Abstract
Tooth agenesis is a common structural birth defect in humans that results from failure of morphogenesis during early tooth development. The homeobox transcription factor Msx1 and the canonical Wnt signaling pathway are essential for “bud to cap” morphogenesis and are causal factors for tooth agenesis. Our recent study suggested that Msx1 regulates Wnt signaling during early tooth development by suppressing the expression of Dkk2 and Sfrp2 in the tooth bud mesenchyme, and it demonstrated partial rescue of Msx1-deficient molar teeth by a combination of DKK inhibition and genetic inactivation of SFRPs. In this study, we found that Sostdc1/Wise, another secreted Wnt antagonist, is involved in regulating the odontogenic pathway downstream of Msx1. Whereas Sostdc1 expression in the developing tooth germ was not increased in Msx1−/− embryos, genetic inactivation of Sostdc1 rescued maxillary molar, but not mandibular molar, morphogenesis in Msx1−/− mice with full penetrance. Since the Msx1−/−;Sostdc1−/− embryos exhibited ectopic Dkk2 expression in the developing dental mesenchyme, similar to Msx1−/− embryos, we generated and analyzed tooth development in Msx1−/−;Dkk2−/− double and Msx1−/−;Dkk2−/−;Sostdc1−/− triple mutant mice. The Msx1−/−;Dkk2−/− double mutants showed rescued maxillary molar morphogenesis at high penetrance, with a small percentage also exhibiting mandibular molars that transitioned to the cap stage. Furthermore, tooth development was rescued in the maxillary and mandibular molars, with full penetrance, in the Msx1−/−;Dkk2−/−;Sostdc1−/− mice. Together, these data reveal 1) that a key role of Msx1 in driving tooth development through the bud-to-cap transition is to control the expression of Dkk2 and 2) that modulation of Wnt signaling activity by Dkk2 and Sostdc1 plays a crucial role in the Msx1-dependent odontogenic pathway during early tooth morphogenesis.
Keywords: cell signaling, growth factors, genetics, odontogenesis, hypodontia, congenital abnormalities
Introduction
To make a tooth, a tooth germ must undergo morphogenesis through the bud, cap, and bell stages, which involves reciprocal interactions between the dental epithelium and mesenchyme (Kwon and Jiang 2018). Initially, the oral epithelium forms a localized thickening, the dental placode, which continues to bud into the underlying mesenchyme and induces mesenchymal condensation around the bud. Prior to the cap stage, the dental mesenchyme induces formation of the primary enamel knot (PEK), which expresses Shh and members of the BMP, FGF, and WNT families, such as Bmp2/4/7, Fgf3/4/9/20, and Wnt3/6/10a/10b, in the distal end of the tooth bud and drives “bud to cap” morphogenesis. Extensive genetic studies demonstrated that tooth development is controlled by an intricate regulatory network consisting of transcription factors and the major signaling pathways: Bmp, Fgf, Shh, and Wnt (Lan et al. 2014). Perturbation in this regulatory network may cause disruption in morphogenesis, in many cases affecting PEK formation and bud-to-cap transition and resulting in tooth agenesis, which can severely affect oral health (Tan et al. 2016).
Transcription factor Msx1 is essential in early tooth morphogenesis. With growth factor Bmp4, it forms the Bmp4-Msx1 positive feedback pathway within the bud-stage dental mesenchyme and promotes bud-to-cap morphogenesis (Chen et al. 1996; Bei et al. 2000; Zhao et al. 2000). The canonical Wnt signaling pathway is also essential in early tooth morphogenesis. Binding of Wnt signals to their receptors Frizzled (Fzd) and Lrp5/6 causes stabilization and translocation of the β-catenin into the nucleus, where β-catenin interacts with DNA-bound Tcf/Lef transcription factors and activates transcription of target genes, such as Lef1 or Axin2 (Nusse and Clevers 2017). Mutations in MSX1, WNT10A, or AXIN2 account for 46% of human tooth agenesis cases (Fournier et al. 2018), and genetic deletion of Msx1 or Lef1, as well as tissue-specific inactivation of β-catenin in the dental epithelium or mesenchyme, causes developmental arrest at the bud stage in mice (Kratochwil et al. 1996; Liu et al. 2008; Chen et al. 2009). Overexpression of secreted Wnt antagonist Dickkopf 1 (Dkk1) in the epithelium consistently results in bud-stage arrest (Liu et al. 2008), while deletion of Sostdc1—otherwise known as Wise, another Wnt antagonist—induces formation of supernumerary teeth caused by dysregulated Wnt signaling activity in the rudimentary dental tissues (Ahn et al. 2010). Dkk1 and Sostdc1 antagonize canonical Wnt signaling by binding to Lrp5/6 and preventing Frz-Lrp5/6 complex formation (Nusse and Clevers 2017). Yet, forced activation of Wnt signaling in the oral epithelium, through constitutive stabilization of β-catenin, results in formation of multiple supernumerary teeth (Järvinen et al. 2006). However, similar activation of β-catenin in the mesenchyme induces ectopic tooth bud-like invaginations in the developing palate but do not result in supernumerary teeth (Chen et al. 2009).
