Abstract
Background and Aim
Nonspecific ileitis is inflammation of the ileum without specific diagnostic features. A minority may go on to develop Crohn's disease, but optimal pathways of further investigation have not been established. This study aimed to identify a cohort of patients with nonspecific ileitis and to determine the value of ileal histology and gastrointestinal ultrasound in identifying/excluding Crohn's disease.
Patients and Methods
In a retrospective analysis, all patients having nonspecific ileitis at colonoscopy from January 2010 to August 2021 were identified. Clinical associations with those subsequently diagnosed with Crohn's disease were examined with specific reference to ileal histology and gastrointestinal ultrasound.
Results
Of 29 638 procedures, 147 patients (0.5%) had nonspecific ileitis. Crohn's disease was subsequently diagnosed in 8 patients (5.4%) at a median of 148 (range 27–603) days after colonoscopy. The presence of chronic inflammation on ileal biopsies was more common in those subsequently diagnosed with Crohn's disease (63% vs 20%; P = 0.0145). On gastrointestinal ultrasound, none of the 26 patients with normal bowel wall thickness (<3 mm) were subsequently diagnosed with Crohn's disease, and repeat ultrasound in 15 patients 1 year later showed no change. Of the nine patients with abnormal sonographic findings, three were diagnostic for Crohn's disease. Repeat ultrasound revealed Crohn's disease in two, while four had resolution of the abnormal findings.
Conclusion
Although ileal histology was of limited value in identifying patients with nonspecific ileitis who were subsequently diagnosed with Crohn's disease, gastrointestinal ultrasound was highly informative. Prospective studies are needed to confirm the value of gastrointestinal ultrasound as a diagnostic and monitoring tool in this setting.
Keywords: gastroenterology, IBD: clinical trials, imaging and advanced technology/applied therapeutics, intestinal disorders, screening and diagnosis
Non‐specific ileitis at ileocolonoscopy may progress to Crohn's disease in a proportion of patients. In this study, we show that the likelihood of this is occurring is low (5.4%). Furthermore, the use of gastrointestinal ultrasound after identifying non‐specific ileitis, may be useful in identifying those that may progress to Crohn's disease.
Introduction
It is common practice to examine the terminal ileum during colonoscopy, 1 and therefore identifying incidental ileitis presents a dilemma regarding diagnosis and necessary action. The spectrum of causes includes Crohn's disease, infection, drugs (in particular, nonsteroidal anti‐inflammatory drugs [NSAIDs]), spondyloarthropathies, vasculitides, neoplasms, and infiltrative diseases. 2 Furthermore, small bowel ulceration has been noted in capsule endoscopy in approximately 10% of healthy controls. 3 In some patients, histological examination of ileal biopsies and the clinical scenario will enable the diagnosis to be made. For instance, Crohn's disease is usually distinguished on the basis of macroscopic and microscopic appearances. However, in situations where minor changes are not diagnostic of Crohn's disease or other conditions, the label of “nonspecific,” “incidentally diagnosed,” or “isolated ileitis” is usually applied.
Overall, the prevalence of nonspecific ileal abnormalities at colonoscopy is less than 2%. For example, in 104 patients undergoing endoscopic evaluation, ileal inflammation, which included cecal, ileocecal vale, and ileal ulcers including those caused by Crohn's disease, infectious, NSAID‐induced, and malignant etiologies, was seen in 7%. Only 28% of these cases had a final diagnosis of a nonspecific cause, accounting for 1.7% of all procedures performed. 4 In a systematic review of patients undergoing colonoscopy for colorectal cancer screening or polyp surveillance, the pooled prevalence rate of incidentally diagnosed terminal ileitis was 1.6%, although there was significant heterogeneity, with studies reporting variable prevalence rates. 5 The pooled prevalence, when repeated without the outlier study that had an exceptionally high prevalence, was 0.7%.
