Abstract
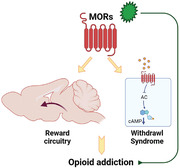
Opioid abuse and addiction have become a global pandemic, posing tremendous health and social burdens. The rewarding effects and the occurrence of withdrawal symptoms are the two mainstays of opioid addiction. Mu‐opioid receptors (MORs), a member of opioid receptors, play important roles in opioid addiction, mediating both the rewarding effects of opioids and opioid withdrawal syndrome (OWS). The underlying mechanism of MOR‐mediated opioid rewarding effects and withdrawal syndrome is of vital importance to understand the nature of opioid addiction and also provides theoretical basis for targeting MORs to treat drug addiction. In this review, we first briefly introduce the basic concepts of MORs, including their structure, distribution in the nervous system, endogenous ligands, and functional characteristics. We focused on the brain circuitry and molecular mechanism of MORs‐mediated opioid reward and withdrawal. The neuroanatomical and functional elements of the neural circuitry of the reward system underlying opioid addiction were thoroughly discussed, and the roles of MOR within the reward circuitry were also elaborated. Furthermore, we interrogated the roles of MORs in OWS, along with the structural basis and molecular adaptions of MORs‐mediated withdrawal syndrome. Finally, current treatment strategies for opioid addiction targeting MORs were also presented.
Keywords: dependence, mu‐opioid receptor, opioid addiction, reward circuitry, withdrawal syndrome
1. Rewarding effects and withdrawal syndrome are two dominant components of opioid addiction.
2. Mu‐opioid receptors (MORs) are highly involved in reward circuitry and the development of withdrawal syndrome.
3. MORs are important targets to treat addiction and withdrawal syndrome.
1. INTRODUCTION
Opioids have been applied for thousands of years in human history to relieve pain. The medicinal application of opioids can be dated back to 1500 B.C. when people used opioids to treat “excessive crying of baby.” 1 Over the last decades, opioids have long been used as the most powerful analgesics and remain the most frequently used analgesics against severe pain. 2 , 3 Currently, opioids are widely used in acute pain 4 , 5 and cancer pain, 6 , 7 , 8 , 9 and are especially noticed and prescribed in chronic pain. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Apart from their analgesic effects, opioids, both prescribed opioid analgesics (morphine, hydrocodone, oxycodone hydrochloride) and illicit opioids (heroin and its analogs) are associated with the propensity for addiction. 19 Drug addiction is characterized by a recurring desire to continue taking the drug despite harmful consequences. 20 , 21 , 22 Five elements of addiction have been identified from the literature, including (1) engaging processes to achieve appetitive effects, (2) preoccupation with the behavior, (3) temporary satiation, (4) loss of control, and (5) harmful consequences. 23
Opioid addiction has become an epidemic and global concern in recent years. It was estimated that 26.8 million people had opioid use disorder globally, with over 100,000 opioid overdose deaths annually. 24 , 25 The prevalence of opioid use disorder is highest in the United States. 25 It was estimated that over 4% of the adult population (more than 10 million Americans) currently misuse prescription opioids. 26 There was an average of five Americans per hour who died from opioid overdose. 27 Moreover, the problem of opioid addiction is even more complicated by the intractable dependence on opioids and the high likelihood of relapse. 28 Tremendous socioeconomic burden, along with the impact on health and well‐being, has been cast on both society and addicted individuals. 29 , 30 , 31 The higher economic cost could be attributed to health care and substance abuse treatment costs, workplace costs due to lost earnings and lost employment, and criminal justice costs. 29 , 32 Moreover, drug addiction and substance abuse have already shed shadows on young people, poisoning their mental and physical health and hindering their social development. 33 , 34 Thus, it is of vital importance to understand the mechanism of opioid addiction and urgent to treat opioid addiction based on related mechanism.
Exogenous and endogenous opioids exert their biological effects via opioid receptors, which belong to the superfamily of seven transmembrane (TM) G‐protein coupled receptors (GPCRs). 35 , 36 , 37 , 38 , 39 Mu‐opioid receptors (MORs), a member of opioid receptors, are dominant in mediating both the analgesic and addictive effects of opioids. 40 , 41 , 42 , 43 , 44 , 45 Opioid addiction is a complex process in which compulsive seeking for rewarding and euphoric effects is initially involved and succeeded by dependence on opioids, which usually leads to the failure of the attempt to quit drug abuse and reinforcement of addiction. 27 , 46 Noticeably, the target of opioids, MORs, play important roles in both the regulation of neural circuitry of the reward system and the cellular adaptions of chronic opioids exposure that cause dependence and withdrawal syndromes. 43 , 47 , 48 , 49 , 50
Currently, most therapeutic strategies for opioid addiction target MORs, which are also the targets for drug development. 51 Thus, considering the central regulatory role of MORs in both reward and dependence aspects of drug addiction, fully understanding the mechanism of MORs in opioid addiction is the cornerstone of effective management of opioid abuse disorders and opioid addiction. In this review, we discuss the structural and functional characteristics of MORs that may have implications for opioid addiction. We thoroughly summarize the circuitry of the brain reward system and the progress in the understanding of the role of MORs within the reward circuitry, as well as the roles of MORs in the development of opioid withdrawal symptoms. Finally, we briefly review current treatment strategies for opioid addiction targeting MORs.
2. MORs
Since ancient times, opioids have been used for their analgesic and psychotropic effects. Despite the therapeutic effects, opioid drugs are also associated with undesired effects, such as addiction (for the definition of addiction, please refer to the Introduction section of this review), dependence (in this review, dependence refers to physical dependence, which manifests as the emergence of withdrawal symptoms when the repeated use of opioids is abruptly stopped or tapered and often pushes users to seek opioids to avoid withdrawal syndrome, resulting in the relapse of opioid misuse and reinforced addiction), 19 tolerance (meaning that an increased dose of opioids is needed to achieve the same therapeutic effects, especially when opioids are taken as analgesics), 52 and abstinence reactions (abstinence means abstaining from the abused drugs, and abstinence reactions here are equal to withdrawal reactions or withdrawal syndrome characterized by symptoms such as muscle and joint pain, diarrhea, cramps, nausea, vomiting, runny nose, insomnia, dysphoria, anxiety, and irritability). 53 For a more detailed review of withdrawal syndrome, refer to Section 4). Both therapeutic and unwanted effects of opioid drugs were exerted through their binding to MORs. In mice with MOR deletion, the analgesic effect of morphine along with its dependence and rewarding effects are simultaneously abolished. 54 Thus, MORs are regarded as the molecular target for opioids such as morphine, fentanyl, methadone, and the notorious heroin that is widely used today.
2.1. Structure of MORs
Classical opioid receptors mainly include three subtypes, the MORs, the delta‐opioid receptors (DORs), and the kappa‐opioid receptors (KORs), which are encoded by OPRM1, OPRD1, and OPRK1, respectively. 43 The discovery of multiple receptors, MORs, DORs, and KORs came from the demonstration of different profiles of pharmacological activity with the prototype agonists morphine, ketazocine, and N‐allylnormetazocine. These receptors belong to the inhibitory GPCR superfamily, which mediates inhibitory signaling upon activation. The typical protein structure of these receptors is characterized by seven TM domains, an extracellular N‐terminus, and an intracellular C‐terminus inside the cells. 55 More recently, cDNA encoding an “orphan” receptor was also identified, which has a high degree of homology to the “classical” opioid receptors. The m1/m2 subdivision of MORs was proposed by Pasternak and colleagues to explain their observations that [3H]‐labeled MORs ligand displayed biphasic binding characteristics. 56 Naloxazone and naloxonazine were reported to abolish the binding of radioligand to the m1‐site 57 (ref: https://www.opioids.com/receptors/index.html)
Alternative splicing is another prominent feature of MORs in cells. Alternative splicing is a regulatory mechanism of gene expression that allows the generation of multiple mRNA species from an individual gene. 58 Beyond the seven highly homogeneous TM regions, divergent alternative splicing occurs at the upstream and downstream terminals of MORs mRNA. 59 Till now, there are 19 transcript variants that have been listed in the Aceview database of NCBI of homo species (UCSC genome browser GRCh37/hg19; for more detailed information, refer to the review by Pasternak and Pan 55 ). In the rat central nervous system (CNS), MOR1, MOR1A, and MOR1B can be detected, and the mRNA levels of MOR1 and MOR1A are significantly higher than those of MOR1B. 60 Moreover, in HEK293 cells expressing these receptors, [D‐Ala2, N‐Me‐Phe4, ‐Gly‐ol5] encephalin (DAMGO)‐induced desensitization was significantly lower for MOR1B than for MOR1 and MOR1A. 60 C‐terminal splicing of MORs might modulate agonist‐induced internalization and re‐sensitization of MORs. The DAMGO‐induced internalization of MOR1B proceeds even faster effect than that of MOR1, followed by rapid recycling of the detected receptor to the cellular surface. 61 After opioid removal, functional recovery (re‐sensitization) of MOR1B was also significantly impaired when compared with MOR1. 62 Other reports also showed that MORs splicing variants are associated with divergent roles for the C‐terminal in morphine‐induced behaviors. In mice with selectively truncated C‐terminal tails encoded by exon 7 transcript variant, morphine‐induced reward and tolerance effects are diminished without notably altering physical dependence, whereas in mice selectively expressing truncated C‐terminal tails encoded by exon 4 transcript variant, morphine tolerance is facilitated, and morphine dependence is reduced without interfering with morphine reward. 63 Interestingly, the effect of morphine on different MORs splicing variant seems to be sex‐dependent. Chronic systemic morphine results in a twofold increase in the levels of spinal cord MOR1B2 and MOR1C1 in male rats, but this effect is completely absent in females, 64 indicating the importance of sex‐specific mechanisms of morphine tolerance and addiction in vivo.
The crystal structure of MOR has been reported by Manglik et al. 65 By using the T4 lysosome fusion protein strategy, the authors obtained the crystal structure of the complex in which MORs bind with the irreversible morphinan antagonist β‐funaltrexamine (β‐FNA). The MORs’ structure generally consists of seven TM alpha‐helices, among which the alpha‐helices are connected by three extracellular loops (ECLs 1–3) and along with three intracellular loops. Also, TM3 is connected to ECL2 by a conserved disulfide bridge bond between C140 and C217. Notably, unlike most GPCR, where the ligand‐binding pocket is buried within the helical bundle by superficial residues in TMs and ECL2, such as M2 and M3 muscarinic acetylcholine receptors, 66 , 67 the binding pocket of MORs for β‐FNA is largely exposed to the portion of the extracellular surface. This observed ligand‐binding pocket may partially explain the rapid dissociation half‐lives for potent opioids such as alvimopan, etorphine, and diprenorphine. 68 , 69 Moreover, MORs were observed to readily dimerize and form oligomers. The homogeneous dimers of MORs are formed through the interface of 28 residues in the structure of TM5 and TM6, and oligomers of MORs dimers are formed through the parallel association mediated by the structure of TM1, TM2, and helix eight; however, the function of the oligomers is still poorly understood.
2.2. Distribution of MORs
MORs are widely distributed in the CNS and peripheral nervous system. In the CNS, MORs can be detected in a variety of brain regions, including the neocortex, hippocampus, striatum, amygdala, thalamus, hypothalamus, periaqueductal gray (PAG), medulla, and pons, where they exert a certain function in local circuits. MORs are suggested to be located in the cerebral neocortex. In the cerebral neocortex, opioid was reported to release at the orbitofrontal cortex after alcohol exposure, and the changes in the opioid of the orbitofrontal cortex correlated significantly with the alcohol‐use behavior. 70 Moreover, in the insular cortex, MORs play an important role to mediate long‐term synaptic depression at the inputs to the dorsolateral (DL) striatum. 71 In the prefrontal cortex (PFC), MORs are also engaged in the regulation of network that controls appetitively motivated behaviors, and the disruption of such network is associated with impulsive appetitive response. 72 In the hippocampus, MORs were mainly found in GABAergic inhibitory interneurons, including parvalbumin (PV)‐expressing basket cells, neuropeptide Y‐expressing interneurons, vasoactive intestinal peptide‐expressing interneurons, somatostatin‐expressing interneurons, and calretinin‐containing interneurons. 73 In addition, in the hippocampus, MORs were also detected in ivy and neurogliaform interneurons. 74 Hippocampal MORs were also reported to be involved in the modulation of sharp waves and ripples and thus the hippocampus‐dependent memory. 75 In the CA3 region of the hippocampus, MORs play a vital role in the acquisition and retrieval of spatial memory. 76 In the striatum, MORs were reported to be expressed by striatal projection neurons in both the matrix and the patches, the two distinct structural divisions of the striatum, and also in cholinergic interneurons. 77 , 78 Selective activation of MORs in cholinergic interneurons in the dorsal lateral striatum caused a strong inhibition of firing activity in the cholinergic interneurons, which was believed to underlie the pathogenesis of dystonia. 78 Striatal MORs have also been shown to contribute to methamphetamine‐induced stereotypy 79 and opioid‐induced locomotor sensitization, 80 suggesting a role of striatal MORs in drug effects. In amygdala intercalated neurons, MORs play a vital role in processing the information between the basolateral complex of the amygdala and central nuclei of the amygdala. 81 The amygdala MOR system has also been declared to regulate reward behavior and appetite behavior. 82
MORs were also reported to be located in major nuclei of the thalamus and hypothalamus. Thalamic MORs are involved in the modulation of pain esthesia. 83 , 84 , 85 , 86 MORs in the parafascicular nucleus of the thalamus were reported to mediate the effects of morphine‐induced antinociception. 87 Hypothalamic MORs, interestingly, are involved in the regulation of the hypothalamic–pituitary–adrenal axis, as evidenced by the fact that the A118G polymorphism of MORs blunted the adrenocorticotropic hormone response to metyrapone. 88 Notably, hypothalamic MORs mediated the effects of drug abuse. MORs were reported to mediate the depression of the hypothalamic hypocretin/orexin arousal system, which may explain the sedation and mental lethargy after morphine exposure. 89 Moreover, MORs on the microglia in the hypothalamus contributed to the neuroinflammation process after alcohol exposure, although glial MORs are not within the central topic of the current review. 90
In more posterior regions like PAG, medulla, and pons, MORs also exist and exert functions. PAG is a midbrain region that is involved in the modulation of nociception, and MORs in the PAG are important targets for analgesia. 91 , 92 , 93 It was reported that the activation of MORs in the ventral PAG could decrease the inhibitory inputs onto the dopamine (DA) neurons in the ventral PAG, the activation of which displayed an antinociceptive effect. 91 Recently, Kandasamy et al. found that positive allosteric modulators targeting MORs in the PAG produced antinociception with reduced levels of morphine‐induced side effects such as reward and respiratory depression. 94 This study demonstrated that positive allosteric modulators of MORs, rather than traditional opioid drugs such as morphine, might be more suitable analgesics with few side effects. MORs in the medulla had two important aspects associated with unwanted effects of morphine‐induced analgesia. For one thing, considering medulla is the home of respiratory centers, MORs activation in the medulla inhibited the respiratory centers, thus mediating the side effect of respiratory depression of morphine. 95 In addition, it was reported that the complex formed by vasopressin 1b receptor, β‐arrestin‐2, and MORs in the rostral ventromedial (VM) medulla mediated morphine tolerance. 96 Similar to MORs in the medulla, MORs in the pons mediated the respiratory depression of morphine. The pre‐Bötzinger complex, a respiratory rhythm‐generating area in the pons, is inhibited upon MORs activation, 97 while the pontine respiratory‐controlling Kölliker‐Fuse neurons, which maintain upper airway patency and a normal respiratory pattern, could be hyperpolarized by MORs, leading to the suppression of post‐inspiratory drive. 98
As for subcellular location, MORs localize in different parts of the neuron, that is, axonal terminals, dendrites and soma, and exhibit distinct functional properties. First, activation of differentially distributed MORs could exert different electrophysiological effects. The activation of MORs in the somatodendric compartment decreases cellular excitability, whereas the activation of MORs in the axonal terminal inhibits neurotransmitter release, resulting in decreased downstream excitation or disinhibition. 99 Second, agonist‐induced MORs desensitization differs between MORs located in the nerve terminals and those in the cell bodies. The high‐efficacy MORs agonist DAMGO, an MOR selective agonist with a Kd of 3.46 nM for native MORs, could induce rapid MOR desensitization at the ventral tegmental area (VTA)
GABAergic neuron bodies but not at the terminals. However, after prolonged treatment (> 7 h) with Met‐enkephalin, one of endogenous opioid peptides isolated from the porcine brain in 1975, both MORs in the terminals and cell bodies exhibit profound desensitization. 100
In the peripheral nervous system, MORs are mainly implicated in nociception. An elucidated description of the detailed role of peripheral MORs can be found in the review by Rauck. 101
2.3. Endogenous MOR ligands
Several endogenous MOR ligands have been reported, including β‐endorphin, Met‐enkephalin, Leu‐enkephalin, and so forth. These endogenous MOR ligands generally contain a Tyr‐Gly‐Gly‐Phe‐Met/Leu sequence at the N‐terminals, which can be recognized as the opioid motif. 40 Another kind of endogenous opioid receptor ligand, endomorphin‐1/2, is characterized by the opioid motif being substituted with peptides Tyr‐Pro‐Trp‐Phe‐NH2 and Tyr‐Pro‐Phe‐Phe‐NH2, and they showed high selectivity and affinity for MORs. 102 Upon binding to MORs, endogenous opioid ligands, that is, β‐endorphin, enkephalin, and endomorphin, can exert distinct functions promptly. Intra‐nucleus accumbens (NAc) administration of β‐endorphin could increase social play behavior, a highly rewarding social interaction in adolescent rodent species, but the administration of met‐enkephalin could not induce similar effects. 103 In the arcuate nucleus of the hypothalamus, β‐endorphin plays an important role in antinociception behavior. 104 In addition, a compensatory increase in enkephalin release during morphine withdrawal could promote a second period of MOR activity, which is responsible for the enhanced naloxone (NX) aversion. 105 Moreover, in the pre‐Bötzinger complex, which is the center of respiratory rhythm generation, endomorphin‐2 could play a promotive role in respiratory depression through MORs. 106 Endogenous opioid peptides can be inactivated by aminopeptidase N and enkephalinases. 40 Inhibition of the degradation of endogenous opioid peptides in both peripheral injured tissues and the CNS could produce analgesic effects. 107 , 108
2.4. Biased signaling of MORs
One of the leading characteristics of MORs is the biased agonism of some MOR ligands, which means that a certain ligand could induce a specific conformational change of the MORs and activate a particular signaling pathway. So far, herkinorin, oliceridine (TRV130), PZM21, mitragynine, and naltrexone (NTX)‐derived compound derivatives have been identified as the biased ligands of MORs. 109 The analgesic and concomitant adverse effects of opioids are mainly mediated through the G‐protein pathway and β‐arrestin pathway, respectively (Figure 1).