Deficiency in Msx1 or Bmp4 in the dental mesenchyme leads to significant reduction of the expression of Wnt-target genes Lef1 and Tcf7 (Jia et al. 2016), whereas Lef1 deficiency or dental mesenchyme-specific deletion of β-catenin does not alter mesenchymal Msx1 or Bmp4 expression (Kratochwil et al. 1996; Chen et al. 2009). A systems biology study identified Wnt and Bmp as the two major mediators of dental epithelial-mesenchymal signaling (O’Connell et al. 2012). These studies suggest that Msx1 is positioned upstream of Wnt signaling activation in early tooth development. Our recent study, based on dental mesenchyme-specific RNA-seq analysis, showed that expression of Dkk2 and Secreted frizzled-related protein 2 (Sfrp2), encoding distinct secreted antagonists of Wnt signaling, was significantly increased in the Msx1−/− mutant embryonic tooth mesenchyme (Jia et al. 2016). However, neither treatment with the DKK inhibitor IIIC3a nor genetic inactivation of Sfrp2 was able to rescue tooth morphogenesis in Msx1−/− embryos, whereas 30% of Msx1−/−;Sfrp2−/−;Sfrp3−/− embryos treated with IIIC3a showed partial rescue of the maxillary molar only (Jia et al. 2016). Thus, the molecular mechanism mediating Msx1 function in tooth morphogenesis and the relationship between Msx1 and Wnt signaling during tooth development require investigation. In this study, we show that complete inactivation of Dkk2 alone was able to rescue maxillary molar morphogenesis in the Msx1−/− mice at high penetrance, which identifies Dkk2 as a crucial downstream target gene in the Msx1-mediated odontogenic pathway. Furthermore, although expression of Sostdc1 in the developing tooth mesenchyme was not increased in the Msx1−/− embryos, we found 1) that inactivation of Sostdc1 also rescued maxillary molar morphogenesis in Msx1−/− mice and 2) that inactivation of Dkk2 and Sostdc1 rescued tooth development in the maxillary and mandibular molars in Msx1−/− mice with full penetrance. These results indicate that Msx1 drives early tooth morphogenesis from the bud to cap stage primarily through controlling Wnt signaling activity.
Materials and Methods
Mouse Strains
Msx1+/−, Sostdc1+/−, and Dkk2+/− mice were used for generating compound mutants (Satokata and Maas 1994; Li et al. 2005; Ahn et al. 2010). Mice were maintained in a CD1 outbred background. A total of 178 mouse samples were used. At least 3 mice, male and female, from embryonic day 12.5 (E12.5) to birth were examined per experiment. Tooth development beyond birth was not examined because Msx1−/− mice develop cleft palate and die at birth. All protocols were prepared before the study and approved by the Institutional Animal Care and Use Committee at the University at Buffalo and Cincinnati Children’s Hospital Medical Center. This study is compliant with the ARRIVE guidelines (Animal Research Reporting of In Vivo Experiments). Additional detailed information about the mice and the experimental procedures is included in the Appendix.
Results
Expression of Lef1 and Sostdc1 during Tooth Morphogenesis in the Msx1-Deficient Maxillary and Mandibular Molars
To address the knowledge gap on the relationship between Msx1 and the Wnt signaling pathway during early tooth development, we analyzed whether expression of Sostdc1 during tooth development is affected by Msx1. We characterized Sostdc1 mRNA expression during tooth morphogenesis in comparison with that of Lef1, a representative Wnt signaling target gene (Kratochwil et al. 1996; Sasaki et al. 2005). When compared with the strong expression in the control embryonic tooth germs, Lef1 expression was markedly reduced at E12.5 to E14.5 in Msx1−/− molars (Fig. 1A–C, A′–C′). In contrast, Sostdc1 signals showed a moderate and gradual decrease in Msx1−/− molars, especially in the mandible (Fig. 1D–F, D′–F′). Real-time quantitative polymerase chain reaction (qRT-PCR) results confirmed the dramatic decrease in Lef1 and moderate decrease in Sostdc1 in the Msx1−/− embryos in both jaws at the bud-to-cap transition (E14.0; Fig. 1G). Interestingly, Sostdc1 and Lef1 exhibited complementary expression patterns. In the distal tip of the tooth germ, Sostdc1 signals were weaker, whereas Lef1 signals were strong and concentrated, as similarly shown in the PEK (Fig. 1A–C, D–F, black arrowheads). However, Sostdc1 signals were strongest whereas Lef1 signals were markedly decreased or even absent in the palatal (for maxilla) or lingual (for mandible) mesenchyme (Fig. 1A–C, D–F, white arrowheads). Whereas the complementary patterns of Lef1 and Sostdc1 expression in the developing tooth germs are consistent with previous findings that Sostdc1 antagonized canonical Wnt signaling during tooth development (Ahn et al. 2010), the moderately decreased expression of Sostdc1 in the Msx1−/− tooth germs indicate that expression of Sostdc1 is regulated differently from that of Dkk2 and Sfrp2, which exhibited significant upregulation in the Msx1−/− tooth mesenchyme (Jia et al. 2016).
Figure 1.