At present, there are no specific published guidelines on how to follow up or monitor patients who are found to have nonspecific ileitis. The worst case scenario is that the lesions are ignored and the opportunity to make a formal diagnosis of Crohn's disease early is lost. In 88 patients undergoing capsule endoscopy and found to have nonspecific enteritis with scattered small erosions or aphthous ulcers with minimal or mild inflammation, 19% were later diagnosed with Crohn's disease. 6 In another retrospective study of 108 patients with isolated terminal ileitis with a median follow‐up time of 54.7 months, 5 (4.6%) developed Crohn's disease after a median of 32.3 months. 7 Another study has shown a progression rate of 14% from isolated ileitis to Crohn's disease, with isolated ileitis cases having excess NOD2 mutations, similar to those with Crohn's disease. 8 In a small study of 29 patients with isolated ileitis, the presence of clinical symptoms was more likely to be associated with progression to Crohn's disease rather than histological findings of chronicity. 9 Furthermore, in the systematic review described above, eight studies had long‐term follow‐up data in 147 patients and the overall risk of progression from incidental terminal ileitis to Crohn's disease was very low, but the authors were not able to quantify the risk. 5 Finally, first‐degree relatives of patients with Crohn's disease have a high risk of nonspecific ileitis or subclinical inflammation that does not progress over time. 10
On the contrary, the finding of nonspecific ileitis situation is prone to over‐investigation. In the capsule study, patients were followed up with endoscopy most commonly, or repeat capsule endoscopy and other small bowel imaging studies, to aid a final diagnosis or the cause for the enteritis. 6 Imaging with barium studies, computed tomography (CT), radiolabeled white cell scans, and visceral angiography have been shown to be useful in defining abnormalities in a single small case series with nonspecific small intestinal ulceration. 11 Applying such techniques to all those with incidental ileal lesions cannot be considered good clinical practice. Simple monitoring methods with good predictive value of who is at higher risk of a serious diagnosis are needed.
We hypothesized that gastrointestinal ultrasound might fulfill this role as a diagnostic predictor. It is a noninvasive modality without radiation that can image the ileum and is often used in the diagnosis and monitoring of patients with Crohn's disease. Features of Crohn's disease on intestinal ultrasound include thickening of the bowel wall, usually >3 mm, increased vascularity on Doppler ultrasound (US), reduced bowel peristalsis, disruption of the bowel wall layers, visualization of penetrating complications associated with Crohn's disease, and extra‐intestinal changes including abdominal lymphadenopathy and mesenteric fatty proliferation. 12 , 13 , 14 , 15 Owing to the benefits of ultrasound compared to other imaging modalities and the risks associated with repeated endoscopic examination, including cost, it would seem to be an appropriate investigation modality to monitor patients with nonspecific ileitis to look for progression, in findings that may suggest a diagnosis of Crohn's disease, or monitoring of regression of ultrasound findings in those with self‐limiting causes of ileitis.
The first step in defining the role of ultrasound is to perform a retrospective review of its impact in such patients. Thus, the aims of this study were, first, to define a cohort of patients with nonspecific ileitis undergoing colonoscopy in our endoscopic service; second, to examine the factors that were associated with a diagnosis of Crohn's disease in follow‐up; and third, to identify those who had been followed up with gastrointestinal ultrasound to determine whether this could be used to risk‐stratify and/or to diagnose patients with Crohn's disease.
Methods
We performed a retrospective review of the endoscopic database of all colonoscopy reports (including procedures performed by gastroenterologists and surgeons) from January 2010 to August 2021 to identify patients with incidental ileal abnormalities. The texts of all colonoscopy reports were searched for terminology that might suggest a diagnosis of nonspecific ileitis. The search terms included nonspecific ileitis, ileitis, ulcerated ileum, ulcer(s) ileum or ileal ulcer (s), aphthous ulcer(s), “erosions ileum” within the same sentence, and “inflammation ileum” within the same sentence. Following the identification of matching reports, each case was reviewed via the electronic clinical record and endoscopic reports to determine whether the patient met the criteria for nonspecific ileitis. Patients with a known diagnosis of Crohn's disease or were concluded to have Crohn's disease based on the endoscopic findings, those with any form of associated colitis, or those with any confirmed infectious causes of ileitis were excluded during this data extraction phase. The remaining patients were considered to have nonspecific ileitis.