FIGURE 1.
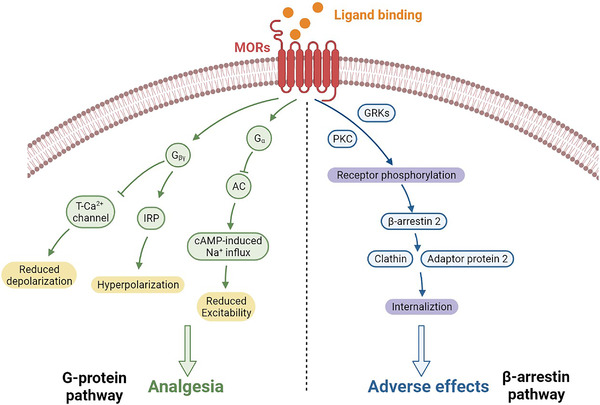
Biased signaling of Mu opioid receptors (MORs). The analgesic effects and adverse effects of MORs ligands are mediated by the G‐protein pathway and β‐arrestin pathway, respectively. The analgesic effects are mediated by G proteins, which inhibit AC, activate IRP channels, inhibit T‐type Ca2+ channels, and finally decrease the excitability of neurons. The adverse effects such as tolerance or respiratory depression are mediated by β‐arrestin 2, which leads to the internalization of the receptors. AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; Ca2+, calcium ion; Na+, sodion; IRP, inwardly rectifying potassium channels; GRKs, G‐protein receptor kinases; PKC, protein kinase C
The analgesic effects of opioids are mainly conducted by an inhibitory subunit of the G‐protein Gi. After being activated by analgesia‐biased ligands, the binding of Gi protein to MORs induces the release of the Gα subunit and Gβγ subunit complex. 110 The α subunit of G protein inhibits the activity of adenylyl cyclase (AC), reduces the intracellular cyclic adenosine monophosphate (cAMP)‐dependent sodion (Na+) influx and eventually represses the excitability of neurons. 111 , 112 , 113 The Gβγ subunit complex activates G‐protein inwardly rectifying potassium channels, promotes cellular hyperpolarization, and can inhibit T‐type calcium channels, decreasing calcium ion (Ca2+) ingress and neural depolarization. 111 , 112 , 113
The adverse effects of opioids, such as tolerance and respiratory depression, 114 are mainly mediated by the β‐arrestin pathway instead. Ligand‐activated MOR is phosphorylated by G‐protein receptor kinases or protein kinase C (PKC). 113 , 115 Phosphorylated MORs thus gain increased affinity to recruit and interact with β‐arrestin 2 (also known as arrestin‐3). The binding of β‐arrestin2 to MORs could uncouple or release the receptor for G‐protein signaling pathways and therefore desensitize the MORs. The MORs/β‐arrestin 2 complex then interacts with clathrin and adaptor protein 2 via the clathrin‐coated pits to endocytose and internalize in the cells. 99 , 116 , 117 , 118 Different opioids have different abilities to promote MORs internalization, with DAMGO, fentanyl, methadone, etorphine and β‐endorphin promoting robust MORs internalization, while morphine, buprenorphine (BUP) and pentazocine promoting relatively weaker internalization. 117 In β‐arrestin knockout (KO) mice, the analgesic effect of morphine was remarkably potentiated and prolonged, 119 and respiratory suppression and acute constipation after morphine administration were attenuated. 120 However, although β‐arrestin KO mice did not develop antinociceptive tolerance, they still became physically dependent on morphine. 121
3. REWARD CIRCUITRY, ADDICTION, AND MORS
Addiction is known as a chronic neurobehavioral disorder where addicted individuals are compulsive to seek and obtain drugs, unable to refrain themselves from taking and become dysphoric, anxious, depressed, or irritable when they do not have access to the abused drugs. 20 Drug addiction is usually a staged process where initially the drug users have recreational or euphoric reactions to the drugs, but upon repeated consumption, they develop compulsive seeking and taking behaviors. 122 Drugs abused often activate the brain reward circuitry, which plays a crucial role in the hedonic regulation of behaviors and initiation of addiction. 123 The neural circuitry of the rewarding effects of abused drugs is extensively distributed in the brain, including the VTA and NAc, and the DA neurons within the VTA and NAc of the brain have been identified as the key components for the rewarding effects of abused drugs. 124 Apart from the VTA and NAc, other brain areas such as the striatum, PFC, thalamus, hypothalamus, and amygdala are also involved in the rewarding effects of abused drugs (Figure 2).
FIGURE 2.
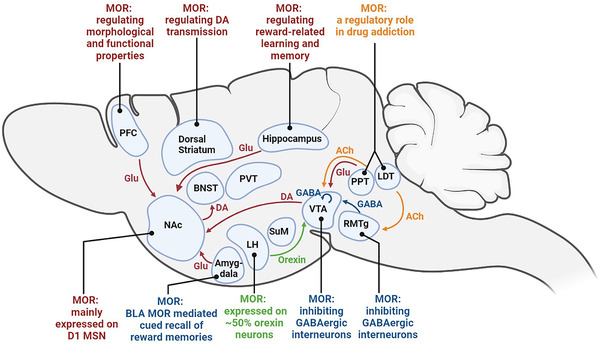
Brain regions within the reward circuitry and the role of MORs. Brain regions and nuclei that participate in reward circuitry and MORs‐mediated rewarding effects are widely distributed in the central nervous system. Ach, acetylcholine; BLA, basolateral amygdala; BNST, bed nuclei of the stria terminalis; DA, dopamine; GABA, γ‐aminobutyric acid; Glu, glutamate; LDT, laterodorsal tegmental nucleus; LH, lateral hypothalamus; NAc, nucleus accumbens; PPT, pedunculopontine tegmental nucleus; PFC, prefrontal cortex; PVT, paraventricular nucleus; RMTg, rostromedial tegmental nucleus; SuM, supramammillary nucleus; VTA, ventral tegmental area
MORs, as key modulators in the reward circuitry, play a major role in the natural reward process, regulating mood states and reward motivation. 125 , 126 Oprm‐1 KO mice demonstrated deficits in social behavior and communication skills, which are deficits usually owned by drug abusers. 127 , 128 , 129 , 130 Moreover, oprm‐1 KO mice showed a decreased motivation for food and sucrose self‐administration 131 and failed to demonstrate an aversion to NX. 132 Considering the wide distribution of MORs in the brain, as expected, opioids and MORs intensively regulate the reward circuitry and are thought to play a role in drug addiction. In the following section, we review the reward circuitry and brain regions that are actively involved, followed by discussing the role of MORs in reward and addiction within each brain area.
3.1. VTA and rostromedial tegmental nucleus (RMTg)
The VTA is a brain region that is generally thought to be the underlying pivot of drug addiction. VTA is characterized by a lack of clear boundaries and heterogeneous cellular architecture, with 60%–65% of the neuronal population being DA, 30%–35% being γ‐amino butyric acid (GABA), and 2%–3% being glutamatergic neurons. 123 , 133 , 134 The activation of VTA DA neurons is the main responder to drug addiction. Tract tracing technology revealed that VTA DA neurons are composed of several subtypes with distinct projections, including projections to the NAc, the PFC, the amygdala and the hippocampus, 135 , 136 among which the subtype projecting to the NAc is involved in drug reward. 123 Administration of cocaine could selectively modify the excitatory synapses of the VTA DA neurons projecting to the NAc shell by increasing the α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor / N‐methyl‐d‐aspartate (NMDA) receptor ratios in these neurons. 137 In addition, cocaine also inhibits the DA transporter (DAT) in the VTA, which also mediates its rewarding effect. 138 , 139 It remains to be elucidated which is the dominant mechanism underlying cocaine addiction, whether modifying VTA‐NAc DA projections or inhibiting VTA DAT. Anatomically, the VTA is composed of the anterior VTA and posterior VTA. It is estimated that the posterior VTA mediates drug rewarding effects more readily than the anterior zone. 124 Apart from opioids (endomorphin‐1), drugs including nicotine, delta‐9‐tetrahydrocannabinol, and cocaine can stimulate rewards arising from the posterior VTA to activate DA neurons within the posterior VTA. 140 , 141 , 142 , 143 While VTA DA neurons are the mainstay for drug reward, it is equally clear that VTA GABAergic neurons are also critical for the reward process. 144 The activation of VTA GABAergic neurons inhibits VTA DA neuron activity and in turn results in a decrease in DA in the NAc and disrupts reward consummatory behavior. 145 GABAergic neurons that critically control the VTA DA are localized at the posterior tip of the VTA, called the RMTg. The RMTg is composed of a relatively pure population of GABAergic neurons, and they project to VTA DA neurons and substantia nigra pars compacta. 146 , 147 Stimulation of RMTg GABAergic neurons could suppress the activity of approximately 90% of DA neurons in the VTA. 148 After reward or reward‐predictive stimuli, the activity of RMTg GABAergic neurons is inhibited. 147
The most prevailing hypothesis for opioid reward depends on the MORs expressed in the VTA GABAergic neurons. In the VTA, MORs are mainly selectively expressed on GABAergic neurons rather than DA neurons. 149 , 150 , 151 , 152 Opioids directly act on the MORs in these GABAergic neurons, resulting in hyperpolarization and a reduced VTA GABAergic neuron firing rate and subsequently disinhibiting the activity of VTA DA neurons. 152 , 153 Investigations into the rewarding effects of intra‐VTA opioid administration verified the role of MORs in the VTA. Intra‐VTA microinjection of the selective MOR agonist DAMGO could induce drug‐associated conditioned place preferences in a dose‐dependent manner. 154 In another study, bilateral microinjections of morphine into the VTA had reinforcing effects of morphine. 155 Administration of the endogenous MOR ligand endomorphine‐1 into the VTA, especially the posterior part of the VTA, could elicit reward and increase locomotor activity. 141 On the contrary, after NX methiodide, the MOR antagonist, was injected into the VTA, conditioned place preference induced by morphine was blocked compared with that of the control. 156
The RMTg, or the posterior tip of the VTA, is predominantly composed of GABAergic neurons, and their major projections are the DA neurons in the VTA. 124 The RMTg is also believed to mediate the rewarding effects of opioid administration. The RMTg GABAergic neurons were thought to provide potent inhibition to the VTA, and stimulation of RMTg GABAergic neurons could inhibit over 90% of the VTA DA neurons. 148 Also, collected data showed that RMTg GABAergic neurons expressed high levels of MORs. 157 After acute exposure to the psychostimulant methamphetamine or aversive stimuli of footshocks, food deprivation or reward omission, the immediate early gene Fos and its product FOS were detected in RMTg GABAergic neurons. 147 , 158 These results suggest the potential role of RMTg in reward processing and drug addiction. Another hypothesis of the MOR‐mediated reward effect arises from RMTg‐involved disinhibition of the VTA. Opioids activate MORs in the GABAergic neurons of the RMTg and inhibit the activation of these GABAergic neurons, resulting in disinhibition of VTA DA neurons and eventually an increased level of DA. Pharmacological studies have revealed the involvement of RMTg in the morphine reward process. After systemic morphine administration, the firing rate of VTA DA neurons increases through the activation of MORs in the RMTg GABAergic neurons. 159 The inhibitory input from the RMTg controls the spontaneous firing activity of the VTA DA neurons. Electrical stimulation of the RMTg could elicit a total suppression of spontaneous activity in about half of the VTA DA neurons, 160 and intravenous morphine could suppress the RMTg‐induced inhibition of DA neurons in vivo. 160 Apart from morphine, the selective MOR agonist DAMGO can also decrease the spontaneous firing rate of RMTg neurons and cause hyperpolarization. 161 Moreover, RMTg infusion of morphine and DAMGO could increase locomotor behavior in rats through MORs. 162 , 163 Apart from opioid‐induced reward effects, MORs in the RMTg GABAergic neurons also regulate ethanol consumption and related conditioned place preference behavior. 164
As mentioned above, VTA DA neurons receive inhibitory inputs from both neighboring GABAergic interneurons within the VTA and GABAergic interneurons in the RMTg. Notably, after being exposed to the endogenous MOR agonist [Met5]encephalin, the input from RMTg is more strongly inhibited than those cells from the local interneurons, with GABA‐A inhibitory postsynaptic currents (IPSCs) of the former decreasing by about 75% and the latter decreasing about 17%. 165 These results suggested that the disinhibition of VTA after morphine exposure is mostly mediated by the GABA (RMTg)‐DA (VTA) pathway rather than the local GABA (VTA)‐DA (VTA) pathway.
3.2. NAc
The major output regulating drug reward from the VTA DA neurons is the NAc, which is part of the ventral striatum. The medium spiny neurons (MSNs) are the subject of VTA DA projections and the predominant cellular population in the NAc. NAc MSNs belong to a heterogeneous group of GABAergic neurons that express different DA receptors, the D1 receptor or D2 receptor. 166 D1 and D2 MSNs have different projections, with D1 MSNs being part of the “direct” pathway, which increases thalamocortical drive‐force, and D2 MSNs constituting the “indirect” pathway, which decreases thalamocortical drive. 123 , 167 Anatomically, the NAc can be segregated into the core, and the shell and MSNs within the different regions have different drug‐induced alterations. 168 The MSNs in the NAc core could discriminate the motivational value of conditioned stimuli through the integration of information and synaptic plasticity at spines on the cellular surface, while MSNs in the NAc shell are involved in the behavioral consequences of repeated administration of addictive drugs. 169 Selective stimulation of D1 receptor‐expressing MSNs of the direct pathway is sufficient to induce persistent reinforcement in both operant and place preference tasks, while activation of D2 MSNs of the indirect pathway could induce transient punishment. 170
In the NAc, MORs are mainly expressed on D1 MSNs of the direct pathway rather than the D2 MSNs of the indirect pathway. 171 By using a bacterial artificial chromosome‐mediated transgenic rescue strategy to re‐express MORs in D1 MSNs of the MOR KO mice, the opioid rewarding model, opiate‐induced striatal DA release, and motivation to self‐administer opiate would be restored. 171 Moreover, morphine infused into the NAc was reported to induce and maintain self‐administration behaviors. 172 , 173 On the contrary, local injection of methylnaloxonium, the MORs selective antagonist, into the NAc could significantly reduce the locomotor activation effect produced by subcutaneous injection of herorin (diacetylmorphine). 174 Other studies have interrogated the role of MORs of the NAc in the addiction other than opioid substances. MORs in the NAc shell contributes to promote binge‐like consumption of palatable foods 145 , and they could also promote alcohol consumption, seeking and conditioned reinforcement by enhancing the incentive motivation. 175 MORs in the NAc participate in the maintenance of local microcircuitry. A recent study demonstrated that a decrease in the copy number of MORs in the NAc resulted in increased inhibitory synaptic transmission in D2 MSN of the NAc as well as an increase in the expression of gephyrin mRNA and the density of inhibitory synaptic. 176 Considering the crucial regulation of D2‐MSN on VTA DA neurons, such alteration caused by MORs copy number changes is supposed to be involved in reward and addiction behavior. A more recent study by Castro et al. reported that MORs regulated reward consumption behavior in mice acting through the circuit from the dorsal raphe to the NAc, and MORs‐mediated inhibition of raphe terminals is necessary and sufficient to determine the consummatory response with the source of endogenous ligands of MORs from NAc enkephalin neurons. 177 This study revealed a novel endogenous opioid circuit that determines state‐dependent reward consumption.