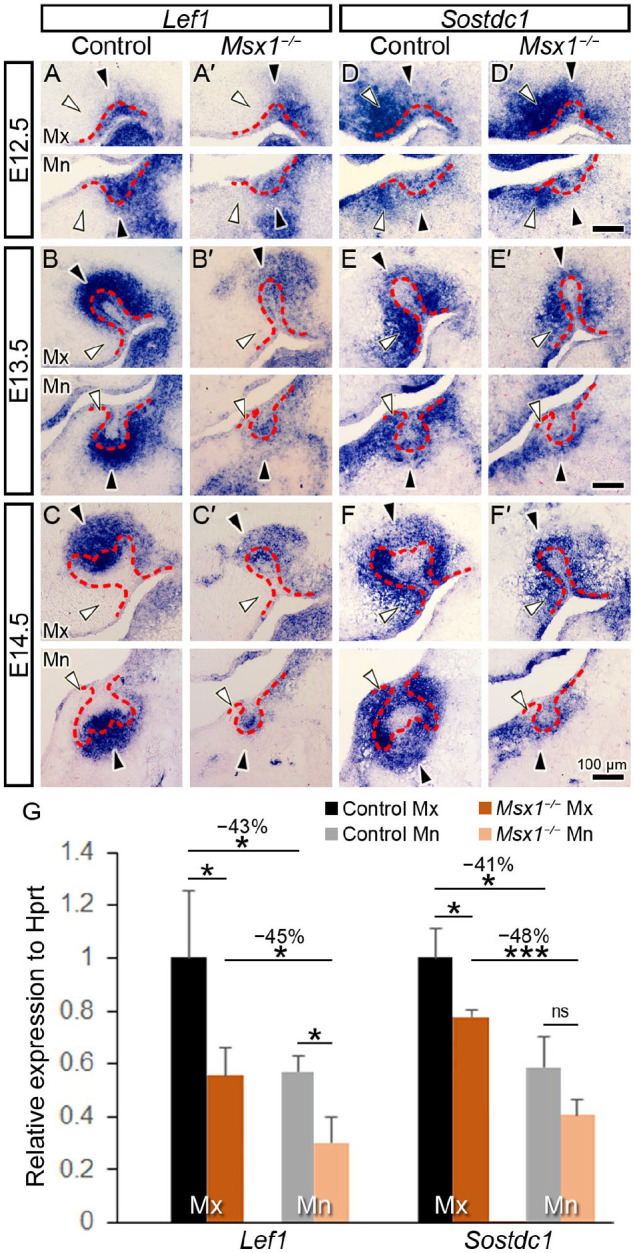
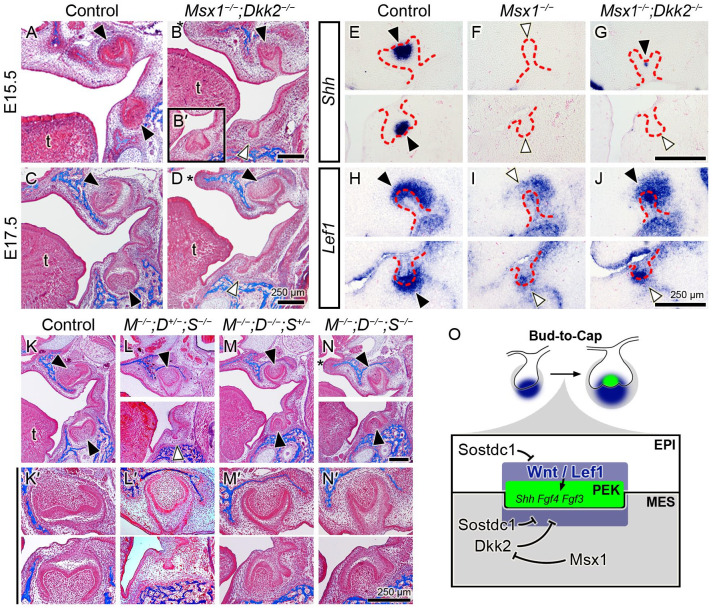
Lef1 and Sostdc1 expression in normal and Msx1−/− tooth morphogenesis. (A–F; A′–F′) Expression pattern of Lef1 and Sostdc1 in frontal sections through the molar tooth germs in the control (A–F) and Msx1−/− (A′–F′) groups. Representative time points for early tooth development were examined: the placode (embryonic day 12.5 [E12.5]), bud (E13.5), and cap (E14.5) stages. In each panel, a white horizontal line divides the upper jaw (maxilla, Mx) and the lower jaw (mandible, Mn); the left side is palatal/lingual and right side is buccal. White arrowhead marks the palatal/lingual mesenchyme; black arrowhead marks the distal end of the tooth germ; red dashed line marks the basal lamina between the dental epithelium and mesenchyme. Scale bars, 100 µm. n = 4 for panels A–C and A′–C′; n = 10 for panels D–F and D′–F′. (G) Real-time quantitative polymerase chain reaction of Lef1 and Sostdc1 in Mx and Mn molar tooth germs in the control and Msx1−/− groups at bud-to-cap transition (at E14.0). Results are expressed as fold change ± SD relative to control Mx. n = 3 for each group. Student t test. *P ≤ 0.05. ***P ≤ 0.001. ns, not significant.
Deletion of Sostdc1 Partially Rescues Tooth Development in Msx1-Deficient Maxillary Molars
Although Sostdc1 expression was not suppressed by Msx1, we hypothesized that Sostdc1 might contribute to the suppression of canonical Wnt signaling in the Msx1−/− tooth germs. To test this, we examined whether Sostdc1 deletion could rescue tooth development in Msx1−/− mice. In contrast to the bud-stage arrest in all Msx1−/− molars, Msx1−/−;Sostdc1−/− compound mutants showed full penetrance (100%) of rescued maxillary molar, which advanced to the bell stage at birth (Fig. 2, Table). Control and Msx1−/−;Sostdc1−/− maxillary molars exhibited histodifferentiation of the dental epithelium and mesenchyme into ameloblasts and odontoblasts, respectively, a key feature of bell-stage morphogenesis (Fig. 2A′–C′). To rule out the possibility that the rescued molar is a supernumerary tooth, which can ectopically form in Sostdc1−/− mice (Ahn et al. 2010), we examined its anteroposterior position through whole-mount analysis of Shh mRNA expression, which marked the tooth germs and the palatal rugae that served as anteroposterior landmarks. Sagittal sections through the first and second molars were also analyzed, where the maxillary nerve (V2) and optic nerve marked the first and second molar positions, respectively (Fig. 2 A–C, G–I). Our data indicated that the rescued Msx1−/−; Sostdc1−/− molars were first molars. However, the rescue remained “partial” because of the marked reduction in the anteroposterior width and height (Fig. 2J).
Figure 2.
