Abstract
Background
Commercial insurance data show that chronic opioid use in opioid-naive patients occurs in 1.5% to 8% of patients undergoing surgical procedures, but little is known about patients with Medicaid.
Methods
Opioid prescription data and medical coding data from 4,788 Medicaid patients who underwent cholecystectomy were analyzed to determine opioid use patterns.
Results
A total of 54.4% of patients received opioids prior to surgery, and 38.8% continued to fill opioid prescriptions chronically; 27.1% of opioid-naive patients continued to get opioids chronically. Patients who received ≥ 50 MME/d had nearly 8 times the odds of chronic opioid use. Each additional opioid prescription filled within 30 days was associated with increased odds of chronic use (odds ratio: 1.71).
Conclusion
Opioid prescriptions are common prior to cholecystectomy in Medicaid patients, and 38.8% of patients continue to receive opioid prescriptions well after surgical recovery. Even 27.1% of opioid-naive patients continued to receive opioid prescriptions chronically.
Highlights
-
•
A total of 54.4% of cholecystectomy Medicaid patients received opioids prior to surgery.
-
•
Nearly 40% continued to fill opioid prescriptions chronically.
-
•
Among opioid-naive patients, 27.1% continue to receive opioids after typical recovery from surgery.
-
•
Patients receiving ≥ 50 MME/d 30 days after surgery had 8 times the odds of chronic opioid use.
-
•
Each additional opioid prescription was associated with increased odds of chronic use (odds ratio: 1.71).
INTRODUCTION
Drug overdose deaths are the primary cause of injury-related death in the United States. Overdose deaths reached an all-time high of 93,000 in 2020 [1,2]. Approximately 70% of these deaths involve opioids [3]. Opioid prescriptions given at the time of surgery may be a gateway to chronic opioid use (COU) and abuse [4]. Most patients take postoperative opioids for a few days; however, some patients continue using opioids long after the indication for postoperative pain control has passed. Cholecystectomy is among the most common general surgical procedures performed in the United States with an estimated 500,000 cases done per year [5]. It is the most common operative procedure performed in Medicaid and self-insured patients [6]. Evaluation of commercial insurance data suggests that COU in previously opioid-naive patients occurs in 1.5% to 8% of patients undergoing common surgical procedures [[7], [8], [9], [10], [11], [12], [13]]. The cost of COU and potential morbidity and mortality associated with misuse is significant in both real and monetary terms [14]. Given the morbidity and costs associated with COU after surgery, the lack of information about Medicaid patients, and concerns about disparities in outcomes, we sought to investigate the prevalence of COU after cholecystectomy in the Medicaid population in South Carolina. We hypothesized that higher opioid prescribing around the time of surgery would be associated with a higher risk of chronic opioid use even in opioid-naive patients.
MATERIALS AND METHODS
Study Design and Population
A retrospective longitudinal cohort study was performed to assess opioid utilization patterns and risk factors for COU following cholecystectomy in Medicaid patients using methods described previously [15]. Deidentified medical and pharmacy claims data for Medicaid patients enrolled between January 2014 and December 2017 who underwent cholecystectomy were reviewed. The dates were chosen as these data include prescriptions written before and after SC initiated a mandatory reporting and prescription tracking system (March 2016) but before SC Medicaid limited acute opioid prescriptions to 5 days (May 2018). We used Medicare part-D data for those patients with dual coverage. Surgeries were identified by using International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM), ICD-10-CM, and Current Procedural Terminology (CPT) codes. Patients with cancer, those with a hospital stay associated with index surgery of greater than 7 days, those having other major surgical procedures at the time of cholecystectomy, those with missing pharmacy claims data, and those without continuous Medicaid coverage during the study period were excluded. Patient data were analyzed starting 6 months before cholecystectomy to 9 months following surgery.
Opioid use was determined through pharmacy dispensing records. In accordance with existing literature filling, a prescription was considered "use." Actual opioid consumption data were not available for analysis. Opioid medications were converted into morphine equivalent daily dose (MME/d) using conversion factors provided by the US Centers for Disease Control and Prevention [16]. We utilized MME/d as it is commonly used to assess risk of overdose. We converted total MME dispensed to MME/d by dividing total MME dispensed over 30 days by 30. We excluded 238 opioid prescriptions involving patches and 1 prescription for an opioid elixir because of data irregularities. Sensitivity analyses performed to assess the impact of these exclusions demonstrated that estimates were robust with no important differences between models that included or excluded elixir and patch opioids.
Comorbid conditions present in the 6 months prior to surgery were identified using ICD-9-CM or ICD-10-CM codes based on Elixhauser definitions [17]. Day of discharge was defined as day 0. The presurgical period was defined as the 90 days leading up to surgery. The exposure period was defined as the 30 days after discharge, which represents the typical time when most patients take opioids for postsurgical pain. The follow-up period was defined as day 31 to 270 (Fig 1). Any opioid prescription filled 90 or more days postdischarge was defined as chronic use. Patients were clustered into opioid use trajectory groups using prescription MME/d values from the 8-month follow-up period. The Medical University of South Carolina Institutional Review Board provided exempt review for this investigation.
Fig 1.
Timeline and definitions, South Carolina Medicaid, 2014–2017.