3.3. Doral striatum
The brain striatal complex can be anatomically divided into the dorsal striatum and the NAc, and the former can further be separated into four sub‐territories with different neurochemical and neuroanatomical properties, the DL, the dorsomedial (DM), the ventrolateral (VL), and the VM 178 , 179 (Figure 3). The corticostriatal network is believed to control heterogeneous decision‐making processes, including both goal‐directed and stimulus bound, highly involved in reward and drug addiction. 180 , 181 , 182 The DL striatum goes through drastic neurochemical and functional changes during drug addiction. In rats that were trained to self‐administer cocaine, glutamate signaling increased in the DL striatum, and antagonism of AMPA receptor increased the efficacy of cue extinction to reduce drug craving. 183 Meanwhile, in rats tolerant to ethanol and nicotine, long‐term depression was occluded in the glutamatergic synapses in the DL striatum, suggesting the contribution of drug addiction to the alteration of synaptic plasticity. 184 The involvement of the DL striatum in stimulus‐response learning was further evidenced by a study where ablation of the neurons of the patch compartment of the DL striatum by dermorphin‐saporin resulted in reduced reinstatement of sucrose self‐administration after sucrose devaluation. 185 Interestingly, the involvement of the DL striatum in drug abuse seems to be influenced by gender as is suggested by the activation of G protein‐coupled estradiol receptor 1 in the DL striatum that could enhance motivation for cocaine and drug‐induced reinstatement in females rather than male rats. 186 , 187 The involvement of the DM striatum has been implicated by the fact that animals with DM striatum excitotoxic lesions selectively affected the behavioral adjustment to a situation involving reward uncertainty, 188 and repeated nicotine administration altered the local field potential in the DM striatum. 189 More recent works revealed the differential involvement of the MSNs of the direct (dMSNs) and indirect pathways (iMSNs) of the DM striatum. In a probabilistic Pavlovian conditioning task, dMSNs were involved in suppressing ongoing licking behavior, while iMSNs contributed to outcome‐dependent behavioral adjustment. 190 More importantly, another study found that optogenetic stimulation of the DM striatum‐external globus pallidus (GPe) iMSNs reduced ethanol‐containing reward‐seeking, whereas optogenetic inhibition of the DM striatum‐GPe iMSNs reversed this change. 191 The activity of MSNs in the DM striatum and related reward behavior is regulated by local astrocytes, at least in part. Kang et al. reported that activation of DM striatum astrocytes decreased the spontaneous excitatory postsynaptic currents (sEPSCs) in dMSNs while increasing sEPSCs in iMSNs and facilitated shifting from habitual to goal‐directed reward‐seeking behavior. 192 Little attention has been given to the regulatory role of the VM and VL striatum in reward and addiction. One possible explanation might be that the VM and VL striatum are in the proximity of the NAc, and the majority of studies focused on the NAc rather than the VM and VL striatum. In fact, generally, the NAc is referred to as the “ventral striatum” in the literature. 193 , 194 , 195 Further studies are needed to elucidate the role of VM and the VL striatum in the control of reward and addiction.
FIGURE 3.
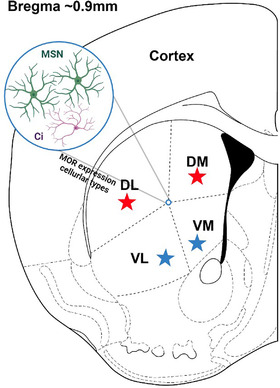
Schematic coronary view of the dorsal thalamus. The dorsal striatum is located dorsally to the nucleus accumbens and can be subdivided into four territories according to the spatial distribution. The four territories of the dorsal striatum include the DL striatum, the DM striatum, the VL striatum, and the VM striatum. MORs are highly expressed on MSNs and Ci in the dorsal striatum. The DL striatum and DM striatum were reported to be involved in the drug addiction process (denoted as red star), while little attention has been given to the role of the VL and VM striatum (denoted as blue star) in drug addiction. Ci, cholinergic interneuron; DL, dorsolateral; DM, dorsomedial; MSN, medium spiny neuron;; VL, ventrolateral; VM, ventromedial
The involvement of dorsal striatum MORs in the regulation of reward and addiction was unraveled by early studies showing that opiate administration could increase the DA concentration in the dorsal caudate nucleus in rats. 196 Further studies demonstrated that in the dorsal striatum, presynaptic activation of MORs reduced glutamate and GABA release, postsynaptic activation of MORs reduced DA release, and MORs mediated the long‐term depression of excitatory inputs to the dorsal striatum. 197 , 198 , 199 , 200 Interestingly, MORs‐dependent modulation of basal DA transmission in the rat dorsal striatum was reported to be region‐specific as evidenced by the observation that DAMGO (an MORs selective agonist) in the rostral and caudal dorsal striatum reduced DA levels, while it increased DA levels in the medial dorsal striatum. 201 Moreover, prolonged treatment with morphine led to reduced dendritic arborization and loss of dendritic spines in the MSNs of the dorsal striatum, which was mediated by D4 receptors on MSNs. 202 In spite of the aforementioned studies, there are currently few studies directly investigating the causal relationship between MORs and drug abuse behaviors. Further studies that directly regulate MORs in different cellular and structural compartments of the dorsal striatum by genetic manipulation methods or optogenetics might provide more knowledge.
3.4. PFC
The NAc not only receives DA inputs from the VTA but also receives glutamatergic inputs from the PFC, the thalamus, the ventral hippocampus, and the amygdala. 203 , 204 Cellular adaptions in the glutamatergic projection from the PFC to the NAc have been discovered in rats withdrawn from cocaine; that is, the altered G protein signaling in the PFC underlies the behavior on drug‐related stimuli, while dysregulated PFC‐NAc synaptic glutamatergic transmission could be the reason for the unmanageable drug‐seeking. 205 The PFC also has a regional preference in its projection to the NAc. The infralimbic (IL) medial PFC (mPFC) largely projects to the shell of the NAc, and the prelimbic (PrL) mPFC prefers the core of the NAc. 204 Optogenetic activation of the IL‐mPFC‐NAc and PrL‐mPFC‐NAc exerts different effects with the former potentiating and the latter inhibiting cocaine craving behavior, respectively. 206 In addition, another study reported that optogenetic stimulation of the PFC‐NAc pathway promoted conditioned reward‐seeking behavior after learning, while activity in activation of the PFC‐paraventricular nucleus (PVT) of the thalamus suppressed both the acquisition and expression of conditioned reward‐seeking. 207 Recently, by means of single‐cell RNA sequencing, a broad impact of cocaine on transcription was observed across the PFC. Especially during the withdrawal phase, the transcriptional impact is extremely prominent. 208
Opioid receptors in the PFC have unneglectable modulatory effects on both the morphological and functional properties of the local network, which subsequently interferes with the output signal of the PFC and thus influences the reward circuitry. A postmortem study found that in opioid drug abusers, the expression of the GluN1 and GluN2B but not the GluN2A subunits of the NMDA receptors was increased in both the mPFC and the lateral PFC. 209 Moreover, further animal studies demonstrated that chronic morphine administration could significantly increase the total dendrite length and dendritic complexity of both PV interneurons and somatostatin interneurons in the mPFC. 210 Another study showed that chronic cocaine administration increased the level of MORs mRNA in the PFC. 211 As for the functional regulatory role of MORs in the PFC, Witkowski et al. found that the activation of MORs expressed in the non‐pyramidal neurons of the mPFC inhibited the voltage‐dependent Na+ currents in a protein kinase A‐ and PKC‐dependent manner, 212 while Olianas discovered that concomitant activation of DORs and MORs in the mPFC potentiated DA D1‐like receptor signaling. 213 DA signaling is indispensable for MORs to exert their modulatory roles. Infusions of DAMGO into the VM PFC could induce augmented sucrose‐reinforced responding, while blocking the D1 receptors of the VM PFC simultaneously attenuated such effect, suggesting that D1 tone plays an enabling or permissive role in the expression of MORs‐elicited effects. 214 This study also demonstrated that simultaneous targeting of the MORs and the DA system might be a more efficacious strategy to counter addiction characterized by dysregulated appetitive motivation.
3.5. Thalamus
Inputs from subregions of the thalamus to the NAc have also been characterized to regulate the drug rewarding process. The PVT and the supramammillary nucleus (SuM) are two widely investigated regions. The PVT is a part of the midline and intralaminar thalamic group and is an interface for brain reward circuits. 215 Previous studies demonstrated that the activation of the PVT is also associated with a predisposition to reinstate to cocaine‐seeking elicited by drug‐related cues, 216 while inactivation of the PVT could prevent the conditioned place preference induced by cocaine. 217 A further two‐photon calcium imaging study by Otis et al. found that PVT neurons projecting to the NAc developed inhibitory responses to reward‐predictive cues coding for both cue‐reward associative information and behavior that were directed by the activity of prefrontal and lateral hypothalamic afferent axons, 218 further confirming the relaying characteristics of PVT‐NAc projection in the reward circuits. The input from the PVT to the NAc mediates the expression of opioid‐withdrawal‐induced physical signs and aversive memory, and activation of this input pathway is sufficient and necessary to mediate behavioral aversion. 219 Chronic morphine exposure could selectively potentiate the excitatory transmission between the PVT and the D2 MSNs in the NAc. 219
The SuM is localized in the posterior hypothalamic and is thought to participate in the drug reward process. 124 The SuM can mediate reward triggered by the GABAA receptor antagonist picrotoxin, nicotine, and the glutamate receptor agonist AMPA. 143 , 220 , 221 The SuM is also reported to interact with the VTA‐NAc system in reward. SuM injections of AMPA were reported to increase extracellular DA in the NAc measured by microdialysis. 220 In addition, intra‐VTA injections of the cholinergic agonist carbachol, which is reported to induce reward effects such as conditioned place preference and vigorous locomotion, could induce significant c‐Fos expression in the SuM, and such an increase in the c‐Fos is positively correlated with vigorous locomotion induced by the intra‐VTA injections of carbachol. 222 These findings suggest the role of SuM in the reward process, but its detailed participation needs further articulation. Although the mRNA of MORs was detected in the SuM, 223 the particular role of SuM MORs in reward and drug addiction remains to be understood in future work.
3.6. Hypothalamus
The hypothalamus, especially the lateral hypothalamus (LH), has also been reported to be involved in drug addiction and reward as is directly evidenced by the observation that deep brain stimulation of the LH reduced cocaine‐seeking behavior. 224 The hypothalamus, including the LH, is enriched in neuropeptides that have important roles in thermoregulation, sleep, feeding, sex drive, and general motivational behavior. 225 As for the role in drug addiction, the most widely recognized LH neuropeptide is orexin, also named hypocretin. Orexins, including orexin A and orexin B, were identified as endogenous ligands for GPCRs of two orexin receptors, OxR1 and OxR2. 226 , 227 Orexins are widely recognized for their crucial regulatory roles in feeding behavior and circadian rhythm as evidenced by the reports that intracerebroventricular injection of orexins induced feeding in rodents, and orexin deficiency caused narcolepsy in humans and animals. 228 , 229 , 230 In addition to feeding and circadian rhythm, orexin neurons in LH are important players in reward processing and drug abuse. 231 Early studies demonstrated that rewarding stimulus would activate orexin neurons in the LH. Harris et al. reported that the activation of LH orexin neurons was linked to preferences for cues associated with drug and food reward, and chemical activation of these groups of neurons reinstated an extinguished drug‐seeking behavior that was completely blocked by prior administration of orexin antagonist. 232 In another report, consistently, chronic exposure to amphetamine for 5 days also activated LH orexin neurons. 225 Moreover, the rewarding‐behavior corresponding activation of orexin neurons was reported to be exclusively located in the LH rather than orexin neurons outside the LH such as in the perifornical or DM hypothalamus, or non‐orexin neurons in the LH. 225 Neuroanatomically, the control of LH orexin neurons over drug addiction is mostly determined by its connectivity with the VTA. Projections from the LH to the VTA affect both DA neurons and GABAergic interneurons in the VTA, the two critical types of neurons in the VTA that regulate reward and addiction as previously mentioned. Borgland et al. reported that orexin A induced potentiation of NMDA‐mediated neurotransmission in VTA DA neuron synapses in vitro and that in vivo administration of an OxR1 antagonist occluded cocaine‐induced potentiation of excitatory currents in VTA DA neurons, suggesting the role of orexin signaling in neural plasticity of VTA DA neurons. 233 In another report, stimulation of LH orexin neurons mainly inhibited VTA DA neurons and activated GABAergic neurons, suggesting that the effect of orexin on VTA DA neurons was strongly mediated by local interneurons. 225
Around 50% of LH orexin neurons express MORs. 234 Early studies in which morphine was injected into the LH enhanced local c‐Fos expression and food consumption confirmed the role of MORs in the regulation of the function of LH. 235 Further study investigating the gene expression profile of LH indicated that chronic morphine exposure significantly altered the gene expression of LH in morphine‐dependent mice, suggesting that LH MORs might contribute to the morphine addictive response. 236 Moreover, upon morphine withdrawal after chronic exposure, the mRNA levels of both MORs and orexin increased. 234 MORs in the LH regulate feeding behavior through orexin neurons; 237 however, the exact relationship and mechanism of MORs and orexin in the regulation of addiction‐related behavior are not clear.
3.7. Amygdala and the extended amygdala (EA)
The amygdala also sends glutamatergic projections to the NA, and its effects are thought to be mediated by specific DA receptors, with D1 agonists but not D2 agonists attenuating amygdaloid inputs. The basolateral amygdala (BLA), which has a crucial role in emotional learning, is critical for the reward modulation. The BLA neuronal response to rewarding cues precedes the reaction of NAc neurons, and cue‐evoked excitation of NAc neurons depends on BLA input. 238 In mice, optogenetic stimulation of the pathway from the BLA to the NAc could reinforce behavioral self‐stimulation of these synaptic inputs, and this effect relies on NAc D1 receptors rather than D2 receptors. 239 On the other hand, optogenetic inhibition of the BLA‐NAc pathway could reduce cue‐evoked intake of sucrose. 239 In drug addiction, incentive motivation often becomes narrowly focused on the particular drug of abuse. Central nucleus of amygdala (CeA) activation could facilitate this kind of narrowing of motivation. When rats are provided the option for intravenous cocaine exposure, optogenetic stimulation of the CeA could intensify that option to become the exclusive focus of pursuit and consumption. 240 The centromedial nucleus of the amygdala (CeMA) also has a role in reward‐related behaviors. Optogenetic activation of GABAergic projection from the CeMA to the VM PFC could produce a positive reward‐like phenotype in real‐time place preference and increase locomotor activity and nose‐poking effort in sucrose operant conditioning. 241
The EA is a basal forebrain macrostructure situated between the amygdala and the striatopalidum, running from the dorsal amygdala through the substantia innominate to the bed nuclei of the stria terminalis (BNST) and the NAc shell 242 , 243 (Figure 4). The EA can be separated into the central EA and the medial EA, with the former part containing the CeA and the lateral portions of the BNST and the medial EA containing the medial nucleus of the amygdala and medial BNST. 244 It remains controversial whether the shell of the NAc is part of the EA. 245 , 246 EA is an important player in drug addiction as well as reward circuitry. EA is highly involved in the control of aversive processes, such as fear responses, stress, anxiety, and particularly opioid withdrawal effects. 247 , 248 It has been demonstrated that the application of NX to the CeA could elicit withdrawal jumping behavior in morphine‐dependent rats, while bilateral electrolytic lesion of the CeA eliminated the withdrawal jumping behavior. 249 Notably, chronic morphine withdrawal increased the firing rate of the PV interneurons in the CeA, while optogenetic inhibition of the activity of CeA PV interneurons attenuated the morphine withdrawal‐induced negative affective states, such as aversive, anxiety, and anhedonic‐like behaviors. 250 This study suggested that the MORs on the PV interneuron might play a major role in CeA‐regulated withdrawal behavior. In addition to the control of aversive situations, EA has also been suggested in reward learning and opioid addiction. When food reward or cocaine was earned by operant responding, the excitatory glutamatergic neurotransmission increased in the ventro‐lateral BNST, 251 suggesting the involvement of the BNST after an appetitive stimulus. The BNST is heavily innervated by mesolimbic DA neurons originating in the VTA, and morphine, nicotine, cocaine, and ethanol could significantly increase the extracellular DA in the BNST, suggesting the sensitivity of the BNST to the DA stimulant actions of drugs of abuse. 252 Blocking the DA D‐1 receptor in the BNST decreased the cocaine reinforcement effect. 253 Chronic activation of MORs in the central EA led to dysregulation of genes clustered into neurogenesis, cell growth, and signaling proteins, suggesting that MORs in the central EA contributed to drug‐induced neural plasticity. 254 Accordingly, microinjections of the opiate receptor antagonist methylnaloxonium into the BNST suppressed heroin self‐administration in dependent rats, further confirming the role of EA MORs in drug addiction.