For those patients deemed to have nonspecific ileitis, the medical records were reviewed, and the data, which included patient demographics, colonoscopy indications, details, and histology details, were extracted. We also reviewed the record for any form of follow‐up imaging or endoscopic assessment that examined the ileum or if they were reviewed in the gastroenterology clinic. In such cases, the records were examined in more detail to determine whether any had been diagnosed with Crohn's disease, which was confirmed on by a senior consultant in the IBD Service.
As part of the gastrointestinal ultrasound subanalysis, only those who had undergone gastrointestinal ultrasound assessment within 6 months (180 days) after colonoscopy were included in the data collection. Records were then reviewed to determine whether follow‐up scanning had been completed. Gastrointestinal ultrasound reports were manually reviewed in the electronic medical record to determine whether the terminal ileum had been examined in patients fulfilling the defined criteria. At a minimum, reports were required to comment on bowel wall thickness and the presence or absence of bowel wall vascularity of the ileum, and this was the data extracted for analysis. A small proportion of patients who had already undergone at least one ultrasound were recruited and followed up prospectively with another gastrointestinal ultrasound to provide longer follow‐up. These patients underwent a systematic gastrointestinal ultrasound examination, with four bowel wall thickness measurements of the terminal ileum, and the bowel wall vascularity was recorded. Recruitment of further patients for this was halted by the COVID‐19 pandemic.
Descriptive statistics were presented for the data analysis. Continuous variables were presented as median and interquartile range (IQR) or range where appropriate. Categorical variables were expressed as frequencies and percentages. The Mann–Whitney U test was used to compare nonparametric data. Fisher's exact test was used to compare categorical data. The data were analyzed using SPSS statistics for Macintosh, Version 27.0 (IBM Corporation, Armonk, NY, USA). This retrospective audit was approved by the Alfred Health Office of Ethics and Research Governance.
Results
Prevalence of nonspecific ileitis at endoscopy
From the 29 638 procedures labeled as colonoscopy, 1153 were found, using the search terms described. Ileal intubation occurred in 79% of diagnostic colonoscopies at our center (data from previous service audits; G. Brown, personal communication). Following review of the clinical record and endoscopy reports, 147 cases (0.5% of total procedures performed) were identified as reporting ileal abnormalities that fulfilled our definition of nonspecific ileitis. The median age was 49 (IQR 34–60) years, and 70 patients were female (48%). In most patients, the indication for undergoing endoscopic evaluation was iron deficiency, with or without anemia (26%). Histopathology samples were taken in all patients, with most having normal histology (47%), followed by acute ileitis (31%), and acute and chronic ileitis (22%). Most diagnoses of ileitis (67%, n = 98) were made from 2016 until study completion (5.5 years) compared with the preceding 6 years.
Figure 1 is a flow diagram of the patient progress and follow‐up through the study. Of the 147 patients fulfilling the criteria for nonspecific ileitis, 66 were followed up with another modality with a median follow‐up time of 370 days (IQR 173–911). Thirty‐five patients were followed up with intestinal ultrasound within 6 months of colonoscopy, and 31 had colonoscopy (52%, n = 16), a gastrointestinal ultrasound that was taken 6 months or longer after the colonoscopy (29%, n = 9), or other cross‐sectional radiology (19%, n = 6) comprising CT enterography (CTE) in 1 and magnetic resonance enterography (MRE) in 5.
Figure 1.
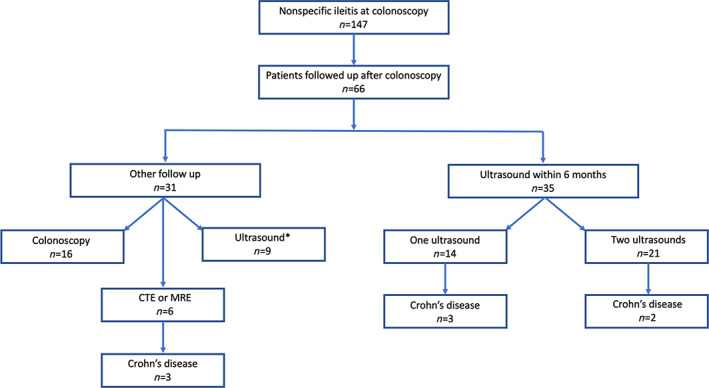
Flow diagram of the patient progress. *Ultrasound not within 6 months.