Exposure and Covariate Definitions
The primary exposures were opioid use during the exposure period as characterized by opioid days (1–5, 6–10, and > 10 days) and mean MME/d (0 < MME < 20, 20 ≤ MME < 50, and MME ≥ 50). MME per day was chosen as the Centers for Disease Control and Prevention provide cutoff risks using this scale. Thirty-day periods were used as schedule II narcotics can only be prescribed for 30 days. MME was reported as average dispensed MME/d. Opioid prescriptions active 90 days prior to admission until 270 days following discharge were collected. The opioid type, strength, quantity, and days' supply of each patient's prescriptions were used to estimate the combined MME for each day. Thirty-day intervals were determined for each patient for the 3 months prior to admission and for the 9 months following discharge. An average MME value was calculated for each 30-day interval by dividing the total MME in the period by 30.
We assessed for risk factors associated with COU using patient demographics, including age on surgery date, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and unknown/other). Baseline opioid use during the 90-day presurgery period was characterized by opioid-naive status or by daily opioid use (> 0–49, 50–89, and ≥ 90 MME/d). We also reported the prescriber type (emergency department, surgeon, primary care provider, or other) for opioid prescriptions filled 14 or fewer days prior to surgery. Opioid use during the presurgery, exposure, and follow-up periods was summarized using mean MME/d and mean opioid days and by the percentage with any dispensed opioids. Opioid use during the exposure period was also characterized by opioid type (long versus short acting; single versus combination opioid medications), number of opioid prescriptions, and the prescriber specialty identified in prescription data. Prescriptions for other medication classes prescribed with opioids which might influence COU were analyzed. These included antipsychotics, antidepressants, benzodiazepines, gabapentin, pregabalin, anxiolytics, muscle relaxants, nonbenzodiazepine sedative/hypnotics, and duloxetine. Nonsteroidal anti-inflammatory drug (NSAID) use, physical therapy, occupational therapy, and acupuncture were also assessed. Surgery and process of care variables evaluated during the exposure period included surgery length of stay, discharge to a skilled nursing or rehabilitation facility, days to first postsurgery visit with the surgeon and primary care provider, total number of outpatient visits, total unique prescribing providers visited, and total unique nonprescribers visited (eg, physical therapists). We also accounted for rehospitalization within 30 days and between 31 and 270 days of discharge after cholecystectomy. Anomalies in our Medicaid data set included missing surgery discharge dates affecting 139 (2.9%) of surgeries. We defined the discharge date to be the same as the admission date for these cases.
Statistical Analyses
Analysis methods have been described previously in detail [15]. In brief, bivariate analyses were performed using t tests, ANOVA, χ2, or Fisher exact tests, as appropriate. Odds ratios (ORs) with 95% confidence intervals (CIs) were determined using logistic regression. Separate models were run for both primary exposures. We used group-based trajectory models to identify clusters of patients with similar patterns of opioid use following the exposure period based on monthly averages of mean MME/d values. These models are based on finite mixture methods. We assumed a zero-inflated Poisson distribution for MME/d values [[18], [19], [20]]. We used SAS PROC TRAJ to compare models with up to fourth-order polynomials and up to 6 groups. We used Bayesian Information Criterion statistics to select the best model based on number of trajectory groups. For each group, we selected the best polynomial fit based on P values for parameter estimates. We then used the predicted group assignment for each patient as the dependent variable in a multinomial logistic model to infer which variables were most strongly associated with predicting group assignment. Analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC).
RESULTS
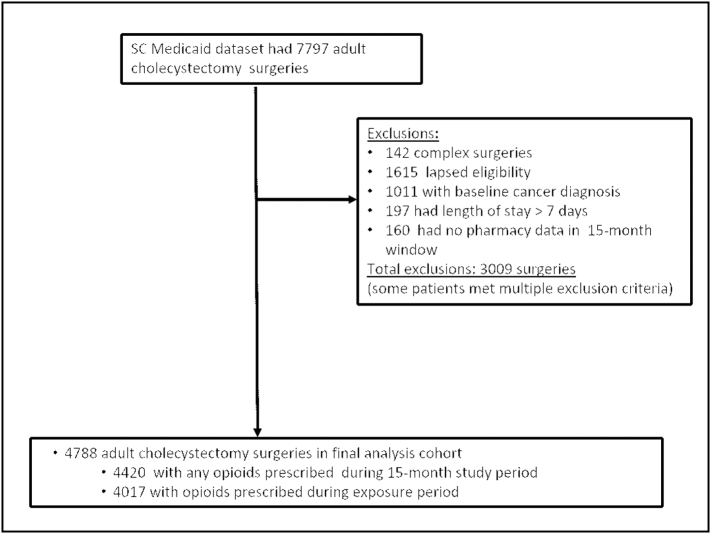
From 2014 to 2017, we identified 4,788 cholecystectomy patients that met inclusion criteria (Table 1 and Fig 2). The mean age of patients was 35 years. The majority (88.8%) were female, and roughly half were white (53.8%). The vast majority (95.7%) of cases were done laparoscopically. Nearly half (47.7%) of surgeries were performed as an outpatient or involved a 1-night hospital stay. Most patients (54.4%) received opioids prior to surgery. Of the 4,788 patients studied, 1,858 (38.8%) continued to fill opioid prescriptions more than 90 days after surgery. Of the 1,248 (26.1%) patients who received opioids in the 2 weeks leading up to surgery, 508 (40.7%) received them from the emergency department, 371 (29.7%) from primary care, and 309 (24.8%) from a surgeon. Patients who received opioids from a primary care provider were significantly more likely to receive opioid prescriptions chronically after surgery. If presurgical opioids came from the ED provider or surgeon, these patients did not have a statistically significant increased risk of receiving chronic opioid prescriptions. The majority (95.1%) of patients who received opioid prescriptions prior to surgery received prescriptions of less than 49 MME/d. Patients who received prescriptions of greater than 50 MME/d before surgery constituted a small group (2.7%) of total patients, but nearly all (93.8%) these patients continued to receive opioids chronically. Almost half of the patients (2,184, 45.6%) were opioid naive prior to surgery. More than 1 in 4 (592, 27.1%) of these patients continued to receive opioid prescriptions well after surgical recovery. Opioid-related overdose treatment was required in 5 patients (0.1%) and occurred between 75 and 215 days after surgery.
