Abstract
Background and Aim
Environmental factors play a key role in development of Crohn's disease (CD), thought to be mediated by changes in the gut microbiota. We aimed to delineate the potential contribution of antibiotic exposure to subsequent development of CD, across diverse geographical populations.
Methods
This case–control study in Australia and three cities in China (Hong Kong, Guangzhou, and Kunming) included four groups: patients with CD, at‐risk individuals including non‐affected first‐degree relatives (FDRs) and household members of CD patients (HM), and unrelated healthy controls (HCs). Environmental risk factors, including childhood antibiotic use and 13 other categories, were assessed using a self‐developed questionnaire. Logistic regression and conditional logistic regression were used to determine environmental factors associated with CD development.
Results
From 2017 to 2019, a total of 254 patients with CD (mean age: 37.98 ± 13.76 years; 58.3% male), 73 FDR (mean age: 49.35 ± 13.28 years; 46.6% male), 122 HMs (including FDR) (mean age: 45.50 ± 13.25 years; 47.5% male), and 78 HC (mean age: 45.57 ± 11.24; 47.4% male) were included. Comparing CD patients with their FDR and HMs, antibiotic use before 18 years old was a risk factor for CD development (adjusted odds ratio [OR] 3.46, 95% confidence interval [CI] 1.38–8.69; P = 0.008). There were no significant differences in other childhood environmental risk factors between CD and their FDR or HMs. Subgroup analysis showed that antibiotic use <18 years old was a risk factor for CD development in the Chinese (adjusted OR 4.80, 95% CI 1.62–12.24; P = 0.005) but not in Australian populations (OR 1.80, 95% CI 0.33–9.95; P = 0.498).
Conclusion
Use of antibiotics <18 years was a risk factor for CD development. Attention should be paid to identifying modifiable environmental risk factors in early childhood, especially in at‐risk families.
Keywords: antibiotics, Crohn's, environmental risk factors
Environmental factors play a key role in development of Crohn's disease (CD). Comparing CD patients with their first‐degree relatives and household members, antibiotic use before 18 years old was a risk factor for CD development (adjusted odds ratio 3.46, 95% confidence interval 1.38–8.69). Attention should be paid to identifying modifiable environmental risk factors in early childhood, especially in at‐risk families.

Introduction
There is a rapid increase in incidence of inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), in newly industrialized countries at the turn of the 21st century. 1 A recent Asia‐Pacific population‐based study in eight Asian countries and Australia demonstrated that Hong Kong and Mainland China had among the highest disease incidence in Asia, while Australia has the equal highest incidence of these diseases in the world. 2 The incidence of IBD in Hong Kong has risen by three‐fold in the past decade. 3 , 4 While there has been a greater awareness of IBD globally, the marked increase in incidence of IBD in newly industrialized countries is likely to relate to environmental exposure, in particular to exposure in early life.
IBD is thought to result from both genetic and environmental factors influencing the gut microbiota to produce an immune response. 5 , 6 So far, positive family history is the strongest identifiable risk factor for IBD development. 7 First‐degree relatives (FDRs) of patients with IBD have a 3–20‐fold increased likelihood of developing the disease than the general population. 8 , 9 Siblings of patients with CD have 35 times increased relative risk of developing CD than the background population. 9 However, familial aggregation is less prevalent in population of emerging disease incidence including Asia, suggesting that environmental factors likely contribute to the change in epidemiologic trends.
Among all factors studied, antibiotic use has been shown to affect the gut microbiome leading to inflammation in the gut. Studies in the West have shown that antibiotic exposure during childhood was associated with an increased risk of subsequent development of CD, 10 , 11 , 12 while the protective effect of antibiotic use was reported for Asians in the Asia‐Pacific Crohn's and Colitis Epidemiology Study (ACCESS) cohort and Middle Eastern migrants in Australia. 2 , 13 A recent systematic review identified that smoking, urban living, appendectomy, tonsillectomy, and antibiotic exposure increased the risk of CD, while physical activity, breastfeeding, and bed sharing were associated with a reduced risk of developing CD. 14
Genetic predisposition plus sharing of putative environmental factors could possibly explain the familial aggregation of IBD. 15 , 16 Genome‐wide association studies have identified more than 200 alleles associated with CD. However, only 8.2–13.1% of heritability was explained by genetic variation, suggesting that other shared environmental and/or epigenetic factors might be involved. 17 , 18 It is therefore important to study the possible environmental factors associated with CD development in both CD patients, their unaffected FDRs, and close household members (HMs), with a view to modifying environmental risk factors that predate CD development.
In this study, we aimed to define the contribution of environmental factors to the increased CD susceptibility in populations at risk for CD (FDRs and HMs), in both Chinese and Caucasian populations by comparing childhood exposures between CD‐affected individuals, FDR, HMs, and healthy controls (HCs).
Methods
Study design and participants
The ENIGMA (Eastern Inflammatory Bowel Disease Gut Microbiota) study consortium comprises IBD specialist centers in Hong Kong (developed economy China), Guangzhou (urban China), Kunming (rural China), and Australia. This case–control, observational study recruited four participant groups: patients with CD, FDRs of patients with CD, HMs of patients with CD, and unrelated HCs (referred to hereafter as HCs). Inclusion criteria included: subjects aged ≥18 years old; competent to provide informed consent, and have lived in the same geographic area for the preceding 6 months. Exclusion criteria included the use of prebiotics, probiotics, or antibiotics in the last 3 months, use of laxatives or anti‐diarrheal drugs in the last 3 months; recent dietary change (e.g. becoming vegetarian/vegan), known current complex infections or sepsis (excluding uncomplicated infections such as influenza), known history of severe organ failure, bowel surgery in the last 6 months (excluding colonoscopy), presence of an ileostomy/stoma, current pregnancy; known contraindications to colonoscopy; and colonoscopy in the last month before sampling.