Associations with a diagnosis of Crohn's disease
Eight of the 147 patients (5.4%) were diagnosed with Crohn's disease. The overall median time from the index colonoscopy to diagnosis was 148 (range 27–603) days. The diagnosis was achieved with gastrointestinal ultrasound in five, and these are discussed below. Two were diagnosed by MRE after and 56 and 603 days, and one by CTE after 43 days. Histology in these three patients revealed acute and chronic ileitis.
A comparison of the demographic and clinical features of the patients according to the diagnosis of Crohn's disease or not is shown in Table 1. The only feature that was significantly associated with the future diagnosis of Crohn's disease was the histopathological finding of acute and chronic ileitis on ileal biopsies at the index colonoscopy (P = 0.0145), as illustrated in Figure 2.
Table 1.
Demographic and clinical features of all patients with nonspecific ileitis (n = 147) by subsequent diagnosis of Crohn's disease or not
| No diagnosis of Crohn's disease | Diagnosed with Crohn's disease | |
|---|---|---|
| Number, n (% of the cohort) | 139 (95%) | 8 (5%) |
| Age, median (IQR), years | 49 (34–60) | 54 (35–65) |
| Female sex, (%) | 48% | 50% |
| Indication for colonoscopy, n (%) | ||
| Altered bowel habit | 7 (5.0%) | 0 (0%) |
| Diarrhea | 14 (10)% | 1 (13)% |
| Iron deficiency ± anemia | 35 (25)% | 3 (38)% |
| Polyp surveillance | 14 (10%) | 0 (0%) |
| Fecal occult blood positive | 10 (7%) | 1 (13%) |
| Abdominal pain | 5 (4%) | 0 (0%) |
| Bleeding per rectum | 23 (17%) | 1 (13%) |
| Other | 31 (22%) | 1 (13%) |
| Ileal histology *, n (%) | ||
| Acute ileitis | 44 (32%) | 1 (13%) |
| Acute and chronic ileitis | 28 (20%) | 5 (62%) |
| Normal histology | 67 (48%) | 2 (25%) |
P = 0.0145 when comparing frequency of the presence versus absence of chronic inflammation according to subsequent diagnosis (Fisher's exact test).
Figure 2.
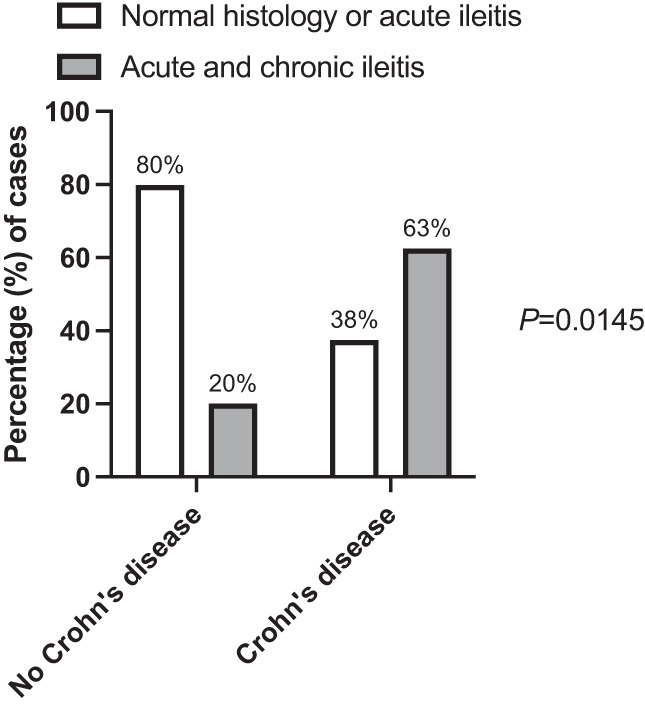
Histological findings of ileal biopsies taken at the index colonoscopy according to the presence or not of chronic inflammatory changes in those with and without a subsequent diagnosis of Crohn's disease.