FIGURE 4.
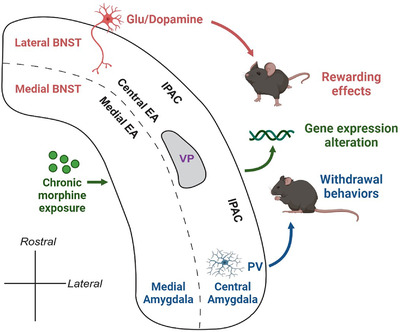
Schematic horizontal view of the extended amygdala (EA) continuum showing the composition of brain regions. The central EA extends from the central nucleus of the amygdala through IPAC to the lateral bed nuclei of the stria terminalis (BNST). Central EA surrounds the VP. The medial EA is located medially to the central EA and contains the medial nucleus of the amygdala and the lateral BNST. Glu and dopaminergic neurotransmission in the BNST is involved in the rewarding aspects of drug addiction, while PV interneurons are involved in withdrawal behaviors of dependent subjects. Chronic morphine exposure could significantly alter the gene expression profiles of EA. Glu, Glutamatergic; IPAC, interstitial nucleus of the posterior limb of the anterior commissure; PV, parvalbumin; VP, ventral pallidum
Evidence exists that MORs in the nuclei of the amygdala and EA contribute to the regulation of local activity and reward‐related behaviors. Prenatal morphine exposure in male rats reduced and increased the density of MORs in the BLA and CeA, respectively, without influencing the MORs level in the BNST. 255 In the BLA, MORs are involved in the encoding of incentive value, which underlies the development of the desire for morphine continuous intake. 256 Another study demonstrated that BLA MORs mediated the cued recall of reward memories, allowing rats to motivate action. 257 Evidence indicates that opioid abuse is associated with NMDA receptor‐dependent plasticity in the CeA and BNST. Beckerman et al. found that GluN1 and MORs were co‐expressed in CeA‐BNST projecting neurons and in the axonal terminals in the BNST, and further deletion of GluN1 in CeA neurons resulted in the decrease in morphine‐induced fos expression in the ventral BNST. 258 This study suggested that NMDA receptors are essential for MORs‐mediated activity in the BNST and opioid addictive behaviors. Moreover, GluR2‐expressing non‐calcium‐permeable AMPA receptors were also reported to be colocalized with MORs in the CeA. 259 However, its implication for reward behaviors remains to be elucidated. MORs activation significantly altered the gene expression profiles in EA, where samples of CeA and BNST were pooled together, 254 where chronic morphine exposure, instead of a single morphine injection, induced overrepresentation of genes governing neurogenesis, cell growth and signaling protein categories from gene ontology analysis. 254
3.8. Hippocampus
The hippocampus is also involved in reward behavior. A population of cells associated with reward has been identified in the hippocampus, and hippocampal activity could be altered under contextual rewarding stimuli. 260 , 261 , 262 The hippocampus also has glutamatergic projections to the NAc, and this kind of innervation is critical for the reward response. Induction of long‐term potential at the synapses of the hippocampus‐NAc inputs could drive conditioned place preference, and the activity of these synapses is required for the response to natural reward. 263 The ventral hippocampus is involved in associative memory and emotional behavior, which are associated with morphine exposure. The morphine‐induced conditioned place preference could significantly facilitate neurogenesis in the ventral dentate gyrus (DG) and increase the dendritic spine density in both the CA1 and DG, 264 suggesting that morphine‐induced reward memory is related to neural and synaptic plasticity in the ventral hippocampus.
The hippocampus is the hub of memory. 265 Reward‐related learning and memory contribute to compulsive drug use and addiction. 266 It was reported that MORs agonists could significantly increase the amplitude of sharp waves and the occurrence of sharp‐wave ripples (a specific electrophysiological activity pattern of the hippocampus underlying the consolidation of memory), as well as increase the network excitability of CA1 regions, suggesting that MORs in the CA1 might contribute to addiction by enhancing drug memory consolidation. 267 Moreover, the adult hippocampus is an active site of neurogenesis where neural stem cells in the DG undergo proliferation and differentiation. 268 Zhang et al. demonstrated that morphine self‐administration, a paradigm mimicking human opiate addiction, increased neural stem cells differentiation and dendritic growth in the adult DG, which is mediated by MORs expressed on neural stem cells. Moreover, they found that conditional overexpression of MORs in DG neural stem cells led to enhanced morphine self‐administration, confirming the role of DG MORs in the establishment of morphine addiction. 269 Furthermore, another two studies reported that cocaine addiction increased the MORs expression and functionality in the rat hippocampus, 270 and adolescent morphine exposure enhanced the MORs‐mediated G‐protein activity in the hippocampus and morphine preference in the conditioned place preference test in adult mice. 271 These two studies provide us with a glimpse of hippocampal MORs alteration under drug addiction situations.
3.9. Laterodorsal tegmental (LDT) nucleus and pedunculopontine tegmental (PPT) nucleus
The LDT nucleus and the PPT nucleus send projections to the VTA, and they are also found to be involved in drug rewarding behavior. 272 , 273 Both the LDT and PPT have heterogeneous cellular subpopulations of cholinergic (acetyltransferase, [ChAT]), GABAergic (glutamic acid decarboxylase [GAD]) and glutamatergic (vesicular glutamate transporters 2) cells. 274 Electrical stimulation of the LDT and PPT could evoke DA increase in the NAc and striatum, respectively, with the former relying on the nicotinic and glutamatergic receptors in the VTA and the latter relying on the nicotinic and glutamatergic receptors in the substantia nigra. 275 , 276 Optogenetic studies have revealed the role of LDT and PPT in rewarding behavior. The LDT preferentially sends synapses onto VTA DA neurons projecting to the NAc lateral shell, and optogenetic stimulation of the LDT‐VTA inputs could elicit reward such as strong conditioned place preference. 277 Furthermore, optogenetic intracranial self‐stimulation of the LDT‐VTA inputs could increase DA in the NAc, and this increase depends on NAc D1 and D2 receptors. 278 On the other hand, in rats, selective optogenetic stimulation of PPT cholinergic inputs to the VTA could result in positive reinforcement. 279 Optogenetic activation of glutamatergic PPT projection to the VTA could preferentially excite VTA DA neurons, and this is sufficient to induce behavioral reinforcement. 280 These results suggest the potential role of LDT and PPT in drug addiction.
Studies regarding MORs in the LDT and PPT and the relevance with reward and addiction are limited. However, the existing studies demonstrate that MORs in LDT and PPT play a regulatory role in drug addiction. An early study by Klitenick et al. reported that bilateral microinjections of DAMGO into the PPT elicited a dose‐dependent increase in motor activity and also an increase in extracellular DA content in the NAc, 281 a core brain region involved in the reward circuitry as previously described. However, this study does not deal with drug addiction behavior. Further study conducted by Corrigall et al. provided direct evidence that infusion of DAMGO into the PPT produced a dose‐related reduction in the number of cocaine self‐administration, suggesting that the MORs in the PPT could influence drug‐reinforced behavior. 282 The influence of PPT or LDT MORs on drug addiction might be mediated by their projection to RMTg. RMTg was reported to receive inputs from LDT and PPT. 146 Wasserman et al. further demonstrated that LDT and PPT cholinergic neurons project to RMTg, and such cholinergic inhibition of RMTg GABA neurons via M4 muscarinic receptors facilitates the opioid inhibition on the same neuron. 157 Future studies are needed to focus on the direct functional influence of MORs on LDT and PPT local circuitry and its relevance to drug addiction.
4. MORS AND OPIOID WITHDRAWAL SYNDROME (OWS)
4.1. OWS
As previously described, MORs play a crucial regulatory role in the reward circuitry through which opioids exert their rewarding and hedonic effects. The rewarding and hedonic effects of drug addiction belong to one important aspect, which initiates and urges drug users to continuously pursue the euphoria provided by the drugs. Noticeably, another indispensable aspect of opioid addiction is the OWS, which usually leads to the failure of the attempt to get rid of the use of opioids and reinforces addiction behavior. 283 In fact, in addition to rewarding effects, negative reinforcement is a recognized model that composes addiction, pointing out that escape or avoidance of negative affect is the principal motive for addictive drug use. 284 In the following part of this review, we will continue to discuss the role of MORs in OWS, followed by introducing current strategies to treat opioid addiction and dependence by targeting MORs.
The severity of OWS varies among different patients depending on the type of the abused opioid, the duration of use, the medication history and family history. 285 , 286 There is a time course of OWS after opioid discontinuation, with the severity of OWS symptoms peaking during the early phase of discontinuation and gradually tails off into the late phase. Abrupt discontinuation from short‐acting opioids such as heroin and oxycodone is responsible for intense OWS that usually begins within 12 h after opioid cessation, peaks at 36–72 h and then gradually calms down during the following 4–7 days. On the contrary, abrupt withdrawal from long‐lasting opioid, such as BUP, usually demonstrates less severe OWS than short‐acting opioids, but the OWS could last for 2 weeks or even more. 27 However, regardless of the categories of the abused opioid, the OWS during the acute phase of discontinuation is usually difficult for the patient to bear, and without proper treatments, many patients are ultimately unable to endure the withdrawal process, resulting in opioid relapse. 287 , 288 Acute withdrawal syndrome is usually composed of symptoms including aches, muscle spasms, abdominal cramps, nausea/vomiting/diarrhea, irritability, insomnia, tachycardia, lacrimation, sweating, and rhinorrhea. 285 , 289 Moreover, emotional deficits such as despair, anxiety, and anhedonia could also develop after opioid withdrawal. 290 , 291 In patients who initially took opioid because of chronic pain, 56.5% of them chose to continuously use opioid in order to avoid withdrawal symptoms. 292 In addition, after short‐term medically supervised withdrawal from opioids without long‐term medication assistance, the relapse rate is as high as 77%, 293 and this is usually associated with death resulting from drug overdose. 294 , 295 Thus, withdrawal symptoms often lead to the failure of opioid discontinuation, reinforce opioid addiction and bring about serious consequences to the abusers.
4.2. The role of MORs in opioid withdrawal
The prolonged exposure to opioid results in multiple adaptive changes in the CNS. The OWS and related dependence on opioid is mainly attributed to alterations in the locus coeruleus (LC), which is mediated by the chronic activation of the MORs (Figure 5). The majority of neurons in the LC are adrenergic neurons or neurons containing norepinephrine (NA), projecting to various regions of the brain, including the PFC, the hippocampus and the amygdala. 296 Under physiological conditions, the LC adrenergic neurons projections can stimulate wakefulness, regulate breath and blood pressure, and maintain alertness. 297 , 298 , 299 MORs are abundant in LC adrenergic neurons. When opioids bind to and activate LC adrenergic MORs, they can inhibit the activity of adenylyl cyclase (AC), thus suppressg the production of cAMP, leading to decreased release of NA eventually. The decreased NA innervation could result in acute opioid effects, such as drowsiness, reduced respiration and blood pressure, and decreased muscle tone. 27 , 299 Upon chronically repeated doses of opioids, adaptions of the LC neurons occur, and the level of cAMP gradually returns to a normal level before opioid exposure; thus, the LC neurons then release the normal amount of NA. However, when opioids are no longer supplied and the inhibitory effect of repeated opioids on LC NA neurons is eliminated, the neurons would produce excessive amount of cAMP, and NA would be excessively released, triggering aches, muscle spasms, abdominal cramps, anxiety, and so forth, the symptoms of OWS. 27 , 299
FIGURE 5.
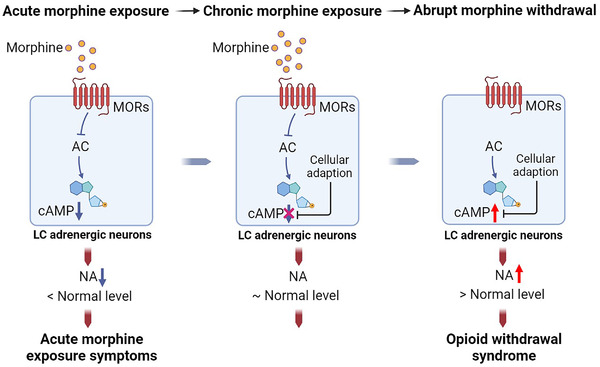
The role of MORs in the development of opioid withdrawal syndrome. MORs are highly expressed in adrenergic neurons in the locus coeruleus (LC). During acute morphine exposure, the activation of MORs by morphine inhibits the activity of adenylyl cyclase (AC), which leads to a decrease in cAMP and subsequent norepinephrine (NA) release. When morphine is chronically administered, adaptation of the LC adrenergic neurons results in the normalization of intracellular cAMP levels. When the supply of morphine stops, the inhibitory effects of morphine on AC diminish, leading to the excess production of cAMP and release of NA, which triggers the occurrence of morphine withdrawal syndrome, including symptoms of aches, muscle spasms, anxiety, and so forth
As previously mentioned, after being activated by opioids, the binding of Gi protein to MORs induces the release of α subunit, and the α subunit inhibits the activity of AC and thus reduces the intracellular levels of cAMP. 110 , 111 , 112 , 113 Sustained activation of LC NA neurons would uncouple MORs from the Gi protein α subunit, resulting in the reduction of the inhibitory effect on AC activity and the re‐upregulation of the AC/cAMP pathway. 300 , 301 The MORs located on the lipid raft are necessary for the superactivation of AC after chronic opioid exposure. After long‐term MOR agonists (morphine, etorphine, and methadone) treatment, the majority of MORs remain on the lipid raft; the treatment of methyl‐beta‐cyclodextrin, a raft‐disrupting agent, could completely blunt the AC superactivation. 302 Apart from the abovementioned Giα uncoupling mechanism, AC superactivation is also mediated by Src kinase. Chronic activation of MORs could further recruit Src kinase to phosphorylate MOR at Tyr336 within the NPXXY motif, and the lack of such Src kinase‐mediated phosphorylation of MORs results in complete blunting of AC activation. 303 , 304
MORs in other regions are also reported to be involved in the generation of withdrawal syndrome. MORs in the dorsal raphe nucleus area (DRN) contribute to the depressive symptoms after opioid abstinence. Genetic KO of MORs in the serotonergic neurons in the DRN before opioid exposure could abolish the development of social withdrawal after opioid withdrawal. 305 This result implies that MORs could regulate serotonergic transmission in the DRN and MORs alteration after chronic opioid exposure may contribute to the psychiatric symptoms during opioid withdrawal. Another study observed that during the extended withdrawal period of 10 days, the expression of MORs in the caudate‐putamen, frontal, and cingulate cortices was increased. 306 After a withdrawal period of 31 days, there was a decrease in the MORs protein level in the striatum, and this was considered to serve as a substrate for relapse to drug‐seeking. 307
5. CURRENT STRATEGIES TO TREAT OPIOID ADDICTION AND DEPENDENCE VIA MORs
Considering the high prevalence of opioid abuse, the intractable withdrawal and dependence situation of opioid abstinence, and the crucial role of MORs in opioid addiction, it is of great importance to treat opioid addiction focusing on MORs with agents with long‐lasting efficiency. The medication treatment of opioid addiction contributes to the prevention of relapse, which can help the addicted individuals to be stable enough to return to work and normal social interaction with periods of abstinence as long as possible. 308 , 309 The MORs are the main targets of treatment of addiction. As previously discussed, both the analgesic and adverse effects of opioids are mediated by MORs. However, currently, the efforts to design an agent that can exert analgesia without the likelihood of being abused have been unsuccessful. 310 The available treatment options for opioid addiction now are mainly focused on the prevention of the development of dependence, the elimination of dependence, and the suppression of withdrawal symptoms. 19 , 299 , 311
Current pharmacological strategies to tackle opioid addiction and dependence can mainly be classified into the following categories: (1) detoxification therapy using opioid antagonists (NTX and NX); (2) opioid substitution therapy using longer‐acting MORs agonists with less euphoric effects (methadone and BUP, etc.); (3) non‐opioid therapies, that is, α2‐adrenergic receptor agonists clonidine and lofexidine 299 , 309 , 312 (Figure 6). However, medications for opioid use disorders are underused. First, only a small percentage of patients who survived opioid overdose received medications for opioid use disorder. 313 In addition, when medications are prescribed, BUP and methadone are frequently given with insufficient dose and/or for too short duration. 19 Clinical trials on agents to treat opioid use disorders/dependence are summarized in Table 1.
FIGURE 6.