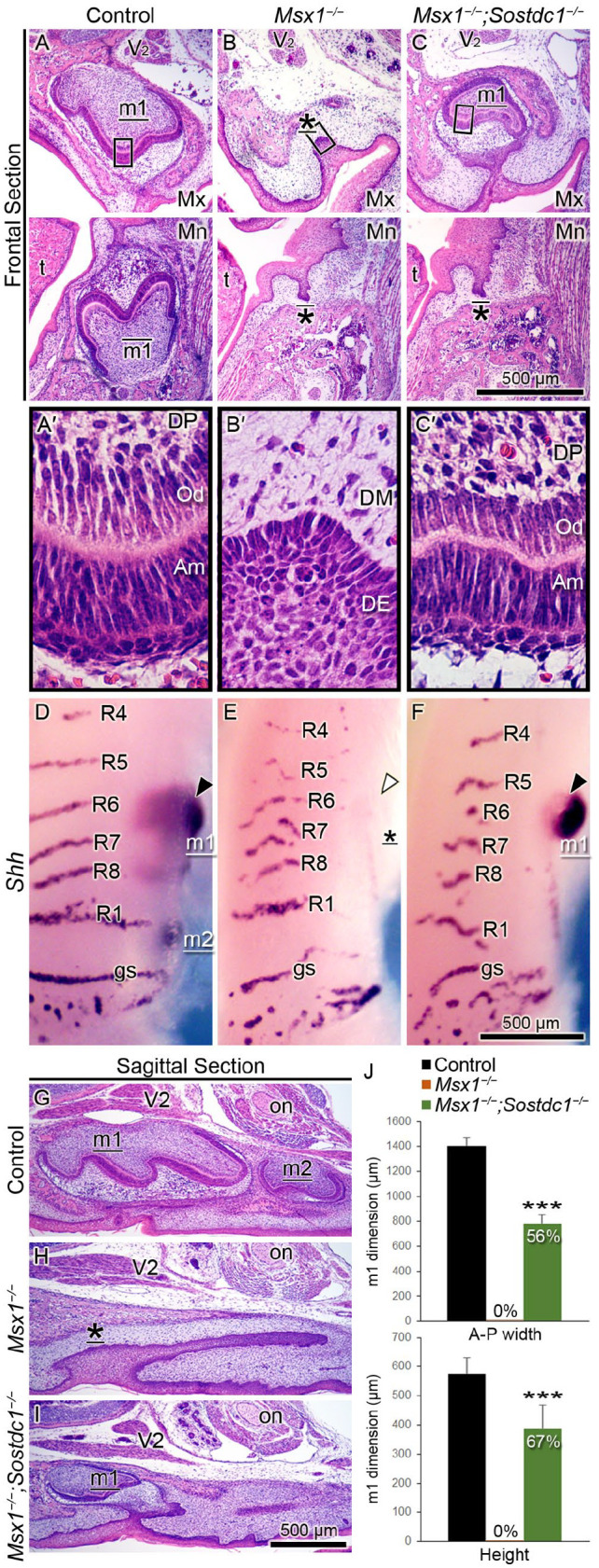
Sostdc1 deletion partially rescues tooth development in Msx1−/− maxillary first molars. (A–C; A′–C′) Hematoxylin and eosin–stained molar tooth germs are shown in frontal sections through the molar tooth germs at birth (P0). (A–C) White horizontal lines divide the maxilla (Mx) and mandible (Mn); the left side is palatal/lingual and the right side is buccal. (A′–C′) High-magnification images of corresponding boxed areas in panels A–C show the histodifferentiation of the ameloblasts (Am), odontoblasts (Od), and dental papilla (DP), as well as the underdifferentiated dental epithelium (DE) and dental mesenchyme (DM). (D–F) Whole-mount visualization of tooth germs in the Mx jaw at embryonic day 16.5 by analyzing Shh mRNA detection, where upper is anterior and lower is posterior. Black and white arrowheads mark Shh-positive and Shh-negative first molar tooth germs, respectively. (G–I) Hematoxylin and eosin–stained molar tooth germs at birth are shown in sagittal sections, where left is anterior and right is posterior. Landmarks used for identification of the tooth germs are the maxillary nerve (V2), which is the second branch of the trigeminal nerve, the fifth cranial nerve, and the optic nerve (on). Tooth germ is labeled m1 (first molar), m2 (second molar), or * (for developmentally arrested tooth bud). These labels are underlined (for Mx) or overlined (for Mn). t, tongue; R, palatal ruga; gs, “geschmacksstreifen” (taste stripes). Scale bars, 500 µm. n = 8 for each of panels A–C; n = 6 for each of panels D–F; n = 10 for each of panels G–I. (J) Measurements of the anteroposterior (A-P) width and height of the tooth germ, showing reduction by 44% in A-P width and by 33% in height. Results are expressed as mean ± SD. n = 10 for each group. Student t test. ***P ≤ 0.001.
Table.
Developmental Rescue of Msx1-Deficient Teeth and Palate by Deletion of Dkk2 and Sostdc1.
| Genotype | Maxilla Molar | Mandible Molar | Palate |
|---|---|---|---|
| Control a | 21/21 | 21/21 | 21/21 |
| Msx1 −/− | 0/7 | 0/7 | 0/7 |
| Msx1−/−;Dkk2+/+;Sostdc1−/−b | 9/9 | 0/9 | 0/9 |
| Msx1−/−;Dkk2−/−;Sostdc1+/+b | 5/6 | 1/6 | 0/6 |
| Msx1−/−;Dkk2+/−;Sostdc1−/− | 6/6 | 0/6 | 6/6 |
| Msx1−/−;Dkk2−/−;Sostdc1+/− | 3/3 | 3/3 | 3/3 |
| Msx1−/−;Dkk2−/−;Sostdc1−/− | 6/6 | 6/6 | 0/6 |
All mice were part of the same breeding scheme. Rescue was determined at E15.5 or later. Tooth rescue was defined as tooth germs that developed to the cap stage or beyond; palate rescue was defined as complete fusion of palatal shelves. All rescues were bilateral. Numbers represent mouse counts: numerator is the rescue count; denominator is the total count.
Control group includes wild type, Msx1+/−, Msx1+/−;Dkk2+/−, Msx1+/−;Sostdc1+/−, and Msx1+/−;Dkk2+/−;Sostdc1+/− genotypes.