Patients investigated with early gastrointestinal ultrasound
The gastrointestinal ultrasound service at our hospital was introduced from 2014, and 35 patients (24%) underwent at least one gastrointestinal ultrasound within 6 months from colonoscopy. Twenty‐one patients had at least one further follow‐up scan after the initial ultrasound.
The age, sex, indications for colonoscopy, and histopathological findings (Table 2) were similar to those in the whole cohort. The median time between colonoscopy and subsequent first ultrasound assessment was 71 (IQR 43–114) days. Five patients were diagnosed with Crohn's disease, three on the first scan and two on the second. The time to diagnosis of Crohn's disease from colonoscopy was 168 (range 27–369) days. Histology revealed acute and chronic ileitis in two, normal histology in two, and acute ileitis in one.
Table 2.
Colonoscopy indications and histology findings for 35 patients with nonspecific ileitis undergoing gastrointestinal ultrasound follow‐up within 6 months
| No diagnosis of Crohn's disease | Diagnosed with Crohn's disease | P‐value | |
|---|---|---|---|
| Number | 30 (86%) | 5 (14%) | |
| Age, median (IQR), years | 47 (34–57) | 62 (35–74) | 0.282 ‡ |
| Female sex, (%) | 60% | 40% | 0.6313 § |
| Indication for colonoscopy, n (%) | |||
| Altered bowel habit | 1 (3%) | 0 (0%) | |
| Diarrhea | 2 (7%) | 1 (20%) | |
| Iron deficiency ± anemia | 14 (47%) | 1 (20%) | |
| Polyp surveillance | 1 (3%) | 0 (0%) | |
| Fecal occult blood positive | 4 (13%) | 1 (20%) | |
| Bleeding per rectum | 4 (13%) | 1 (20%) | |
| Other | 4 (13%) | 1 (20%) | |
| Ileal histology † , n (%) | |||
| Acute ileitis | 13 (43.3%) | 1 (20%) | 0.586 † |
| Acute and chronic ileitis | 7 (23.3%) | 2 (40%) | |
| Normal histology | 10 (33.3%) | 2 (40%) | |
| Sonographic findings | |||
| Bowel wall thickness, median (range) mm | 2.0 (1.3–4.0) | 5.0 (4.4–6.7) | 0.0002 ‡ |
| Bowel wall vascularity | 3 (10%) | 5 (100%) | 0.0002 § |
Presence versus absence of chronic inflammation according to subsequent diagnosis (Fisher's exact test).
Mann–Whitney U test.
Fisher's exact test.
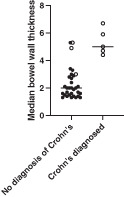
The bowel wall thickness at the first gastrointestinal ultrasound (n = 35) according to the future diagnosis of Crohn's disease is shown in Figure 3. The median bowel wall thickness for those diagnosed with Crohn's disease (either on the first or subsequent scan) was significantly higher on the first scan at 5.0 (range 4.4–6.7) mm compared with those who were not diagnosed with Crohn's disease at 2.0 (range 1.3–4.0) mm (P = 0.0002). All patients who were diagnosed with Crohn's disease had evidence of increased bowel wall vascularity on Doppler US at the first ultrasound.
Figure 3.