Table 1.
Demographic and clinical characteristics for cholecystectomy, SC Medicaid 2014–2017
| Measure | Level | Chronic⁎ | Nonchronic | Total | P value |
|---|---|---|---|---|---|
| Unique patients, n (%) | 1858 (38.8%) | 2930 (61.2%) | 4788 | <.0001 | |
| Age | Mean (SD) | 37.8 (13.0) | 33.1 (12.6) | 34.9 (12.9) | |
| Age category, n (%) | 18–34 y | 896 (48.2%) | 1884 (64.3%) | 2780 (58.1%) | <.0001 |
| 35–54 y | 711 (38.3%) | 819 (28.0%) | 1530 (32.0%) | ||
| 55–64 y | 199 (10.7%) | 162 (5.5%) | 361 (7.5%) | ||
| 65 + y | 52 (2.8%) | 65 (2.2%) | 117 (2.4%) | ||
| Sex, n (%) | Male | 264 (14.2%) | 272 (9.3%) | 536 (11.2%) | <.0001 |
| Female | 1594 (85.8%) | 2658 (90.7%) | 4252 (88.8%) | ||
| Race ethnicity, n (%) | White | 1061 (57.1%) | 1517 (51.8%) | 2578 (53.8%) | |
| Black | 545 (29.3%) | 1072 (36.7%) | 1617 (33.8%) | ||
| Hispanic | 32 (1.7%) | 83 (2.8%) | 115 (2.4%) | ||
| Unknown/other | 220 (11.8%) | 258 (8.8%) | 478 (10.0%) | ||
| Baseline measures (90 d presurgery period), n (%) | |||||
| Opioid naive† | 0 d | 592 (27.1%) | 1592 (72.9%) | 2184 (45.6%) | <.0001 |
| Opioid use during 30 d before surgery | > 0–49 MME/d | 1146 (46.3%) | 1330 (53.7%) | 2476 (95.1%) | <.0001 |
| 50–89 MME/d | 77 (92.8%) | 6 (7.2%) | 83 (3.2%) | ||
| ≥ 90MME/d | 43 (95.6%) | 2 (4.4%) | 45 (1.7%) | ||
| Total | 1266 (48.6%) | 1338 (51.3%) | 2604 (100%) | ||
| Prescriber type for opioids ≤ 14 d before surgery‡‡ | Emergency department | 205 (40.4%) | 303 (59.7%) | 508 (40.7%) | <.0001 |
| Primary care provider | 242 (65.2%) | 129 (34.8%) | 371 (29.7%) | <.0001 | |
| Surgery | 156 (50.5%) | 153 (49.5%) | 309 (24.8%) | .65 | |
| Other | 129 (62.6%) | 77 (37.4%) | 206 (16.5%) | .0005 | |
| Total | 644 (51.6%) | 604 (48.4%) | 1248 (100%) | ||
| Opioid use | |||||
| Mean opioid days [mean (SD)] | 90 d presurgery‡ | 22.8 (31.2) | 3.8 (9.4) | 11.2 (22.8) | <.0001 |
| Exposure period⁎⁎ | 10.8 (10.2) | 4.6 (4.3) | 7.0 (7.8) | <.0001 | |
| ≥ 90 d postsurgery | 48.7 (60.8) | 0 | 18.9 (44.7) | <.0001 | |
| Mean MME [mean (SD)] | 90 d presurgery | 11.8 (26.6) | 1.7 (6.1) | 5.9 (18.5) | <.0001 |
| Exposure period | 18.7 (28.6) | 6.9 (6.8) | 11.5 (19.5) | <.0001 | |
| ≥ 90 d postsurgery | 13.3 (27.9) | 0 (0) | 5.6 (19.3) | <.0001 | |
| Percent with opioid use | 90 d presurgery | 68.1% | 45.7% | 54.4% | <.0001 |
| Exposure period | 88.1% | 81.3% | 83.9% | <.0001 | |
| ≥ 90 d postsurgery | 100.0% | 0.0% | 38.7% | <.0001 | |
| Other medications or alternative pain treatments during exposure period, n (%) | |||||
| NSAIDS, APAP | 208 (11.2%) | 179 (6.1%) | 387 (8.1%) | <.0001 | |
| Antidepressants | 323 (17.4%) | 245 (8.4%) | 568 (11.9%) | <.0001 | |
| Antipsychotics | 78 (4.2%) | 54 (1.8%) | 132 (2.8%) | <.0001 | |
| Gabapentin | 144 (7.8%) | 47 (1.6%) | 191 (4.0%) | <.0001 | |
| Pregabalin | 29 (1.6%) | 3 (0.1%) | 32 (0.7%) | <.0001 | |
| Benzodiazepines | 275 (14.8%) | 114 (3.9%) | 389 (8.1%) | <.0001 | |
| Selected sedatives/hypnotics†† | 77 (4.1%) | 65 (2.2%) | 142 (3.0%) | .0002 | |
| Muscle relaxants | 140 (7.5%) | 57 (2.0%) | 197 (4.1%) | <.0001 | |
| Duloxetine | 39 (2.1%) | 21 (0.7%) | 60 (1.3%) | <.0001 | |
| Alternative pain treatments | Physical therapy | 25 (0.5%) | 17 (0.6%) | 42 (0.9%) | .007 |
| Occupational therapy | 8 (0.4%) | 7 (0.2%) | 15 (0.3%) | .18 | |
| Surgery and process of care variables | |||||
| Rehospitalization, n (%) | ≤ 30 d of discharge | 87 (4.7%) | 95 (3.2%) | 182 (3.8%) | .01 |
| 31–270 d postdischarge | 329 (17.7%) | 143 (4.9%) | 472 (9.9%) | <.0001 | |
| Skilled nursing and Rehab facilities, n (%) | 2 (0.04%) | 2 (0.1%) | 4 (0.1%) | .64 | |
| Hospital length of stay, n (%) | 2 or more nights | 980 (52.7%) | 1535 (52.4%) | 2515 (52.5%) | .81 |
| 1 night or same day | 878 (47.3%) | 1395 (47.6%) | 2273 (47.7%) | ||