For consistency, Msx1−/−;Sostdc1−/− and Msx1−/−;Dkk2−/− were indicated as Msx1−/−;Dkk2+/+;Sostdc1−/− and Msx1−/−;Dkk2−/−;Sostdc1+/+, respectively.
Forced activation of canonical Wnt signaling in the oral epithelium can induce supernumerary teeth in Msx1−/− mice, manifested as multiple ectopically positioned odontoma-like tissues that form without going through the normal “bud to cap to bell” morphogenesis (Wang et al. 2009). We assayed for various odontogenic molecular markers to verify whether the rescue of the Msx1−/−;Sostdc1−/− molar involved normal bud-to-cap morphogenesis. At early cap stage (E14.25), expression of PEK markers Shh, Fgf4, and Fgf3 was markedly downregulated in the maxillary and mandibular molars in the Msx1−/− mutants, and their expression was considerably restored in the Msx1−/−;Sostdc1−/− double mutants but only in the maxillary molars (Fig. 3A–I). Of note, Fgf3 expression in the dental mesenchyme showed similar marked downregulation in the maxillary and mandibular Msx1−/− molars and was rescued only in the maxillary molars in the Msx1−/−;Sostdc1−/− embryos (Fig. 3G–I). These results indicate that the Msx1−/−;Sostdc1−/− maxillary molar developed through bud-to-cap morphogenesis and PEK formation similar to control mice. To examine whether the rescue of maxillary molar morphogenesis involved recovery of Wnt signaling, we examined Lef1 expression at the bud-to-cap transition (at E14.0). When compared with the marked reduction of Lef1 signals in the Msx1−/− molar, where residual Lef1 was higher in the maxilla than in the mandible, Msx1−/−;Sostdc1−/− maxillary molars showed considerable restoration of Lef1 expression in the dental epithelium and mesenchyme (Fig. 3J–L). qRT-PCR analysis showed that as compared with the significantly downregulated Lef1 expression in the Msx1−/− molars in both jaws, Msx1−/−;Sostdc1−/− molars showed significant recovery (+48%) in the maxilla but not the mandible (Fig. 3P). When testing whether the rescue involved downregulation of ectopic Dkk2 expression, we found that ectopic Dkk2 signals in the Msx1−/− molar mesenchyme versus the control molars remained upregulated in the Msx1−/−;Sostdc1−/− molars, in the maxilla and mandible, which was confirmed with qRT-PCR. Taken together, our results indicate that although Msx1−/−Sostdc1−/− embryos exhibited increased expression of Dkk2 in the tooth mesenchyme similar to that of Msx1−/− embryos, the complete inactivation of Sostdc1 restored Wnt signaling activity to a sufficient level to drive tooth morphogenesis through the bud-to-cap transition in the Msx1−/−;Sostdc1−/− maxillary molar germs as compared with Msx1−/− maxillary molars.
Figure 3.
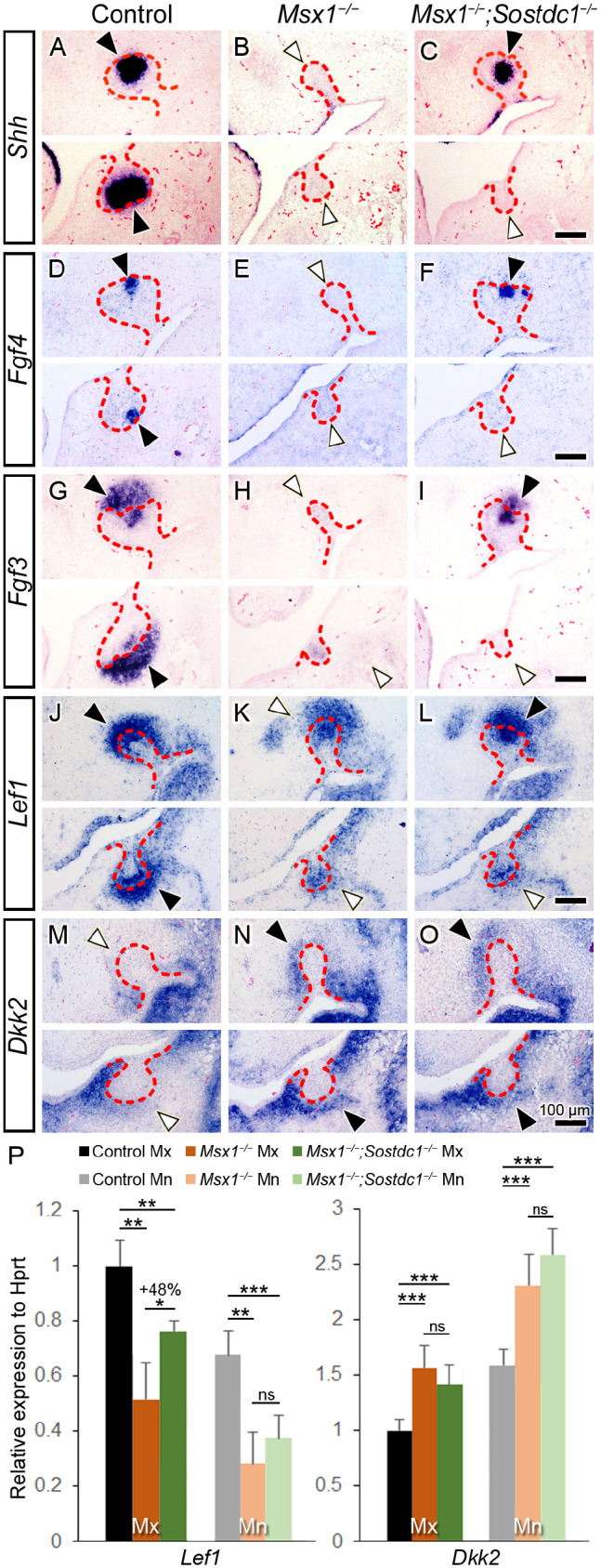
Sostdc1 deletion partially restores Wnt signaling and bud-to-cap transition in Msx1−/− maxillary molars. (A–O) Molecular marker assay in control, Msx1−/−, and Msx1−/−;Sostdc1−/− tooth development at around the bud-to-cap transition (embryonic day 13.75 [E13.75] to E14.25). Primary enamel knot markers Shh (A–C) and Fgf4 (D–F) and a marker for primary enamel knot and dental mesenchyme, Fgf3 (G–I), at the early cap stage (at E14.25). (J–L) Wnt signaling marker Lef1 at bud-to-cap transition (at E14.0). (M–O) Secreted Wnt inhibitor Dkk2 at the late bud stage (at E13.75). In each panel, a white horizontal line divides the maxillary (Mx) and mandibular (Mn) molars; the left side is palatal/lingual and the right side is buccal. The distal end of a tooth germ is marked with an arrowhead: black, stronger mRNA expression; white, weaker. The red dashed line marks the boundary between the dental epithelium and mesenchyme. Scale bars, 100 µm. n = 4 for panels A–O. (P) Real-time quantitative polymerase chain reaction of Lef1 and Dkk2 expression in Mx and Mn molar tooth germs at bud-to-cap transition (at E14.0). Results are expressed as fold change ± SD relative to control Mx. n = 3–5 for Lef1 expression; n = 4–6 for Dkk2 expression. Student t test. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. ns, not significant.