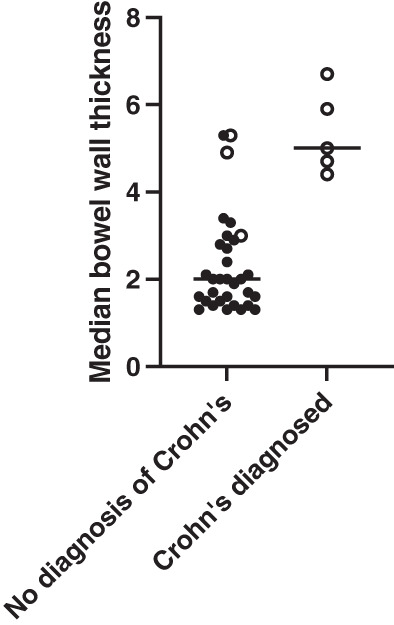
Scatterplot of median bowel wall thickness at first ultrasound within 6 months of colonoscopy by subsequent diagnosis of Crohn's disease or not. Bolded circles represent patients without increased vascularity on Doppler US, and open circles represent those with increased vascularity on Doppler US.
Twenty‐one patients had a repeat gastrointestinal ultrasound, with a median time of 387 (IQR 235–801) days between the two ultrasounds (second ultrasound 476 days from index colonoscopy). In 15 patients with normal ultrasound findings on the initial scan, the median bowel wall thickness remained stable between both assessments, 1.7 (1.5–2.8) versus 2.4 (IQR 1.9–3.2) mm (P = 0.062). In six patients, the initial gastrointestinal ultrasound showed increased bowel wall thickness, five of whom had increased vascularity. Two of these patients were diagnosed with Crohn's disease based on the findings of the second scan. The sonographic findings from the first to the second examination are shown in Figure 4. All the patients who were not diagnosed with Crohn's disease had reduction of the bowel wall thickness and loss of increased vascularity on the follow‐up scan. Both patients diagnosed with Crohn's disease had persistently increased vascularity on Doppler US, whereas in one the bowel wall thickness worsened and in the other it improved.
Figure 4.
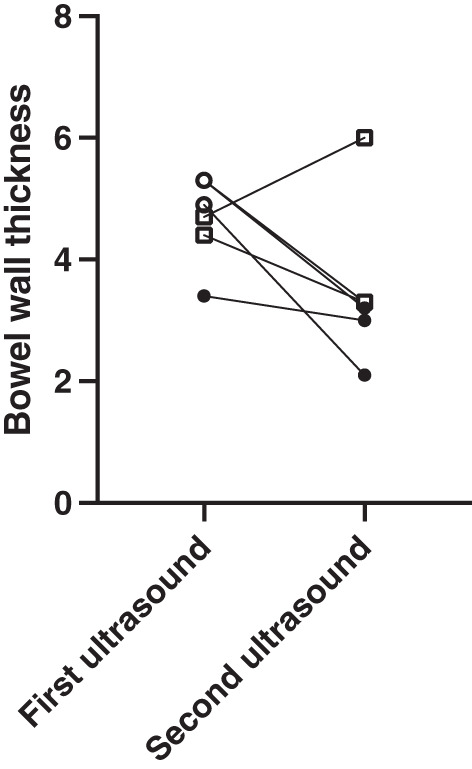
Patients undergoing two ultrasound examinations with abnormal findings at baseline. Circles represent patients not diagnosed with Crohn's disease, and squares represent those who were diagnosed with Crohn's disease. Open symbols represent those with increased vascularity on Doppler US and closed those without.
Discussion
Nonspecific ileitis is usually incidentally and unexpectedly found at ileocolonoscopy and presents as a diagnostic challenge. The major issue in clinical practice, and the one addressed in the current study, is whether such a finding represents evolving Crohn's disease, because effective management early in the course of the disease will likely prevent ongoing tissue destruction and improve outcomes. In our study, the rate of incidental ileal lesions was low, and the progression from nonspecific changes to Crohn's disease occurred in a small percentage of patients. The presence of chronic inflammation at ileal histology were more likely to be seen in patients progressing to Crohn's disease compared to those that do not, but more than one in three did not have this feature. The more recent introduction of gastrointestinal ultrasound was helpful in assessing patients with nonspecific ileitis, with increased bowel wall thickness with or without increased vascularity was highly predictive of a subsequent diagnosis of Crohn's disease and, where uncertain, follow‐up ultrasound readily evaluated resolution of bowel wall thickening, making progression to Crohn's disease highly unlikely.
