prediction models in this setting, we developed a useful model to predict the generalization of OoMG in patients with purely ocular symptoms at disease onset. All the predictors are easily, rapidly, and routinely available in the clinical practice. The nomogram based on the study model can better inform both patients and physicians the risk of generalization in a personalized manner, thereby improving the clinical decision-making process and providing risk stratification in future clinical trials. Further prospective data are in need to validate or update the prediction model.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221104508 for Prediction of the generalization of myasthenia gravis with purely ocular symptoms at onset: a multivariable model development and validation by Feng Li, Hongbin Zhang, Ya Tao, Frauke Stascheit, Jiaojiao Han, Feng Gao, Hongbo Liu, Alberto Carmona-Bayonas, Zhongmin Li, Jens-C. Rueckert, Andreas Meisel and Song Zhao in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors wish to thank all patients for participating in this study. FL thanks China Scholarship Council for the financial support during his study in Germany.
Footnotes
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines after the approval by the Ethics Committees of both Charite University Hospital Berlin (EA1/025/11) and the First Affiliated Hospital of Zhengzhou University (2020-KY-231). Written informed consent for the use of clinical data was waived for the Chinese cohort, and it was not necessary for the German cohort according to the § 25 of State Hospital Act of Berlin. All authors give their consent for the publication of the research findings.
Author contributions: Feng Li: Data curation; Formal analysis; Methodology; Resources; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Hongbin Zhang: Data curation; Resources; Writing – review & editing.
Ya Tao: Data curation; Resources; Writing – original draft.
Frauke Stascheit: Data curation; Resources; Writing – review & editing.
Jiaojiao Han: Data curation; Resources; Writing – review & editing.
Feng Gao: Data curation; Resources; Writing – review & editing.
Hongbo Liu: Data curation; Resources; Writing – review & editing.
Alberto Carmona-Bayonas: Formal analysis; Methodology; Software; Validation; Writing – review & editing.
Zhongmin Li: Data curation; Resources; Writing – original draft.
Jens-C. Rueckert: Conceptualization; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Andreas Meisel: Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Song Zhao: Conceptualization; Methodology; Supervision; Writing – review & editing.
ORCID iDs: Andreas Meisel  https://orcid.org/0000-0001-7233-5342
https://orcid.org/0000-0001-7233-5342
Song Zhao  https://orcid.org/0000-0002-4658-3921
https://orcid.org/0000-0002-4658-3921
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AM reports personal fees from Alexion, Bristol Myers Squipp, Grifols, and Hormosan and research grants from Alexion and Octapharma outside the submitted work. The remaining authors have nothing to disclose.
Data sharing statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Feng Li, Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; Department of Surgery, Competence Center of Thoracic Surgery, Charite University Hospital Berlin, Berlin, Germany.
Hongbin Zhang, Department of Surgery, Competence Center of Thoracic Surgery, Charite University Hospital Berlin, Berlin, Germany.
Ya Tao, Department of Obstetrics, The First Affiliated Hospital of Zhengzhou University, Obstetric Emergency and Critical Care Medicine of Henan Province, Zhengzhou, China.
Frauke Stascheit, Department of Neurology, Integrated Center for Myasthenia Gravis, NeuroCure Clinical Research Center, Center for Stroke Research Berlin, Charité – University Medicine Berlin, Berlin, Germany.
Jiaojiao Han, Department of Neuroimmunology, Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China.
Feng Gao, Department of Neuroimmunology, Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China.
Hongbo Liu, Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
Alberto Carmona-Bayonas, Hospital Universitario Morales Meseguer, Universidad de Murcia, Instituto Murciano de Investigación Biosanitaria, Murcia, Spain.
Zhongmin Li, Department of Surgery, Competence Center of Thoracic Surgery, Charite University Hospital Berlin, Berlin, Germany.
Jens-C. Rueckert, Department of Surgery, Competence Center of Thoracic Surgery, Charite University Hospital Berlin, 10117, Berlin, Germany
Andreas Meisel, Department of Neurology, Integrated Center for Myasthenia Gravis, NeuroCure Clinical Research Center, Center for Stroke Research Berlin, Charité – University Medicine Berlin, Berlin, Germany.
Song Zhao, Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan, China.