| No. unique providers visited (claims data) | Mean (SD) | 1.3 (2.1) | 0.8 (1.5) | 1.0 (1.7) | <.0001 |
| No. distinct prescribers (pharmacy data) | Mean (SD) | 1.3 (0.8) | 0.9 (0.6) | 1.07 (0.7) | <.0001 |
| No. unique nonprescribers visited | Mean (SD) | 0.03 (0.2) | 0.02 (0.15) | 0.03 (0.2) | .10 |
Use of opioids ≥ 90 days after discharge from procedure is the measure of "chronic use."
Opioid-naive for analyses defined as 0 opioid day during 90 days prior to surgery.
Presurgical period = 90 days prior to procedure.
Exposure period = 30 days after discharge from procedure.
This category included nonbenzodiazepine sedative/hypnotics and selected anxiolytics.
Patients with ≥ 1 prescription from given source; patients could have multiple prescribers.
Fig 2.
Cohort diagram.
Chronic opioid users were prescribed opioids at a higher rate during the exposure period (30 days after surgery) compared to those who did not continue to receive opioids (88.1% vs 81.3%, P < .0001). Chronic opioid users were slightly older (mean age 37.8 vs 33.1, P < .0001) and were more likely to be co-prescribed medications including antidepressants (17.4% vs 8.4%, P < .0001), benzodiazepines (14.8% vs 3.9%, P < .0001), antipsychotics (4.2% vs 1.8%, P < .0001), and muscle relaxants (7.5% vs 2.0%, P < .0001). In addition, chronic users had a higher comorbidity burden (3.2 vs 2.3 comorbidities, P < .0001) (Table 2).
Table 2.
Comorbidities by chronic opioid outcome, n (%)
| Condition⁎ | Chronic opioid use† | Nonchronic use | Total | P value |
|---|---|---|---|---|
| Congestive heart failure | 87 (4.7%) | 89 (3.0%) | 176 (3.7%) | .0035 |
| Valvular disease | 103 (5.5%) | 82 (2.8%) | 185 (3.9%) | < .0001 |
| Pulmonary circulation disease | 29 (1.6%) | 32 (1.1%) | 61 (1.3%) | .1858 |
| Peripheral vascular disease | 71 (3.8%) | 60 (2.0%) | 131 (2.7%) | .0004 |
| Hypertension without complications | 705 (37.9%) | 832 (28.4%) | 1537 (32.1%) | < .0001 |
| Hypertension with complications | 127 (6.8%) | 137 (4.7%) | 264 (5.5%) | .0018 |
| Paralysis | 23 (1.2%) | 31 (1.1%) | 54 (1.1%) | .5765 |
| Other neurological disorders | 196 (10.5%) | 201 (6.9%) | 397 (8.3%) | < .0001 |
| Chronic pulmonary disease | 518 (27.9%) | 583 (19.9%) | 1101 (23.0%) | < .0001 |
| Diabetes w/o chronic complications | 265 (14.3%) | 287 (9.8%) | 552 (11.5%) | < .0001 |
| Diabetes w/ chronic complications | 150 (8.1%) | 140 (4.8%) | 290 (6.1%) | < .0001 |
| Hypothyroidism | 171 (9.2%) | 168 (5.7%) | 339 (7.1%) | < .0001 |
| Renal failure | 56 (3.0%) | 61 (2.1%) | 117 (2.4%) | .044 |
| Liver disease | 265 (14.3%) | 298 (10.2%) | 563 (11.8%) | < .0001 |
| Peptic ulcer disease | 25 (1.3%) | 28 (1.0%) | 53 (1.1%) | .2565 |
| Acquired immune deficiency syndrome | 12 (0.6%) | 9 (0.3%) | 21 (0.4%) | .1143 |
| Rheumatoid arthritis | 107 (5.8%) | 64 (2.2%) | 171 (3.6%) | < .0001 |
| Coagulopathy | 47 (2.5%) | 52 (1.8%) | 99 (2.1%) | .077 |
| Obesity | 617 (33.2%) | 920 (31.4%) | 1537 (32.1%) | .193 |
| Weight loss | 96 (5.2%) | 113 (3.9%) | 209 (4.4%) | .0351 |
| Fluid and electrolyte disorders | 327 (17.6%) | 403 (13.8%) | 730 (15.2%) | .0004 |
| Chronic blood loss anemia | 102 (5.5%) | 300 (10.2%) | 402 (8.4%) | < .0001 |
| Deficiency anemias | 313 (16.8%) | 478 (16.3%) | 791 (16.5%) | .632 |
| Alcohol abuse | 78 (4.2%) | 70 (2.4%) | 148 (3.1%) | .0006 |
| Substance use disorder | 232 (12.5%) | 197 (6.7%) | 429 (9.0%) | < .0001 |
| Psychoses | 325 (17.5%) | 350 (11.9%) | 675 (14.1%) | < .0001 |
| Depression | 16 (0.9%) | 11 (0.4%) | 27 (0.6%) | .0454 |
| Sleep apnea | 203 (10.9%) | 160 (5.5%) | 363 (7.6%) | < .0001 |
| Mean number of comorbidities | 3.2 (2.8) | 2.3 (2.3) | 2.7 (2.5) | < 0.0001 |
Elixhauser comorbidities as defined by Quan et al.16 Sleep apnea as defined by Oliva et al.22