Overall, the prevalence of nonspecific ileitis in our cohort was low (0.5% of procedures). This prevalence rate is similar to that of nonspecific ileitis reported in a systematic review of patients undergoing ileocolonoscopy for bowel cancer screening and polyp surveillance. 5 However, it should be noted that this included all procedures at our institution including surveillance procedures for those with known inflammatory bowel disease. Furthermore, with the way data were extracted, using a label of colonoscopy defined by the treating clinician, we were unable to determine the rate of complete colonoscopy to the cecum and ileal intubation in this cohort. However, previous audit of our colonoscopy service, which included gastroenterologists, colorectal surgeons, and trainees, indicated that almost 80% of patients have their ileum entered. Cases may have been missed during the data extraction phase depending on labeling and description of ileitis by the treating clinician or when the clinical records were reviewed for nonspecific ileitis. We saw a trend toward an increase in detection of nonspecific ileitis in the more recent procedures, potentially highlighting that there was increased awareness to document the observation of nonspecific ileitis. It is therefore likely that the overall prevalence of nonspecific ileitis in our cohort may in fact be higher than documented.
Overall, the rate of patients progressing to Crohn's disease from nonspecific ileal findings was small (5.4% of overall cases), and diagnosis often was made within the first year from the date of the colonoscopy. Three were diagnosed with cross‐sectional imaging including CTE and MRE, and the rest were diagnosed with gastrointestinal ultrasound. The colonoscopist's description of the appearances were not discriminating (data not shown). Ileal biopsies were taken for routine histopathology in all of the cases, but, while the presence of chronic inflammatory changes were statistically more likely to be associated with subsequent diagnosis for Crohn's disease, their predictive value in the individual patient was low because even normal histology was the initial finding in one‐quarter of those with Crohn's disease. Such a finding is not unexpected given the patchy nature of Crohn's disease. Indeed, the poor predictive value of ileal histology has been previously reported. 9 Hence, histology of ileal biopsies should not be used to risk‐stratify patients for more intensive follow‐up unless diagnostic features (such as granulomas) are present.
Gastrointestinal ultrasound was useful in discriminating those patients developing Crohn's disease from those who did not, based on bowel wall thickness, which was significantly higher in those subsequently diagnosed with Crohn's disease. However, a thickened ileal wall is insufficient for a diagnosis of Crohn's disease because the abnormalities (including increased vascularity) got resolved in some patients in whom a clear diagnosis could not be made on the basis of the initial ultrasound. This highlights the fact that abnormal findings on early assessment with ultrasound should not be overinterpreted and that repeated scans should be performed to determine whether there is progression, suggesting Crohn's disease, or regression of findings. Given the majority of cases of Crohn's disease were diagnosed within the first year after colonoscopy, but some up to the 2‐year mark, it seems appropriate to perform an early ultrasound after colonoscopy to identify patients who may have Crohn's disease and certainly consider a repeat scan at least 12 months' later, especially in cases with abnormal findings on the initial scan.
There are limitations, especially related to the retrospective nature of the study. Apart from issues of data extraction and reporting described above, is likely that the diagnosis of Crohn's disease was missed in a proportion of cases who did not become symptomatic over their period of observation or were lost to follow‐up. In support of this possibility, the proportion of patients in the latter cohort who were subjected to gastrointestinal ultrasound was nearly three‐fold higher than in the total cohort. This may have been due to selection bias in who was scanned, or may reflect the largely subclinical nature of early Crohn's disease. In this way, the use of proactive ultrasound assessment of all patients with nonspecific ileitis could be supported. Ideally, these issues warrant a prospective study of patients with nonspecific ileitis with protocolized reporting and follow‐up investigation where gastrointestinal ultrasound soon after the colonoscopy and again in 6–12 months would be central. Finally, prospectively following patients and understanding their risk factors for Crohn's disease, such as family history, other medical conditions, and medication history, would help clarify the etiology or risks for progression of nonspecific ileitis findings.