References
- 1. Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers 2019; 5: 30. [DOI] [PubMed] [Google Scholar]
- 2. Al-Haidar M, Benatar M, Kaminski HJ. Ocular myasthenia. Neurol Clin 2018; 36: 241–251. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki S, Murai H, Imai T, et al. Quality of life in purely ocular myasthenia in Japan. BMC Neurol 2014; 14: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Lott LB, Kerber KA, Lee PP, et al. Diplopia-related ambulatory and emergency department visits in the United States, 2003-2012. JAMA Ophthalmol 2017; 135: 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol 2003; 60: 243–248. [DOI] [PubMed] [Google Scholar]
- 6. Blum S, Lee D, Gillis D, et al. Clinical features and impact of myasthenia gravis disease in Australian patients. J Clin Neurosci 2015; 22: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 7. Aguirre F, Villa AM. Prognosis of ocular myasthenia gravis in an Argentinian population. Eur Neurol 2018; 79: 113–117. [DOI] [PubMed] [Google Scholar]
- 8. Kamarajah SK, Sadalage G, Palmer J, et al. Ocular presentation of myasthenia gravis: a natural history cohort. Muscle Nerve 2018; 57: 622–627. [DOI] [PubMed] [Google Scholar]
- 9. Bever CT, Jr, Aquino AV, Penn AS, et al. Prognosis of ocular myasthenia. Ann Neurol 1983; 14: 516–519. [DOI] [PubMed] [Google Scholar]
- 10. Hong YH, Kwon SB, Kim BJ, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci 2008; 273: 10–14. [DOI] [PubMed] [Google Scholar]
- 11. Teo KY, Tow SL, Haaland B, et al. Low conversion rate of ocular to generalized myasthenia gravis in Singapore. Muscle Nerve 2018; 57: 756–760. [DOI] [PubMed] [Google Scholar]
- 12. Benatar M, McDermott MP, Sanders DB, et al. Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): a randomized, controlled trial. Muscle Nerve 2016; 53: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li F, Li Z, Chen Y, et al. Thymectomy in ocular myasthenia gravis before generalization results in a higher remission rate. Eur J Cardiothorac Surg 2020; 57: 478–487. [DOI] [PubMed] [Google Scholar]
- 14. Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg 2013; 145: 1319–1324. [DOI] [PubMed] [Google Scholar]
- 15. Wong SH, Petrie A, Plant GT. Ocular myasthenia gravis: toward a risk of generalization score and sample size calculation for a randomized controlled trial of disease modification. J Neuroophthalmol 2016; 36: 252–258. [DOI] [PubMed] [Google Scholar]
- 16. Goldstein AJ, Oliva I, Honarpisheh H, et al. A tour of the thymus: a review of thymic lesions with radiologic and pathologic correlation. Can Assoc Radiol J 2015; 66: 5–15. [DOI] [PubMed] [Google Scholar]
- 17. Farrugia ME, Cleary M, Carmichael C. A retrospective study of acetylcholine receptor antibody positive ocular myasthenia in the West of Scotland. J Neurol Sci 2017; 382: 84–86. [DOI] [PubMed] [Google Scholar]
- 18. Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed. New York: Springer, 2015. [Google Scholar]
- 19. R Core Team. R: a language and environment for statistical computing (R version 3.6.1 (2019-07-05)). Vienna: R Foundation for Statistical Computing. [Google Scholar]
- 20. Therneau TM. A package for survival analysis in R. 2.38 ed, 2015, https://rdrr.io/cran/survival/f/inst/doc/survival.pdf.
- 21. Bender A, Groll A, Scheipl F. A generalized additive model approach to time-to-event analysis. Stat Model 2018; 18: 299–321. [Google Scholar]
- 22. Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol 2011; 73: 3–36. [Google Scholar]
- 23. Breheny P, Burchett W. Visualization of regression models using visreg. R J 2017; 9: 56–71. [Google Scholar]
- 24. Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162: W1–W73. [DOI] [PubMed] [Google Scholar]
- 25. Apinyawasisuk S, Chongpison Y, Jariyakosol S, et al. Factors affecting generalization of ocular myasthenia gravis in patients with positive acetylcholine receptor antibody. Am J Ophthalmol 2020; 209: 10–17. [DOI] [PubMed] [Google Scholar]
- 26. Verma R, Wolfe GI, Kupersmith MJ. Ocular myasthenia gravis – how effective is low dose prednisone long term? J Neurol Sci 2021; 420: 117274. [DOI] [PubMed] [Google Scholar]
- 27. Abukhalil F, Mehta B, Saito E, et al. Gender and ethnicity based differences in clinical and laboratory features of myasthenia gravis. Autoimmune Dis 2015; 2015: 197893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nacu A, Andersen JB, Lisnic V, et al. Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity 2015; 48: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi J, Huan X, Zhou L, et al. Comorbid autoimmune diseases in patients with myasthenia gravis: a retrospective cross-sectional study of a Chinese Cohort. Front Neurol 2021; 12: 790941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Misra UK, Kalita J, Singh VK, et al. A study of comorbidities in myasthenia gravis. Acta Neurol Belg 2020; 120: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005; 57: 444–448. [DOI] [PubMed] [Google Scholar]
- 32. Clifford KM, Hobson-Webb LD, Benatar M, et al. Thymectomy may not be associated with clinical improvement in MuSK myasthenia gravis. Muscle Nerve 2019; 59: 404–410. [DOI] [PubMed] [Google Scholar]
- 33. Gregersen PK, Kosoy R, Lee AT, et al. Risk for myasthenia gravis maps to a (151) Pro->Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol 2012; 72: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asmail A, Kesler A, Drory VE, et al. Effect of ethnic origin and gender on the clinical manifestations of myasthenia gravis among the Jewish population in Israel. J Neuroimmunol 2017; 307: 47–52. [DOI] [PubMed] [Google Scholar]
- 35. Ling CS, Shen ML, Wang Y, et al. The associations of HLA-DRB1 gene polymorphisms with late-onset myasthenia gravis: a meta-analysis. Neurol Sci 2020; 41: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 36. Lefvert AK, Zhao Y, Ramanujam R, et al. PTPN22 R620W promotes production of anti-AChR autoantibodies and IL-2 in myasthenia gravis. J Neuroimmunol 2008; 197: 110–113. [DOI] [PubMed] [Google Scholar]
- 37. Renton AE, Pliner HA, Provenzano C, et al. A genome-wide association study of myasthenia gravis. JAMA Neurol 2015; 72: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong X, Xiang M, Cheng X, et al. PTPN22 R620W polymorphism is associated with myasthenia gravis risk: a systematic review and meta-analysis. Med Sci Monit 2015; 21: 2567–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Utsugisawa K, Nagane Y, Akaishi T, et al. Early fast-acting treatment strategy against generalized myasthenia gravis. Muscle Nerve 2017; 55: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221104508 for Prediction of the generalization of myasthenia gravis with purely ocular symptoms at onset: a multivariable model development and validation by Feng Li, Hongbin Zhang, Ya Tao, Frauke Stascheit, Jiaojiao Han, Feng Gao, Hongbo Liu, Alberto Carmona-Bayonas, Zhongmin Li, Jens-C. Rueckert, Andreas Meisel and Song Zhao in Therapeutic Advances in Neurological Disorders