Recruitment and setting
The study took place across Australia, Hong Kong, Guangzhou, and Kunming, between October 2017 and December 2019. Subjects were recruited cases from a weekly IBD out‐patient clinic at a major metropolitan hospital in Melbourne, Australia, a major acute hospital in Hong Kong, and two regional hospitals from Mainland China (Guangzhou [GZ] and Kunming [KM]). Study investigators identified eligible participants, explained the study, answered participant questions, obtained written consent forms, and booked participants' research visit (see study visits). If FDRs or HMs had attended the IBD clinic, we recruited them in the same manner described for cases. Alternatively, we asked cases to provide contact details of eligible FDRs and/or HMs and invited them to participate via phone. Unrelated HCs were recruited from general gastroenterology clinics via phone or in person in Australia, via mass media in Hong Kong and from visitors visiting regional hospitals attending health talks in regional hospitals in Guangzhou and Kunming.
Study visits
Researchers met with participants and collected demographic and clinical information via interview and review of electronic medical records. Experienced researchers assessed questionnaire data for clarity and completeness.
Measurement tools
Demographic and clinical information were collected using a custom‐built form. For all participants, age, weight, gender, smoking status (smoker, ex‐smoker, non‐smoker), family history of IBD, appendectomy history, co‐morbid illnesses, proton pump inhibitor use, food supplements including prebiotics, probiotics, or antibiotics within 3 months of recruitment were collected.
For CD cases, the following additional information was collected: disease duration, severity and extent of disease (characterized according to the Montreal classification), dose and duration of current medications, self‐reported drug compliance, and disease activity based on the CD activity index (CDAI).
Environmental factors were collected using a self‐developed questionnaire. Since there was no validated environmental questionnaire to be used in both China and Australia, we developed a questionnaire, the ENIGMA Environmental Questionnaire (EEQ) based on the Asian Environmental Questionnaire (AEQ), which was derived from the International Organization for study of Inflammatory Bowel Disease (IOIBD). Although the questionnaire for IOIBD and AEQ were not formally validated, they have been previously used in other studies of IBD cohorts. 2 , 19 , 20 , 21 In this study, we developed a cross‐culturally valid and linguistically comparable environmental questionnaire in Chinese and English for ENIGMA study. It was used in geographically different populations including Hong Kong Chinese, Mainland Chinese, and Australians. Three versions of EEQ were developed: the English version, the Chinese (Hong Kong) and Chinese (Mainland) version.
The original AEQ was demonstrated to be valid for use in Hong Kong Chinese population. In Australia, a small number of existing items were removed or modified and new items were added for the different culture. In the EEQ, demographic and dietary components in the original AEQ were removed as these would be assessed separately in the ENIGMA study. Six steps were undertaken in the questionnaire development: (i) new item generation through literature review; (ii) linguistic validation from Chinese (Hong Kong) to English using forward and backward translation; (iii) cultural adaptation with the Australia team; (iv) translation of modified items from English to Chinese (Hong Kong); (v) combining modified items with other questions in Chinese (Hong Kong); (vi) pilot testing and cognitive interview; and (vii) revision, harmonization, and finalization. Regarding translation involved in the development process, the English and Chinese (Hong Kong) versions were translated first through a vigorous process of linguistic validation. 19 , 22 After the Chinese (Hong Kong) version was produced, the questionnaire was then sent to the study sites in Mainland China (Guangzhou and Kunming) for translation and cultural adaptation. Questions in the EEQ were related to different areas: (i) ethnicity and the country and city of birth; (ii) family history of IBD; (iii) method of delivery and breastfed history; (iv) education and job; (v) air pollutants in working and living area; (vi) environmental conditions since birth till currently, including antibiotic use, living areas, water source, hot water supply, toilet conditions, keeping animals, self‐smoking, family smoking, and migration. The conditions would be answered in each of the age category: 0–12 months, >12 months–5 years, >5–10 years, >10–18 years, and at the time of filling in the questionnaire (Appendix EEQ).
Statistical analysis
All collected clinical information and data were entered into an electronic Case Report Form (eCRF) specifically designed for this study, and stored in a web‐based database, the Research Electronic Data Capture (REDCap) database. REDCap is a secure, HIPPA‐compliant web application for building and managing eCRF, questionnaires, and databases. Continuous variables were presented as mean ± SD, while categorical data were presented as number (percentage). Table S1, Supporting information outlines the group comparisons that were made. A paired comparison between CD participants and their FDRs or HMs was made using McNenmars test for categorical variables and Wilcoxon test (differences between groups symmetrical) or Sign test (differences between groups asymmetrical). An unpaired comparison between CD participants and HCs was made using χ 2 test for categorical variables or Mann–Whitney U test for continuous variables. Logistic regression (unmatched comparisons) and conditional logistic regression (matched comparisons) were conducted to identify environmental factors associated with CD development, as appropriate. All variables with P value <0.1 in univariate analyses and factors including age and gender were included in the multivariable logistic regression models. All regression analyses were stratified by country on the basis that multiple differences exist between countries. All statistical tests were two‐sided. Statistical significance was taken as P < 0.05. Statistical analyses were conducted in IBM SPSS version 25 software (IBM Corp., Armonk, NY, USA). This study was approved by the ethics committee of each individual hospital that participated.
Results
From 2017 to 2019, a total of 254 CD patients (100 in Australia, 98 in Hong Kong, 56 from Mainland China: GZ and KM), 73 FDRs (28 in Australia, 25 in Hong Kong, and 20 in Mainland China: GZ and KM), 122 HMs of CD patients (including FDR) (50 in Australia, 38 in Hong Kong and 34 in Mainland China: GZ and KM), and 78 unrelated HCs (20 in Australia, 22 in Hong Kong and 36 in Mainland China: GZ and KM) were enrolled into the study. Mean age at recruitment was 38.0 ± 13.8 years, 49.4 ± 13.3, 45.6 ± 13.3, and 45.6 ± 11.2 years for CD patients, FDR, HMs, and HCs, respectively. There was no difference in gender distribution between CD, FDR, and HMs and HCs. The majority of the FDR were either parents (30.1%) or siblings (38.4%) of the CD patients. All recruited subjects completed the EEQ environmental questionnaire. Table 1 shows the baseline characteristics of the participants.
Table 1.