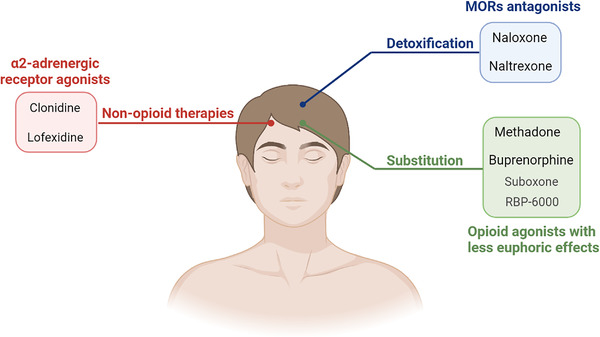
Strategies to treat opioid addiction and dependence. Current strategies to treat opioid addiction mainly involve detoxification therapy followed by maintenance of opioid substitution therapy. Detoxification therapies harness MOR antagonists such as naloxone and naltrexone to reverse the acute intoxication effects. Substitution therapies include dose‐monitored opioid agonists, methadone and buprenorphine (with formulations of Suboxone and RBP‐6000), with lasting and less euphoric effects to reduce withdrawal syndrome. α2‐Adrenergic receptor agonists, including clonidine and lofexidine, are non‐opioid therapies targeting the withdrawal symptoms caused by norepinephrine hyperactivity during opioid abstinence
TABLE 1.
Clinical trials on agents to treat opioid use disorder/opioid withdrawal syndrome
| Agents | Category | Interventions | Main results | Ref. |
|---|---|---|---|---|
| Naloxone (NX) | Mu opioid receptors (MORs) antagonist | Orally taken NX | Orally taken NX improved symptoms of opioid associated constipation and reduced laxative use | 343 , 344 |
| Intravenously taken NX | Intravenous NX reversed the morphine‐induced respiratory depression in healthy volunteers | 345 | ||
| Sustained‐release NX capsule, orally taken | Successful treatment of opioid‐induced constipation without comprising the desired opioid effects | 346 | ||
| Naltrexone (NTX) | MORs antagonist | Extended‐release (XR) NTX, intramuscular injection | XR NTX was associated with a lower rate of opioid relapse than usual treatment | 347 |
| Injectable XR NTX | Injectable XR NTX significantly increased opioid‐free days and decreased craving for opioids in patients with opioid dependence disorder | 348 | ||
| Injectable XR NTX versus oral NTX | Injectable XR NTX was associated with twice the rate of treatment retention at 6 months, compared with oral NTX | 349 | ||
| Methadone | MORs agonist | Daily oral methadone hydrochloride | Both moderate‐ and high‐dose methadone resulted in decreased illicit opioid use. The high‐dose group had significantly greater decreases in illicit opioid use | 350 , 351 |
| Methadone maintenance therapy | Methadone maintenance therapy was efficacious in reducing heroin use and human immunodeficiency virus (HIV) risk behaviors | 352 | ||
| High‐dose and low‐dose methadone | Compared with low‐dose methadone, levomethadyl acetate, buprenorphine (BUP), and high‐dose methadone substantially reduced the use of illicit opioids | 353 | ||
| BUP | MORs agonist | BUP, administered three times a week | BUP substantially reduced the use of illicit opioids, compared with low‐dose methadone | 353 |
| Sublingual tablets consisting of BUP and NX or BUP alone | BUP and NX in combination and BUP alone were safe and reduced the use of opiates and the craving for opiates among opiate‐addicted patients | 354 | ||
| Continuing treatment was 24‐mg BUP‐NX per day for 9 weeks and then tapered to week 12 versus short‐term detoxification was 14‐mg BUP‐NX per day and then tapered to Day 14 | Continuing treatment with BUP‐NX improved the outcome, compared with short‐term detoxification in opioid‐addicted youth | 355 | ||
| Sublingual BUP‐NX tablets, followed by four BUP implants | Compared with placebo, BUP implants resulted in less opioid use over 16 weeks among persons with opioid dependence | 356 | ||
| Sublingual BUP‐NX tablets, followed by four BUP implants | BUP implants did not result in an inferior likelihood of remaining a responder | 357 | ||
| BUP taper versus ongoing BUP maintenance therapy | Tapering was less efficacious than ongoing maintenance treatment in patients with prescription opioid dependence who received BUP therapy in primary care | 358 | ||
| A novel, weekly, subcutaneous BUP depot formulation, CAM2038 | CAM2038 was safely tolerated and produced immediate and sustained opioid blockade and withdrawal suppression | 359 | ||
| Daily BUP hydrochloride with NX hydrochloride versus XR NTX | XR NTX was as effective as BUP‐NX in maintaining short‐term abstinence from heroin | 360 | ||
| Intramuscular XR‐NTX versus daily self‐administered BUP‐NX sublingual film | It was more difficult to initiate patients to XR‐NTX than BUP‐NX, which negatively affected overall relapse. Once initiated, both medications were equally safe and effective | 361 | ||
| Monthly subcutaneous injection of BUP‐XR | Participants' percentage abstinence was significantly higher in BUP‐XR groups than in the placebo group. Treatment with BUP‐XR was also well‐tolerated | 338 | ||
| BUP‐NX versus XR‐NTX | BUP‐NX was more cost‐effective than XR NTX to prevent opioid relapse in patients with opioid use disorder | 362 | ||
| Clonidine | α2‐adrenergic receptor agonist | Clonidine, orally taken | Clonidine was efficacious to block acute opiate‐withdrawal symptoms | 340 |
| Clonidine versus methadone detoxification | Clonidine may not be superior to methadone in terms of the number of patients able to achieve abstinence | 363 | ||
| Opioid‐dependent patients who maintained abstinence during Weeks 5 and 6 were continued on BUP and randomly assigned to receive clonidine or placebo for 14 weeks | Clonidine was an adjunctive maintenance treatment that increased the duration of abstinence | 364 | ||
| Lofexidine | α2‐adrenergic receptor agonist | BUP detoxification versus lofexidine detoxification | BUP is at least as effective as lofexidine opiate detoxification treatment | 365 |
| Lofexidine–NTX versus placebo–NTX | Patients with lofexidine–NTX had higher opioid abstinence rates and improved relapse outcomes as well as attenuated stress and drug cue‐induced opioid craving response | 366 | ||
| Lofexidine and clonidine were each tested as pretreatments once in combination with intramuscular NX | Neither lofexidine nor clonidine suppressed the subjective discomfort of opioid withdrawal or significantly reduced other autonomic signs of opioid withdrawal | 367 |
Typical opioid use disorder treatment involves detoxification therapy followed by maintenance of opioid substitution therapy, with the former aiming at the reversal of the intoxication caused by opioid overuse and the latter aiming at progressive reduction in OWS and relapse. 310 , 314 The detoxification therapy involves opioid antagonists. NX is a potent non‐selective MORs competitive antagonist that can reverse the acute intoxicating effects of opioid overdose such as respiratory depression. 315 NX can exert its effects rapidly. Intramuscular or intravenous administration of NX could restore respiratory depression within 1 to 2 min. 310 Apart from intramuscular or intravenous administration, intranasal NX was reported to be as effective as intravenous NX in reversing both the depressive effects on the CNS and respiratory depression caused by opioid overuse. 316 Intranasal NX provides a convenient approach to administer, which could be extremely useful in some emergency situations. NTX is another MORs antagonist. Studies have found that extended‐release (XR) NTX is effective in maintaining short‐term withdrawal from heroin, 317 , 318 and its economic costs are also acceptable. 319
Opioid substitution therapy involves dose‐controlled, long‐acting opioid agonists with less euphoric effects, which could reduce withdrawal syndrome and inhibit craving for opioids. Methadone and BUP are the two most commonly used agents in opioid substitution therapy. Methadone is a full MORs agonist and remains the gold standard in the treatment of opioid addiction. 320 It has a long half‐life with an average around 22 h, 321 and its efficacy varies with different doses. Lower dosages (20–40 mg/day) of methadone are sufficient to suppress the emergence of opioid withdrawal symptoms, but it may not be enough to block the craving for opioids. 310 , 320 It was demonstrated that a daily dosage ranging from 60 to 100 mg is more effective than a lower dosage in reducing the use of heroin and cocaine during the treatment. 322 In addition to the decrease in opioid use, in a 17‐year longitudinal cohort study, methadone maintenance therapy is also associated with lower rates of offending crime. 323
BUP is a partial MORs agonist with a high affinity for MORs, 324 while it is also an invert agonist at KORs and an antagonist at DORs. 325 In addition, BUP also acts as a full agonist at a fourth category of receptors, the opioid receptor‐like 1 (ORL1, also known as NOP). 326 The interaction of BUP with MORs is characterized by four aspects, including (1) low efficacy or partial agonism, meaning that the maximal effect of BUP is less than that of a full MORs agonist, (2) high affinity, meaning that BUP is difficult to displace from the MORs, (3) high potency, meaning that low doses of BUP might be enough to elicit the same degree of effects by high doses of other agonists, and (4) slow dissociation, meaning that BUP has a long duration of action. 327 Because of its nature as a partial MOR agonist and slow dissociation, BUP is associated with less sedation and euphoria effects than methadone, and it also has long‐term action to treat withdrawal symptoms and decrease mortality. 328 Moreover, BUP does not activate biased signaling of MORs, avoiding the activation of the β‐arrestin pathway and thus diminishing the adverse effects of MORs activation when morphine was co‐administered with BUP. 325 , 329 Notably, BUP also activates ORL1, which complicates its action through MORs. OLR1 has high sequence similarity with other opioid receptors and is coupled with similar second messengers. 330 Lutfy et al. reported that the concomitant activation of OLR1 compromised the MOR‐mediated effects of BUP. 331 This was evidenced by their finding that in ORL1 KO mice, the antinociceptive effect of BUP was markedly enhanced. 332 They further demonstrated that activation of ORL1 by BUP also compromised the rewarding effects of BUP as evidenced by BUP behaving as a full MORs agonist and inducing greater rewarding effects in mice lacking ORL1. 333 Compared with the lower dose of BUP (less than 16 mg/day), a higher dose of BUP (16–32 mg/day) is more efficient to achieve better retention in treatment as suggested by a meta‐analysis. 334 This dose‐dependent effect of BUP could involve its role at ORL1 as suggested by the fact that BUP concentration‐dependently displaced the specific binding of nociception, the endogenous ligand of ORL1. 333 The regulatory importance of ORL1 on the activity of MORs encouraged the development of ORL1/MORs (also called NOP/MOP) bifunctional agonists in order to achieve analgesia without causing addiction and abuse. 335 Fortunately, efforts have been conducted to develop and test such agents. Early investigation illustrated that SR16435, an ORL1/MORs bifunctional agonist, was more potent than morphine in attenuating pain with slower development of tolerance in mice. 336 Recent advances also provided preclinical evidence that AT‐121, which had partial agonist activity at both ORL1 and MORs, exerted morphine‐like analgesia without causing side effects such as respiratory depression, abuse potential, and physical dependence. 337 Currently, the most frequently used formulation of BUP is named Suboxone (BUP:NX, 4:1), which has very low potential to be abused. 299 , 310 Recently, a monthly administered, XR BUP therapy, referred to as RBP‐6000 or BUP‐XR, was found to be applicable to achieve abstinence and was also well‐tolerated. 338 This monthly formulation represents an advance in the treatment of opioid abuse that both enhances the benefits of BUP and reduces the risks of BUP.
The α2‐adrenergic receptor agonists are the representatives of non‐opioid therapies, and they can target the withdrawal symptoms caused by NA hyperactivity during opioid abstinence through autoreceptor feedback inhibition. 339 Clonidine and lofexidine are the two mainstay α2‐adrenergic receptor agonists used to treat opioid addiction, and both have shown efficacy in treating opioid withdrawal. 340 , 341 The efficacy difference is not detected between clonidine and lofexidine, but clonidine is associated with unwanted hypotension than lofexidine more frequently. 342
6. CONCLUSION AND FUTURE PERSPECTIVES
The dominant roles of opioids in analgesia and their strong euphoric temptation determine that opioid abuse and addiction would still be a prevalent problem in the future. MORs play a protagonist part in the reward system regulation and dependence development caused by long‐term adaption, regardless of these effects being wanted or unwanted. MORs also bear the significance of being the target of relieving opioid addiction and dependence. With a more elaborate understanding of the circuitry contributions and signal transduction characteristics of MORs, more effective drugs will be developed to overcome opioids abuse. A possible option is to prepare a monoclonal antibody that targets MORs and stabilizes MORs in a certain conformation. However, concerns of the mode of administration and related adverse effects still exist. In addition, the development of drug screening would help us find more analgesics with mild or even no risks of being addicted. In conclusion, MORs are central to opioid addiction; at the same time, they present us with a possible gate to treat drug abuse and dependence in the future.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Z.J.J. and S.C.G. searched and screened the literature, wrote the draft, and drew the figures and tables. J.M.D. and L.L. assisted in the search of literature, the drawing of figures, and the critical revision of the article. Y.X.M. and Z.N.C. critically revised the article and figures, supervised the coordination and were in charge of correspondence. Z.J.J. and S.C.G. contributed equally to this article. All the authors have agreed to the final submitted version of this article.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Figures in this article were created with the aid of Biorender. This manuscript was supported by National Basic Research Program of China (Grant Number 2015CB553701), the National Science and Technology Major Project (Grant Number 2019ZX09732001), and the Key Military Logistics Research Project (Grant Number BWSXXXX09).
Zhang J‐J, Song C‐G, Dai J‐M, Li L, Yang X‐M, Chen Z‐N. Mechanism of opioid addiction and its intervention therapy: Focusing on the reward circuitry and mu‐opioid receptor. MedComm. 2022;3:e148. 10.1002/mco2.148
Jia‐Jia Zhang and Chang‐Geng Song contributed equally to the study.
Contributor Information
Xiang‐Min Yang, Email: yxiangmind@163.com.
Zhi‐Nan Chen, Email: znchen@fmmu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review, as no new data were created or analyzed.