Use of opioids ≥ 90 days after discharge from procedure is the measure of "chronic use."
Multivariable generalized linear model analysis demonstrated that patients who received ≥ 50 MME/d in the 30 days after surgery had nearly 8 times the odds of chronic opioid use compared to those who received no opioids (OR: 7.76, 95% CI: 3.71–16.26). Those receiving between 20 and 50 MME/d had nearly double the odds for chronic use (OR: 1.88, 95% CI: 1.31–2.71) (Table 3). Patients receiving ≤ 20 MME/d had lower risk of COU (OR 0.56, 95% CI: 0.44–0.72) compared to those who received no opioids. Patients who received opioids prior to surgery had 73% greater odds for chronic use (OR: 1.73, 95% CI: 1.50–1.98). Each additional opioid prescription filled during the exposure period was associated with increased odds of chronic use (OR: 1.71, 95% CI: 1.48–1.98). Those who were rehospitalized > 30 days but ≤ 270 days following discharge had more than 3 times greater odds for chronic use: (OR: 3.69, 95% CI: 2.92–4.66). During the 1-month postsurgery exposure period, prescribing of gabapentin concomitant with opioids was associated with nearly twice the odds of COU (OR: 1.97, 95% CI: 1.31–2.95); for benzodiazepines and muscle relaxants, the odds were 80% higher (OR: 1.80, 95% CI: 1.37–2.38) and 61% (OR: 1.61, 95% CI: 1.11–2.34), respectively. Patients with severe depression (OR 1.21, 95% CI; 1.02–1.43) and sleep apnea (OR; 1.33, 95% CI: 1.01–1.74) also had significantly greater odds of receiving opioids chronically. A separate model using the number of opioid days during the 1-month postsurgery period as the primary exposure produced similar results (Table 3).
Table 3.
Odds of chronic use⁎ following cholecystectomy predicted by logistic model
| Variable | Level |
Opioid exposure model |
|
|---|---|---|---|
|
Odds ratio (95% CI) | |||
| Opioid days exposure | MME exposure | ||
| Opioid naive† | No versus yes | 1.75 (1.52–2.01) | 1.73 (1.5–1.98) |
| Opioid days in 30 d following discharge | 1–5 vs 0 days | 0.60 (0.47–0.77) | |
| 6–10 vs 0 days | 0.70 (0.53–0.93) | ||
| 10 + vs 0 days | 2.42 (1.73–3.4) | ||
| Mean daily MME in 30 d following discharge | 0 < MME < 20 vs 0 MME | 0.56 (0.44–0.72) | |
| 20 ≤ MME < 50 vs 0 MME | 1.88 (1.31–2.71) | ||
| MME ≥ 50 vs 0 MME | 7.76 (3.71–16.3) | ||
| Number of opioid prescriptions during exposure period | Per additional prescription | 1.44 (1.24–1.68) | 1.71 (1.48–1.98) |
| Age category | 35–54 vs 18–30 | 1.21 (1.03–1.42) | 1.2 (1.02–1.41) |
| 55–64 vs 18–30 | 1.33 (0.99–1.79) | 1.36 (1.01–1.82) | |
| 65 + vs 18–30 | 1.30 (0.82–2.07) | 1.25 (0.79–1.98) | |
| Race/ethnicity | Minority race/ethnicity versus white | 0.90 (0.78–1.03) | 0.93 (0.81–1.07) |
| Sex | Female versus male | 0.91 (0.72–1.14) | 0.95 (0.75–1.19) |
| Other medications during exposure period | NSAIDS, APAP | 1.61 (1.26–2.06) | 1.56 (1.22–2) |
| Antipsychotics | 1.05 (0.66–1.65) | 1.16 (0.73–1.82) | |
| Antidepressants | 1.10 (0.88–1.39) | 1.1 0(0.88–1.39) | |
| Gabapentin | 1.77 (1.18–2.66) | 1.97 (1.31–2.95) | |
| Pregabalin | 3.05 (0.86–10.8) | 3.03 (0.84–10.9) | |
| Benzodiazepines | 1.93 (1.47–2.55) | 1.8 (1.37–2.38) | |
| Selected sedatives/hypnotics | 0.80 (0.53–1.22) | 0.76 (0.50–1.16) | |
| Muscle relaxants | 1.55 (1.07–2.26) | 1.61 (1.11–2.34) | |
| Duloxetine | 1.19 (0.63–2.23) | 1.13 (0.59–2.14) | |
| Rehospitalization | During exposure period | 0.66 (0.45–0.97) | 0.68 (0.46–1.01) |
| 31–270 d after discharge | 3.84 (3.03–4.85) | 3.69 (2.92–4.66) | |
| Comorbid conditions‡ | Depression | 1.20 (1.01–1.43) | 1.21 (1.02–1.43) |
| Sleep apnea | 1.38 (1.06–1.80) | 1.33 (1.01–1.74) | |
Use of opioids ≥ 90 days after discharge from procedure indicates "chronic use."