Baseline characteristics and environmental factors of all subjects in the Australian and Chinese cohorts
| Australia | China (Hong Kong, Guangzhou, and Kunming) | |||||||
|---|---|---|---|---|---|---|---|---|
| Crohn's disease (n = 100) | Household members (including FDR) (n = 50) | FDR (n = 28) | Healthy controls (n = 20) | Crohn's disease (n = 154) | Household members (including FDR) (n = 72) | FDR (n = 45) | Healthy controls (n = 58) | |
| Age (mean ± SD) | 38.57 ± 14.08 | 45.74 ± 13.51 | 50.86 ± 13.20 | 52.30 ± 13.91 | 37.59 ± 13.57 | 45.34 ± 13.17 | 48.41 ± 13.39 | 43.25 ± 9.21 |
| Weight in kg (mean ± SD) | 72.91 ± 16.63 | 81.00 ± 18.40 | 74.81 ± 12.39 | 78.36 ± 18.35 | 67.10 ± 25.64 | 77.10 ± 32.67 | 80.52 ± 34.56 | 75.74 ± 34.75 |
| Height in cm (mean ± SD) | 170.85 ± 10.65 | 170.51 ± 9.42 | 169.07 ± 6.88 | 170.60 ± 11.49 | 165.22 ± 18.86 | 163.92 ± 7.39 | 163.74 ± 8.09 | 163.43 ± 23.74 |
| Gender (male) n, % | 48, 48.48 | 25, 50.00 | 9, 32.14 | 8, 40.00 | 99, 64.71 | 33, 45.83 | 25, 55.56 | 29, 50.00 |
| Smoking n, % | 39, 39.00 | 19, 38.00 | 6, 21.43 | 8, 40.00 | 37, 24.18 | 18, 25.00 | 14, 31.11 | 17, 29.31 |
| Alcohol n, % | 92, 92.00 | 43, 86.00 | 24, 85.71 | 17, 85.00 | 59, 38.82 | 26, 36.11 | 16, 35.56 | 25, 43.10 |
| Family history of IBD n, % | 34, 34.00 | 15, 30.00 | 23, 85.19 | 2, 10.00 | 8, 5.19 | 53, 73.61 | 38, 84.44 | 0, 0 |
| History of bowel resection beyond 6 months n, % | 35, 35.00 | 1, 2.00 | 1, 3.57 | 0, 0 | 14, 9.09 | 0, 0 | 0, 0 | 0, 0 |
| History of malignancy within 5 years n, % | 3, 3.00 | 5, 10.00 | 2, 7.14 | 1, 5.00 | 1, 0.65 | 0, 0 | 0, 0 | 0, 0 |
| Use of PPI/H2RB/antacids n, % | 11, 11.00 | 3, 6.00 | 3, 10.71 | 3 15.00 | 21, 14.09 | 7, 10.00 | 5, 11.63 | 2, 3.57 |
| Delivered by Caesarean section n, % | 14, 14.14 | 3, 6.12 | 2, 7.14 | 2, 11.10 | 16, 10.96 | 2, 2.86 | 2, 4.76 | 4, 6.90 |
| Breastfed when being a baby n, % | 80, 81.63 | 37, 78.72 | 20, 74.07 | 17, 85.00 | 85, 60.28 | 56, 83.58 | 34, 85.00 | 44, 77.19 |
| Breastfed duration when being a baby (among being breastfed at baby stage), months (mean ± SD) | 8.60 ± 6.63 | 7.85 ± 4.79 | 8.56 ± 5.64 | 6.62 ± 3.40 | 10.89 ± 5.93 | 11.91 ± 6.59 | 10.78 ± 3.91 | 10.32 ± 4.21 |
| Education with University level n, % | 64, 64.00 | 32, 64.00 | 19, 67.86 | 15, 75.00 | 88, 58.28 | 31, 43.06 | 16, 35.56 | 26, 44.83 |
| Living or working near construction area n, % | 16, 16.00 | 5, 10.00 | 3, 10.71 | 5, 25.00 | 64, 42.38 | 31, 43.06 | 21, 46.67 | 33, 56.90 |
| Living or working near mining n, % | 3, 3.00 | 1, 2.00 | 0, 0 | 1, 5.00 | 2, 1.32 | 2, 2.78 | 1, 2.22 | 2, 3.45 |
| Living or working near furnaces n, % | 0, 0 | 1, 2.00 | 0, 0 | 0, 0 | 1, 0.66 | 0, 0 | 0, 0 | 0, 0 |
| Living or working near factories n, % | 4, 4.00 | 3, 6.00 | 2, 7.14 | 1, 5.00 | 21, 13.91 | 9, 12.50 | 2, 4.44 | 3, 5.17 |
| Living or working near busy highways n, % | 26, 26.00 | 18, 36.00 | 9, 32.14 | 5, 25.00 | 33, 21.85 | 10, 13.89 | 4, 8.89 | 10, 17.24 |
| Burn coal as fuel n, % | 0, 0 | 1, 2.00 | 0, 0 | 0, 0 | 7, 4.64 | 3, 4.17 | 3, 6.67 | 3, 5.17 |
| >50% Smog occur in living area in pass 6 months n, % | 12, 12.12 | 7, 14.29 | 1, 3.70 | 4, 20.00 | 12, 8.05 | 7, 9.86 | 3, 6.98 | 6, 10.34 |
| Antibiotics use n, % | ||||||||
| 0–12 months | 23, 23.23 | 10, 20.00 | 8, 28.57 | 3, 15.00 | 30, 20.27 | 8, 11.27 | 3, 6.82 | 15, 25.86 |
| >12 months–5 years | 59, 59.00 | 30, 60.00 | 17, 60.71 | 9, 45.00 | 66, 44.59 | 18, 25.00 | 11, 24.44 | 27, 46.55 |
| >5–10 years | 69, 69.00 | 33, 66.00 | 19, 67.86 | 15, 75.00 | 80, 52.98 | 26, 36.11 | 16, 35.56 | 35, 60.34 |
| >10–18 years | 81, 81.00 | 44, 88.00 | 23, 82.14 | 14, 70.00 | 113, 74.83 | 39, 54.17 | 26, 57.78 | 43, 74.14 |
| Currently | 81, 81.00 | 39, 78.00 | 25, 89.29 | 16, 80.00 | 76, 50.33 | 24, 33.33 | 11, 24.44 | 20, 34.48 |