REFERENCES
- 1. Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391‐5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corder G, Castro DC, Bruchas MR, Scherrer G. Endogenous and exogenous opioids in pain. Annu Rev Neurosci. 2018;41:453‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McQuay H. Opioids in pain management. Lancet. 1999;353(9171):2229‐2232. [DOI] [PubMed] [Google Scholar]
- 4. Carr DB, Goudas LC. Acute pain. Lancet. 1999;353(9169):2051‐2058. [DOI] [PubMed] [Google Scholar]
- 5. Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in children in the emergency department. Lancet. 2016;387(10013):83‐92. [DOI] [PubMed] [Google Scholar]
- 6. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence‐based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58‐e68. [DOI] [PubMed] [Google Scholar]
- 7. Arthur J, Bruera E. Balancing opioid analgesia with the risk of nonmedical opioid use in patients with cancer. Nat Rev Clin Oncol. 2019;16(4):213‐226. [DOI] [PubMed] [Google Scholar]
- 8. Portenoy RK, Ahmed E. Principles of opioid use in cancer pain. J Clin Oncol. 2014;32(16):1662‐1670. [DOI] [PubMed] [Google Scholar]
- 9. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943‐1953. [DOI] [PubMed] [Google Scholar]
- 11. Volkow ND, McLellan AT. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253‐1263. [DOI] [PubMed] [Google Scholar]
- 12. Volkow N, Benveniste H, McLellan AT. Use and misuse of opioids in chronic pain. Annu Rev Med. 2018;69:451‐465. [DOI] [PubMed] [Google Scholar]
- 13. Tobin DG, Lockwood MB, Kimmel PL, et al. Opioids for chronic pain management in patients with dialysis‐dependent kidney failure. Nat Rev Nephrol. 2022;18(2):113‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reuben DB, Alvanzo AA, Ashikaga T, et al. National institutes of health pathways to prevention workshop: the role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295‐300. [DOI] [PubMed] [Google Scholar]
- 15. Nadeau SE. Opioids for chronic noncancer pain: to prescribe or not to prescribe‐What is the question? Neurology. 2015;85(7):646‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: pharmacotherapy and therapeutic monitoring with opioid analgesia. J Am Acad Dermatol. 2015;73(4):575‐582. quiz 583–584. [DOI] [PubMed] [Google Scholar]
- 17. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15):1624‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276‐286. [DOI] [PubMed] [Google Scholar]
- 19. Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208‐216. [DOI] [PubMed] [Google Scholar]
- 20. Zou Z, Wang H, d'Oleire Uquillas F, Wang X, Ding J, Chen H. Definition of substance and non‐substance addiction. Adv Exp Med Biol. 2017;1010:21‐41. [DOI] [PubMed] [Google Scholar]
- 21. Camí J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975‐986. [DOI] [PubMed] [Google Scholar]
- 22. Leshner AI. Science‐based views of drug addiction and its treatment. JAMA. 1999;282(14):1314‐1316. [DOI] [PubMed] [Google Scholar]
- 23. Sussman S, Sussman AN. Considering the definition of addiction. Int J Environ Res Public Health. 2011;8(10):4025‐4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. [DOI] [PubMed] [Google Scholar]
- 26. Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58:143‐159. [DOI] [PubMed] [Google Scholar]
- 27. Kosten TR, Baxter LE. Review article: effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am J Addict. 2019;28(2):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wegman MP, Altice FL, Kaur S, et al. Relapse to opioid use in opioid‐dependent individuals released from compulsory drug detention centres compared with those from voluntary methadone treatment centres in Malaysia: a two‐arm, prospective observational study. Lancet Glob Health. 2017;5(2):e198‐e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose. Med Care. 2013;54(10):901‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55‐70. [DOI] [PubMed] [Google Scholar]
- 31. Peacock A, Bruno R, Gisev N, et al. New psychoactive substances: challenges for drug surveillance. Lancet. 2019;394(10209):1668‐1684. [DOI] [PubMed] [Google Scholar]
- 32. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657‐667. [DOI] [PubMed] [Google Scholar]
- 33. Stockings E, Hall WD, Lynskey M, et al. Prevention, early intervention, harm reduction, and treatment of substance use in young people. Lancet Psychiatry. 2016; 3(3):280‐296. [DOI] [PubMed] [Google Scholar]
- 34. Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry. 2016;3(3):251‐264. [DOI] [PubMed] [Google Scholar]
- 35. Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Opioid receptor types and subtypes: the delta receptor as a model. Annu Rev Pharmacol Toxicol. 1996;36:379‐401. [DOI] [PubMed] [Google Scholar]
- 36. Mafi A, Kim SK. The atomistic level structure for the activated human κ‐opioid receptor bound to the full Gi protein and the MP1104 agonist. Proc Natl Acad Sci USA. 2020;117(11):5836‐5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laugwitz KL, Offermanns S, Spicher K, Schultz G. Mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH‐SY5Y cells. Neuron. 1993;10(2):233‐242. [DOI] [PubMed] [Google Scholar]
- 38. Gillis A, Kliewer A, Kelly E, et al. Critical assessment of G protein‐biased agonism at the μ‐opioid receptor. Trends Pharmacol Sci. 2020;41(12):947‐959. [DOI] [PubMed] [Google Scholar]
- 39. Koehl A, Hu H, Maeda S, et al. Structure of the μ‐opioid receptor‐G(i) protein complex. Nature. 2018;558(7711):547‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stein C. Opioid receptors. Annu Rev Med. 2016;67:433‐451. [DOI] [PubMed] [Google Scholar]
- 41. Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23(4):737‐746. [DOI] [PubMed] [Google Scholar]
- 42. Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine‐induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu‐opioid‐receptor gene. Nature. 1996;383(6603):819‐823. [DOI] [PubMed] [Google Scholar]
- 43. Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19(8):499‐514. [DOI] [PubMed] [Google Scholar]
- 44. Corder G, Tawfik VL, Wang D, et al. Loss of μ opioid receptor signaling in nociceptors, but not microglia. Nat Med. 2017;23(2):164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corder G, Doolen S, Donahue RR, et al. Constitutive μ‐opioid receptor activity leads to long‐term endogenous analgesia and dependence. Science. 2013;341(6152):1394‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koob GF. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021;73(1):163‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Narita M, Funada M, Suzuki T. Regulations of opioid dependence by opioid receptor types. Pharmacol Ther. 2001;89(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 48. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148‐155. [DOI] [PubMed] [Google Scholar]
- 49. Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38(4):217‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manglik A, Lin H, Aryal DK, et al. Structure‐based discovery of opioid analgesics with reduced side effects. Nature. 2016;537(7619):185‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia‐when is enough too much? A review of opioid‐induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558‐1568. [DOI] [PubMed] [Google Scholar]
- 53. Ballantyne JC, Sullivan MD, Koob GF. Refractory dependence on opioid analgesics. Pain. 2019;160(12):2655‐2660. [DOI] [PubMed] [Google Scholar]
- 54. Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine‐induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu‐opioid‐receptor gene. Nature. 1996;383(6603):819‐823. [DOI] [PubMed] [Google Scholar]
- 55. Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65(4):1257‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martin WR. Opioid antagonists. Pharmacol Rev. 1967;19(4):463‐521. [PubMed] [Google Scholar]
- 57. Corbett A, McKnight S, Henderson G. Opioid receptors. Accessed April 30, 2022. https://www.opioids.com/receptors/index.html
- 58. Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol. 2017;18(7):437‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wei LN, Loh HH. Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Annu Rev Pharmacol Toxicol. 2011;51:75‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oldfield S, Braksator E, Rodriguez‐Martin I, et al. C‐terminal splice variants of the mu‐opioid receptor: existence, distribution and functional characteristics. J Neurochem. 2008;104(4):937‐945. [DOI] [PubMed] [Google Scholar]
- 61. Koch T, Schulz S, Schroder H, Wolf R, Raulf E, Hollt V. Carboxyl‐terminal splicing of the rat mu opioid receptor modulates agonist‐mediated internalization and receptor resensitization. J Biol Chem. 1998;273(22):13652‐13657. [DOI] [PubMed] [Google Scholar]
- 62. Tanowitz M, Hislop JN, von Zastrow M. Alternative splicing determines the post‐endocytic sorting fate of G‐protein‐coupled receptors. J Biol Chem. 2008;283(51):35614‐35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu J, Lu Z, Narayan A, et al. Alternatively, spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Invest. 2017;127(4):1561‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verzillo V, Madia PA, Liu NJ, Chakrabarti S, Gintzler AR. Mu‐opioid receptor splice variants: sex‐dependent regulation by chronic morphine. J Neurochem. 2014;130(6):790‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manglik A, Kruse AC, Kobilka TS, et al. Crystal structure of the micro‐opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kruse AC, Hu J, Pan AC, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482(7386):552‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haga K, Kruse AC, Asada H, et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482(7386):547‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kurowski M, Rosenbaum JS, Perry DC, Sadee W. [3H]‐etorphine and [3H]‐diprenorphine receptor binding in vitro and in vivo: differential effect of Na+ and guanylyl imidodiphosphate. Brain Res. 1982;249(2):345‐352. [DOI] [PubMed] [Google Scholar]
- 69. Cassel JA, Daubert JD, DeHaven RN. [(3)H]Alvimopan binding to the micro opioid receptor: comparative binding kinetics of opioid antagonists. Eur J Pharmacol. 2005;520(1‐3):29‐36. [DOI] [PubMed] [Google Scholar]
- 70. Mitchell JM, O'Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4(116):116ra6. [DOI] [PubMed] [Google Scholar]
- 71. Munoz B, Fritz BM, Yin F, Atwood BK. Alcohol exposure disrupts mu opioid receptor‐mediated long‐term depression at insular cortex inputs to dorsolateral striatum. Nat Commun. 2018;9(1):1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baldo BA. Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci. 2016;39(6):366‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12(2):119‐136. [DOI] [PubMed] [Google Scholar]
- 74. Krook‐Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of mu‐opioid receptor modulation. J Neurosci. 2011;31(42):14861‐14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giannopoulos P, Papatheodoropoulos C. Effects of mu‐opioid receptor modulation on the hippocampal network activity of sharp wave and ripples. Br J Pharmacol. 2013;168(5):1146‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meilandt WJ, Barea‐Rodriguez E, Harvey SA. Role of hippocampal CA3 mu‐opioid receptors in spatial learning and memory. J Neurosci. 2004;24(12):2953‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Märtin A, Calvigioni D, Tzortzi O, Fuzik J, Wärnberg E, Meletis K. A spatiomolecular map of the striatum. Cell Rep. 2019;29(13):4320‐4333.e5. [DOI] [PubMed] [Google Scholar]
- 78. Ponterio G, Tassone A, Sciamanna G, et al. Enhanced mu opioid receptor‐dependent opioidergic modulation of striatal cholinergic transmission in DYT1 dystonia. Mov Disord. 2018;33(2):310‐320. [DOI] [PubMed] [Google Scholar]
- 79. Horner KA, Hebbard JC, Logan AS, Vanchipurakel GA, Gilbert YE. Activation of mu opioid receptors in the striatum differentially augments methamphetamine‐induced gene expression and enhances stereotypic behavior. J Neurochem. 2012;120(5):779‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tao YM, Yu C, Wang WS, et al. Heteromers of μ opioid and dopamine D(1) receptors modulate opioid‐induced locomotor sensitization in a dopamine‐independent manner. Br J Pharmacol. 2017;174(17):2842‐2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blaesse P, Goedecke L, Bazelot M, Capogna M, Pape HC, Jungling K. mu‐opioid receptor‐mediated inhibition of intercalated neurons and effect on synaptic transmission to the central amygdala. J Neurosci. 2015;35(19):7317‐7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith CM, Walker LL, Leeboonngam T, McKinley MJ, Denton DA, Lawrence AJ. Endogenous central amygdala mu‐opioid receptor signaling promotes sodium appetite in mice. Proc Natl Acad Sci USA. 2016;113(48):13893‐13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Selley DE, Cao CC, Liu Q, Childers SR. Effects of sodium on agonist efficacy for G‐protein activation in mu‐opioid receptor‐transfected CHO cells and rat thalamus. Br J Pharmacol. 2000;130(5):987‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pozza DH, Potes CS, Barroso PA, Azevedo L, Castro‐Lopes JM, Neto FL. Nociceptive behaviourr upon modulation of mu‐opioid receptors in the ventrobasal complex of the thalamus of rats. Pain. 2010;148(3):492‐502. [DOI] [PubMed] [Google Scholar]
- 85. Nakamura A, Hasegawa M, Minami K, et al. Differential activation of the mu‐opioid receptor by oxycodone and morphine in pain‐related brain regions in a bone cancer pain model. Br J Pharmacol. 2013;168(2):375‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hoot MR, Sim‐Selley LJ, Selley DE, Scoggins KL, Dewey WL. Chronic neuropathic pain in mice reduces μ‐opioid receptor‐mediated G‐protein activity in the thalamus. Brain Res. 2011;1406:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tamaddonfard E, Erfanparast A. Role of μ‐opioid receptor in parafascicular nucleus of thalamus on morphine‐induced antinociception in a rat model of acute trigeminal pain. Vet Res Forum. 2017;8(1):29‐34. [PMC free article] [PubMed] [Google Scholar]
- 88. Ducat E, Ray B, Bart G, et al. Mu‐opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic‐pituitary‐adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict Biol. 2013;18(2):325‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li Y, van den Pol AN. Mu‐opioid receptor‐mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28(11):2814‐2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shrivastava P, Cabrera MA, Chastain LG, et al. Mu‐opioid receptor and delta‐opioid receptor differentially regulate microglial inflammatory response to control proopiomelanocortin neuronal apoptosis in the hypothalamus: effects of neonatal alcohol. J Neuroinflammation. 2017;14(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Datta U, Kelley LK, Middleton JW, Gilpin NW. Positive allosteric modulation of the cannabinoid type‐1 receptor (CB1R) in periaqueductal gray (PAG) antagonizes anti‐nociceptive and cellular effects of a mu‐opioid receptor agonist in morphine‐withdrawn rats. Psychopharmacology (Berl). 2020;237(12):3729‐3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fullerton EF, Rubaharan M, Karom MC, Hanberry RI, Murphy AZ. Advanced age attenuates the antihyperalgesic effect of morphine and decreases μ‐opioid receptor expression and binding in the rat midbrain periaqueductal gray in male and female rats. Neurobiol Aging. 2021;98:78‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li C, Sugam JA, Lowery‐Gionta EG, et al. Mu opioid receptor modulation of dopamine neurons in the periaqueductal gray/dorsal raphe: a role in regulation of pain. Neuropsychopharmacology. 2016;41(8):2122‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kandasamy R, Hillhouse TM, Livingston KE, et al. Positive allosteric modulation of the mu‐opioid receptor produces analgesia with reduced side effects. Proc Natl Acad Sci USA. 2021;118(16):e2000017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94(7):961‐972. [PubMed] [Google Scholar]
- 96. Koshimizu TA, Honda K, Nagaoka‐Uozumi S, et al. Complex formation between the vasopressin 1b receptor, β‐arrestin‐2, and the μ‐opioid receptor underlies morphine tolerance. Nat Neurosci. 2018;21(6):820‐833. [DOI] [PubMed] [Google Scholar]
- 97. Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non‐analgesic effects of opioids: opioid‐induced respiratory depression. Curr Pharm Des. 2012;18(37):5994‐6004. [DOI] [PubMed] [Google Scholar]
- 98. Levitt ES, Abdala AP, Paton JF, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory‐controlling Kölliker‐Fuse neurons and suppresses post‐inspiratory drive. J Physiol. 2015;593(19):4453‐4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams JT, Ingram SL, Henderson G, et al. Regulation of mu‐opioid receptors: desensitization. Pharmacol Rev. 2013;65(1):223‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lowe JD, Bailey CP. Functional selectivity and time‐dependence of mu‐opioid receptor desensitization at nerve terminals in the mouse ventral tegmental area. Br J Pharmacol. 2015;172(2):469‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rauck RL. Treatment of opioid‐induced constipation: focus on the peripheral mu‐opioid receptor antagonist methylnaltrexone. Drugs. 2013;73(12):1297‐1306. [DOI] [PubMed] [Google Scholar]
- 102. Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu‐opiate receptor. Nature. 1997;386(6624):499‐502. [DOI] [PubMed] [Google Scholar]
- 103. Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens mu‐opioid receptors mediate social reward. J Neurosci. 2011;31(17):6362‐6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sun YG, Lundeberg T, Yu LC. Involvement of endogenous beta‐endorphin in antinociception in the arcuate nucleus of hypothalamus in rats with inflammation. Pain. 2003;104(1‐2):55‐63. [DOI] [PubMed] [Google Scholar]
- 105. Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149(3):642‐649. [DOI] [PubMed] [Google Scholar]
- 106. Qi J, Li H, Zhao TB, et al. Inhibitory effect of endomorphin‐2 binding to the mu‐opioid receptor in the rat pre‐botzinger complex on the breathing activity. Mol Neurobiol. 2017; 54(1): 461‐469. [DOI] [PubMed] [Google Scholar]
- 107. Schreiter A, Gore C, Labuz D, et al. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB J. 2012;26(12):5161‐5171. [DOI] [PubMed] [Google Scholar]
- 108. Roques BP, Fournie‐Zaluski MC, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov. 2012;11(4):292‐310. [DOI] [PubMed] [Google Scholar]
- 109. Madariaga‐Mazon A, Marmolejo‐Valencia AF, Li Y, Toll L, Houghten RA, Martinez‐Mayorga K. Mu‐opioid receptor biased ligands: a safer and painless discovery of analgesics? Drug Discov Today. 2017;22(11):1719‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rasmussen SG, DeVree BT, Zou Y, et al. Crystal structure of the beta2 adrenergic receptor‐Gs protein complex. Nature. 2011;477(7366):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Watson H. Biological membranes. Essays Biochem. 2015;59:43‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Manglik A, Kim TH, Masureel M, et al. Structural insights into the dynamic process of beta2‐adrenergic receptor signaling. Cell. 2015;161(5):1101‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chan HCS, McCarthy D, Li J, Palczewski K, Yuan S. Designing safer analgesics via mu‐opioid receptor pathways. Trends Pharmacol Sci. 2017;38(11):1016‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Trang T, Al‐Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and poppies: the good. J Neurosci. 2015;35(41):13879‐13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Luttrell LM, Lefkowitz RJ. The role of beta‐arrestins in the termination and transduction of G‐protein‐coupled receptor signals. J Cell Sci. 2002;115(Pt 3):455‐465. [DOI] [PubMed] [Google Scholar]
- 116. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Connor M, Osborne PB, Christie MJ. Mu‐opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143(6):685‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299‐343. [DOI] [PubMed] [Google Scholar]
- 119. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta‐arrestin 2. Science. 1999;286(5449):2495‐2498. [DOI] [PubMed] [Google Scholar]