Opioid-naive for analyses defined as 0 opioid day.
Only statistically significant comorbid conditions reported.
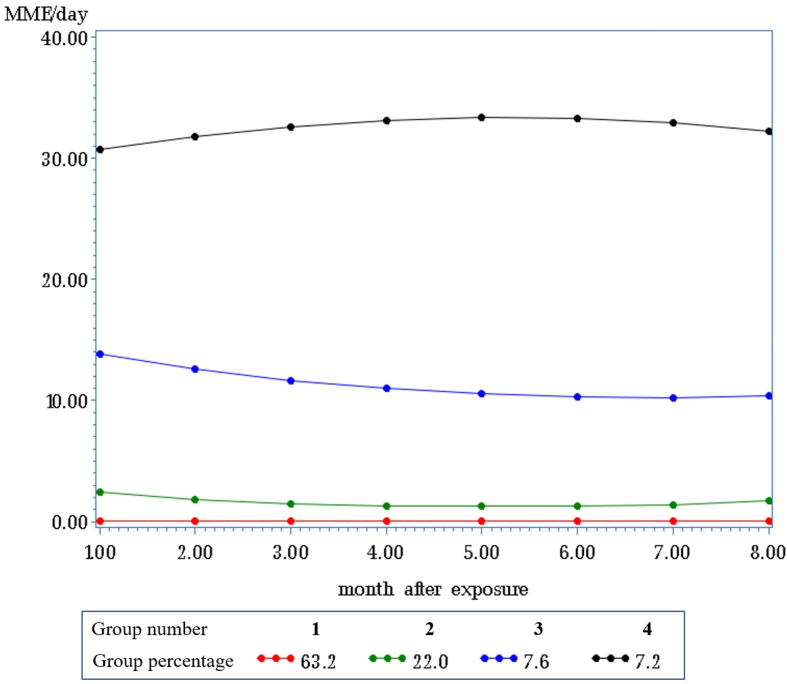
Group-based trajectory analysis demonstrated 4 opioid patterns stratified by MME/d: little to no use (63.2%), low use (22.0%), medium use (7.6%), and high use (7.2%) (Fig 3). Univariate analyses revealed that medium- and high-use groups were somewhat more likely to be male. Patients in the low-, medium-, and high-use groups were older, were more likely to have concomitant medications prescribed with opioids, were more likely to be rehospitalized, and had the highest number of prescribers during the exposure period (Supplementary Table 2).
Fig 3.
Group-based trajectory analyses of cholecystectomy, South Carolina Medicaid, 2014–2017.
In multinomial models predicting group membership (Supplementary Table 3), patients in the high-use group were more likely to be male, to have 1 or more substance use disorders, and to suffer from chronic conditions. Risk factors that predicted inclusion in COU groups include rehospitalization; non-naive opioid status prior to surgery; 10 or more mean opioid days and ≥ 50 MME/d; multiple opioid prescriptions; and prescriptions for NSAIDs, gabapentin, benzodiazepines, or muscle relaxants during the immediate postoperative period.
DISCUSSION
Cholecystectomy is among the most common operations performed in the United States [5,21]. It is the most common operation performed in Medicaid and self-insured patients, but little is known about opioid use associated with cholecystectomy in this population [6]. This study reveals high opioid prescribing before and sustained prescribing after cholecystectomy in SC Medicaid patients. Of particular concern are patients who were opioid naive prior to surgery. More than a quarter of these patients (27.1%) continued to receive opioids chronically. Surgery may be an initial exposure gateway to chronic opioid use [4]. In a related study, Brummett et al. reported that less than 6% of patients became persistent opioid users after cholecystectomy. Their study used a commercial insurance data source and a 180-day time frame postsurgery, included only patients who were opioid naive and who had postsurgery opioid exposure, and excluded those with subsequent surgeries. For comparison purposes, we analyzed a subset of surgeries in our study that most closely matched these more restrictive criteria and applied the shorter 180-day postoperative time frame. We found that 18.8% of previously opioid-naive Medicaid patients in this subset continued to receive opioids chronically as compared to the commercially insured patients (6%) in the cohort of Brummett et al [12]. Although a more direct comparison of these studies is not feasible, these findings suggest the possibility of clinically relevant differences in chronic opioid use between patients who have commercial insurance and those who have Medicaid in SC.