| Before 18 years | 88, 88.00 | 46, 92.00 | 24, 85.71 | 16, 80.00 | 125, 82.78 | 44, 61.11 | 29, 64.44 | 47, 81.03 |
| Before 10 years | 75, 75.00 | 38, 76.00 | 21, 75.00 | 15, 75.00 | 88, 58.28 | 30, 41.67 | 19, 42.22 | 35, 60.34 |
| Living in city n, % | ||||||||
| 0–12 months | 69, 69.00 | 33, 66.00 | 24, 85.71 | 13, 65.00 | 108, 71.52 | 37, 51.39 | 22, 48.89 | 31, 54.39 |
| >12 months–5 years | 70, 70.00 | 30, 60.00 | 23, 82.14 | 12, 60.00 | 108, 71.52 | 39, 54.17 | 24, 53.33 | 32, 55.17 |
| >5–10 years | 71, 71.00 | 30, 60.00 | 22, 78.57 | 12, 60.00 | 112, 74.17 | 43, 59.72 | 25, 55.56 | 31, 54.39 |
| >10–18 years | 72, 72.00 | 35, 70.00 | 22, 78.57 | 13, 65.00 | 123, 81.46 | 56, 78.87 | 33, 75.00 | 44, 75.86 |
| Currently | 77, 77.00 | 37, 74.00 | 25, 89.29 | 15, 75.00 | 135, 90.00 | 62, 86.11 | 38, 84.44 | 51, 87.93 |
| Before 18 years | 77, 77.00 | 37, 74.00 | 25, 89.29 | 14, 70.00 | 126, 83.44 | 56, 77.78 | 33, 73.33 | 44, 75.86 |
| Before 10 years | 74, 74.00 | 33, 66.00 | 24, 85.71 | 13, 65.00 | 114, 75.50 | 45, 62.50 | 26, 57.78 | 32, 55.17 |
| Running water at home n, % | ||||||||
| 0–12 months | 96, 96.00 | 47, 94.00 | 28, 100 | 20, 100 | 107, 71.33 | 39, 54.17 | 19, 42.22 | 31, 53.45 |
| >12 months–5 years | 98, 98.00 | 48, 96.00 | 28, 100 | 20, 100 | 114, 75.50 | 45, 62.50 | 24, 53.33 | 32, 55.17 |
| >5–10 years | 99, 99.00 | 49, 98.00 | 28, 100 | 20, 100 | 121, 80.13 | 49, 68.06 | 28, 62.22 | 34, 58.62 |
| >10–18 years | 100, 100 | 49, 98.00 | 27, 96.43 | 20, 100 | 130, 86.09 | 63, 87.50 | 38, 84.44 | 44, 75.86 |
| Currently | 100, 100 | 50, 100 | 28, 100 | 20, 100 | 148, 98.01 | 67, 95.71 | 42, 97.67 | 58, 100.00 |
| Before 18 years | 100, 100 | 49, 98.00 | 28, 100 | 20, 100 | 130, 86.09 | 64, 88.89 | 38, 84.44 | 45, 77.59 |
| Before 10 years | 100, 100 | 49, 98.00 | 28, 100 | 20, 100 | 121, 80.13 | 51, 70.83 | 29, 64.44 | 35, 60.34 |
| Hot water from tap n, % | ||||||||
| 0–12 months | 92, 92.00 | 45, 90.00 | 24, 85.71 | 24, 95.00 | 66, 43.71 | 18, 25.00 | 8, 17.78 | 8, 13.79 |
| >12 months–5 years | 93, 93.00 | 46, 92.00 | 24, 85.71 | 24, 95.00 | 70, 46.36 | 19, 26.39 | 9, 20.00 | 9, 15.52 |
| >5–10 years | 93, 93.00 | 47, 94.00 | 24, 85.71 | 24, 95.00 | 78, 51.66 | 22, 30.56 | 11, 24.44 | 10, 17.24 |
| >10–18 years | 97, 97.00 | 48, 96.00 | 25, 89.29 | 20, 100 | 97, 64.24 | 33, 45.83 | 18, 40.00 | 23, 40.35 |
| Currently | 99, 99.00 | 50, 100 | 27, 96.43 | 20, 100 | 120, 79.47 | 59, 81.94 | 38, 84.44 | 41, 70.69 |
| Before 18 years | 97, 97.00 | 48, 96.00 | 25, 89.29 | 20, 100 | 97, 64.24 | 35, 48.61 | 19, 42.22 | 23, 39.66 |
| Before 10 years | 94, 94.00 | 47, 94.00 | 24, 85.71 | 24, 95.00 | 78, 51.66 | 23, 31.94 | 11, 24.44 | 10, 17.24 |
| Flushing toilet at home n, % | ||||||||
| 0–12 months | 93, 93.00 | 44, 88.00 | 23, 82.14 | 16, 80.00 | 64, 42.38 | 17, 23.61 | 9, 20.00 | 17, 29.31 |
| >12 months–5 years | 93, 93.00 | 45, 90.00 | 23, 82.14 | 16, 80.00 | 72, 47.68 | 20, 27.78 | 11, 24.44 | 18, 31.03 |
| >5–10 years | 97, 97.00 | 47, 94.00 | 26, 92.86 | 18, 90.00 | 85, 56.29 | 28, 38.89 | 14, 31.11 | 21, 36.21 |
| >10–18 years | 100, 100 | 49, 98.00 | 26, 92.86 | 20, 100 | 103, 68.21 | 36, 50.00 | 19, 42.22 | 22, 37.93 |
| Currently | 100, 100 | 50, 100 | 28, 100 | 20, 100 | 121, 80.13 | 50, 69.44 | 31, 68.89 | 31, 53.45 |
| Before 18 years | 100, 100 | 49, 98.00 | 27, 96.43 | 20, 100 | 104, 68.87 | 36, 50.00 | 19, 42.22 | 23, 39.66 |
| Before 10 years | 98, 98.00 | 47, 94.00 | 26, 92.86 | 18, 90.00 | 86, 56.95 | 28, 38.89 | 14, 31.11 | 21, 36.21 |
| Keep animal at home n, % | ||||||||
| 0–12 months | 51, 51.00 | 30, 60.00 | 15, 53.57 | 11, 55.00 | 36, 24.16 | 24, 33.33 | 14, 31.11 | 24, 41.38 |