Deletion of Dkk2 Partially Rescues Tooth Development in Msx1-Deficient Maxillary Molars
Whereas our previous study showed that intraperitoneal injection of pregnant Msx1+/− females with the DKK inhibitor IIIC3a from gestational day 11 through 13 could not rescue tooth morphogenesis in Msx1−/− embryos, we recently obtained Dkk2−/− mice to directly examine whether Dkk2 is a key downstream target gene of the Msx1-mediated odontogenic pathway. At later stages when molar tooth development advanced to the early (E15.5) and late (E17.5) bell stages in control mice, the Msx1−/−;Dkk2−/− double mutants showed rescued maxillary molars at high penetrance (5 of 6 samples), with 1 of 6 samples also showing mandibular molar development to the cap stage but not as advanced as the bell-stage morphogenesis in the maxillary molars (Fig. 4A–D, B′; Table). We analyzed the molecular markers for PEK formation (Shh) and Wnt signaling activity (Lef1) at the bud-to-cap transition. Shh expression, which was present in the control and absent in the Msx1−/− molars in both jaws, was restored in the Msx1−/−;Dkk2−/− in the maxillary molar germs (Fig. 4E–G). Lef1 expression, which was strong in the control but markedly reduced in the Msx1−/− molars in both jaws, showed considerable recovery in the Msx1−/−;Dkk2−/− maxillary molar and a minor recovery in the mandibular molar (Fig. 4H–J). These results indicate that the significantly increased expression of Dkk2 in the Msx1−/− tooth mesenchyme (Fig. 3M, N, P; Appendix Fig. 1) is a major contributor to the bud stage developmental arrest of the Msx1−/− tooth germs.
Figure 4.
Compound deletion of Dkk2 and Sostdc1 rescues tooth development in Msx1−/− maxillary and mandibular molars. (A–D) Trichrome-stained mouse embryo heads from control and Msx1−/−;Dkk2−/− groups, shown in frontal sections through the molar tooth germs at embryonic days 15.5 (E15.5) and E17.5. (B, D) Deletion of Dkk2 partially rescues tooth development in the Msx1−/− maxillary molar. (B′) Inset in panel B is from a different embryo, showing a rare case of rescue in the mandibular molar. (E–J) Molecular marker assay in control, Msx1−/−, and Msx1−/−;Dkk2−/− tooth development. (E–G) Primary enamel knot (PEK) marker Shh at the early cap stage (at E14.25). (H–J) Wnt signaling marker Lef1 at bud-to-cap transition (at E14.0). Trichrome-stained frontal sections of embryo heads (K–N) and high magnification of their molar tooth germs (K′–N′) at E18.5. M, D, and S stand for Msx1, Dkk2, and Sostdc1. In each panel, the left side is palatal/lingual and the right side is buccal. In panels A–D and K–N, the black arrowhead indicates morphogenesis beyond the bud-to-cap transition, and the white arrow indicates bud-stage arrest. In panels E–J, the distal end of the tooth germ is marked with an arrowhead: black, stronger mRNA expression within the 3 genotypes; white, weaker. Red dashed lines mark the boundary between the dental epithelium and mesenchyme. A white horizontal line in panels E–J, L, N, and K′–N′ divides the maxilla and mandible, imaged from distinct sections from the same embryo. Asterisk in panels B, D, and N marks cleft palate. t, tongue. Scale bar, 250 µm. n = 5, 2, 3, 1 for panels A–D; n = 4 for panels E–G; n = 6 for panels H–J; n = 4, 2, 3, 2 for panels K–N. (O) Schematic diagram illustrating the deduced molecular regulatory network involving Msx1, Dkk2, Sostdc1, and Wnt signaling/Lef1 at the bud-to-cap transition.