- 120. Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta‐arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195‐1201. [DOI] [PubMed] [Google Scholar]
- 121. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu‐opioid receptor desensitization by beta‐arrestin‐2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720‐723. [DOI] [PubMed] [Google Scholar]
- 122. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cooper S, Robison AJ, Mazei‐Robison MS. Reward circuitry in addiction. Neurotherapeutics. 2017;14(3):687‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pellissier LP, Gandía J, Laboute T, Becker JAJ, Le Merrer J. μ opioid receptor, social behaviour and autism spectrum disorder: reward matters. Br J Pharmacol. 2018;175(14):2750‐2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nummenmaa L, Saanijoki T, Tuominen L, et al. μ‐opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9(1):1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL. Autistic‐like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology. 2014;39(9):2049‐2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bland AR, Ersche KD. Deficits in recognizing female facial expressions related to social network in cocaine‐addicted men. Drug Alcohol Depend. 2020;216:108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tobler PN, Preller KH, Campbell‐Meiklejohn DK, et al. Shared neural basis of social and non‐social reward deficits in chronic cocaine users. Soc Cogn Affect Neurosci. 2016;11(6):1017‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu‐opioid receptor‐deficient mice. Eur J Neurosci. 2007;25(11):3398‐3405. [DOI] [PubMed] [Google Scholar]
- 132. Skoubis PD, Matthes HW, Walwyn WM, Kieffer BL, Maidment NT. Naloxone fails to produce conditioned place aversion in mu‐opioid receptor knock‐out mice. Neuroscience. 2001;106(4):757‐763. [DOI] [PubMed] [Google Scholar]
- 133. Nair‐Roberts RG, Chatelain‐Badie SD, Benson E, White‐Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1‐6):321‐353. [DOI] [PubMed] [Google Scholar]
- 135. Beier KT, Steinberg EE, DeLoach KE, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input‐output mapping. Cell. 2015;162(3):622‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nguyen C, Mondoloni S, Le Borgne T, et al. Nicotine inhibits the VTA‐to‐amygdala dopamine pathway to promote anxiety. Neuron. 2021;109(16):2604‐2615.e9. [DOI] [PubMed] [Google Scholar]
- 137. Lammel S, Ion DI, Roeper J, Malenka RC. Projection‐specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Beuming T, Kniazeff J, Bergmann ML, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11(7):780‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Calipari ES, Juarez B, Morel C, et al. Dopaminergic dynamics underlying sex‐specific cocaine reward. Nat Commun. 2017;8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26(18):4901‐4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin‐1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22(16):7225‐7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self‐administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin‐3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005;313(1):134‐145. [DOI] [PubMed] [Google Scholar]
- 143. Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26(3):723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bouarab C, Thompson B, Polter AM. VTA GABA neurons at the interface of stress and reward. Front Neural Circuits. 2019;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73(6):1184‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513(6):566‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61(5):786‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31(32):11457‐11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dilts RP, Kalivas PW. Autoradiographic localization of mu‐opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488(1‐2):311‐327. [DOI] [PubMed] [Google Scholar]
- 150. Garzon M, Pickel VM. Plasmalemmal mu‐opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41(4):311‐328. [DOI] [PubMed] [Google Scholar]
- 151. Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7(7):e42365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Steffensen SC, Stobbs SH, Colago EE, et al. Contingent and non‐contingent effects of heroin on mu‐opioid receptor‐containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202(1):139‐151. [DOI] [PubMed] [Google Scholar]
- 154. Narita M, Matsushima Y, Niikura K, et al. Implication of dopaminergic projection from the ventral tegmental area to the anterior cingulate cortex in μ‐opioid‐induced place preference. Addict Biol. 2010;15(4):434‐447. [DOI] [PubMed] [Google Scholar]
- 155. Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980; 12(6):965‐968. [DOI] [PubMed] [Google Scholar]
- 156. Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111(6):1324‐1334. [DOI] [PubMed] [Google Scholar]
- 157. Wasserman DI, Tan JM, Kim JC, Yeomans JS. Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine‐induced locomotion. Eur J Neurosci. 2016;44(1):1761‐1770. [DOI] [PubMed] [Google Scholar]
- 158. Lavezzi HN, Parsley KP, Zahm DS. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: psychostimulant‐elicited Fos expression and collateralization. Brain Struct Funct. 2012;217(3):719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jalabert M, Bourdy R, Courtin J, et al. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci USA. 2011;108(39):16446‐16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37(5):1164‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Matsui A, Williams JT. Opioid‐sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31(48):17729‐17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Steidl S, Dhillon ES, Sharma N, Ludwig J. Muscarinic cholinergic receptor antagonists in the VTA and RMTg have opposite effects on morphine‐induced locomotion in mice. Behav Brain Res. 2017;323:111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kotecki L, Hearing M, McCall NM, et al. GIRK channels modulate opioid‐induced motor activity in a cell type‐ and subunit‐dependent manner. J Neurosci. 2015;35(18):7131‐7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fu R, Chen X, Zuo W, et al. Ablation of mu opioid receptor‐expressing GABA neurons in rostromedial tegmental nucleus increases ethanol consumption and regulates ethanol‐related behaviors. Neuropharmacology. 2016;107:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron. 2014;82(6):1346‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor‐regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429‐1432. [DOI] [PubMed] [Google Scholar]
- 167. Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed). 2013;5:273‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213(1‐2):17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15(6):816‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cui Y, Ostlund SB, James AS, et al. Targeted expression of mu‐opioid receptors in a subset of striatal direct‐pathway neurons restores opiate reward. Nat Neurosci. 2014;17(2):254‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. David V, Cazala P. Anatomical and pharmacological specificity of the rewarding effect elicited by microinjections of morphine into the nucleus accumbens of mice. Psychopharmacology. 2000;150(1):24‐34. [DOI] [PubMed] [Google Scholar]
- 173. Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237(2):429‐440. [DOI] [PubMed] [Google Scholar]
- 174. Amalric M, Koob GF. Low doses of methylnaloxonium in the nucleus accumbens antagonize hyperactivity induced by heroin in the rat. Pharmacol Biochem Behav. 1985;23(3):411‐415. [DOI] [PubMed] [Google Scholar]
- 175. Richard JM, Fields HL. Mu‐opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol consumption, self‐administration and cue‐induced reinstatement. Neuropharmacology. 2016;108:14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Toddes C, Lefevre EM, Brandner DD, Zugschwert L, Rothwell PE. μ‐opioid receptor (Oprm1) copy number influences nucleus accumbens microcircuitry and reciprocal social behaviors. J Neurosci. 2021;41(38):7965‐7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Castro DC, Oswell CS, Zhang ET, et al. An endogenous opioid circuit determines state‐dependent reward consumption. Nature. 2021;598(7882):646‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive‐sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247‐291. [DOI] [PubMed] [Google Scholar]
- 179. Todtenkopf MS, Stellar JR, Williams EA, Zahm DS. Differential distribution of parvalbumin immunoreactive neurons in the striatum of cocaine sensitized rats. Neuroscience. 2004;127(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 180. Delgado MR. Reward‐related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70‐88. [DOI] [PubMed] [Google Scholar]
- 181. Cox J, Witten IB. Striatal circuits for reward learning and decision‐making. Nat Rev Neurosci. 2019;20(8):482‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision‐making. J Neurosci. 2007;27(31):8161‐8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bender BN, Torregrossa MM. Dorsolateral striatum dopamine‐dependent cocaine seeking is resistant to pavlovian cue extinction in male and female rats. Neuropharmacology. 2021;182:108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Abburi C, Wolfman SL, Metz RA, Kamber R, McGehee DS, McDaid J. Tolerance to ethanol or nicotine results in increased ethanol self‐administration and long‐term depression in the dorsolateral striatum. eNeuro. 2016;3(4):112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Jenrette TA, Logue JB, Horner KA. Lesions of the patch compartment of dorsolateral striatum disrupt stimulus‐response learning. Neuroscience. 2019;415:161‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Quigley JA, Logsdon MK, Graham BC, Beaudoin KG, Becker JB. Activation of G protein‐coupled estradiol receptor 1 in the dorsolateral striatum enhances motivation for cocaine and drug‐induced reinstatement in female but not male rats. Biol Sex Differ. 2021;12(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Quigley JA, Becker JB. Activation of G‐protein coupled estradiol receptor 1 in the dorsolateral striatum attenuates preference for cocaine and saccharin in male but not female rats. Horm Behav. 2021;130:104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Torres C, Glueck AC, Conrad SE, Morón I, Papini MR. Dorsomedial striatum lesions affect adjustment to reward uncertainty, but not to reward devaluation or omission. Neuroscience. 2016;332:13‐25. [DOI] [PubMed] [Google Scholar]
- 189. Licheri V, Eckernäs D, Bergquist F, Ericson M, Adermark L. Nicotine‐induced neuroplasticity in striatum is subregion‐specific and reversed by motor training on the rotarod. Addict Biol. 2020;25(3):e12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Shin JH, Kim D, Jung MW. Differential coding of reward and movement information in the dorsomedial striatal direct and indirect pathways. Nat Commun. 2018;9(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hong SI, Kang S, Chen JF, Choi DS. Indirect medium spiny neurons in the dorsomedial striatum regulate ethanol‐containing conditioned reward seeking. J Neurosci. 2019;39(36):7206‐7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kang S, Hong SI, Lee J, et al. Activation of astrocytes in the dorsomedial striatum facilitates transition from habitual to goal‐directed reward‐seeking behavior. Biol Psychiatry. 2020;88(10):797‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Chen G, Cuzon Carlson VC, Wang J, et al. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35(10):1739‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Miendlarzewska EA, Aberg KC, Bavelier D, Schwartz S. Prior reward conditioning dampens hippocampal and striatal responses during an associative memory task. J Cogn Neurosci. 2021;33(3):402‐421. [DOI] [PubMed] [Google Scholar]
- 195. Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J Neurosci. 2016;36(18):5047‐5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274‐5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Muñoz B, Fritz BM, Yin F, Atwood BK. Alcohol exposure disrupts mu opioid receptor‐mediated long‐term depression at insular cortex inputs to dorsolateral striatum. Nat Commun. 2018;9(1):1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Mamaligas AA, Cai Y, Ford CP. Nicotinic and opioid receptor regulation of striatal dopamine D2‐receptor mediated transmission. Sci Rep. 2016;6:37834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Birdsong WT, Jongbloets BC, Engeln KA, Wang D, Scherrer G, Mao T. Synapse‐specific opioid modulation of thalamo‐cortico‐striatal circuits. eLife. 2019;8:e45146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Atwood BK, Kupferschmidt DA, Lovinger DM. Opioids induce dissociable forms of long‐term depression of excitatory inputs to the dorsal striatum. Nat Neurosci. 2014;17(4):540‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Campos‐Jurado Y, Martí‐Prats L, Zornoza T, Polache A, Granero L, Cano‐Cebrián MJ. Regional differences in mu‐opioid receptor‐dependent modulation of basal dopamine transmission in rat striatum. Neurosci Lett. 2017;638:102‐108. [DOI] [PubMed] [Google Scholar]
- 202. Rivera A, Suárez‐Boomgaard D, Miguelez C, et al. Dopamine D(4) receptor is a regulator of morphine‐induced plasticity in the rat dorsal striatum. Cells. 2021;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25‐52. [DOI] [PubMed] [Google Scholar]
- 204. Sesack SR, Grace AA. Cortico‐basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal‐accumbens glutamate transmission. Neuron. 2005;45(5):647‐650. [DOI] [PubMed] [Google Scholar]
- 206. Ma YY, Lee BR, Wang X, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse‐based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Otis JM, Namboodiri VM, Matan AM, et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543(7643):103‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Bhattacherjee A, Djekidel MN, Chen R, Chen W, Tuesta LM, Zhang Y. Cell type‐specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat Commun. 2019;10(1):4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Daneshparvar H, Sadat‐Shirazi MS, Fekri M, et al. NMDA receptor subunits change in the prefrontal cortex of pure‐opioid and multi‐drug abusers: a post‐mortem study. Eur Arch Psychiatry Clin Neurosci. 2019;269(3):309‐315. [DOI] [PubMed] [Google Scholar]
- 210. Wang X, Liu P, Ma L, Wang F. Opioid signal transduction regulates the dendritic morphology of somatostatin and parvalbumin interneurons in the medial prefrontal cortex. Neuroreport. 2019;30(8):592‐599. [DOI] [PubMed] [Google Scholar]
- 211. Sun H, Luessen DJ, Kind KO, Zhang K, Chen R. Cocaine self‐administration regulates transcription of opioid peptide precursors and opioid receptors in rat caudate putamen and prefrontal cortex. Neuroscience. 2020;443:131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Witkowski G, Szulczyk P. Opioid mu receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A‐ and C‐dependent mechanism. Brain Res. 2006;1094(1):92‐106. [DOI] [PubMed] [Google Scholar]
- 213. Olianas MC, Dedoni S, Onali P. Potentiation of dopamine D1‐like receptor signaling by concomitant activation of δ‐ and μ‐opioid receptors in mouse medial prefrontal cortex. Neurochem Int. 2012;61(8):1404‐1416. [DOI] [PubMed] [Google Scholar]
- 214. Selleck RA, Giacomini J, Buchholtz BD, Lake C, Sadeghian K, Baldo BA. Modulation of appetitive motivation by prefrontal cortical mu‐opioid receptors is dependent upon local dopamine D1 receptor signaling. Neuropharmacology. 2018;140:302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Zhou K, Zhu Y. The paraventricular thalamic nucleus: a key hub of neural circuits underlying drug addiction. Pharmacol Res. 2019;142:70‐76. [DOI] [PubMed] [Google Scholar]
- 216. James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235‐242. [DOI] [PubMed] [Google Scholar]
- 217. Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend. 2014;134:387‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Otis JM, Zhu M, Namboodiri VMK, et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue‐reward processing. Neuron. 2019;103(3):423‐431.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530(7589):219‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24(25):5758‐5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology. 2005;30(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ikemoto S, Witkin BM, Morales M. Rewarding injections of the cholinergic agonist carbachol into the ventral tegmental area induce locomotion and c‐Fos expression in the retrosplenial area and supramammillary nucleus. Brain Res. 2003;969(1‐2):78‐87. [DOI] [PubMed] [Google Scholar]
- 223. Minami M, Onogi T, Toya T, et al. Molecular cloning and in situ hybridization histochemistry for rat mu‐opioid receptor. Neurosci Res. 1994;18(4):315‐322. [DOI] [PubMed] [Google Scholar]
- 224. Levy D, Shabat‐Simon M, Shalev U, Barnea‐Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward‐related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27(51):14179‐14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73(6):759‐768. [DOI] [PubMed] [Google Scholar]
- 226. Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6‐25. [DOI] [PubMed] [Google Scholar]
- 227. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1‐2):71‐75. [DOI] [PubMed] [Google Scholar]
- 228. Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345‐354. [DOI] [PubMed] [Google Scholar]
- 229. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171‐181. [DOI] [PubMed] [Google Scholar]
- 230. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573‐585. [DOI] [PubMed] [Google Scholar]
- 231. Cason AM, Smith RJ, Tahsili‐Fahadan P, Moorman DE, Sartor GC, Aston‐Jones G. Role of orexin/hypocretin in reward‐seeking and addiction: implications for obesity. Physiol Behav. 2010;100(5):419‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Harris GC, Wimmer M, Aston‐Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556‐559. [DOI] [PubMed] [Google Scholar]
- 233. Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589‐601. [DOI] [PubMed] [Google Scholar]
- 234. Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191(1):137‐145. [DOI] [PubMed] [Google Scholar]
- 235. Li D, Olszewski PK, Shi Q, et al. Effect of opioid receptor ligands injected into the rostral lateral hypothalamus on c‐fos and feeding behavior. Brain Res. 2006;1096(1):120‐124. [DOI] [PubMed] [Google Scholar]
- 236. Befort K, Filliol D, Darcq E, et al. Gene expression is altered in the lateral hypothalamus upon activation of the mu opioid receptor. Ann N Y Acad Sci. 2008;1129:175‐184. [DOI] [PubMed] [Google Scholar]
- 237. Ardianto C, Yonemochi N, Yamamoto S, et al. Opioid systems in the lateral hypothalamus regulate feeding behavior through orexin and GABA neurons. Neuroscience. 2016;320:183‐193. [DOI] [PubMed] [Google Scholar]
- 238. Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward‐seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Stuber GD, Sparta DR, Stamatakis AM, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Warlow SM, Robinson MJF, Berridge KC. Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J Neurosci. 2017;37(35):8330‐8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Seo DO, Funderburk SC, Bhatti DL, et al. A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior. J Neurosci. 2016;36(42):10831‐10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185‐205. [DOI] [PubMed] [Google Scholar]
- 243. Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus‐amygdala continuum in human and monkey. J Comp Neurol. 1991;309(4):445‐485. [DOI] [PubMed] [Google Scholar]
- 244. Oler JA, Birn RM, Patriat R, et al. Evidence for coordinated functional activity within the extended amygdala of non‐human and human primates. NeuroImage. 2012;61(4):1059‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Baidoo N, Leri F. Extended amygdala, conditioned withdrawal and memory consolidation. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113:110435. [DOI] [PubMed] [Google Scholar]
- 246. Zahm DS. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Crit Rev Neurobiol. 1998;12(3):245‐265. [DOI] [PubMed] [Google Scholar]