| >12 months–5 years | 61, 61.00 | 34, 68.00 | 20, 71.43 | 14, 70.00 | 50, 33.11 | 29, 40.28 | 18, 40.00 | 31, 53.45 |
| >5–10 years | 72, 72.00 | 37, 74.00 | 23, 82.14 | 14, 70.00 | 55, 36.42 | 36, 50.00 | 22, 48.89 | 32, 56.14 |
| >10–18 years | 82, 82.00 | 41, 82.00 | 24, 85.71 | 17, 85.00 | 53, 35.10 | 28, 38.89 | 19, 42.22 | 27, 46.55 |
| Currently | 70, 70.00 | 41, 82.00 | 20, 71.43 | 15, 75.00 | 27, 18.12 | 13, 18.06 | 9, 20.00 | 14, 24.14 |
| Before 18 years | 86, 86.00 | 45, 90.00 | 25, 89.29 | 18, 90.00 | 80, 52.98 | 43, 59.72 | 27, 60.00 | 43, 74.14 |
| Before 10 years | 76, 84.44 | 39, 88.64 | 24, 88.89 | 14, 87.50 | 68, 48.92 | 40, 57.97 | 24, 57.14 | 38, 71.70 |
| Self‐Smoking n, % | ||||||||
| >5–10 years | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 2, 1.32 | 1, 1.39 | 1, 2.22 | 2, 3.45 |
| >10–18 years | 19, 19.00 | 13, 26.00 | 3, 10.71 | 4, 20.00 | 22, 14.57 | 14, 19.44 | 9, 20.00 | 15, 25.86 |
| Currently | 33, 33.00 | 17, 34.00 | 7, 25.00 | 9, 45.00 | 16, 10.67 | 15, 20.83 | 10, 22.22 | 14, 24.14 |
| Before 18 year | 19, 19.00 | 13, 26.00 | 3, 10.71 | 4, 20.00 | 22, 14.57 | 14, 19.44 | 9, 20.00 | 16, 27.59 |
| Before 10 year | 0, 0 | 0, 0 | 0, 0 | 0, 0 | 2, 1.32 | 1, 1.39 | 1, 2.22 | 2, 3.45 |
| Family smoking n, % | ||||||||
| 0–12 months | 47, 47.00 | 28, 56.00 | 20, 71.43 | 8, 45.00 | 69, 46.31 | 39, 54.17 | 24, 53.33 | 36, 62.07 |
| >12 months–5 years | 49, 49.00 | 29, 58.00 | 20, 71.43 | 10, 50.00 | 71, 47.02 | 39, 54.93 | 24, 54.55 | 37, 63.79 |
| >5–10 years | 45, 45.00 | 28, 56.00 | 18, 64.29 | 8, 40.00 | 73, 48.67 | 40, 55.56 | 26, 57.78 | 34, 59.65 |
| >10–18 years | 41, 41.00 | 23, 46.00 | 16, 57.14 | 7, 35.00 | 72, 47.68 | 39, 54.17 | 24, 53.33 | 34, 58.62 |
| Currently | 35, 35.00 | 14, 28.00 | 14, 50.00 | 4, 20.00 | 44, 29.14 | 17, 23.61 | 8, 17.78 | 22, 37.93 |
| Before 18 years | 52, 52.00 | 32, 64.00 | 20, 71.43 | 12, 60.00 | 83, 54.97 | 46, 63.89 | 28, 62.22 | 41, 70.69 |
| Before 10 years | 50, 50.00 | 31, 62.00 | 20, 71.43 | 11, 55.00 | 76, 50.33 | 42, 58.33 | 27, 60.00 | 38, 65.52 |
| Migration n, % | ||||||||
| 0–12 months | 20, 20.00 | 15, 30.00 | 6, 21.43 | 6, 30.00 | 14, 9.33 | 2, 2.78 | 1, 2.22 | 4, 6.90 |
| >12 months–5 years | 22, 22.00 | 15, 30.61 | 6, 22.22 | 4, 20.00 | 14, 9.27 | 2, 2.78 | 1, 2.22 | 5, 8.62 |
| >5–10 years | 19, 19.00 | 12, 24.00 | 5, 17.86 | 5, 25.00 | 5, 3.31 | 3, 4.17 | 2, 4.44 | 4, 6.90 |
| >10–18 years | 20, 20.00 | 10, 20.00 | 7, 25.00 | 4, 20.00 | 11, 7.28 | 4, 5.56 | 2, 4.44 | 3, 5.17 |
| Currently | 25, 25.00 | 14, 28.00 | 9, 32.14 | 7, 35.00 | 12, 7.95 | 3, 4.17 | 3, 6.67 | 3, 5.17 |
| Before 18 years | 28, 28.00 | 17, 34.00 | 8, 28.57 | 7, 35.00 | 24, 15.89 | 5, 6.94 | 3, 6.67 | 9, 15.52 |
| Before 10 years | 23, 23.00 | 16, 32.00 | 6, 21.43 | 7, 35.00 | 15, 9.93 | 3, 4.17 | 2, 4.44 | 6, 10.34 |
FDR, first‐degree relatives; IBD, inflammatory bowel disease.
Antibiotic exposure before the age of 18 years is a risk factor for CD development
The relationship of childhood antibiotic exposure was evaluated in CD patients and their FDR and HMs across all regions. Overall, 21.6, 50.4, 59.4, and 77.3% of CD patients were exposed to antibiotic at 0–12 months of age, 12 months–5 years, 5–10 years, and 10–18 years, respectively. Significantly more CD patients were exposed to antibiotics before 18 years old compared with their FDR and HMs (84.86 vs 73.3%; P = 0.011). A matched conditional logistic regression was conducted in order to identify environmental factors associated with CD development between all CD patients and their FDR and HMs. Antibiotic use before 18 years old was a risk factor for CD development (adjusted odds ratio [OR] 3.46, 95% confidence interval [CI] 1.38–8.69; P = 0.008) (Fig. 1, Table S1).