More than half (54.4%) of patients in this study received opioid prescriptions prior to surgery, but the data are not sufficient to identify the clinical indications for these prescriptions. Most prescriptions were for less than 49 MME/d. Of the 1,248 (26.1%) patients who received opioids in the 2 weeks leading up to surgery, those who received them from a primary care provider had a statistically significant increased risk of continuing to receive opioids 90–270 days after discharge. If preoperative opioid prescriptions came from an ED provider or surgeon, no statistically significant increased risk of chronic opioid prescribing was seen. The meaning of these findings is not clear. Some surgeons provide prescriptions intended for postoperative use prior to surgery, so patients do not have to worry about picking up a prescription in the postoperative period. The results of our study suggest that those prescriptions may not contribute significantly to COU. The primary indication to perform cholecystectomy is pain related to the gallbladder. Accordingly, patients may have received opioid prescriptions from primary care or ED providers while awaiting referral to a surgeon. The prescription information available in our data set does not provide indications for opioid prescriptions, so the data are insufficient to determine why preoperative prescriptions from primary care are associated with increased odds of chronic opioid prescribing relative to when these prescriptions are provided by an ED provider or surgeon. Our data suggest that all providers who care for surgical patients should be aware of the risks of opioid prescribing and use nonopioid pain management regimens when possible.
Regardless of provider type, patients who received ≥ 50 MME/d after surgery had nearly 8 times the odds of chronic opioid use compared to those who received no opioids following surgery (OR: 7.76, 95% CI: 3.71–16.26). These may represent patients with known pain control problems or significant comorbidities. Even patients who received fairly low (20–50 MME/d) amounts of opioids after surgery had nearly double the odds of chronic opioid use (OR: 1.88, 95% CI: 1.31–2.71) (Table 3). In addition, patients who received opioids before surgery had a 73% greater odds of continuing to receive opioid prescriptions chronically (OR: 1.73, 95% CI: 1.50–1.98). Each additional opioid prescription filled within 30 days of discharge was associated with increased odds of chronic use (OR: 1.71, 95% CI: 1.48–1.98). Our data are insufficient to determine the causative factors of these associations, but knowledge that the associations exist may help prescribers tailor prescribing practices more intelligently.
We identified 4 major trajectories of opioid prescribing after surgery: none, low, medium, and high. Patients in the high-prescribing group were more likely to be male, to have 1 or more substance use disorder diagnosis, and to suffer from chronic comorbid conditions. Risk factors during the exposure period that predicted inclusion in chronic use groups include rehospitalization; non-naive opioid status prior to surgery; 10 or more mean opioid days during exposure period; ≥ 50 MME/d prescribed during the immediate postoperative period; multiple opioid prescriptions; and prescriptions for NSAIDs, gabapentin, benzodiazepines, or muscle relaxants. Knowledge of these factors may help providers identify patients at high risk for chronic opioid use after surgery.
These data suggest that efforts to use opioid-sparing pain management practices should occur both before and after surgery as both time frames may impact the odds of long-term opioid use. Chronic opioid prescribing should be avoided when possible as it is known to result in more hospitalizations and provider visits [13]. The economic impact of chronic opioid use is substantial. Persistent opioid use is associated with $1,500 to $2,700 in additional health care spending with an additional $200 per month of sustained spending beyond 6 months following surgery [22]. In addition, patients may go on to misuse or abuse prescriptions and may transition to illicit drug use.
The high incidence of biliary disease in the United States, the significant cost of chronic opioid use, the high rate of opioid prescribing in Medicaid patients shown in this study, and the role of taxpayers in funding these patients suggest that additional efforts are needed to improve prescribing practices in the perioperative period to focus on nonopioid pain control measures. By using opioid-sparing prescribing methods, providers may be able to decrease the risk of chronic opioid use after surgery. Targeting risk mitigation to specific patient populations at highest risk for chronic opioid use may be beneficial. South Carolina initiated efforts to improve prescribing in 2018 through the South Carolina Surgical Quality Collaborative. Member facilities successfully decreased opioid prescribing after common general surgical procedures including cholecystectomy. Between 2018 and 2020, the average number of opioids prescribed was cut in half for most procedures by the Collaborative. Given the high level of opioid prescriptions seen in this study, Medicaid patients may benefit from targeted efforts to use opioid-sparing techniques perioperatively.
This study has several limitations. The claims data do not provide adequate information to determine the specific reasons why patients received opioids prior to or after surgery. This was an association study only. Causation should not be inferred from this study. There is insufficient information from our data set to make determinations about the actual causes of continued opioid prescribing after cholecystectomy. We use the term chronic opioid use as this term is consistent with existing literature; however, data are only available on dispensed opioid prescriptions which may not represent actual opioid consumption. The accuracy of administrative claims data is dependent on chart documentation and coding practices, both of which have limitations and potential for error. Though included in our model, postoperative provider visit data are difficult to interpret because of the global period associated with surgical charges. Methodologic differences and inconsistencies in variable definitions exist in the literature, making comparisons between studies limited. Calculation of MME/d was done mathematically and may not accurately reflect the actual MME consumed per day.