- 247. Bupesh M, Abellán A, Medina L. Genetic and experimental evidence supports the continuum of the central extended amygdala and a mutiple embryonic origin of its principal neurons. J Comp Neurol. 2011;519(17):3507‐3531. [DOI] [PubMed] [Google Scholar]
- 248. Waraczynski M. Toward a systems‐oriented approach to the role of the extended amygdala in adaptive responding. Neurosci Biobehav Rev. 2016;68:177‐194. [DOI] [PubMed] [Google Scholar]
- 249. Calvino B, Lagowska J, Ben‐Ari Y. Morphine withdrawal syndrome: differential participation of structures located within the amygdaloid complex and striatum of the rat. Brain Res. 1979;177(1):19‐34. [DOI] [PubMed] [Google Scholar]
- 250. Wang L, Shen M, Jiang C, Ma L, Wang F. Parvalbumin interneurons of central amygdala regulate the negative affective states and the expression of corticotrophin‐releasing hormone during morphine withdrawal. Int J Neuropsychopharmacol. 2016;19(11):pyw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Dumont EC, Mark GP, Mader S, Williams JT. Self‐administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8(4):413‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20(20):Rc102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Epping‐Jordan MP, Markou A, Koob GF. The dopamine D‐1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784(1‐2):105‐115. [DOI] [PubMed] [Google Scholar]
- 254. Befort K, Filliol D, Ghate A, et al. Mu‐opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27(11):2973‐2984. [DOI] [PubMed] [Google Scholar]
- 255. Vathy I, Slamberová R, Rimanóczy A, Riley MA, Bar N. Autoradiographic evidence that prenatal morphine exposure sex‐dependently alters mu‐opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(3):381‐393. [DOI] [PubMed] [Google Scholar]
- 256. Wassum KM, Cely IC, Balleine BW, Maidment NT. Micro‐opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci. 2011;31(5):1591‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lichtenberg NT, Wassum KM. Amygdala mu‐opioid receptors mediate the motivating influence of cue‐triggered reward expectations. Eur J Neurosci. 2017;45(3):381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Beckerman MA, Glass MJ. The NMDA‐NR1 receptor subunit and the mu‐opioid receptor are expressed in somatodendritic compartments of central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp Neurol. 2012;234(1):112‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Beckerman MA, Glass MJ. Ultrastructural relationship between the AMPA‐GluR2 receptor subunit and the mu‐opioid receptor in the mouse central nucleus of the amygdala. Exp Neurol. 2011;227(1):149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Tryon VL, Penner MR, Heide SW, King HO, Larkin J, Mizumori SJY. Hippocampal neural activity reflects the economy of choices during goal‐directed navigation. Hippocampus. 2017;27(7):743‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Olafsdottir HF, Barry C, Saleem AB, Hassabis D, Spiers HJ. Hippocampal place cells construct reward related sequences through unexplored space. eLife. 2015;4:e06063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Gauthier JL, Tank DW. A dedicated population for reward coding in the hippocampus. Neuron. 2018;99(1):179‐193.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. LeGates TA, Kvarta MD, Tooley JR, et al. Reward behaviour is regulated by the strength of hippocampus‐nucleus accumbens synapses. Nature. 2018;564(7735):258‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Alvandi MS, Bourmpoula M, Homberg JR, Fathollahi Y. Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats. Addict Biol. 2017;22(6):1883‐1894. [DOI] [PubMed] [Google Scholar]
- 265. Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017;20(11):1434‐1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward‐related learning and memory. Annu Rev Neurosci. 2006;29:565‐598. [DOI] [PubMed] [Google Scholar]
- 267. Giannopoulos P, Papatheodoropoulos C. Effects of μ‐opioid receptor modulation on the hippocampal network activity of sharp wave and ripples. Br J Pharmacol. 2013;168(5):1146‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Zhang H, Jia M, Wang XW, et al. Dentate gyrus μ‐opioid receptor‐mediated neurogenic processes are associated with alterations in morphine self‐administration. Sci Rep. 2019;9(1):1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. De Sa Nogueira D, Bourdy R, Filliol D, Romieu P, Befort K. Hippocampal mu opioid receptors are modulated following cocaine self‐administration in rat. Eur J Neurosci. 2021;53(10):3341‐3349. [DOI] [PubMed] [Google Scholar]
- 271. Kota D, Alajaji M, Bagdas D, Selley DE, Sim‐Selley LJ, Damaj MI. Early adolescent nicotine exposure affects later‐life hippocampal mu‐opioid receptors activity and morphine reward but not physical dependence in male mice. Pharmacol Biochem Behav. 2018;173:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Watabe‐Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole‐brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858‐873. [DOI] [PubMed] [Google Scholar]
- 273. Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat‐anatomical substratum for integrative functions. J Comp Neurol. 2005;490(3):270‐294. [DOI] [PubMed] [Google Scholar]
- 274. Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29(2):340‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12(10):3596‐3604. [DOI] [PubMed] [Google Scholar]
- 276. Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17(4):751‐762. [DOI] [PubMed] [Google Scholar]
- 277. Lammel S, Lim BK, Ran C, et al. Input‐specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Steidl S, O'Sullivan S, Pilat D, Bubula N, Brown J, Vezina P. Operant responding for optogenetic excitation of LDTg inputs to the VTA requires D1 and D2 dopamine receptor activation in the NAcc. Behav Brain Res. 2017; 333: 161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Xiao C, Cho JR, Zhou C, et al. Cholinergic mesopontine signals govern locomotion and reward through dissociable midbrain pathways. Neuron. 2016;90(2):333‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Yoo JH, Zell V, Wu J, et al. Activation of pedunculopontine glutamate neurons is reinforcing. J Neurosci. 2017;37(1):38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Klitenick MA, Kalivas PW. Behavioral and neurochemical studies of opioid effects in the pedunculopontine nucleus and mediodorsal thalamus. J Pharmacol Exp Ther. 1994;269(1):437‐448. [PubMed] [Google Scholar]
- 282. Corrigall WA, Coen KM, Adamson KL, Chow BL. Manipulations of mu‐opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self‐administration in rats. Psychopharmacology. 1999;145(4):412‐417. [DOI] [PubMed] [Google Scholar]
- 283. Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci. 2017;19(3):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33‐51. [DOI] [PubMed] [Google Scholar]
- 285. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35(2):253‐259. [DOI] [PubMed] [Google Scholar]
- 286. Farrell M. Opiate withdrawal. Addiction. 1994;89(11):1471‐1475. [DOI] [PubMed] [Google Scholar]
- 287. Jarvis BP, Holtyn AF, Subramaniam S, et al. Extended‐release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113(7):1188‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):Cd002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Vernon MK, Reinders S, Mannix S, Gullo K, Gorodetzky CW, Clinch T. Psychometric evaluation of the 10‐item short opiate withdrawal scale‐gossop (SOWS‐Gossop) in patients undergoing opioid detoxification. Addict Behav. 2016;60:109‐116. [DOI] [PubMed] [Google Scholar]
- 290. Welsch L, Bailly J, Darcq E, Kieffer BL. The negative affect of protracted opioid abstinence: progress and perspectives from rodent models. Biol Psychiatry. 2020;87(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Goeldner C, Lutz PE, Darcq E, et al. Impaired emotional‐like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69(3):236‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Weiss RD, Potter JS, Griffin ML, et al. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J Subst Abuse Treat. 2014;47(2):140‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Nunes EV, Gordon M, Friedmann PD, et al. Relapse to opioid use disorder after inpatient treatment: protective effect of injection naltrexone. J Subst Abuse Treat. 2018;85:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ravndal E, Amundsen EJ. Mortality among drug users after discharge from inpatient treatment: an 8‐year prospective study. Drug Alcohol Depend. 2010;108(1‐2):65‐69. [DOI] [PubMed] [Google Scholar]
- 295. Seaman SR, Brettle RP, Gore SM. Mortality from overdose among injecting drug users recently released from prison: database linkage study. BMJ. 1998;316(7129):426‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Mazei‐Robison MS, Nestler EJ. Opiate‐induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med. 2012;2(7):a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113‐168. [DOI] [PubMed] [Google Scholar]
- 298. Foote SL, Bloom FE, Aston‐Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63(3):844‐914. [DOI] [PubMed] [Google Scholar]
- 299. Rehni AK, Jaggi AS, Singh N. Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets. CNS Neurol Disord Drug Targets. 2013;12(1):112‐125. [DOI] [PubMed] [Google Scholar]
- 300. Liu JG, Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38(1‐2):1‐19. [DOI] [PubMed] [Google Scholar]
- 301. Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25(4):210‐218. [DOI] [PubMed] [Google Scholar]
- 302. Zhao H, Loh HH, Law PY. Adenylyl cyclase superactivation induced by long‐term treatment with opioid agonist is dependent on receptor localized within lipid rafts and is independent of receptor internalization. Mol Pharmacol. 2006;69(4):1421‐1432. [DOI] [PubMed] [Google Scholar]
- 303. Rehni AK, Singh N. Modulation of src‐kinase attenuates naloxone‐precipitated opioid withdrawal syndrome in mice. Behav Pharmacol. 2011;22(2):182‐190. [DOI] [PubMed] [Google Scholar]
- 304. Zhang L, Zhao H, Qiu Y, Loh HH, Law PY. Src phosphorylation of micro‐receptor is responsible for the receptor switching from an inhibitory to a stimulatory signal. J Biol Chem. 2009;284(4):1990‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Lutz PE, Ayranci G, Chu‐Sin‐Chung P, et al. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive‐like behaviors during heroin abstinence. Neuropsychopharmacology. 2014;39(11):2694‐2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Seip‐Cammack KM, Reed B, Zhang Y, Ho A, Kreek MJ. Tolerance and sensitization to chronic escalating dose heroin following extended withdrawal in Fischer rats: possible role of mu‐opioid receptors. Psychopharmacology. 2013;225(1):127‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Blackwood CA, Hoerle R, Leary M, et al. Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence‐induced incubation of drug seeking after escalated oxycodone self‐administration. Molecular neurobiology. 2019;56(5):3603‐3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88(2‐3):188‐196. [DOI] [PubMed] [Google Scholar]
- 309. Wang SC, Chen YC, Lee CH, Cheng CM. Opioid addiction, genetic susceptibility, and medical treatments: a review. Int J Mol Sci. 2019;20(17):4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the united states: an update. Am J Psychiatry. 2016;173(1):18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559‐574. [DOI] [PubMed] [Google Scholar]
- 312. Bhalla S, Andurkar SV, Gulati A. Neurobiology of opioid withdrawal: role of the endothelin system. Life Sci. 2016;159:34‐42. [DOI] [PubMed] [Google Scholar]
- 313. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2011(9):Cd005031. [DOI] [PubMed] [Google Scholar]
- 315. Goodrich PM. Naloxone hydrochloride: a review. AANA J. 1990;58(1):14‐16. [PubMed] [Google Scholar]
- 316. Sabzghabaee AM, Eizadi‐Mood N, Yaraghi A, Zandifar S. Naloxone therapy in opioid overdose patients: intranasal or intravenous? A randomized clinical trial. Arch Med Sci. 2014;10(2):309‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Solli KK, Latif ZE, Opheim A, et al. Effectiveness. Addiction. 2018;113(10):1840‐1849. [DOI] [PubMed] [Google Scholar]
- 318. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended‐release naltrexone vs daily buprenorphine‐naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Hartung DM, McCarty D, Fu R, Wiest K, Chalk M, Gastfriend DR. Extended‐release naltrexone for alcohol and opioid dependence: a meta‐analysis of healthcare utilization studies. J Subst Abuse Treat. 2014;47(2):113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 321. Inturrisi CE. Pharmacology of methadone and its isomers. Minerva Anestesiol. 2005;71(7‐8):435‐437. [PubMed] [Google Scholar]
- 322. Faggiano F, Vigna‐Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):Cd002208. [DOI] [PubMed] [Google Scholar]
- 323. Russolillo A, Moniruzzaman A, McCandless LC, Patterson M, Somers JM. Associations between methadone maintenance treatment and crime: a 17‐year longitudinal cohort study of Canadian provincial offenders. Addiction. 2018;113(4):656‐667. [DOI] [PubMed] [Google Scholar]
- 324. Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70(2):S13‐27. [DOI] [PubMed] [Google Scholar]
- 325. Davis MP, Pasternak G, Behm B. Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs. 2018;78(12):1211‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like‐1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther. 2006;318(1):206‐213. [DOI] [PubMed] [Google Scholar]
- 327. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long‐acting formulations. J Addict Med. 2019;13(2):93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104(7):1193‐1200. [DOI] [PubMed] [Google Scholar]
- 329. Raehal KM, Bohn LM. The role of beta‐arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60(1):58‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Schröder W, Lambert DG, Ko MC, Koch T. Functional plasticity of the N/OFQ‐NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol. 2014;171(16):3777‐3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2(4):395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Lutfy K, Eitan S, Bryant CD, et al. Buprenorphine‐induced antinociception is mediated by mu‐opioid receptors and compromised by concomitant activation of opioid receptor‐like receptors. J Neurosci. 2003;23(32):10331‐10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K. The role of the opioid receptor‐like (ORL1) receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience. 2008;155(3):597‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31(1):8‐18. [DOI] [PubMed] [Google Scholar]
- 335. Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4(2):214‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/μ‐opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther. 2013;346(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Ding H, Kiguchi N, Yasuda D, et al. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med. 2018;10(456):eaar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778‐790. [DOI] [PubMed] [Google Scholar]
- 339. Ayanga D, Shorter D, Kosten TR. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin Pharmacother. 2016;17(17):2307‐2318. [DOI] [PubMed] [Google Scholar]
- 340. Gold MS, Redmond DE Jr, Kleber HD. Clonidine blocks acute opiate‐withdrawal symptoms. Lancet. 1978;2(8090):599‐602. [DOI] [PubMed] [Google Scholar]
- 341. Howells C, Allen S, Gupta J, Stillwell G, Marsden J, Farrell M. Prison based detoxification for opioid dependence: a randomised double blind controlled trial of lofexidine and methadone. Drug Alcohol Depend. 2002;67(2):169‐176. [DOI] [PubMed] [Google Scholar]
- 342. Gowing L, Farrell MF, Ali R, White JM. Alpha2‐adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;(3):Cd002024. [DOI] [PubMed] [Google Scholar]
- 343. Robinson BA, Johansson L, Shaw J. Oral naloxone in opioid‐associated constipation. Lancet. 1991;338(8766):581‐582. [DOI] [PubMed] [Google Scholar]
- 344. Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid‐associated constipation. Pain. 2000;84(1):105‐109. [DOI] [PubMed] [Google Scholar]
- 345. Olofsen E, van Dorp E, Teppema L, et al. Naloxone reversal of morphine‐ and morphine‐6‐glucuronide‐induced respiratory depression in healthy volunteers: a mechanism‐based pharmacokinetic‐pharmacodynamic modeling study. Anesthesiology. 2010;112(6):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 346. Sanders M, Jones S, Löwenstein O, Jansen JP, Miles H, Simpson K. New formulation of sustained release naloxone can reverse opioid induced constipation without compromising the desired opioid effects. Pain Med. 2015;16(8):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 347. Lee JD, Friedmann PD, Kinlock TW, et al. Extended‐release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374(13):1232‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended‐release naltrexone for opioid dependence: a double‐blind. Lancet. 2011;377(9776):1506‐1513. [DOI] [PubMed] [Google Scholar]
- 349. Sullivan MA, Bisaga A, Pavlicova M, et al. A randomized trial comparing extended‐release injectable suspension and oral naltrexone, both combined with behavioral therapy. Am J Psychiatry. 2019;176(2):129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose‐response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 351. Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate‐ vs high‐dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281(11):1000‐1005. [DOI] [PubMed] [Google Scholar]
- 352. Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180‐day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303‐1310. [DOI] [PubMed] [Google Scholar]
- 353. Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343(18):1290‐1297. [DOI] [PubMed] [Google Scholar]
- 354. Fudala PJ, Bridge TP, Herbert S, et al. Office‐based treatment of opiate addiction with a sublingual‐tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949‐958. [DOI] [PubMed] [Google Scholar]
- 355. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short‐term buprenorphine‐naloxone for treatment of opioid‐addicted youth: a randomized trial. JAMA. 2008;300(17):2003‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576‐1583. [DOI] [PubMed] [Google Scholar]
- 357. Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, Vocci FJ. Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA. 2016;316(3):282‐290. [DOI] [PubMed] [Google Scholar]
- 358. Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O'Connor PG. Primary care‐based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Walsh SL, Comer SD, Lofwall MR, et al. Effect of buprenorphine weekly depot (CAM2038) and hydromorphone blockade in individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):894‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended‐release naltrexone vs daily buprenorphine‐naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended‐release naltrexone versus buprenorphine‐naloxone for opioid relapse prevention (X:bOT): a multicentre. Lancet. 2018;391(10118):309‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Murphy SM, McCollister KE, Leff JA, et al. Cost‐effectiveness of buprenorphine‐naloxone versus extended‐release naltrexone to prevent opioid relapse. Ann Intern Med. 2019;170(2):90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Washton AM, Resnick RB. Clonidine versus methadone for opiate detoxification. Lancet. 1980;2(8207):1297. [DOI] [PubMed] [Google Scholar]
- 364. Kowalczyk WJ, Phillips KA, Jobes ML, et al. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am J Psychiatry. 2015;172(8):760‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Raistrick D, West D, Finnegan O, Thistlethwaite G, Brearley R, Banbery J. A comparison of buprenorphine and lofexidine for community opiate detoxification: results from a randomized controlled trial. Addiction. 2005;100(12):1860‐1867. [DOI] [PubMed] [Google Scholar]
- 366. Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress‐induced and cue‐induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190(4):569‐574. [DOI] [PubMed] [Google Scholar]
- 367. Walsh SL, Strain EC, Bigelow GE. Evaluation of the effects of lofexidine and clonidine on naloxone‐precipitated withdrawal in opioid‐dependent humans. Addiction. 2003;98(4):427‐439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review, as no new data were created or analyzed.