In conclusion, this audit of incidental findings at ileocolonoscopy of nonspecific ileitis has a low chance of progressing to a diagnosis of Crohn' disease necessitating a risk‐stratification protocol. Clinical and histological features are unreliable, but gastrointestinal ultrasound appears to provide the potential to improve such targeting of follow‐up. Prospective studies are required to determine the true place of gastrointestinal ultrasound in investigative algorithms in such patients.
Declaration of Conflict of Interest: None.
References
- 1. Marshall JB, Barthel JS. The frequency of total colonoscopy and terminal ileal intubation in the 1990s. Gastrointest. Endosc. 1993; 39: 518–20. [DOI] [PubMed] [Google Scholar]
- 2. DiLauro S, Crum‐Cianflone NF. Ileitis: when it is not Crohn's disease. Curr. Gastroenterol. Rep. 2010; 12: 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujimori S. Asymptomatic small intestinal ulcerative lesions: obesity and Helicobacter pylori are likely to be risk factors. World J. Gastroenterol. 2021; 27: 4484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toshniwal J, Chawlani R, Thawrani A et al. All ileo‐cecal ulcers are not Crohn's: Changing perspectives of symptomatic ileocecal ulcers. World J. Gastrointest. Endosc. 2017; 9: 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agrawal M, Bento‐Miranda M, Walsh S, Narula N, Colombel JF, Ungaro RC. Prevalence and progression of incidental terminal ileitis on non‐diagnostic colonoscopy: a systematic review and meta‐analysis. J. Crohns Colitis. 2021; 15: 1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sihag S, Tan B, Semenov S et al. Development of significant disease in a cohort of patients with non‐specific enteritis on capsule endoscopy: clinical suspicion and a high base line Lewis score are predictive of Crohn's disease. BMC Gastroenterol. 2020; 20: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tse CS, Deepak P, Smyrk TC, Raffals LE. Isolated acute terminal ileitis without preexisting inflammatory bowel disease rarely progresses to Crohn's disease. Dig. Dis. Sci. 2017; 62: 3557–62. [DOI] [PubMed] [Google Scholar]
- 8. O'Donnell S, Crotty PL, O'Sullivan M et al. Isolated active ileitis: is it a mild subtype of Crohn's disease? Inflamm. Bowel Dis. 2013; 19: 1815–22. [DOI] [PubMed] [Google Scholar]
- 9. Courville EL, Siegel CA, Vay T, Wilcox AR, Suriawinata AA, Srivastava A. Isolated asymptomatic ileitis does not progress to overt Crohn disease on long‐term follow‐up despite features of chronicity in ileal biopsies. Am. J. Surg. Pathol. 2009; 33: 1341–7. [DOI] [PubMed] [Google Scholar]
- 10. Sorrentino D, Avellini C, Geraci M et al. Tissue studies in screened first‐degree relatives reveal a distinct Crohn's disease phenotype. Inflamm. Bowel Dis. 2014; 20: 1049–56. [DOI] [PubMed] [Google Scholar]
- 11. Bryant TH, Jackson JE. The radiographic appearances of non‐specific small intestinal ulceration. Clin. Radiol. 2002; 57: 117–22. [DOI] [PubMed] [Google Scholar]
- 12. Pascu M, Roznowski AB, Müller HP, Adler A, Wiedenmann B, Dignass AU. Clinical relevance of transabdominal ultrasonography and magnetic resonance imaging in patients with inflammatory bowel disease of the terminal ileum and large bowel. Inflamm. Bowel Dis. 2004; 10: 373–82. [DOI] [PubMed] [Google Scholar]
- 13. Tarján Z, Tóth G, Györke T, Mester A, Karlinger K, Makó EK. Ultrasound in Crohn's disease of the small bowel. Eur. J. Radiol. 2000; 35: 176–82. [DOI] [PubMed] [Google Scholar]
- 14. Pallotta N, Tomei E, Viscido A et al. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm. Bowel Dis. 2005; 11: 146–53. [DOI] [PubMed] [Google Scholar]
- 15. Calabrese E, La Seta F, Buccellato A et al. Crohn's disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm. Bowel Dis. 2005; 11: 139–45. [DOI] [PubMed] [Google Scholar]