Combined Inactivation of Dkk2 and Sostdc1 Rescues Development of Maxillary and Mandibular Molars in Msx1-Deficient Mice
Given that Sostdc1 and Dkk2 regulate canonical Wnt signaling in a similar fashion and that inactivation of either Sostdc1 or Dkk2 resulted in rescue of the development of the maxillary molar but not as effectively that of the mandibular molar in the Msx1−/− embryos, we next investigated whether combined inactivation of Dkk2 and Sostdc1 could rescue mandibular molar morphogenesis in the Msx1−/− embryos. Similar to the Msx1−/−;Sostdc1−/− mice, all Msx1−/−;Dkk2+/−;Sostdc1−/− mice showed full penetrance of rescue only in the maxillary molars. In contrast, Msx1−/−;Dkk2−/−;Sostdc1+/− and Msx1−/−;Dkk2−/−; Sostdc1−/− mice showed full penetrance of rescue of the maxillary and mandibular molars (Fig. 4K–N, K′–N′; Table). With our previous report showing significantly increased expression of Sfrp1 and Sfrp2, in addition to Dkk2, in the mandibular molar mesenchyme in Msx1−/− embryos (Jia et al. 2016), these data indicate that Msx1 drives tooth morphogenesis through the bud-to-cap transition primarily through regulating canonical Wnt signaling activity by controlling expression of the secreted Wnt antagonists. Of note, Msx1−/− mice exhibited complete penetrance of cleft palate and tooth bud arrest (Satokata and Maas 1994), and Msx1−/−;Dkk2+/−;Sostdc1−/− and Msx1−/−;Dkk2−/−;Sostdc1+/− exhibited successful palate fusion; however, all Msx1−/−;Dkk2−/−;Sostdc1−/− exhibited cleft palate without any substantial improvement, despite mice with each of these 3 compound mutant genotypes exhibiting rescued maxillary molars. This indicates that palate development and maxillary molar morphogenesis are independently regulated by Msx1 interaction with the Wnt signaling pathway.
Discussion
The current study advances our knowledge of the molecular mechanism underlying tooth development by demonstrating Dkk2 and Sostdc1 as effectors of Msx1 in early tooth morphogenesis. We provide for the first time genetic evidence that Msx1 promotes Wnt signaling to drive tooth morphogenesis by suppressing Dkk2 expression in the dental mesenchyme, demonstrated by the ectopic Dkk2 expression in the Msx1−/− tooth bud mesenchyme and the developmental rescue of Msx1−/−;Dkk2−/− molar teeth (Fig. 4O). In addition, this study identifies a previously unknown role of Sostdc1 in the tooth developmental arrest in Msx1−/− mice. In contrast to Dkk2 and Sfrp2, whose expression was significantly increased in the molar tooth mesenchyme in Msx1−/− embryos (Jia et al. 2016), we found that expression of Sostdc1 was moderately decreased in the Msx1−/− molar germs, as compared with control embryos. Previous studies have shown that BMP4 induced Sostdc1 expression in cultured tooth bud explants (Laurikkala et al. 2003) and that Bmp4 expression was reduced in the Msx1−/− molar mesenchyme (Chen et al. 1996; Jia et al. 2013). Thus, the moderate decrease in Sostdc1 expression in Msx1−/− molar germs was likely a secondary consequence of reduced Bmp4 activity.
Sostdc1 was initially proposed to function as an inhibitor of Bmp signaling in tooth development (Laurikkala et al. 2003). However, extensive genetic interaction assays with various signaling pathway components, including Lrp5/6 (Wnt), Bmpr1a (Bmp), and Fgf10 or Fgfr1/2 (Fgf), indicate that Sostdc1 regulates tooth development primarily through antagonizing Lrp5/6-mediated canonical Wnt signaling (Ahn et al. 2010). The complementary expression patterns of Sostdc1 and Lef1 during normal tooth morphogenesis (Fig. 1) and the recovery of Lef1 expression in the molar germs in the Msx1−/−;Sostdc1−/− and Msx1−/−;Dkk2−/− mouse embryos (Figs. 3 and 4) indicate that the rescue of tooth development in these mice are mediated through recovery of Wnt signaling activity. Although expression of Sostdc1 expression was moderately decreased whereas that of Dkk2 was significantly increased in the Msx1−/− molar germs, genetic inactivation of Sostdc1 or Dkk2 similarly rescued maxillary molar morphogenesis in the Msx1−/− mice. We previously showed that expression of Sfrp2 was also significantly increased in the Msx1−/− molar mesenchyme and that genetic inactivation of Sfrp2 and Sfrp3 combined with treatment with the DKK inhibitor IIIC3a was able to partly rescue maxillary molar development (Jia et al. 2016). Together, these results indicate that the bud-stage tooth development arrest in the Msx1−/− embryos resulted from the combined inhibitory effects on Wnt signaling activity by several Wnt antagonists, including Dkk2, Sfrp2, and Sostdc1, and that Msx1 controls tooth development through the bud-to-cap transition primarily through regulating expression of these genes encoding secreted Wnt antagonists.
Tooth agenesis and orofacial clefts are two of the most common craniofacial birth defects that occasionally co-occur in patients as well as in animal models (Phan et al. 2016). While cleft palate may result from primary defects in the developing palatal shelves or secondary effects of structural abnormality in surrounding structures (Bush and Jiang 2012), Msx1 plays critical primary roles in regulating palate and tooth development (Zhang et al. 2002). We observed that Msx1−/−;Dkk2+/−; Sostdc1−/− and Msx1−/−;Dkk2−/−;Sostdc1+/− mice exhibited fused secondary palate, in contrast to the full penetrance of cleft palate in Msx1−/− mice. While this study has not specifically investigated the relationship between Msx1 and Dkk2/Sostdc1 in palate development, the rescue of palate morphogenesis in Msx1−/−;Dkk2+/−;Sostdc1−/− and Msx1−/−;Dkk2−/−; Sostdc1+/− mice suggests that the combined inactivation of Dkk2 and Sostdc1 in the developing palate also modulates Msx1-dependent developmental processes during palate morphogenesis. Although Dkk2−/− or Sostdc1−/− mutant mice do not show cleft palate, recent studies have implicated misregulation of Dkk2 and combined activities of Dkk2 and Sostdc1 in contributing to cleft palate in the Pax9−/− mutant mice through inhibition of Wnt signaling (Jia et al. 2017; Li et al. 2017).