In conclusion, more than half (54.4%) of South Carolina Medicaid patients who underwent cholecystectomy between 2014 and 2017 received opioids prior to surgery. The majority (75.2%) of preoperative prescriptions came from nonsurgeon providers. Almost 40% of patients continue to receive opioid prescriptions well after surgical recovery. Higher MME/d postoperative prescriptions were associated with higher odds of continuing to receive opioids chronically. Even among patients who did not receive any opioids prior to surgery, 27.1% continued to fill opioid prescriptions 90–270 days after surgery.
Author Contribution
Study conception and design: Lockett, Ward, Ball, McCauley, Taber, Gebregziabher, Mauldin.
Acquisition of data: Ball, Ward.
Analysis and interpretation of data: Lockett, Ward, Ball, McCauley, Taber, Gebregziabher, Cina, Basco, Mauldin.
Drafting of manuscript: Lockett, Ward, Ball, McCauley, Taber, Gebregziabher, Cina, Basco, Mauldin.
Critical revision: Lockett, Ward, Ball.
Conflict of Interest
S Ball, R Ward, and M Lockett receive salary support from the SC Department of Health and Human Services grant. All additional authors have no disclosures.
Funding Source
Data used in this project were received through a contract (A201912450A) with the SC Department of Health and Human Services for Drug Utilization Review services and supported in part by the NIH National Institute on Drug Abuse grant DA0036566 NIH and the National Center for Advancing Translational Sciences through grant number UL1 TR001450. None of the funding agencies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethics Approval
The Medical University of South Carolina Institutional Review Board provided exempt review for this investigation.
Acknowledgments
This effort would not have been possible without the knowledge and assistance of the South Carolina Department of Health and Human Services.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sopen.2022.05.009.
Appendix A. Supplementary data
Supplementary Tables: Cholecystectomy phenotype, trajectory group demographics, trajectory group membership
References
- 1.Hedegaard H., Miniño A.M., Warner M. National Center for Health Statistics; Hyattsville, MD: 2020. Drug overdose deaths in the United States, 1999–2018.pdf icon NCHS data brief, no 356. [Google Scholar]
- 2.https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 3.Wilson N., Kariisa M., Seth P., et al. Drug and opioid-involved overdose deaths-United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waljee J.F., Li L., Brummett C.M., Englesbe M.J. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017;265(4):728–730. doi: 10.1097/SLA.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Rep. 2017;(102) [PubMed] [Google Scholar]
- 6.Lopez-Gonzalez L., Pickens G.T., Washington R., Weiss A.J. Agency for Healthcare Research and Quality; Rockville, MD: 2014. Characteristics of Medicaid and uninsured hospitalizations, 2012. (HCUP statistical brief #183). [PubMed] [Google Scholar]
- 7.Lee J.S., Parashar V., Miller J.B., et al. Opioid prescribing after curative-intent surgery: a qualitative study using the theoretical domains framework. Ann Surg Oncol. 2018;25(7):1843–1851. doi: 10.1245/s10434-018-6466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbaugh C.M., Lee J.S., Hu H.M., et al. Persistent opioid use among pediatric patients after surgery. Pediatrics. 2018;141(1) doi: 10.1542/peds.2017-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brescia A.A., Waljee J.F., Hu H.M., et al. Impact of prescribing on new persistent opioid use after cardiothoracic surgery. Ann Thorac Surg. 2019;108(4):1107–1113. doi: 10.1016/j.athoracsur.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santosa K.B., Hu H.M., Brummett C.M., et al. New persistent opioid use among older patients following surgery: a Medicare claims analysis. Surgery. 2020;167(4):732–742. doi: 10.1016/j.surg.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hah J.M., Bateman B.T., Ratliff J., Curtin C., Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1569–1587. doi: 10.1213/ANE.0000000000002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummett C.M., Waljee J.F., Goesling J., et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6) doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard 1 2 3 Ryan, Gunaseelan 2 3 Vidhya, Brummett 2 4 Chad, Waljee 1 2 3 Jennifer, Englesbe 1 2 3 Michael, Telem 1 3 Dana. New persistent opioid use after inguinal hernia repair. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.S., Vu J.V., Edelman A.L., et al. Health care spending and new persistent opioid use after surgery. Ann Surg. 2020;272(1):99–104. doi: 10.1097/SLA.0000000000003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basco W.T., Ward R.C., Taber D.J., Simpson K.N., Gebregziabher M., Cina R.A., et al. Patterns of dispensed opioids after tonsillectomy in children and adolescents in South Carolina, United States, 2010–2017. Int J Pediatr Otorhinolaryngol. 2021;143:110636. doi: 10.1016/j.ijporl.2021.110636. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Centers for Disease Control and Prevention Opioid overdose data resources. 2019. https://www.cdc.gov/drugoverdose/resources/data.html
- 17.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.-C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 18.Nagin D. Harvard University Press; Cambridge, Mass: 2005. Group-based modeling of development. [Google Scholar]
- 19.Jones B.L., Nagin D.S. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35(4):542–571. [Google Scholar]
- 20.Nagin D.S., Odgers C.L. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6(1):109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 21.Characteristics of oOperating rRoom pProcedures in U.S Hospitals 2011—statistical brief #170. www.hcup-us.ahrq.gov
- 22.Florence C.S., Zhou C., Luo F., Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901–906. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables: Cholecystectomy phenotype, trajectory group demographics, trajectory group membership