Figure 1.
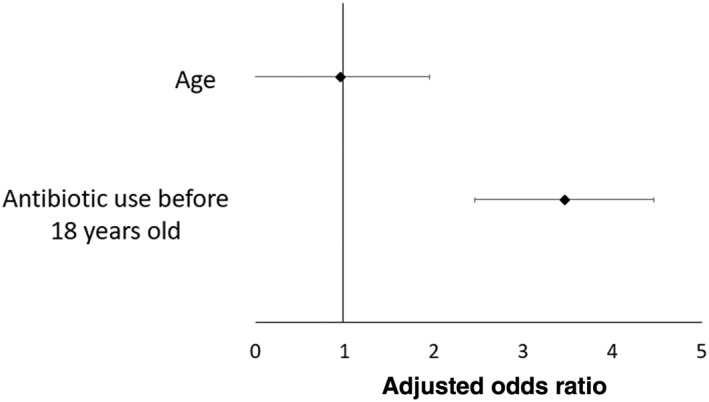
Multivariate matched analysis comparing environmental risk factors between all Crohn's disease patients and their first‐degree relatives and household members.
Further subgroup analysis was performed in Australian and the Chinese cohort, respectively. There was no significant difference in terms of proportion of CD patients and their FDR and HMs being exposed to antibiotics at all age groups. Comparing Australian CD patients with their FDR and HMs, matched multivariate analysis showed that only current living or working near busy highways was the only protective factor against CD development (adjusted OR 0.07, 95% CI 0.01–0.99; P = 0.049) (Fig. 2, Table S2). While in the Chinese cohort, significantly more CD patients were exposed to antibiotic at all age groups (birth till 18 years old) compared with their FDR and HMs (birth–12 months: 20.3 vs 11.3%; >12 months–5 years: 44.6 vs 26%; >5 years–10 years: 53 vs 36%; >10–18 years: 74.8 vs 54.2%). A matched multivariate analysis showed that antibiotic use before 18 years old was a risk factor for CD development (adjusted OR 4.80, 95% CI 1.62–14.24; P = 0.005). (Fig. 3, Table S3).
Figure 2.
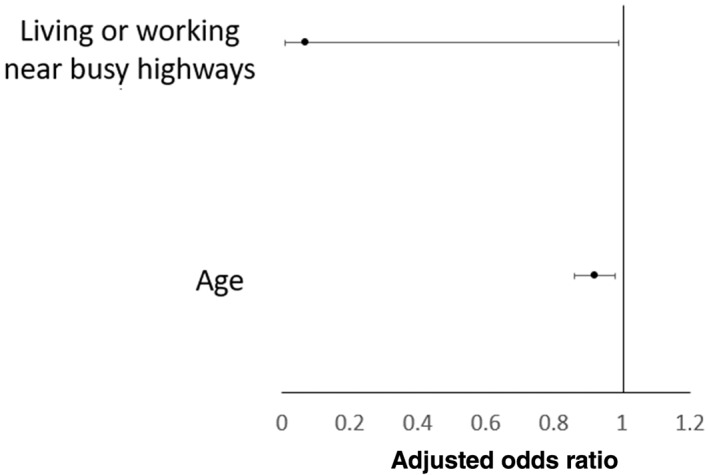
Multivariate matched analysis comparing environmental risk factors between Australian Crohn's disease and their first‐degree relatives and household members.
Figure 3.
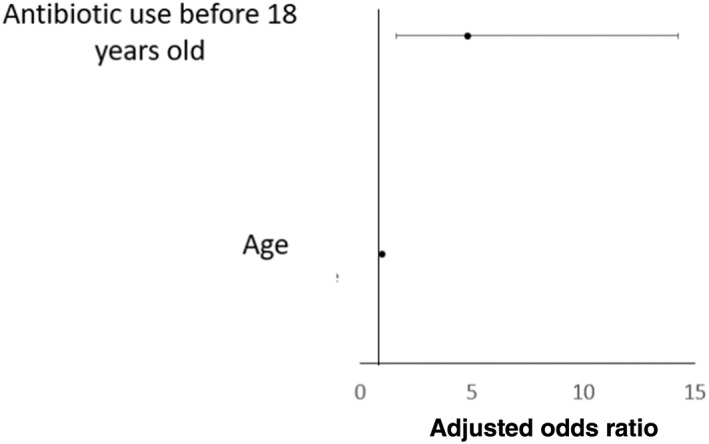
Multivariate matched analysis comparing environmental risk factors between Chinese Crohn's disease (CD) and their first‐degree relatives and household members of CD subjects.
Family history of CD is a risk factor for CD development in the Australian cohort but not in the Chinese cohort
The role of family history of IBD as a risk factor for CD development 7 was examined. Across regions, 16.5 and 2.6% of the CD patients and unrelated HCs had a positive family history of IBD, although this was not statistically significant (P = 0.597).
An unpaired analysis was performed to identify environmental factors associated with CD development between all CD patients and HCs. In the multivariable logistic regression model, current living or working near a construction site (adjusted OR 0.49, 95% CI 0.25–0.99; P = 0.046) was protective against CD development, while having a flush toilet between 10 and 18 years old was a risk factor for CD development (adjusted OR 6.20, 95% CI 1.17–32.88; P = 0.032) (Fig. 4, Table S4). In the Australian cohort, significantly more CD patients had a positive family history of IBD compared with unrelated HCs (34 vs 10%; P = 0.048), although it was not significant in multivariable logistic regression (Table S5). Similarly, family history of IBD was not a risk factor for CD development in the Chinese cohort by unpaired multivariable logistic regression (Table S6).
Figure 4.
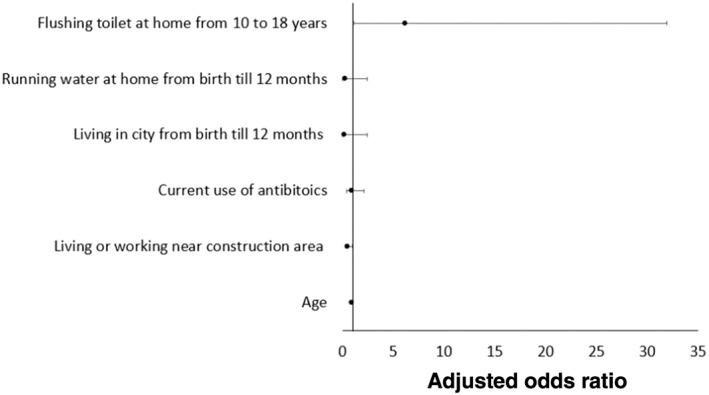
Multivariate matched analysis comparing environmental risk factors between all Crohn's disease patients and unrelated healthy controls.