Remarkably, our study reveals that the molecular mechanisms underlying tooth development are not identical between the maxillary and mandibular molars. An asymmetrical tooth rescue between jaws in the Msx1−/−;Sostdc1−/− and Msx1−/−; Dkk2−/− mice was similarly observed in our previous rescue study in the Msx1−/−Sfrp2−/−Sfrp3−/− mouse treated with IIIC3a. This may be explained, at least partly, through the difference in the intrinsic level of certain tooth developmental antagonists, such as Dkk2, Osr2, Sfrp1/2, and Wif1, between the maxillary and mandibular molars, which may set a different molecular environment for tooth development, possibly leading to a different susceptibility to certain gene mutations between the jaws (Jia et al. 2013; Kwon et al. 2017). From a clinical perspective, this study may provide a key understanding of why certain teeth in humans, such as the permanent second premolars or maxillary lateral incisors, are more susceptible than other teeth to genetic mutations and show a higher prevalence of tooth agenesis (Fournier et al. 2018). It would be meaningful to dissect the molecular landscape of different teeth within the same organism to gain insights into the site-specific developmental programs employed in organogenesis. Our rescue studies also identify secreted Wnt antagonists as potential therapeutic targets for preventing or correcting congenital dental anomalies. The recent development of a monoclonal antibody therapy to treat osteoporosis by targeting sclerostin (SOST), a paralog of SOSTDC1 that similarly antagonizes canonical Wnt signaling through Lrp5/6 disruption (MacNabb et al. 2016), sets a good example of how our research can provide the groundwork for developing new strategies for resolving birth defects and for regenerative therapies.
Author Contributions
J.-M. Lee, C. Qin, R. Jiang, H.-J.E. Kwon, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; O.H. Chai, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; Y. Lan, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211070583 for MSX1 Drives Tooth Morphogenesis Through Controlling Wnt Signaling Activity by J.-M. Lee, C. Qin, O.H. Chai, Y. Lan, R. Jiang and H.-J.E. Kwon in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Dental and Craniofacial Research (2T32DE023526 to J.-M. Lee, R01DE018401 and R01DE027046 to R. Jiang, 1R03DE030985 to H.-J.E. Kwon) and the National Center for Advancing Translational Sciences (5KL2TR0013-05 to H.-J.E. Kwon) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: R. Jiang  https://orcid.org/0000-0001-7842-4696
https://orcid.org/0000-0001-7842-4696
H.-J.E. Kwon  https://orcid.org/0000-0001-6538-6045
https://orcid.org/0000-0001-6538-6045
References
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. 2010. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 137(19):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei M, Kratochwil K, Maas RL. 2000. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 127(21):4711–4718. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. 2012. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 139(2):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. 2009. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 334(1):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. 1996. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 122(10):3035–3044. [DOI] [PubMed] [Google Scholar]
- Fournier BP, Bruneau MH, Toupenay S, Kerner S, Berdal A, Cormier-Daire V, Hadj-Rabia S, Coudert AE, de La Dure-Molla M. 2018. Patterns of dental agenesis highlight the nature of the causative mutated genes. J Dent Res. 97(12):1306–1316. [DOI] [PubMed] [Google Scholar]
- Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. 2006. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc Natl Acad Sci U S A. 103(49):18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Kwon HE, Lan Y, Zhou J, Liu H, Jiang R. 2016. Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Dev Biol. 420(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Zhou J, Fanelli C, Wee Y, Bonds J, Schneider P, Mues G, D’Souza RN. 2017. Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero. Development. 144(20):3819–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Zhou J, Gao Y, Baek JA, Martin JF, Lan Y, Jiang R. 2013. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development. 140(2):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. 1996. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 10(11):1382–1394. [DOI] [PubMed] [Google Scholar]
- Kwon HE, Jia S, Lan Y, Liu H, Jiang R. 2017. Activin and Bmp4 signaling converge on Wnt activation during odontogenesis. J Dent Res. 96(10):1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HE, Jiang R. 2018. Development of teeth. In: Carlson BM, editor. Reference module in biomedical sciences. Amsterdam (Netherlands): Elsevier. doi: 10.1016/B978-0-12-801238-3.64113-2 [DOI] [Google Scholar]
- Lan Y, Jia S, Jiang R. 2014. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 25–26:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjärvi L, Thesleff I, Itoh N. 2003. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 264(1):91–105. [DOI] [PubMed] [Google Scholar]
- Li C, Lan Y, Krumlauf R, Jiang R. 2017. Modulating Wnt signaling rescues palate morphogenesis in Pax9 mutant mice. J Dent Res. 96(11):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, et al. 2005. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 37(9):945–952. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. 2008. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 313(1):210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNabb C, Patton D, Hayes JS. 2016. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J Osteoporos. 2016:6217286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Clevers H. 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 169(6):985–999. [DOI] [PubMed] [Google Scholar]
- O’Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, Koo S, Kamiya N, Ingber DE, Park PJ, et al. 2012. A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal. 5(206):ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan M, Conte F, Khandelwal KD, Ockeloen CW, Bartzela T, Kleefstra T, van Bokhoven H, Rubini M, Zhou H, Carels CE. 2016. Tooth agenesis and orofacial clefting: genetic brothers in arms? Hum Genet. 135(12):1299–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Xu X, Han J, Bringas P, Jr, Maeda T, Slavkin HC, Grosschedl R, Chai Y. 2005. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 278(1):130–143. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. 1994. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 6(4):348–356. [DOI] [PubMed] [Google Scholar]
- Tan H, Peres KG, Peres MA. 2016. Retention of teeth and oral health-related quality of life. J Dent Res. 95(12):1350–1357. [DOI] [PubMed] [Google Scholar]
- Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, et al. 2009. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 136(11):1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. 2002. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 129(17):4135–4146. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, Hu Y, Fromm SH, Chen Y. 2000. Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech Dev. 99(1–2):29–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211070583 for MSX1 Drives Tooth Morphogenesis Through Controlling Wnt Signaling Activity by J.-M. Lee, C. Qin, O.H. Chai, Y. Lan, R. Jiang and H.-J.E. Kwon in Journal of Dental Research