Discussion
Our study has addressed the hypothesis that environmental factor plays a role in the development of CD, across different ethnic and geographic areas, encompassing high, intermediate, and emerging rates of CD. Antibiotic exposure before the age of 18 years was a risk factor for CD development compared with non‐affected FDR and HMs. The risk of CD in FDRs of CD patients is almost eight times higher compared with families without any history of IBD. 23 People living in the same family share similar lifestyle and environment. Our research findings supported the emerging hypothesis that environmental exposures in early life are implicated in disease etiology and, secondly, that modulation of these exposures can potentially modulate the risk of IBD development later in life.
There were differences in environmental risk factors for CD development in the Australian and the Chinese cohort. Comparing CD patients with their FDR and HMs in Australia and China, we showed that antibiotic use before 18 years old was a risk factor for CD development in the Chinese cohort but not in the Australian cohort. There have been conflicting environmental risk factors for CD development between the East and the West, for example, smoking and early life exposure to antibiotics had been associated with increased risk of CD in the West, but contradictory findings have been noted in the East. 24 In our study, a different effect of early life exposure to antibiotics has been noted in at‐risk population (FDR and HMs) in the East and the West, as opposed to previous findings compared with unrelated HCs. 2 The difference in environmental factors in the two cohorts suggested that environmental factors had a different effect in the East and the West and possibly explained the difference in the underlying disease pathways. Alternatively, antibiotic exposure may be so common in some populations that risk may relate to susceptibility more than exposure. Further studies are needed in different geographical locations and different ethnicities in order to identify modifiable environmental factors in different populations.
Family aggregation of IBD has been well recognized. We noted that significantly more CD patients in Australia had a positive family history compared with HCs. The reported prevalence of associated family history in the Asian population was less than that reported in the West. 25 , 26 , 27 The risk of developing IBD in FDR is 30–100 times higher than that of the general population. However, the mode of inheritance does not follow simple Mendelian rules. 28 However, there has been rapid increase in IBD incidence in Asian and newly industrialized countries. 1 Genetic factors could possibly partially explain this rise. Family history of IBD comprises both genetic and environmental influences. Thus, for the first time, we reported the potential modifiable environmental risk factors for CD among family members, with a hope to modulate their risk of developing CD in the future. However, current evidence suggests that dietary factors might exert different effects in the East versus West. While these results might suggest differences in the underlying disease pathways, it is important to keep in mind that a reliable comparison is complicated by different traditions in prescribing antibiotics in East versus West, as well as access to over‐the‐counter antibiotics.
Our study is the first to directly compare environmental risk factors in CD cases to their FDR and HMs in both Western and Chinese cohorts. Given the rapid increase in the burden of IBD in Asia, a preventive strategy is urgently needed to avoid overloading the healthcare system. Limitations in our study include missing data, as missing data are inevitable in a questionnaire study. Besides, findings, especially on questions regarding early lifetime factors, like antibiotics use, are likely to be subjected to recall bias. Moreover, some of the important questions regarding antibiotic use and CD development, for example, route, class, and duration of antibiotics, specific susceptible period to antibiotics, and interaction with other environmental factors, could not be answered in the current context. Finally, patients with CD might take antibiotics for their disease before a diagnosis of CD is made.
In conclusion, we report for the first time that the use of antibiotics before 18 years old was a risk factor for CD development compared with non‐affected FDR and HMs. This observed association indicates that early childhood factors may modulate the risk of IBD later in life even in at‐risk population. Attention should be paid to identifying potential modifiable environmental risk factors in at‐risk family members in the future.
Supporting information
Table S1. Univariate and multivariate matched analysis comparing environmental risk factors between all CD patients and their first‐degree relatives and household members.
Table S2. Univariate and multivariate matched analysis comparing environmental risk factors between Australian CD and their first‐degree relatives and household members.
Table S3. Univariate and multivariate matched analysis comparing environmental risk factors between Chinese CD and their first‐degree relatives and household members of CD subjects.
Table S4. Univariate and multivariate matched analysis comparing environmental risk factors between all CD patients and unrelated healthy controls.
Table S5. Univariate and multivariate matched analysis comparing environmental risk factors between Chinese CD patients and unrelated healthy controls.
Table S6. Univariate and multivariate matched analysis comparing environmental risk factors between Australian CD patients and unrelated healthy controls.
Appendix S1. Environmental Questionnaire‐IBD.
Declaration of conflict of interest: Dr. Joyce W Y Mak received research grants from Janssen. Prof. Siew C Ng has served as speaker for Janssen, Abbvie, Takeda, Ferring, Tilotts, Menarini, Pfizer and has received research grants from Olympus, Ferring, Janssen, and Abbvie. She is a scientific co‐founder of GenieBiome limited and holds provisional patents for Therapeutic and Diagnostic Use of Bacteria in COVID‐19 Infection, Therapeutic and Prophylactic Use of Microorganisms and Fecal Virome and Fecal Fungome and Therapeutic Efficacy of Fecal Microbiota Transplantation in recurrent clos difficile infections. Other authors have no conflicts of interests to declare.
Author contribution: Joyce W Y Mak and Jessica Y L Ching had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for the study concept and design. Joyce W Y Mak, Yang Sun, Amy L Wilson‐O'Brien, Annalise Stanley, Xiaoqing Lin, Jessica Y L Ching, Junkun Niu, Amy L Hamilton, Rui Feng, Whitney Tang, Leo Or, Ming Hu Chen, and Yinglei Mao were responsible for data acquisition. Gina L Trakman, Winnie Y Y Lin, Amy L Wilson‐O'Brien, Jessica Y L Ching, Annalise Stanley, Amy L Hamilton, Whitney Tang, Leo Or, Mark Morrison, Michael A Kamm, and Siew C Ng were responsible for questionnaire development. Joyce W Y Mak and Jessica Y L Ching were responsible for analysis of data. Michael A Kamm and Siew C Ng were responsible for critical revision of the manuscript. All authors were responsible for the interpretation of data, the drafting, and approval of the final version for submission.
Financial support: This study is supported by the Leona M. and Harry B. Helmsley Charitable Trust and Croucher Senior Medical Research Fellowship. The Translational Research Institute is funded by a grant from the Australian Federal Government.
References
- 1. Ng SC, Shi HY, Hamidi N et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet. 2018; 390: 2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Tang W, Leong RW et al. Environmental risk factors in inflammatory bowel disease: a population‐based case‐control study in Asia‐Pacific. Gut. 2015; 64: 1063–71. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Bernstein CN, Vatn MH et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013; 62: 630–49. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Leung WK, Shi HY et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a Territory‐Wide Population‐Based Registry in Hong Kong. Inflamm. Bowel Dis. 2016; 22: 1954–60. [DOI] [PubMed] [Google Scholar]
- 5. de Lange KM, Moutsianas L, Lee JC et al. Genome‐wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017; 49: 256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2010; 6: 339–46. [PMC free article] [PubMed] [Google Scholar]
- 7. Torres J, Burisch J, Riddle M, Dubinsky M, Colombel JF. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 2016; 65: 1061–9. [DOI] [PubMed] [Google Scholar]
- 8. Orholm M, Munkholm P, Langholz E, Nielsen OH, Sorensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N. Engl. J. Med. 1991; 324: 84–8. [DOI] [PubMed] [Google Scholar]
- 9. Probert CS, Jayanthi V, Hughes AO, Thompson JR, Wicks AC, Mayberry JF. Prevalence and family risk of ulcerative colitis and Crohn's disease: an epidemiological study among Europeans and south Asians in Leicestershire. Gut. 1993; 34: 1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn's disease and ulcerative colitis. Am. J. Gastroenterol. 2011; 106: 2133–42. [DOI] [PubMed] [Google Scholar]
- 11. Virta L, Auvinen A, Helenius H, Huovinen P, Kolho KL. Association of repeated exposure to antibiotics with the development of pediatric Crohn's disease–a nationwide, register‐based Finnish case‐control study. Am. J. Epidemiol. 2012; 175: 775–84. [DOI] [PubMed] [Google Scholar]
- 12. Hviid A, Svanstrom H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011; 60: 49–54. [DOI] [PubMed] [Google Scholar]
- 13. Ko Y, Kariyawasam V, Karnib M et al. Inflammatory bowel disease environmental risk factors: a population‐based case‐control study of middle eastern migration to Australia. Clin. Gastroenterol. Hepatol. 2015; 13: 1453–63 e1. [DOI] [PubMed] [Google Scholar]
- 14. Piovani D, Danese S, Peyrin‐Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta‐analyses. Gastroenterology. 2019; 157: 647–59 e4. [DOI] [PubMed] [Google Scholar]
- 15. Torres J, Mehandru S, Colombel JF, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017; 389: 1741–55. [DOI] [PubMed] [Google Scholar]
- 16. Ungaro R, Mehandru S, Allen PB, Peyrin‐Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389: 1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu JZ, van Sommeren S, Huang H et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015; 47: 979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jostins L, Ripke S, Weersma RK et al. Host‐microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012; 491: 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halfvarson J, Jess T, Magnuson A et al. Environmental factors in inflammatory bowel disease: a co‐twin control study of a Swedish‐Danish twin population. Inflamm. Bowel Dis. 2006; 12: 925–33. [DOI] [PubMed] [Google Scholar]
- 20. Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease – a population based study 2007–2009. J. Crohns Colitis. 2013; 7: 79–88. [DOI] [PubMed] [Google Scholar]
- 21. Vind I, Riis L, Jespersgaard C et al. Genetic and environmental factors as predictors of disease severity and extent at time of diagnosis in an inception cohort of inflammatory bowel disease, Copenhagen County and City 2003–2005. J. Crohns Colitis. 2008; 2: 162–9. [DOI] [PubMed] [Google Scholar]
- 22. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross‐cultural adaptation of self‐report measures. Spine. 2000; 25: 3186–91. [DOI] [PubMed] [Google Scholar]
- 23. Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population‐based cohort study 1977–2011. Am. J. Gastroenterol. 2015; 110: 564–71. [DOI] [PubMed] [Google Scholar]
- 24. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J. Gastroenterol. Hepatol. 2020; 35: 380–9. [DOI] [PubMed] [Google Scholar]
- 25. Park JB, Yang SK, Byeon JS et al. Familial occurrence of inflammatory bowel disease in Korea. Inflamm. Bowel Dis. 2006; 12: 1146–51. [DOI] [PubMed] [Google Scholar]
- 26. Kuwahara E, Asakura K, Nishiwaki Y et al. Effects of family history on inflammatory bowel disease characteristics in Japanese patients. J. Gastroenterol. 2012; 47: 961–8. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Bopanna S, Kedia S et al. Familial aggregation of inflammatory bowel disease in patients with ulcerative colitis. Intest. Res. 2017; 15: 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thjodleifsson B, Sigthorsson G, Cariglia N et al. Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology. 2003; 124: 1728–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate and multivariate matched analysis comparing environmental risk factors between all CD patients and their first‐degree relatives and household members.
Table S2. Univariate and multivariate matched analysis comparing environmental risk factors between Australian CD and their first‐degree relatives and household members.
Table S3. Univariate and multivariate matched analysis comparing environmental risk factors between Chinese CD and their first‐degree relatives and household members of CD subjects.
Table S4. Univariate and multivariate matched analysis comparing environmental risk factors between all CD patients and unrelated healthy controls.
Table S5. Univariate and multivariate matched analysis comparing environmental risk factors between Chinese CD patients and unrelated healthy controls.
Table S6. Univariate and multivariate matched analysis comparing environmental risk factors between Australian CD patients and unrelated healthy controls.
Appendix S1. Environmental Questionnaire‐IBD.


